Prevention of Pneumococcal Disease Among Infants and Children --- Use of 13-Valent Pneumococcal Conjugate Vaccine and 23-Valent Pneumococcal Polysaccharide Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP) (original) (raw)
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
J. Pekka Nuorti, MD
Cynthia G. Whitney, MD
Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases
This report originated in the Division of Bacterial Diseases, Rana Hajjeh, MD, Director, and the National Center for Immunization and Respiratory Diseases, Anne Schuchat, MD, Director.
Corresponding preparer: Cynthia G. Whitney, MD, National Center for Immunization and Respiratory Diseases, CDC, 1600 Clifton Rd, NE, MS C-23, Atlanta GA 30333. Telephone: 404-639-4927; Fax: 404-639-3970; E-mail: cwhitney@cdc.gov.
SUMMARY
On February 24, 2010, a 13-valent pneumococcal polysaccharide-protein conjugate vaccine (PCV13 [Prevnar 13, Wyeth Pharmaceuticals Inc., marketed by Pfizer Inc.]) was licensed by the Food and Drug Administration (FDA) for prevention of invasive pneumococcal disease (IPD) caused among infants and young children by the 13 pneumococcal serotypes covered by the vaccine and for prevention of otitis media caused by serotypes also covered by the 7-valent pneumococcal conjugate vaccine formulation (PCV7 [Prevnar, Wyeth]). PCV13 contains the seven serotypes included in PCV7 (serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F) and six additional serotypes (serotypes 1, 3, 5, 6A, 7F, and 19A). PCV13 is approved for use among children aged 6 weeks--71 months and supersedes PCV7, which was licensed by FDA in 2000.
This report summarizes recommendations approved by the Advisory Committee on Immunization Practices (ACIP) on February 24, 2010, for the use of PCV13 to prevent pneumococcal disease in infants and young children aged <6 years. Recommendations include 1) routine vaccination of all children aged 2--59 months, 2) vaccination of children aged 60--71 months with underlying medical conditions, and 3) vaccination of children who received ≥1 dose of PCV7 previously ([CDC. Licensure of a 13-valent pneumococcal conjugate vaccine [PCV13] and recommendations for use among children---Advisory Committee on Immunization Practices [ACIP], 2010. MMWR 2010;59:258--61)](mm5909a2.htm ?s%5Fcid=mm5909a2%5Fw). Recommendations also are provided for targeted use of the 23-valent pneumococcal polysaccharide vaccine (PPSV23, formerly PPV23) in children aged 2--18 years with underlying medical conditions that increase their risk for contracting pneumococcal disease or experiencing complications of pneumococcal disease if infected.
The ACIP recommendation for routine vaccination with PCV13 and the immunization schedules for children aged ≤59 months who have not received any previous PCV7 or PCV13 doses are the same as those published previously for PCV7 (CDC. Preventing pneumococcal disease among infants and young children: recommendations of the Advisory Committee on Immunization Practices [ACIP]. MMWR 2000;49[No. RR-9]; CDC. Updated recommendation from the Advisory Committee on Immunization Practices [ACIP] for use of 7-valent pneumococcal conjugate vaccine [PCV7] in children aged 24--59 months who are not completely vaccinated. MMWR 2008;57:343--4), with PCV13 replacing PCV7 for all doses. For routine immunization of infants, PCV13 is recommended as a 4-dose series at ages 2, 4, 6, and 12--15 months. Infants and children who have received ≥1 dose of PCV7 should complete the immunization series with PCV13. A single supplemental dose of PCV13 is recommended for all children aged 14--59 months who have received 4 doses of PCV7 or another age-appropriate, complete PCV7 schedule. For children who have underlying medical conditions, a supplemental PCV13 dose is recommended through age 71 months. Children aged 2--18 years with underlying medical conditions also should receive PPSV23 after completing all recommended doses of PCV13.
Introduction
Streptococcus pneumoniae (pneumococcus) remains a leading cause of serious illness, including bacteremia, meningitis, and pneumonia among children and adults worldwide. It is also a major cause of sinusitis and acute otitis media (AOM). In February 2000, a 7-valent pneumococcal polysaccharide-protein conjugate vaccine (PCV7; Prevnar, Wyeth) was licensed by the Food and Drug Administration (FDA) for use among infants and young children in the United States (1). In prelicensure randomized clinical trials, PCV7 was demonstrated to be safe and highly efficacious against invasive pneumococcal disease (IPD), moderately efficacious against pneumonia, and somewhat effective in reducing otitis media episodes and related office visits (2--4). On the basis of the results of these clinical trials, in2000, ACIP recommended routine use of PCV7 for all children aged 2--23 months and for children aged 24--59 months who are at increased risk for pneumococcal disease (e.g., children with anatomic or functional asplenia, sickle cell disease (SCD), HIV infection or other immunocompromising condition, or chronic illness including chronic heart or lung disease, cerebrospinal fluid leaks, and diabetes mellitus) (1). In 2007, ACIP revised its recommendation for routine use to include all children aged 2--59 months (5). National Immunization Survey data indicate that in 2009, PCV7 coverage among children aged 19--35 months was 92.6% for ≥3 doses and 80.4% for ≥4 doses (6).
The safety, efficacy, and effectiveness in practice of PCV7 and other pneumococcal conjugate vaccines has been established in multiple settings in both industrialized and developing countries (7). In 2007, the World Health Organization (WHO) recommended that all countries incorporate pneumococcal conjugate vaccines in their national infant immunization programs (8).
On February 24, 2010, a new 13-valent pneumococcal polysaccharide-protein conjugate vaccine (PCV13 [Prevnar13], Wyeth Pharmaceuticals, Inc., marketed by Pfizer, Inc.) was approved by FDA for prevention of IPD caused among infants and young children by the 13 serotypes in the vaccine (9). PCV13 is formulated and manufactured using the same processes as PCV7 and was licensed by FDA on the basis of studies demonstrating safety and an ability comparable to that of PCV7 to elicit antibodies protective against IPD (10). PCV13 is approved for use among children aged 6 weeks--71 months and replaces PCV7, which is made by the same manufacturer. PCV13 contains the seven serotypes included in PCV7 (serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F) and six additional serotypes (1, 3, 5, 6A, 7F, and 19A). PCV13 also is approved for the prevention of otitis media caused by the seven serotypes also covered by PCV7; no efficacy data for prevention of otitis media are available for the six additional serotypes.
This report summarizes the recommendations approved by ACIP on February 24, 2010, for the prevention of pneumococcal disease among infants and children aged ≤18 years (11) and replaces the previous ACIP recommendations for preventing pneumococcal disease in children (1,5,12). It also provides updated information regarding changes in the epidemiology of pneumococcal disease in the United States after the routine PCV7 infant vaccination program began in 2000.
Background
Clinical Efficacy of Pneumococcal Conjugate Vaccines
The efficacy of pneumococcal conjugate vaccines (PCVs) was evaluated in randomized, controlled trials among children aged <2 years. A prelicensure clinical efficacy trial of PCV7 conducted among 37,868 healthy children at a health maintenance organization in northern California indicated that PCV7 was 97.4% (95% confidence interval [CI] = 82.7%--99.9%) efficacious against IPD caused by vaccine serotypes (the primary endpoint) among fully vaccinated infants (2). A recently updated systematic review by the Cochrane Collaboration included results from five randomized, controlled trials to evaluate PCVs (including PCV7 and experimental 9-valent and 11-valent vaccine formulations) against IPD and/or pneumonia. The trials conducted in various settings in both industrialized countries (U.S. general population [_2_] and Native American children [_13_]) and developing countries (South Africa [_14_], the Gambia [_15_], and the Philippines [_16_]) included 113,044 children aged <2 years (17). PCVs were demonstrated to be efficacious in preventing IPD, X-ray--confirmed pneumonia, and clinically diagnosed pneumonia. Among healthy children aged <2 years, the pooled PCV vaccine efficacy estimate was 80% (95% CI = 58%--90%) for vaccine-type IPD, 58% (95% CI = 29%--75%) for IPD caused by all serotypes, 27% (95% CI = 15%--36%) for chest X-ray--confirmed pneumonia meeting WHO criteria (18), and 6% (95% CI = 2%--9%) for clinical pneumonia.
In a clinical trial conducted in South Africa, a 9-valent investigational PCV was administered to infants as a 3-dose schedule at age 6, 10, and 14 weeks without a booster dose. This vaccine prevented IPD among HIV-infected children, although the point estimate was somewhat lower (65%; 95% CI = 24%--86%) than among HIV-uninfected children (83%; 95% CI = 39%--97%) (14). After a 6-year follow-up, vaccine efficacy against IPD declined substantially among HIV-infected children but not among healthy children (19).
Before PCV7 introduction, Streptococcus pneumoniae was detected in 28%--55% of middle-ear aspirates among children with AOM (1). In a randomized, clinical trial conducted in Finland in which the bacterial etiology of AOM was determined by myringotomy, the efficacy of PCV7 in preventing culture-confirmed, vaccine serotype AOM episodes was 57% (95% CI = 44%--67%) (4); the overall net reduction in AOM caused by any pneumococcal serotype was 34% (95% CI = 21%--45%). Overall, PCV7 prevented 6%--7% of all AOM episodes in the clinical trials (2,4,20); reductions also were observed for the outcomes of frequent otitis media (9%) and tympanostomy tube placement (20%) (2).
Updated Safety Data from PCV7 Postmarketing Studies
A systematic review of 42 pre- and postmarketing infant studies did not identify major safety problems with PCV7 or other PCVs (21). In general, PCV7 injection-site reactions were mild and self-limited. The incidence of high fever was <1%. Mild local and systemic reactions were sometimes more frequent after the second and third vaccination than after the first vaccination. A small increase in hospitalizations for reactive airway disease was observed among PCV7 and PCV9 recipients compared with controls in two large clinical trials (2,14). However, a 3-year follow-up study of safety outcomes among subjects in the U.S. IPD efficacy study did not demonstrate an association of PCV7 with increased health-care use for reactive airway disease (22).
According to data from the Vaccine Adverse Event Reporting System (VAERS), a U.S. passive reporting system for adverse events occurring after immunization, the majority of reports received during the first 2 years after PCV7 licensure among children were minor adverse events similar to those observed previously in prelicensure clinical trials (23). Approximately 31.5 million PCV7 doses were distributed during this time period, and VAERS received 4,154 reports of events that had occurred within 3 months of receiving PCV7 (rate: 13 reports per 100,000 PCV7 doses distributed). In 74.3% of reports, the child had received other vaccines concurrently with PCV7. Serious events were described in 608 (14.6%) reports, consistent with the frequency of serious adverse events (14.2%) reported to VAERS for other childhood vaccines (24).
Epidemiology of Pneumococcal Disease Among Children Aged <5 Years After Routine PCV7 Immunization
Invasive Pneumococcal Disease
Effectiveness data from observational postmarketing studies of the U.S. routine infant PCV7 immunization program have been consistent with the results of prelicensure randomized clinical trials (25--29). In the United States, major changes have occurred in the epidemiology of pneumococcal disease after routine infant vaccination with PCV7 began in 2000 (7,30). Substantial decreases were observed in the incidence rates of invasive pneumococcal disease, including pneumococcal meningitis (31,32) among young children.
Data from the Active Bacterial Core Surveillance [ABCs], an active population- and laboratory-based surveillance system (http://www.cdc.gov/abcs/index.html) indicate that the overall incidence of IPD among children aged <5 years decreased from approximately 99 cases per 100,000 population during 1998--1999 to 21 cases per 100,000 population in 2008 (rate difference: 78 cases per 100,000 population; percentage reduction: 79%) (Figure 1) (CDC, unpublished data, 2009). The reductions in overall IPD resulted from a 99% decrease in disease caused by the seven serotypes in PCV7 and serotype 6A, a serotype against which PCV7 provides some cross-protection (28). The decreases have been offset partially by increases in IPD caused by nonvaccine serotypes, in particular 19A (33,34). In the general U.S. population, the overall rates of IPD have leveled off and remained at approximately 22--25 annual cases per 100,000 children aged <5 years since 2002 (34). Although the absolute rate increase in IPD attributable to 19A in the general population has been small (approximately five cases per 100,000 population) compared with the decreases in PCV7-type disease (35--37), surveillance of one small population (Alaska Native children living in a remote region) showed a reduced overall vaccine benefit because of an increase in IPD caused by non-PCV7 types, particularly serotype 19A (38,39).
Trends in Antimicrobial Resistance
The emergence of pneumococcal strains resistant to penicillin and other antibiotics complicates the treatment of pneumococcal disease and might reduce the effectiveness of recommended treatment regimens. Before PCV7 was introduced, five of the seven serotypes included in PCV7 (6B, 9V, 14, 19F, and 23F) accounted for approximately 80% of penicillin-nonsusceptible isolates (1). Following routine PCV7 use, the incidence of IPD caused by penicillin-resistant strains decreased 57% overall and 81% among children aged <2 years. These decreases were a result of declines in nonsusceptible PCV7 serotypes. (40). Decreases also were observed for erythromycin-resistant strains and those resistant to multiple antibiotics. However, IPD caused by penicillin-nonsusceptible non-PCV7 serotypes has increased, and most of the resistant infections now are caused by serotype 19A (33,35,37,40--42). In addition, the emergence of multidrug-resistant serotype 19A strains causing meningitis and other severe invasive infections (31,43), pneumococcal mastoiditis (44), and treatment failures for otitis media have been reported (45).
Trends in Noninvasive Pneumococcal Disease
Decreases in rates of hospitalizations and ambulatory care visits for community-acquired pneumonia have been reported consistently among children aged <2 years after PCV7 introduction (46--49). From pre-PCV7 baseline (1997--1999) to 2006, the rate of hospitalizations for pneumonia attributable to all causes decreased 35% (from 12.5 to 8.1 cases per 1,000 population) among children aged <2 years (46). Compared with the average annual number of pneumonia admissions during 1997--1999, this rate reduction represented an estimated 36,300 fewer pneumonia hospitalizations in 2006, when an estimated 67,400 total hospitalizations for all causes of pneumonia occurred among children aged <2 years in the United States. No similar reduction in pneumonia hospitalizations has been observed in children aged 2--4 years.
An estimated 13 million episodes of AOM occur annually in the United States among children aged <5 years (50,51). Population-based studies using various national and regional administrative and insurance databases have reported decreases in rates of ambulatory visits for otitis media (52,53), rates of frequent otitis media (defined as three episodes in 6 months or four episodes in 1 year) and tympanostomy-tube placement (54) among young children following PCV7 introduction. Although the observed trends in health-care use for otitis media might have been affected by factors other than PCV7 (e.g., secular trend or changes in coding or clinical practices), even modest vaccine-associated reductions in otitis media would result in substantial health benefits because of the substantial burden of disease (51).
Indirect Effects of the PCV7 Vaccination Program in Unvaccinated Populations
Substantial evidence has accumulated to demonstrate that routine infant PCV7 vaccination has reduced transmission of PCV7 serotypes, resulting in a reduced incidence of IPD among unvaccinated persons of all ages, including infants too young to be vaccinated and elderly persons (7,27,30,55,56). Among persons aged 18--49 years, 50--64 years, and ≥65 years, overall rates of IPD have decreased 34%, 14%, and 37% respectively from 1998--1999 to 2008; decreases in rates of disease caused by PCV7 serotypes ranged from 90% to 93% (CDC, unpublished data,2009).
The measured indirect effects on noninvasive pneumococcal disease have been less clear (49). However, a time-series analysis of national hospital discharge data during 1997--2004 indicated a statistically significant decrease after PCV7 introduction in rates of all-cause pneumonia hospitalizations among young adults but not among other adult age groups (47).
Invasive Pneumococcal Disease Caused by Serotypes Covered in PCV13
ABCs data indicate that in 2008, a total of 61% of IPD cases among children aged <5 years were attributable to the serotypes covered in PCV13, with serotype 19A accounting for 43% of cases; PCV7 serotypes caused <2% of cases (Figure 2). Three of the six additional serotypes, (19A, 7F, and 3) accounted for 99% of IPD cases, serotypes 1 and 5 together caused 0.6% of cases, and serotype 6A caused 0.6% of cases. In age groups ≥5 years, the serotypes covered in PCV7 caused from 4% to 7%, and the serotypes in PCV13 caused 43%--66% of IPD cases, respectively (Figure 3).
In 2008, children aged <24 months accounted for more than two thirds of all IPD cases among children aged <5 years; overall rates were highest among children aged <12 months and 12--24 months (rate: 39 and 32 cases per 100,000 population, respectively) (Table 1). Among children aged >24 months, rates decreased markedly with age. Rates of all IPD and IPD caused by serotypes covered by PCV13 were twice as high in black children as in white children. However, no difference was found between the proportion of IPD cases caused by PCV13 serotypes in black children compared with white children (CDC, unpublished data, 2009).
Projections from active surveillance data to the U.S. population indicate that in 2008, an estimated 4,100 cases of IPD (rate: 20 cases per 100,000 population) occurred among children aged <5 years in the United States; PCV13 serotypes caused an estimated 2,500 cases (rate: 12 cases per 100,000 population) (CDC, unpublished data, 2009).
Children at Increased Risk for Pneumococcal Infections
Rates of pneumococcal infections in the United States vary among demographic groups, with higher rates occurring among infants, young children, elderly persons, Alaska Natives, and certain American Indian populations. Although racial disparities have diminished since PCV7 was introduced (57,58), black children continue to have higher rates of IPD compared with white children (Table 1). The risk for IPD is highest among persons who have congenital or acquired immunodeficiency, abnormal innate immune response, HIV-infection, or absent or deficient splenic function (e.g., SCD or congenital or surgical asplenia) (1,12). Children with cochlear implants are also at substantially increased risk for pneumococcal meningitis (59,60).
Several studies have evaluated antibody responses to PCV7 among children with SCD and among HIV-infected children (1,61). The antibody responses among infants with SCD generally have been comparable to infants without SCD (62--64). For HIV-infected children, the antibody responses to various PCV formulations have been slightly lower but generally are comparable to those in HIV-uninfected children (65,66). Studies of small numbers of children with SCD and HIV infection suggested that PCV7 is safe and immunogenic when administered to children aged ≤13 years (1,65). In addition, a multicenter study indicated that a schedule of 2 doses of PCV7 followed by 1 dose of 23-valent pneumococcal polysaccharide vaccine (PPSV23, formerly PPV23) was safe and immunogenic in highly active antiretroviral therapy (HAART)--treated HIV-infected children and adolescents aged 2--19 years who had not received PCV7 in infancy (however, 75% of subjects had received PPSV23 previously) (67). In addition, PCV7 was as immunogenic among low birth weight and preterm infants as among normal birth weight and full-term infants (68).
After the introduction and widespread use of HAART in the United States, rates of IPD among HIV-infected children decreased, but whether further declines have occurred after routine PCV7 vaccination is unclear, and rates remain elevated compared with those for HIV-uninfected children (69). Rates among children with SCD have decreased substantially following PCV7 introduction but still remain higher than among healthy children, particularly among older children with SCD (70,71).
During 2006--2008, of 475 IPD cases in children aged 24--59 months in the ABCs surveillance population of approximately 18 million persons, 51 (11%) cases occurred in children with underlying medical conditions that are indications for PPSV23 (Table 2). Of these 51 cases, 23 (45%) were caused by PCV13 serotypes (Table 3). The 11 serotypes included in PPSV23 but not in PCV13 (serotype 6A is not included in PPSV23) caused an additional eight (16%) cases (CDC, unpublished data, 2009).
13-Valent Pneumococcal Conjugate Vaccine
Vaccine Composition
PCV13 (Prevnar13) contains polysaccharides of the capsular antigens of S. pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F, individually conjugated to a nontoxic diphtheria cross-reactive material (CRM) carrier protein (CRM197). A 0.5-mL PCV13 dose contains approximately 2.2 _μ_g of polysaccharide from each of 12 serotypes and approximately 4.4 _μ_g of polysaccharide from serotype 6B; the total concentration of CRM197 is approximately 34 _μ_g. The vaccine contains 0.02% polysorbate 80 (P80), 0.125 mg of aluminum as aluminum phosphate (AlPO4) adjuvant, 5mL of succinate buffer, and no thimerosal preservative (9). Except for the addition of six serotypes, P80, and succinate buffer, the formulation of PCV13 is the same as that of PCV7.
Evaluation of PCV13 Immunogenicity
The immunogenicity of PCV13 was evaluated in a randomized, double-blind trial (Study 004) in which 663 healthy U.S. infants received at least 1 dose of PCV13 or PCV7 according to the routine immunization schedule (at ages 2, 4, 6, and 12--15 months) (10). To compare PCV13 antibody responses with those for PCV7, criteria for noninferior immunogenicity after 3 and 4 doses of PCV13 (pneumococcal IgG antibody concentrations measured by enzyme-linked immunosorbent assay [ELISA]) were defined for the seven serotypes common to PCV7 and PCV13 and for the six additional serotypes in PCV13. Functional antibody responses were evaluated by opsonophagocytosis assay in a subset of the study population (10). Evaluation of these immunologic parameters indicated that PCV13 induced levels of antibodies that were comparable to those induced by PCV7 and shown to be protective against IPD (10). PCV13 immunogenicity data are not yet available for children in the specific groups at increased risk for pneumococcal disease.
Immune Responses After the 3-Dose Infant Series among Healthy Infants
Among infants receiving the 3-dose primary infant series, responses to ten of the PCV13 serotypes met the prespecified primary endpoint criterion (percentage of subjects achieving an IgG seroresponse of ≥0.35 _μ_g/mL 1 month after the third dose) (_72_--74). Responses to shared serotypes 6B and 9V and new serotype 3 did not meet this criterion (Table 4). For serotypes 6B and 9V, however, the differences were small. Among PCV13 recipients, the IgG seroresponse rate for serotype 3 was 63.5%; for the other additional serotypes, the seroresponse rate ranged from 89.7% (serotype 5) to 98.4% (serotypes 7F and 19A). Detectable opsonophygocytic antibodies (OPA) to serotypes 6B, 9V, and 3 indicated the presence of functional antibodies (74,75). The percentages of subjects with an OPA antibody titer ≥1:8 were similar for the seven common serotypes among PCV13 recipients (range: 90%--100%) and PCV7 recipients (range: 93%--100%); the proportion of PCV13 recipients with an OPA antibody titer ≥1:8 was >90% for all of the 13 serotypes (10).
Immune Responses After the Fourth Dose Among Healthy Children
After the fourth dose, the noninferiority criterion for IgG geometric mean concentrations (GMCs) was met for 12 of the 13 serotypes; the noninferiority criterion was not met for the response to serotype 3 (Table 5). For the seven common serotypes, the IgG GMCs achieved after the 4-dose series were somewhat lower for PCV13 than for PCV7, except for serotype 19F (Table 5). Detectable OPA antibodies were present for all serotypes after the fourth dose; the percentage of PCV13 recipients with an OPA titer ≥1:8 ranged from 97% to 100% for the 13 serotypes and was 98% for serotype 3. Following the fourth dose, the IgG GMCs and OPA geometric mean titers (GMTs) were higher for all 13 serotypes compared with those after the third dose.
Antibody Responses to PCV13 Booster Dose Among Toddlers Who Received 3 Doses of Either PCV7 or PCV13 as Infants
In a randomized, double-blind trial conducted in France, 613 infants were randomly assigned to three groups in a 2:1:1 ratio: 1) PCV13 at ages 2, 3, 4, and 12 months [PCV13/PCV13] or 2) PCV7 at ages 2, 3, and 4 months followed by PCV13 at age 12 months [PCV7/PCV13] or 3) PCV7 at ages 2, 3, 4, and 12 months [PCV7/PCV7] (Study 008) (10). A single PCV13 dose administered at age 12 months to children who had received 3 doses of PCV7 resulted in higher IgG GMCs to all six additional serotypes compared with IgG GMCs after 3 PCV13 doses administered to infants at 2, 3, and 4 months. One month after the 12-month dose, the IgG GMCs for the seven common serotypes were similar among all three groups. For five of the six additional serotypes, IgG GMCs among PCV7/PCV13 recipients were somewhat lower than among PCV13/PCV13 recipients; for serotype 3, GMC was somewhat higher among the PCV7/PCV13 group (Table 6). The clinical relevance of these lower antibody responses is not known (9).
After the 12-month dose of PCV13, the percentage of children with OPA antibody titers ≥1:8 for the six additional serotypes were comparable regardless of whether the children had received PCV7 or PCV13 in infancy. The OPA GMTs among PCV7/PCV13 recipients also were similar to those among PCV13/PCV13 recipients (Figure 4) (Study 008) (10).
Immune Responses Among Previously Unvaccinated Older Infants and Children
In an open-label, nonrandomized and noncontrolled study of PCV13 conducted in Poland (Study 3002), children aged 7--11 months, 12--23 months, and 24--71 months who had not received pneumococcal conjugate vaccine doses previously were administered 1, 2, or 3 doses of PCV13 according to age-appropriate immunization schedules (10). Descriptive analyses suggest that these three schedules resulted in antibody responses to each of the 13 serotypes that were comparable to the IgG GMCs achieved after the 3-dose infant PCV13 series in the U.S. immunogenicity trial (Study 004), except for serotype 1, for which IgG GMC was lower among children aged 24--71 months (1.78 _μ_g/mL compared with 2.03 _μ_g/mL) in the U.S. study (10). Compared with the immune responses after 4 doses of PCV13, the responses induced by the recommended catch-up schedules among children aged ≥7 months might result in lower antibody concentrations for some serotypes The clinical relevance of these lower antibody responses is not known (9).
Adverse Reactions After Administration of PCV13 in Clinical Trials
The safety of PCV13 was assessed in 13 clinical trials in which approximately 15,000 doses were administered to 4,729 healthy children aged 6 weeks--15 months using various 3-dose primary infant schedules (at ages 2, 4, and 6 months; 2, 3, and 4 months; and 6, 10, and 14 weeks) with a booster dose at 12--15 months, concomitantly with other routine pediatric vaccines. Three primary safety studies were conducted in the United States. In these studies, 1,908 children received at least 1 dose of PCV13 concomitantly with routine U.S. pediatric vaccinations. The comparison group of 2,760 children received at least 1 dose of PCV7. Supportive data for safety outcomes were provided by a study among 354 children aged 7--71 months, who received at least 1 dose of PCV13 (9). No safety or immunogenicity studies for PVC13 have been completed among infants born prematurely, children aged ≥72 months, or children who have underlying medical conditions that increase the risk for pneumococcal disease.
The most commonly reported (in ≥20% of subjects) solicited adverse reactions that occurred within 7 days after each dose of PCV13 were injection-site reactions, fever, decreased appetite, irritability, and increased or decreased sleep (9). The incidence and severity of solicited local reactions at the injection site (pain, tenderness, erythema, and induration/swelling) and solicited systemic reactions (irritability, drowsiness/increased sleep, decreased appetite, fever, and restless or decreased sleep) were similar in the PCV13 and PCV7 groups (Table 7). The frequency of adverse reactions was similar after each vaccine dose in the series and is described in the PCV13 package insert (9). The frequency of unsolicited adverse events was also similar in the two groups. The following unsolicited adverse events occurred in >1% of infants and toddlers: diarrhea, vomiting, and rash. Reactions occurring in <1% of infants and toddlers following PCV13 included crying, hypersensitivity reaction (including face edema, dyspnea, and bronchospasm), seizures (including febrile seizures), and urticaria or urticaria-like rash. The most commonly reported serious adverse events included bronchiolitis, gastroenteritis, and pneumonia. Serious adverse events reported following vaccination occurred among 8.2% of PCV13 recipients and 7.2% of PCV7 recipients. No statistically significant differences in types or rates of serious adverse events or unanticipated adverse events were identified (9). These data suggest that the safety profiles of PCV13 and PCV7 are comparable.
The safety of a supplemental dose of PCV13 was evaluated in an open-label study in which 284 healthy U.S. children aged 15--59 months who had received 3 or 4 doses of PCV7 previously received 1 or 2 doses of PCV13; children aged 15--23 months received 2 PCV13 doses, and children aged 24--59 months received 1 PCV13 dose (9). The incidence and severity of solicited local reactions and systemic adverse reactions that occurred within 7 days after 1 dose of PCV13 among children aged 15--59 months who had received 4 PCV7 doses were comparable to those among children receiving their fourth dose of PCV13 (see Tables 7 and 8 in PCV13 package insert) (9).
Certain rare adverse events that were observed during PCV7 postmarketing surveillance are included in the PCV13 package insert (9) although they were not observed in the PCV13 clinical trials: hypotonic-hyporesponsive episode, apnea, anaphylactic/anaphylactoid reaction including shock, angioneurotic edema, erythema multiforme, injection-site dermatitis, injection-site pruritus, injection-site urticaria, and lymphadenopathy localized to the region of the injection site. The causal relation of these events to vaccination is unknown.
Vaccine Administration
PCV13 is administered intramuscularly as a 0.5-mL dose and is available in latex-free, single-dose, prefilled syringes. PCV13 has been administered concurrently with vaccines containing the following antigens with no adverse effects on immunogenicity or safety: diphtheria, tetanus, acellular pertussis, Haemophilus influenzae type b, inactivated poliomyelitis, rotavirus, hepatitis B, meningococcal serogroup C, measles, mumps, rubella, and varicella (9). PCV13 can be administered at the same time as other routine childhood vaccinations if administered in a separate syringe at a separate injection site. The safety and efficacy of concurrent administration of PCV13 and PPV23 has not been studied, and concurrent administration is not recommended.
Precautions and Contraindications
Before administering PCV13, vaccination providers should consult the package insert for precautions, warnings, and contraindications (9). Vaccination with PCV13 is contraindicated in persons known to have a severe allergic reaction (e.g., anaphylaxis) to any component of PCV13 or PCV7 or to any diphtheria toxoid-containing vaccine. Before PCV13 administration, all precautions should be taken to prevent allergic or any other adverse reactions, including a review of the patient's vaccination history for possible sensitivity to the vaccine or similar vaccines and for previous vaccination-related adverse reactions to determine the presence of any contraindication to vaccination with PCV13 and to allow an assessment of risks and benefits.
Apnea following intramuscular vaccination has been observed in some infants born prematurely. Decisions about when to administer an intramuscular vaccine, including PCV13, to infants born prematurely should be based on consideration of the individual infant's medical status and potential benefits and possible risks of vaccination (9).
All vaccines can be administered to persons with minor acute illness (e.g. diarrhea or mild upper-respiratory tract infection with or without fever). Persons with moderate or severe acute illness should be vaccinated as soon as the acute illness has improved, after screening for contraindications.
Adverse events after receipt of any vaccine, even if it is not clear that the vaccine caused the adverse event, should be reported to the Vaccine Adverse Event Reporting System (VAERS). Two methods can be used to report to VAERS: 1) online reporting through the secure VAERS internet site (https://vaers.hhs.gov) is encouraged; 2) a reporting form can be downloaded at http://vaers.hhs.gov/esub/index and sent via fax to 877-721-0366 or mailed to VAERS, P.O. Box 1100, Rockville, MD 20849 when completed. Providers can contact VAERS at telephone 1-800-822-7967 or by e-mail at info@vaers.org to request a reporting form or obtain assistance in reporting.
23-Valent Pneumococcal Polysaccharide Vaccine
The 23-valent pneumococcal polysaccharide vaccine (PPSV23) (Pneumovax23, marketed by Merck & Company, Inc.) was licensed in the United States in 1983 and contains 23 capsular polysaccharide antigens of S. pneumoniae: 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, and 33F. One 0.5-mL dose of PPSV23 contains 25 _µ_g of each polysaccharide in isotonic saline solution with 0.25% phenol as a preservative.
Since 1997, ACIP has recommended use of PPSV23 for persons aged ≥2 years who have certain underlying medical conditions (Table 2) (12). Children with these conditions are at increased risk for IPD and might have infections caused by a broader range of serotypes than healthy children (76). Therefore, since 2000, children aged ≥2 years with underlying medical conditions have been recommended to receive PPSV23 after PCV7 (1). PPSV23 is not recommended to be used alone for this group of children because conjugate vaccines have several advantages over PPSV23, including immunologic priming and induction of immunologic memory, reduction in nasopharyngeal carriage of vaccine type pneumococci, likely greater effectiveness against serotypes currently causing most IPD, and evidence for effectiveness against noninvasive syndromes including nonbacteremic pneumonia and otitis media (61,77).
Data regarding safety of PPSV23 when administered after PCV13 are not available. However, clinical experience of PPSV23 use since 1983 has not raised safety concerns, and the reported safety profile for PPSV23 in children appears similar to adults; the most common adverse reactions following PPSV23 vaccination are mild local reactions (e.g., pain at the injection site, erythema, and swelling) that usually resolve within 48 hours of vaccination (12,78). Immunogenicity data on sequential vaccination with PCV13 followed by PPSV23 are not available, but studies on sequential vaccination with PCV7 followed by PPSV23 have demonstrated that PPSV23 elicits robust booster responses for the seven serotypes in common with PCV7 (79). Limited and inconclusive data are available on the efficacy and effectiveness of PPSV23 among children with underlying medical conditions (78), and the clinical effectiveness of PPSV23 among children who have received PCV7 or PCV13 is unknown. No safety or immunogenicity data are available regarding the vaccine sequence of PPSV23 followed by PCV13; limited safety and immunogenity data are available for PPSV23 followed by PCV7 among HIV-infected children and adolescents (67).
Few studies have evaluated the immunogenicity and safety of >1 dose of PPSV23 in children systematically (78,79). Recent studies evaluating immune responses elicited by PPSV23 among adults (80,81) have reported a concern that vaccination with unconjugated pneumococcal polysaccharide antigens might induce hyporesponsiveness (i.e., lower antibody response) on rechallenge with pneumococcal antigens, including PCV7, although the clinical relevance of this immunologic observation is unknown (79). No data are available to indicate whether administration of PPSV23 to children before or after PCV13 might result in hyporesponsiveness to subsequent doses of PCV13.
Recommendations for Use of PCV13 and PPSV23
ACIP recommends use of PCV13 for 1) all children aged 2--59 months and 2) children aged 60--71 months with underlying medical conditions that increase their risk for pneumococcal disease or complications (Table 2).
No Previous PCV7/PCV13 Vaccination
- The ACIP recommendation for routine vaccination with PCV13 and the vaccination schedules for infants and toddlers through age 59 months who have not received any previous PCV7 or PCV13 doses are the same as those previously published for PCV7 (Table 8) (1,5). PCV13 is recommended as a 4-dose series at ages 2, 4, 6, and 12--15 months.
Infants Aged 2--6 Months
- The primary infant series consists of 3 doses of PCV13. Infants receiving their first dose at age ≤6 months should receive 3 doses of PCV13 at intervals of approximately 8 weeks (the minimum interval is 4 weeks). The fourth (booster) dose is recommended at age 12--15 months and at least 8 weeks after the third dose (Table 8).
- Newborns should begin the schedule at age 2 months, although the first dose can be administered as early as 6 weeks. For prematurely born infants (i.e., <37 weeks' gestation) who are medically stable enough to be vaccinated, PCV13 should be administered at the recommended chronologic age concurrent with other routine vaccinations.
Children Aged ≥7 Months
- Healthy children aged 7--59 months who have not been vaccinated with PCV7 or PCV13 previously should receive 1--3 doses of PCV13, depending on their age at the time when vaccination begins and whether underlying medical conditions are present. Children aged 24--71 months with underlying medical conditions should receive 2 doses of PCV13 (Table 8). Interruption of the vaccination schedule does not require reinstitution of the entire series or the addition of extra doses.
Infants Aged 7--11 Months
- Three doses are recommended. The first 2 doses should be administered with an interval of at least 4 weeks between doses. The third dose should be administered at age 12--15 months, at least 8 weeks after the second PCV13 dose.
Children Aged 12--23 Months
- Two doses are recommended, with an interval of at least 8 weeks between doses.
Children Aged >24 Months
- Unvaccinated healthy children aged 24--59 months should receive a single dose of PCV13.
- Unvaccinated children aged 24--71 months with underlying medical conditions (Table 2) should receive 2 doses of PCV13 with an interval of at least 8 weeks between doses.
Children Vaccinated Previously with PCV7 or PCV13
Incomplete PCV7/PCV13 Vaccination
Children Aged <24 Months
- Infants and children aged <24 months who have received ≥1 dose of PCV7 should complete the vaccination series with PCV13 (Tables 9 and 10).
- Children aged 12--23 months who have received 3 doses of PCV7 before age 12 months are recommended to receive 1 dose of PCV13, administered at least 8 weeks after the most recent dose of PCV7 (Tables 9 and 10). This dose will constitute their fourth and final PCV dose, completing the series for the PCV7 serotypes and eliciting an immune response to the six additional serotypes.
- No additional PCV13 doses are recommended for children aged 12--23 months who received 2--3 doses of PCV7 before age 12 months and at least 1 dose of PCV13 at age ≥12 months.
Children Aged >24 Months
- Similar to the previous ACIP recommendation for use of PCV7 (5), 1 dose of PCV13 is recommended for all healthy children aged 24--59 months with any incomplete PCV schedule (PCV7 or PCV13) before age 24 months (Table 11).
- For children aged 24--71 months with underlying medical conditions who have received any incomplete schedule of <3 doses of PCV (PCV7 or PCV13) before age 24 months, 2 doses of PCV13 are recommended (Table 11).
- For children with underlying medical conditions who have received 3 doses of PCV (PCV7 or PCV13), a single dose of PCV13 is recommended through age 71 months. The minimum interval between doses is 8 weeks.
Complete PCV7 Vaccination
- A single supplemental dose of PCV13 is recommended for all children aged 14--59 months who have received 4 doses of PCV7 or another age-appropriate, complete PCV7 schedule.
- For children who have underlying medical conditions, a single supplemental PCV13 dose is recommended through 71 months. This includes children who have received PPSV23 previously. PCV13 should be administered at least 8 weeks after the most recent dose of PCV7 or PPSV23. This will constitute the final dose of PCV for these children (Tables 9 and 11).
Children Aged 6--18 Years With Certain High-Risk Conditions
- A single dose of PCV13 may be administered for children aged 6--18 years who have not received PCV13 previously and are at increased risk for invasive pneumococcal disease because of anatomic or functional asplenia, including sickle cell disease, immunocompromising conditions such as HIV-infection, cochlear implant, or cerebrospinal fluid leaks, regardless of whether they have previously received PCV7 or PPSV23.
- Routine use of PCV13 is not recommended for healthy children aged ≥5 years.
Administration of PPSV23 After PCV7 Or PCV13 Among Children Aged 2--18 Years Who Are at Increased Risk for Pneumococcal Disease
- Children aged ≥2 years with underlying medical conditions (Table 2) should receive PPSV23 after completing all recommended doses of PCV13. These children should be administered 1 dose of PPSV23 at age ≥2 years and at least 8 weeks after the most recent dose of PCV13 (Table 12).
- Children who have received PPSV23 previously also should receive recommended PCV13 doses.
- Children aged 24--71 months with underlying medical conditions who received <3 doses of PCV7 before age 24 months should receive a series of 2 doses of PCV13 followed by 1 dose of PPSV23 administered ≥8 weeks later.
- Children aged 24--71 months with underlying medical conditions who received any incomplete schedule of 3 doses of PCV7 before age 24 months should receive 1 dose of PCV13 followed by 1 dose of PPSV23 administered ≥8 weeks later.
- When elective splenectomy, immunocompromising therapy, or cochlear implant placement is being planned, PCV13 and/or PPSV23 vaccination should be completed at least 2 weeks before surgery or initiation of therapy.
Revaccination With PPSV23 Among Children at Highest Risk
- A second dose of PPSV23 is recommended 5 years after the first dose of PPSV23 for children who have anatomic or functional asplenia, including SCD, HIV infection, or other immunocompromising condition (Table 12). No more than 2 PPSV23 doses are recommended.
American Indian/Alaska Native Children
- Routine use of PPSV23 after PCV7 or PCV13 is not recommended for American Indian/Alaska Native children aged ≥24 months without underlying medical conditions. However, in special situations, public health authorities might recommend use of PPSV23 after PCV7 or PCV13 for American Indian/Alaska Native children who are living in areas/communities in which risk for invasive pneumococcal disease is increased.
Public Health Considerations
Evaluation of PCV13 Cost-Effectiveness
Two independent analyses of PCV13 cost-effectiveness reviewed according to ACIP guidelines were presented for consideration by ACIP (82,83). Although these studies used different modeling approaches and assumptions about potential vaccine effects, their conclusions were largely consistent with each other. A CDC study using a cohort model to evaluate cost-effectiveness among infants and young children did not include the potential indirect PCV13 effects among unvaccinated groups in the analysis because of uncertainty regarding their magnitude and timing and the fact that varying assumptions about indirect effects were demonstrated to have major impact on PCV7 cost-effectiveness estimates (84). The results suggested that from a societal perspective, routine infant vaccination of a single birth cohort with PCV13 replacing PCV7 was likely cost-saving with over 142millionsavedincludingproductivitygains(82).The1−dosesupplementalPCV13vaccinationwasdeterminedtobecomparableincost−effectivenesstootheracceptedpublichealthinterventions,costingapproximately142 million saved including productivity gains (82). The 1-dose supplemental PCV13 vaccination was determined to be comparable in cost-effectiveness to other accepted public health interventions, costing approximately 142millionsavedincludingproductivitygains(82).The1−dosesupplementalPCV13vaccinationwasdeterminedtobecomparableincost−effectivenesstootheracceptedpublichealthinterventions,costingapproximately20,200 per discounted quality adjusted life-year (QALY) saved (range in sensitivity analyses: 11,200−−11,200--11,200−−35,500 per discounted QALY saved). Another study sponsored by the PCV13 manufacturer used a decision-analytic Markov model that also predicted substantial net savings for the routine PCV13 program compared with PCV7 (83). The 1-dose supplemental PCV13 program was found to be cost-saving under the assumption that the occurrence of indirect effects would be accelerated by ≥6 months as the supplemental dose would reduce nasopharyngeal carriage of certain PCV13 serotypes among older children; when indirect effects were not included, the supplemental dose program was still considered cost-effective.
Postlicensure Monitoring
Because PCV13 was licensed by FDA on the basis of safety and immunogenicity studies alone, without efficacy data, postlicensure monitoring of vaccine effectiveness will be particularly important in addition to the usual postlicensure monitoring of safety. Also, because wide use of PCV13 can be expected to alter the distribution of S. pneumoniae serotypes, continued monitoring of the epidemiologic patterns of pneumococcal disease will remain necessary (85). FDA and CDC will conduct postlicensure monitoring for adverse events associated with PCV13 using VAERS data and CDC will conduct a case-control study of vaccine effectiveness using ABCs data; the manufacturer will also conduct postlicensure studies.
In June 2009, the Council of State and Territorial Epidemiologists adopted a new position statement and case definition for national surveillance of IPD and recommended enhanced surveillance to track the effects of PCV13 vaccination program (86). Cases of IPD among all ages are now reportable in many states, and health departments in these states forward the data to CDC through the National Notifiable Diseases Surveillance System. The national surveillance case definition for invasive S. pneumoniae disease is isolation of S. pneumoniae from a normally sterile body site (e.g., blood, cerebrospinal fluid, or, less commonly, joint, pleural or pericardial fluid). Epidemiologically important data (e.g., demographics, information on underlying conditions associated with increased risk of IPD, vaccination status, and antibiotic susceptibility testing results) should be reported for each case to track prevalence and geographic distribution of antibiotic resistance patterns.
In addition, public health officials might want to track changes in serotypes among IPD cases in their jurisdiction. A method for polymerase chain reaction (PCR)-based serotyping of S. pneumoniae isolates is now available for use by state and territorial public health laboratories. The PCR method or conventional serotyping can be used for distinguishing whether the detected cases are caused by serotypes included in PCV13 (87,88).
References
- CDC. Preventing pneumococcal disease among infants and young children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2000;49(No. RR-9).
- Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J 2000;19:187--95.
- Black SB, Shinefield HR, Ling S, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr Infect Dis J 2002;21:810--5.
- Eskola J, Kilpi T, Palmu A, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med 2001;344:403--9.
- CDC. Updated recommendation from the Advisory Committee on Immunization Practices (ACIP) for use of 7-valent pneumococcal conjugate vaccine (PCV7) in children aged 24--59 months who are not completely vaccinated. MMWR 2008;57:343--4.
- CDC. National, state, and local area vaccination coverage among children aged 19--35 months---United States, 2009. MMWR 2010;59:1171--1177.
- Grijalva CG, Griffin MR. Population-based impact of routine infant immunization with pneumococcal conjugate vaccine in the USA. Expert Rev Vaccines 2008;7:83--95.
- World Health Organization. Pneumococcal conjugate vaccine for childhood immunization---WHO position paper. Wkly Epidemiol Rec 2007;12:93--104.
- Food and Drug Administration. Product approval information---licensing action, package insert: Prevnar 13 (pneumococcal 13-valent conjugate vaccine), Pfizer. Rockville, MD: US Department of Health and Human Services, Food and Drug Administration; 2010. Available at http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm201667.htm
- Food and Drug Administration. Prevnar 13: clinical review of new product license application. Rockville, MD: US Department of Health and Human Services, Food and Drug Administration; 2010. Available at http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm201667.htm.
- CDC. Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children---Advisory Committee on Immunization Practices (ACIP), 2010. MMWR 2010;59:258--61.
- CDC. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 1997;46(No. RR-8).
- O'Brien KL, Moulton LH, Reid R, et al. Efficacy and safety of seven-valent conjugate pneumococcal vaccine in American Indian children: group randomised trial. Lancet 2003;362:355--61.
- Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, Pierce N. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med 2003;349:1341--8.
- Cutts FT, Zaman SMA, Enwere G, et al. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet 2005;365:1139--46.
- Lucero MG, Nohynek H, Williams G, et al. Efficacy of an 11-valent pneumococcal conjugate vaccine against radiologically confirmed pneumonia among children less than 2 years of age in the Philippines: a randomized, double-blind, placebo-controlled trial. Pediatr Infect Dis J 2009;28:455--62.
- Lucero MG, Dulalia VE, Nillos LT, et al. Pneumococcal conjugate vaccines for preventing vaccine-type invasive pneumococcal disease and X-ray defined pneumonia in children less than two years of age. Cochrane Database Syst Rev 2009:CD004977.
- Hansen J, Black S, Shinefield H, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than 5 years of age for prevention of pneumonia: updated analysis using World Health Organization standardized interpretation of chest radiographs. Pediatr Infect Dis J 2006;25:779--81.
- Madhi SA, Adrian P, Kuwanda L, et al. Long-term immunogenicity and efficacy of a 9-valent conjugate pneumococcal vaccine in human immunodeficient virus infected and non-infected children in the absence of a booster dose of vaccine. Vaccine 2007;25:2451--7.
- Fireman B, Black SB, Shinefield HR, Lee J, Lewis E, Ray P. Impact of the pneumococcal conjugate vaccine on otitis media. Pediatr Infect Dis J 2003;22:10--6.
- Destefano F, Pfeifer D, Nohynek H. Safety profile of pneumococcal conjugate vaccines: systematic review of pre- and post-licensure data. Bull World Health Organ 2008;86:373--80.
- Food and Drug Administration. Clinical review of new product license application (PLA 92-0279), Prevnar pneumococcal 7-valent conjugate vaccine. Rockville, MD: US Department of Health and Human Services, Food and Drug Administration; 2000. Available at http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm180017.htm
- Wise RP, Iskander J, Pratt RD, et al. Postlicensure safety surveillance for 7-valent pneumococcal conjugate vaccine. JAMA 2004;292:1702--10.
- Zhou W, Pool V, Iskander JK, et al. Surveillance for safety after immunization: Vaccine Adverse Event Reporting System (VAERS)---United States, 1991--2001. MMWR 2003;52(No. SS-1).
- Kaplan SL, Mason EO Jr, Wald ER, et al. Decrease of invasive pneumococcal infections in children among 8 children's hospitals in the United States after the introduction of the 7-valent pneumococcal conjugate vaccine. Pediatrics 2004;113:443--9.
- Mahon BE, Hsu K, Karumuri S, Kaplan SL, Mason EO Jr, Pelton SI. Effectiveness of abbreviated and delayed 7-valent pneumococcal conjugate vaccine dosing regimens. Vaccine 2006;24:2514--20.
- Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med 2003;348:1737--46.
- Whitney CG, Pilishvili T, Farley MM, et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet 2006;368:1495--502.
- Hsu K, Pelton S, Karumuri S, Heisey-Grove D, Klein J. Population-based surveillance for childhood invasive pneumococcal disease in the era of conjugate vaccine. Pediatr Infect Dis J 2005;24:17--23.
- Whitney CG, Moore, MR. Direct and indirect effectiveness and safety of pneumococcal conjugate vaccine in practice. In: Siber GR, Klugman KP, Makela PH, eds. Pneumococcal vaccines: the impact of conjugate vaccine. Washington, DC: ASM Press; 2008:353--68.
- Hsu HE, Shutt KA, Moore MR, et al. Effect of pneumococcal conjugate vaccine on pneumococcal meningitis. N Engl J Med 2009;360:244--56.
- Tsai CJ, Griffin MR, Nuorti JP, Grijalva CG. Changing epidemiology of pneumococcal meningitis after the introduction of pneumococcal conjugate vaccine in the United States. Clin Infect Dis 2008;46:1664--72.
- Moore MR, Gertz RE, Jr, Woodbury RL, et al. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J Infect Dis 2008;197:1016--27.
- Pilishvili T, Lexau C, Farley MM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 2010;201:32--41.
- Hicks L, Harrison L, Flannery B, et al. Incidence of pneumococcal disease due to non--pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998--2004. J Infect Dis 2007;196:1346--54.
- Byington CL, Samore MH, Stoddard GJ, et al. Temporal trends of invasive disease due to Streptococcus pneumoniae among children in the Intermountain West: emergence of nonvaccine serogroups. Clin Infect Dis 2005;41:21--9.
- Hsu KK, Shea KM, Stevenson AE, Pelton SI. changing serotypes causing childhood invasive pneumococcal disease: Massachusetts, 2001--2007. Pediatr Infect Dis J 2010;4:289-93.
- Singleton RJ, Hennessy TW, Bulkow LR, et al. Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska Native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA 2007;297:1784--92.
- Wenger JD, Zulz T, Bruden D, et al. Invasive pneumococcal disease in Alaskan children: impact of the seven-valent pneumococcal conjugate vaccine and the role of water supply. Pediatr Infect Dis J 2010;29:251--6.
- Kyaw MH, Lynfield R, Schaffner W, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med 2006;354:1455--63.
- Dagan R, Klugman KP. Impact of conjugate pneumococcal vaccines on antibiotic resistance. Lancet Infect Dis 2008;8:785--95.
- Messina AF, Katz-Gaynor K, Barton T, et al. Impact of the pneumococcal conjugate vaccine on serotype distribution and antimicrobial resistance of invasive Streptococcus pneumoniae isolates in Dallas, TX, children from 1999 through 2005. Pediatr Infect Dis J 2007;26:461--7.
- Pelton SI, Huot H, Finkelstein JA, et al. Emergence of 19A as virulent and multidrug resistant Pneumococcus in Massachusetts following universal immunization of infants with pneumococcal conjugate vaccine. Pediatr Infect Dis J 2007;26:468-72.
- Ongkasuwan J, Valdez TA, Hulten KG, Mason EO Jr, Kaplan SL. Pneumococcal mastoiditis in children and the emergence of multidrug-resistant serotype 19A isolates. Pediatrics 2008;122:34--9.
- Pichichero ME, Casey JR. Emergence of a multiresistant serotype 19A pneumococcal strain not included in the 7-valent conjugate vaccine as an otopathogen in children. JAMA 2007;298:1772--8.
- CDC. Pneumonia hospitalizations among young children before and after introduction of pneumococcal conjugate vaccine---United States, 1997--2006. MMWR 2009;58:1--4.
- Grijalva CG, Nuorti JP, Arbogast PG, Martin SW, Edwards KM, Griffin MR. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet 2007;369:1179--86.
- Zhou F, Kyaw MH, Shefer A, Winston CA, Nuorti JP. Health care utilization for pneumonia in young children after routine pneumococcal conjugate vaccine use in the United States. Arch Pediatr Adolesc Med 2007;161:1162--8.
- Nelson JC, Jackson M, Yu O, et al. Impact of the introduction of pneumococcal conjugate vaccine on rates of community acquired pneumonia in children and adults. Vaccine 2008;26:4947--54.
- O'Brien MA, Prosser LA, Paradise JL, et al. New vaccines against otitis media: projected benefits and cost-effectiveness. Pediatrics 2009;123:1452--63.
- Ray GT, Pelton SI, Klugman KP, Strutton DR, Moore MR. Cost-effectiveness of pneumococcal conjugate vaccine: an update after 7 years of use in the United States. Vaccine 2009;27:6483--94.
- Grijalva CG, Poehling KA, Nuorti JP, et al. National impact of universal childhood immunization with pneumococcal conjugate vaccine on outpatient medical care visits in the United States. Pediatrics 2006;118:865--73.
- Zhou F, Shefer A, Kong Y, Nuorti JP. Trends in acute otitis media-related health care utilization by privately insured young children in the United States, 1997--2004. Pediatrics 2008;121:253--60.
- Poehling KA, Szilagyi PG, Grijalva CG, et al. Reduction of frequent otitis media and pressure-equalizing tube insertions in children after introduction of pneumococcal conjugate vaccine. Pediatrics. 2007;119:707--15.
- Poehling KA, Talbot TR, Griffin MR, et al. Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine. JAMA 2006;295:1668--74.
- Lexau CA, Lynfield R, Danila R, et al. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA 2005;294:2043--51.
- Flannery B, Schrag S, Bennett NM, et al. Impact of childhood vaccination on racial disparities in invasive Streptococcus pneumoniae infections [see comment]. JAMA 2004;291:2197--203.
- Talbot TR, Poehling KA, Hartert TV, et al. Elimination of racial differences in invasive pneumococcal disease in young children after introduction of the conjugate pneumococcal vaccine. Pediatr Infect Dis J 2004;23:726--31.
- Reefhuis J, Honein MA, Whitney CG, et al. Risk of bacterial meningitis in children with cochlear implants. N Engl J Med 2003;349:435--45.
- Whitney CG. Cochlear implants and meningitis in children. Pediatr Infect Dis J 2004;23:767--8.
- Black S, Eskola J, Whitney C, Shinefield H. Pneumococcal conjugate vaccine and pneumococcal common protein vaccines. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. 5th ed. Philadelphia, PA: WB Saunders Company; 2008:531--68.
- O'Brien KL, Swift AJ, Winkelstein JA, et al. Safety and immunogenicity of heptavalent pneumococcal vaccine conjugated to CRM197 among infants with sickle cell disease. Pediatrics 2000;106:965--72.
- Reinert P, Benkerrou M, de Montalembert M, et al. Immunogenicity and safety of a pneumococcal conjugate 7-valent vaccine in infants with sickle cell disease. Pediatr Infect Dis J 2007;26:1105--9.
- Vernacchio L, Neufeld EJ, MacDonald K, et al. Combined schedule of 7-valent pneumococcal conjugate vaccine followed by 23-valent pneumococcal vaccine in children and young adults with sickle cell disease. J Pediatr 1998;133:275--8.
- Bliss SJ, O'Brien KL, Janoff EN, et al. The evidence for using conjugate vaccines to protect HIV-infected children against pneumococcal disease. Lancet Infect Dis 2008;8:67--80.
- Nachman S, Kim S, King J, et al. Safety and immunogenicity of a heptavalent pneumococcal conjugate vaccine in infants with human immunodeficiency virus type 1 infection. Pediatrics 2003;112(1 Pt 1):66--73.
- Abzug MJ, Pelton SI, Song LY, et al. Immunogenicity, safety, and predictors of response after a pneumococcal conjugate and pneumococcal polysaccharide vaccine series in human immunodeficiency virus-infected children receiving highly active antiretroviral therapy. Pediatr Infect Dis J 2006;25:920--9.
- Shinefield H, Black S, Ray P, Fireman B, Schwalbe J, Lewis E. Efficacy, immunogenicity and safety of heptavalent pneumococcal conjugate vaccine in low birth weight and preterm infants. Pediatr Infect Dis J 2002;21:182--6.
- Steenhoff AP, Wood SM, Rutstein RM, Wahl A, McGowan KL, Shah SS. Invasive pneumococcal disease among human immunodeficiency virus-infected children, 1989--2006. Pediatr Infect Dis J 2008;27:886--91.
- Adamkiewicz TV, Silk BJ, Howgate J, et al. Effectiveness of the 7-Valent pneumococcal conjugate vaccine in children with sickle cell disease in the first decade of life. Pediatrics 2008;121:562--9.
- Halasa NB, Shankar SM, Talbot TR, et al. Incidence of invasive pneumococcal disease among individuals with sickle cell disease before and after the introduction of the pneumococcal conjugate vaccine. Clin Infect Dis 2007;44:1428--33.
- Jodar L, Butler J, Carlone G, et al. Serological criteria for evaluation and licensure of new pneumococcal conjugate vaccine formulations for use in infants. Vaccine 2003;21:3265--72.
- Siber GR, Chang I, Baker S, et al. Estimating the protective concentration of anti-pneumococcal capsular polysaccharide antibodies. Vaccine 2007;25:3816--26.
- World Health Organization. Pneumococcal conjugate vaccines: recommendations for the production and control of pneumococcal conjugate vaccines. WHO Technical Report Series No. 927. Geneva, Switzerland: World Health Organization; 2005.
- Romero-Steiner S, Frasch C, Concepcion N, et al. Multilaboratory evaluation of a viability assay for measurement of opsonophagocytic antibodies specific to the capsular polysaccharides of Streptococcus pneumoniae. Clin Diagn Lab Immunol 2003;10:1019--24.
- Hausdorff WP, Feikin DR, Klugman KP. Epidemiological differences among pneumococcal serotypes. Lancet Infect Dis 2005;5:83--93.
- O'Brien KL, Santosham M. Potential impact of conjugate pneumococcal vaccines on pediatric pneumococcal diseases. Am J Epidemiol 2004;159:634--44.
- Jackson LA, Neuzil KM. Pneumococcal polysaccharide vaccines. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. 5th ed. Philadelphia, PA: WB Saunders Company; 2008:569--604.
- O'Brien KL, Hochman M, Goldblatt D. Combined schedules of pneumococcal conjugate and polysaccharide vaccines: is hyporesponsiveness an issue? Lancet Infect Dis 2007;7:597--606.
- de Roux A, Schmole-Thoma B, Siber GR, et al. Comparison of pneumococcal conjugate polysaccharide and free polysaccharide vaccines in elderly adults: conjugate vaccine elicits improved antibacterial immune responses and immunological memory. Clin Infect Dis 2008;46:1015--23.
- Torling J, Hedlund J, Konradsen HB, Ortqvist A. Revaccination with the 23-valent pneumococcal polysaccharide vaccine in middle-aged and elderly persons previously treated for pneumonia. Vaccine 2003;22:96--103.
- Messonnier M, Zhou F, Nuorti JP. Cost-effectiveness of 13-valent pneumococcal conjugate vaccine among infants and children in the United States [Abstract no. 182]. Presented at the 7th International Symposium on Pneumococci and Pneumococcal Diseases (ISPPD-7), Tel Aviv, Israel; March 14--18, 2010.
- Rubin J, McGarry L, Strutton D, et al. Public health and economic impact of 13-valent pneumococcal conjugate vaccine (PCV13) in the U.S. Infectious Disease Society of America (IDSA) 47th Annual Meeting 2009, Philadelphia, Pennsylvania; October 29--November 1, 2009.
- Rozenbaum MH, Hoek AJ, Hak E, Postma MJ. Huge impact of assumptions on indirect effects on the cost-effectiveness of routine infant vaccination with 7-valent conjugate vaccine (Prevnar). Vaccine 2010;28:2367--9.
- Schuchat A, Bell BP. Monitoring the impact of vaccines postlicensure: new challenges, new opportunities. Expert Rev Vaccines 2008;7:437--56.
- Council of State and Territorial Epidemiologists. Enhancing state-based surveillance for invasive pneumococcal disease. CSTE Position Statement 2009--ID-06. Atlanta, GA: Council of State and Territorial Epidemiologists; 2009. Available at http://www.cste.org/ps2009/09-ID-06.pdf.
- Pai R, Moore MR, Pilishvili T, Gertz RE, Whitney CG, Beall B. Postvaccine genetic structure of Streptococcus pneumoniae serotype 19A from children in the United States. J Infect Dis 2005;192:1988--95.
- CDC. PCR deduction of pneumococcal serotypes, 2008. Atlanta, GA: US Department of Health and Human Services, CDC; 2008. Available at http://www.cdc.gov/ncidod/biotech/strep/pcr.htm.
FIGURE 1. Incidence* of invasive pneumococcal disease (IPD) among children aged <18 years, by age group --- United States, Active Bacterial Core surveillance areas, 1998--1999 and 2008
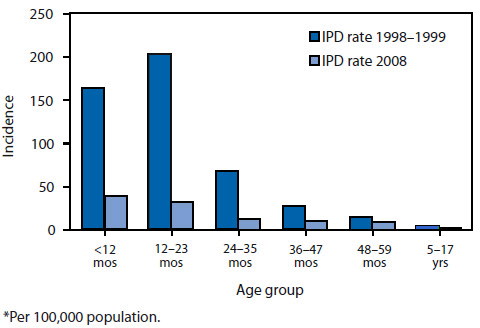
*Per 100,000 population.
Alternate Text: This figure shows the incidence per 100,000 population of cases of IPD among children aged <18 years for 1998-1999 and 2008. A substantially greater proportion of cases occurred among children aged <23 months during 1998-1999 compared with 2008.
FIGURE 2. Proportion of cases of invasive pneumococcal disease among children aged <5 years, by vaccine serotype --- United States, Active Bacterial Core surveillance areas, 2008
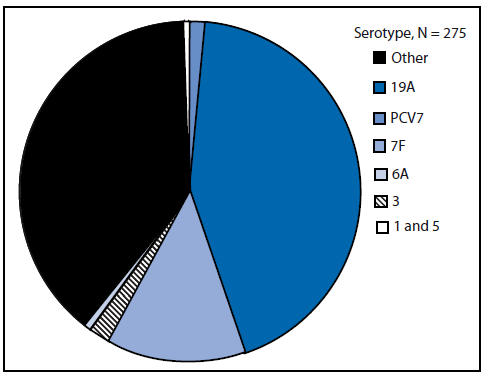
Abbreviation: PCV7 = 7-valent pneumococcal polysaccharide-protein conjugate vaccine.
Alternate Text: This figure shows the proportion of cases of invasive pneumococcal disease for 2008 among children aged <5 years, by vaccine serotype.
FIGURE 3. Proportion of cases of invasive pneumococcal disease caused by serotypes in different vaccine formulations, by age group --- United States, Active Bacterial Core surveillance areas, 2008
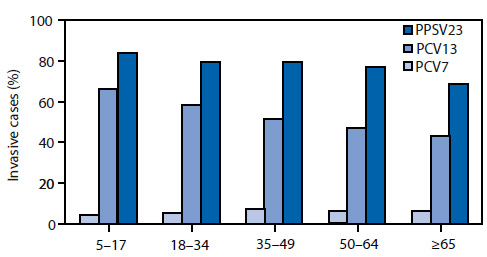
Abbreviations: PCV7 = 7-valent pneumococcal polysaccharide-protein conjugate vaccine, PCV13 = 13-valent pneumococcal polysaccharide-protein conjugate vaccine, and PPSV23 = 23-valent pneumococcal polysaccharide vaccine.
Alternate Text: This figure shows the proportion of cases of invasive pneumococcal disease caused by serotypes in three different vaccine formulations, by age group. The three vaccines are the 7-valent pneumococcal polysaccharide-protein conjugate vaccine (PCV7), the 13-valent pneumococcal polysaccharide-protein conjugate vaccine (PCV13), and the 23-valent pneumococcal polysaccharide vaccine (PPSV23).
| TABLE 1. Rates* **of invasive pneumococcal disease (IPD) among children aged <5 years, by age, race, and vaccine serotype group, --- Active Bacterial Core surveillance (ABCs),**† 10 U.S. sites, 2008 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Serotype group Age (yrs) | All races | White | Black | ||||||
| All IPD | PCV13 types | Non-PCV13 types | All IPD | PCV13 types | Non-PCV13 types | All IPD | PCV13 types | Non-PCV13 types | |
| <1 | 39.2 | 23.8 | 15.5 | 33.3 | 20.3 | 13.0 | 65.6 | 40.0 | 25.6 |
| 1 | 32.4 | 15.9 | 16.5 | 27.9 | 13.7 | 14.2 | 47.4 | 23.2 | 24.2 |
| 2 | 12.6 | 8.8 | ---§ | 7.1 | 5.0 | --- | 28.0 | 19.4 | --- |
| 3 | 10.8 | 7.3 | --- | 6.6 | 4.5 | --- | 24.0 | 16.3 | --- |
| 4 | 9.2 | 7.7 | --- | 9.8 | 8.1 | --- | --- | --- | --- |
| All <5 | 21.0 | 12.7 | 8.2 | 17.0 | 10.3 | 6.7 | 34.9 | 21.2 | 13.7 |
| Abbreviation: PCV13 = 13-valent pneumococcal polysaccharide-protein conjugate vaccine. Source: CDC, Active Bacterial Core surveillance (ABCs), unpublished data, 2009. * Per 100,000 population. † Information about ABCs is available at http://www.cdc.gov/abcs/index.html. § Indicates too few cases in the cell to calculate rates. For races other than black and white, the number of cases was too low to calculate rates in individual 1-year age strata. Among children of other races aged <5 years, overall rates were 14.6 for all IPD, 8.9 for PCV13 types, and 5.7 for non-PCV13 types. |
| TABLE 2. Underlying medical conditions that are indications for pneumococcal vaccination among children, by risk group | |
|---|---|
| Risk group | Condition |
| Immunocompetent children | Chronic heart disease* |
| Chronic lung disease† | |
| Diabetes mellitus | |
| Cerebrospinal fluid leaks | |
| Cochlear implant | |
| Children with functional or anatomic asplenia | Sickle cell disease and other hemoglobinopathies Congenital or acquired asplenia, or splenic dysfunction |
| Children with immunocompromising conditions | HIV infection |
| Chronic renal failure and nephrotic syndrome | |
| Diseases associated with treatment with immunosuppressive drugs or radiation therapy, including malignant neoplasms, leukemias, lymphomas and Hodgkin disease; or solid organ transplantation | |
| Congenital immunodeficiency§ | |
| Source: Advisory Committee on Immunization Practices, 2010. * Particularly cyanotic congenital heart disease and cardiac failure. † Including asthma if treated with high-dose oral corticosteroid therapy. § Includes B- (humoral) or T-lymphocyte deficiency; complement deficiencies, particularly C1, C2, C3, and C4 deficiency; and phagocytic disorders (excluding chronic granulomatous disease). |
| TABLE 3. Number and proportion of children aged 24--59 months with invasive pneumococcal disease (IPD), by PPSV23 indication and serotype group --- Active Bacterial Core Surveillance (ABCs), 10 U.S. sites, 2006--2008 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Serotype Group | All IPD | PCV13 | Serotypes included in PPSV23 but not in PCV13* | Other serotypes | Unknown serotypes | ||||
| No. | No. | (%) | No. | (%) | No. | (%) | No. | (%) | |
| All Cases | 475 | 276 | 58.1 | 73 | 15.4 | 54 | 11.4 | 72 | 15.2 |
| No underlying condition† | 424 | 253 | 59.7 | 65 | 15.3 | 40 | 9.4 | 66 | 15.6 |
| Any ACIP indication | 51 | 23 | 45.1 | 8 | 15.7 | 14 | 27.5 | 6 | 11.8 |
| Sickle cell disease or asplenia§ | 11 | 3 | 27.3 | 3 | 27.3 | 5 | 45.5 | 0 | 0 |
| HIV/AIDS | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chronic illness¶ | 3 | 1 | 33.3 | 0 | 0 | 1 | 33.3 | 1 | 33.3 |
| Other immunocompromising condition¶ | 37 | 19 | 51.4 | 5 | 13.5 | 8 | 21.6 | 5 | 13.5 |
| Abbreviations: PPSV23 = 23-valent pneumococcal polysaccharide vaccine, PCV13 = 13-valent pneumococcal polysaccharide-protein conjugate vaccine, and ACIP = Advisory Committee on Immunization Practices. * The 11 serotypes included in PPSV23 but not in PCV13; serotype 6A is not included in PPSV23. † Absence of underlying medical conditions listed in Table 2. § Includes other hemoglobinopathies, congenital or acquired asplenia, or splenic dysfunction. ¶ Does not include HIV, AIDS, sickle cell disease, hemoglobinopathies, or splenic dysfunction. |
| TABLE 4. Percentage of infants with pneumococcal IgG ≥0.35 μg/mL 1 month following the third infant dose --- noninferiority study (004), United States | ||||
|---|---|---|---|---|
| Vaccine serotype | PCV13 (n† = 249--252) | PCV7 (n = 250--252) | Difference* (%PCV13 - PCV7) | 95% CI for the difference in proportions |
| Common serotypes | ||||
| 4 | 94.4 | 98.0 | -3.6 | (-7.3-- -0.1) |
| 6B§ | 87.3 | 92.8 | -5.5 | (-10.9-- -0.1) |
| 9V§ | 90.5 | 98.4 | -7.9 | (-12.4-- -4.0) |
| 14 | 97.6 | 97.2 | 0.4 | (-2.7--3.5) |
| 18C | 96.8 | 98.4 | -1.6 | (-4.7--1.2) |
| 19F | 98.0 | 97.6 | 0.4 | (-2.4--3.4) |
| 23F | 90.5 | 94.0 | -3.6 | (-8.5--1.2) |
| 6 additional serotypes in PCV13 | ||||
| 1 | 95.6 | ¶ | 2.8 | (-1.3--7.2) |
| 3§ | 63.5 | ¶ | -29.3 | (-36.2--22.4) |
| 5 | 89.7 | ¶ | -3.1 | (-8.3--1.9) |
| 6A | 96.0 | ¶ | 3.2 | (-0.8--7.6) |
| 7F | 98.4 | ¶ | 5.6 | (1.9--9.7) |
| 19A | 98.4 | ¶ | 5.6 | (1.9--9.7) |
| Abbreviations: PCV13 = 13-valent pneumococcal polysaccharide-protein conjugate vaccine, PCV7 = 7-valent pneumococcal polysaccharide-protein conjugate vaccine, and CI = confidence interval. Source: Food and Drug Administration clinical review of PCV13 (10). * Difference in proportions (PCV13-PCV7 reference value) expressed as a difference in percentages. † N = range of subjects with a determinate IgG antibody concentration by enzyme-linked immunosorbent assay (ELISA) to a given serotype. § Serotype did not meet the prespecified primary endpoint criterion. ¶ For the additional serotypes, the reference value is serotype 6B in the PCV7 group. Noninferiority was defined as the lower limit of the 2-sided 95% CI for the difference in proportions of >-10%. |
| TABLE 5. Pneumococcal IgG geometric mean concentrations (μg/mL) 1 month following the fourth (booster) dose of pneumococcal conjugate vaccine, noninferiority study (004), United States | ||||
|---|---|---|---|---|
| Vaccine serotype | PCV13 (n† = 232--236) | PCV7 (n = 222--223) | GMC ratio* (PCV13/PCV7) | 95% CI for the GMC ratio |
| Common serotypes | ||||
| 4 | 3.7 | 5.5 | 0.7 | (0.6--0.8) |
| 6B | 11.5 | 15.6 | 0.7 | (0.6--0.9) |
| 9V | 2.6 | 3.6 | 0.7 | (0.6--0.9) |
| 14 | 9.1 | 12.7 | 0.7 | (0.6--0.9) |
| 18C | 3.2 | 4.7 | 0.7 | (0.6--0.8) |
| 19F | 6.6 | 5.6 | 1.2 | (1.0--1.4) |
| 23F | 5.1 | 7.8 | 0.7 | (0.5--0.8) |
| 6 additional serotypes in PCV13 | ||||
| 1 | 5.1 | ¶ | 1.4 | (1.2--1.7) |
| 3§ | 0.9 | ¶ | 0.3 | (0.2--0.3) |
| 5 | 3.7 | ¶ | 1.0 | (0.9--1.2) |
| 6A | 8.2 | ¶ | 2.3 | (1.9--2.7) |
| 7F | 5.7 | ¶ | 1.6 | (1.3--1.9) |
| 19A | 8.6 | ¶ | 2.4 | (2.0--2.8) |
| Abbreviations: PCV13 = 13-valent pneumococcal polysaccharide-protein conjugate vaccine, PCV7 = 7-valent pneumococcal polysaccharide-protein conjugate vaccine, GMC = geometric mean concentrations, and CI = confidence interval. Source: Food and Drug Administration clinical review of PCV13 (10). * GMC ratio: PCV13 to PCV7 reference. † N = range of subjects with a determinate IgG antibody concentration by enzyme-linked immunosorbent assay (ELISA) to a given serotype. § Serotype did not meet the prespecified noninferiority criteria. ¶ For the additional serotypes, the reference value is serotype 9V in the PCV7 group. Noninferiority was defined as a lower limit of the 2-sided 95% CI for the GMC ratio (PCV13 group/PCV7 group) >0.5. |
FIGURE 4. Opsonophygocytic antibody (OPA) responses (GMTs) to six additional serotypes after 4 doses of PCV13 and 3 doses of PCV7 followed by 1 dose of PCV13*
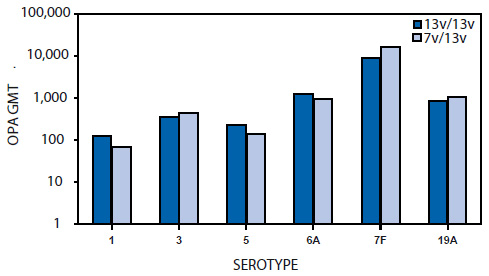
Abbreviations: PCV7 = 7-valent pneumococcal polysaccharide-protein conjugate vaccine, PCV13 = 13-valent pneumococcal polysaccharide-protein conjugate vaccine,
Source: Food and Drug Administration. PCV13 clinical review (10).
*In both groups, the proportion of subjects with OPA titers ≥1:8 was ≥97.8%.
Alternate Text: This figure shows OPA responses to six additional serotypes after receipt of 4 doses of the 13-valent pneumococcal polysaccharide-protein conjugate vaccine (PCV13) and 3 doses of the 7-valent pneumococcal polysaccharide-protein conjugate vaccine (PCV7), followed by 1 dose of PCV13.
| TABLE 6. Pneumococcal IgG antibody geometric mean concentrations (μg/mL) 1 month after the 12-month dose among children previously administered 3 doses of either PCV13 or PCV7 | |||
|---|---|---|---|
| Vaccine serotype | PCV13/PCV13 after dose at 12 mos* (n† = 233--236) | PCV7/PCV13 after dose at 12 mos* (n = 108--113) | PCV7/PCV7 after dose at 12 mos* (n = 111--127) |
| Serotypes common to PCV7 and PCV13 | |||
| 4 | 4.2 | 4.0 | 4.9 |
| 6B | 9.0 | 10.3 | 9.6 |
| 9V | 2.6 | 2.3 | 3.2 |
| 14 | 9.5 | 7.8 | 10.8 |
| 18C | 2.3 | 2.4 | 2.8 |
| 19F | 5.2 | 3.7 | 4.1 |
| 23F | 3.0 | 3.1 | 3.7 |
| Six additional serotypes in PCV13 | |||
| 1 | 4.1 | 1.8 | NA |
| 3 | 1.0 | 1.3 | NA |
| 5 | 3.3 | 1.1 | NA |
| 6A | 6.1 | 2.6 | NA |
| 7F | 4.5 | 3.7 | NA |
| 19A | 9.5 | 5.3 | NA |
| Abbreviations: PCV13 = 13-valent pneumococcal polysaccharide-protein conjugate vaccine, PCV7 = 7-valent pneumococcal polysaccharide-protein conjugate vaccine, and NA = not applicable. * A randomized, controlled trial conducted in France using a 3-dose infant series given at age 2, 3, 4 months and a toddler dose at age 12 months (Study 008). Data are from the Food and Drug Administration PCV13 clinical review (10). † N = range of subjects with a determinate IgG antibody concentration by enzyme-linked immunosorbent assay (ELISA) to a given serotype. |
| TABLE 7. Reported frequencies of adverse events occurring in >1% of recipients following administration of PCV13 or PCV7 in 13 combined clinical trials | ||
|---|---|---|
| Adverse event | % | |
| PCV 13* | PCV 7† | |
| Injection-site reaction* | (n = 4,729) | (n = 2,760) |
| Pain/Tenderness | 48.8 | 54.4 |
| Erythema (any) | 46.6 | 46.6 |
| Induration/swelling | 35.3 | 37.1 |
| Erythema (>2.4 cm but <7.0 cm) | ||
| Infant series | 4.6 | 4.5 |
| Toddler dose | 13.6 | 12.8 |
| Older children (aged 2--5 yrs) | 37.8 | NA |
| Induration/swelling (>2.4 cm but <7.0 cm) | ||
| Infant series | 7.4 | 6.2 |
| Toddler dose | 12.4 | 11.3 |
| Older children (aged 2--5 yrs) | 25.0 | NA |
| Pain/tenderness interfering with movement | 8.0 | 8.7 |
| Irritability* | 70.0 | 68.4 |
| Drowsiness/increased sleep* | 59.2 | 58.3 |
| Decreased appetite* | 38.7 | 48.0 |
| Fever* | 36.9 | 46.7 |
| Restless sleep/Decreased sleep* | 36.0 | 34.4 |
| Fever >39º C* | 5.3 | 7.4 |
| Diarrhea | 3.1 | 3.0 |
| Vomiting | 1.8 | 2.0 |
| Rash | 1.1 | 1.6 |
| Abbreviations: PCV13 = 13-valent pneumococcal polysaccharide-protein conjugate vaccine, PCV7 = 7-valent pneumococcal polysaccharide-protein conjugate vaccine, and NA = data not available. * Solicited adverse events from 13 combined clinical trials among healthy infants and children aged 6 weeks--16 months and 354 children aged 7--71 months. Data were obtained daily for 4 or 7 days after each vaccination and represent the highest frequency after any dose in the infant series, the toddler dose, or a dose given to older children who had not received PCV previously. The frequencies of solicited adverse reactions after each vaccine dose in the series were similar and are available in the PCV13 package insert (9). |
| TABLE 8. Recommended schedule for use of 13-valent pneumococcal conjugate vaccine (PCV13) among previously unvaccinated infants and children by age at time of first vaccination | ||
|---|---|---|
| Age at first dose (mos) | Primary PCV13 series* | PCV13 booster dose† |
| 2--6 | 3 doses | 1 dose at 12--15 mos |
| 7--11 | 2 doses | 1 dose at 12--15 mos |
| 12--23 | 2 doses | NA |
| 24--59 in healthy children | 1 dose | NA |
| 24--71 in children with certain chronic diseases or immunocompromising conditions§ | 2 doses | NA |
| Abbreviation: NA = not applicable * Minimum interval between doses is 8 weeks except for children vaccinated at age <12 months, for whom minimum interval between doses is 4 weeks. Minimum age for administration of first dose is 6 weeks. † Administered at least 8 weeks after the previous dose. § See Table 2 for a complete list of conditions. |
| TABLE 9. Recommended schedule for administering doses of 13-valent pneumococcal conjugate vaccine (PCV13) to children aged <24 months by PCV vaccination history and age --- Advisory Committee on Immunization Practices, United States, 2010 | ||
|---|---|---|
| Age at this visit (mos) | Vaccination history: total number of PCV7 and/or PCV13 doses received previously | Recommended PCV13 regimen* |
| 2--6 mos | 0 doses | 3 doses, 8 weeks apart; fourth dose at age 12--15 mos |
| 1 dose | 2 doses, 8 weeks apart; fourth dose at age 12--15 mos | |
| 2 doses | 1 dose, 8 weeks after the most recent dose; fourth dose at age 12--15 mos | |
| 7--11 mos | 0 doses | 2 doses, 8 weeks apart; third dose at 12--15 mos |
| 1 or 2 doses before age 7 mos | 1 dose at age 7--11 mos, with a second dose at 12--15 mos, ≥8 weeks later | |
| 12--23 mos | 0 doses | 2 doses, ≥8 weeks apart |
| 1 dose before age 12 mos | 2 doses, ≥8 weeks apart | |
| 1 dose at ≥12 mos | 1 dose, ≥8 weeks after the most recent dose† | |
| 2 or 3 doses before age 12 mos | 1 dose,≥8 weeks after the most recent dose† | |
| 4 doses of PCV7 or other age-appropriate, complete PCV7 schedule | 1 supplemental dose ≥8 weeks after the most recent dose | |
| Abbreviation: PCV7 = 7-valent pneumococcal polysaccharide-protein conjugate vaccine. * Minimum interval between doses is 8 weeks except for children vaccinated at age <1 year, for whom minimum interval between doses is 4 weeks. † No additional PCV13 doses are indicated for children aged 12--23 months who have received 2 or 3 doses of PCV7 before age 12 months and at least 1 dose of PCV13 at age ≥12 months. |
| TABLE 10. Recommended transition from 7-valent pneumococcal polysaccharide-protein conjugate vaccine (PCV7) to 13-valent pneumococcal conjugate vaccine (PCV13) in the routine immunization schedule among infants and children, according to number of previous PCV7 doses received | ||||
|---|---|---|---|---|
| Infant series | Booster dose | Supplemental PCV13 dose | ||
| 2 mos | 4 mos | 6 mos | ≥12 mos* | 14--59 mos† |
| PCV7 | PCV13 | PCV13 | PCV13 | NA |
| PCV7 | PCV7 | PCV13 | PCV13 | NA |
| PCV7 | PCV7 | PCV7 | PCV13 | NA |
| PCV7 | PCV7 | PCV7 | PCV7 | PCV13 |
| Abbreviation: NA = not applicable * No additional PCV13 doses are indicated for children aged 12-- 23 months who have received 2 or 3 doses of PCV7 before age 12 months and at least 1 dose of PCV13 at age ≥12 months. † For children with underlying medical conditions (see Table 2), a supplemental PCV13 dose is recommended through age 71 months. |
| TABLE 11. Recommended schedule for administering doses of 13-valent pneumococcal conjugate vaccine (PCV13) to children aged ≥24 months by PCV vaccination history and age | ||
|---|---|---|
| Age at this visit (mos) | Vaccination history: total number of PCV7 and/or PCV13 doses received previously before age 24 months | Recommended PCV13 regimen* |
| 24--59 mos in healthy children | Unvaccinated or any incomplete schedule | 1 dose, ≥ 8 weeks after the most recent dose |
| 4 doses of PCV7 or other age-appropriate, complete PCV7 schedule | 1 supplemental dose, ≥8 weeks after the most recent dose | |
| 24--71 mos in children with underlying medical conditions† | Unvaccinated or any incomplete schedule of <3 doses | 2 doses, the first dose ≥8 weeks after the most recent dose and a second dose ≥ 8 weeks later |
| Any incomplete schedule of 3 doses | 1 dose, ≥ 8 weeks after the most recent dose | |
| 4 doses of PCV7 or other age-appropriate complete PCV7 schedule | 1 supplemental dose, ≥8 weeks after the most recent dose | |
| Abbreviation: PCV7 = 7-valent pneumococcal polysaccharide-protein conjugate vaccine. * Minimum interval between doses is 8 weeks. † For list of conditions, see Table 2. |
| TABLE 12. Schedule for vaccination using 23-valent polysaccharide vaccine (PPSV23) after 13-valent pneumococcal conjugate vaccine (PCV13) for children aged ≥2 years with underlying medical conditions | ||
|---|---|---|
| Group | Schedule for PPSV23 | Revaccination with PPSV23 |
| Children who are immunocompromised, have sickle cell disease, or functional or anatomic asplenia | 1 dose of PPSV23 administered at age ≥2 yrs and ≥8 weeks after last indicated dose of PCV13 | 1 dose 5 years after the first dose of PPSV23 |
| Immunocompetent children with chronic illness* | 1 dose of PPSV23 administered at age ≥2 yrs and ≥8 weeks after last indicated dose of PCV13 | Not recommended |
| * Chronic heart disease, chronic lung disease, diabetes mellitus, cerebrospinal fluid leaks, or cochlear implant. |
ACIP Pneumococcal Vaccines Working Group, 2007--2010
**Chairs:**Michael Marcy MD, Los Angeles, California (2008--present) and Julie Morita, MD, Chicago, Illinois (2007--2008).
Members: Kathleen Neuzil, MD, Lisa Jackson, MD, Seattle, Washington; Nancy Bennett, MD, Rochester, New York; Carol Baker, MD, Houston, Texas; Shalini Desai, MD, Toronto, Ontario, Canada; Dale Morse, MD, Albany, New York; Mary Glode, MD, Denver, Colorado; William Schaffner, MD, Nashville, Tennessee; Richard Zimmerman, MD, Pittsburgh, Pennsylvania; Jane Zucker, MD, New York, New York, Lorry Rubin, MD, New Hyde Park, New York; Doug Campos-Outcalt, MD, Phoenix, Arizona; Lucia Lee, MD, Rockville, Maryland; Kristin Nichol, MD, Minneapolis, Minnesota; Raymond Strikas, MD, District of Columbia; Farukh Khambaty, PhD, Bethesda, Maryland; Jay Butler, MD, Thomas Hennessy, MD, Anchorage, Alaska; William Atkinson, MD, Angela Calugar, MD, Carol Friedman*, MD, Jane Gidudu, MD, Jessica Henry, MPH, Beth Hibbs, MPH, Hajime Kamiya, MD, Matthew Moore, MD, Gina Mootrey, MD, George Nelson, MD, Pekka Nuorti, MD, Jennifer Rosen, MD, Sandra Romero-Steiner, PhD, Cynthia Whitney, MD, Atlanta, Georgia.
*Deceased.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of Health and Human Services.
References to non-CDC sites on the Internet are provided as a service to MMWR readers and do not constitute or imply endorsement of these organizations or their programs by CDC or the U.S. Department of Health and Human Services. CDC is not responsible for the content of pages found at these sites. URL addresses listed in MMWR were current as of the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.