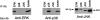Involvement of guanosine triphosphatases and phospholipase C-gamma2 in extracellular signal-regulated kinase, c-Jun NH2-terminal kinase, and p38 mitogen-activated protein kinase activation by the B cell antigen receptor - PubMed (original) (raw)
Involvement of guanosine triphosphatases and phospholipase C-gamma2 in extracellular signal-regulated kinase, c-Jun NH2-terminal kinase, and p38 mitogen-activated protein kinase activation by the B cell antigen receptor
A Hashimoto et al. J Exp Med. 1998.
Abstract
Mitogen-activated protein (MAP) kinase family members, including extracellular signal-regulated kinase (ERK), c-Jun NH2-terminal kinase ( JNK), and p38 MAP kinase, have been implicated in coupling the B cell antigen receptor (BCR) to transcriptional responses. However, the mechanisms that lead to the activation of these MAP kinase family members have been poorly elucidated. Here we demonstrate that the BCR-induced ERK activation is reduced by loss of Grb2 or expression of a dominant-negative form of Ras, RasN17, whereas this response is not affected by loss of Shc. The inhibition of the ERK response was also observed in phospholipase C (PLC)-gamma2-deficient DT40 B cells, and expression of RasN17 in the PLC-gamma2-deficient cells completely abrogated the ERK activation. The PLC-gamma2 dependency of ERK activation was most likely due to protein kinase C (PKC) activation rather than calcium mobilization, since loss of inositol 1,4,5-trisphosphate receptors did not affect ERK activation. Similar to cooperation of Ras with PKC activation in ERK response, both PLC-gamma2-dependent signal and GTPase are required for BCR-induced JNK and p38 responses. JNK response is dependent on Rac1 and calcium mobilization, whereas p38 response requires Rac1 and PKC activation.
Figures
Figure 1
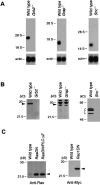
Disruption of Grb2, Grap, and Shc genes in DT40 cells. (A) RNA expression of Grb2, Grap, and Shc was analyzed by Northern blot analysis using each chicken cDNA probe (top) or β-actin (bottom) (reference 64). Positions of 28S and 18S RNA are shown. (B) Grb2, Grap, and Shc protein expression in wild-type and targeted DT40 cells. Each protein was detected by Western blotting analysis using anti-Grb2, anti-Grap, and anti-Shc Abs. (C) Expression of dominant-negative Ras and dominant-negative Rac1.
Figure 2
Cross-reactivity of various Abs. DT40 cell extracts were immunoprecipitated by anti-ERK2 Ab, anti-p38 Ab, and anti-JNK Ab. These immunoprecipitates were separated by 9% SDS-PAGE gels, transferred to nitrocellulose membranes, and incubated with indicated Abs (Blot Ab).
Figure 3
BCR-induced ERK2 activation in various DT40 cells. Various DT40 cells were stimulated with M4 (4 μg/ ml) for indicated time. ERK2 was immunoprecipitated and the precipitates were assayed for kinase activity using GST–Elk1 fusion protein as a substrate. The kinase reaction products were resolved by 12.5% SDS-PAGE and their phosphorylation was quantified by autoradiography.
Figure 4
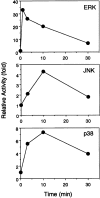
Time kinetics of ERK2, JNK, and p38 responses upon BCR engagement. After BCR stimulation, DT40 cell lysates were immunoprecipitated with anti-ERK2 Ab, anti-JNK1 Ab, or anti-p38 Ab. The kinase activities were assayed using GST–Elk1, GST–c-Jun, and GST–ATF2 as substrates for ERK2, JNK, and p38, respectively.
Figure 5
Involvement of PLC-γ2 and Ras on BCR-induced ERK2 activation. BCR-stimulated DT40 cells were lysed and immunoprecipitated with anti-ERK2 Ab. Stimulation conditions and in vitro kinase assay were described as in Fig. 3.
Figure 6
Effect of RasN17 and Rac1N17 on JNK and p38 responses. DT40 cells were stimulated with M4 (4 μg/ml) for indicated times. Cell lysates were immunoprecipitated with anti-JNK1 Ab and anti-p38 Ab, and kinase activities were assayed using GST–c-Jun and GST–ATF2 as substrates, respectively. After electrophoresis, the labeled GST–c-Jun and GST–ATF2 bands were visualized by autoradiography.
Figure 7
BCR-induced JNK and p38 responses in PLC-γ2– and IP3R-deficient DT40 cells. Cells were stimulated with M4 (4 μg/ml) for indicated times and cell lysates were immunoprecipitated with anti-JNK1 and anti-p38 Ab. The kinase assays were done as in Fig. 6.
Similar articles
- BLNK required for coupling Syk to PLC gamma 2 and Rac1-JNK in B cells.
Ishiai M, Kurosaki M, Pappu R, Okawa K, Ronko I, Fu C, Shibata M, Iwamatsu A, Chan AC, Kurosaki T. Ishiai M, et al. Immunity. 1999 Jan;10(1):117-25. doi: 10.1016/s1074-7613(00)80012-6. Immunity. 1999. PMID: 10023776 - Platelet-derived growth factor activates p38 mitogen-activated protein kinase through a Ras-dependent pathway that is important for actin reorganization and cell migration.
Matsumoto T, Yokote K, Tamura K, Takemoto M, Ueno H, Saito Y, Mori S. Matsumoto T, et al. J Biol Chem. 1999 May 14;274(20):13954-60. doi: 10.1074/jbc.274.20.13954. J Biol Chem. 1999. PMID: 10318806 - Differential activation of the ERK, JNK, and p38 mitogen-activated protein kinases by CD40 and the B cell antigen receptor.
Sutherland CL, Heath AW, Pelech SL, Young PR, Gold MR. Sutherland CL, et al. J Immunol. 1996 Oct 15;157(8):3381-90. J Immunol. 1996. PMID: 8871635 - Regulation of the phospholipase C-gamma2 pathway in B cells.
Kurosaki T, Maeda A, Ishiai M, Hashimoto A, Inabe K, Takata M. Kurosaki T, et al. Immunol Rev. 2000 Aug;176:19-29. doi: 10.1034/j.1600-065x.2000.00605.x. Immunol Rev. 2000. PMID: 11043765 Review.
Cited by
- Identification of a phospholipase C-gamma1 (PLC-gamma1) SH3 domain-binding site in SLP-76 required for T-cell receptor-mediated activation of PLC-gamma1 and NFAT.
Yablonski D, Kadlecek T, Weiss A. Yablonski D, et al. Mol Cell Biol. 2001 Jul;21(13):4208-18. doi: 10.1128/MCB.21.13.4208-4218.2001. Mol Cell Biol. 2001. PMID: 11390650 Free PMC article. - Dual role of the adaptor protein SLP-65: organizer of signal transduction and tumor suppressor of pre-B cell leukemia.
Herzog S, Storch B, Jumaa H. Herzog S, et al. Immunol Res. 2006;34(2):143-55. doi: 10.1385/IR:34:2:143. Immunol Res. 2006. PMID: 16760574 Review. - Molecular underpinning of B-cell anergy.
Yarkoni Y, Getahun A, Cambier JC. Yarkoni Y, et al. Immunol Rev. 2010 Sep;237(1):249-63. doi: 10.1111/j.1600-065X.2010.00936.x. Immunol Rev. 2010. PMID: 20727040 Free PMC article. Review. - Aberrant B cell receptor signaling from B29 (Igbeta, CD79b) gene mutations of chronic lymphocytic leukemia B cells.
Gordon MS, Kato RM, Lansigan F, Thompson AA, Wall R, Rawlings DJ. Gordon MS, et al. Proc Natl Acad Sci U S A. 2000 May 9;97(10):5504-9. doi: 10.1073/pnas.090087097. Proc Natl Acad Sci U S A. 2000. PMID: 10792036 Free PMC article. - Deficiency of BLNK hampers PLC-gamma2 phosphorylation and Ca2+ influx induced by the pre-B-cell receptor in human pre-B cells.
Taguchi T, Kiyokawa N, Takenouch H, Matsui J, Tang WR, Nakajima H, Suzuki K, Shiozawa Y, Saito M, Katagiri YU, Takahashi T, Karasuyama H, Matsuo Y, Okita H, Fujimoto J. Taguchi T, et al. Immunology. 2004 Aug;112(4):575-82. doi: 10.1111/j.1365-2567.2004.01918.x. Immunology. 2004. PMID: 15270728 Free PMC article.
References
- DeFranco AL. The complexity of signaling pathways activated by the BCR. Curr Opin Immunol. 1997;9:296–308. - PubMed
- Reth M, Wienands J. Initiation and processing of signals from the B cell antigen receptor. Annu Rev Immunol. 1997;15:453–479. - PubMed
- Kurosaki T. Molecular mechanisms in B cell antigen receptor signaling. Curr Opin Immunol. 1997;9:309–318. - PubMed
- Pleiman CM, D'Ambrosio D, Cambier JC. The B–cell antigen receptor complex: structure and signal transduction. Immunol Today. 1994;15:393–399. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous