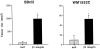Adenoviral gene transfer of beta3 integrin subunit induces conversion from radial to vertical growth phase in primary human melanoma - PubMed (original) (raw)
Adenoviral gene transfer of beta3 integrin subunit induces conversion from radial to vertical growth phase in primary human melanoma
M Y Hsu et al. Am J Pathol. 1998 Nov.
Abstract
Expression of the beta3 subunit of the alphavbeta3 vitronectin receptor on melanoma cells is associated with tumor thickness and the ability to invade and metastasize. To address the role of alphavbeta3 in the complex process of progression from the nontumorigenic radial to the tumorigenic vertical growth phase of primary melanoma, we examined the biological consequences of overexpressing alphavbeta3 in early-stage melanoma cells using an adenoviral vector for gene transfer. Overexpression of functional alphavbeta3 in radial growth phase primary melanoma cells 1) promotes both anchorage-dependent and -independent growth, 2) initiates invasive growth from the epidermis into the dermis in three-dimensional skin reconstructs, 3) prevents apoptosis of invading cells, and 4) increases tumor growth in vivo. Thus, alphavbeta3 serves diverse biological functions during the progression from the nontumorigenic radial growth phase to the tumorigenic and-invasive vertical growth phase primary melanoma.
Figures
Figure 1.
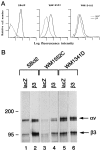
Induction of β3 expression by β3/Ad5. A: Cell surface expression of virally transduced β3 integrin subunit. Virus-infected melanoma cells were trypsinized, sequentially incubated with anti-β3 MAb (SSA6) and FITC-conjugated goat anti-mouse IgG, and analyzed by flow cytometry. The x axis indicates relative fluorescence intensity (log units); the y axis shows the relative cell number. - - - and ——, lacZ/Ad5- and β3/Ad5-infected cells, respectively. B: Complex formation of virus-induced β3 with endogenous αv. Cell lysates of infected melanoma cells were prepared after metabolic labeling, normalized for radioactivity, immunoprecipitated with β3-specific SSA6 MAb, and subjected to electrophoresis. Gels were fixed, dried, and exposed to x-ray film.
Figure 2.
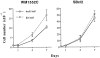
In vitro growth of melanoma cells after β3 overexpression. Two days after viral infection, 2 × 105 cells were seeded into six-well tissue culture plates. Cell growth was monitored on days 1, 4, and 7 using a Coulter counter. Average cell number from triplicate wells was plotted for WM1552 and SBcl2 cells.
Figure 3.
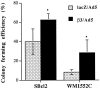
Cell growth in soft agar of melanoma cells overexpressing β3. Melanoma cells were infected at a multiplicity of infection of 20 and, 2 days later, resuspended in 0.25% agar, seeded on an acellular layer, and fed regularly. After 3 to 4 weeks of culture, colony-forming efficiency was determined as the percentage of cells forming colonies containing four or more cells. Ten randomly chosen high-power fields were examined for each condition. *Statistical significance using Student’s _t_-tests; P values were 0.009 and 0.007 for SBcl2 and WM1552C cells, respectively.
Figure 4.
Effect of β3 overexpression on melanoma invasion and survival in three-dimensional skin reconstructs. Virus-infected SBcl2 melanoma cells were incorporated into the epidermis of skin reconstructs as described in Materials and Methods. Mature reconstructs were harvested, fixed, and embedded in paraffin. β3/Ad5-infected SBcl2 cells grew in an invasive pattern reminiscent of VGP primary melanoma (A and C), whereas lacZ/Ad5-infected cells spread horizontally, resembling RGP primary melanoma (B and D). Control virus-infected cells at the dermal/epidermal junction displayed apoptotic features, including nucleus condensation, membrane blebbing, and presence of apoptotic bodies. β3/Ad5-infected cells were completely negative for staining by the ApopTag in situ apoptosis detection kit (E), whereas lacZ/Ad5-infected cells stained positive (F). Magnification, ×100 (A, B, E, and F) and ×259 (C and D).
Figure 5.
Tumorigenicity of melanoma cells overexpressing β3 subunit. Ad5-infected cells were trypsinized and resuspended in growth medium. SCID mice were injected subcutaneously with 3 × 10 cells/mouse in a volume of 100 μl (five mice/group), and tumor size was determined at day 7. Student’s _t_-tests confirmed statistically significant differences between groups with P values <0.05 (SBcl2, P = 0.006; WM1552C, P = 0.001).
Comment in
- Role of the beta3 integrin subunit in human primary melanoma progression: multifunctional activities associated with alpha(v)beta3 integrin expression.
Seftor RE. Seftor RE. Am J Pathol. 1998 Nov;153(5):1347-51. doi: 10.1016/s0002-9440(10)65719-7. Am J Pathol. 1998. PMID: 9811323 Free PMC article. Review. No abstract available.
Similar articles
- Osteonectin/SPARC induction by ectopic beta(3) integrin in human radial growth phase primary melanoma cells.
Sturm RA, Satyamoorthy K, Meier F, Gardiner BB, Smit DJ, Vaidya B, Herlyn M. Sturm RA, et al. Cancer Res. 2002 Jan 1;62(1):226-32. Cancer Res. 2002. PMID: 11782382 - Adenoviral transduction efficiency of ovarian cancer cells can be limited by loss of integrin beta3 subunit expression and increased by reconstitution of integrin alphavbeta3.
Brüning A, Köhler T, Quist S, Wang-Gohrke S, Moebus VJ, Kreienberg R, Runnebaum IB. Brüning A, et al. Hum Gene Ther. 2001 Mar 1;12(4):391-9. doi: 10.1089/10430340150504019. Hum Gene Ther. 2001. PMID: 11242531 - Role of the beta3 integrin subunit in human primary melanoma progression: multifunctional activities associated with alpha(v)beta3 integrin expression.
Seftor RE. Seftor RE. Am J Pathol. 1998 Nov;153(5):1347-51. doi: 10.1016/s0002-9440(10)65719-7. Am J Pathol. 1998. PMID: 9811323 Free PMC article. Review. No abstract available. - Molecular role(s) for integrins in human melanoma invasion.
Seftor RE, Seftor EA, Hendrix MJ. Seftor RE, et al. Cancer Metastasis Rev. 1999;18(3):359-75. doi: 10.1023/a:1006317125454. Cancer Metastasis Rev. 1999. PMID: 10721490 Review.
Cited by
- PIM kinases as therapeutic targets against advanced melanoma.
Shannan B, Watters A, Chen Q, Mollin S, Dörr M, Meggers E, Xu X, Gimotty PA, Perego M, Li L, Benci J, Krepler C, Brafford P, Zhang J, Wei Z, Zhang G, Liu Q, Yin X, Nathanson KL, Herlyn M, Vultur A. Shannan B, et al. Oncotarget. 2016 Aug 23;7(34):54897-54912. doi: 10.18632/oncotarget.10703. Oncotarget. 2016. PMID: 27448973 Free PMC article. - Life isn't flat: taking cancer biology to the next dimension.
Smalley KS, Lioni M, Herlyn M. Smalley KS, et al. In Vitro Cell Dev Biol Anim. 2006 Sep-Oct;42(8-9):242-7. doi: 10.1290/0604027.1. In Vitro Cell Dev Biol Anim. 2006. PMID: 17163781 Review. - Integrin alphav-mediated inactivation of p53 controls a MEK1-dependent melanoma cell survival pathway in three-dimensional collagen.
Bao W, Strömblad S. Bao W, et al. J Cell Biol. 2004 Nov 22;167(4):745-56. doi: 10.1083/jcb.200404018. J Cell Biol. 2004. PMID: 15557124 Free PMC article. - Tumor Cell Adhesion As a Risk Factor for Sentinel Lymph Node Metastasis in Primary Cutaneous Melanoma.
Meves A, Nikolova E, Heim JB, Squirewell EJ, Cappel MA, Pittelkow MR, Otley CC, Behrendt N, Saunte DM, Lock-Andersen J, Schenck LA, Weaver AL, Suman VJ. Meves A, et al. J Clin Oncol. 2015 Aug 10;33(23):2509-15. doi: 10.1200/JCO.2014.60.7002. Epub 2015 Jul 6. J Clin Oncol. 2015. PMID: 26150443 Free PMC article. - CAR T cells targeting αvβ3 integrin are effective against advanced cancer in preclinical models.
Wallstabe L, Mades A, Frenz S, Einsele H, Rader C, Hudecek M. Wallstabe L, et al. Adv Cell Gene Ther. 2018 Sep;1(2):e11. doi: 10.1002/acg2.11. Epub 2018 Jul 10. Adv Cell Gene Ther. 2018. PMID: 30420973 Free PMC article.
References
- Clark WH, Jr, Elder DE, Guerry D, Epstein MN, Greene MH, Van Horn M: A study of tumor progression: the precursor lesions of superficial spreading and nodular melanoma. Hum Pathol 1984, 15:1147-1165 - PubMed
- Clark WH, Jr, Elder DE, Guerry D, IV, Braitman LE, Trock BJ, Schultz D, Synnestvedt M, Halpern AC: Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst 1989, 82:626-627 - PubMed
- Herlyn M, Thurin J, Balaban G, Bennicelli JL, Herlyn D, Elder DE, Bondi E, Guerry D, Nowell P, Clark WH, Koprowski H: Characteristics of cultured human melanocytes isolated from different stages of tumor progression. Cancer Res 1985, 45:5670-5676 - PubMed
- Herlyn M, Balaban G, Bennicelli J, Guerry D, Halaban R, Herlyn D, Elder DE, Maul GG, Steplewski Z, Nowell PC, Clark WH, Jr, Koprowski H: Primary melanoma cells of the vertical growth phase: similarities to metastatic cells. J Natl Cancer Inst 1985, 74:283-289 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical