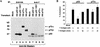Viral oncoproteins discriminate between p53 and the p53 homolog p73 - PubMed (original) (raw)
Viral oncoproteins discriminate between p53 and the p53 homolog p73
M C Marin et al. Mol Cell Biol. 1998 Nov.
Abstract
p73 is a recently identified member of the p53 family. Previously it was shown that p73 can, when overproduced in p53-defective tumor cells, activate p53-responsive promoters and induce apoptosis. In this report we describe the generation of anti-p73 monoclonal antibodies and confirm that two previously described p73 isoforms are produced in mammalian cells. Furthermore, we show that these two isoforms can bind to canonical p53 DNA-binding sites in electrophoretic mobility shift assays. Despite the high degree of similarity between p53 and p73, we found that adenovirus E1B 55K, simian virus 40 T, and human papillomavirus E6 do not physically interact with p73. The observation that viral oncoproteins discriminate between p53 and p73 suggests that the functions of these two proteins may differ under physiological conditions. Furthermore, they suggest that inactivation of p73 may not be required for transformation.
Figures
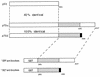
FIG. 1
GST-p73 fusion proteins used to generate monoclonal antibodies. Shown schematically are p53, p73α, and p73β. Mice were immunized with GST fused to either the C terminus of p73α (ER mice) or the C terminus of p73β (GC mice). The p73β cDNA lacks exon 13, resulting in a frameshift at residue 495. p73β therefore contains five residues (black box) not present in p73α.
FIG. 2
Characterization of anti-p73 antibodies. (A) p73α and p73β 35S-labelled in vitro translation products were immunoprecipitated with either control (anti-T; PAb419) or anti-p73 (ER13, ER15, and GC15) antibodies, as indicated, under native (lanes 3 to 6 and 11 to 14) or denaturing (lanes 7 to 10 and 15 to 18) conditions. Two microliters of the indicated in vitro translation product was loaded directly in lanes 1 and 2. (B) p73α and p73β were cotranslated in vitro in the presence of [35S]methionine and immunoprecipitated with either control (anti-T; PAb419) or anti-p73 (ER13, ER15, and GC15) antibodies, as indicated, under native (lanes 2 to 5) or denaturing (lanes 6 to 10) conditions. Two microliters of the indicated in vitro translation product was loaded directly in lanes 1, 11, and 12. (C) p51A, p53, and p73β 35S-labelled in vitro translation products were immunoprecipitated with either control (anti-T; PAb419) or anti-p73 (ER13, ER15, and GC15) antibodies, as indicated. Two microliters of the indicated in vitro translation product was loaded directly in lanes 1 to 3. For panels A to C, proteins were resolved by SDS-polyacrylamide gel electrophoresis and detected by fluorography. (D) GST-p53 (lanes 1 to 5), GST-p73β (aa 380 to 499) (lanes 6 to 10), and GST-p73α (aa 380 to 637) (lanes 11 to 15) were produced in bacteria, purified by using glutathione-Sepharose, resolved by SDS-polyacrylamide gel electrophoresis in preparative wells, and transferred to a membrane. The membrane was cut into strips and immunoblotted with either control (anti-T; PAb419) or anti-p73 (ER13, ER15, and GC15) antibodies, as indicated. Bound antibody was detected colorimetrically with an alkaline phosphatase-conjugated antimouse antibody.
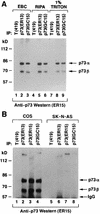
FIG. 3
Identification of p73α and p73β in cell extracts. (A) COS cells were lysed in the presence of 0.5% Nonidet P-40 (EBC buffer) (lanes 1 to 3), in radioimmunoprecipitation assay buffer (lanes 4 to 6), or in the presence of 1% Triton (lanes 7 to 9) and immunoprecipitated (IP) with a control antibody (anti-T [PAb419]), anti-p73 (ER13), or anti-p73 (GC15) as indicated. Antibodies were cross-linked to protein A-Sepharose prior to immunoprecipitation. (B) COS cells (lanes 1 to 4) and SK-N-AS cells (lanes 5 to 8) were lysed in EBC buffer and immunoprecipitated with a control antibody (anti-T [PAb419]), anti-p73 (ER13), anti-p73 (ER15), or anti-p73 (GC15) as indicated. For both panels, Bound proteins were resolved by electrophoresis in an SDS–8% polyacrylamide gel and immunoblotted with anti-p73 (ER15) by using a horseradish peroxidase-conjugated antimouse antibody and enhanced chemiluminescence.
FIG. 4
p73α and p73β protein levels in neuroblastoma cell lines. Cell extracts prepared from the indicated cell lines were resolved by electrophoresis in an SDS–8% polyacrylamide gel and immunoblotted with an anti-p73 antibody (ER15). Each lane contained ∼300 μg of total cell extract. Bound antibody was detected by using a horseradish peroxidase-conjugated antimouse antibody and enhanced chemiluminescence.
FIG. 5
p73 binds to canonical p53 DNA-binding sites. A 32P-radiolabelled p53 DNA-binding site was incubated with p53 in vitro translation product (A, lanes 4 to 9), p73α in vitro translation product (A, lanes 10 to 15, and B, lanes 1 to 5), p73β in vitro translation product (B, lanes 6 to 10), or unprogrammed reticulocyte lysate (A, lanes 1 to 3) and subjected to electrophoretic mobility shift analysis. Binding reactions were carried out in the presence of a vast molar excess of unlabelled specific or nonspecific competitor DNA where indicated. Anti-p53, anti-p73, or a control antibody (anti-T) was added prior to the electrophoretic mobility shift assay as indicated. The asterisk indicates a nonspecific complex.
FIG. 6
Differential binding of p53 and p73 to E1B 55K. (A) 293 human embryonic kidney cells were lysed and immunoprecipitated (IP) with the indicated antibodies. Specifically bound proteins were resolved by electrophoresis in an SDS–10% polyacrylamide gel and detected by immunoblotting with anti-p73 antibody (left panel) or anti-p53 antibody (right panel). Bound antibodies were detected by using a horseradish peroxidase-conjugated antimouse antibody and enhanced chemiluminescence. The asterisk indicates faster-migrating species, previously noted by others, that interact with anti-p53 antibodies. (B) 293 human embryonic kidney cells were transfected with 1 or 10 μg, as indicated by the triangles, of expression plasmids encoding HA-tagged p53 (lanes 2, 7, and 8) or HA-tagged p73α (lanes 4, 5, 9, and 10) or with 10 μg of the backbone expression plasmid (lanes 1 and 6). Cell extracts were prepared and immunoprecipitated with anti-HA (lanes 1 to 5) or anti-E1B 55K antibodies (lanes 6 to 10). Specifically bound proteins were resolved by electrophoresis in an SDS–10% polyacrylamide gel and detected by immunoblotting with anti-HA antibody (12CA5) by using a horseradish peroxidase-conjugated antimouse antibody and enhanced chemiluminescence. The asterisks indicate nonspecific bands.
FIG. 7
E6 targets p53, but not p73, for degradation in vitro. (A) p53, p73α, and p73β 35S-labelled in vitro translation products were incubated with immobilized GST-E6 (lanes 4 to 12) or GST (lanes 13 to 15) as indicated. Each binding reaction mixture contained 40 μl (lanes 4, 7, 10, and 13 to 15), 20 μl (lanes 5, 8, and 11), or 10 μl (lanes 6, 9, and 12) of in vitro translation product. Specifically bound proteins were resolved by SDS-polyacrylamide gel electrophoresis and detected by fluorography. Five microliters of the indicated in vitro translation product was loaded directly in lanes 1 to 3. (B) p53, p73α, and p73β 35S-labelled in vitro translation products were incubated at 30°C with a lysate prepared from bacteria producing GST-E6 (right panel) or GST-E4 (left panel). The proteins were then resolved by electrophoresis in an SDS–10% polyacrylamide gel and detected by fluorography.
FIG. 8
Differential binding of p53 and p73 to SV40 T. (A) COS cells, which stably produce SV40 T, were transfected with expression plasmids encoding the indicated HA-tagged proteins or with the backbone expression plasmid (Mock). Cell extracts were prepared and immunoprecipitated (IP) with anti-HA (lanes 1 to 5) or anti-T (lanes 6 to 10) antibodies. Specifically bound proteins were resolved by electrophoresis in an SDS–10% polyacrylamide gel and detected by immunoblotting with anti-HA antibody (12CA5) by using a horseradish peroxidase-conjugated antimouse antibody and enhanced chemiluminescence. (B) SAOS2 cells were transfected a CAT reporter plasmid containing a minimal promoter consisting of two p53-binding sites upstream of a TATA box together with expression plasmids for wild-type p53 or p73α. In each case, the amount of CAT activity, in the absence of T antigen, was set to 100%. Where indicated, the cells were also transfected with a plasmid encoding wild-type (wt) or mutant (mut) T antigen. Error bars represent one standard deviation.
Similar articles
- Functional inactivation of p73, a homolog of p53 tumor suppressor protein, by human papillomavirus E6 proteins.
Park JS, Kim EJ, Lee JY, Sin HS, Namkoong SE, Um SJ. Park JS, et al. Int J Cancer. 2001 Mar 15;91(6):822-7. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1130>3.0.co;2-0. Int J Cancer. 2001. PMID: 11275986 - The emerging p53 gene family.
Kaelin WG Jr. Kaelin WG Jr. J Natl Cancer Inst. 1999 Apr 7;91(7):594-8. doi: 10.1093/jnci/91.7.594. J Natl Cancer Inst. 1999. PMID: 10203277 Review. - Regulation of the p53 homolog p73 by adenoviral oncogene E1A.
Das S, El-Deiry WS, Somasundaram K. Das S, et al. J Biol Chem. 2003 May 16;278(20):18313-20. doi: 10.1074/jbc.M211704200. Epub 2003 Mar 13. J Biol Chem. 2003. PMID: 12639967 - Inactivation of p53 but not p73 by adenovirus type 5 E1B 55-kilodalton and E4 34-kilodalton oncoproteins.
Roth J, König C, Wienzek S, Weigel S, Ristea S, Dobbelstein M. Roth J, et al. J Virol. 1998 Nov;72(11):8510-6. doi: 10.1128/JVI.72.11.8510-8516.1998. J Virol. 1998. PMID: 9765388 Free PMC article. - The p53 gene family.
Kaelin WG Jr. Kaelin WG Jr. Oncogene. 1999 Dec 13;18(53):7701-5. doi: 10.1038/sj.onc.1202955. Oncogene. 1999. PMID: 10618710 Review.
Cited by
- p73 as a Tissue Architect.
Maeso-Alonso L, López-Ferreras L, Marques MM, Marin MC. Maeso-Alonso L, et al. Front Cell Dev Biol. 2021 Jul 23;9:716957. doi: 10.3389/fcell.2021.716957. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34368167 Free PMC article. Review. - Combining Oncolytic Virotherapy with p53 Tumor Suppressor Gene Therapy.
Bressy C, Hastie E, Grdzelishvili VZ. Bressy C, et al. Mol Ther Oncolytics. 2017 Mar 21;5:20-40. doi: 10.1016/j.omto.2017.03.002. eCollection 2017 Jun 16. Mol Ther Oncolytics. 2017. PMID: 28480326 Free PMC article. Review. - IER3 is a crucial mediator of TAp73β-induced apoptosis in cervical cancer and confers etoposide sensitivity.
Jin H, Suh DS, Kim TH, Yeom JH, Lee K, Bae J. Jin H, et al. Sci Rep. 2015 Feb 10;5:8367. doi: 10.1038/srep08367. Sci Rep. 2015. PMID: 25666857 Free PMC article. - Regulatory feedback loop between TP73 and TRIM32.
Gonzalez-Cano L, Hillje AL, Fuertes-Alvarez S, Marques MM, Blanch A, Ian RW, Irwin MS, Schwamborn JC, Marín MC. Gonzalez-Cano L, et al. Cell Death Dis. 2013 Jul 4;4(7):e704. doi: 10.1038/cddis.2013.224. Cell Death Dis. 2013. PMID: 23828567 Free PMC article. - Eukaryotic translation elongation factor 1-alpha 1 inhibits p53 and p73 dependent apoptosis and chemotherapy sensitivity.
Blanch A, Robinson F, Watson IR, Cheng LS, Irwin MS. Blanch A, et al. PLoS One. 2013 Jun 14;8(6):e66436. doi: 10.1371/journal.pone.0066436. Print 2013. PLoS One. 2013. PMID: 23799104 Free PMC article.
References
- Bargonetti J, Manfredi J J, Chen X, Marshak D R, Prives C. A proteolytic fragment from the central region of p53 has marked sequence-specific DNA-binding activity when generated from wild-type but not oncogenic mutant p53 protein. Genes Dev. 1993;7:2565–2574. - PubMed
- Bargonetti J, Reynisdottir I, Friedman P N, Prives C. Site-specific binding of wild-type p53 to cellular DNA is inhibited by SV40 T antigen and mutant p53. Genes Dev. 1992;6:1886–1898. - PubMed
- Braithwaite A W, Blair G E, Nelson C C, McGovern J, Bellett A J. Adenovirus E1b-58 kD antigen binds to p53 during infection of rodent cells: evidence for an N-terminal binding site on p53. Oncogene. 1991;6:781–787. - PubMed
- Caput, D. Unpublished data.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous