New spinosaurids from the Wessex Formation (Early Cretaceous, UK) and the European origins of Spinosauridae (original) (raw)
Introduction
Spinosaurids are among the most distinctive, unusual and controversial of theropods; they are characterised by an elongate, laterally compressed rostrum, sub-conical dentition and, in a subset of taxa, a dorsal sail formed by elongate neural spines. Their unusual cranial (and in derived forms, postcranial) morphology is atypical of non-avian theropods, and multiple lines of evidence point to an ability to exploit semi-aquatic niches1,2,3,4,5,6,7,8. A “generalist” or varied diet may have included a range of terrestrial and aquatic prey9,10,[11](#ref-CR11 "Hone, D. W. E. & Holtz, T. R. Jr. A century of spinosaurs—a review and revision of the Spinosauridae with comments on their ecology. Acta Geol. Sin. Engl. Ed. 91, 1120–1132. https://doi.org/10.1111/1755-6724.13328
(2017)."),[12](/articles/s41598-021-97870-8#ref-CR12 "Schade, M., Rauhut, O. W. & Evers, S. W. Neuroanatomy of the spinosaurid Irritator challengeri (Dinosauria: Theropoda) indicates potential adaptations for piscivory. Sci. Rep. 10, 1–9 (2020).") and was potentially influenced by individual size[13](/articles/s41598-021-97870-8#ref-CR13 "Cuff, A. R. & Rayfield, E. J. Feeding mechanics in spinosaurid theropods and extant crocodilians. PLoS ONE 8, e65295 (2013).") or habitat[14](/articles/s41598-021-97870-8#ref-CR14 "Alonso, A. & Canudo, J. I. On the spinosaurid theropod teeth from the early Barremian (Early Cretaceous) Blesa Formation (Spain). Hist. Biol. 28, 823–834 (2016)."). It has been suggested that spinosaurids became increasingly aquatic during their evolution[15](/articles/s41598-021-97870-8#ref-CR15 "Arden, T. M. S., Klein, C. G., Zouhri, S. & Longrich, N. R. Aquatic adaptation in the skull of carnivorous dinosaurs (Theropoda: Spinosauridae) and the evolution of aquatic habits in spinosaurids. Cretaceous Res. 93, 275–284.
https://doi.org/10.1016/j.cretres.2018.06.013
(2019).") and that highly modified taxa like _Spinosaurus_ engaged in specialised underwater pursuit predation[7](/articles/s41598-021-97870-8#ref-CR7 "Ibrahim, N. et al. Semiaquatic adaptations in a giant predatory dinosaur. Science 345, 1613–1616 (2014)."),[8](/articles/s41598-021-97870-8#ref-CR8 "Ibrahim, N. et al. Tail-propelled aquatic locomotion in a theropod dinosaur. Nature 581, 67–70 (2020)."). However, the sequence by which semiaquatic adaptations were acquired, and the degree of specialisation to aquatic life in _Spinosaurus_ and other spinosaurids, remain debatable and the topic of ongoing research[16](#ref-CR16 "Barker, C. T., Naish, D., Newham, E., Katsamenis, O. L. & Dyke, G. Complex neuroanatomy in the rostrum of the Isle of Wight theropod Neovenator salerii. Sci. Rep. 7, 3749.
https://doi.org/10.1038/s41598-017-03671-3
(2017)."),[17](#ref-CR17 "Henderson, D. M. A buoyancy, balance and stability challenge to the hypothesis of a semi-aquatic Spinosaurus Stromer, 1915 (Dinosauria: Theropoda). PeerJ 6, e5409 (2018)."),[18](#ref-CR18 "Hone, D. W. E. & Holtz, T. R. Jr. Comment on: Aquatic adaptation in the skull of carnivorous dinosaurs (Theropoda: Spinosauridae) and the evolution of aquatic habits in spinosaurids. Cretaceous Res. 93, 275–284 (2019)."),[19](/articles/s41598-021-97870-8#ref-CR19 "Hone, D. W. E. & Holtz, T. R. Jr. Evaluating the ecology of Spinosaurus: Shoreline generalist or aquatic pursuit specialist?. Palaeontologia Electron. 23, a03 (2021).").The majority of spinosaurid material comes from Early and “mid” Cretaceous strata, although isolated dental remains suggest persistence of the group into the Late Cretaceous (Santonian)20. Views on how spinosaurids relate to other theropods, and on the relationships within Spinosauridae itself, are controversial. The majority of recent studies find spinosaurids to be nested within Megalosauroidea21,22,23, a position requiring an origin during the Early Jurassic at least. An alternative is that they are outside a Megalosauridae + Allosauroidea clade24. Spinosauridae itself is agreed by most to consist of the two clades Baryonychinae and Spinosaurinae[15](/articles/s41598-021-97870-8#ref-CR15 "Arden, T. M. S., Klein, C. G., Zouhri, S. & Longrich, N. R. Aquatic adaptation in the skull of carnivorous dinosaurs (Theropoda: Spinosauridae) and the evolution of aquatic habits in spinosaurids. Cretaceous Res. 93, 275–284. https://doi.org/10.1016/j.cretres.2018.06.013
(2019)."),[21](/articles/s41598-021-97870-8#ref-CR21 "Carrano, M. T., Benson, R. B. & Sampson, S. D. The phylogeny of Tetanurae (Dinosauria: Theropoda). J. Syst. Paleontol. 10, 211–300 (2012)."),[24](#ref-CR24 "Rauhut, O. W. & Pol, D. Probable basal allosauroid from the early Middle Jurassic Cañadón Asfalto Formation of Argentina highlights phylogenetic uncertainty in tetanuran theropod dinosaurs. Sci. Rep. 9, 1–9 (2019)."),[25](#ref-CR25 "Sereno, P. C. et al. A long-snouted predatory dinosaur from Africa and the evolution of spinosaurids. Science 282, 1298–1302 (1998)."),[26](/articles/s41598-021-97870-8#ref-CR26 "Benson, R. B. A description of Megalosaurus bucklandii (Dinosauria: Theropoda) from the Bathonian of the UK and the relationships of Middle Jurassic theropods. Zool. J. Linn. Soc. 158, 882–935 (2010)."), although the number of valid taxa within these clades remains uncertain. Several—typically based on dental or fragmentary material—have been considered _nomina dubia_ or subsumed into synonymy by some workers (e.g.[25](/articles/s41598-021-97870-8#ref-CR25 "Sereno, P. C. et al. A long-snouted predatory dinosaur from Africa and the evolution of spinosaurids. Science 282, 1298–1302 (1998)."),[27](/articles/s41598-021-97870-8#ref-CR27 "Smyth, R. S., Ibrahim, N. & Martill, D. M. Sigilmassasaurus is Spinosaurus: A reappraisal of African spinosaurines. Cretaceous Res. 114, 1520 (2020).")). Support for the spinosaurine/baryonychine dichotomy may not, however, be as strong as conventionally supposed[22](/articles/s41598-021-97870-8#ref-CR22 "Evers, S. W., Rauhut, O. W., Milner, A. C., McFeeters, B. & Allain, R. A reappraisal of the morphology and systematic position of the theropod dinosaur Sigilmassasaurus from the “middle” Cretaceous of Morocco. PeerJ 3, e1323 (2015).") and baryonychines may be paraphyletic to a monophyletic Spinosaurinae[28](/articles/s41598-021-97870-8#ref-CR28 "Sales, M. A. F. & Schultz, C. L. Spinosaur taxonomy and evolution of craniodental features: Evidence from Brazil. PLoS ONE 12, e0187070.
https://doi.org/10.1371/journal.pone.0187070
(2017).").Discussions of spinosaurid phylogeny have frequently been coupled with evaluations of the clade’s biogeographical history, in part because spinosaurines exhibit a strong Gondwanan signal and baryonychines a mostly Laurasian one (though neither clade is restricted to these two regions). However, rigorous attempts to reconstruct the clade’s palaeogeographic patterns have yet to be undertaken, largely due to the phylogenetic instability of spinosaurid taxa . Sereno et al.25 proposed an ancestral pan-Pangaean distribution followed by a Laurasian baryonychine and Gondwanan spinosaurine divergence driven by the opening of the Tethys. More recent discoveries (including of the Iberian Vallibonavenatrix, initially identified as a spinosaurine) provide complications for this model, rendering the biogeographical history of the clade unresolved23.
In the UK, spinosaurid fossils are restricted to the Lower Cretaceous Wealden Supergroup (see “Geological Context” below), a fossiliferous succession of mudstones, sandstones and siltstones well known for its dinosaurs and other vertebrates that is mostly exposed in the English south-east and Isle of Wight29,30. The partial holotype skeleton (NHMUK PV R9951) of _Baryonyx walkeri_—one of the world’s best spinosaurid specimens and the first to reveal the true appearance of members of this group—is from the Upper Weald Clay Formation (Barremian) of Surrey1,31. Its discovery precipitated the referral of various isolated Wealden Supergroup elements to this taxon; these include specimens from other Upper Weald Clay locations in Surrey, as well as the upper Berriasian–lower Valanginian Ashdown and Valanginian Wadhurst Clay formations of East Sussex1,32. Teeth referred to the nomen dubium “_Suchosaurus cultridens_”, from the Valanginian Grinstead Clay Formation of West Sussex, have also been attributed to Baryonyx33,34, although other work has favoured an indeterminate baryonychine position30,35,36.
Wealden Group spinosaurid remains have also been reported from the Isle of Wight, specifically from the Barremian Wessex Formation. Published material has, until now, consisted only of isolated teeth37,38 and the single dorsal vertebra IWCMS 2012.56339. Due to the temporal overlap of the Upper Weald Clay and Wessex Formations (both are Barremian40), these were previously assumed to belong to Baryonyx or a close relative; indeed, the teeth and vertebra have been referred to cf. Baryonyx/Baryonyx sp. and Baryonyx cf. walkeri respectively30,38,41. Attention has been drawn to differences in enamel ornamentation that exist between these isolated teeth and the teeth of the B. walkeri holotype, leading to suggestions that they might represent an additional baryonychine taxon30,38,41. The presence of multiple spinosaurids based on the presence of several tooth morphotypes has also been put forward for other palaeoecosystems (e.g.42). However, variation in spinosaurid crown ornamentation has uncertain taxonomic value within Spinosauridae43 and may be influenced by both tooth position and ontogeny44,45.
The fragmentary and incomplete remains of two new baryonychine spinosaurid specimens were recovered at Chilton Chine on the Isle of Wight’s southwest coast and are herein named Ceratosuchops inferodios gen. et sp. nov. and Riparovenator milnerae gen. et sp. nov. (Figs. 1, 2). Both include partial skulls, the latter being associated with a series of caudal vertebrae (see supplementary information (SI) 1 for allocation of the material recovered and brief osteological descriptions). Surprisingly, both specimens differ from the broadly contemporaneous B. walkeri and from each other, and our interpretation demonstrates the presence of multiple spinosaurid taxa within the Wealden Supergroup. In this article, we explore their position within Spinosauridae via a new phylogenetic analysis and use this to re-evaluate spinosaurid palaeobiogeography. Finally, we discuss the possible implications of these new taxa for baryonychine diversity and ecology. A more detailed work on the osteology of the Wealden Group spinosaurids (including the additional spinosaurid elements recovered from Chilton Chine that could not be attributed to either taxon) is in preparation.
Figure 1
Known material referred to the baryonychines Ceratosuchops inferodios (rear) and Riparovenator milnerae (front) recovered at Chilton Chine (Isle of Wight, UK). White bones represent recovered elements. The arrangement of the elements in the caudal series is estimated; their relative position in the true series, and relationship with respect to each other (bar for those of the largely articulated mid-caudal series), are estimated. Image credit: Dan Folkes (CC-BY 4.0). Scale bar: 100 cm.
Figure 2
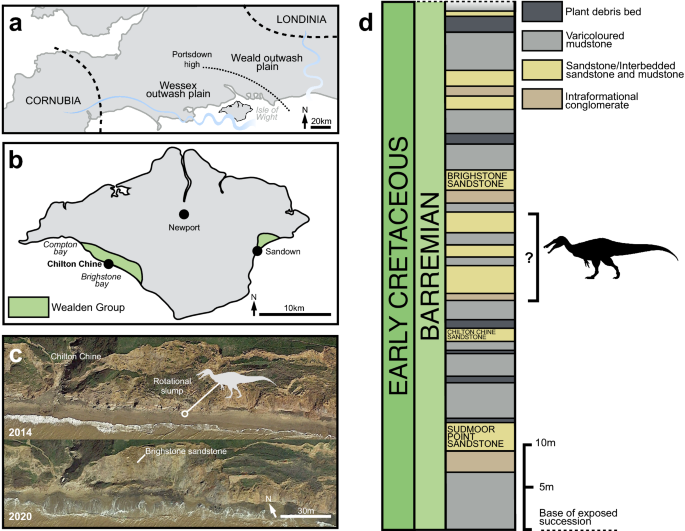
(modified from Penn et al., 2020); (b) map of the Isle Wight, highlighting the location of Chilton Chine and Wealden Group outcrops; (c) aerial photographs of Chilton Chine, highlighting the approximate position of the in situ material referred to Riparovenator milnerae (see SI) and the extensive coastal processes affecting the locality since the initial discoveries (map data: Google, Landsat/Copernicus, TerraMetrics, Maxmar Technologies); (d) schematic lithological log of the base of the exposed Wessex Fm. at Brighstone Bay (modified from Sweetman et al. 2014), highlighting approximate position of the R. milnerae in situ material. Silhouette credit: Dan Folkes (CC-BY 4.0).
Locality information and stratigraphy of Chilton Chine. (a) Schematic palaeogeographic map of the Wessex and Weald sub-basins of southern England
Institutional abbreviations
IWCMS: Dinosaur Isle Museum (Isle of Wight County Museum Service), Sandown, UK; ML: Museu de Lourinhã, Lourinhã, Portugal; MNN: Musée National du Niger, Niamey, Republic of Niger; MSNM: Museo di Storia Naturale di Milano; NHMUK: Natural History Museum, London, UK.
Geological context
The Lower Cretaceous Wealden Supergroup of southern England includes a succession of principally non-marine strata, accumulated between the late Berriasian and early Aptian. These are mainly deposited in two sub-basins: the larger Weald sub-basin of south-eastern England (where the strata are subdivided into the younger Weald Clay Group and older Hastings Group), and the smaller Wessex sub-basin of the Isle of Wight and central-southern England (composed of the younger Wealden Group and older Purbeck Limestone Group)40 (Fig. 2a). The Wealden Group on the Isle of Wight crops out along the southwest coast and less extensively so along the southeast coast (Fig. 2b), with both areas revealing exposures of the entirely Barremian Wessex Formation and overlying late Barremian-early Aptian Vectis Formation46,47. The Wessex Formation is formed of sandstones and varicoloured mudstones with interspersed plant debris beds. These sediments are mostly of alluvial origin and were deposited by a perennial, moderately sized, highly sinuous, west-to-east flowing river; some represent lacustrine environments as well40,46,47.
The new spinosaurid material reported here was collected at beach level, between 2013 and 2017, some of which originated from an exposure of the Wessex Formation located just east of Chilton Chine. The latter is a coastal geological feature situated approximately 1 km from Brighstone on the island’s southwest coast (Fig. 2b, c). The strata at Chilton Chine have experienced a substantial rotational slump, and additional, smaller slumps have further complicated the area’s stratigraphy. The braincase and caudal series referred to Riparovenator were recovered in situ and in close association (c. 10 m), likely from an unnamed layer between the Brighstone and Chilton Chine Sandstones (Fig. 2d); additional elements were recovered as isolated surface finds (see also SI). The sandstone matrix surrounding this specimen is largely fine-grained but does include small clasts; comparatively little plant debris (usually typical of the plant debris beds) was present during preparation. The Ceratosuchops premaxillae, braincase and referred postorbital were recovered from isolated sandstone blocks found on the foreshore, and as such their precise original stratigraphic location remains uncertain.
Systematic palaeontology
- DINOSAURIA Owen, 1842.
- THEROPODA Marsh, 1881.
- TETANURAE Gauthier, 1986.
- SPINOSAURIDAE Stromer, 1915.
- BARYONYCHINAE Charig and Milner, 1986, sensu Sereno et al., 1998.
- CERATOSUCHOPSINI clade nov.
- LSID urn:lsid:zoobank.org:act:D2370EE2-B5B3-4921-B2F6-FA5207CE85BF.
Definition: The most inclusive branch-based clade containing Ceratosuchops inferodios but not Baryonyx walkeri and Spinosaurus aegyptiacus.
Included taxa: Ceratosuchops inferodios; Riparovenator milnerae; Suchomimus tenerensis Sereno et al., 1998.
Diagnosis: postorbital facet of frontal dorsoventrally thick (height more than 40% of length) and excavated by a deep, longitudinal slot; well-defined and strongly curved anterior margins of supratemporal fossa; occipital surface of the basisphenoid collateral oval scars excavated.
Genus Ceratosuchops nov.
LSID urn:lsid:zoobank.org:act:5EB49885-7AF9-45DF-854A-C75A1AED16A1.
Etymology: kératos (Greek, κέρας)—“horn”, prominent postorbital boss and rugose orbital brow; soûkhos (Greek, Σοῦχος)—“crocodile”; óps (Greek, ὄψ)—“face”.
Type species: Ceratosuchops inferodios.
Diagnosis: as for type and only species.
Ceratosuchops inferodios sp. nov.
LSID urn:lsid:zoobank.org:act:1957EEF7-F3DD-49FF-BB90-82F53EF8E34A.
Etymology: īnfernus (Latin)—underworld, hell; erodiós (Greek, ερωδιός)—heron, in reference to its presumed heron-like ecology.
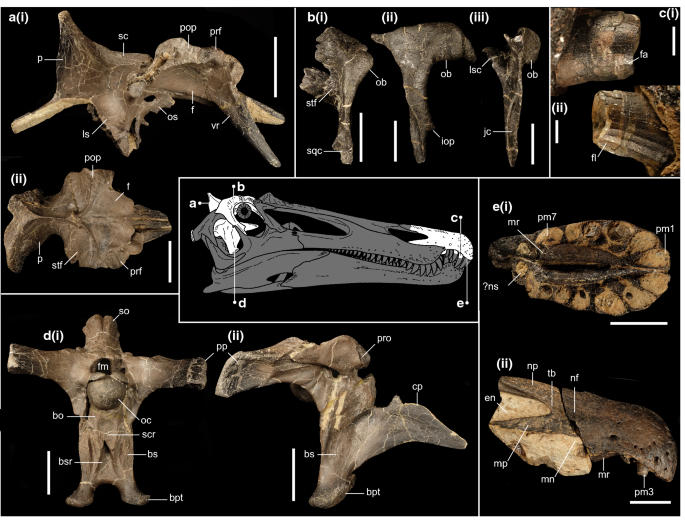
Holotype: Associated premaxillary bodies (IWCMS 2014.95.5) and posterior premaxillary fragment (IWCMS 2021.30); a near complete but disarticulated braincase (IWCMS 2014.95.1-3) (Fig. 3).
Figure 3
Cranial material of Ceratosuchops inferodios. (a) Holotype skull roof fragment (IWCMS 2014.95.1), in (i) right lateral and (ii) dorsal views; (b) referred right postorbital (IWCMS 2014.95.4), in (i) dorsal, (ii) lateral and (iii) posterior views; (c) close up of holotype in situ Rpm3 (IWCMS 2014.95.5) in (i) labial and (ii) lingual views; (d) holotype basicranium (IWCMS 2014.95.3), in (i) posterior (rearticulated with the supraoccipital + left otoccipital fragment IWCMS 2014.95.2) and (ii) right lateral views; (e) holotype premaxillae (IWCMS 2014.95.5, 2021.30), in (i) ventral and (ii) right lateral views. bo basioccipital, bs basisphenoid, bpt basipterygoid process, bsr basisphenoid recess, cp cultriform process, en external naris, f frontal, fa faceting, fl fluting, fm foramen magnum, iop infraorbital process, jc jugal contact, ls laterosphenoid, lsc laterosphenoid contact, mn maxillary notch, mp maxillary process, mr median ridge, nf narial fossa, np nasal process, ns nasal sinus, ob orbital boss, oc occipital condyle, os orbitosphenoid, p parietal, pop postorbital process, pm(n) premaxillary tooth/alveolus (tooth position), prf prefrontal, pro prootic, sc sagittal crest, scr subcondylar recess, so supraoccipital, sqf squamosal contact, stf supratemporal fossa, tb tuberosity, vp ventral process of the prefrontal. Skull reconstruction credit: Dan Folkes (CC-BY 4.0). Scale bars a–b, d–e: 50 mm; c: 5 mm.
Referred material: Right postorbital (IWCMS 2014.95.4) (Fig. 3).
Diagnosis: Baryonychine distinguished by the presence of the following unique traits: premaxillae displaying a pair of bilaterally located antenarial tuberosities; narrow (reversal of the ancestral megalosauroid condition) and ventrally restricted subcondylar recess of the basioccipital; oval scars of the basisphenoid excavated by deep, elongate sulci; anteroposteriorly thick interbasipterygoidal web; supraoccipital dorsal process possessing a gently curving posteroventral surface in coronal section.
This taxon can be further separated from other baryonychines by the following combination of traits: presence of narial fossae on the premaxilla (as in cf. Suchomimus but not Baryonyx); short subnarial (maxillary) process of the premaxilla (as in Baryonyx but not cf. Suchomimus); lack of premaxillary sagittal crest (as in Baryonyx but not cf. Suchomimus); curved anterior margin of the dorsal facet of the paroccipital process (angular in Baryonyx and probably Riparovenator); posterolaterally directed paroccipital processes of the otoccipitals (more laterally directed in Baryonyx); exoccipital components of the occipital condyle closely spaced (as in Riparovenator and cf. Suchomimus but not Baryonyx); subcondylar recess lacking mediolaterally thick lateral crests (as in cf. Suchomimus but not Baryonyx or Riparovenator); relatively stout supraoccipital dorsal process (as in Baryonyx but not cf. Suchomimus); lack of a dorsal extension of the basisphenoid recess under the basioccipital apron (recess extends dorsally in Baryonyx and Riparovenator).
Type locality and type horizon: Wessex Fm. (Barremian), Chilton Chine, near Brighstone (Isle of Wight, UK).
Genus Riparovenator gen. nov.
LSID urn:lsid:zoobank.org:act:9F4B6370-138E-443E-9650-DE6134FD9CC0.
Etymology: Rīpārius (Latin)–relating to the riverbank; vēnātor (Latin) –hunter.
Type species: Riparovenator milnerae.
Diagnosis: as for type and only species.
Riparovenator milnerae sp. nov.
LSID urn:lsid:zoobank.org:act:791F5DA4-1BDB-47DC-8ABF-BC24F14722B1.
Etymology: In honour of Angela Milner and her contributions to spinosaurid palaeobiology (and palaeontology as whole).
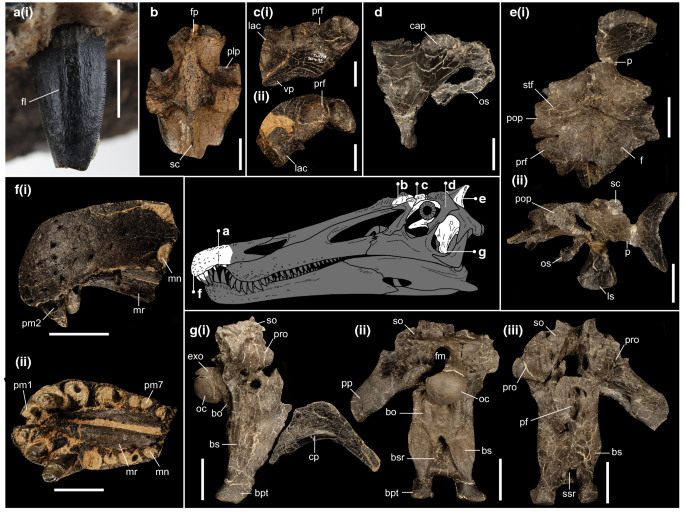
Holotype: Associated premaxillary bodies (IWCMS 2014.95.6); a disarticulated braincase (IWCMS 2014.96.1, 2; 2020.448.1, 2); a left “preorbital” fragment (partial lacrimal and prefrontal) (IWCMS 2014.96.3) (Fig. 4).
Figure 4
Cranial material of Riparovenator milnerae. (a) Close up of holotype in situ RpmVII (IWCMS 2014.95.6), in labial view; (b) referred posterior nasal fragment (IWCMS 2014.95.7) in dorsal view; (c) holotype left preorbital fragment (IWCMS 2014.96.3) in (i) lateral and (ii) anterodorsal view; (d) holotype right laterosphenoid (IWCMS 2014.96.2) in lateral view; (e) holotype skull roof and associated left laterosphenoid (IWCMS 2014.96.1) in (i) dorsal and (ii) left lateral views; (f) holotype premaxillary bodies (IWCMS 2014.95.6) in (i) left lateral and (ii) ventral views; (g) holotype basicranium (IWCMS 2020.448.1) in (i) right lateral (with fractured cultriform process IWCMS 2020.448.2), (ii) posterior and (ii) anterior views. bo basioccipital, bs basisphenoid, bpt basipterygoid process, bsr basisphenoid recess, cap capitate process of the laterosphenoid, cp cultriform process, exo exoccipital, f frontal, fl fluting, fm foramen magnum, fp frontal process, lac lacrimal, ls laterosphenoid, mn maxillary notch, mr median ridge, oc occipital condyle, os orbitosphenoid, p parietal, plp posterolateral process, pop postorbital process, pm(n) premaxillary tooth/alveolus (tooth position), prf prefrontal, pro prootic, sc sagittal crest, scr subcondylar recess, so supraoccipital, ssr subsellar recess, stf supratemporal fossa, vp ventral process of the prefrontal. Skull reconstruction credit: Dan Folkes (CC-BY 4.0). Scale bars (a): 5 mm; (b–d): 20 mm; (e–g): 50 mm.
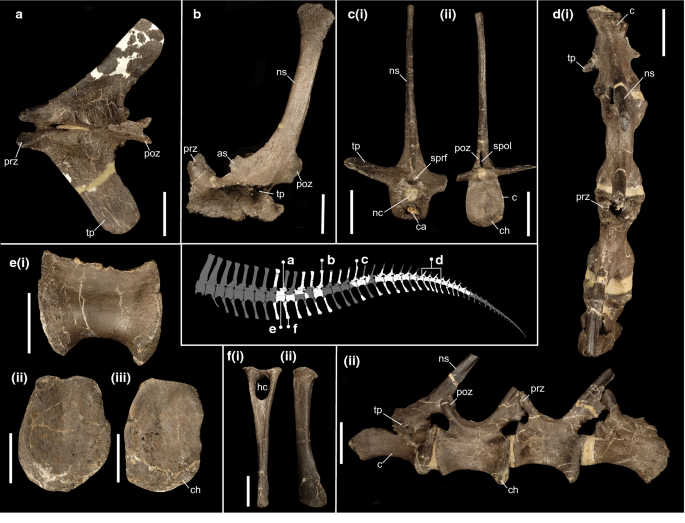
Referred material. A posterior nasal fragment (IWCMS 2014.95.7) (Fig. 4); an extensive caudal axial series (IWCMS 2020.447.1-39) (Fig. 5).
Figure 5
Caudal material referred to Riparovenator milnerae. (a) Anterior neural arch (IWCMS 2020.447.3) in dorsal view; (b) anterior neural arch (IWCMS 2020.447.2) in left lateral view; (c) partial middle vertebra (IWCMS 2020.447.8) in (i) anterior and (ii) posterior views; (d) articulated mid-caudal series (IWCMS 2020.447.12) in (i) dorsal and (ii) left lateral views; (e) anterior centrum (IWCMS 2020.447.5) in (i) left lateral, (ii) anterior and (iii) posterior views; (f) anterior chevron (IWCMS 2020.447.20) in (i) anterior and (ii) left lateral views. as anterior spur, c centrum, ca cavity, ch chevron contact, h haemal canal, nc neural canal, ns neural spine, poz postzygapophysis, prz prezygapophysis, spof spinopostygapophyseal fossa, sprf spinoprezygapophyseal fossa, tp transverse process. Tail reconstruction credit: Dan Folkes (CC-BY 4.0). Scale bars: 50 mm.
Diagnosis: Baryonychine distinguished by the presence of the following unique traits: notched dorsal orbital margin between prefrontal and postorbital process of the frontal; deeply inset facial nerve (CN VII) foramen that is largely obscured from lateral view; deep subcondylar recess (depth over 1/3 of its mediolateral width; depth less than 1/5 in other baryonychines); reduced exposure of ventral surface of the basipterygoid processes in lateral view.
This taxon can be further separated from other baryonychines by the following combination of traits: curved dorsal margin of the frontal process of the nasal in lateral view (margin effectively straight in Baryonyx); straight dorsal margin of the dorsum sellae (V-shaped in Baryonyx and Ceratosuchops); exoccipital components of the occipital condyle closely spaced (as in Ceratosuchops and cf. Suchomimus but not Baryonyx); mediolaterally thick crests bordering the subcondylar recess (as in Baryonyx but not Ceratosuchops or cf. Suchomimus); lateral margins of the basipterygoid processes concave in ventral view.
Type locality and type horizon: Between the Chilton Chine and Brighstone Sandstones, Wessex Fm. (Barremian), Chilton Chine, near Brighstone (Isle of Wight, UK).
Results
Parsimony analysis
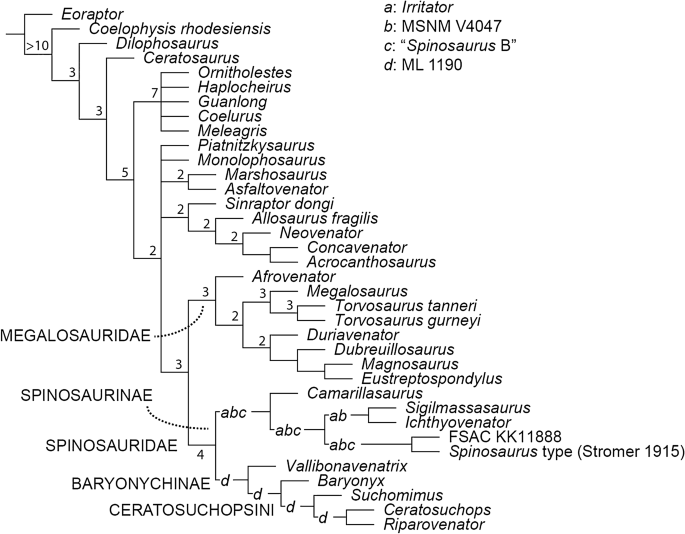
The parsimony analysis of 1810 characters and 40 Operational Taxonomic Units (OTUs) found 2660 shortest trees of 2448 steps each (CI = 0.4939; RI = 0.4551). The strict consensus is partially resolved, and we find support for the monophyly of Coelurosauria, Allosauroidea and Megalosauria. Among spinosaurids, the strict consensus topology weakly supports a dichotomous Baryonychinae-Spinosaurinae split, although their in-group relationships are completely unresolved. Pruning rogue spinosaurid OTUs (recovered by the TNT command pcrprune), improved in-group resolution but did little to alter the poor nodal (Bremer) support (Fig. 6). Jackknife resampling (see SI) also weakly supports the above-mentioned dichotomy, although it is unable to resolve the relationships between most of the spinosaurine in-group.
Figure 6
Phylogenetic relationships of Spinosauridae, based on parsimony analyses. Reduced consensus tree following a posteriori pruning of rogue spinosaurid OTUs. Values at nodes indicate the Bremer support values following pruning of rogue spinosaurid OTUs as well as select fragmentary taxa (see main text). Letters represent potential placement of rogue spinosaurid OTUs: a, Irritator; b, MSNM V4047; c, “Spinosaurus B”; d, ML 1190.
These results are in accordance with the majority of previous works regarding the well supported monophyly of Spinosauridae[11](/articles/s41598-021-97870-8#ref-CR11 "Hone, D. W. E. & Holtz, T. R. Jr. A century of spinosaurs—a review and revision of the Spinosauridae with comments on their ecology. Acta Geol. Sin. Engl. Ed. 91, 1120–1132. https://doi.org/10.1111/1755-6724.13328
(2017)."),[21](/articles/s41598-021-97870-8#ref-CR21 "Carrano, M. T., Benson, R. B. & Sampson, S. D. The phylogeny of Tetanurae (Dinosauria: Theropoda). J. Syst. Paleontol. 10, 211–300 (2012).") and the sister-group relationship between Baryonychinae and Spinosaurinae[15](/articles/s41598-021-97870-8#ref-CR15 "Arden, T. M. S., Klein, C. G., Zouhri, S. & Longrich, N. R. Aquatic adaptation in the skull of carnivorous dinosaurs (Theropoda: Spinosauridae) and the evolution of aquatic habits in spinosaurids. Cretaceous Res. 93, 275–284.
https://doi.org/10.1016/j.cretres.2018.06.013
(2019)."),[21](/articles/s41598-021-97870-8#ref-CR21 "Carrano, M. T., Benson, R. B. & Sampson, S. D. The phylogeny of Tetanurae (Dinosauria: Theropoda). J. Syst. Paleontol. 10, 211–300 (2012)."),[24](#ref-CR24 "Rauhut, O. W. & Pol, D. Probable basal allosauroid from the early Middle Jurassic Cañadón Asfalto Formation of Argentina highlights phylogenetic uncertainty in tetanuran theropod dinosaurs. Sci. Rep. 9, 1–9 (2019)."),[25](#ref-CR25 "Sereno, P. C. et al. A long-snouted predatory dinosaur from Africa and the evolution of spinosaurids. Science 282, 1298–1302 (1998)."),[26](/articles/s41598-021-97870-8#ref-CR26 "Benson, R. B. A description of Megalosaurus bucklandii (Dinosauria: Theropoda) from the Bathonian of the UK and the relationships of Middle Jurassic theropods. Zool. J. Linn. Soc. 158, 882–935 (2010).") (the synapomorphies of these clades are listed in the [SI](/articles/s41598-021-97870-8#MOESM3)). However, the results of the Templeton test (discussed below; see also [SI](/articles/s41598-021-97870-8#MOESM1)) and poor nodal support reveal that the relationships of the spinosaurid in-group remain elusive and are hindered by the incompleteness of the sampled OTUs (see below). With respect to the broader affinities of Spinosauridae, our results generally conform to those of Cau[48](/articles/s41598-021-97870-8#ref-CR48 "Cau, A. The assembly of the avian body plan: A 160-million-year long process. Bollettino della Società Paleontologica Italiana 57, 1–25 (2018).") and Rauhut and Pol[24](/articles/s41598-021-97870-8#ref-CR24 "Rauhut, O. W. & Pol, D. Probable basal allosauroid from the early Middle Jurassic Cañadón Asfalto Formation of Argentina highlights phylogenetic uncertainty in tetanuran theropod dinosaurs. Sci. Rep. 9, 1–9 (2019).") in that they support the dissolution of the “traditional” avetheropodan node within Tetanurae, recovering instead an early-branching position for Coelurosauria, and (in this case) a polytomous Carnosauria that includes Allosauroidea and Megalosauroidea. In contrast, we find Megalosauridae to retain a traditional sister-group relationship with Spinosauridae.Bayesian analysis
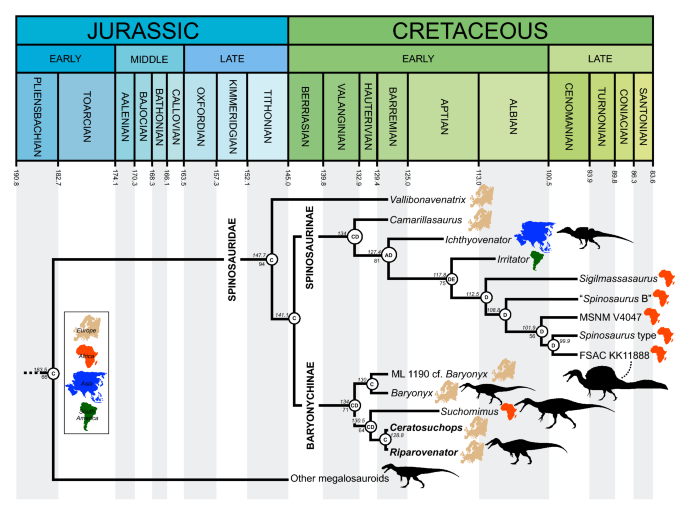
The Maximum Clade Credibility Tree (MCCT) obtained by the Bayesian inference analysis is in overall agreement with the strict consensus of the shortest trees recovered by the parsimony analysis, though resolution is substantially improved (Fig. 7, S1). The MCCT indicates that the [megalosaurid-spinosaurid] divergence occured at over 180 Mya and thus requires a c. 36 MY ghost lineage pre-dating an Early Cretaceous radiation of Spinosauridae. The monophyly of Spinosauridae is strongly supported (pp = 0.94), and relatively strong support is recovered for Baryonychinae (pp = 0.71) and Spinosaurinae (pp of the subclade excluding Camarillasaurus = 0.81). Within Baryonychinae, the two Wessex Fm. specimens are recovered as sister taxa and form a moderately supported clade with Suchomimus (pp = 0.64). Within Spinosaurinae, the hypothesis that all North African spinosaurines form a clade (to the exclusion of non-African taxa) is weak, with almost all in-group nodes possessing pp values < 50. The MCCT topology is thus equivocal on the distinction between Sigilmassasaurus and Spinosaurus. Of additional note is the early-branching position of Vallibonavenatrix outside the baryonychine-spinosaurine clade.
Figure 7
Time-calibrated phylogenetic relationships of Spinosauridae, based on the Maximum Clade Credibility Tree inferred by the Bayesian analysis (see SI for extended figure). Numbers at nodes represent node age (top, in million years) and posterior probability values > 50% (bottom). Letters at nodes refer to the most likely ancestral area reconstructed. Geologic timescale from Walker et al. (2018). A Asia, B North America, C Europe, D Africa, E South America. Silhouette credits: _Riparovenator_—Dan Folkes (CC-BY 4.0); Baryonyx, Megalosaurus, _Suchomimus_—Scott Hartman/Phylopic (CC-BY-NC-SA 3.0); FSAC KK 11,888—Scott Hartman; _Ichthyovenator_—Alex Vieira (CC-BY-NC-SA 4.0).
Discussion
Taxonomic interpretation
Ceratosuchops inferodios and Riparovenator milnerae represent new Wealden Group theropod taxa, differing from the other broadly contemporaneous spinosaurid Baryonyx walkeri in numerous anatomical features (see Diagnosis, Appendices 1 and 2).
Our taxonomic interpretation nevertheless overlaps with several vexing and interrelated issues: the relative maturity of the Wessex Formation taxa and the broadly coeval Baryonyx is unknown, as is the scope of variation amongst spinosaurid cranial characters. Regarding the former, no unambiguous methodology is currently available to distinguish the ontogenetic status of non-coelurosaurian theropods[49](/articles/s41598-021-97870-8#ref-CR49 "Griffin, C. T. et al. Assessing ontogenetic maturity in extinct saurian reptiles. Biol. Rev. https://doi.org/10.1111/brv.12666
(2020)."): while similar fusion patterns (e.g. premaxillae, nasals) and relatively comparable body sizes suggest overarching similitudes between these Wealden Supergroup specimens, we accept that there are caveats to these indicators of maturity[49](/articles/s41598-021-97870-8#ref-CR49 "Griffin, C. T. et al. Assessing ontogenetic maturity in extinct saurian reptiles. Biol. Rev.
https://doi.org/10.1111/brv.12666
(2020)."). Multi-element histological sectioning of the more complete _Baryonyx_ type specimen could nonetheless prove an informative starting point. We are cognisant that some of the differences we report for our two new baryonychine taxa are associated with individual and/or ontogenetic variation in better-sampled theropods (Appendix [1](/articles/s41598-021-97870-8#MOESM1)); indeed, intraspecific variation is seen to increase throughout the extreme ontogenetic series of the tyrannosaurid _Tyrannosaurus_[50](/articles/s41598-021-97870-8#ref-CR50 "Carr, T. D. A high-resolution growth series of Tyrannosaurus rex obtained from multiple lines of evidence. PeerJ 8, e9192 (2020)."), for instance. Meanwhile, the presence of a mosaic of states in the Wessex Fm. pair, regarded as “mature” and “immature” in other theropods, could be interpreted as revealing high variation in the order of ontogenetic character maturation (sequence polymorphism) within a single taxon (as seen in non-tetanuran theropods[51](/articles/s41598-021-97870-8#ref-CR51 "Griffin, C. T. & Nesbitt, S. J. Anomalously high variation in postnatal development is ancestral for dinosaurs but lost in birds. Proc. Natl. Acad. Sci. 113, 14757–14762 (2016).")); conversely, it is unknown whether spinosaurids were affected by such variability and there are currently no good reasons to assume that all are applicable to the clade. Simply put, the interpretation of spinosaurid cranial characters requires larger samples before we can properly assess the tempo and sequence of ontogenetic character state maturation and scope of intraspecific variation. In the absence of these data, we opt to name the two Wessex Fm. taxa given the presence of unique characters and character combinations.Osteologically, the Wessex Fm. spinosaurid pair provides welcome new cranial and caudal information, all of which will be expanded upon in future work. Of note is the rugose brow region of the skull in both specimens: in Ceratosuchops, this terminates posteriorly in an enlarged postorbital boss (Fig. 3b). Given the similarity in frontal postorbital facet shape in Riparovenator and cf. Suchomimus (MNN GDF 214), a similarly built postorbital (and thus brow region) is inferred. Such cranial ornamentation suggests a role in socio-sexual signalling[11](/articles/s41598-021-97870-8#ref-CR11 "Hone, D. W. E. & Holtz, T. R. Jr. A century of spinosaurs—a review and revision of the Spinosauridae with comments on their ecology. Acta Geol. Sin. Engl. Ed. 91, 1120–1132. https://doi.org/10.1111/1755-6724.13328
(2017)."), although “overbuilt” brow regions may have also had a role in intraspecific antagonism[52](/articles/s41598-021-97870-8#ref-CR52 "Sereno, P. C. & Brusatte, S. L. Basal abelisaurid and carcharodontosaurid theropods from the Lower Cretaceous Elrhaz Formation of Niger. Acta Palaeontol. Pol. 53, 15–46 (2008)."); biomechanical analyses, such as Finite Element analysis (FEA) could shed light on this interpretation. Several previously diagnostic cranial features in _Baryonyx_, such as the deep occiput (sensu[11](/articles/s41598-021-97870-8#ref-CR11 "Hone, D. W. E. & Holtz, T. R. Jr. A century of spinosaurs—a review and revision of the Spinosauridae with comments on their ecology. Acta Geol. Sin. Engl. Ed. 91, 1120–1132.
https://doi.org/10.1111/1755-6724.13328
(2017).")), are here interpreted as obsolescent given our recovered topologies and taxonomic interpretation of the new finds; others, for instance the cruciform process of the nasal (sensu[11](/articles/s41598-021-97870-8#ref-CR11 "Hone, D. W. E. & Holtz, T. R. Jr. A century of spinosaurs—a review and revision of the Spinosauridae with comments on their ecology. Acta Geol. Sin. Engl. Ed. 91, 1120–1132.
https://doi.org/10.1111/1755-6724.13328
(2017).")), require refinement to take into account differing morphologies. In the caudal series, the relatively elongate neural spines of _Riparovenator_ in particular push back the appearance of a dorsoventrally deep tail in spinosaurids, this feature being previously known from the Aptian in _Ichthyovenator_ and _Suchomimus_.Spinosaurid diversity in the Wealden Supergroup
Theropods appear rare in the Wealden Supergroup generally30, despite the strata being the subject of enthusiastic collecting efforts and producing numerous specimens of herbivorous dinosaurs53. As new Wealden Group taxa, Ceratosuchops and Riparovenator are thus substantial additions for the British theropod record, sharing the Wessex Fm. with at least three other mid-to-large theropods: the 7–8 m allosauroid Neovenator53,54, an indeterminate tetanuran55, and the 4–5 m tyrannosauroid Eotyrannus56. These new spinosaurid finds corroborate previous suggestions that Early Cretaceous Wealden Supergroup faunas were inhabited by more than one baryonychine taxon30,38,41, a discovery with potential implications for ecological separation within the clade, and for Spinosauridae as a whole.
The presence of more than one baryonychine taxon in the British Wealden was previously suggested from the discovery of at least two spinosaurid dental morphotypes30,38,57. However, it has been argued27 that the presence of multiple spinosaurid dental morphotypes within deposits containing a single known taxon was indicative of a “significant degree of subtle heterodonty” within the given putative taxon, rather than the presence of multiple taxa. Spinosaurids did exhibit heterodonty, but the discovery of these new Wessex Fm. baryonychines, coupled with the occurrence of at least two taxa and multiple dental morphotypes (representing baryonychines and spinosaurines) from the Early Cretaceous deposits of the Iberian Peninsula58,59,60,61,62, paints a more complex picture. Diagnostic Iberian taxa from the Barremian specifically include Baryonyx, Vallibonavenatrix and possibly Camarillasaurus (see below), though their remains currently occur in non-overlapping formations and the true taxonomic diversity of the Iberian sample remains elusive61,62. The validity of Iberian spinosaurine dental morphotypes has also been questioned27, despite there being some quantitative support for this referral (e.g.63) and the presence of unambiguous spinosaurine synapomorphies (carinae lacking serrations sensu Hendrickx et al.64). The presence of more than one spinosaurid taxon is also indicated by dental variation present in a Lower Cretaceous assemblage from Tunisia65. We are aware of, and reject, claims that the profound variation present in this sample—in this case, the presence of denticulated and non-denticulated morphotypes—can be explained by intraspecific variation27 in the absence of a clear morphological gradient or variably denticulated in situ dental series. Isolated Iberian spinosaurid teeth are known to possess variably denticulated carinae62; however their taxonomic implications are presently unclear.
Returning to the British Wealden Supergroup, labial and lingual enamel ornamentation (e.g. Fig. 3c) can be observed in both Wessex Formation taxa, as it can in isolated baryonychine teeth from this formation. This is a trait shared with “_Suchosaurus_” crowns from the neighbouring Weald sub-basin (and the Nigerien Suchomimus)64 and is distinct from the largely lingually fluted condition of Baryonyx walkeri64 (the caveat being the aforementioned variability and incomplete understanding of spinosaurid enamel ornamentation more generally). Regardless, assuming our taxonomic interpretation is correct, the discovery of the new Wessex Formation specimens renders the presence of Baryonyx in the Wessex Formation ambiguous. Indeed, the dorsal vertebra IWCMS 2012.563 previously referred to Baryonyx30 differs in its prominent spinodiapophyseal lamina and taller neural spine (potential damage to the Baryonyx type dorsal vertebrae notwithstanding)30. We argue that the specimen is best identified as an indeterminate baryonychine or spinosaurid; this can be extended to other isolated Wealden Supergroup spinosaurid material previously referred to “_Baryonyx_”.
Ecological demands require that large predators occur at low taxonomic diversity66. Despite this, numerous Jurassic and Cretaceous dinosaur assemblages include two or more comparably sized, morphologically similar, sympatric theropods67; examples are present in various geological formations worldwide (e.g.,21,68,69,70,71,72,73,74,75). The presence of two or more spinosaurid taxa in the same geological unit is therefore not without precedent and may in fact be typical76—the above mentioned Early Cretaceous of Iberia appears to testify this diversity. Indeed, when present, spinosaurids may have been locally abundant, perhaps when environmental circumstances benefited their specialised niche[11](/articles/s41598-021-97870-8#ref-CR11 "Hone, D. W. E. & Holtz, T. R. Jr. A century of spinosaurs—a review and revision of the Spinosauridae with comments on their ecology. Acta Geol. Sin. Engl. Ed. 91, 1120–1132. https://doi.org/10.1111/1755-6724.13328
(2017)."),[20](/articles/s41598-021-97870-8#ref-CR20 "Hone, D., Xu, X. & Wang, D. A probable baryonychine (Theropoda: Spinosauridae) tooth from the Upper Cretaceous of Henan Province, China. Vertebrata PalAsiatica 48, 19–26 (2010)."), a feature potentially driving high diversity within the clade. As such, the possible presence of broadly coeval spinosaurid taxa in the British Wealden Supergroup may represent the norm based on our knowledge of other assemblages. A view popular in the Mesozoic dinosaur literature is that large theropod taxa can only coexist when anatomical traits indicative of resource partitioning are identifiable[77](#ref-CR77 "Henderson, D. M. Skull and tooth morphology as indicators of niche partitioning in sympatric Morrison Formation theropods. Gaia 15, 219–226 (2000)."),[78](#ref-CR78 "Farlow, J. O. & Holtz, T. R. The fossil record of predation in dinosaurs. Paleontol. Soc. Pap. 8, 251–266 (2002)."),[79](#ref-CR79 "Barrett, P. M. & Rayfield, E. J. Ecological and evolutionary implications of dinosaur feeding behaviour. Trends Ecol. Evol. 21, 217–224 (2006)."),[80](/articles/s41598-021-97870-8#ref-CR80 "Brusatte, S. L., Carr, T. D. & Norell, M. A. The osteology of Alioramus, a gracile and long-snouted tyrannosaurid (Dinosauria: Theropoda) from the Late Cretaceous of Mongolia. Bull. Am. Mus. Nat. Hist. 2012, 1–197 (2012)."). This view obviously has merit, but is not incompatible with the possibility that similar, closely related taxa can co-exist and even overlap in ecological requirements; niche separation may be temporal (seasonal or daily), spatial (between habitats within ecosystems), or conditional. It should also be noted that those baryonychines inhabiting the regions represented by the Wessex and Weald sub-basins may have been separated in habitat choice (possible sub-basin habitat differences or climates have been previously suggested[40](/articles/s41598-021-97870-8#ref-CR40 "Batten, D. J. Wealden Geology in English Wealden Fossils (ed D. J. Batten) 7–14 (Palaeontological Association Field Guide to Fossils 14, 2011)."),[81](/articles/s41598-021-97870-8#ref-CR81 "Radley, J. D. & Allen, P. The southern English Wealden (non-marine Lower Cretaceous): Overview of palaeoenvironments and palaeoecology. Proc. Geol. Assoc. 123, 382–385 (2012)."),[82](/articles/s41598-021-97870-8#ref-CR82 "Raven, T. J., Barrett, P. M., Pond, S. B. & Maidment, S. C. Osteology and taxonomy of British wealden supergroup (Berriasian–Aptian) Ankylosaurs (Ornithischia, Ankylosauria). J. Vertebr. Paleontol. 40, 6956 (2020).")). Nonetheless, these hypotheses assume _Baryonyx_, _Ceratosuchops_ and _Riparovenator_ were contemporaries and subject to interspecific interactions. Alternatively, given the variable and intermittent sedimentation of fluvial systems[83](/articles/s41598-021-97870-8#ref-CR83 "Robinson, S. A. & Hesselbo, S. P. Fossil-wood carbon-isotope stratigraphy of the non-marine Wealden Group (Lower Cretaceous, southern England). J. Geol. Soc. 161, 133–145 (2004)."), the expanse of time deposited within the Upper Weald Clay and exposed Wessex Fms., and the difficulties correlating their respective stratigraphies, it remains possible one or more of these spinosaurids were separated by geological time.Phylogenetic analyses
We have determined through a large-scale phylogenetic analysis that the Wessex Formation specimens are more closely related to (a hypodigm OTU coding of) the Nigerien Suchomimus tenerensis than to Baryonyx, forming with it the newly recognised clade Ceratosuchopsini. This clade, and its internal relationships, is recovered under Bayesian and reduced consensus search strategies as well as through jackknife resampling (see SI), suggesting a potentially stable topology with albeit limited support. However, the extent to which the recovered topology is influenced by ontogenetically controlled character states is uncertain (see above). Additional characters, not currently included in the clade’s diagnosis, may further unite the Ceratosuchopsini, (e.g. shallow rise of the parietal nuchal crest; see Appendix 1); comparisons with sufficient, adequately preserved spinosaurid material would nevertheless be required.
Comparing the consensus trees produced by the two phylogenetic methodologies reveals grossly congruent topologies. However, some of the finer aspects of the ingroup relationships remain debatable: while Baryonychinae is recovered regardless of the phylogenetic methodology employed, we cannot reject alternative suboptimal scenarios where baryonychines form a paraphyletic grade to spinosaurines; constrained topologies forcing the Wessex Fm. pair outside of a Baryonyx + Suchomimus node, Ceratosuchopsini outside of a Baryonyx + Spinosaurinae node, and Baryonyx outside of a Ceratosuchopsini + Spinosaurinae node, required 2, 4 and 6 extra steps respectively, with all results insignificant under the Templeton test (see SI). This echoes previous works22,[28](/articles/s41598-021-97870-8#ref-CR28 "Sales, M. A. F. & Schultz, C. L. Spinosaur taxonomy and evolution of craniodental features: Evidence from Brazil. PLoS ONE 12, e0187070. https://doi.org/10.1371/journal.pone.0187070
(2017).") questioning the robusticity of the traditional spinosaurid in-group dichotomy. Similarly, differing topologies recovered within Spinosaurinae indicate a continued lack of in-group resolution. This impacts the perceived number of coeval North African spinosaurines, a topic that has attracted considerable debate in recent years[7](/articles/s41598-021-97870-8#ref-CR7 "Ibrahim, N. et al. Semiaquatic adaptations in a giant predatory dinosaur. Science 345, 1613–1616 (2014)."),[22](/articles/s41598-021-97870-8#ref-CR22 "Evers, S. W., Rauhut, O. W., Milner, A. C., McFeeters, B. & Allain, R. A reappraisal of the morphology and systematic position of the theropod dinosaur Sigilmassasaurus from the “middle” Cretaceous of Morocco. PeerJ 3, e1323 (2015)."),[27](/articles/s41598-021-97870-8#ref-CR27 "Smyth, R. S., Ibrahim, N. & Martill, D. M. Sigilmassasaurus is Spinosaurus: A reappraisal of African spinosaurines. Cretaceous Res. 114, 1520 (2020)."),[84](/articles/s41598-021-97870-8#ref-CR84 "McFeeters, B. New mid-cervical vertebral morphotype of Spinosauridae from the Kem Kem Group of Morocco. Vertebr. Anat. Morphol. Palaeontol. 8, 182–193 (2020)."). The polytomous, parsimony-based strict consensus, coupled with the insignificance of the constrained analysis performed to group North African specimens (see [SI](/articles/s41598-021-97870-8#MOESM1)), are unable to support or reject the existence of a spinosaurine subclade incorporating all North African material—a topology required to corroborate the proposed synonymy of these specimens (i.e. _Spinosaurus_ sensu Ibrahim et al.[7](/articles/s41598-021-97870-8#ref-CR7 "Ibrahim, N. et al. Semiaquatic adaptations in a giant predatory dinosaur. Science 345, 1613–1616 (2014).")). This is somewhat echoed in our Bayesian analysis: a North African subclade is recovered, but its support is weak. Thus, the proposed synonymy of _Spinosaurus_ and _Sigilmassasaurus_[27](/articles/s41598-021-97870-8#ref-CR27 "Smyth, R. S., Ibrahim, N. & Martill, D. M. Sigilmassasaurus is Spinosaurus: A reappraisal of African spinosaurines. Cretaceous Res. 114, 1520 (2020).") is regarded as equivocal here. It should be noted that these results might be affected by our decision to employ a composite OTU for _Sigilmassasaurus_, otherwise known from highly fragmentary type material; future analyses may consider removal of this OTU. Moreover, a better understanding of North African spinosaurine relationships requires the discovery of more complete individuals regardless.Other minor topological differences include the Bayesian recovery of the Portuguese spinosaurid ML 1190 with the Baryonyx walkeri type specimen, a result differing from Arden et al.’s[15](/articles/s41598-021-97870-8#ref-CR15 "Arden, T. M. S., Klein, C. G., Zouhri, S. & Longrich, N. R. Aquatic adaptation in the skull of carnivorous dinosaurs (Theropoda: Spinosauridae) and the evolution of aquatic habits in spinosaurids. Cretaceous Res. 93, 275–284. https://doi.org/10.1016/j.cretres.2018.06.013
(2019).") parsimony-driven placement of this specimen as an indeterminate spinosaurid. Our Bayesian findings are instead consistent with the specimen’s original referral to _Baryonyx_[36](/articles/s41598-021-97870-8#ref-CR36 "Mateus, O., Araujo, R., Natário, C. & Castanhinha, R. A new specimen of the theropod dinosaur Baryonyx from the early Cretaceous of Portugal and taxonomic validity of Suchosaurus. Zootaxa 2827, 54–68 (2011)."). The low posterior probabilities for this grouping hinder support for this association, as does the instability of ML 1190 in our parsimony analysis, but these likely originate from the fragmentary nature of the latter OTU in particular. Meanwhile, _Vallibonavenatrix_—initially described as a spinosaurine[23](/articles/s41598-021-97870-8#ref-CR23 "Malafaia, E. et al. A new spinosaurid theropod (Dinosauria: Megalosauroidea) from the upper Barremian of Vallibona, Spain: Implications for spinosaurid diversity in the Early Cretaceous of the Iberian Peninsula. Cretaceous Res. 106, 104221 (2020).")—is recovered as a baryonychine (Fig. [6](/articles/s41598-021-97870-8#Fig6)) or a basal spinosaurid outside the baryonychine-spinosaurine spilt (Fig. [7](/articles/s41598-021-97870-8#Fig7)), indicating an unstable position within Spinosauridae. Elsewhere, the recovery of _Camarillasaurus_ as a spinosaurine (originally described as a ceratosaur[85](/articles/s41598-021-97870-8#ref-CR85 "Sánchez-Hernández, B. & Benton, M. J. Filling the ceratosaur gap: A new ceratosaurian theropod from the Early Cretaceous of Spain. Acta Palaeontol. Pol. 59, 581–600 (2014).")), lends further support to recently published reinterpretations of this taxon’s systematic position[61](/articles/s41598-021-97870-8#ref-CR61 "Malafaia, E., Gasulla, J., Escaso, F., Narvaéz, I. & Ortega, F. An update of the spinosaurid (Dinosauria: Theropoda) fossil record from the Lower Cretaceous of the Iberian Peninsula: distribution, diversity, and evolutionary history. J. Iber. Geol. 46, 431–444 (2020)."),[76](/articles/s41598-021-97870-8#ref-CR76 "Samathi, A., Sander, P. M. & Chanthasit, P. A spinosaurid from Thailand (Sao Khua Formation, Early Cretaceous) and a reassessment of Camarillasaurus cirugedae from the Early Cretaceous of Spain. Hist. Biol. 1–15 (2021)."),[86](/articles/s41598-021-97870-8#ref-CR86 "Rauhut, O. W., Canudo, J. I. & Castanera, D. A reappraisal of the Early Cretaceous theropod dinosaur Camarillasaurus from Spain in Program and Abstracts XVII Conference of the EAVP (European Association of Vertebrate Paleontologists) pp. 96 (2019)."). This interpretation may help explain the presence of spinosaurine-like teeth recovered from the same deposits[58](/articles/s41598-021-97870-8#ref-CR58 "Sánchez-Hernández, B., Benton, M. J. & Naish, D. Dinosaurs and other fossil vertebrates from the Late Jurassic and Early Cretaceous of the Galve area, NE Spain. Palaeogeogr. Palaeoclimatol. Palaeoecol. 249, 180–215 (2007)."). The “wildcard” status of _Irritator_ within Spinosaurinae in our parsimony-driven analysis is perhaps unexpected given its well-preserved cranial material. Whilst derived apomorphies in the dentition and narial region (that are absent in baryonychines) support its association to other spinosaurine OTUs with overlapping osteologies (e.g. MSNM V4047, _Spinosaurus_ holotype), its unstable nature is likely explained by the inability to compare it to other taxa known predominantly from postcranial material.Our phylogenetic analyses involved a substantial character list and large sample of spinosaurid OTUs, improving upon most previous studies that used comparatively smaller datasets. It also represents a first attempt to reconstruct spinosaurid palaeogeographic patterns using Bayesian methods. However, some spinosaurid OTUs could only be scored based on initial reports rather than first-hand observation or detailed monographs. This was the case for the Suchomimus OTU, for instance, and we favour using a hypodigm until the holotype specimen is thoroughly inventoried and described. We are nevertheless cognisant of the problematic inclusion of composite OTUs87,88. The lack of thorough descriptive works for many spinosaurid specimens remains a running hindrance in the study of the clade’s systematics and taxonomy, and is exacerbated by the incomplete nature of most specimens. This incompleteness likely contributes to the labile positions of various spinosaurid OTUs as well as the moderate to poor in-group node support throughout the consensus topologies.
Spinosaurid palaeobiogeography
From a palaeogeographical perspective, our analysis supports a European origin of Spinosauridae generally consistent with the Laurasian origin model suggested by Milner33, with regionalisation and vicariance explaining the subsequent distribution of genus-level taxa. The Dispersal-Extinction-Cladogenesis (DEC) model supports the expansion of spinosaurid distribution during the first half of the Early Cretaceous to Asia and Western Gondwana, followed by progressive contraction of their distribution into the “mid” Cretaceous. In contrast, our findings are not consistent with Sereno et al.’s25 suggestion of an ancestral cosmopolitan distribution for spinosaurids followed by a vicariance-led divergence of Laurasian baryonychines and Gondwanan spinosaurines, and a single dispersal event invoked to explain the presence of Suchomimus in Africa. Our results instead indicate a European origin followed by at least two Early Cretaceous migrations to Africa, these leading, respectively, to Suchomimus and a clade within Spinosaurinae. An opposite direction of dispersal89,90 is not consistent with our results. This European origin and recovery of Camarillasaurus as an early-branching spinosaurine would appear to simplify the “complex” palaeogeographic pattern suggested by the presence of putative Iberian spinosaurines23. The Aptian extinction of the Eurasian Spinosauridae is potentially contradicted by evidence indicating their persistence well into the Late Cretaceous of Asia20. The highly fragmentary specimen (a single tooth) was not, however, included among our OTUs, and its omission has likely influenced the tempo of the clade’s perceived Eurasian extinction. Importantly, its potential baryonychine affinities may complicate the clade’s palaeogeographic patterns.
Methods
Phylogenetic analysis
Each of the new Wessex Formation specimens was entered into a phylogenetic dataset based on a modified version of the Cau48 analysis implemented by Dal Sasso et al.91, focusing on non-coelurosaurian tetanurans (see SI for further details). The Triassic saurischian Eoraptor was used as a root for the assessment of character polarity. The dataset was analysed using maximum parsimony as the tree search strategy using TNT version 1.592. The search strategy involved 100 “New Technology” search analyses using the default setting, followed by a series of “Traditional Search” analyses exploring the tree islands found during the first round. Nodal support was calculated saving all trees up to 10 steps longer than the shortest topologies found and using the “Bremer Supports” function of TNT. In an attempt to improve tree resolution, rogue spinosaurid OTUs (Irritator, ML 1190, MSNM V4047 and “Spinosaurus B”) were identified using the command pcrprune93. Alternative relationships not recovered in the shortest found topologies were then enforced in TNT, and the statistical significance of their step difference from the most parsimonious trees found was calculated using the Templeton test94 implemented in TNT (see SI).
Bayesian inference analysis
The phylogenetic dataset was analysed integrating the morphological data matrices with absolute ages of the least inclusive stratigraphic range including each terminal unit. The Sampled Ancestor Fossilized Birth Death Skyline Model (SAFBD;95) implemented in BEAST 2.4.4.96,97 was used as the tree model. In our analysis, rate variation across traits was modeled using the multi-gamma parameter (implemented for the analysis of morphological data in BEAST 2). The rate variation across branches was modeled using the relaxed log-normal clock model, with the number of discrete rate categories that approximate the rate distribution set as n − 1 (with n the number of branches), the mean clock rate using default setting, and not setting to normalize the average rate. Since the character matrix includes autapomorphies of the sampled taxa, the Lewis98 model was not conditioned to variable characters only. Stratigraphic information for the taxa was converted to geochronological ages. Stratigraphic data and age constraints for each terminal were obtained from the Paleobiology Database (http://paleobiodb.org/), checked against the International Chronostratigraphic Chart (http://stratigraphy.org/), and included as uniform priors for tip-dating. The extant taxon included (the avian Meleagris) calibrates the height for the tip-date setting (the uniform prior setting used for incorporating uncertainty in the age of the fossil taxa requires at least one terminal taxon to have the tip age fixed to a value, see95). The analysis used four replicate runs of 10 million generations, with sampling every 1000 generations, that were subsequently combined using LogCombiner 1.7.3 (included in the BEAST package)96,97. In the analyses, burnin was set at 20%. Convergence and effective sample sizes of every numerical parameter among the different analyses were identified using Tracer (included in the BEAST package). The root age of the tree model was conservatively set as a uniform prior spanning between the age of the oldest in-group taxa and 252 Mya (near the Permian–Triassic boundary), which consistently pre-dates the diversification of all theropod branches.
We used the MCCT resulting from the Bayesian analysis as a temporally calibrated phylogenetic framework for palaeobiogeographic reconstruction, inferring ancestral geographic placement of nodes using RASP (Reconstruct Ancestral State in Phylogenies)99. The distribution range of the taxa was a priori divided into five areas: Asia (A), North America (B), Europe (C), Africa (D), and South America (E). Each terminal taxon was scored for the geographic area character state according to the continent(s) it was recovered in. Biogeographic inferences on the phylogenetic frameworks were obtained by applying the Dispersal-Extinction-Cladogenesis model (DEC)100, with no a priori constraints on range and dispersal parameters.
Referencess
- Charig, A. J. & Milner, A. C. Baryonyx walkeri, a fish-eating dinosaur from the Wealden of Surrey. Bull. Nat. Hist. Museum Geol. Ser. 53, 11–70 (1997).
Google Scholar - Amiot, R. et al. Oxygen isotope composition of continental vertebrate apatites from Mesozoic formations of Thailand; environmental and ecological significance. Geol. Soc. Lond. Spec. Publ. 315, 271–283 (2009).
Article ADS Google Scholar - Amiot, R. et al. Oxygen isotope evidence for semi-aquatic habits among spinosaurid theropods. Geology 38, 139–142 (2010).
Article ADS CAS Google Scholar - Hassler, A. et al. Calcium isotopes offer clues on resource partitioning among Cretaceous predatory dinosaurs. Proc. R. Soc. B Biol. Sci. 285, 20180197 (2018).
Article CAS Google Scholar - Aureliano, T. et al. Semi-aquatic adaptations in a spinosaur from the Lower Cretaceous of Brazil. Cretaceous Res 90, 283–295 (2018).
Article Google Scholar - McCurry, M. R. et al. The repeated evolution of dental apicobasal ridges in aquatic-feeding mammals and reptiles. Biol. J. Lin. Soc. 127, 245–259 (2019).
Article Google Scholar - Ibrahim, N. et al. Semiaquatic adaptations in a giant predatory dinosaur. Science 345, 1613–1616 (2014).
Article ADS CAS PubMed Google Scholar - Ibrahim, N. et al. Tail-propelled aquatic locomotion in a theropod dinosaur. Nature 581, 67–70 (2020).
Article ADS CAS PubMed Google Scholar - Ruiz-Omeñaca, J. I., Canudo, J. I., Cruzado-Caballero, P., Infante, P. & Moreno-Azanza, M. Baryonychine teeth (Theropoda: Spinosauridae) from the Lower Cretaceous of La Cantalera (Josa, NE Spain). Kaupia 14, 59–63 (2005).
Google Scholar - Bertin, T. A catalogue of material and review of the Spinosauridae. PalArch’s J. Vertebr. Palaeontol. 7, 1–39 (2010).
Google Scholar - Hone, D. W. E. & Holtz, T. R. Jr. A century of spinosaurs—a review and revision of the Spinosauridae with comments on their ecology. Acta Geol. Sin. Engl. Ed. 91, 1120–1132. https://doi.org/10.1111/1755-6724.13328 (2017).
Article Google Scholar - Schade, M., Rauhut, O. W. & Evers, S. W. Neuroanatomy of the spinosaurid Irritator challengeri (Dinosauria: Theropoda) indicates potential adaptations for piscivory. Sci. Rep. 10, 1–9 (2020).
Article CAS Google Scholar - Cuff, A. R. & Rayfield, E. J. Feeding mechanics in spinosaurid theropods and extant crocodilians. PLoS ONE 8, e65295 (2013).
Article ADS CAS PubMed PubMed Central Google Scholar - Alonso, A. & Canudo, J. I. On the spinosaurid theropod teeth from the early Barremian (Early Cretaceous) Blesa Formation (Spain). Hist. Biol. 28, 823–834 (2016).
Article Google Scholar - Arden, T. M. S., Klein, C. G., Zouhri, S. & Longrich, N. R. Aquatic adaptation in the skull of carnivorous dinosaurs (Theropoda: Spinosauridae) and the evolution of aquatic habits in spinosaurids. Cretaceous Res. 93, 275–284. https://doi.org/10.1016/j.cretres.2018.06.013 (2019).
Article Google Scholar - Barker, C. T., Naish, D., Newham, E., Katsamenis, O. L. & Dyke, G. Complex neuroanatomy in the rostrum of the Isle of Wight theropod Neovenator salerii. Sci. Rep. 7, 3749. https://doi.org/10.1038/s41598-017-03671-3 (2017).
Article ADS CAS PubMed PubMed Central Google Scholar - Henderson, D. M. A buoyancy, balance and stability challenge to the hypothesis of a semi-aquatic Spinosaurus Stromer, 1915 (Dinosauria: Theropoda). PeerJ 6, e5409 (2018).
Article PubMed PubMed Central Google Scholar - Hone, D. W. E. & Holtz, T. R. Jr. Comment on: Aquatic adaptation in the skull of carnivorous dinosaurs (Theropoda: Spinosauridae) and the evolution of aquatic habits in spinosaurids. Cretaceous Res. 93, 275–284 (2019).
Article Google Scholar - Hone, D. W. E. & Holtz, T. R. Jr. Evaluating the ecology of Spinosaurus: Shoreline generalist or aquatic pursuit specialist?. Palaeontologia Electron. 23, a03 (2021).
Google Scholar - Hone, D., Xu, X. & Wang, D. A probable baryonychine (Theropoda: Spinosauridae) tooth from the Upper Cretaceous of Henan Province, China. Vertebrata PalAsiatica 48, 19–26 (2010).
Google Scholar - Carrano, M. T., Benson, R. B. & Sampson, S. D. The phylogeny of Tetanurae (Dinosauria: Theropoda). J. Syst. Paleontol. 10, 211–300 (2012).
Article Google Scholar - Evers, S. W., Rauhut, O. W., Milner, A. C., McFeeters, B. & Allain, R. A reappraisal of the morphology and systematic position of the theropod dinosaur Sigilmassasaurus from the “middle” Cretaceous of Morocco. PeerJ 3, e1323 (2015).
Article PubMed PubMed Central Google Scholar - Malafaia, E. et al. A new spinosaurid theropod (Dinosauria: Megalosauroidea) from the upper Barremian of Vallibona, Spain: Implications for spinosaurid diversity in the Early Cretaceous of the Iberian Peninsula. Cretaceous Res. 106, 104221 (2020).
Article Google Scholar - Rauhut, O. W. & Pol, D. Probable basal allosauroid from the early Middle Jurassic Cañadón Asfalto Formation of Argentina highlights phylogenetic uncertainty in tetanuran theropod dinosaurs. Sci. Rep. 9, 1–9 (2019).
Article CAS Google Scholar - Sereno, P. C. et al. A long-snouted predatory dinosaur from Africa and the evolution of spinosaurids. Science 282, 1298–1302 (1998).
Article ADS CAS PubMed Google Scholar - Benson, R. B. A description of Megalosaurus bucklandii (Dinosauria: Theropoda) from the Bathonian of the UK and the relationships of Middle Jurassic theropods. Zool. J. Linn. Soc. 158, 882–935 (2010).
Article Google Scholar - Smyth, R. S., Ibrahim, N. & Martill, D. M. Sigilmassasaurus is Spinosaurus: A reappraisal of African spinosaurines. Cretaceous Res. 114, 1520 (2020).
Article Google Scholar - Sales, M. A. F. & Schultz, C. L. Spinosaur taxonomy and evolution of craniodental features: Evidence from Brazil. PLoS ONE 12, e0187070. https://doi.org/10.1371/journal.pone.0187070 (2017).
Article CAS PubMed PubMed Central Google Scholar - Martill, D. M. & Naish, D. Dinosaurs of the Isle of Wight (Palaeontological Association, 2001).
Google Scholar - Naish, D. Theropod dinosaurs in English Wealden Fossils (ed D. J. Batten) 526–559 (The Palaeontological Association, 2011).
- Charig, A. J. & Milner, A. C. Baryonyx, a remarkable new theropod dinosaur. Nature 324, 359–361 (1986).
Article ADS CAS PubMed Google Scholar - Turmine-Juhel, P. et al. Microvertebrates from the Wadhurst Clay Formation (Lower Cretaceous) of Ashdown Brickworks, East Sussex, UK. Proc. Geol. Assoc. 130, 752–769 (2019).
Article Google Scholar - Milner, A. C. Fish-eating theropods: A short review of the systematics, biology and palaeobiogeography of spinosaurs in Actas de las II Jornadas Internacionales sobre Paleontología de Dinosaurios y su Entorno: Salas de los Infantes (Burgos, España), septiembre de 2001 (ed. Huerta Hurtado, P. & Torcida Férnandes-Baldor, F.). 129–138 (2003).
- Buffetaut, E. Spinosaurs before Stromer: early finds of spinosaurid dinosaurs and their interpretations in Dinosaurs and Other Extinct Saurians: A Historical Perspective (eds R. T. J. Moody, E. Buffetaut, D. Naish, & D. M. Martill) 175–188 (The Geological Society of London Vol. 343, 2010).
- Salisbury, S. W. & Naish, D. Crocodilians in English Wealden Fossils (ed D. J. Batten) 305–369 (The Palaeontological Association, 2011).
- Mateus, O., Araujo, R., Natário, C. & Castanhinha, R. A new specimen of the theropod dinosaur Baryonyx from the early Cretaceous of Portugal and taxonomic validity of Suchosaurus. Zootaxa 2827, 54–68 (2011).
Article Google Scholar - Martill, D. M. & Hutt, S. Possible baryonychid dinosaur teeth from the Wessex Formation (Lower Cretaceous, Barremian) of the Isle of Wight, England. Proceedings of the Geologists' Association 107, 81–84 (1996).
Article Google Scholar - Naish, D., Hutt, S. & Martill, D. M. Saurischian dinosaurs 2: Theropods in Dinosaurs of the Isle of Wight (eds D. M. Martill & D. Naish) 242–309 (The Palaeontological Association, 2001).
- Hutt, S. & Newbery, P. An exceptional theropod vertebra from the Wessex Formation (Lower Cretaceous) Isle of Wight, England. Proc. Isle Wight Nat. Hist. Archaeol. Soc. 20, 61–76 (2004).
Google Scholar - Batten, D. J. Wealden Geology in English Wealden Fossils (ed D. J. Batten) 7–14 (Palaeontological Association Field Guide to Fossils 14, 2011).
- Naish, D. & Martill, D. M. Dinosaurs of Great Britain and the role of the Geological Society of London in their discovery: Basal Dinosauria and Saurischia. J. Geol. Soc. 164, 493–510 (2007).
Article ADS Google Scholar - Richter, U., Mudroch, A. & Buckley, L. G. Isolated theropod teeth from the Kem Kem beds (early Cenomanian) near Taouz, Morocco. Paläontol. Z. 87, 291–309 (2013).
Article Google Scholar - Canudo, J. I & Ruiz-Omeñaca, J. I. Los restos directos de dinosaurios terópodos (excluyendo Aves) en España in Dinosaurios y otros reptiles mesozoicos en España (ed. Peréz-Lorente) 347–374 (Fundación Patrimonio Paleontológico de La Rioja, Instituto de Estudios Riojanos, Universidad de La Rioja, Logroño, 2003).
- Fowler, D. Recently rediscovered baryonychine teeth (Dinosauria: Theropoda): New morphologic data, range extension & similarity to Ceratosaurus. J. Vertebr. Paleontol. 27, 76A-76A (2007).
Google Scholar - Hendrickx, C., Mateus, O. & Buffetaut, E. Morphofunctional analysis of the quadrate of Spinosauridae (Dinosauria: Theropoda) and the presence of Spinosaurus and a second spinosaurine taxon in the Cenomanian of North Africa. PLoS ONE 11, e0144695 (2016).
Article PubMed PubMed Central CAS Google Scholar - Sweetman, S. C. The Wealden of the Isle of Wight in English Wealden Fossils (ed D. J. Batten) 52–78 (Palaeontological Association Field Guide to Fossils 14, 2011).
- Radley, J. D. & Allen, P. The Wealden (non-marine Lower Cretaceous) of the Wessex Sub-basin, southern England. Proc. Geol. Assoc. 123, 319–373 (2012).
Article Google Scholar - Cau, A. The assembly of the avian body plan: A 160-million-year long process. Bollettino della Società Paleontologica Italiana 57, 1–25 (2018).
Google Scholar - Griffin, C. T. et al. Assessing ontogenetic maturity in extinct saurian reptiles. Biol. Rev. https://doi.org/10.1111/brv.12666 (2020).
Article PubMed Google Scholar - Carr, T. D. A high-resolution growth series of Tyrannosaurus rex obtained from multiple lines of evidence. PeerJ 8, e9192 (2020).
Article Google Scholar - Griffin, C. T. & Nesbitt, S. J. Anomalously high variation in postnatal development is ancestral for dinosaurs but lost in birds. Proc. Natl. Acad. Sci. 113, 14757–14762 (2016).
Article ADS CAS PubMed PubMed Central Google Scholar - Sereno, P. C. & Brusatte, S. L. Basal abelisaurid and carcharodontosaurid theropods from the Lower Cretaceous Elrhaz Formation of Niger. Acta Palaeontol. Pol. 53, 15–46 (2008).
Article Google Scholar - Brusatte, S. L., Benson, R. B. J. & Hutt, S. The osteology of Neovenator salerii (Dinosauria: Theropoda) from the Wealden Group (Barremian) of the Isle of Wight. Vol. 162 (Monograph of the Palaeontographical Society, 2008).
- Hutt, S., Martill, D. M. & Barker, M. J. The first European allosaurid dinosaur (Lower Cretaceous, Wealden Group, England). Neues Jahrbuch für Geologie und Paläontologie-Monatshefte, 635–644 (1996).
- Benson, R. B., Brusatte, S. L., Hutt, S. & Naish, D. A new large basal tetanuran (Dinosauria: Theropoda) from the Wessex Formation (Barremian) of the Isle of Wight, England. J. Vertebr. Paleontol. 29, 612–615 (2009).
Article Google Scholar - Hutt, S., Naish, D., Martill, D. M., Barker, M. J. & Newbery, P. A preliminary account of a new tyrannosauroid theropod from the Wessex Formation (Early Cretaceous) of southern England. Cretaceous Res. 22, 227–242 (2001).
Article Google Scholar - Buffetaut, E. The spinosaurid dinosaur Baryonyx (Saurischia, Theropoda) in the Early Cretaceous of Portugal. Geol. Mag. 144, 1021–1025 (2007).
Article ADS Google Scholar - Sánchez-Hernández, B., Benton, M. J. & Naish, D. Dinosaurs and other fossil vertebrates from the Late Jurassic and Early Cretaceous of the Galve area, NE Spain. Palaeogeogr. Palaeoclimatol. Palaeoecol. 249, 180–215 (2007).
Article Google Scholar - Canudo, J. I. et al. Primera evidencia de dientes aislados atribuidos a Spinosauridae (Theropoda) en el Aptiano inferior (Cretácico Inferior) de Europa: Formación Arcillas de Morella (España). Ameghiniana 45, 649–662 (2008).
Google Scholar - Gasca, J. M., Díaz-Martínez, I., Moreno-Azanza, M., Canudo, J. I. & Alonso, A. A hypertrophied ungual phalanx from the lower Barremian of Spain: Implications for the diversity and palaeoecology of Spinosauridae (Theropoda) in Iberia. Cretaceous Res. 84, 141–152 (2018).
Article Google Scholar - Malafaia, E., Gasulla, J., Escaso, F., Narvaéz, I. & Ortega, F. An update of the spinosaurid (Dinosauria: Theropoda) fossil record from the Lower Cretaceous of the Iberian Peninsula: distribution, diversity, and evolutionary history. J. Iber. Geol. 46, 431–444 (2020).
Article Google Scholar - Isasmendi, E., Sáez-Benito, P., Torices, A., Navarro-Lorbés, P. & Pereda-Suberbiola, X. New insights about theropod palaeobiodiversity in the Iberian Peninsula and Europe: Spinosaurid teeth (Theropoda, Megalosauroidea) from the Lower Cretaceous of La Rioja (Spain). Cretaceous Res. 116, 104600 (2020).
Article Google Scholar - Alonso, A., Gasca, J., Navarro-Lorbés, P., Rubio, C. & Canudo, J. A new contribution to our knowledge of the large-bodied theropods from the Barremian of the Iberian Peninsula: The “Barranco del Hocino” site (Spain). J. Iber. Geol. 44, 7–23 (2018).
Article Google Scholar - Hendrickx, C., Mateus, O., Araújo, R. & Choiniere, J. The distribution of dental features in non-avian theropod dinosaurs: Taxonomic potential, degree of homoplasy, and major evolutionary trends. Palaeontol. Electron. 22, 1–110 (2019).
Google Scholar - Fanti, F., Cau, A., Martinelli, A. & Contessi, M. Integrating palaeoecology and morphology in theropod diversity estimation: A case from the Aptian-Albian of Tunisia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 410, 39–57 (2014).
Article Google Scholar - Molnar, R. E. Variation in theory and in theropods in Dinosaur Systematics: Approaches and Perspectives (eds K. Carpenter & P. J. Currie) 71–79 (Cambridge Univeristy Press, 1990).
- Van Valkenburgh, B. & Molnar, R. E. Dinosaurian and mammalian predators compared. Paleobiology 28, 527–543 (2002).
Article Google Scholar - Russell, D. A. Tyrannosaurs from the Late Cretaceous of western Canada. Natl. Museum Nat. Sci. Publ. Paleontol. 1, 1–34 (1970).
Google Scholar - Farlow, J. O. & Planka, E. R. Body size overlap, habitat partitioning and living space requirements of terrestrial vertebrate predators: Implications for the paleoecology of large theropod dinosaurs. Hist. Biol. 16, 21–40 (2002).
Article Google Scholar - Currie, P. J. Cranial anatomy of tyrannosaurid dinosaurs from the Late Cretaceous of Alberta, Canada. Acta Palaeontol. Pol. 48, 191 (2003).
Google Scholar - Rauhut, O. W. Osteology and relationships of a new theropod dinosaur from the Middle Jurassic of Patagonia. Palaeontology 48, 87–110 (2005).
Article Google Scholar - Novas, F. E., Chatterjee, S., Rudra, D. K. & Datta, P. New Aspects of Mesozoic Biodiversity 45–62 (Springer, Berlin, 2010).
Book Google Scholar - Hone, D. W. et al. A new, large tyrannosaurine theropod from the Upper Cretaceous of China. Cretaceous Res. 32, 495–503 (2011).
Article Google Scholar - Cau, A., Dalla Vecchia, F. M. & Fabbri, M. A thick-skulled theropod (Dinosauria, Saurischia) from the Upper Cretaceous of Morocco with implications for carcharodontosaurid cranial evolution. Cretaceous Res. 40, 251–260 (2013).
Article Google Scholar - Gianechini, F. A. et al. A New Furileusaurian Abelisaurid from La Invernada (Upper Cretaceous, Santonian, Bajo De La Carpa Formation), Northern Patagonia, Argentina. J. Vertebr. Paleontol. 40, 7151 (2021).
Google Scholar - Samathi, A., Sander, P. M. & Chanthasit, P. A spinosaurid from Thailand (Sao Khua Formation, Early Cretaceous) and a reassessment of Camarillasaurus cirugedae from the Early Cretaceous of Spain. Hist. Biol. 1–15 (2021).
- Henderson, D. M. Skull and tooth morphology as indicators of niche partitioning in sympatric Morrison Formation theropods. Gaia 15, 219–226 (2000).
Google Scholar - Farlow, J. O. & Holtz, T. R. The fossil record of predation in dinosaurs. Paleontol. Soc. Pap. 8, 251–266 (2002).
Article Google Scholar - Barrett, P. M. & Rayfield, E. J. Ecological and evolutionary implications of dinosaur feeding behaviour. Trends Ecol. Evol. 21, 217–224 (2006).
Article PubMed Google Scholar - Brusatte, S. L., Carr, T. D. & Norell, M. A. The osteology of Alioramus, a gracile and long-snouted tyrannosaurid (Dinosauria: Theropoda) from the Late Cretaceous of Mongolia. Bull. Am. Mus. Nat. Hist. 2012, 1–197 (2012).
Article Google Scholar - Radley, J. D. & Allen, P. The southern English Wealden (non-marine Lower Cretaceous): Overview of palaeoenvironments and palaeoecology. Proc. Geol. Assoc. 123, 382–385 (2012).
Article Google Scholar - Raven, T. J., Barrett, P. M., Pond, S. B. & Maidment, S. C. Osteology and taxonomy of British wealden supergroup (Berriasian–Aptian) Ankylosaurs (Ornithischia, Ankylosauria). J. Vertebr. Paleontol. 40, 6956 (2020).
Article Google Scholar - Robinson, S. A. & Hesselbo, S. P. Fossil-wood carbon-isotope stratigraphy of the non-marine Wealden Group (Lower Cretaceous, southern England). J. Geol. Soc. 161, 133–145 (2004).
Article ADS CAS Google Scholar - McFeeters, B. New mid-cervical vertebral morphotype of Spinosauridae from the Kem Kem Group of Morocco. Vertebr. Anat. Morphol. Palaeontol. 8, 182–193 (2020).
Google Scholar - Sánchez-Hernández, B. & Benton, M. J. Filling the ceratosaur gap: A new ceratosaurian theropod from the Early Cretaceous of Spain. Acta Palaeontol. Pol. 59, 581–600 (2014).
Google Scholar - Rauhut, O. W., Canudo, J. I. & Castanera, D. A reappraisal of the Early Cretaceous theropod dinosaur Camarillasaurus from Spain in Program and Abstracts XVII Conference of the EAVP (European Association of Vertebrate Paleontologists) pp. 96 (2019).
- Palci, A., Caldwell, M. W. & Papazzoni, C. A. A new genus and subfamily of mosasaurs from the Upper Cretaceous of northern Italy. J. Vertebr. Paleontol. 33, 599–612 (2013).
Article Google Scholar - Madzia, D. & Cau, A. Inferring ‘weak spots’ in phylogenetic trees: Application to mosasauroid nomenclature. PeerJ 5, e3782 (2017).
Article PubMed PubMed Central Google Scholar - Gheerbrant, E. & Rage, J.-C. Paleobiogeography of Africa: How distinct from Gondwana and Laurasia?. Palaeogeogr. Palaeoclimatol. Palaeoecol. 241, 224–246 (2006).
Article Google Scholar - Canudo, J. I. et al. What Iberian dinosaurs reveal about the bridge said to exist between Gondwana and Laurasia in the Early Cretaceous. Bull. Soc. Géol. France 180, 5–11 (2009).
Article Google Scholar - Dal Sasso, C., Maganuco, S. & Cau, A. The oldest ceratosaurian (Dinosauria: Theropoda), from the Lower Jurassic of Italy, sheds light on the evolution of the three-fingered hand of birds. PeerJ 6, e5976 (2018).
Article Google Scholar - Goloboff, P. A. & Catalano, S. A. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics 32, 221–238 (2016).
Article PubMed Google Scholar - Goloboff, P. A. & Szumik, C. A. Identifying unstable taxa: Efficient implementation of triplet-based measures of stability, and comparison with Phyutility and RogueNaRok. Mol. Phylogenet. Evol. 88, 93–104 (2015).
Article PubMed Google Scholar - Schmidt-Lebuhn, A. N. TNT script for the Templeton Test (2016).
- Gavryushkina, A., Welch, D., Stadler, T. & Drummond, A. J. Bayesian inference of sampled ancestor trees for epidemiology and fossil calibration. PLoS Comput. Biol. 10, e1003919 (2014).
Article ADS PubMed PubMed Central Google Scholar - Drummond, A. J., Suchard, M. A., Xie, D. & Rambaut, A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 29, 1969–1973 (2012).
Article CAS PubMed PubMed Central Google Scholar - Bouckaert, R. et al. BEAST 2: A software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 10, e1003537 (2014).
Article PubMed PubMed Central CAS Google Scholar - Lewis, P. O. A likelihood approach to estimating phylogeny from discrete morphological character data. Syst. Biol. 50, 913–925 (2001).
Article CAS PubMed Google Scholar - Yu, Y., Harris, A. J., Blair, C. & He, X. RASP (reconstruct ancestral state in phylogenies): A tool for historical biogeography. Mol. Phylogenet. Evol. 87, 46–49 (2015).
Article PubMed Google Scholar - Ree, R. H. & Smith, S. A. Maximum likelihood inference of geographic range evolution by dispersal, local extinction, and cladogenesis. Syst. Biol. 57, 4–14 (2008).
Article PubMed Google Scholar
Acknowledgements
We thank Martin Munt and Alex Peaker (Dinosaur Isle Museum, Sandown) for access to specimens and help with the stratigraphic relationship of the fossils; Gary Blackwell, Martin New, Eleanor Foxwell (Dinosaur Isle Museum) and Shaun Smith for preparation of the material; Martin Munt, Gary Blackwell and Alex Peaker, as well as volunteers Eleanor Foxwell, Callum Atkinson, Steve Hutt and Trudie Wilson, who helped excavate the site in 2017; Tim Medland, Andrew Cocks, Jon and Lou Moody, and Shaun Smith for donating their finds to the museum; Kai Bailey for finding and preparing remains attributable to Ceratosuchops; Replicate 3D for providing printed models; Susie Maidment (Natural History Museum, London) for providing access to the Baryonyx type specimen; Serjoscha Evers (University of Fribourg), Simone Maganuco (Natural History Museum of Milan) and Marco Schade (University of Greifswald) for providing additional imagery and discussion of Suchomimus, spinosaurid material from the Kem Kem beds, and Irritator respectively; Christophe Hendrickx for comments regarding spinosaurid dental variation, and John Radley for discussions regarding Wealden geology. We also thank Dan Folkes (University of Bristol), Scott Hartman (University of Wisconsin-Maddison) and Alex Vieira for the skeletals and silhouettes used in the figures. The program TNT was made available thanks to the Willi Hennig Society. We would like to extend out gratitude to the editorial team at Scientific Reports, and also to the reviewers for the thorough comments that helped improve this manuscript. This study was funded by the Engineering and Physical Science Research Council (EPSRC) (Grant No. 2283360) and Institute for Life Sciences (University of Southampton). The acquisition of material was supported by the Friends of Dinosaur Isle (Charity No. 1170688).
Author information
Authors and Affiliations
- Institute for Life Sciences, University of Southampton, University Road, Southampton, SO17 1BJ, UK
Chris T. Barker, Claire E. Clarkin & Neil J. Gostling - Faculty of Engineering and Physical Sciences, University of Southampton, University Road, Southampton, SO17 1BJ, UK
Chris T. Barker - School of Biological and Behavioural Sciences, Queen Mary University of London, Mile End Road, London, E1 4NS, UK
David W. E. Hone - School of Biological Sciences, Faculty of Environment and Life Sciences, University of Southampton, University Road, Southampton, SO17 1BJ, UK
Darren Naish, Claire E. Clarkin & Neil J. Gostling - 43125, Parma, Italy
Andrea Cau - School of Environment, Geography and Geosciences, University of Portsmouth, Burnaby Road, Portsmouth, PO1 3QL, UK
Jeremy A. F. Lockwood - Department of Earth Sciences, Natural History Museum, Cromwell Road, London, SW7 5BD, UK
Jeremy A. F. Lockwood - Hessle, HU13 0HD, UK
Brian Foster - Bioengineering Science Research Group, Faculty of Engineering and Physical Sciences, University of Southampton, Southampton, UK
Philipp Schneider - High-Performance Vision Systems, Center for Vision, Automation and Control, AIT Austrian Institute of Technology, Vienna, Austria
Philipp Schneider
Authors
- Chris T. Barker
You can also search for this author inPubMed Google Scholar - David W. E. Hone
You can also search for this author inPubMed Google Scholar - Darren Naish
You can also search for this author inPubMed Google Scholar - Andrea Cau
You can also search for this author inPubMed Google Scholar - Jeremy A. F. Lockwood
You can also search for this author inPubMed Google Scholar - Brian Foster
You can also search for this author inPubMed Google Scholar - Claire E. Clarkin
You can also search for this author inPubMed Google Scholar - Philipp Schneider
You can also search for this author inPubMed Google Scholar - Neil J. Gostling
You can also search for this author inPubMed Google Scholar
Contributions
C.T.B., D.N. and N.J.G. conceived the study. B.F. and J.F.A.L. found and donated material from Chilton Chine; B.F. prepared cranial elements. C.T.B. wrote the first drafts (main text and SI), compiled Appendix 1 and 2, and created Figs. 2, 3, 4, 5 and 7; AC compiled the character list and scored the OTUs used in the phylogenetic analyses; C.T.B., A.C. and D.E.H. edited the scores for the spinosaurid OTUs. A.C. conducted the phylogenetic analyses, drafted the methods and results sections, and created Figs. 6 and S1 and S2. All authors contributed to the interpretation of the specimens and editing of the manuscript and its SI.
Corresponding authors
Correspondence toChris T. Barker or Neil J. Gostling.
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Barker, C.T., Hone, D.W.E., Naish, D. et al. New spinosaurids from the Wessex Formation (Early Cretaceous, UK) and the European origins of Spinosauridae.Sci Rep 11, 19340 (2021). https://doi.org/10.1038/s41598-021-97870-8
- Received: 28 April 2021
- Accepted: 26 August 2021
- Published: 29 September 2021
- DOI: https://doi.org/10.1038/s41598-021-97870-8