KDEL receptor (Erd2p)-mediated retrograde transport of the cholera toxin A subunit from the Golgi involves COPI, p23, and the COOH terminus of Erd2p - PubMed (original) (raw)
KDEL receptor (Erd2p)-mediated retrograde transport of the cholera toxin A subunit from the Golgi involves COPI, p23, and the COOH terminus of Erd2p
I Majoul et al. J Cell Biol. 1998.
Abstract
A cholera toxin mutant (CTX-K63) unable to raise cAMP levels was used to study in Vero cells the retrograde transport of the toxin A subunit (CTX-A-K63), which possesses a COOH-terminal KDEL retrieval signal. Microinjected GTP-gamma-S inhibits the internalization as well as Golgi-ER transport of CTX-A-K63. The appearance of CTX-A-K63 in the Golgi induces a marked dispersion of Erd2p and p53 but not of the Golgi marker giantin. Erd2p is translocated under these conditions most likely to the intermediate compartment as indicated by an increased colocalization of Erd2p with mSEC13, a member of the mammalian coat protein II complex. IgGs as well as Fab fragments directed against Erd2p, beta-COP, or p23, a new member of the p24 protein family, inhibit or block retrograde transport of CTX-A-K63 from the Golgi without affecting its internalization or its transport to the Golgi. Anti-Erd2p antibodies do not affect the binding of CTX-A to Erd2p, but inhibit the CTX-K63-induced translocation of Erd2p and p53.
Figures
Figure 1
SDS-PAGE (reducing conditions) of Fab fragments prepared from IgGs directed against the EAGE peptide of β-COP, and the cytoplasmic tails of p23 or Erd2p. Note the complete disappearance of uncleaved IgG (large arrow, IgG heavy chains) and the appearance of a low molecular mass double band (small arrows) representing Fab and Fc fragments respectively. M, molecular mass markers.
Figure 2
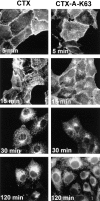
Time-dependent transport of wild-type CTX-A and mutant CTX-A (CTX-A–K63) from the plasma membrane to the ER in Vero cells after incubation with either wild-type CTX or CTX–K63, in which serine63 of the A subunit has been replaced by lysine. The initial concentration of the toxins was 0.5 μg/ml. Binding and initiation of uptake of wild-type or mutated CTX was performed as given in the Materials and Methods section (under “Methods”).
Figure 3
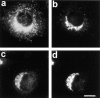
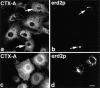
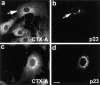
Dispersion of Erd2p (a), but not of giantin (b) 40 min after start of uptake of CTX–K63 (0.5 μg/ml). (c and d) Distribution of Erd2p and giantin, respectively, in control cells incubated in the absence of CTX–K63. The experiment was performed as given in the legend to Fig. 2. Bar, 10 μm.
Figure 4
Uptake of CTX–K63 leads to a concomitant dispersion of Erd2p and p53 in Vero cells. Vero cells were incubated with 0.5 μg/ml CTX–K63 and the uptake started as in Fig. 2. At zero time (top panels) and after 50 min (bottom panels) cells were fixed and immunostained for Erd2p and p53 using Cy2-labeled anti-Erd2p (rabbit) and a mouse anti-p53 antibody. Note that Erd2p as well as p53 change from a cap-like to a more circular distribution in the presence of the KDEL cargo (CTX-A–K63). Bar, 10 μm.
Figure 5
Distribution of Erd2p and mSEC13 in Vero cells under steady-state conditions. Bar, 10 μm.
Figure 6
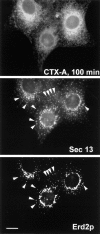
Retrograde transport of CTX-A–K63 induces an increased colocalization (arrows) of Erd2p and mSEC13. Cells were fixed and analyzed 100 min after start of CTX–K63 uptake by using triple immunofluorescence. Note that the distribution of mSEC13 shows only minor changes in the presence of CTX–K63 (compare with Fig. 5), but that Erd2p exhibits a shift from a Golgi-like distribution towards punctated structures costaining for mSEC13. Bar, 10 μm.
Figure 7
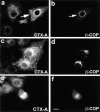
Fab fragments directed against the cytoplasmic tail of Erd2p inhibit retrograde transport of CTX-A–K63 from the Golgi to the ER in Vero cells. The Fab fragments were microinjected 10–15 min before uptake of CTX– K63 was initiated as given in the Materials and Methods section. The cells were further incubated for 120 min. The cells were then fixed and immunostained for CTX-A (a and c). The microinjected cells (a and b, arrows) were identified by the immunofluorescence of Cy2-labeled anti–Erd2p Fab fragments that had been mixed at a 1:3 ratio with non-labeled anti– Erd2p Fab fragments (b and d). Note the retention of CTX-A–K63 in perinuclear structures in cells microinjected with the anti–Erd2p Fab fragments. a and b show the results of laser scan microscopy; c and d show the results of conventional immunofluorescence microscopy. Bar, 10 μm.
Figure 8
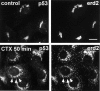
Antibodies against Erd2p inhibit the CTX-K63–induced dispersion of Erd2p and of p53. The experiment was performed as in Fig. 7. 50 min after start of CTX– K63 uptake, the cells were fixed and processed for staining with anti-Erd2p and anti-p53 (triple immunofluorescence). (a) microinjected cell stained by microinjected Cy2-labeled anti–Erd2p Fab fragments; (b) staining for Erd2p after fixation and permeabilization with Cy3-labeled anti-Erd2p antibodies; (c) staining for p53 using mouse anti-p53 and an AMCA-labeled secondary anti–mouse IgG antibody. Note the strong reduction of dispersion of Erd2p and p53 in the anti-Erdp–injected cell. Bar, 10 μm.
Figure 9
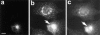
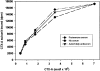
Binding of CTX-A to Erd2p of Golgi membranes from Sf9 cells is not affected by antibodies directed against the cytoplasmic tail of Erd2p. Erd2p was overexpressed in Sf9 cells using Bacculovirus infection. Golgi membranes from overexpressing Sf9 cells were prepared and permeabilized as given in the Materials and Methods section and incubated at pH 7.2 with preimmune serum, antiserum directed against the cytoplasmic tail of Erd2p, or without serum. The pH was then adjusted to 6.0 and the binding of increasing concentrations of 125I–CTX-A was measured. Blanks without membranes (ranging from ∼200 dpm to ∼1,800 dpm for the different concentrations of labeled CTX-A used) were subtracted. The binding curves obtained after the three different preincubation conditions show only minor (<5%) differences.
Figure 10
Fab fragments from IgGs directed against the EAGE peptide of β-COP inhibit the translocation of CTX-A–K63 from the Golgi to the ER in Vero cells. Fab fragments were microinjected 10–15 min before starting the uptake of CTX– K63 as in Fig. 2. Incubation of the cells was continued for another 120 min. The cells were then fixed and processed for double immunofluorescence. Microinjected cells are identified by immunofluorescence of Cy2-labeled anti–β-COP Fab fragments that had been mixed with the unlabeled anti–β-COP Fab fragments at a 1:3 ratio. a, c, and e show CTX-A–K63; b, d, and f β-COP. a and b were obtained by laser scan microscopy (microinjected cell indicated by arrow); c–f were obtained by conventional immunofluorescence microscopy. Bar, 10 μm.
Figure 11
Fab fragments from IgGs directed against the COOH-terminal tail of p23 inhibit the retrograde transport of CTX-A–K63 from the Golgi to the ER in Vero cells. The Fab fragments were microinjected 10–15 min before starting CTX–K63 uptake. The cells were further incubated for 120 min as in Fig. 2 followed by fixation and double-immunofluorescence microscopy. (a and c) CTX-A–K63; (b and d) p23. Microinjected cells are identified using Cy2-labeled anti–p23 Fab fragments that mixed with unlabeled anti-p23 at a 1:3 ratio. a and b were obtained by laser scan microscopy (microinjected cell indicated by arrows); c and d were obtained by conventional immunofluorescence microscopy. Bar, 10 μm.
Figure 12
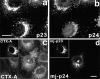
Comparison of effects of antibodies directed against the COOH termini of p23 and p24 on retrograde transport of CTX-A–K63 from the Golgi to the ER. (a and b) Vero cells that were neither treated with CTX– K63 nor microinjected, were fixed, permeabilized, and then immunostained for p24 or p23 using Cy5- and Cy2- labeled primary antibodies, respectively. Both antibodies stain similar structures, mainly representing Golgi membranes. (c and d) Vero cells were loaded with CTX–K63 as given in Fig. 2. About 20 min after the start of CTX– K63 uptake, antibodies against p24 were microinjected. As control, cells from the same coverslip were injected with antibodies directed against the COOH terminus of p23 (inset). Cells were then incubated for another 80 min, fixed, and then analyzed for the distribution of CTX-A–K63 (c) or p24 (d) or p23 (inset in d) by laser scan immunofluorescence microscopy. The cell microinjected with the anti-p24 antibodies is marked by an asterisk. Bar, 10 μm.
Similar articles
- Dissociation of coatomer from membranes is required for brefeldin A-induced transfer of Golgi enzymes to the endoplasmic reticulum.
Scheel J, Pepperkok R, Lowe M, Griffiths G, Kreis TE. Scheel J, et al. J Cell Biol. 1997 Apr 21;137(2):319-33. doi: 10.1083/jcb.137.2.319. J Cell Biol. 1997. PMID: 9128245 Free PMC article. - Transport of an external Lys-Asp-Glu-Leu (KDEL) protein from the plasma membrane to the endoplasmic reticulum: studies with cholera toxin in Vero cells.
Majoul IV, Bastiaens PI, Söling HD. Majoul IV, et al. J Cell Biol. 1996 May;133(4):777-89. doi: 10.1083/jcb.133.4.777. J Cell Biol. 1996. PMID: 8666663 Free PMC article. - Immunocytochemical localization of beta-COP to the ER-Golgi boundary and the TGN.
Griffiths G, Pepperkok R, Locker JK, Kreis TE. Griffiths G, et al. J Cell Sci. 1995 Aug;108 ( Pt 8):2839-56. doi: 10.1242/jcs.108.8.2839. J Cell Sci. 1995. PMID: 7593324 - Evidence for a COP-I-independent transport route from the Golgi complex to the endoplasmic reticulum.
Girod A, Storrie B, Simpson JC, Johannes L, Goud B, Roberts LM, Lord JM, Nilsson T, Pepperkok R. Girod A, et al. Nat Cell Biol. 1999 Nov;1(7):423-30. doi: 10.1038/15658. Nat Cell Biol. 1999. PMID: 10559986 - Pathogenic Effects of Impaired Retrieval between the Endoplasmic Reticulum and Golgi Complex.
Kokubun H, Jin H, Aoe T. Kokubun H, et al. Int J Mol Sci. 2019 Nov 9;20(22):5614. doi: 10.3390/ijms20225614. Int J Mol Sci. 2019. PMID: 31717602 Free PMC article. Review.
Cited by
- Differential induction of two p24delta putative cargo receptors upon activation of a prohormone-producing cell.
Kuiper RP, Waterham HR, Rötter J, Bouw G, Martens GJ. Kuiper RP, et al. Mol Biol Cell. 2000 Jan;11(1):131-40. doi: 10.1091/mbc.11.1.131. Mol Biol Cell. 2000. PMID: 10637296 Free PMC article. - Quantitative ER <--> Golgi transport kinetics and protein separation upon Golgi exit revealed by vesicular integral membrane protein 36 dynamics in live cells.
Dahm T, White J, Grill S, Füllekrug J, Stelzer EH. Dahm T, et al. Mol Biol Cell. 2001 May;12(5):1481-98. doi: 10.1091/mbc.12.5.1481. Mol Biol Cell. 2001. PMID: 11359937 Free PMC article. - Receptor for retrograde transport in the apicomplexan parasite Toxoplasma gondii.
Pfluger SL, Goodson HV, Moran JM, Ruggiero CJ, Ye X, Emmons KM, Hager KM. Pfluger SL, et al. Eukaryot Cell. 2005 Feb;4(2):432-42. doi: 10.1128/EC.4.2.432-442.2005. Eukaryot Cell. 2005. PMID: 15701805 Free PMC article. - The cargo receptors Surf4, endoplasmic reticulum-Golgi intermediate compartment (ERGIC)-53, and p25 are required to maintain the architecture of ERGIC and Golgi.
Mitrovic S, Ben-Tekaya H, Koegler E, Gruenberg J, Hauri HP. Mitrovic S, et al. Mol Biol Cell. 2008 May;19(5):1976-90. doi: 10.1091/mbc.e07-10-0989. Epub 2008 Feb 20. Mol Biol Cell. 2008. PMID: 18287528 Free PMC article. - Rab6 coordinates a novel Golgi to ER retrograde transport pathway in live cells.
White J, Johannes L, Mallard F, Girod A, Grill S, Reinsch S, Keller P, Tzschaschel B, Echard A, Goud B, Stelzer EH. White J, et al. J Cell Biol. 1999 Nov 15;147(4):743-60. doi: 10.1083/jcb.147.4.743. J Cell Biol. 1999. PMID: 10562278 Free PMC article.
References
- Barlowe C, d'Enfert C, Schekman R. Purification and characterization of SAR1p, a small GTP-binding protein required for transport vesicle formation from the endoplasmic reticulum. J Biol Chem. 1993;268:873–879. - PubMed
- Barthel A, Nickel W, Tonko C, Söling HD. Sorting and budding of constitutive secretory vesicles in hepatocytes and hepatoma cells. Adv Enzyme Regul. 1995;35:283–292. - PubMed
- Belden WJ, Barlowe C. Erv25p, a component of COP II-coated vesicles, forms a complex with emp24p that is required for efficient endoplasmic reticulum to Golgi transport. J Biol Chem. 1996;271:26939–26946. - PubMed
- Blum R, Feick P, Puype M, Vanderkerckhove J, Klengel R, Mystainczyk W, Schulz I. Tmp21 and p24A. Two type I proteins enriched in pancreatic microsomal membranes, are members of a family involved in vesicular trafficking. J Biol Chem. 1996;27:17183–17189. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous