Nerve growth factor-dependent activation of NF-kappaB contributes to survival of sympathetic neurons - PubMed (original) (raw)
Nerve growth factor-dependent activation of NF-kappaB contributes to survival of sympathetic neurons
S B Maggirwar et al. J Neurosci. 1998.
Abstract
Neurotrophins activate multiple signaling pathways in neurons. However, the precise roles of these signaling molecules in cell survival are not well understood. In this report, we show that nerve growth factor (NGF) activates the transcription factors NF-kappaB and AP-1 in cultured sympathetic neurons. Activated NF-kappaB complexes were shown to consist of heterodimers of p50 and Rel proteins (RelA, as well as c-Rel), and NF-kappaB activation was found to occur independently of de novo protein synthesis but in a manner that required the action of the proteasome complex. Treatment with the NF-kappaB inhibitory peptide SN50 in the continuous presence of NGF resulted in dose-dependent induction of cell death. Under the conditions used, SN50 was shown to selectively inhibit NF-kappaB activation but not the activation of other cellular transcription factors such as AP-1 and cAMP response element-binding protein. Cells treated with SN50 exhibited morphological and biochemical hallmarks of apoptosis, and the kinetics of cell killing were accelerated relative to death induced by NGF withdrawal. Finally, experiments were conducted to test directly whether NF-kappaB could act as a survival factor for NGF-deprived neurons. Microinjection of cells with an expression plasmid encoding NF-kappaB (c-Rel) resulted in enhanced neuronal survival after withdrawal of NGF, whereas cells that were transfected with a vector encoding a mutated derivative of c-Rel lacking the transactivation domain underwent cell death to the same extent as control cells. Together, these findings suggest that the activation of NF-kappaB/Rel transcription factors may contribute to the survival of NGF-dependent sympathetic neurons.
Figures
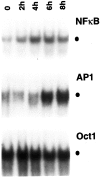
Fig. 1.
Effect of NGF on various transcription factors in sympathetic neurons. NGF was withdrawn from primary cultures of rat sympathetic neurons for 14 hr, after which NGF (100 ng/ml) was added back to the culture for the indicated time periods. Nuclear extracts were prepared from these cells and were then subjected to EMSA using32P-radiolabeled DNA probes as indicated. The specific binding of protein complexes to the different probes is indicated by the filled circle. The results shown are representative of more than three independent experiments; essentially identical results were also obtained when cells were exposed to 50 ng/ml NGF (data not shown).
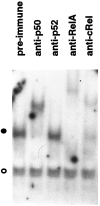
Fig. 2.
NGF-induced NF-κB contains prototypical complexes. Nuclear extracts from SCG neurons maintained in the presence of NGF were subjected to EMSA in the presence of the indicated antisera. The filled circle indicates the specific NF-κB protein–DNA complex; the open circle shows the formation of a nonspecific protein–DNA complex. The results shown are representative of more than three independent experiments.
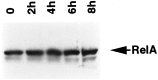
Fig. 3.
NGF-mediated activation of NF-κB is independent of de novo synthesis of RelA. Cultures of SCG neurons were deprived of NGF for 14 hr, followed by incubation with NGF for the indicated periods. Whole-cell extracts were then subjected to immunoblot analysis using antibodies specific for RelA. The results shown are representative of two independent experiments.
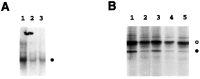
Fig. 4.
Activation of NF-κB by NGF requires the action of the proteasome complex and involves proteolysis of IκB-α.A, SCG neurons maintained in the continuous presence of NGF were treated with the proteasome inhibitors ALLN (50 μ
m
) (lane 2) or MG132 (50 μ
m
) (lane 3), or with medium alone (lane 1), for 2 hr. Nuclear extracts prepared from these cells were subjected to EMSA analysis to detect_κ_B-binding proteins. The filled circle indicates the specific NF-κB–DNA complex.B, SCG neurons were metabolically labeled for 1 hr with 300 μCi/ml [35S]methionine–[35S]cysteine in the presence of NGF (100 ng/ml). The pulse-labeled cells were then lysed and extracted immediately (lane 1) or cold-chased for 2 hr in either the absence (lane 2) or presence of MG132 (50 μ
m
) (lane 3), SN50M (100 μg/ml) (lane 4), or SN50 (100 μg/ml) (lane 5). Whole-cell extracts were isolated and then subjected to immunoprecipitation using IκB-α-specific antiserum as described in Materials and Methods. The _filled circle_indicates IκB-α, whereas the open circle denotes a nonspecific protein. IκB-α levels were quantitated by densitometric analysis of this autoradiogram (NIH Image software) and normalized to the level of the nonspecific protein (open circle). Based on this analysis, IκB-α levels had declined to 18% of starting levels within 2 hr of NGF withdrawal (compare lanes 1, 2). IκB-α degradation was unaffected by addition of the SN50M or SN50 peptides (IκB-α declined to 19 and 15% of its starting level, respectively) (lanes 4,5), but the protein was protected by addition of the proteasome inhibitor MG132 (IκB-α remained at 62% of its initial level) (lane 3). The results shown are representative of two (B) or three (A) independent experiments.
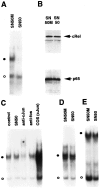
Fig. 5.
Selective inhibition of NF-κB in sympathetic neurons by synthetic peptide SN50. A, Cultures of SCG neurons maintained in the continuous presence of NGF for 5 d were treated with either SN50M or SN50 at a concentration of 75 μg/ml for 4 hr. Nuclear extracts isolated from these cells were used to perform EMSA for analysis of κ_B-binding proteins. The_filled circle indicates the specific NF-κB–DNA complex; the open circle denotes a nonspecific protein–DNA binding complex. B, Whole-cell extracts of SN50M- and SN50-treated neurons maintained in the presence of NGF were subjected to immunoblotting analyses by using anti-RelA and anti-c-RelA antibodies. C, Nuclear extracts of SN50- or SN50M-treated (control) neurons were subjected to EMSA using a 32P-radiolabeled DNA probe corresponding to a consensus AP-1 binding site. The filled circle denotes the AP-1–DNA complex. EMSA was also performed in the presence of anti-c-Jun and anti-c-Fos antibodies (as indicated), and nuclear extracts from c-Jun transfected COS-1 cells were included as a control (COScJun). D, E, EMSA was also performed using nuclear extracts of SN50- and SN50M-treated neurons (as indicated) with radiolabeled oligonucleotide probes specific for CREB (D) and OCT (E) transcription factors. The results shown are representative of a minimum of either two (B) or three (all other panels) experiments.
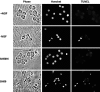
Fig. 6.
SN50 induces cell death with characteristic features of apoptosis. Cultured neurons were either maintained in the continuous presence of NGF, deprived of NGF, or treated with NGF with SN50 or SN50M peptides (100 μg/ml each). After 24 hr, cells were fixed, analyzed for TUNEL reactivity, and stained with Hoechst dye to label nuclei. Phase-contrast, TUNEL, and Hoechst images of the same field of view are shown for each treatment. _Arrowheads_indicate examples of cells deprived of NGF or treated with SN50 that have condensed chromatin and TUNEL reactivity.
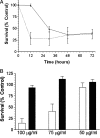
Fig. 7.
Death of sympathetic neurons treated with SN50 is time- and dose-dependent. A, Neuronal cultures were either deprived of NGF (black line, filled triangles) or treated with 100 μg/ml SN50 in the presence of NGF (dashed line, open squares). Survival was assayed by MTT reduction at 12, 30, 48, and 72 hr after a single application of SN50. Data represents the mean ± SEM from three independent experiments. B, Neuronal cultures were treated with a single application of 50, 75, or 100 μg/ml SN50 (open bars) or SN50M (filled bars), both in the presence of NGF. Viability was assayed after 48 hr of treatment by Nissl staining as described in Materials and Methods. The results represent the mean ± SEM of at least three independent experiments. For both A and_B_, results are expressed as a percentage of the survival of control neurons maintained in the continuous presence of NGF.
Fig. 8.
A, Overexpression of c-Rel promotes survival in NGF-deprived neurons. Shown are images of NGF-deprived neurons after injection with either c-Rel or c-Rel(1–359) expression vectors. Neurons were microinjected and then deprived of NGF for 48 hr. Cells were then stained with Hoechst dye and visualized by phase-contrast and fluorescence microscopy. Shown are phase-contrast views, rhodamine-positive cells (injected cells), and Hoechst-stained nuclei in one field of view for each injected DNA.Arrowheads for c-Rel injections indicate healthy looking injected cells with normal nuclear chromatin. Arrowheads_for c-Rel(1–359) indicate injected cells containing condensed nuclear chromatin. B, Overexpression of c-Rel is sufficient to prevent neuronal cell death caused by NGF deprivation. Cultured neurons were microinjected with plasmids encoding either c-Rel, c-Rel(1–359), or LacZ, and then deprived of NGF for 48 hr as described in Materials and Methods. Survival was assayed on the basis of cellular morphology (as viewed by phase-contrast microscopy) and by the presence or absence of condensed chromatin (detected with Hoechst dye). The results (mean ± SEM) from at least three independent experiments are shown. Survival for c-Rel-injected cells was significantly different from LacZ- or c-Rel(1–359)-injected neurons (two-tailed_t test; p < 0.0001 in both cases).
Similar articles
- Analysis of the NF-kappa B and PI 3-kinase/Akt survival pathways in nerve growth factor-dependent neurons.
Sarmiere PD, Freeman RS. Sarmiere PD, et al. Mol Cell Neurosci. 2001 Sep;18(3):320-31. doi: 10.1006/mcne.2001.1021. Mol Cell Neurosci. 2001. PMID: 11591132 - Mechanism for biphasic rel A. NF-kappaB1 nuclear translocation in tumor necrosis factor alpha-stimulated hepatocytes.
Han Y, Brasier AR. Han Y, et al. J Biol Chem. 1997 Apr 11;272(15):9825-32. doi: 10.1074/jbc.272.15.9825. J Biol Chem. 1997. PMID: 9092517 - Hepatitis B virus HBx protein activates transcription factor NF-kappaB by acting on multiple cytoplasmic inhibitors of rel-related proteins.
Su F, Schneider RJ. Su F, et al. J Virol. 1996 Jul;70(7):4558-66. doi: 10.1128/JVI.70.7.4558-4566.1996. J Virol. 1996. PMID: 8676482 Free PMC article. - [The Rel/NF-kappa-B transcription factors: complex role in cell regulation].
Bottex-Gauthier C, Pollet S, Favier A, Vidal DR. Bottex-Gauthier C, et al. Pathol Biol (Paris). 2002 Apr;50(3):204-11. doi: 10.1016/s0369-8114(02)00289-4. Pathol Biol (Paris). 2002. PMID: 11980335 Review. French. - Regulation of DNA binding by Rel/NF-kappaB transcription factors: structural views.
Chen FE, Ghosh G. Chen FE, et al. Oncogene. 1999 Nov 22;18(49):6845-52. doi: 10.1038/sj.onc.1203224. Oncogene. 1999. PMID: 10602460 Review.
Cited by
- Brimonidine prevents axonal and somatic degeneration of retinal ganglion cell neurons.
Lambert WS, Ruiz L, Crish SD, Wheeler LA, Calkins DJ. Lambert WS, et al. Mol Neurodegener. 2011 Jan 13;6(1):4. doi: 10.1186/1750-1326-6-4. Mol Neurodegener. 2011. PMID: 21232114 Free PMC article. - Nuclear factor-kappa B family member RelB inhibits human immunodeficiency virus-1 Tat-induced tumor necrosis factor-alpha production.
Kiebala M, Polesskaya O, Yao Z, Perry SW, Maggirwar SB. Kiebala M, et al. PLoS One. 2010 Jul 29;5(7):e11875. doi: 10.1371/journal.pone.0011875. PLoS One. 2010. PMID: 20686703 Free PMC article. - Activation of NF-kappaB is essential for hepatocyte growth factor-mediated proliferation and tubulogenesis.
Müller M, Morotti A, Ponzetto C. Müller M, et al. Mol Cell Biol. 2002 Feb;22(4):1060-72. doi: 10.1128/MCB.22.4.1060-1072.2002. Mol Cell Biol. 2002. PMID: 11809798 Free PMC article. - Transcriptional Regulation of the Sodium-activated Potassium Channel SLICK (KCNT2) Promoter by Nuclear Factor-κB.
Tomasello DL, Gancarz-Kausch AM, Dietz DM, Bhattacharjee A. Tomasello DL, et al. J Biol Chem. 2015 Jul 24;290(30):18575-83. doi: 10.1074/jbc.M115.643536. Epub 2015 Jun 21. J Biol Chem. 2015. PMID: 26100633 Free PMC article. - Rel/Nuclear factor-kappa B apoptosis pathways in human cervical cancer cells.
Shehata MF. Shehata MF. Cancer Cell Int. 2005 Apr 27;5(1):10. doi: 10.1186/1475-2867-5-10. Cancer Cell Int. 2005. PMID: 15857509 Free PMC article.
References
- Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072:129–157. - PubMed
- Arsura M, Wu M, Sonenshein GE. TGF β1 inhibits NF-κB/Rel activity inducing apoptosis of B cells: transcriptional activation of IκBα. Immunity. 1996;5:31–40. - PubMed
- Beg AA, Baltimore D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science. 1996;274:782–784. - PubMed
- Behl C, Davis JB, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid β protein toxicity. Cell. 1994;77:817–827. - PubMed
- Bertrand F, Atfi A, Cadoret A, L’Allemain G, Robin H, Lascols O, Capeau J, Cherqui G. A role for nuclear factor κB in the antiapoptotic function of insulin. J Biol Chem. 1998;273:2931–2938. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS34400/NS/NINDS NIH HHS/United States
- ES07026/ES/NIEHS NIH HHS/United States
- R01 ES007026/ES/NIEHS NIH HHS/United States
- R01 NS034400/NS/NINDS NIH HHS/United States
- P01 MH57556/MH/NIMH NIH HHS/United States
- T32 ES007026/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials