The cancer growth suppressor gene mda-7 selectively induces apoptosis in human breast cancer cells and inhibits tumor growth in nude mice - PubMed (original) (raw)
The cancer growth suppressor gene mda-7 selectively induces apoptosis in human breast cancer cells and inhibits tumor growth in nude mice
Z Z Su et al. Proc Natl Acad Sci U S A. 1998.
Abstract
A differentiation induction subtraction hybridization strategy is being used to identify and clone genes involved in growth control and terminal differentiation in human cancer cells. This scheme identified melanoma differentiation associated gene-7 (mda-7), whose expression is up-regulated as a consequence of terminal differentiation in human melanoma cells. Forced expression of mda-7 is growth inhibitory toward diverse human tumor cells. The present studies elucidate the mechanism by which mda-7 selectively suppresses the growth of human breast cancer cells and the consequence of ectopic expression of mda-7 on human breast tumor formation in vivo in nude mice. Infection of wild-type, mutant, and null p53 human breast cancer cells with a recombinant type 5 adenovirus expressing mda-7, Ad.mda-7 S, inhibited growth and induced programmed cell death (apoptosis). Induction of apoptosis correlated with an increase in BAX protein, an established inducer of programmed cell death, and an increase in the ratio of BAX to BCL-2, an established inhibitor of apoptosis. Infection of breast carcinoma cells with Ad.mda-7 S before injection into nude mice inhibited tumor development. In contrast, ectopic expression of mda-7 did not significantly alter cell cycle kinetics, growth rate, or survival in normal human mammary epithelial cells. These data suggest that mda-7 induces its selective anticancer properties in human breast carcinoma cells by promoting apoptosis that occurs independent of p53 status. On the basis of its selective anticancer inhibitory activity and its direct antitumor effects, mda-7 may represent a new class of cancer suppressor genes that could prove useful for the targeted therapy of human cancer.
Figures
Figure 1
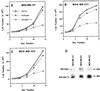
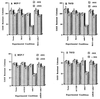
Effect of Ad. Vec, Ad.β-gal, and Ad._mda-_7 S on the growth of normal breast and breast cancer cells. The indicated cell types were infected with 100 pfu/cell of Ad.Vec, Ad.β-gal, or Ad._mda-_7 S, and cell growth was determined over a 14-day period. Triplicate samples varied by ≤10%. Similar results (±15%) were obtained in two additional replicate studies. In the lower panels, β-galactosidase activity was assayed in HMEC (A), HBL-100 (B), T47D (C), and MCF-7 (D) cells 48 hr after infection with the Ad.β-gal.
Figure 2
Effect of Ad. _mda-_7 S on growth and BAX protein levels in human breast cancer cells. The experimental growth protocol was as described in the legend to Fig. 1. The breast carcinoma cell lines analyzed include MDA-MB-157 (A), MDA-MB-231 (B), and MDA-MB-453 (C). D provides immunoblot analyses of BAX expression 2 days after infection of the indicated breast cancer cell line with 100 pfu/cell of Ad.Vec or Ad._mda-_7 S. Coomassie blue staining of gels indicated equal protein loading.
Figure 3
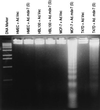
Induction of nucleosomal DNA degradation in human breast cancer cells, but not in normal breast epithelial cells, infected with Ad._mda-_7 S. The indicated cell types were infected with 100 pfu/cell of Ad.Vec or Ad._mda-_7 (S) and were analyzed for nucleosomal DNA degradation 4 days after infection.
Figure 4
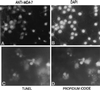
Nuclear localization of _mda-_7 and induction of a positive TUNEL reaction in MCF-7 cells infected with Ad. _mda-_7 S. MCF-7 cells were doubly stained with Anti-_MDA-_7 antibody (A) and 4′,6-diamidino-2-phenylindole (DAPI) (B) 2 days after infection with 100pfu/cell of Ad._mda-_7 S. The position of two mitotic cells stained with Anti-_mda-_7 antibody are shown in A, and the corresponding 4′,6-diamidino-2-phenylindole counterstain is indicated in B (arrows label metaphase chromosomes). MCF-7 cells 4 days after infection with 100 pfu/cell of Ad._mda-_7 S were doubly stained by the TUNEL method (C) and propidium iodide (D).
Figure 5
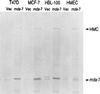
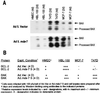
Immunoprecipitation of _MDA-_7 and an HMC protein with an _MDA-_7 mAb. The indicated cell lines were infected with 100 pfu/cell of Ad.Vec or Ad._mda-_7 for 4 days and were labeled with [35S]methionine, and the levels of the _MDA-_7 and HMC proteins were determined by immunoprecipitation analysis. Coomassie blue staining of gels indicated equal protein loading.
Figure 6
Expression of Bcl-2, Bax, and Bak in normal breast epithelial and breast carcinoma cells infected with Ad. _mda-_7 S or Ad.Vec. (A) Immunoblot analyses of BAX protein in normal mammary epithelial and cancer cells. Equal amounts of whole cell protein (verified by Coomassie blue staining) from 2- and 4-day cell cultures infected with 100 pfu/cell of Ad.Vec or Ad._mda-_7 S were resolved by SDS/PAGE (4–20%), were immunoblotted, and were probed with Anti-BAX mAb. Note that both intact BAX and processed BAX proteins are visible in the breast cancer cell lines 2 days after Ad._mda-_7 infection. Low levels of BAX in 4-day MCF-7 is caused by proteolytic degradation because the cells in this study were 70% apoptotic by 4 days after infection with Ad._mda-_7 S. (B) Tabular compilation of protein levels of the Bcl-2 gene family members, BCL-2 and BAK, 4 days after infection with 100 pfu/cell of Ad._mda-_7 S or Ad.Vec in normal breast epithelial cells and breast carcinoma cell lines.
Figure 7
Effect of inducible _mda-_7 expression alone and in combination with Bcl-2 or Ad E1B expression on colony formation in MCF-7 and T47D cells. Cells were transfected with a pMAMneo-_mda-_7 and a pSFFV-bcl-2 [MCF-7 (A) or T47D (B)] or a pCMVE1B [MCF-7 (C) and T47D (D)] expression plasmid, alone and in combination, and were grown in medium containing 300 μg of G418 per milliliter and in the presence or absence of 1 × 10−6 M dexamethasone (DEX). Colonies were enumerated after ≈3–4 weeks of growth ± SD for five replicate plates. Similar results have been obtained ±15% in a replicate study (data not shown).
Figure 8
Effect of _mda-_7 on the growth of MCF-7 cells in nude mice. MCF-7 cells were uninfected (CONTROL) or infected with 100 pfu/cell of Ad.Vec or Ad._mda-_7 S and were incubated for 4 days at 37°C. Cells were removed with trypsin, were mixed with Matrigel (1:1), and were injected into nude mice (106 cells/animal). Results are shown as tumor weight in grams ± SD. Statistical significance was determined with a Student’s t test using the computer program
sigma stat
(Jandel, San Rafael, CA), and a P value of <0.05 is indicated by an asterisk. Two additional tumorigenicity studies have been performed with qualitatively similar results (data not shown).
Similar articles
- A combinatorial approach for selectively inducing programmed cell death in human pancreatic cancer cells.
Su Z, Lebedeva IV, Gopalkrishnan RV, Goldstein NI, Stein CA, Reed JC, Dent P, Fisher PB. Su Z, et al. Proc Natl Acad Sci U S A. 2001 Aug 28;98(18):10332-7. doi: 10.1073/pnas.171315198. Proc Natl Acad Sci U S A. 2001. PMID: 11526239 Free PMC article. - Tumor-suppressive effects by adenovirus-mediated mda-7 gene transfer in non-small cell lung cancer cell in vitro.
Saeki T, Mhashilkar A, Chada S, Branch C, Roth JA, Ramesh R. Saeki T, et al. Gene Ther. 2000 Dec;7(23):2051-7. doi: 10.1038/sj.gt.3301330. Gene Ther. 2000. PMID: 11175318 - The cancer growth suppressing gene mda-7 induces apoptosis selectively in human melanoma cells.
Lebedeva IV, Su ZZ, Chang Y, Kitada S, Reed JC, Fisher PB. Lebedeva IV, et al. Oncogene. 2002 Jan 24;21(5):708-18. doi: 10.1038/sj.onc.1205116. Oncogene. 2002. PMID: 11850799 - mda-7/IL-24, a novel cancer selective apoptosis inducing cytokine gene: from the laboratory into the clinic.
Fisher PB, Gopalkrishnan RV, Chada S, Ramesh R, Grimm EA, Rosenfeld MR, Curiel DT, Dent P. Fisher PB, et al. Cancer Biol Ther. 2003 Jul-Aug;2(4 Suppl 1):S23-37. Cancer Biol Ther. 2003. PMID: 14508078 Review. - Restoring apoptosis as a strategy for cancer gene therapy: focus on p53 and mda-7.
Lebedeva IV, Su ZZ, Sarkar D, Fisher PB. Lebedeva IV, et al. Semin Cancer Biol. 2003 Apr;13(2):169-78. doi: 10.1016/s1044-579x(02)00134-7. Semin Cancer Biol. 2003. PMID: 12654260 Review.
Cited by
- Interleukin-24 Immunobiology and Its Roles in Inflammatory Diseases.
Zhong Y, Zhang X, Chong W. Zhong Y, et al. Int J Mol Sci. 2022 Jan 6;23(2):627. doi: 10.3390/ijms23020627. Int J Mol Sci. 2022. PMID: 35054813 Free PMC article. Review. - Molecular targets and signaling pathways regulated by interleukin (IL)-24 in mediating its antitumor activities.
Panneerselvam J, Munshi A, Ramesh R. Panneerselvam J, et al. J Mol Signal. 2013 Dec 30;8(1):15. doi: 10.1186/1750-2187-8-15. J Mol Signal. 2013. PMID: 24377906 Free PMC article. - RGD-IL-24, a novel tumor-targeted fusion cytokine: expression, purification and functional evaluation.
Xiao B, Li W, Yang J, Guo G, Mao XH, Zou QM. Xiao B, et al. Mol Biotechnol. 2009 Feb;41(2):138-44. doi: 10.1007/s12033-008-9115-y. Epub 2008 Oct 25. Mol Biotechnol. 2009. PMID: 18953678 - mda-7/IL-24: multifunctional cancer-specific apoptosis-inducing cytokine.
Gupta P, Su ZZ, Lebedeva IV, Sarkar D, Sauane M, Emdad L, Bachelor MA, Grant S, Curiel DT, Dent P, Fisher PB. Gupta P, et al. Pharmacol Ther. 2006 Sep;111(3):596-628. doi: 10.1016/j.pharmthera.2005.11.005. Epub 2006 Feb 7. Pharmacol Ther. 2006. PMID: 16464504 Free PMC article. Review. - Insights into glutamate transport regulation in human astrocytes: cloning of the promoter for excitatory amino acid transporter 2 (EAAT2).
Su ZZ, Leszczyniecka M, Kang DC, Sarkar D, Chao W, Volsky DJ, Fisher PB. Su ZZ, et al. Proc Natl Acad Sci U S A. 2003 Feb 18;100(4):1955-60. doi: 10.1073/pnas.0136555100. Epub 2003 Feb 10. Proc Natl Acad Sci U S A. 2003. PMID: 12578975 Free PMC article.
References
- Jiang H, Lin J, Fisher P B. Mol Cell Differ. 1994;2:221–239.
- Waxman S, editor. Differentiation Therapy. Vol. 10. Rome: Ares Serono Symposia Publications; 1995. pp. 1–531.
- Jiang H, Lin J J, Su Z-z, Goldstein N I, Fisher P B. Oncogene. 1995;11:2477–2486. - PubMed
- Jiang H, Fisher P B. Mol Cell Differ. 1993;1:285–299.
- Jiang H, Lin J, Su Z-z, Kerbel R S, Herlyn M, Weissman R B, Welch D, Fisher P B. Oncogene. 1995;10:1855–1864. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA72994/CA/NCI NIH HHS/United States
- R01 CA035675/CA/NCI NIH HHS/United States
- CA35675/CA/NCI NIH HHS/United States
- R01 GM031452/GM/NIGMS NIH HHS/United States
- GM31452/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous