Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals - PubMed (original) (raw)
Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals
J P Pearson et al. J Bacteriol. 1999 Feb.
Abstract
Many gram-negative bacteria communicate by N-acyl homoserine lactone signals called autoinducers (AIs). In Pseudomonas aeruginosa, cell-to-cell signaling controls expression of extracellular virulence factors, the type II secretion apparatus, a stationary-phase sigma factor (sigmas), and biofilm differentiation. The fact that a similar signal, N-(3-oxohexanoyl) homoserine lactone, freely diffuses through Vibrio fischeri and Escherichia coli cells has led to the assumption that all AIs are freely diffusible. In this work, transport of the two P. aeruginosa AIs, N-(3-oxododecanoyl) homoserine lactone (3OC12-HSL) (formerly called PAI-1) and N-butyryl homoserine lactone (C4-HSL) (formerly called PAI-2), was studied by using tritium-labeled signals. When [3H]C4-HSL was added to cell suspensions of P. aeruginosa, the cellular concentration reached a steady state in less than 30 s and was nearly equal to the external concentration, as expected for a freely diffusible compound. In contrast, [3H]3OC12-HSL required about 5 min to reach a steady state, and the cellular concentration was 3 times higher than the external level. Addition of inhibitors of the cytoplasmic membrane proton gradient, such as azide, led to a strong increase in cellular accumulation of [3H]3OC12-HSL, suggesting the involvement of active efflux. A defined mutant lacking the mexA-mexB-oprM-encoded active-efflux pump accumulated [3H]3OC12-HSL to levels similar to those in the azide-treated wild-type cells. Efflux experiments confirmed these observations. Our results show that in contrast to the case for C4-HSL, P. aeruginosa cells are not freely permeable to 3OC12-HSL. Instead, the mexA-mexB-oprM-encoded efflux pump is involved in active efflux of 3OC12-HSL. Apparently the length and/or degree of substitution of the N-acyl side chain determines whether an AI is freely diffusible or is subject to active efflux by P. aeruginosa.
Figures
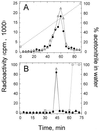
FIG. 1
HPLC analysis of tritium-labeled material extracted from P. aeruginosa PAO1 that had been loaded with [3H]3OC12-HSL (A) or with [3H]C4-HSL (B) compared with [3H]3OC12-HSL and [3H]C4-HSL, respectively. Symbols: ●, material extracted from cells; ▵, 3H-AI; □, percent (volume/volume) acetonitrile in water.
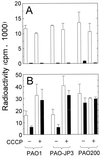
FIG. 2
Accumulation of radiolabeled compounds by P. aeruginosa. (A) [14C]leucine accumulation in strain PAO1 treated with chloramphenicol (5 mM); (B) accumulation of [3H]3OC12-HSL (circles) and [3H]C4-HSL (triangles) in strain PAO1; (C) accumulation of [3H]3OC12-HSL (circles) and [3H]C4-HSL (triangles) in mexAB-orpM mutant strain PAO200. Closed symbols, non-azide-treated cells; open symbols, cells treated with sodium azide (30 mM). Accumulation of each compound was determined as described in Materials and Methods. Data are the averages (± SDs) of results from two to five independent experiments.
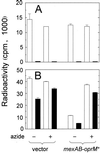
FIG. 3
Efflux of [3H]C4-HSL (A) and [3H]3OC12-HSL (B) from P. aeruginosa PAO1 (wild type), PAO-JP3 (lasR rhlR), or PAO200 (mexABA-oprM). Open bars, radioactivity loaded in cells; closed bars, radioactivity remaining in cells after suspension in AI-free buffer. Cells were de-energized with CCCP (250 μM) as indicated. Data are the averages (± SDs) of results from two to four independent experiments.
FIG. 4
Efflux of [3H]C4-HSL (A) and [3H]3OC12-HSL (B) from P. aeruginosa PAO200 in the presence (labeled mexAB-oprM+ [pPS952]) or absence (labeled vector [pUCP21T]]) of mexAB-oprM. Open bars, radioactivity loaded in cells; closed bars, radioactivity remaining in cells after suspension in AI-free buffer. Cells were de-energized with sodium azide (30 mM) as indicated. Data are the averages (± SDs) of results from two independent experiments.
Similar articles
- Paraoxonase-2 deficiency enhances Pseudomonas aeruginosa quorum sensing in murine tracheal epithelia.
Stoltz DA, Ozer EA, Ng CJ, Yu JM, Reddy ST, Lusis AJ, Bourquard N, Parsek MR, Zabner J, Shih DM. Stoltz DA, et al. Am J Physiol Lung Cell Mol Physiol. 2007 Apr;292(4):L852-60. doi: 10.1152/ajplung.00370.2006. Epub 2006 Nov 22. Am J Physiol Lung Cell Mol Physiol. 2007. PMID: 17122353 - Pseudomonas aeruginosa N-3-Oxododecanoyl Homoserine Lactone Disrupts Endothelial Integrity by Activating the Angiopoietin-Tie System.
Shin J, Ahn SH, Oh DJ. Shin J, et al. Cell Biochem Biophys. 2024 Jun;82(2):1555-1566. doi: 10.1007/s12013-024-01307-8. Epub 2024 May 18. Cell Biochem Biophys. 2024. PMID: 38762714 - Induction of neutrophil chemotaxis by the quorum-sensing molecule N-(3-oxododecanoyl)-L-homoserine lactone.
Zimmermann S, Wagner C, Müller W, Brenner-Weiss G, Hug F, Prior B, Obst U, Hänsch GM. Zimmermann S, et al. Infect Immun. 2006 Oct;74(10):5687-92. doi: 10.1128/IAI.01940-05. Infect Immun. 2006. PMID: 16988244 Free PMC article. - Dominant role of paraoxonases in inactivation of the Pseudomonas aeruginosa quorum-sensing signal N-(3-oxododecanoyl)-L-homoserine lactone.
Teiber JF, Horke S, Haines DC, Chowdhary PK, Xiao J, Kramer GL, Haley RW, Draganov DI. Teiber JF, et al. Infect Immun. 2008 Jun;76(6):2512-9. doi: 10.1128/IAI.01606-07. Epub 2008 Mar 17. Infect Immun. 2008. PMID: 18347034 Free PMC article. - Acyl-homoserine lactone binding to and stability of the orphan Pseudomonas aeruginosa quorum-sensing signal receptor QscR.
Oinuma K, Greenberg EP. Oinuma K, et al. J Bacteriol. 2011 Jan;193(2):421-8. doi: 10.1128/JB.01041-10. Epub 2010 Nov 19. J Bacteriol. 2011. PMID: 21097632 Free PMC article.
Cited by
- Harnessing DNA computing and nanopore decoding for practical applications: from informatics to microRNA-targeting diagnostics.
Takiguchi S, Takeuchi N, Shenshin V, Gines G, Genot AJ, Nivala J, Rondelez Y, Kawano R. Takiguchi S, et al. Chem Soc Rev. 2025 Jan 2;54(1):8-32. doi: 10.1039/d3cs00396e. Chem Soc Rev. 2025. PMID: 39471098 Free PMC article. Review. - Effect of phenylalanine arginyl β-naphthylamide on the imipenem resistance, elastase production, and the expression of quorum sensing and virulence factor genes in Pseudomonas aeruginosa clinical isolates.
Zolpirani FH, Ghaemi EA, Yasaghi M, Nikokar I, Ardebili A. Zolpirani FH, et al. Braz J Microbiol. 2024 Sep;55(3):2715-2726. doi: 10.1007/s42770-024-01426-7. Epub 2024 Jun 27. Braz J Microbiol. 2024. PMID: 38926315 Free PMC article. - Synthetic quorum-sensing circuit to control consortial biofilm formation and dispersal in a microfluidic device.
Hong SH, Hegde M, Kim J, Wang X, Jayaraman A, Wood TK. Hong SH, et al. Nat Commun. 2012 Jan 3;3:613. doi: 10.1038/ncomms1616. Nat Commun. 2012. PMID: 22215088 Free PMC article. - Protein modulator of multidrug efflux gene expression in Pseudomonas aeruginosa.
Daigle DM, Cao L, Fraud S, Wilke MS, Pacey A, Klinoski R, Strynadka NC, Dean CR, Poole K. Daigle DM, et al. J Bacteriol. 2007 Aug;189(15):5441-51. doi: 10.1128/JB.00543-07. Epub 2007 Jun 1. J Bacteriol. 2007. PMID: 17545281 Free PMC article. - Efflux-mediated resistance to fluoroquinolones in gram-negative bacteria.
Poole K. Poole K. Antimicrob Agents Chemother. 2000 Sep;44(9):2233-41. doi: 10.1128/AAC.44.9.2233-2241.2000. Antimicrob Agents Chemother. 2000. PMID: 10952561 Free PMC article. Review. No abstract available.
References
- Chapon-Herve V, Akrim M, Latifi A, Williams P, Lazdunski A, Bally M. Regulation of the xcp secretion pathway by multiple quorum-sensing modulons in Pseudomonas aeruginosa. Mol Microbiol. 1997;24:1169–1178. - PubMed
- Davies D G, Parsek M R, Pearson J P, Iglewski B H, Costerton J W, Greenberg E P. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–298. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI033713/AI/NIAID NIH HHS/United States
- AI33713/AI/NIAID NIH HHS/United States
- T32 AI007362/AI/NIAID NIH HHS/United States
- 5T32AI07362/AI/NIAID NIH HHS/United States
- R37 AI033713/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous