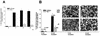The Syk protein tyrosine kinase is essential for Fcgamma receptor signaling in macrophages and neutrophils - PubMed (original) (raw)
The Syk protein tyrosine kinase is essential for Fcgamma receptor signaling in macrophages and neutrophils
F Kiefer et al. Mol Cell Biol. 1998 Jul.
Abstract
The cytoplasmic protein tyrosine kinase Syk has two amino-terminal SH2 domains that engage phosphorylated immunoreceptor tyrosine-based activation motifs in the signaling subunits of immunoreceptors. Syk, in conjunction with Src family kinases, has been implicated in immunoreceptor signaling in both lymphoid and myeloid cells. We have investigated the role of Syk in Fcgamma receptor (FcgammaR)-dependent and -independent responses in bone marrow-derived macrophages and neutrophils by using mouse radiation chimeras reconstituted with fetal liver cells from Syk-/- embryos. Chimeric mice developed an abdominal hemorrhage starting 2 to 3 months after transplantation that was ultimately lethal. Syk-deficient neutrophils derived from the bone marrow were incapable of generating reactive oxygen intermediates in response to FcgammaR engagement but responded normally to tetradecanoyl phorbol acetate stimulation. Syk-deficient macrophages were defective in phagocytosis induced by FcgammaR but showed normal phagocytosis in response to complement. The tyrosine phosphorylation of multiple cellular polypeptides, including the FcgammaR gamma chain, as well as Erk2 activation, was compromised in Syk-/- macrophages after FcgammaR stimulation. In contrast, the induction of nitric oxide synthase in macrophages stimulated with lipopolysaccharide and gamma interferon was not dependent on Syk. Surprisingly, Syk-deficient macrophages were impaired in the ability to survive or proliferate on plastic petri dishes. Taken together, these results suggest that Syk has specific physiological roles in signaling from FcgammaRs in neutrophils and macrophages and raise the possibility that in vivo, Syk is involved in signaling events other than those mediated by immunoreceptors.
Figures
FIG. 1
Transplantation model for the generation of _Syk_−/− bone marrow macrophages. Timed intercrosses of mice heterozygous for a Syk null allele were sacrificed at midgestation. Fetal liver (FL) of recovered embryos was used to reconstitute lethally irradiated recipients. Part of the remaining embryos served to confirm the genotype at the Syk locus by Southern blotting. Starting at 6 weeks after the bone marrow graft, the extent of recipient repopulation was monitored from a drop of peripheral (periph.) blood by using the GPI isoenzyme marker. Donor embryos were of the isoenzyme type GPI-1AA, while recipients were of the GPI-1BB isoenzyme type. The peripheral blood GPI type of all recipients was completely converted to the donor type. A perfused liver sample taken at the same time served as a negative control. The mixed isoenzyme type of spleen and thymus is due to graft-derived functional precursors (GPI-1AA) and host-derived stromal components (GPI-1BB).
FIG. 2
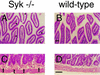
Diffuse bleeding in the intestinal mucosa of bone marrow chimeras transplanted with _Syk_−/− fetal liver. Mice were reconstituted with _Syk_−/− (A and C) or wild-type (B and D) fetal liver. A and B are transverse sections through intestinal villi, while C and D show longitudinal sections through the lamina propria. Mice reconstituted with Syk-deficient bone marrow frequently show dilation of the central villus vessel (arrowheads in A) and display diffuse bleeding at discrete spots in the lamina propria (arrows in C). Pictures show eosin-hematoxilin-stained 5-μm sections; the bar corresponds to 250 μm.
FIG. 3
Quantitative analysis of H2O2 production by neutrophils during the oxidative burst following IgG or TPA stimulation. Primary bone marrow neutrophils were derived from either wild-type or _Syk_−/− bone marrow chimeras. Cells were incubated with IgG-opsonized zymosan particles, and H2O2 production was determined by using horseradish peroxidase-catalyzed oxidation of scopoletin. The ability to generate a response to an FcγR-independent signal was tested by exposure of an equivalent cell sample to 0.1 μM TPA. Each bar represents the mean and error range of two independent experiments.
FIG. 4
Syk-deficient macrophages plated on petri dishes display reduced adherence and proliferation. The pictures on the left are phase-contrast images of wild-type and Syk−/− bone marrow after a 10-day culture period in plastic petri dishes in the presence of M-CSF. Those on the right are phase-contrast micrographs of the same cultures after collection of the adherent fraction and reseeding onto glass coverslips. Bar, 500 μm.
FIG. 5
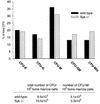
Colony assay of bone marrow derived from wild-type and Syk−/− fetal liver repopulated recipients. Single-cell bone marrow suspensions were seeded into semisolid medium in the presence of IL-1α, IL-3, IL-11, Kit ligand, and erythropoietin. After 10 days, colonies were typed and counted. CFU-E, erythrocyte burst-forming unit; CFU-G, granulocyte CFU; CFU-M, macrophage CFU; CFU-GM, granulocyte-macrophage CFU; CFU-Mix, multilineage CFU (colonies consist of erythrocytes and at least two additional myeloid lineages).
FIG. 6
The capacity of _Syk_−/− macrophages to bind to IgG-RBC is not impaired. IgG-RBC were exposed to wild-type and _Syk_−/− macrophages on glass coverslips and allowed to bind at 0°C for 10 min. Free RBC were removed, and the percentage of cells that had bound two or more opsonized particles was determined. BSA-coated RBC served as a control for nonspecific binding. The corresponding phase micrographs are on the right. Bound IgG-RBC are visible as perfectly round, sharply demarcated, bright circles. The diagram represents the average of five independent experiments. n is the total number of cells evaluated. Bar, 500 μm.
FIG. 7
Phagocytosis of IgG-RBC is selectively impaired in Syk-deficient macrophages. (A) Time course of phagocytosis of IgG-RBC by wild-type and _Syk_−/− macrophages. IgG-RBC were exposed to wild-type and _Syk_−/− macrophages on glass coverslips, and the percentage of cells that had taken up opsonized particles was determined after the indicated incubation times. Both wild-type and Syk-deficient macrophages showed maximal phagocytosis after 10 min; however, the extent of uptake was greatly reduced in Syk-deficient cells. (B) Quantitative analysis of the capacity of wild-type and _Syk_−/− macrophages to phagocytose IgG-RBC. IgG-RBC were allowed to bind to wild-type and _Syk_−/− macrophages, and after incubation at 37°C for the indicated intervals, incompletely phagocytosed RBC were lysed by hypotonic shock. The bars depict the percentage of macrophages that had taken up two or more opsonized particles. The photographs show the corresponding phase micrographs. n is the total number of evaluated cells. Bar, 500 μm.
FIG. 8
_Syk_−/− macrophages display normal phagocytosis of serum-coated particles. Wild-type and _Syk_−/− macrophages on glass coverslips were tested for the capacity to phagocytose opsonized zymosan particles in the presence of the fluorescent dye Lucifer Yellow. After 10 min of incubation, successful completion of the phagocytic process was determined by colocalization of zymosan and trapped Lucifer Yellow in sealed phagosomes. The percentage of phagocytosis-positive macrophages is depicted in the bar graph. The photographs show differential interference contrast (DIC) and corresponding fluorescence pictures. Arrows indicate phagocytic events. Bar, 500 μm.
FIG. 9
Syk-deficient macrophages fail to phosphorylate specific proteins on tyrosine in response to FcγR stimulation. Bone marrow-derived macrophages on tissue culture dishes were stimulated with IgG-RBC (see Fig. 6 and 7). After the indicated incubation times, cells were lysed and either analyzed directly by blotting with antiphosphotyrosine antibody (left panel) or immunoprecipitated (IP) with antiphosphotyrosine (pTyr) antibody prior to immunoblotting. WT, wild type. The values on the left are molecular masses in kilodaltons.
FIG. 10
(A) FcγR γ-chain phosphorylation is not detectable in Syk-deficient macrophages. The presence of Syk (top row) and the FcγR γ chain (bottom row) and their tyrosine-phosphorylated forms in bone marrow macrophages stimulated with IgG-RBC was tested by Western blotting. FcγR γ-chain samples were separated on a 15% gel. IP, immunoprecipitation; WT, wild type. (B) Syk phosphorylates a peptide corresponding to the ITAM of the FcR γ chain efficiently in vitro. Lck- and Myc-tagged versions of Syk and ZAP-70 were expressed transiently in Cos-1 cells. The kinases were immunoprecipitated from cell lysates by using either anti-Myc (Syk, ZAP-70) or anti-Lck antibodies, and immune complex kinase reactions were performed in the presence of a peptide corresponding to the first ITAM of the TCR ζ chain or the ITAM of the FcR γ chain. A peptide derived from the carboxy-terminal sequence of Syk served as a control substrate. Reaction products were separated on a 16.5% Tricine gel and visualized by autoradiography. The values on the left are molecular masses in kilodaltons.
FIG. 11
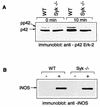
(A) _Syk_−/− macrophages display reduced phosphorylation of p42 Erk-2 after FcγR engagement. Activation of Erk-2 MAPK was visualized by the phosphorylation-induced mobility shift. Macrophages were allowed to bind to IgG-RBC, and after the indicated incubation times, cells were lysed and tested for the presence of doubly threonine-tyrosine-phosphorylated forms of Erk-2 by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. (B) Induction of NOS2 (iNOS) by LPS-gamma interferon is normal in Syk-deficient macrophages. Macrophage cultures were induced with 10-μg/ml LPS and 2-U/ml gamma interferon for 16 h. At the end of the incubation period, cells were lysed and probed for the presence of NOS2 by Western blotting. WT, wild type.
Similar articles
- A critical role for Syk in signal transduction and phagocytosis mediated by Fcgamma receptors on macrophages.
Crowley MT, Costello PS, Fitzer-Attas CJ, Turner M, Meng F, Lowell C, Tybulewicz VL, DeFranco AL. Crowley MT, et al. J Exp Med. 1997 Oct 6;186(7):1027-39. doi: 10.1084/jem.186.7.1027. J Exp Med. 1997. PMID: 9314552 Free PMC article. - Syk-dependent signaling pathways in neutrophils and macrophages are indispensable in the pathogenesis of anti-collagen antibody-induced arthritis.
Ozaki N, Suzuki S, Ishida M, Harada Y, Tanaka K, Sato Y, Kono T, Kubo M, Kitamura D, Encinas J, Hara H, Yoshida H. Ozaki N, et al. Int Immunol. 2012 Sep;24(9):539-50. doi: 10.1093/intimm/dxs078. Int Immunol. 2012. PMID: 22914861 - Differential kinase requirements in human and mouse Fc-gamma receptor phagocytosis and endocytosis.
Huang ZY, Barreda DR, Worth RG, Indik ZK, Kim MK, Chien P, Schreiber AD. Huang ZY, et al. J Leukoc Biol. 2006 Dec;80(6):1553-62. doi: 10.1189/jlb.0106019. Epub 2006 Sep 11. J Leukoc Biol. 2006. PMID: 16921024 - The molecular dissection of Fc gamma receptor mediated phagocytosis.
Indik ZK, Park JG, Hunter S, Schreiber AD. Indik ZK, et al. Blood. 1995 Dec 15;86(12):4389-99. Blood. 1995. PMID: 8541526 Review. - Tyrosine phosphorylation and Fcgamma receptor-mediated phagocytosis.
Strzelecka A, Kwiatkowska K, Sobota A. Strzelecka A, et al. FEBS Lett. 1997 Jan 2;400(1):11-4. doi: 10.1016/s0014-5793(96)01359-2. FEBS Lett. 1997. PMID: 9000504 Review.
Cited by
- Maternal antibodies facilitate Amyloid-β clearance by activating Fc-receptor-Syk-mediated phagocytosis.
Illouz T, Nicola R, Ben-Shushan L, Madar R, Biragyn A, Okun E. Illouz T, et al. Commun Biol. 2021 Mar 12;4(1):329. doi: 10.1038/s42003-021-01851-6. Commun Biol. 2021. PMID: 33712740 Free PMC article. - Back to the future: oral targeted therapy for RA and other autoimmune diseases.
O'Shea JJ, Laurence A, McInnes IB. O'Shea JJ, et al. Nat Rev Rheumatol. 2013 Mar;9(3):173-82. doi: 10.1038/nrrheum.2013.7. Epub 2013 Feb 19. Nat Rev Rheumatol. 2013. PMID: 23419429 Free PMC article. Review. - Immune complexes and late complement proteins trigger activation of Syk tyrosine kinase in human CD4(+) T cells.
Chauhan AK, Moore TL. Chauhan AK, et al. Clin Exp Immunol. 2012 Feb;167(2):235-45. doi: 10.1111/j.1365-2249.2011.04505.x. Clin Exp Immunol. 2012. PMID: 22235999 Free PMC article. - The selective inhibition of the Syk tyrosine kinase ameliorates experimental autoimmune arthritis.
Káposztás E, Balogh L, Mócsai A, Kemecsei É, Jakus Z, Németh T. Káposztás E, et al. Front Immunol. 2023 Dec 4;14:1279155. doi: 10.3389/fimmu.2023.1279155. eCollection 2023. Front Immunol. 2023. PMID: 38111569 Free PMC article. - Large-scale death of retinal astrocytes during normal development is non-apoptotic and implemented by microglia.
Puñal VM, Paisley CE, Brecha FS, Lee MA, Perelli RM, Wang J, O'Koren EG, Ackley CR, Saban DR, Reese BE, Kay JN. Puñal VM, et al. PLoS Biol. 2019 Oct 18;17(10):e3000492. doi: 10.1371/journal.pbio.3000492. eCollection 2019 Oct. PLoS Biol. 2019. PMID: 31626642 Free PMC article.
References
- Beaven M A, Baumgartner R A. Downstream signals initiated in mast cells by Fc epsilon RI and other receptors. Curr Opin Immunol. 1996;8:766–772. - PubMed
- Cheng A M, Chan A C. Protein tyrosine kinases in thymocyte development. Curr Opin Immunol. 1997;9:528–533. - PubMed
- Cheng A M, Rowley B, Pao W, Hayday A, Bolen J B, Pawson T. Syk tyrosine kinase required for mouse viability and B-cell development. Nature. 1995;378:303–306. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous