Bordetella pertussis, the Causative Agent of Whooping Cough, Evolved from a Distinct, Human-Associated Lineage of B. bronchiseptica (original) (raw)
Abstract
Bordetella pertussis, B. bronchiseptica, B. parapertussishu, and B. parapertussisov are closely related respiratory pathogens that infect mammalian species. B. pertussis and B. parapertussishu are exclusively human pathogens and cause whooping cough, or pertussis, a disease that has resurged despite vaccination. Although it most often infects animals, infrequently B. bronchiseptica is isolated from humans, and these infections are thought to be zoonotic. B. pertussis and B. parapertussishu are assumed to have evolved from a _B. bronchiseptica_–like ancestor independently. To determine the phylogenetic relationships among these species, housekeeping and virulence genes were sequenced, comparative genomic hybridizations were performed using DNA microarrays, and the distribution of insertion sequence elements was determined, using a collection of 132 strains. This multifaceted approach distinguished four complexes, representing B. pertussis, B. parapertussishu, and two distinct B. bronchiseptica subpopulations, designated complexes I and IV. Of the two B. bronchiseptica complexes, complex IV was more closely related to B. pertussis. Of interest, while only 32% of the complex I strains were isolated from humans, 80% of the complex IV strains were human isolates. Comparative genomic hybridization analysis identified the absence of the pertussis toxin locus and dermonecrotic toxin gene, as well as a polymorphic lipopolysaccharide biosynthesis locus, as associated with adaptation of complex IV strains to the human host. Lipopolysaccharide structural diversity among these strains was confirmed by gel electrophoresis. Thus, complex IV strains may comprise a human-associated lineage of B. bronchiseptica from which B. pertussis evolved. These findings will facilitate the study of pathogen host-adaptation. Our results shed light on the origins of the disease pertussis and suggest that the association of B. pertussis with humans may be more ancient than previously assumed.
Synopsis
Bordetella pertussis causes whooping cough, which kills 300,000 persons annually, and is reemerging despite vaccination. This human-restricted species is closely related to the respiratory pathogens B. parapertussishu, which is also human restricted, and B. bronchiseptica, which infects a broad range of mammals. Based on its limited genetic diversity and lack of historical descriptions, it has been suggested that the association between B. pertussis and humans is recent. In this study, the authors examined the genetic diversity and evolutionary relationships of these three Bordetella species. Their results suggest that B. parapertussis evolved from an animal-associated lineage of B. bronchiseptica, while B. pertussis evolved from a distinct B. bronchiseptica lineage that may already have had a preference for hominids up to 2.5 million years ago. Extant members of this newly identified B. bronchiseptica lineage were found to circulate in human populations. Comparisons of gene content revealed genomic features that are shared by and specific to B. pertussis and the B. bronchiseptica human-associated lineage and that may be important for association with the human host. These two lineages also have differences in key virulence genes that may reflect immune competition in the human host. By elucidating the evolutionary origins of human-adapted Bordetella, this study sets the stage for identification of key molecular events in host adaptation.
Introduction
The genus Bordetella is composed of several species, of which three are exclusively respiratory pathogens of mammalian hosts: B. bronchiseptica, B. pertussis, and B. parapertussis (henceforth referred to as the mammalian bordetellae). B. bronchiseptica causes chronic and often asymptomatic respiratory tract infections in a wide variety of mammals. It is only sporadically isolated from humans [1,2], particularly from immunocompromised individuals, and human infections have been considered to be zoonotic [3]. B. parapertussis consists of two distinct lineages: one found in humans and the other found in sheep (B. parapertussishu and B. parapertussisov respectively) [4]. B. pertussis and B. parapertussishu have been isolated only from humans and cause acute, transient infections and disease, designated whooping cough or pertussis. Pertussis is especially severe in young, unvaccinated children and has reemerged in recent years in vaccinated populations [5–7]. Previous research indicated that B. pertussis and B. parapertussishu independently evolved from a _B. bronchiseptica_–like ancestor [8.9], and comparison of the genomes of the three isolates chosen for sequencing suggested that the time to the last common ancestor (LCA) for B. pertussis and B. parapertussishu and for B. bronchiseptica was 0.7 to 3.5 and 0.27 to 1.4 million years, respectively [10]. Despite their different host tropisms, the mammalian bordetellae are very closely related [8,9]. Analysis of their genome sequences revealed that the adaptation of B. pertussis and B. parapertussishu to the human host was accompanied by extensive genome decay [10].
Their differences in host tropism in contrast to their close genetic relationships make the mammalian bordetellae attractive candidates for the study of host-adaptation. Such studies are facilitated by the availability of genome sequences of B. bronchiseptica, B. pertussis, and B. parapertussishu [10]. So far, only a single representative of each species has been sequenced, and it is important to determine their relationships to the Bordetella population as a whole. To that end and to identify genetic events that may be associated with host adaptation, we used a combination of multilocus sequence typing (MLST) [11], comparative genomic hybridization (CGH) with whole-genome microarrays [12], and the distribution of several insertion sequence elements (ISEs) to characterize 132 mammalian Bordetella strains with diverse host associations. This work identified two B. bronchiseptica lineages, the first of which is composed of mainly strains of animal origin and includes the B. bronchiseptica strain from which the genome sequence has been determined. The second lineage, comprising strains mainly of human origin, is more closely related to B. pertussis than the first lineage. Comparison of the two B. bronchiseptica lineages to B. pertussis revealed genetic differences that may be associated with adaptation to the human host.
Results
Population Structure of the Mammalian Bordetellae, Based on Multilocus Sequence Typing
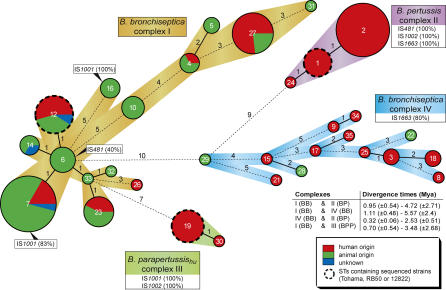
To determine the relationships between the mammalian bordetellae, we determined the partial sequences of seven housekeeping genes from 132 strains (Table S1 and http://pubmlst.org/bordetella). We observed 32 sequence types (STs) among the 132 Bordetella isolates. Allele segments were divided into five equally sized subloci, and a minimum spanning tree (MST) algorithm was used to cluster the subloci [13]. Complexes were defined as groups of strains differing at fewer than five of 35 subloci with a minimum of two STs per complex. Using this criterion, strains could be assigned to one of four complexes, designated complexes I through IV (Figure 1).
Figure 1. Minimum Spanning Tree of B. bronchiseptica, B. pertussis, and B. parapertussis.
The tree was based on the sequence of seven housekeeping genes. Individual genes were split into five subloci, and a categorical clustering was performed. In the minimum spanning tree, sequence types sharing the highest number of single locus variants were connected first. Each circle represents a sequence type (ST) the size of which is related to the number of isolates within that particular ST. Colors within circles indicate host distribution. The numbers between connected STs represent the number of different subloci between those STs. The clonal complexes (I, II, III, and IV) are indicated by colored strips between connected STs. ST16 (B. bronchiseptica complex I) harbors the B. parapertussisov strains. STs containing strains of which the genome has been sequenced (B. pertussis Tohama, B. parapertussis 12822 or B. bronchiseptica RB50) are indicated by a thickset, dashed line. The distribution of the insertion sequence elements IS_481_, IS_1001_, IS_1002,_ and IS_1663_ is shown in boxes (see also Table S1); numbers between parentheses indicate the percentage of strains that contained the ISE as determined by PCR amplification. The divergence times between B. bronchiseptica complexes I and IV and B. pertussis complex II are shown.
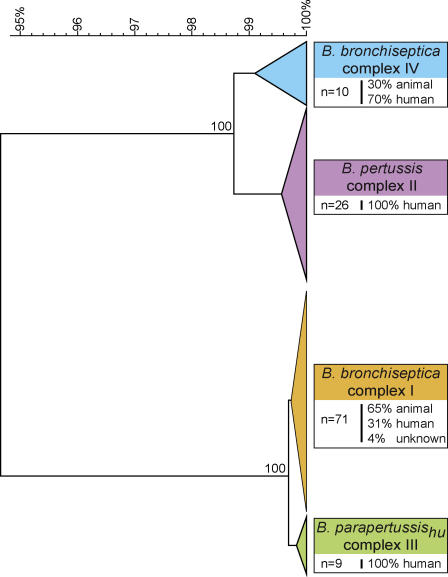
Complexes II and III contained the B. pertussis and B. parapertussishu isolates, respectively. Both of these complexes showed very limited genetic diversity (H = 0.65 and 0.35, respectively), as described previously [8,9]. B. bronchiseptica was divided into two distinct populations, designated complexes I and IV, respectively. The genetic diversity of these two complexes (H = 2.16 and 2.45, respectively) was much higher than that of complexes II and III. Complex I contained the majority of the B. bronchiseptica strains (76 of 91 strains), including the sequenced RB50 strain. In addition, it contained the B. parapertussisov isolates in the study population (ST16). B. bronchiseptica complex IV was more closely related to B. pertussis than was B. bronchiseptica complex I. Furthermore, the host species associations of the two complexes were quite distinct. Of the B. bronchiseptica complex IV isolates, 80% were isolated from humans, while this was the case for only 32% of the complex I isolates. It should be noted that human B. bronchiseptica isolates were overrepresented in our strain collection, in comparison with their occurrence in natural populations. However, the human complex IV isolates originated from different continents, comprising North America, South America, and Europe. Previously, phylogenetic analysis based on CGH suggested the existence of a distinct B. bronchiseptica lineage that was closely related to B. pertussis [12].
The relationships among the mammalian bordetellae inferred from housekeeping gene sequences were confirmed by an analysis based on the pertactin gene (prn), which codes for a surface-associated virulence factor involved in adherence [14,15]. A UPGMA tree was constructed from the aligned prn sequence data, and the topology of this tree was very similar to the MLST tree (Figure 2). B. bronchiseptica strains are grouped into two lineages, corresponding to complex I and IV in the MLST tree. Also, B. bronchiseptica complex IV and B. pertussis strains clustered together in one branch, which was supported by bootstrapping. B. parapertussishu comprised a separate branch within a larger cluster that also contained the B. bronchiseptica complex I strains. The B. parapertussisov strains were indistinguishable from B. bronchiseptica complex I strains, as was observed in the MLST tree.
Figure 2. UPGMA Tree Based on the Analysis of the Pertactin Gene of Bordetella Isolates Used in the MLST Analysis.
The DNA segment coding for the extracellular domain of pertactin (P.69) was used for analysis, with the exclusion of the repeat regions 1 and 2. Bootstrap values are shown for the nodes separating the complexes and are based on 500 bootstrap replicates. The scale indicates the genetic distance along the branches. Colors of the branches indicate the four complexes defined by MLST. The number of strains of each branch is shown in boxes, as well as the host distribution.
Distribution of Insertion Sequence Elements
The distribution of ISEs has been used to reveal evolutionary relationships among the Bordetella population [9]. Toward this end, we screened our strain collection for the presence of IS_481_, IS_1001_, IS_1002_, and IS_1663_ using PCR (Table S1). The distribution of the ISEs was mapped onto the MST (see Figure 1). IS_481_ was detected in all B. pertussis strains but not in any other species, with the exception of two B. bronchiseptica isolates, both from a horse (B1975, B0230, ST6), consistent with previous observations [9,16]. IS_1001_ was detected in all B. parapertussishu and B. parapertussisov strains. Additionally, IS_1001_ was detected in most (21 of 25) B. bronchiseptica strains belonging to ST7 in complex I, but not in other STs, including STs in complex II and IV. IS_1002_ was detected only in B. pertussis and in B. parapertussishu strains, confirming previous observations [4,9]. IS_1663_ [10] was detected in all B. pertussis isolates but also in ten of 13 B. bronchiseptica complex IV strains. The three complex IV strains in which IS_1663_ was not detected belonged to STs 18 and 21.
Divergence Times of Complexes
Under the assumption that the mutation rate in prokaryotes is relatively constant, the time since descent from the LCA can be estimated using pairwise mean allele distances (K S) [17,18]. The clock rates described by Whittam [19] and by Guttman and Dykhuizen [20] were used to estimate a range of divergence times between complexes. Calculations indicated that B. pertussis and B. bronchiseptica complex IV separated approximately 0.3 to 2.5 million years ago (Mya), which suggests a more recent divergence time than B. pertussis and B. bronchiseptica complex I, estimated at 1.1 to 5.6 Mya. B. parapertussishu and B. bronchiseptica complex I diverged between 0.7 and 3.5 Mya according to our calculations. The divergence times of combinations of complexes is shown in Figure 1.
Gene Content of Strains from B. bronchiseptica Complexes I and IV
The high percentage of complex IV strains of human origin compared to the percentage of those in complex I suggested a preference for the human host in complex IV strains. To identify genetic events that may have played a role in host adaptation or host restriction, we used CGH with Bordetella DNA microarrays [17,18].
Genomic DNA from 26 B. bronchiseptica complex I and 13 complex IV strains was hybridized to microarrays (CGH data files have been deposited in ArrayExpress, accession E-TABM-32). Significance Analysis of Microarrays (SAM) was used to identify probes with statistically significant log intensity ratio differences between the two complexes (Table S2). This approach detected sequences that have been deleted more often in one of the two complexes, as well as DNA sequences diverging from the reference sequences in one of the two complexes.
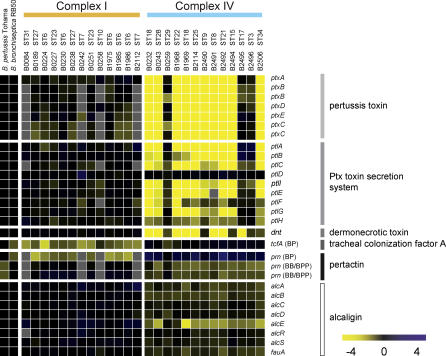
Thirty-one probes, representing 29 genes and an ISE, hybridized more strongly or more frequently to the genomes of complex IV strains than to those of complex I. Two of these probes represented the B. pertussis homologs of the virulence genes prn and tcfA, encoding pertactin and tracheal colonization factor, respectively [15,21] (Figure 3). Sequence analysis confirmed that the B. bronchiseptica complex IV prn sequences were more similar to those of B. pertussis than to those of the B. bronchiseptica complex I (see Figure 2).
Figure 3. Gene Content of the Differentially Hybridizing Virulence Loci between B. bronchiseptica Complex I and IV, as Determined by CGH.
Each column represents one strain. Strain numbers and STs are indicated above the columns. Each row represents one ORF (in B. bronchiseptica RB50 gene order), ORF designations are shown to the right of the rows. In the case of tcfA and prn, the origins of the probes are indicated between parentheses. The BP probe of tcfA was 100% similar to B. pertussis Tohama and 85.1% similar to B. bronchiseptica RB50. The BP prn probe was 100% similar to B. pertussis Tohama and 86% similar to B. parapertussis 12822 and B. bronchiseptica RB50. The BB/BPP prn probes were both 100% similar to B. parapertussis 12822 (BPP) and B. bronchiseptica RB50 (BB) and 86% similar to B. pertussis Tohama. The yellow-black-blue color scale indicates the hybridization value relative to the reference; references are B. bronchiseptica RB50, B. parapertussis 12822, and B. pertussis Tohama. For B. bronchiseptica RB50 and B. pertussis Tohama, the data in the figure are based on the genomic sequences. Yellow indicates decreased hybridization, black indicates hybridization values comparative to the references, and blue indicates gene duplications. Intermediate values indicate partial deletions or sequence divergence. Missing data are represented in gray.
Sixteen of these 31 probes had been identified previously as _B. pertussis_–specific [12]. In most cases, these probes hybridized to nine or more complex IV strains but not to any complex I or III strain. The genes represented by these probes encode diverse functions involved in metabolism, transport, regulation, and transposition (Table S2). With the possible exception of BP0703, which encodes a TonB-dependent iron receptor, no obvious virulence genes were observed among them. Most of the 16 genes were located in small clusters along the B. pertussis Tohama chromosome. The presence of these genes in B. bronchiseptica complex IV and B. pertussis but not in B. bronchiseptica complex I or B. parapertussishu suggests that they were acquired by the common ancestor of B. pertussis and B. bronchiseptica complex IV.
We also identified 248 probes, representing 237 genes, that exhibited significantly stronger hybridization to complex I genomes than to complex IV genomes (Table S2). Sixty-eight (27%) corresponded to genes associated with mobile elements such as prophages, while many of the other probes represented genes involved in metabolic, transport, and regulatory functions. Surprisingly, several virulence-associated genes were found to be missing or divergent in the complex IV strains, as compared to complex I strains. These included the B. bronchiseptica homologs of tcfA and prn, Bvg-regulated intermediate phase gene A (bipA), the alcaligin biosynthesis locus (alcA/E), the pertussis toxin synthesis and transport locus (ptx/ptl), the dermonecrotic toxin gene (dnt), and the lipopolysaccharide (LPS) biosynthetic locus (Figures 3 and 4).
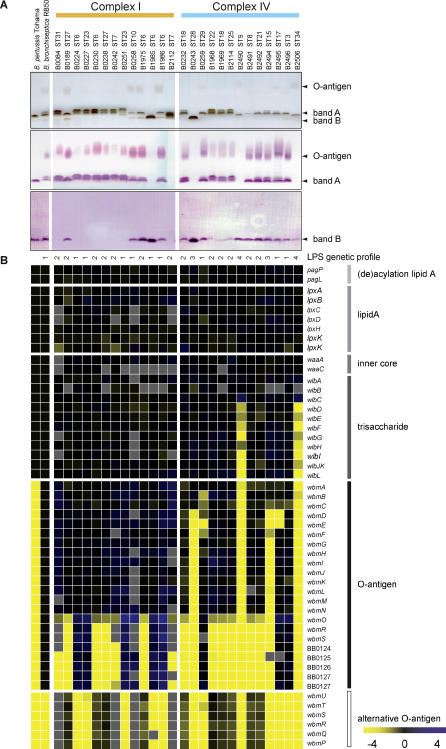
Figure 4. Expression of LPS by B. bronchiseptica Complex I and Complex IV Strains and Gene Content Variation at the LPS Biosynthesis Locus.
(A) Top: Electrophoretic LPS profiles obtained by tricine-SDS-PAGE and silver staining. Middle: Western blot of the same samples with mAb 36G3, which detects band A. Bottom: Western blot of the same samples with mAb BL8, which detects band B.
(B) Gene content of the LPS biosynthesis locus as determined by CGH. See Figure 3 for details. For B. bronchiseptica RB50 and B. pertussis Tohama, the data in the figure are based on the genomic sequences. The genes wbmPQRSTU represent an alternative LPS O-antigen biosynthesis sublocus that is orthologous to the genes found in B. parapertussis 12822 [10] and B. bronchiseptica C7635E [26]. LPS genetic profiles as described in the text are indicated at the top of the columns. Color scale as in Figure 3. Missing data are represented in gray.
Interestingly, ten of 13 complex IV strains harbored deletions in the ptx and ptl loci, which encode pertussis toxin (Ptx) and its secretion machinery, respectively (Figure 3) [22,23]. While conditions under which Ptx is expressed by either B. parapertussis or B. bronchiseptica have not been found, the structural genes are generally conserved among these species [24], suggesting selective pressure to retain the ability to produce functional Ptx under certain circumstances. The complex IV strains in which ptx/ptl was still present (ST3/17/29) were tested for expression of Ptx by immunoblotting, and these strains were found not to express Ptx under the growth conditions used (unpublished data). Another distinguishing characteristic of the complex IV strains was the deletion of the dermonecrotic toxin gene (dnt) in 8 of 13 strains. In contrast, this gene was detected in all B. pertussis, B. parapertussishu, B. parapertussisov, and B. bronchiseptica complex I strains.
LPS Genetic and Structural Diversity in B. bronchiseptica
The genetic structure of the LPS biosynthesis locus differed between complex I and IV strains. The LPS molecules of Gram-negative bacteria usually consist of three, covalently linked, major domains: the lipid A, the branched chain oligosaccharide core, and the hydrophilic O-antigen. A number of genetic loci have been implicated in the synthesis of these domains in Bordetella, such as the lpx locus (lipid A), the waa locus (inner core), the wlb locus (outer core), and the wbm locus (O-antigen) [25–29].
B. pertussis LPS usually consists of lipid A and an inner core, to which the outer core (a trisaccharide) is attached; this is also referred to as band A. Certain B. pertussis strains produce only band B, which is identical to band A except that it lacks the trisaccharide [30]. The O-antigen, which is added to the trisaccharide, is found only in B. bronchiseptica and B. parapertussis. This structure is missing in B. pertussis due to the deletion of the genes wbmA-U [26]. In Figure 4, the gene content of the LPS locus is shown for 13 complex I and 13 complex IV strains and for B. pertussis Tohama and B. bronchiseptica RB50. Four LPS gene content profiles, designated LPS 1–4, could be distinguished among the B. bronchiseptica strains. Strains with the LPS 1 profile had an LPS gene composition similar to RB50, characterized by the absence of wbmPQRSTU. The LPS 2 profile was characterized by the absence of the genes wbmORS and BB0124–BB0127 and the presence of an alternative O-antigen locus, comprising wbmPQRSTU, orthologous to the B. parapertussis 12822 genes [10]. Both of these genotypes appear competent for the production of a full length LPS. Strains with the LPS 3 profile lacked wbmD-U and BB0124-BB0127, suggesting that they may not produce O-antigen. The LPS 4 profile was similar to the LPS 3 profile but additionally lacked wbmABC and wlbD-L, suggesting that strains of this genotype may be deficient for the production of trisaccharide as well as O-antigen. The deletion in the O-antigen genes of LPS 4 strains was similar to that observed in the O-antigen genes of B. pertussis Tohama. All complex I strains displayed either an LPS 1 or an LPS 2 profile. Nine of 13 complex IV strains had either an LPS 1 or an LPS 2 profile, while four complex IV strains showed more extensive deletions, resulting in LPS 3 and LPS 4 profiles.
To study the effect of these deletions on LPS production, proteinase K–treated cell lysates were analyzed by Tricine-SDS-PAGE, followed by silver staining or immunoblotting with monoclonal antibodies (mAbs) directed against either band A or band B (mAbs 36G3 and BL-8, respectively [31,32]) (Figure 4). Silver-staining showed that all complex I strains produced band A, except for B1985, which produced band B, and B2112, which produced a band migrating at a position between bands A and B. These strains showed no obvious deletions at their wlb or wbm loci, and the fact that they did not produce band A and O-antigen may be attributed to point mutations, e.g., in their wlb locus, or to regulatory effects. These results were confirmed by immunoblotting with mAb 36G3. The epitope in the trisaccharide that is recognized by this mAb was also present in the O-antigenic repeats, as was described previously [33]. In general, most strains that produced band A also produced an additional band just above band A. This extra band was also recognized by 36G3 and therefore is likely derived from band A.
The nine complex IV strains with LPS 1 or 2 profiles all produced band A. The two strains with the LPS 4 profile, B2490 and B2506, produced a band smaller than band A, which failed to be recognized by mAb 36G3. Of the two strains with an LPS 3 profile, one strain (B0243) produced only band B and no O-antigen. Unexpectedly, the other strain, B2494, produced band A and O-antigen as detected by immunoblotting, indicating that this strain contains as yet uncharacterized O-antigen biosynthesis genes. Silver staining also suggested that the LPS 4 strains may produce O-antigen, although the O-antigen failed to be recognized by mAb 36G3.
Discussion
Although it has long been speculated that B. pertussis evolved from a B. bronchiseptica strain [8,9], a specific lineage has not been identified. Here we identify and characterize such a B. bronchiseptica lineage. Analysis of MLST data from the mammalian bordetellae identified four distinct complexes. Complex I and IV comprised B. bronchiseptica strains, while complex II and III comprised the human pathogens B. pertussis and B. parapertussishu, respectively. Our results suggest that B. pertussis and B. parapertussishu evolved from complexes I and IV, respectively, indicating that adaptation to humans occurred as two independent events, consistent with previous data [9,10].
The population structure of the mammalian bordetellae inferred from MLST data largely corresponded with a maximum parsimony phylogeny derived from a previous CGH study [12], with the exception of the relationship of B. parapertussisov and B. parapertussishu. In the current study, B. parapertussishu and B. parapertussisov are clearly derived from different STs in complex I. Further, in contrast to B. parapertussishu, B. parapertussisov is actually part of B. bronchiseptica complex I. The closer relationship between the sheep- and human-derived B. parapertussis lineages that was inferred from CGH data may be an artifact of long-branch attraction in the maximum parsimony tree [34].
Consistent with previous studies [8,9,35], B. pertussis and B. parapertussishu showed a relatively low degree of genetic diversity, suggesting that they evolved recently or encountered a recent evolutionary bottleneck. Of the three B. pertussis STs observed, two were found exclusively before 1960, whereas all modern strains belong to ST2. The temporal shift in B. pertussis STs is consistent with our previous studies on antigenic shifts that show major changes in the B. pertussis population after the introduction of mass vaccination against pertussis in the 1950s and 1960s [35,36].
Most human disease is by far caused by B. pertussis, and we therefore focused on the relationship of B. bronchiseptica complex IV with B. pertussis. B. bronchiseptica complex IV strains were found to be more closely related to B. pertussis than to the complex I B. bronchiseptica strains. A tree based on prn nucleotide sequences also suggested a closer relationship of complex IV strains to B. pertussis than to complex I strains. A number of other features of complex IV strains were consistent with their close relationship with B. pertussis. Most complex IV strains were isolated from humans (80%), while the majority of complex I strains were of animal origin (68%). Almost all B. bronchiseptica complex IV strains were isolated from patients with whooping cough symptoms. Further, complex IV strains and B. pertussis shared an IS element, IS_1663,_ that was not found outside these two lineages. The sharing of an IS element may be explained by either vertical or horizontal transfer. The former suggests a common ancestry, while the latter would point to niche sharing of B. pertussis and B. bronchiseptica complex IV. It seems unlikely that the association of complex IV strains with humans is due to a sampling artifact, as the strains analyzed were from widely separated geographic regions, including North America, South America, and Europe. Thus, these strains were not epidemiologically related.
Three STs (ST12, ST23, and ST27) found in complex I also contained a high percentage of human strains (55%, 43%, and 77%, respectively). All other, nonhuman isolates of these STs were collected from domesticated animals. Thus, both complex I and IV contain B. bronchiseptica strains that are well adapted to the human host. However, the particular relevance of the human-associated lineage in complex IV appears to be its evolutionary relationship with B. pertussis. The high frequency of human isolates observed in complex IV may be due to the close interaction of humans with the animal hosts in which these strains reside or to the fact that complex IV strains are better adapted to a human environment than B. bronchiseptica complex I strains. In either case, the B. bronchiseptica complex IV infections of humans would be zoonotic. Another intriguing possibility is that B. bronchiseptica complex IV strains are to a large extent adapted to the human host and primarily transmitted between humans.
Microarray-based CGH revealed 29 genes and IS_1663_ to be more frequently present in, or more similar to, B. pertussis orthologs in B. bronchiseptica complex IV than to complex I strains. Of these genes, 16 were unique to B. pertussis and B. bronchiseptica complex IV, suggesting they were acquired after the common ancestor of complex IV and B. pertussis diverged from complex I. With the exception of prn and tcfA, which hybridized more strongly to the _B. pertussis_–derived probe, no known virulence genes were identified among these 30 genes. Conversely, 237 genes were absent or divergent in complex IV compared to complex I strains, suggesting that the B. bronchiseptica complex IV genome is decaying, as has been assumed for B. pertussis and B. parapertussishu. Genome decay has been associated with host restriction or niche change in a number of pathogens such as Yersinia pestis [37,38] and Burkholderia mallei [39] and has been suggested to be a driving force of host restriction for B. pertussis and B. parapertussis as well [10]. Likewise, the apparent preference of complex IV strains for humans may also be associated with genome decay. Because putative complex IV–specific sequences were not represented on the microarray used here, we were unable to address the possibility that complex IV strains have acquired, through lateral transfer, genetic loci that may have promoted host preference. However, gene acquisition appears to have been a rare event in the evolution of B. pertussis and B. parapertussis from B. bronchiseptica complex I [10].
Differences between complex IV strains and B. pertussis were observed with respect to three major virulence factors, Ptx, Dnt, and LPS. All B. bronchiseptica complex I strains examined contained intact Ptx genes. Although conditions under which the Ptx genes are expressed in B. bronchiseptica have not been identified, their conservation suggests that they may confer a selective advantage in the ecology of complex I strains. In contrast to complex I strains, the genes encoding Ptx and its secretion apparatus were deleted from most complex IV strains (10 of 13). In the strains that did retain the Ptx locus, no in vitro expression of Ptx was observed, even though these strains were closely related to B. pertussis.
Another characteristic that sets complex IV strains apart from all other mammalian bordetellae is that in eight of 13 strains, dnt was deleted. Dnt is an intracellular toxin that activates the small GTPase Rho through deamidation or polyamination [40]. It has been shown that Dnt is important for turbinate atrophy and the colonization of the upper respiratory tract by B. bronchiseptica in pigs [41].
Based on CGH, four LPS genetic profiles were distinguished. The LPS genetic locus was generally more polymorphic in complex IV strains than in complex I strains, and deletions were observed in the O-antigen and trisaccharide biosynthesis genes in some complex IV strains. In complex IV strains with the LPS 4 profile, the extent of deletion in the O-antigen genes was very similar to that seen in B. pertussis Tohama. B. pertussis does not produce repetitive O-antigen as it lacks the wbm genes but makes a lipo-oligosaccharide that consists of lipid A to which a single trisaccharide is attached [26]. Like B. pertussis, four complex IV strains lacked the O-antigen genes known to be present in the sequenced genomes of B. bronchiseptica and B. parapertussishu. Despite the absence of these genes, in at least one of these strains O-antigen was detected by immunoblotting, suggesting that this strain carries LPS genes distinct from those in RB50 or 12822. The two LPS 4 strains, both isolated from humans, also lacked the genes required for biosynthesis of the trisaccharide and failed to produce trisaccharide detectable by immunoblotting. The absence of the trisaccharide is intriguing in view of the fact that it was found to be otherwise conserved in all Bordetella strains analyzed.
It seems likely that in addition to gene loss and acquisition, differences in gene regulation have significantly contributed to host adaptation [42]. The differences observed between complex IV strains and B. pertussis, particularly with respect to Ptx, Dnt, and LPS, may be due to differences in niches occupied. Another possibility is that these differences have arisen in response to immune competition between B. bronchiseptica complex IV strains and B. pertussis. Gupta and co-workers [43] provided evidence that immunodominant surface antigens are organized into nonoverlapping combinations as a result of selection by the host immune system. This process could also have driven the inactivation and conservation of virulence factors in mammalian bordetellae infecting the same host. In a similar vein, Bjørnstad and Harvill [44] hypothesized that, since B. pertussis and B. parapertussishu both infect humans, they may have evolved to evade cross-immunity by the other pathogen. The authors propose that immune competition provides an explanation for differences observed between B. pertussis and B. parapertussishu. For example, B. pertussis but not B. parapertussishu expresses Ptx, although both contain the required genes. Conversely, B. parapertussishu expresses O-antigen, while the corresponding genes have been deleted from B. pertussis. Similarly, the deletion of the genes for Ptx, Dnt, and genes involved in trisaccharide syntheses by complex IV strains may have been driven by immune competition with B. pertussis and possibly also with B. parapertussishu .
The origin of the disease whooping cough is still a mystery. Although the disease has very typical symptoms in children and was one of the major causes of child mortality before the introduction of vaccination, the first written reference to the disease in Europe is found in 1540 [45]. The first description of an epidemic, which occurred in Paris, was given by Baillon in 1578 [46]. Particularly interesting are the observations made by Nils Rosen von Rosenstein in 1766, who wrote [47], “The hooping cough never appeared in Europe originally, but was transported thither from other parts of the world by means of merchandise, seamen and animals. Its first appearance in Sweden cannot be determined with any certainty; but in France it began in the year 1414.” In contrast, 16th- and 17th-century descriptions of the disease and epidemics in Europe are documented frequently in the literature [46]. The absence of references to pertussis-like symptoms in the ancient literature has been taken as evidence that the association of B. pertussis with humans is of recent origin.
We propose that the association of B. pertussis with humans is, in fact, ancient but that the introduction of B. pertussis into Europe may be more recent. Complex IV strains showed a degree of diversity that was comparable to complex I strains (2.16 and 2.45, respectively), and thus, assuming that complex IV strains are primarily adapted to the human host, this association must be ancient. Parkhill et al. [10] previously estimated the time to the LCA of a B. bronchiseptica complex I strain (RB50) and B. pertussis to be 0.7 to 3.5 Mya, based on the mean number of synonymous substitutions per synonymous site of orthologous gene pairs. Our data indicate that current B. pertussis strains expanded clonally from the B. pertussis_–_B. bronchiseptica complex IV LCA 0.32 to 2.53 Mya, further supporting an ancient association of B. pertussis with humans. However, we cannot rule out the possibility that more recent human-associated ancestors of B. pertussis are extinct or undiscovered. Such recent ancestors would indicate a more recent origin of B. pertussis.
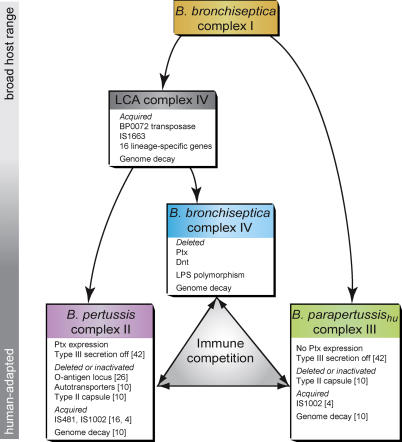
Although it is tempting to speculate that the LCA of B. pertussis and B. bronchiseptica complex IV was associated with humans, the possibility remains that this association emerged after the split with B. pertussis. A possible evolutionary scenario (Figure 5) represents the adaptation of an ancestral B. bronchiseptica complex I strain to humans or their hominid ancestors. From this lineage, the LCA of B. bronchiseptica complex IV and B. pertussis evolved, subsequently giving rise to B. bronchiseptica complex IV and B. pertussis.
Figure 5. Model of the Evolution of the Mammalian Bordetellae.
The bar on the left indicates increasing degrees of adaptation to the human host. Arrows indicate descent; double arrows between complexes indicate possible within-host immune competition. In boxes, genetic events are shown that may have played a role in speciation and niche adaptation. Numbers between parentheses refer to references. See text for details.
Recent emergence of a pathogenic clone from a more ancient human-associated progenitor species has been proposed as the mechanism for the origin of Mycobacterium tuberculosis [48]. Although previous genetic analysis had suggested that M. tuberculosis emerged as little as 20,000 years ago, phylogenetic analysis of M. tuberculosis and a closely related but more diverse group of smooth tubercle bacilli indicated that this more broadly defined species has been associated with hominids for up to 3 million years.
Yersinia pestis, the causative agent of plague, is a clone that evolved from Y. pseudotuberculosis 1,500 to 20,000 years ago, shortly before the first known pandemics of human plague, and its recent origin is further suggested by the complete lack of polymorphism in housekeeping genes [18]. Similarly, B. pertussis also shows limited diversity. However, in contrast to Y. pestis, which reveals absolutely no polymorphisms in housekeeping genes, we observed three STs in B. pertussis. This may suggest an older origin of B. pertussis compared to Y. pestis, although other factors, such as population size and bottlenecks, could also explain these differences. The most plausible explanation from our data is that the association of B. pertussis with humans originated in the LCA of B. pertussis and B. bronchiseptica complex IV. Based on that assumption, the apparent emergence of pertussis in Europe within the last 500 years may be attributable to import via travel or migration or to the recent acquisition by B. pertussis of the ability to cause more severe, whooping cough–like symptoms. Although most of the B. bronchiseptica complex IV strains in our collection were isolated from patients suspected to have pertussis, we know little of the severity of the symptoms caused by these strains. It is conceivable that B. bronchiseptica preceded B. pertussis in Europe and that its disease was not documented because of its relatively mild and nonspecific course.
The work presented here places the three sequenced mammalian Bordetella strains within a phylogenetic context, thereby facilitating rational selection of strains for further genomic sequencing. In particular, sequencing of one or more members of complex IV may shed more light on processes involved in host adaptation and immune competition. Further, the identification of a B. bronchiseptica lineage which circulates in human populations may be important for public health. In recent years, whole cell vaccines have been replaced by acellular vaccines comprised of one to five antigens derived from B. pertussis [49]. The acellular vaccines induce a less cross-reactive immune response compared to whole cell vaccines [50] and may therefore result in an increase in B. parapertussis and B. bronchiseptica infections in vaccinated human populations.
Materials and Methods
Bacterial strains.
A total of 132 Bordetella isolates were used in this study: 91 B. bronchiseptica, 9 B. parapertussishu, 3 B. parapertussisov, and 29 B. pertussis isolates (see Table S1). The three strains from which the genome sequence has been determined, B. bronchiseptica RB50_, B. pertussis_ Tohama and B. parapertussis 12822 [10], were included. The collection included clinical isolates from humans and a broad range of animal species. Strains were grown on Bordet Gengou (BD, Franklin Lakes, New Jersey, United States) agar supplemented with 15% sheep blood at 37 °C for 2 to 5 days. Chromosomal DNA was isolated using the Wizard Genomic DNA Purification Kit (Promega, Madison, Wisconsin, United States), according to the manufacturers' protocol for Gram-negative bacteria.
DNA sequencing.
The nucleotide sequences were determined for internal regions of seven housekeeping genes for all strains (http://pubmlst.org/bordetella [51]). The nucleotide sequence of the prn region encoding the extracellular domain of the surface-associated autotransporter pertactin, P.69, was determined for 116 strains, with the exclusion of the repeat regions 1 and 2 [35]. These regions are comprised of amino acids repeats and are highly polymorphic due to insertion or deletion of the repeat unit. Primer information is listed in Table S3.
Detection of ISEs.
The distribution of IS_481,_ IS_1001,_ IS_1002,_ and IS_1663_ was determined for all strains using PCR amplification (see Table S1). For PCR amplification of IS_481,_ IS_1001,_ and IS_1002,_ primers were used that have been described previously [4,52]. Primer characteristics are listed in Table S3.
LPS SDS-PAGE and Western blotting.
BG-agar grown bacteria were harvested, boiled in 1× sample buffer (7.5% glycerol, 0.125 M Tris-HCl [pH 6.8], 1.5% SDS), and treated with proteinase K [33]. Tricine-SDS-PAGE was then performed in 4% stacking and 16% separating gels, as previously described by Lesse et al. [53]. Silver staining was performed as described by Tsai and Frasch [54]. LPS was transferred to PVDF membranes (Amersham Biosciences, Buckinghamshire, United Kingdom) and blocked with 0.5% (w/v) Protifar nonfat dried milk, 0.5% bovine serum albumin (w/v), and 0.1% Tween 20 in PBS. Immunoblotting was performed with monoclonal antibodies 36G3 and BL-8, directed against band A and band B LPS, respectively [31,32].
Sequence data analysis.
Analysis of nucleotide sequence data was performed using Bionumerics software package version 4.0 beta 4 (Applied Maths, Sint-Martens-Latem, Belgium). The Bordetella MLST database can be accessed at http://pubmlst.org/bordetella [51].
For each locus in the MLST analysis, the allele sequences for all strains were trimmed to a uniform length, and an allele number was assigned to each unique allele sequence. The combination of the allele numbers at the seven loci defines the ST or allelic profile of each strain. Construction of trees based on allelic profiles may not accurately reflect the true genetic distance because both single and multiple nucleotide polymorphisms are given equal weight. Consequently, the degree of sequence difference between two alleles is not quantitatively reflected in the MLST profile. Conversely, tree construction based on concatenated allele sequences does not take into account the introduction of clustered multiple base substitutions due to a single recombinational event. As a result, trees based on MLST sequences often contain long branches, incorrectly suggesting a large genetic distance. Therefore, we used a method designated as split-MLST, in which each locus is split into a user-defined number of equally sized subloci (D. A. Diavatopoulos, P. Vauterin, L. Vauterin, F. R. Mooi, and L. M. Schouls, unpublished data). Using this method, the sensitivity of categorical clustering could be increased, without the perturbing effect of recombination. The topology of the tree appeared to vary if the number of subloci per MLST locus was lower than five. However, above the value four, increasing the number of subloci had no significant effect on the topology of the tree, and we therefore selected the lowest possible split value, five, resulting in a total of 35 subloci.
The genetic diversity for each complex was calculated using the Shannon-Weiner index of diversity (H) using the following formula:

where Pi is the frequency of the _i_th type [55].
For estimation of divergence times between complexes, we calculated the pairwise mean distance (Ks) between alleles using DNASP 4.00 [56]. The divergence time was calculated using the following formula:

where Ks is the number of synonymous substitutions per synonymous site and r is the molecular clock rate of Escherichia coli as determined by Whittam [19] or by Guttman and Dykhuizen [20]. We used these two rates to calculate a range of divergence times. The divergence time was first calculated for each combination of STs between complexes, and from these the averaged age between complexes was calculated.
Comparative genomic hybridization.
The preparation of PCR product-based microarrays and the comparative genomic hybridization was performed essentially as described by Cummings et al. (12). This study employed a new array design that contained all of the probes from the first array plus 1,417 additional probes that brought the theoretical ORF coverage of these arrays up to 97.4% for B. pertussis Tohama, 98.5% for B. bronchiseptica RB50, and 97.9% for B. parapertussis 12822. Like the previously used array probes, these additional probes were PCR products with a size of less than 300 base pairs and amplified from the sequenced reference genomes with ORF-specific oligonucleotides (Illumina, San Diego, California, United States) designed with Microarray Architect (C. A. Cummings and D. A. Relman, unpublished data).
A total of 26 complex I and 13 complex IV strains were hybridized to the arrays. The genomic DNA of B. bronchiseptica was labeled with Cy5 and hybridized to the array in conjunction with a Cy3-labeled genomic DNA reference comprising the three sequenced mammalian Bordetella genomes (B. pertussis Tohama, B. parapertussis 12822, and B. bronchiseptica RB50). For the list of strains analyzed by CGH, see Table S1. Labeled probes were purified using the Cyscribe GFX Purification Kit (Amersham Biosciences, Freiburg, Germany) following the manufacturer's protocol for probes produced by the CyScribe First-Strand cDNA Labelling Kit. After purification, the test and reference-labeled DNA samples were concentrated to less than 8.5 μl using a Savant SpeedVac SVC-100H. The test and reference samples were combined and 150 μg of yeast tRNA (Invitrogen Life Technologies, San Diego, California, United States) was added to block nonspecific binding. The probe volume was adjusted to 24 μl with water and then 5.1 μl of 20× SSC (1× SSC = 0.15 M NaCl plus 0.015 M sodium citrate) and 0.9 μl of 10% sodium dodecyl sulfate (SDS) were added. Thirty microliters of the probe was added to the array and covered with a 25 × 40 mm No. 1 glass coverslip. Hybridization was performed in GeneMachines Hybchambers (Genomic Solutions, Ann Arbor, Michigan, United States) with 2× 30 μl of 3× SSC to maintain humidity and incubated at 65 °C overnight.
Arrays were washed in 0.5× SSC, 0.03% SDS for 30 s, 0.1× SSC, 0.01% SDS for 30 s, 0.05× SSC, 0.005% SDS for 1 min, and 0.025× SSC for 1 min. The first wash was performed at 65 °C, and the remaining washes were performed at room temperature. Slides were dried using a Quick-Dry Filtered Air Gun (Matrix Technologies Corporation, Hudson, New Hampshire, United States). Images were acquired on a PerkinElmer ScanArray 4000XL scanner using Scanarray Express software (PerkinElmer Life and Analytical Sciences, Inc., Boston, Massachusetts, United States). Images were analyzed with GenePix Pro software (Axon Instruments, Union City, California, United States).
Processed two-color array image data were submitted to an in-house microarray database. Data were extracted using filters to eliminate automatically and manually flagged spots and spots with very low background subtracted signal intensity (<150) in the reference channel. B. bronchiseptica complex I and complex IV enriched sequences were identified using the Significance Analysis for Microarrays software (SAM) [57]. The probes were analyzed using 26 complex I and 13 complex IV strains that were hybridized to the arrays. SAM analysis was run using the two-class option with KNN missing value imputation. In addition to a statistically significant difference, a 2-fold difference in mean signal intensity ratio for each probe was also required.
Supporting Information
Dataset S1. CGH Data of the Mammalian Bordetellae.
(5.0 MB XLS)
Dataset S2. CGH Data of the Differentially Hybridizing Probes between Complexes I and IV as Identified by SAM.
(207 KB XLS)
Table S1. Characteristics of the Strains Used in the MLST Analysis.
(16 KB PDF)
Table S2. Probes That Hybridized Differentially to B. bronchiseptica Complex I and IV Genomes as Determined by SAM Analysis.
(19 KB PDF)
Table S3. Primer Characteristics for the Genes Used in Multilocus Sequence Typing, Pertactin Sequencing, and the Detection of the Insertion Sequence Elements.
(41 KB PDF)
Accession Numbers
The nucleotide sequences of pertactin have been deposited in GenBank (http://www.ncbi.nlm.nih.gov/Genbank) under accession numbers DQ141700 through DQ141711 and DQ141713 through DQ141816.
Acknowledgments
We are grateful to Dr. Geoffrey Foster (SAC Veterinary Science Division, Inverness) and to Dr. Gary Sanders (Centers for Disease Control and Prevention, Atlanta, Georgia, United States) for providing B. bronchiseptica strains. We thank Dr. Eric Harvill (Penn State University, Pennsylvania, United States) for sharing unpublished data and discussions and Ing. Marjolein van Gent, Ing. Betsy Kuipers, and Ing. Hendrik-Jan Hamstra for assistance and introduction to LPS work. We also thank Dr. Martin Maiden and Dr. Keith Jolley for assistance with setting up the Bordetella MLST database. This work was supported by a travel grant from Netherlands Organization for Scientific Research (NWO). CAC was supported by an American Lung Association Research Training Fellowship. DAR received grant support from the National Institutes of Health (grants AI54970 and AI057188).
Abbreviations
CGH
comparative genomic hybridization
ISE
insertion sequence element
LCA
last common ancestor
LPS
lipopolysaccharide
mAb
monoclonal antibody
Mya
million years ago
MLST
multilocus sequence typing
MST
minimum spanning tree
ST
sequence type
Footnotes
Competing interests. The authors have declared that no competing interests exist.
Author contributions. DAD and FRM conceived and designed the experiments. DAD performed the experiments. DAD, CAC, LMS, and MMB analyzed the data. CAC and LMS contributed reagents/materials/analysis tools. DAD, CAC, LMS, DAR, and FRM wrote the paper.
References
- Gueirard P, Weber C, Le Coustumier A, Guiso N. Human Bordetella bronchiseptica infection related to contact with infected animals: Persistence of bacteria in host. J Clin Microbiol. 1995;33:2002–2006. doi: 10.1128/jcm.33.8.2002-2006.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauwens JE, Spach DH, Schacker TW, Mustafa MM, Bowden RA. Bordetella bronchiseptica pneumonia and bacteremia following bone marrow transplantation. J Clin Microbiol. 1992;30:2474–2475. doi: 10.1128/jcm.30.9.2474-2475.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolfrey BF, Moody JA. Human infections associated with Bordetella bronchiseptica . Clin Microbiol Rev. 1991;4:243–255. doi: 10.1128/cmr.4.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zee A, Groenendijk H, Peeters M, Mooi FR. The differentiation of Bordetella parapertussis and Bordetella bronchiseptica from humans and animals as determined by DNA polymorphism mediated by two different insertion sequence elements suggests their phylogenetic relationship. Int J Syst Bacteriol. 1996;46:640–647. doi: 10.1099/00207713-46-3-640. [DOI] [PubMed] [Google Scholar]
- Mooi FR, van Loo IH, King AJ. Adaptation of Bordetella pertussis to vaccination: A cause for its reemergence? Emerg Infect Dis. 2001;7((3 Suppl)):526–528. doi: 10.3201/eid0707.017708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowcroft NS, Britto J. Whooping cough—A continuing problem. BMJ. 2002;324:1537–1538. doi: 10.1136/bmj.324.7353.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orenstein WA. Pertussis in adults: Epidemiology, signs, symptoms, and implications for vaccination. Clin Infect Dis. 1999;28((Suppl 2)):S147–S150. doi: 10.1086/515061. [DOI] [PubMed] [Google Scholar]
- Musser JM, Hewlett EL, Peppler MS, Selander RK. Genetic diversity and relationships in populations of Bordetella spp. J Bacteriol. 1986;166:230–237. doi: 10.1128/jb.166.1.230-237.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zee A, Mooi F, van Embden J, Musser J. Molecular evolution and host adaptation of Bordetella spp.: Phylogenetic analysis using multilocus enzyme electrophoresis and typing with three insertion sequences. J Bacteriol. 1997;179:6609–6617. doi: 10.1128/jb.179.21.6609-6617.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhill J, Sebaihia M, Preston A, Murphy LD, Thomson N, Harris DE, et al. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica . Nat Genet. 2003;35:32–40. doi: 10.1038/ng1227. [DOI] [PubMed] [Google Scholar]
- Maiden MC, Bygraves JA, Feil E, Morelli G, Russell JE, Urwin R, et al. Multilocus sequence typing: A portable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad Sci U S A. 1998;95:3140–3145. doi: 10.1073/pnas.95.6.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings CA, Brinig MM, Lepp PW, Van De PS, Relman DA. Bordetella species are distinguished by patterns of substantial gene loss and host adaptation. J Bacteriol. 2004;186:1484–1492. doi: 10.1128/JB.186.5.1484-1492.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouls LM, van der Heide HGJ, Vauterin L, Vauterin P, Mooi FR. Multiple-locus variable-number tandem repeat analysis of Dutch Bordetella pertussis strains reveals rapid genetic changes with clonal expansion during the late 1990s. J Bacteriol. 2004;186:5496–5505. doi: 10.1128/JB.186.16.5496-5505.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leininger E, Roberts M, Kenimer JG, Charles IG, Fairweather N, Novotny P, et al. Pertactin, an Arg-Gly-Asp-containing Bordetella pertussis surface protein that promotes adherence of mammalian cells. Proc Natl Acad Sci U S A. 1991;88:345–349. doi: 10.1073/pnas.88.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles IG, Dougan G, Pickard D, Chatfield S, Smith M, Novotny P, et al. Molecular cloning and characterization of protective outer membrane protein P.69 from Bordetella pertussis . Proc Natl Acad Sci U S A. 1989;86:3554–3558. doi: 10.1073/pnas.86.10.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLafferty MA, Harcus DR, Hewlett EL. Nucleotide sequence and characterization of a repetitive DNA element from the genome of Bordetella pertussis with characteristics of an insertion sequence. J Gen Microbiol. 1988;134((Pt 8)):2297–2306. doi: 10.1099/00221287-134-8-2297. [DOI] [PubMed] [Google Scholar]
- Jukes TH, Cantor CR. Mammalian protein metabolism. New York: Academic; 1969. 132. p. [Google Scholar]
- Achtman M, Zurth K, Morelli G, Torrea G, Guiyoule A, Carniel E. Yersinia pestis, the cause of plague, is a recently emerged clone of Yersinia pseudotuberculosis . Proc Natl Acad Sci U S A. 1999;96:14043–14048. doi: 10.1073/pnas.96.24.14043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittam TS. Escherichia and Salmonella cellular and molecular biology. Washington (DC): American Society of Microbiologists; 1996. 2,720 p. [Google Scholar]
- Guttman DS, Dykhuizen DE. Clonal divergence in Escherichia coli as a result of recombination, not mutation. Science. 1994;266:1380–1383. doi: 10.1126/science.7973728. [DOI] [PubMed] [Google Scholar]
- Finn TM, Stevens LA. Tracheal colonization factor: A Bordetella pertussis secreted virulence determinant. Mol Microbiol. 1995;16:625–634. doi: 10.1111/j.1365-2958.1995.tb02425.x. [DOI] [PubMed] [Google Scholar]
- Kotob SI, Hausman SZ, Burns DL. Localization of the promoter for the ptl genes of Bordetella pertussis, which encode proteins essential for secretion of pertussis toxin. Infect Immun. 1995;63:3227–3230. doi: 10.1128/iai.63.8.3227-3230.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss AA, Johnson FD, Burns DL. Molecular characterization of an operon required for pertussis toxin secretion. Proc Natl Acad Sci U S A. 1993;90:2970–2974. doi: 10.1073/pnas.90.7.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arico B, Rappuoli R. Bordetella parapertussis and Bordetella bronchiseptica contain transcriptionally silent pertussis toxin genes. J Bacteriol. 1987;169:2847–2853. doi: 10.1128/jb.169.6.2847-2853.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe T, White KA, Allen AG, Peacock M, Raetz CR, Maskell DJ. Bordetella pertussis waaA encodes a monofunctional 2-keto-3-deoxy-D-manno-octulosonic acid transferase that can complement an Escherichia coli waaA mutation. J Bacteriol. 1999;181:2648–2651. doi: 10.1128/jb.181.8.2648-2651.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston A, Allen AG, Cadisch J, Thomas R, Stevens K, Churcher CM, et al. Genetic basis for lipopolysaccharide O-antigen biosynthesis in Bordetellae. Infect Immun. 1999;67:3763–3767. doi: 10.1128/iai.67.8.3763-3767.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston A, Maskell D. The molecular genetics and role in infection of lipopolysaccharide biosynthesis in the Bordetellae. J Endotoxin Res. 2001;7:251–261. [PubMed] [Google Scholar]
- Allen AG, Isobe T, Maskell DJ. Identification and cloning of waaF (rfaF) from Bordetella pertussis and use to generate mutants of Bordetella spp. with deep rough lipopolysaccharide. J Bacteriol. 1998;180:35–40. doi: 10.1128/jb.180.1.35-40.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A, Maskell D. The identification, cloning and mutagenesis of a genetic locus required for lipopolysaccharide biosynthesis in Bordetella pertussis . Mol Microbiol. 1996;19:37–52. doi: 10.1046/j.1365-2958.1996.354877.x. [DOI] [PubMed] [Google Scholar]
- Peppler MS. Two physically and serologically distinct lipopolysaccharide profiles in strains of Bordetella pertussis and their phenotype variants. Infect Immun. 1984;43:224–232. doi: 10.1128/iai.43.1.224-232.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D, Peppler MS, Brodeur BR. Immunological characterization of the lipooligosaccharide B band of Bordetella pertussis . Infect Immun. 1992;60:2718–2725. doi: 10.1128/iai.60.7.2718-2725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman JT, Kuipers B, Vogel ML, Hamstra HJ, Nagel J. Description of a hybridoma bank towards Bordetella pertussis toxin and surface antigens. Microb Pathog. 1990;8:377–382. doi: 10.1016/0882-4010(90)90024-k. [DOI] [PubMed] [Google Scholar]
- van den Akker WM. Lipopolysaccharide expression within the genus Bordetella: Influence of temperature and phase variation. Microbiology. 1998;144((Pt 6)):1527–1535. doi: 10.1099/00221287-144-6-1527. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Cases in which parsimony or compatibility methods will be positively misleading. Systematic Biol. 1978;27:401–410. [Google Scholar]
- van Loo IH, van der Heide HG, Nagelkerke NJ, Verhoef J, Mooi FR. Temporal trends in the population structure of Bordetella pertussis during 1949–1996 in a highly vaccinated population. J Infect Dis. 1999;179:915–923. doi: 10.1086/314690. [DOI] [PubMed] [Google Scholar]
- Mooi FR, van Oirschot H, Heuvelman K, van der Heide HG, Gaastra W, Willems RJ. Polymorphism in the Bordetella pertussis virulence factors P.69/pertactin and pertussis toxin in The Netherlands: Temporal trends and evidence for vaccine-driven evolution. Infect Immun. 1998;66:670–675. doi: 10.1128/iai.66.2.670-675.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhill J, Wren BW, Thomson NR, Titball RW, Holden MT, Prentice MB, et al. Genome sequence of Yersinia pestis, the causative agent of plague. Nature. 2001;413:523–527. doi: 10.1038/35097083. [DOI] [PubMed] [Google Scholar]
- Chain PS, Carniel E, Larimer FW, Lamerdin J, Stoutland PO, Regala WM, et al. Insights into the evolution of Yersinia pestis through whole-genome comparison with Yersinia pseudotuberculosis . Proc Natl Acad Sci U S A. 2004;101:13826–13831. doi: 10.1073/pnas.0404012101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierman WC, DeShazer D, Kim HS, Tettelin H, Nelson KE, Feldblyum T, et al. Structural flexibility in the Burkholderia mallei genome. Proc Natl Acad Sci U S A. 2004;101:14246–14250. doi: 10.1073/pnas.0403306101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashimoto T, Katahira J, Cornejo WR, Masuda M, Fukuoh A, Matsuzawa T, et al. Identification of functional domains of Bordetella dermonecrotizing toxin. Infect Immun. 1999;67:3727–3732. doi: 10.1128/iai.67.8.3727-3732.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockmeier SL, Register KB, Magyar T, Lax AJ, Pullinger GD, Kunkle RA. Role of the dermonecrotic toxin of Bordetella bronchiseptica in the pathogenesis of respiratory disease in swine. Infect Immun. 2002;70:481–490. doi: 10.1128/IAI.70.2.481-490.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo S, Yuk MH, Huang LL, Miller JF. Regulation of type III secretion in Bordetella . Mol Microbiol. 2004;52:1201–1214. doi: 10.1111/j.1365-2958.2004.04053.x. [DOI] [PubMed] [Google Scholar]
- Gupta S, Maiden MC, Feavers IM, Nee S, May RM, Anderson RM. The maintenance of strain structure in populations of recombining infectious agents. Nat Med. 1996;2:437–442. doi: 10.1038/nm0496-437. [DOI] [PubMed] [Google Scholar]
- Bjornstad ON, Harvill ET. Evolution and emergence of Bordetella in humans. Trends Microbiol. 2005;13:355–359. doi: 10.1016/j.tim.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Major RH. Classic descriptions of disease. Springfield (Illinois): C. C. Thomas; 1945. [Google Scholar]
- Lapin JH. Whooping cough. Springfield (Illinois): C. C. Thomas; 1943. [Google Scholar]
- Still GF. The history of paediatrics. London: Oxford University Press; 1931. 526. p. [Google Scholar]
- Gutierrez MC, Brisse S, Brosch R, Fabre M, Omais B, Marmiesse M, et al. Ancient origin and gene mosaicism of the progenitor of Mycobacterium tuberculosis . PLoS Pathog. 2005;1:e5. doi: 10.1371/journal.ppat.0010005. DOI: 10.1371/journal.ppat.0010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards KM, Decker MD. Pertussis vaccine. In: Plotkin SA, Orenstein WA, editors. Vaccines. Philadelphia: WB Saunders; 2004. pp. 2708–2720. [Google Scholar]
- David S, van Furth R, Mooi FR. Efficacies of whole cell and acellular pertussis vaccines against Bordetella parapertussis in a mouse model. Vaccine. 2004;22:1892–1898. doi: 10.1016/j.vaccine.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Jolley KA, Chan MS, Maiden MC. mlstdbNet—Distributed multi-locus sequence typing (MLST) databases. BMC Bioinformatics. 2004;5:86. doi: 10.1186/1471-2105-5-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zee A, Agterberg C, Peeters M, Schellekens J, Mooi FR. Polymerase chain reaction assay for pertussis: Simultaneous detection and discrimination of Bordetella pertussis and Bordetella parapertussis . J Clin Microbiol. 1993;31:2134–2140. doi: 10.1128/jcm.31.8.2134-2140.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesse AJ, Campagnari AA, Bittner WE, Apicella MA. Increased resolution of lipopolysaccharides and lipooligosaccharides utilizing tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Immunol Methods. 1990;126:109–117. doi: 10.1016/0022-1759(90)90018-q. [DOI] [PubMed] [Google Scholar]
- Tsai CM, Frasch CE. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Margalef R. Information theory in ecology. General Systems. 1958;3:36–71. [Google Scholar]
- Rozas J, Sanchez-DelBarrio JC, Messeguer X, Rozas R. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics. 2003;19:2496–2497. doi: 10.1093/bioinformatics/btg359. [DOI] [PubMed] [Google Scholar]
- Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dataset S1. CGH Data of the Mammalian Bordetellae.
(5.0 MB XLS)
Dataset S2. CGH Data of the Differentially Hybridizing Probes between Complexes I and IV as Identified by SAM.
(207 KB XLS)
Table S1. Characteristics of the Strains Used in the MLST Analysis.
(16 KB PDF)
Table S2. Probes That Hybridized Differentially to B. bronchiseptica Complex I and IV Genomes as Determined by SAM Analysis.
(19 KB PDF)
Table S3. Primer Characteristics for the Genes Used in Multilocus Sequence Typing, Pertactin Sequencing, and the Detection of the Insertion Sequence Elements.
(41 KB PDF)