Regulation of exit from quiescence by p27 and cyclin D1-CDK4 - PubMed (original) (raw)
Regulation of exit from quiescence by p27 and cyclin D1-CDK4
M H Ladha et al. Mol Cell Biol. 1998 Nov.
Abstract
The synthesis of cyclin D1 and its assembly with cyclin-dependent kinase 4 (CDK4) to form an active complex is a rate-limiting step in progression through the G1 phase of the cell cycle. Using an activated allele of mitogen-activated protein kinase kinase 1 (MEK1), we show that this kinase plays a significant role in positively regulating the expression of cyclin D1. This was found both in quiescent serum-starved cells and in cells expressing dominant-negative Ras. Despite the observation that cyclin D1 is a target of MEK1, in cycling cells, activated MEK1, but not cyclin D1, is capable of overcoming a G1 arrest induced by Ras inactivation. Either wild-type or catalytically inactive CDK4 cooperates with cyclin D1 in reversing the G1 arrest induced by inhibition of Ras activity. In quiescent NIH 3T3 cells expressing either ectopic cyclin D1 or activated MEK1, cyclin D1 is able to efficiently associate with CDK4; however, the complex is inactive. A significant percentage of the cyclin D1-CDK4 complexes are associated with p27 in serum-starved activated MEK1 or cyclin D1 cell lines. Reduction of p27 levels by expression of antisense p27 allows for S-phase entry from quiescence in NIH 3T3 cells expressing ectopic cyclin D1, but not in parental cells.
Figures
FIG. 1
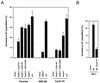
Expression of activated MEK1 (MEK-EE) and cyclin D1 in NIH 3T3 cells. (A) Parental NIH 3T3 cells and their cyclin D1 and MEK-EE derivatives were rendered quiescent by serum starvation or maintained in a cycling asynchronous state. Under these two conditions, the levels of endogenous and exogenous MEK1, cyclin D1, and CDK4 were determined by Western blot analysis of whole-cell lysates. (B) Same as panel A, except that the cell cycle distribution of the cells was monitored by fluorescence-activated cell sorting analysis. (C) Lysates were prepared from serum-starved and asynchronous cultures of parental NIH 3T3 cells and the cyclin D1 and MEK-EE derivatives. Immunoprecipitation (IP) with antibody to CDK4 were performed. Immune complexes were assayed for CDK4-associated kinase activity with recombinant glutathione _S_-transferase (GST)–Rb as substrate. NRS, normal rabbit serum.
FIG. 2
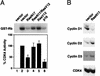
Relative ability of stable expression of cyclin D1 or MEK-EE to reverse the cell cycle arrest induced by RasN17. (A) The indicated cell lines were transfected with pMT-ΔBam (control plasmid; similar results were obtained with pcDNA3 and pCMV), pMT-RasN17, pCMV-CDK4, pRc/CMV-CDK4K35M (catalytically inactive CDK4), or pcDNA3-p16 together with a plasmid encoding the CD20 cell surface marker. The transfected population was identified by staining with fluorescein isothiocyanate-conjugated anti-CD20 antibody, and DNA content (2N, 4N) was monitored by staining with propidium iodide. The cell cycle distribution of the transfected population was determined by two-color flow cytometry. The cells were cultured in DMEM–5% BCS throughout the experiment. The absolute changes in the percentages of cells in G1 compared to control transfections are shown with the mean standard error from at least three independent experiments. In control transfected cultures, the G1 population was approximately 40%. WT, wild type. (B) Same as panel A except that Rb−/− 3T3 cells, cultured in DMEM–10% fetal bovine serum, were used. The expression plasmid for Rb was pCMV-Rb. The absolute changes in the percentages of cells in G1 compared to control transfections are shown with the mean standard error from at least three independent experiments.
FIG. 3
Effect of RasN17 expression on CDK4 activity and D cyclin levels. (A) NIH 3T3 cells, cultured in DMEM–5% BCS, were transfected with plasmids encoding the indicated proteins or vector alone together with a plasmid encoding the surface marker CD20. Forty-two hours later, transfected cells were isolated by magnetic sorting with Dynabeads (see Materials and Methods). Extracts were prepared, normalized for protein content, and subjected to immunoprecipitation for CDK4. CDK4-associated kinase was measured by using glutathione _S_-transferase (GST)–Rb as a substrate. In lane 1, control normal rabbit serum was used. Lane 2 is the control (pMT-ΔBam, vector-alone transfection; the same results were obtained when pcDNA3 was used as the vector control) set to 100% kinase activity. The bar graph represents the relative kinase activity with the mean standard error from at least three independent experiments. A representative autoradiogram is shown. (B) Same as panel A except that cell extracts were used for Western blot analysis for cyclin D1, cyclin D2, cyclin D3, and CDK4. Indicated proteins were detected by enhanced chemiluminescence.
FIG. 4
The effect of activated MEK1 on CDK4 activity and cyclin D1 levels in cells transiently expressing RasN17. (A) NIH 3T3 cells expressing ectopic cyclin D1 or MEK-EE or cells infected with control virus were transiently transfected with control plasmid (pMT-ΔBam), pMT-RasN17, or pcDNA3-p16. The transfected population, cultured in DMEM–5% BCS, was magnetically sorted, and CDK4 kinase assays were performed as described for Fig. 3B. Kinase activity for vector control transfections was set to 100% as indicated. The bar graph represents the relative kinase activity with the mean standard error from at least three independent experiments. A representative autoradiogram is shown. GST, glutathione _S_-transferase. (B) Same as panel A except that cell lysates were used to assess the relative amounts of cyclin D1 protein present as a function of RasN17 expression by Western blot analysis.
FIG. 5
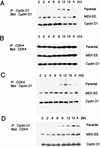
Association between cyclin D1 and CDK4. (A) Parental NIH 3T3 cells and their MEK-EE and cyclin D1 derivatives were rendered quiescent by serum starvation. Cells were then restimulated by serum addition. At the indicated times, lysates were prepared and immunoprecipitations (IP) for cyclin D1 were performed. Immune complexes were resolved in a denaturing gel, and Western blot analysis was performed for cyclin D1. Lysates prepared from cycling asynchronous cultures (designated A) were also analyzed. (B) Same as panel A except that CDK4 was analyzed. (C and D) Same as above except that CDK4 immunoprecipitates were analyzed for associated cyclin D1 and cyclin D1 immunoprecipitates were analyzed for associated CDK4. The experiments performed for each of the blots shown in panels A, B, C, and D were performed in parallel. Indicated proteins were detected by enhanced chemiluminescence, and the exposure time in each of the panels is the same; thus, the relative amounts of cyclin D1 associated with CDK4 and vice versa can be compared. The results are representative of at least five independent experiments.
FIG. 6
CDK4 kinase activity during G1. Parental NIH 3T3 cells and their MEK-EE and cyclin D1 derivatives were rendered quiescent by serum starvation. Cells were stimulated to reenter the cell cycle by serum addition. At the indicated times, lysates were prepared and normalized for protein content, and immunoprecipitations for CDK4 were performed. Immune complexes were then assayed for CDK4-associated kinase activity with glutathione _S_-transferase–Rb as substrate. Cycling asynchronous cultures (designated A) were also assayed. Control immunoprecipitations from asynchronous cultures with normal rabbit serum (N) are shown. The results are representative of at least three independent experiments.
FIG. 7
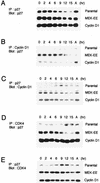
Association of p27 with cyclin D1 and CDK4. (A) Parental NIH 3T3 cells and their MEK-EE and cyclin D1 derivatives were rendered quiescent by serum starvation. Cells were then restimulated by serum addition. At the indicated times, lysates were prepared and immunoprecipitations (IP) for p27 were performed. Immune complexes were resolved in a denaturing gel, and Western blot analysis was performed for p27. Lysates prepared from cycling asynchronous cultures (designated A) were also analyzed. (B, C, D, and E) Same as panel A except that antibodies used for immunoprecipitation and Western blot analysis (Blot) were as indicated. The experiments for each of the blots shown in panels A, B, C, D, and E were performed in parallel. Indicated proteins were detected by enhanced chemiluminescence, and the exposure time in each of the panels is the same; thus, a comparison of the relative amounts of p27 associated with cyclin D1 and CDK4 can be made. The results are representative of at least five independent experiments.
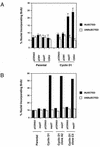
FIG. 8
Effect of microinjection of antisense p27-encoding plasmid into quiescent cells on S-phase entry. (A) Vector plasmids, pcDNA3 and pCMV, or plasmids encoding either antisense p27 (pCMV5-asp27) or CDK4 (pCMV-CDK4 together with a plasmid encoding GFP [pcDNA3-GFP]) were microinjected into serum-starved NIH 3T3 cells (Parental) or their cyclin D1 derivative. BrdU was added to the cultures, and cells were subsequently stained for incorporated BrdU as described in Materials and Methods. The percentages of nuclei staining positive for both BrdU and GFP are shown (filled bars). Also shown is the percent BrdU incorporation for uninjected cells (open bars). Shown are the means plus standard errors for three independent experiments. (B) Same as panel A except that two clonal derivatives of the NIH 3T3-cyclin D1 lines, A2 and C3, were used. The results are for one experiment.
References
- Albanese C, Johnson J, Watanabe G, Eklund N, Vu D, Arnold A, Pestell R G. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem. 1995;270:23589–23597. - PubMed
- Alessandrini A, Chiaur D S, Pagano M. Regulation of the cyclin-dependent kinase inhibitor p27 by degradation and phosphorylation. Leukemia. 1997;11:342–345. - PubMed
- Baldin V, Lukas J, Marcote M J, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812–821. - PubMed
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous