Complement-dependent clearance of apoptotic cells by human macrophages - PubMed (original) (raw)
Complement-dependent clearance of apoptotic cells by human macrophages
D Mevorach et al. J Exp Med. 1998.
Abstract
Apoptotic cells are rapidly engulfed by phagocytes, but the receptors and ligands responsible for this phenomenon are incompletely characterized. Previously described receptors on blood- derived macrophages have been characterized in the absence of serum and show a relatively low uptake of apoptotic cells. Addition of serum to the phagocytosis assays increased the uptake of apoptotic cells by more than threefold. The serum factors responsible for enhanced uptake were identified as complement components that required activation of both the classical pathway and alternative pathway amplification loop. Exposure of phosphatidylserine on the apoptotic cell surface was partially responsible for complement activation and resulted in coating the apoptotic cell surface with C3bi. In the presence of serum, the macrophage receptors for C3bi, CR3 (CD11b/CD18) and CR4 (CD11c/CD18), were significantly more efficient in the uptake of apoptotic cells compared with previously described receptors implicated in clearance. Complement activation is likely to be required for efficient uptake of apoptotic cells within the systemic circulation, and early component deficiencies could predispose to systemic autoimmunity by enhanced exposure to and/or aberrant deposition of apoptotic cells.
Figures
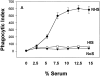
Figure 1
Heat-labile serum factor(s) augment the phagocytosis of apoptotic cells in a concentration-, time-, and temperature-dependent fashion. (A) Apoptotic murine thymocytes were offered to human macrophages in the presence of 15% normal non– heat-inactivated human serum (NHS), 15% normal heat-inactivated (56°C for 30 min) serum (HIS), or without (NoS) the addition of serum, as described in Materials and Methods. The results are expressed as PI, and the mean ± SD of five experiments is shown. (B) Phagocytosis of apoptotic cells was compared at 4°C and 37°C. (C–G) Wright stain of human macrophages after exposure to either murine apoptotic thymocytes (C and D) or autologous human apoptotic neutrophils (E–G) in the presence (C, F, and G) or absence (D and E) of 15% human serum. Original magnification: ×400. Arrows, some of the apoptotic cells bound or engulfed.
Figure 1
Heat-labile serum factor(s) augment the phagocytosis of apoptotic cells in a concentration-, time-, and temperature-dependent fashion. (A) Apoptotic murine thymocytes were offered to human macrophages in the presence of 15% normal non– heat-inactivated human serum (NHS), 15% normal heat-inactivated (56°C for 30 min) serum (HIS), or without (NoS) the addition of serum, as described in Materials and Methods. The results are expressed as PI, and the mean ± SD of five experiments is shown. (B) Phagocytosis of apoptotic cells was compared at 4°C and 37°C. (C–G) Wright stain of human macrophages after exposure to either murine apoptotic thymocytes (C and D) or autologous human apoptotic neutrophils (E–G) in the presence (C, F, and G) or absence (D and E) of 15% human serum. Original magnification: ×400. Arrows, some of the apoptotic cells bound or engulfed.
Figure 1
Heat-labile serum factor(s) augment the phagocytosis of apoptotic cells in a concentration-, time-, and temperature-dependent fashion. (A) Apoptotic murine thymocytes were offered to human macrophages in the presence of 15% normal non– heat-inactivated human serum (NHS), 15% normal heat-inactivated (56°C for 30 min) serum (HIS), or without (NoS) the addition of serum, as described in Materials and Methods. The results are expressed as PI, and the mean ± SD of five experiments is shown. (B) Phagocytosis of apoptotic cells was compared at 4°C and 37°C. (C–G) Wright stain of human macrophages after exposure to either murine apoptotic thymocytes (C and D) or autologous human apoptotic neutrophils (E–G) in the presence (C, F, and G) or absence (D and E) of 15% human serum. Original magnification: ×400. Arrows, some of the apoptotic cells bound or engulfed.
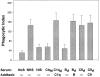
Figure 2
Both classical and alternative complement components are required for efficient uptake of apoptotic cells. The efficiency of phagocytosis of autologous neutrophils was compared in 15% human serum that was selectively deficient in the complement components C1q, C3, Factor B, or C9 (Sigma Chemical Co.). To verify that a specific complement component was responsible for the effect observed, add-back experiments were performed with the missing factor (C1q, 250 μg/ml; Factor B, 250 μg/ml; or C9, 60 μg/ml [Sigma Chemical Co.]). d, deficient. The mean ± SD of four experiments is shown.
Figure 3
Autologous apoptotic cells activate serum complement and are coated with the C3 breakdown product, C3bi. Lymphocytes (A–D) or neutrophils (E) were untreated (A) or induced to undergo apoptosis (B–E) and incubated with 15% autologous serum for 1 h as described in Materials and Methods. Lymphocytes were stained with PE-conjugated murine anti–human-C3bi (Quidel; bold line) or PE-IgG2bκ as isotype control (dotted line). In C and D, apoptotic lymphocytes were incubated with 0.4 or 1.6 mM annexin V, respectively (provided by Dr. A.E. Gharavi, Louisiana State University Medical Center, New Orleans, LA), for 10 min at 4°C followed by incubation with RPMI containing 15% autologous serum for 1 h/37°C/5% CO2, and then stained for C3bi as in A and B. In E, neutrophils were induced to undergo apoptosis, incubated with 15% autologous serum for 1 h, and then analyzed by two-color flow cytometry as shown. The percent staining in each compartment is shown. These results are representative of four experiments.
Figure 3
Autologous apoptotic cells activate serum complement and are coated with the C3 breakdown product, C3bi. Lymphocytes (A–D) or neutrophils (E) were untreated (A) or induced to undergo apoptosis (B–E) and incubated with 15% autologous serum for 1 h as described in Materials and Methods. Lymphocytes were stained with PE-conjugated murine anti–human-C3bi (Quidel; bold line) or PE-IgG2bκ as isotype control (dotted line). In C and D, apoptotic lymphocytes were incubated with 0.4 or 1.6 mM annexin V, respectively (provided by Dr. A.E. Gharavi, Louisiana State University Medical Center, New Orleans, LA), for 10 min at 4°C followed by incubation with RPMI containing 15% autologous serum for 1 h/37°C/5% CO2, and then stained for C3bi as in A and B. In E, neutrophils were induced to undergo apoptosis, incubated with 15% autologous serum for 1 h, and then analyzed by two-color flow cytometry as shown. The percent staining in each compartment is shown. These results are representative of four experiments.
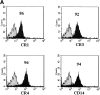
Figure 4
The complement receptors CR3 and CR4 are predominantly responsible for uptake of apoptotic cells in the presence of serum. (A) The expression of the complement receptors was examined on day 7 human macrophages by flow cytometry using specific mouse mAbs (black histogram) as described in Materials and Methods. The percentage of positive cells compared with the appropriate isotype control (grey histogram) is shown for each antibody. (B) Macrophages were preincubated with the antibody or tetrapeptide shown for 15 min at 4°C in the presence of 15% autologous human serum. The PI in the presence of the inhibitor was calculated from three separate experiments, and results were compared with the serum control by Student's t test. Inhibition by antibodies to CR3 (P = 0.0001), CR4 (P = 0.0001), annexin V (ANN; P = 0.001), and RGDS (P = 0.01) was statistically significant, whereas neither anti-CR1, anti-CD36, anti-CD14 (61D3 and 63D3), nor the control tetrapeptide (RGES) significantly inhibited uptake in the presence of serum.
Figure 4
The complement receptors CR3 and CR4 are predominantly responsible for uptake of apoptotic cells in the presence of serum. (A) The expression of the complement receptors was examined on day 7 human macrophages by flow cytometry using specific mouse mAbs (black histogram) as described in Materials and Methods. The percentage of positive cells compared with the appropriate isotype control (grey histogram) is shown for each antibody. (B) Macrophages were preincubated with the antibody or tetrapeptide shown for 15 min at 4°C in the presence of 15% autologous human serum. The PI in the presence of the inhibitor was calculated from three separate experiments, and results were compared with the serum control by Student's t test. Inhibition by antibodies to CR3 (P = 0.0001), CR4 (P = 0.0001), annexin V (ANN; P = 0.001), and RGDS (P = 0.01) was statistically significant, whereas neither anti-CR1, anti-CD36, anti-CD14 (61D3 and 63D3), nor the control tetrapeptide (RGES) significantly inhibited uptake in the presence of serum.
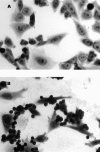
Figure 5
CR3 is responsible for recognition of opsonized apoptotic cells. CHO cells or CHO cells stably transfected with human CR3 (CHO-R3; provided by Drs. R.R. Ingalls and D.T. Golenbock, Boston Medical Center, Boston, MA [23]) were Wright stained and examined for uptake of apoptotic thymocytes in the absence (A) or presence (B) of serum. Original magnification: ×400. (C) The results of four experiments using serum deficient in C3 (C3d) or C9 (C9d) are shown.
Figure 5
CR3 is responsible for recognition of opsonized apoptotic cells. CHO cells or CHO cells stably transfected with human CR3 (CHO-R3; provided by Drs. R.R. Ingalls and D.T. Golenbock, Boston Medical Center, Boston, MA [23]) were Wright stained and examined for uptake of apoptotic thymocytes in the absence (A) or presence (B) of serum. Original magnification: ×400. (C) The results of four experiments using serum deficient in C3 (C3d) or C9 (C9d) are shown.
Similar articles
- Opsonization of apoptotic cells. Implications for uptake and autoimmunity.
Mevorach D. Mevorach D. Ann N Y Acad Sci. 2000;926:226-35. doi: 10.1111/j.1749-6632.2000.tb05615.x. Ann N Y Acad Sci. 2000. PMID: 11193038 - The role of CR3 (CD11b/CD18) and CR4 (CD11c/CD18) in complement-mediated phagocytosis and podosome formation by human phagocytes.
Lukácsi S, Nagy-Baló Z, Erdei A, Sándor N, Bajtay Z. Lukácsi S, et al. Immunol Lett. 2017 Sep;189:64-72. doi: 10.1016/j.imlet.2017.05.014. Epub 2017 May 26. Immunol Lett. 2017. PMID: 28554712 - Opsonization of apoptotic cells and its effect on macrophage and T cell immune responses.
Kim SJ, Gershov D, Ma X, Brot N, Elkon KB. Kim SJ, et al. Ann N Y Acad Sci. 2003 Apr;987:68-78. doi: 10.1111/j.1749-6632.2003.tb06034.x. Ann N Y Acad Sci. 2003. PMID: 12727625 Review. - Phagocytosis of opsonized apoptotic cells: roles for 'old-fashioned' receptors for antibody and complement.
Hart SP, Smith JR, Dransfield I. Hart SP, et al. Clin Exp Immunol. 2004 Feb;135(2):181-5. doi: 10.1111/j.1365-2249.2003.02330.x. Clin Exp Immunol. 2004. PMID: 14738443 Free PMC article. Review.
Cited by
- Insight into the role of phosphatidylserine in complement-mediated synapse loss in Alzheimer's disease.
Sokolova D, Childs T, Hong S. Sokolova D, et al. Fac Rev. 2021 Feb 24;10:19. doi: 10.12703/r/10-19. eCollection 2021. Fac Rev. 2021. PMID: 33718936 Free PMC article. Review. - Interaction of the Factor H Family Proteins FHR-1 and FHR-5 With DNA and Dead Cells: Implications for the Regulation of Complement Activation and Opsonization.
Kárpáti É, Papp A, Schneider AE, Hajnal D, Cserhalmi M, Csincsi ÁI, Uzonyi B, Józsi M. Kárpáti É, et al. Front Immunol. 2020 Jul 16;11:1297. doi: 10.3389/fimmu.2020.01297. eCollection 2020. Front Immunol. 2020. PMID: 32765490 Free PMC article. - Protein therapeutics and their lessons: Expect the unexpected when inhibiting the multi-protein cascade of the complement system.
Schmidt CQ, Smith RJH. Schmidt CQ, et al. Immunol Rev. 2023 Jan;313(1):376-401. doi: 10.1111/imr.13164. Epub 2022 Nov 18. Immunol Rev. 2023. PMID: 36398537 Free PMC article. Review. - Maturation and localization of macrophages and microglia during infection with a neurotropic murine coronavirus.
Templeton SP, Kim TS, O'Malley K, Perlman S. Templeton SP, et al. Brain Pathol. 2008 Jan;18(1):40-51. doi: 10.1111/j.1750-3639.2007.00098.x. Epub 2007 Oct 12. Brain Pathol. 2008. PMID: 17935605 Free PMC article. - Regulatory natural autoantibodies to apoptotic cells: pallbearers and protectors.
Silverman GJ. Silverman GJ. Arthritis Rheum. 2011 Mar;63(3):597-602. doi: 10.1002/art.30140. Arthritis Rheum. 2011. PMID: 21360488 Free PMC article.
References
- Surh CD, Sprent J. T cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994;372:100–103. - PubMed
- Savill J, Dransfield I, Hogg N, Haslett C. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature. 1990;343:170–173. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials