Synaptogenesis via dendritic filopodia in developing hippocampal area CA1 - PubMed (original) (raw)
Synaptogenesis via dendritic filopodia in developing hippocampal area CA1
J C Fiala et al. J Neurosci. 1998.
Abstract
To determine the role of dendritic filopodia in the genesis of excitatory synaptic contacts and dendritic spines in hippocampal area CA1, serial section electron microscopy and three-dimensional analysis of 16 volumes of neuropil from nine male rat pups, aged postnatal day 1 (P1) through P12, were performed. The analysis revealed that numerous dendritic filopodia formed asymmetric synaptic contacts with axons and with filopodia extending from axons, especially during the first postnatal week. At P1, 22 +/- 5.5% of synapses occurred on dendritic filopodia, with 19 +/- 5.9% on filopodia at P4, 20 +/- 8.0% at P6, decreasing to 7.2 +/- 4.7% at P12 (p < 0.02). Synapses were found at the base and along the entire length of filopodia, with many filopodia exhibiting multiple synaptic contacts. In all, 162 completely traceable dendritic filopodia received 255 asymmetric synaptic contacts. These synapses were found at all parts of filopodia with equal frequency, usually occurring on fusiform swellings of the diameter. Most synaptic contacts (53 +/- 11%) occurred directly on dendritic shafts during the first postnatal week. A smaller but still substantial portion (32 +/- 12%) of synapses were on shafts at P12 (p < 0.036). There was a highly significant (p < 0.0002) increase in the proportion of dendritic spine synapses with age, rising from just 4.9 +/- 4.3% at P1 to 37 +/- 14% at P12. The concurrence of primarily shaft and filopodial synapses in the first postnatal week suggests that filopodia recruit shaft synapses that later give rise to spines through a process of outgrowth.
Figures
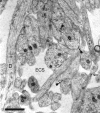
Fig. 1.
Representative CA1 neuropil from postnatal day 1 (R53) has large amounts of extracellular space (ECS). A dendrite (D) with well organized microtubules gives rise to a dendritic filopodium (df), which continues on adjacent serial sections. An asymmetric synapse (s) on a stubby dendritic protrusion is evident at the top of the figure. Also in evidence are nonsynaptic surface specializations (solid square arrows). The tip of an axonal filopodium (af), identified by tracing it back to its axonal origin, has a surface specialization or possibly a nascent synapse (open arrow) where it contacts the tip of a dendritic filopodium (f), also identified through series. Scale bar, 1 μm.
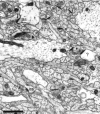
Fig. 2.
Representative CA1 neuropil from postnatal day 4 (R48a). A dendritic filopodium (df) emerges from the middle of a dendrite (D). The entire filopodium is reconstructed in Figure 5. A synapse on an apparently stubby profile is actually at the base (B) of a filopodium that could be traced on adjacent serial sections. A shaft synapse (sh) has synaptic vesicles in a docked position, suggesting the synapse is functional. The profile in the upper left corner (f) makes synaptic contact with an axon containing docked vesicles. This synapse occurs in the middle of a dendritic filopodium that could be followed for 70 sections to its tip, at which there was no synaptic contact. Scale bar, 1 μm.
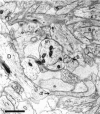
Fig. 3.
Neuropil from postnatal day 6 (R43b) showing varicosities (V) with interposed thin regions that give some dendrites a beaded appearance. Some varicosities had a watery cytoplasm (W) with organelles compressed to the periphery, suggesting that they might have undergone swelling. A synapse (s) located on a dendritic shaft has the appearance of a symmetric synapse with equally thin densities on both membranes. Asymmetric synaptic contacts on two dendritic filopodia profiles (f) were identified through serial sections. Note that these profiles might be mistaken for dendritic spines if they were viewed only on this section. Scale bar, 1 μm.
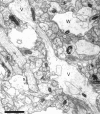
Fig. 4.
The neuropil from postnatal day 12 (R45a) is much denser than in the first postnatal week, with many more profiles of small processes. Asymmetric synapses are seen on thin dendritic spines (t), as well as on stubby spines (s) and dendritic shafts (sh). Many dendritic spines at this age do not have the typical characteristics of adult CA1 spines. An atypical thin spine (a) receives synaptic contacts from two different axons. Multiple synapses are rarely identifiable on single sections. The atypical stubby (as) in the lower left has only one of its two synapses visible on this section, whereas two synapses (arrows) are apparent on the atypical stubby (as) in the inset. One postsynaptic process (?) could not be traced to its origin and, therefore, could not be unequivocally identified. Scale bar, 1 μm.
Fig. 5.
Three-dimensional reconstruction of the dendritic filopodium whose origin is shown in Figure 2. The filopodium is ∼11 μm long from origin to tip. Filopodia of this length were rarely captured in series of only 100 ultrathin sections. At its narrowest point (solid arrow) the filopodium has a diameter of ∼0.1 μm, whereas the maximum diameter is ∼1 μm. The filopodium is enfolded in stubby protrusions from the axon, one of which (star) appears to make a synaptic contact with the filopodium (F) (inset, star arrow). Scale bar in inset, 0.5 μm; 3D scale bar, 1 μm.
Fig. 6.
A montage of four sections from postnatal day 6 (R44a) showing a filopodium (f) emerging from a dendrite (D), and terminating in a bulbous head (h) that has two synapses from different axons. The filopodium is similar in shape to an atypical spine (see Materials and Methods), but its unusual length (∼3 μm) is more characteristic of a filopodium. In addition, the “neck” has a variable diameter, a dark cytoplasm suggesting a dense accumulation of actin, and exhibits a surface specialization at one point along its length where it contacts another dendritic process (arrow). Scale bar, 1 μm.
Fig. 7.
A montage of two sections from P1 (R55) showing a vesicle-filled process (P) emerging from a bend in a dendrite (D), all of which is outlined in_white_. This process shares more characteristics with growth processes than most dendritic filopodia (compare with Figs. 1and 2), suggesting that it might represent a budding branch of the dendrite. Note the mitochondrian (arrow) partly invades the base of the process. Mitochondria were never observed in filopodia. Scale bar, 1 μm.
Fig. 8.
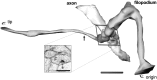
Top. Interaction between axonal and dendritic filopodia at postnatal day 4 (R48a). A, An axon has a broad protrusion with a grainy cytoplasm indicative of actin. This actin-filled region is devoid of microtubules and ends in a narrow process (af) identical in appearance to dendritic filopodia except for the presence of synaptic vesicles (black arrows). There are two synapses at the base of this axonal filopodium, one (blue arrow) with a dendrite shaft and another (red arrow) with the tip of a dendritic filopodium (df). B, Three-dimensional reconstruction of segments of the same axon and dendrite with the interacting filopodia that are shown in A. Scale bars, 1 μm.
Fig. 10.
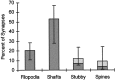
Distribution of synapse locations in CA1 during the first postnatal week. Bars represent the mean percentages of synapses in each class. Light shading indicates mean percentages for atypical spines and stubbies (see Materials and Methods for details). Error bars show the minimum and maximum values for combined means measured in 12 series from seven animals aged P1, P4, and P6.
Fig. 11.
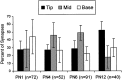
The location of synapses on 162 complete filopodia as a percent of the total number (n) of synapses on these filopodia at each age. There were more tip locations at P12 (F(3,12) = 6.21;p < 0.009), but apparent trends in mid and base locations were not statistically significant (p < 0.07 and p < 0.26, respectively).
Fig. 12.
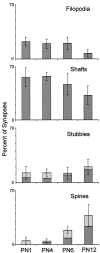
Distribution of synapse locations by age as revealed by the mean percentage of synapses at each location.Light regions depict percentages with atypical characteristics, (see Materials and Methods for details). Error bars indicate SDs.
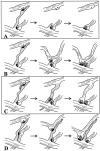
Fig. 13.
Possible mechanisms by which filopodia might induce shaft synapses. A, Synapses form on the receptive surface of filopodia, but only those near the base of filopodia are stabilized into shaft synapses. B, Filopodia retract, pulling the presynaptic partner to the dendrite. C, Dendritic filopodia guide axonal filopodia to the dendrite where synapses are stabilized by subsequent maturation of the axonal filopodium into an axon branch. D, Dendritic filopodia act as conduits along which synapses move to the dendrite shaft.
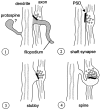
Fig. 14.
Genesis of most excitatory synapses may involve four stages. 1, Initial induction of a synapse by a filopodium. In early stages of development, atypical or “protospines” are apparent, but their ultimate fate is unclear.2, Retraction of the filopodia and stabilization of the synapse on the dendrite shaft. 3, Emergence of a stubby protrusion beneath the synapse. 4, Outgrowth of protrusion into a mature spine.
Similar articles
- Synaptogenesis on mature hippocampal dendrites occurs via filopodia and immature spines during blocked synaptic transmission.
Petrak LJ, Harris KM, Kirov SA. Petrak LJ, et al. J Comp Neurol. 2005 Apr 4;484(2):183-90. doi: 10.1002/cne.20468. J Comp Neurol. 2005. PMID: 15736233 - LTP enhances synaptogenesis in the developing hippocampus.
Watson DJ, Ostroff L, Cao G, Parker PH, Smith H, Harris KM. Watson DJ, et al. Hippocampus. 2016 May;26(5):560-76. doi: 10.1002/hipo.22536. Epub 2015 Oct 23. Hippocampus. 2016. PMID: 26418237 Free PMC article. - [On the function of dendritic filopodia].
Portera Cailliau C, Yuste R. Portera Cailliau C, et al. Rev Neurol. 2001 Dec 16-31;33(12):1158-66. Rev Neurol. 2001. PMID: 11785056 Review. Spanish. - [Three-dimentional organization of synapses and astroglia in the hippocampus of rats and ground squirrels: new structural and functional paradigms of the synapse function].
Popov VI, Medvedev NI, Rogachevskiĭ VV, Ignat'ev DA, Stewart MG, Fesenko EE. Popov VI, et al. Biofizika. 2003 Mar-Apr;48(2):289-308. Biofizika. 2003. PMID: 12723356 Review. Russian.
Cited by
- Dendritic Spine Plasticity: Function and Mechanisms.
Runge K, Cardoso C, de Chevigny A. Runge K, et al. Front Synaptic Neurosci. 2020 Aug 28;12:36. doi: 10.3389/fnsyn.2020.00036. eCollection 2020. Front Synaptic Neurosci. 2020. PMID: 32982715 Free PMC article. - Cofilin 1-mediated biphasic F-actin dynamics of neuronal cells affect herpes simplex virus 1 infection and replication.
Xiang Y, Zheng K, Ju H, Wang S, Pei Y, Ding W, Chen Z, Wang Q, Qiu X, Zhong M, Zeng F, Ren Z, Qian C, Liu G, Kitazato K, Wang Y. Xiang Y, et al. J Virol. 2012 Aug;86(16):8440-51. doi: 10.1128/JVI.00609-12. Epub 2012 May 23. J Virol. 2012. PMID: 22623803 Free PMC article. - Neurotrophic Factors and Dendritic Spines.
von Bohlen Und Halbach O. von Bohlen Und Halbach O. Adv Neurobiol. 2023;34:223-254. doi: 10.1007/978-3-031-36159-3_5. Adv Neurobiol. 2023. PMID: 37962797 - Neddylation inhibition impairs spine development, destabilizes synapses and deteriorates cognition.
Vogl AM, Brockmann MM, Giusti SA, Maccarrone G, Vercelli CA, Bauder CA, Richter JS, Roselli F, Hafner AS, Dedic N, Wotjak CT, Vogt-Weisenhorn DM, Choquet D, Turck CW, Stein V, Deussing JM, Refojo D. Vogl AM, et al. Nat Neurosci. 2015 Feb;18(2):239-51. doi: 10.1038/nn.3912. Epub 2015 Jan 12. Nat Neurosci. 2015. PMID: 25581363 - Pannexin 1 Regulates Dendritic Protrusion Dynamics in Immature Cortical Neurons.
Sanchez-Arias JC, Candlish RC, van der Slagt E, Swayne LA. Sanchez-Arias JC, et al. eNeuro. 2020 Aug 26;7(4):ENEURO.0079-20.2020. doi: 10.1523/ENEURO.0079-20.2020. Print 2020 Jul/Aug. eNeuro. 2020. PMID: 32737184 Free PMC article.
References
- Berry M, Hollingworth T, Flinn RM, Anderson EM. The growth of Purkinje cell dendrites of the rat –a quantitative study. J Anat. 1972;111:491–493. - PubMed
- Collin C, Miyaguchi K, Segal M. Dendritic spine density and LTP induction in cultured hippocampal slices. J Neurophysiol. 1997;77:1614–1623. - PubMed
- Cooper MW, Smith SJ. A real-time analysis of growth cone-target cell interactions during the formation of stable contacts between hippocampal neurons in culture. J Neurobiol. 1992;23:814–828. - PubMed
- Cotman C, Taylor D, Lynch G. Ultrastructural changes in synapses in the dentate gyrus of the rat during development. Brain Res. 1973;63:205–213. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 MH/DA 57351/MH/NIMH NIH HHS/United States
- P30 HD018655/HD/NICHD NIH HHS/United States
- R01 DA057351/DA/NIDA NIH HHS/United States
- R01 NS021184/NS/NINDS NIH HHS/United States
- R01 NS033574/NS/NINDS NIH HHS/United States
- R37 NS021184/NS/NINDS NIH HHS/United States
- NS21184/NS/NINDS NIH HHS/United States
- NS33374/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous