Characterization of neurotrophin and Trk receptor functions in developing sensory ganglia: direct NT-3 activation of TrkB neurons in vivo - PubMed (original) (raw)
Characterization of neurotrophin and Trk receptor functions in developing sensory ganglia: direct NT-3 activation of TrkB neurons in vivo
I Fariñas et al. Neuron. 1998 Aug.
Abstract
Spinal sensory ganglia have been shown to contain neuronal subpopulations with different functions and neurotrophin dependencies. Neurotrophins act, in large part, through Trk receptor tyrosine kinases: nerve growth factor (NGF) via TrkA, brain-derived neurotrophic factor (BDNF) and neurotrophin-4/5 (NT-4/5) via TrkB, and neurotrophin-3 (NT-3) via TrkC. In the present paper, we use antibodies to TrkA, TrkB, and TrkC to characterize their expression patterns and to determine which subpopulations of cells are lost in mice lacking individual neurotrophins or Trk receptors. Despite previous reports of Trk receptor mRNAs in neural crest cells, we detect Trk receptor proteins only in neurons and not in neural crest cells or neuronal precursors. Comparisons of neonatal mice deficient in NT-3 or its cognate receptor TrkC have shown that there is a much greater deficiency in spinal sensory neurons in the former, suggesting that NT-3 may activate receptors in addition to TrkC. Using the same antibodies, we show that, during the major period of neurogenesis, NT-3 is required to maintain neurons that express TrkB in addition to those that express TrkC but is not essential for neurons expressing TrkA. Results also indicate that survival of cells expressing both receptors can be maintained by activation of either one alone. NT-3 can thus activate more than one Trk receptor in vivo, which when coexpressed are functionally redundant.
Figures
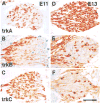
Figure 1. TrkA, TrkB, and TrkC Expression in Early DRGs Is Highly Dynamic
Immunostaining of thoracolumbar DRGs from E11 (A–C) and E13 (D–F) wild-type embryos with antibodies specific to TrkA (A and D), TrkB (B and E), or TrkC (C and F) shows the highly dynamic expression of these receptors over this 2 day period. Scale bar, 100 μm.
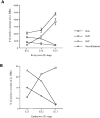
Figure 2. Development of TrkA-, TrkB-, and TrkC-Expressing Cells in Early DRGs
(A) Graph depicts numbers of cells in the L1 DRG expressing individual Trk receptors or neurofilament antigen at E11, E12, and E13. The numbers of cells positive for each Trk were counted in the L1 DRGs of at least two different embryos. Numbers for neurofilament-expressing cells were determined earlier and published in a separate publication (Fariñas et al., 1996). (B) Graph showing the percentage of neurons expressing each Trk in DRGs of different embryonic stages as quantified by division by the total number of neurons (neurofilament-expressing cells) present in these ganglia at each stage (Fariñas et al., 1996). Each point in the graph represents the percentage of Trk-positive neurons in the L1 ganglia.
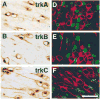
Figure 3. Trk Proteins Are Found in Developing Sensory Neurons but Not in Precursors
Immunostaining of lumbar DRGs of E11 embryos with antibodies specific to TrkA (A and D), TrkB (B and E), and TrkC (C and F) illustrate the neuronal morphology of cells expressing each of the Trk receptors. Sections (D–F) through lumbar DRGs of E11 embryos stained with each anti-Trk (red) and with anti-BrdU (green) show a complete lack of colocalization between the nucleotide and each Trk receptor. Scale bar, 20 μm.
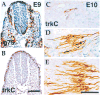
Figure 4. Absence of Trk Expression in Neural Crest and Appearance with Neurogenesis in DRGs
Consecutive transverse sections through the body of an E9 embryo stained for p75NTR to reveal the migrating neural crest (A) and for TrkC (B) demonstrate that neural crest cells do not express the TrkC protein. Neural crest cells were also immunonegative for TrkB and TrkA (data not shown). DRGs in an E10 embryo at successive levels in the caudorostral direction were stained with anti-TrkC antibodies (C–E). Coincident with the spatiotemporal progress of the neurogenic process, the number of cells positive for TrkC increases in the caudal-to-rostral direction. Even in the most caudal DRGs, where as few as one positive cell can be observed, the morphology of the labeled cells is clearly neuronal (C). Scale bars, 190 μm (A and B); 70 μm (C–E).
Figure 5. TrkB- and TrkC- but Not TrkA-Positive Neurons Die in Lumbar DRGs of E11 NT-3–Deficient Embryos
Pyknotic figures (arrows) can be observed that are positive for TrkC (A) and TrkB (C) but not for TrkA (E). Double labeling for either TrkC ([B], red) or TrkB ([D], red) and TUNEL (green) in lumbar DRGs of E11 NT-3–deficient embryos reveals cells positive for both a Trk and TUNEL (arrows). Scale bar, 20 μm.
Figure 6. TrkB- and TrkC-Positive Cells Do Not Die in TrkC- and TrkB-Deficient DRGs, Respectively
(A–C) Confocal microscopy of lumbar DRGs (middle portion of the ganglion) in a wild-type E11 embryo stained with anti-TrkB ([A], green) and anti-TrkC ([B], red) shows in a combined image that some cells express both receptors (C). Section through a lumbar DRG of a TrkC-deficient E11 embryo stained with antibodies to TrkB (D). Notice that, in spite of the presence of numerous pyknotic figures (arrows), none are positive for TrkB. Section through a lumbar DRG of a TrkB-deficient E11 embryo was stained with antibodies to TrkC (E). Only a few pyknotic profiles are observed (arrows), none of which are positive for TrkC. Scale bar, 20 μm.
Similar articles
- Binding of neurotrophin-3 to p75LNGFR, TrkA, and TrkB mediated by a single functional epitope distinct from that recognized by trkC.
Rydén M, Ibáñez CF. Rydén M, et al. J Biol Chem. 1996 Mar 8;271(10):5623-7. doi: 10.1074/jbc.271.10.5623. J Biol Chem. 1996. PMID: 8621424 - Expression of Trk receptors in the developing mouse trigeminal ganglion: in vivo evidence for NT-3 activation of TrkA and TrkB in addition to TrkC.
Huang EJ, Wilkinson GA, Fariñas I, Backus C, Zang K, Wong SL, Reichardt LF. Huang EJ, et al. Development. 1999 May;126(10):2191-203. doi: 10.1242/dev.126.10.2191. Development. 1999. PMID: 10207144 Free PMC article. - Absence of sensory neurons before target innervation in brain-derived neurotrophic factor-, neurotrophin 3-, and TrkC-deficient embryonic mice.
Liebl DJ, Tessarollo L, Palko ME, Parada LF. Liebl DJ, et al. J Neurosci. 1997 Dec 1;17(23):9113-21. doi: 10.1523/JNEUROSCI.17-23-09113.1997. J Neurosci. 1997. PMID: 9364058 Free PMC article. - Role of neurotrophins and trk receptors in the development and maintenance of sensory neurons: an overview.
Lindsay RM. Lindsay RM. Philos Trans R Soc Lond B Biol Sci. 1996 Mar 29;351(1338):365-73. doi: 10.1098/rstb.1996.0030. Philos Trans R Soc Lond B Biol Sci. 1996. PMID: 8730773 Review. - Structural and functional properties of the TRK family of neurotrophin receptors.
Barbacid M. Barbacid M. Ann N Y Acad Sci. 1995 Sep 7;766:442-58. doi: 10.1111/j.1749-6632.1995.tb26693.x. Ann N Y Acad Sci. 1995. PMID: 7486690 Review.
Cited by
- Identification of Neuronal Cells in Sciatic Nerves of Adult Rats.
Liu Y, Zhou S, Zhao L, Gu X. Liu Y, et al. Front Cell Neurosci. 2022 Mar 25;16:816814. doi: 10.3389/fncel.2022.816814. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35401123 Free PMC article. - A local source of FGF initiates development of the unmyelinated lineage of sensory neurons.
Hadjab S, Franck MC, Wang Y, Sterzenbach U, Sharma A, Ernfors P, Lallemend F. Hadjab S, et al. J Neurosci. 2013 Nov 6;33(45):17656-66. doi: 10.1523/JNEUROSCI.1090-13.2013. J Neurosci. 2013. PMID: 24198358 Free PMC article. - The neurotrophin receptor p75 regulates gustatory axon branching and promotes innervation of the tongue during development.
Fei D, Huang T, Krimm RF. Fei D, et al. Neural Dev. 2014 Jun 24;9:15. doi: 10.1186/1749-8104-9-15. Neural Dev. 2014. PMID: 24961238 Free PMC article. - Interaction of Brn3a and HIPK2 mediates transcriptional repression of sensory neuron survival.
Wiggins AK, Wei G, Doxakis E, Wong C, Tang AA, Zang K, Luo EJ, Neve RL, Reichardt LF, Huang EJ. Wiggins AK, et al. J Cell Biol. 2004 Oct 25;167(2):257-67. doi: 10.1083/jcb.200406131. Epub 2004 Oct 18. J Cell Biol. 2004. PMID: 15492043 Free PMC article. - Dynamic expression of neurotrophic factor receptors in postnatal spinal motoneurons and in mouse model of ALS.
Zhang J, Huang EJ. Zhang J, et al. J Neurobiol. 2006 Jul;66(8):882-95. doi: 10.1002/neu.20269. J Neurobiol. 2006. PMID: 16680759 Free PMC article.
References
- Acheson A, Conover JC, Fandl JP, DeChiara TM, Russell M, Thadani A, Squinto SP, Yancopoulous GD, Lindsay RM. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature. 1995;374:450–453. - PubMed
- Airaksinen MS, Koltzenburg M, Lewin GR, Masu Y, Helbig C, Wolf E, Brem G, Toyka KV, Thoenen H, Meyer M. Specific subtypes of cutaneous mechanoreceptors require neurotrophin-3 following peripheral target innervation. Neuron. 1996;16:287–295. - PubMed
- Alexiades MR, Cepko CL. Subsets of retinal progenitors display temporally regulated and distinct biases in the fates of their progeny. Development. 1997;124:1119–1131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 NS016033/NS/NINDS NIH HHS/United States
- P01 NS016033-180014/NS/NINDS NIH HHS/United States
- P50 MH048200-070005/MH/NIMH NIH HHS/United States
- MH48200/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials