Nitrogen (original) (raw)
Data Zone | Discovery | Facts | Appearance & Characteristics | Uses | Abundance & Isotopes | References
The chemical element nitrogen is classed as a gas and a nonmetal. It was discovered in 1772 by Daniel Rutherford and independently by Carl Scheele.

Data Zone
| Classification: | Nitrogen is a gas and a nonmetal |
|---|---|
| Color: | colorless |
| Atomic weight: | 14.0067 |
| State: | gas |
| Melting point: | -210.1 oC, 63.05 K |
| Boiling point: | -195.8 oC, 77.4 K |
| Electrons: | 7 |
| Protons: | 7 |
| Neutrons in most abundant isotope: | 7 |
| Electron shells: | 2,5 |
| Electron configuration: | 1s2 2s2 2p3 |
| Density @ 20oC: | 0.0012506 g/cm3 |
Show more, including: Heats, Energies, Oxidation,
Reactions, Compounds, Radii, Conductivities
| Atomic volume: | 17.3 cm3/mol |
|---|---|
| Structure: | hcp: hexagonal close-packed |
| Specific heat capacity | 1.04 J g-1 K-1 |
| Heat of fusion | 0.720 kJ mol-1 of N2 |
| Heat of atomization | 473 kJ mol-1 |
| Heat of vaporization | 5.57 kJ mol-1 of N2 |
| 1st ionization energy | 1402.3 kJ mol-1 |
| 2nd ionization energy | 2856 kJ mol-1 |
| 3rd ionization energy | 4578 kJ mol-1 |
| Electron affinity | -6.75 kJ mol-1 |
| Minimum oxidation number | -3 |
| Min. common oxidation no. | -3 |
| Maximum oxidation number | 5 |
| Max. common oxidation no. | 5 |
| Electronegativity (Pauling Scale) | 3.04 |
| Polarizability volume | 1.1 Å3 |
| Reaction with air | none |
| Reaction with 15 M HNO3 | none |
| Reaction with 6 M HCl | none |
| Reaction with 6 M NaOH | none |
| Oxide(s) | N2O, NO, NO2, N2O5 |
| Hydride(s) | NH3 (ammonia), N2H4 (hydrazine), HN3 (hydrazoic acid) |
| Chloride(s) | NCl3 |
| Atomic radius | 65 pm |
| Ionic radius (1+ ion) | – |
| Ionic radius (2+ ion) | – |
| Ionic radius (3+ ion) | 30 pm |
| Ionic radius (1- ion) | – |
| Ionic radius (2- ion) | – |
| Ionic radius (3- ion) | – |
| Thermal conductivity | 0.02583 W m-1 K-1 |
| Electrical conductivity | – |
| Freezing/Melting point: | -210.1 oC, 63.05 K |
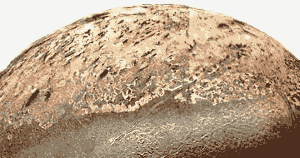
Nasa Image. Triton’s nitrogen geysers push black particles miles above the surface. These are blown by winds on Triton and form black deposits on the satellite’s surface. (See Facts, below.)
Oxygen is removed from air by burning phosphorus, to leave a much higher concentration of nitrogen.

Liquid nitrogen condenses water vapor from the surrounding air. NASA.
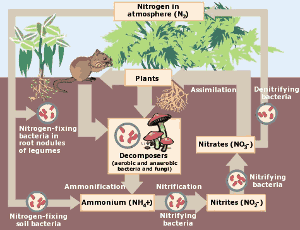
The nitrogen cycle. Click here for larger image. (Courtesy of the Environmental Protection Agency)
Discovery of Nitrogen
In 1674 the English physician John Mayow demonstrated that air is not a single element, it is made up of different substances. He did this by showing that only a part of air is combustible. Most of it is not. (1)
Almost a century later, Scottish chemist Joseph Black carried out more detailed work on air. After removing oxygen and carbon dioxide, part of the air remained.
Black used burning phosphorus as the final step in oxygen removal. (Burning phosphorus has a very high affinity for oxygen and is efficient at removing it completely.) Black then assigned further study of the gases in air to his doctoral student, Daniel Rutherford. (2)
Rutherford built on Black’s work and in a series of steps thoroughly removed oxygen and carbon dioxide from air. He showed that, like carbon dioxide, the residual gas could not support combustion or living organisms. Unlike carbon dioxide, however, nitrogen was insoluble in water and alkali solutions. Rutherford reported his discovery in 1772 of ‘noxious air,’ which we now call nitrogen. (3)
Swedish pharmacist Carl Scheele discovered nitrogen independently, calling it spent air.
Scheele absorbed oxygen in a number of ways, including using a mixture of sulfur and iron filings and burning phosphorus. After removing the oxygen, he reported a residual gas which would not support combustion and had between two-thirds and three-quarters of the volume of the original air. Scheele published his results in 1777, although it is thought the work was carried out in 1772. (4)
Although Rutherford and Scheele are now jointly credited with nitrogen’s discovery, it appears to have been discovered earlier by Henry Cavendish, but not published.
Prior to 1772 (the precise date is unknown – Priestley refers to it in his work “Experiments and Observations Made in and Before the Year 1772”) Cavendish wrote to Joseph Priestley describing ‘burnt air’.
The ‘burnt air’ had been prepared by passing air repeatedly over red hot charcoal (removing the oxygen) and then bubbling the remaining gas through a solution of caustic potash (potassium hydroxide) which would have removed the carbon dioxide.
Cavendish wrote: “The specific gravity of this air was found to differ very little from that of common air; of the two, it seemed rather lighter. It extinguished flame, and rendered common air unfit for making bodies burn in the same manner as fixed air, but in a less degree, as a candle which burnt about 80″ in pure common air, and which went out immediately in common air mixed with 6/55 of fixed air, burnt about 26″ in common air mixed with the same portion of this burnt air.” (5)
In 1790 the French chemist Jean-Antoine-Claude Chaptal named the element ‘nitrogen’ after experiments showed it to be a constituent of nitre, as potassium nitrate was called then.
Interesting Facts about Nitrogen
- About 2.5 percent of the weight of living organisms comes from nitrogen in organic molecules.
- Many of the molecules of life contain nitrogen. It is the fourth most abundant element in the human body.
- The nitrogen compound nitroglycerin can be used for relief of angina, a life threatening heart condition.
- Neptune’s satellite Triton has five mile high, nitrogen-powered geysers.
Like Earth, Triton’s atmosphere is mainly nitrogen, but Triton is so cold the nitrogen sits on the surface as a rock-hard solid. The solid nitrogen allows the feeble light arriving from the sun to pass through it. Dark impurities in the nitrogen ice or in darker rocks below the ice warm up slightly in the sunlight, melting and vaporizing the solid nitrogen, which eventually breaks through the solid nitrogen as geysers which push ice particles one to five miles above Triton’s frozen surface. - Nitrogen is the seventh most abundant element in the universe.
- In 1919, the world learned for the first time that atomic nuclei could be disintegrated. Ernest Rutherford reported that he had bombarded nitrogen gas with alpha-particles (helium nuclei) and found hydrogen was produced. (Further research by Patrick Blackett showed that the alpha particles had transmuted nitrogen-14 to oxygen-17 plus hydrogen.)
- The universe’s nitrogen was made, and is being made, by the CNO cycle in stars heavier than our sun. (See image below)
Nitrogen and the CNO Cycle
When the universe’s first generation of stars was born, they contained only the elements made in the big bang: hydrogen, helium, and a tiny amount of lithium.
As these stars burned, they synthesized heavier elements, such as carbon. Supernovae then spread the heavier elements out into galaxies where more stars were born.
Carbon from supernovae plays a crucial role in the way many second and higher generation stars burn. In stars whose mass is higher than about 1.1 – 1.5 times that of our sun, carbon-12 catalyzes the fusion of hydrogen to helium – i.e. carbon-12 takes part in the fusion reaction, but is not consumed by it.
As you can see on the left, carbon-12 is regenerated at the end of each cycle, the net result of which is that four hydrogen nuclei are consumed and one helium nucleus is produced. This reaction is called the CNO cycle.
Over time, each carbon-12 nucleus can take part in a very large number of cycles. A proportion of nitrogen made during the CNO cycle evades further reaction. At the end of a star’s life, this nitrogen may be distributed into the galaxy. In our solar system the nitrogen from a star that died billions of years ago ended up as an essential element in proteins and DNA and formed about 80 percent of our planet’s atmosphere.

What will liquid nitrogen do to an air filled balloon?
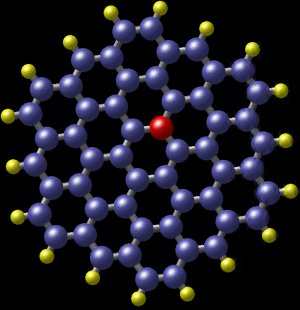
A polycyclic aromatic hydrocarbon with nitrogen. The blue balls are carbon atoms and the yellow balls are hydrogen atoms. The red ball shows the position of a nitrogen atom which fits almost perfectly within the molecule. This molecule was detected in the spiral galaxy M81, some 12 million light years from Earth. (Image: Nasa)
Appearance and Characteristics
Harmful effects:
Nitrogen is non-toxic under normal conditions.
Direct skin contact with liquid nitrogen causes severe frostbite.
Decompression in divers or astronauts can cause the ‘bends’ – a potentially fatal condition when nitrogen bubbles form in the bloodstream.
Characteristics:
Nitrogen is a colorless, odorless, tasteless, diatomic and generally inert gas at standard temperature and pressure.
At atmospheric pressure, nitrogen is liquid between 63 K and 77 K.
Liquids colder than this are considerably more expensive to make than liquid nitrogen is.
Uses of Nitrogen
Nitrogen is used to produce ammonia (Haber process) and fertilizers, vital for current food production methods. It is also used to manufacture nitric acid (Ostwald process).
In enhanced oil recovery, high pressure nitrogen is used to force crude oil that would otherwise not be recovered out of oil wells. Nitrogen’s inert qualities find use in the chemical and petroleum industries to blanket storage tanks with an inert layer of gas.
Liquid nitrogen is used as a refrigerant. Superconductors for practical technologies should ideally have no electrical resistance at temperatures higher than 63 K because this temperature is achievable relatively cheaply using liquid nitrogen. Lower temperatures come with a much higher price tag.
While elemental nitrogen is not very reactive, many of nitrogen’s compounds are unstable.
Oxides naturally form in steel during welding and these weaken the weld. Nitrogen can be used to exclude oxygen during welding, resulting in better welds.
In the natural world, the nitrogen cycle is of crucial importance to living organisms. Nitrogen is taken from the atmosphere and converted to nitrates through lightning storms and nitrogen fixing bacteria. The nitrates fertilize plant growth where the nitrogen becomes bound in amino acids, DNA and proteins. It can then be eaten by animals. Eventually the nitrogen from the plants and animals returns to the soil and atmosphere and the cycle repeats.
Abundance and Isotopes
Abundance earth’s crust: 19 parts per million by weight, 28 parts per million by moles
Abundance solar system: 1,000 ppm by weight, 90 ppm by moles
Cost, pure: $0.4 per 100g
Cost, bulk: $ per 100g
Source: Commercially, nitrogen is obtained from liquid air by fractional distillation. Earth’s atmosphere contains in the region of 4 quadrillion tons (4 x 1015) of nitrogen.
Isotopes: Nitrogen has 12 isotopes whose half-lives are known, with mass numbers 11 to 19. Naturally occurring nitrogen is a mixture of two isotopes, 14N and 15N with natural abundances of 99.6% and 0.4% respectively.

References
- Alexander Findlay, Chemistry in the Service of Man., (2007) p46. Findlay Press.
- Aaron John Ihde, The Development of Modern Chemistry., (2007) p38. Dover Publications.
- Jonathan Shectman, Groundbreaking Scientific Experiments, Inventions, and Discoveries of the 18th Century., (2003) p78. Greenwood Publishing Group.
- Ida Freund, The Experimental Basis Of Chemistry – Suggestions For A Series Of Experiments Illustrative Of The Fundamental Principles Of Chemistry., (2007) p145. Caffin Press.
- George Wilson, The Life of Henry Cavendish., (1851) p28. The Cavendish Society. (pdf – large download 31 MB).
- Royston M. Roberts, Serendipity, Accidental Discoveries in Science., (1989) p89. John Wiley and Sons.
Cite this Page
For online linking, please copy and paste one of the following:
or
To cite this page in an academic document, please use the following MLA compliant citation:
"Nitrogen." Chemicool Periodic Table. Chemicool.com. 08 Oct. 2012. Web.
https://www.chemicool.com/elements/nitrogen.html.