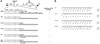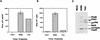Mutations of the human immunodeficiency virus type 1 p6Gag domain result in reduced retention of Pol proteins during virus assembly - PubMed (original) (raw)
Mutations of the human immunodeficiency virus type 1 p6Gag domain result in reduced retention of Pol proteins during virus assembly
X F Yu et al. J Virol. 1998 Apr.
Abstract
One of the crucial steps in the assembly of the human immunodeficiency virus type 1 (HIV-1) and other retroviruses is the incorporation and retention of all the key viral enzymes in released virions. The viral enzymes protease, reverse transcriptase, and integrase of HIV-1 are initially synthesized as Gag-Pol fusion polyproteins. It has been shown that the incorporation of Gag-Pol polyproteins during virus assembly requires the Gag domains that are shared by the Gag and Gag-Pol precursors. We now report that truncation of the C-terminal p6 domain of HIV-1 Gag, which is present in the Gag precursor but not in the Gag-Pol precursor, drastically reduced the amount of Pol proteins in the mutant virions. Mutations in the lentivirus conserved motif P(T/S)APP in p6 also drastically reduced the amount of Pol proteins in mutant virions. The steady-state levels of Gag-Pol precursors and cleaved Pol proteins in the transfected cells were not affected by mutations in p6. The incorporation of unprocessed Gag-Pol precursors into p6 mutant virions was detected when the viral protease was mutated, suggesting that the interactions among mutant Gag molecules and Gag-Pol precursors were not significantly affected. These results suggest that the p6 domain of HIV-1 Gag may play an important role in recruiting or retaining cleaved Pol proteins during virus assembly.
Figures
FIG. 1
Construction of HIV-1 mutants. (A) The top diagram shows the genome organization of the HIV-1 parental construct HXB2. Mutants were constructed as described in the text. The TAA construct contains a premature stop codon that truncates all but one amino acid of p6. The LTALL construct contains amino acid substitutions of leucine for prolines 7, 10, and 11 in HIV-1 p6, and the amino acid sequences expressed from the overlapping pol open reading frame in LTALL are intact. The constructs PTAP−, PR−, and PR−/PTAP− have been described previously (13). Diagonally striped boxes, Pol domains in Gag-Pol precursor. (B) The nucleotide sequences and corresponding amino acid sequences in Gag (p6) and Pol for the wild type (WT) and the p6-mutant constructs are shown. Nucleotides and amino acids that differ from those in the wild-type sequences are underlined.
FIG. 2
Analysis of virus production by p24 assay, RT assay, and immunoblot. Virions were purified and analyzed as previously described (40). (A) In the p24 assay, bars represent averages from five replicates and error bars show standard deviations; error bars for TAA are too small to be seen. (B) In the RT assay, bars represent averages from triplicates and error bars show standard deviations; error bars for COS-7 and TAA are too small to be seen. (C and D) For immunoblots, viral lysates were separated by SDS-12% PAGE, transferred onto nitrocellulose filters, and blotted with an HIV-1-positive human serum. (D) Alternatively, lysates were blotted with a MAb against HIV-1 RT (purchased from Biotechnology Transfer, Inc. [Columbia, Md.]) or an antiserum to HIV-1 IN (catalog no. 757; obtained through the AIDS Research Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases). The arrow indicates the position of the p6-truncated Gag precursor molecule. Lane C, viral lysate from COS cells transfected with an HIV-1 protease-mutant virus which contains only unprocessed Pr55Gag and Pr160Gag-Pol precursors.
FIG. 2
Analysis of virus production by p24 assay, RT assay, and immunoblot. Virions were purified and analyzed as previously described (40). (A) In the p24 assay, bars represent averages from five replicates and error bars show standard deviations; error bars for TAA are too small to be seen. (B) In the RT assay, bars represent averages from triplicates and error bars show standard deviations; error bars for COS-7 and TAA are too small to be seen. (C and D) For immunoblots, viral lysates were separated by SDS-12% PAGE, transferred onto nitrocellulose filters, and blotted with an HIV-1-positive human serum. (D) Alternatively, lysates were blotted with a MAb against HIV-1 RT (purchased from Biotechnology Transfer, Inc. [Columbia, Md.]) or an antiserum to HIV-1 IN (catalog no. 757; obtained through the AIDS Research Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases). The arrow indicates the position of the p6-truncated Gag precursor molecule. Lane C, viral lysate from COS cells transfected with an HIV-1 protease-mutant virus which contains only unprocessed Pr55Gag and Pr160Gag-Pol precursors.
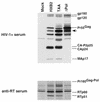
FIG. 3
Immunoblot of intracellular viral proteins in transfected COS-7 cells. Cell lysates from mock-, HXB2-, and TAA-transfected COS-7 cells were electrophoresed and transferred to nitrocellulose filters. Lysates on one filter were blotted with the human HIV-1-positive serum (upper panel), and those on the other were blotted with the MAb against HIV-1 RT (lower panel). Arrow in upper panel points to a truncated Gag precursor molecule.
FIG. 4
Intracellular and virion-associated viral proteins from transfected COS-7 cells. (A) Cell and viral lysates (left and right panels, respectively) were obtained from mock-, wild-type-, and LTALL-transfected COS-7 cells and were blotted with the human HIV-1-positive serum. (B) Viral lysates were obtained from mock-, wild-type-, and PTAP−-transfected COS-7 cells and blotted with the human HIV-1-positive serum (upper panel) or the MAb against HIV-1 RT (lower panel). (C) Viral lysates were obtained from mock-, PR−-, and PR−/PTAP−-transfected COS-7 cells and were blotted with the human HIV-1-positive serum (upper panel) or the MAb against HIV-1 RT (lower panel). The amounts of viral proteins (p24 equivalent) for PR− and PR−/PTAP− are indicated.
FIG. 4
Intracellular and virion-associated viral proteins from transfected COS-7 cells. (A) Cell and viral lysates (left and right panels, respectively) were obtained from mock-, wild-type-, and LTALL-transfected COS-7 cells and were blotted with the human HIV-1-positive serum. (B) Viral lysates were obtained from mock-, wild-type-, and PTAP−-transfected COS-7 cells and blotted with the human HIV-1-positive serum (upper panel) or the MAb against HIV-1 RT (lower panel). (C) Viral lysates were obtained from mock-, PR−-, and PR−/PTAP−-transfected COS-7 cells and were blotted with the human HIV-1-positive serum (upper panel) or the MAb against HIV-1 RT (lower panel). The amounts of viral proteins (p24 equivalent) for PR− and PR−/PTAP− are indicated.
FIG. 4
Intracellular and virion-associated viral proteins from transfected COS-7 cells. (A) Cell and viral lysates (left and right panels, respectively) were obtained from mock-, wild-type-, and LTALL-transfected COS-7 cells and were blotted with the human HIV-1-positive serum. (B) Viral lysates were obtained from mock-, wild-type-, and PTAP−-transfected COS-7 cells and blotted with the human HIV-1-positive serum (upper panel) or the MAb against HIV-1 RT (lower panel). (C) Viral lysates were obtained from mock-, PR−-, and PR−/PTAP−-transfected COS-7 cells and were blotted with the human HIV-1-positive serum (upper panel) or the MAb against HIV-1 RT (lower panel). The amounts of viral proteins (p24 equivalent) for PR− and PR−/PTAP− are indicated.
Similar articles
- Proline residues in human immunodeficiency virus type 1 p6(Gag) exert a cell type-dependent effect on viral replication and virion incorporation of Pol proteins.
Dettenhofer M, Yu XF. Dettenhofer M, et al. J Virol. 1999 Jun;73(6):4696-704. doi: 10.1128/JVI.73.6.4696-4704.1999. J Virol. 1999. PMID: 10233929 Free PMC article. - Effects of human immunodeficiency virus type 1 transframe protein p6* mutations on viral protease-mediated Gag processing.
Chiu HC, Wang FD, Chen YA, Wang CT. Chiu HC, et al. J Gen Virol. 2006 Jul;87(Pt 7):2041-2046. doi: 10.1099/vir.0.81601-0. J Gen Virol. 2006. PMID: 16760407 - Effects of mutations in Pr160gag-pol upon tRNA(Lys3) and Pr160gag-plo incorporation into HIV-1.
Mak J, Khorchid A, Cao Q, Huang Y, Lowy I, Parniak MA, Prasad VR, Wainberg MA, Kleiman L. Mak J, et al. J Mol Biol. 1997 Jan 31;265(4):419-31. doi: 10.1006/jmbi.1996.0742. J Mol Biol. 1997. PMID: 9034361 - Proteolytic processing of foamy virus Gag and Pol proteins.
Flügel RM, Pfrepper KI. Flügel RM, et al. Curr Top Microbiol Immunol. 2003;277:63-88. doi: 10.1007/978-3-642-55701-9_3. Curr Top Microbiol Immunol. 2003. PMID: 12908768 Review. - The packaging and maturation of the HIV-1 Pol proteins.
Hill M, Tachedjian G, Mak J. Hill M, et al. Curr HIV Res. 2005 Jan;3(1):73-85. doi: 10.2174/1570162052772942. Curr HIV Res. 2005. PMID: 15638725 Review.
Cited by
- How HIV-1 Gag Manipulates Its Host Cell Proteins: A Focus on Interactors of the Nucleocapsid Domain.
Klingler J, Anton H, Réal E, Zeiger M, Moog C, Mély Y, Boutant E. Klingler J, et al. Viruses. 2020 Aug 13;12(8):888. doi: 10.3390/v12080888. Viruses. 2020. PMID: 32823718 Free PMC article. Review. - Efficient particle production by minimal Gag constructs which retain the carboxy-terminal domain of human immunodeficiency virus type 1 capsid-p2 and a late assembly domain.
Accola MA, Strack B, Göttlinger HG. Accola MA, et al. J Virol. 2000 Jun;74(12):5395-402. doi: 10.1128/jvi.74.12.5395-5402.2000. J Virol. 2000. PMID: 10823843 Free PMC article. - Evolutionarily conserved requirement for core binding factor beta in the assembly of the human immunodeficiency virus/simian immunodeficiency virus Vif-cullin 5-RING E3 ubiquitin ligase.
Han X, Liang W, Hua D, Zhou X, Du J, Evans SL, Gao Q, Wang H, Viqueira R, Wei W, Zhang W, Yu XF. Han X, et al. J Virol. 2014 Mar;88(6):3320-8. doi: 10.1128/JVI.03833-13. Epub 2014 Jan 3. J Virol. 2014. PMID: 24390335 Free PMC article. - Functional complementation of nucleocapsid and late domain PTAP mutants of human immunodeficiency virus type 1 during replication.
Nikolaitchik OA, Gorelick RJ, Leavitt MG, Pathak VK, Hu WS. Nikolaitchik OA, et al. Virology. 2008 Jun 5;375(2):539-49. doi: 10.1016/j.virol.2008.02.026. Epub 2008 Mar 19. Virology. 2008. PMID: 18353416 Free PMC article. - Higher-order oligomerization targets plasma membrane proteins and HIV gag to exosomes.
Fang Y, Wu N, Gan X, Yan W, Morrell JC, Gould SJ. Fang Y, et al. PLoS Biol. 2007 Jun;5(6):e158. doi: 10.1371/journal.pbio.0050158. PLoS Biol. 2007. PMID: 17550307 Free PMC article.
References
- Ahsari-Lari M A, Dohehower L A, Gibbs R A. Analysis of human immunodeficiency virus type 1 (HIV-1) integrase mutants. Virology. 1995;211:332–335. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials