Nitric oxide in the human cardiovascular system—SKB lecture 1997 (original) (raw)
Furchgott & Zawadzki demonstrated that the ability of acetylcholine to relax rabbit aorta is dependent on the presence of an intact endothelium [1]. If the endothelium is removed, the vessel no longer relaxes to acetylcholine but still responds to glyceryl trinitrate. This ‘endothelium-dependent relaxation’ to acetylcholine is mediated by the release of a labile substance which Furchgott named endothelium-derived relaxing factor, or EDRF.
Endothelium-dependent relaxation in human vessels
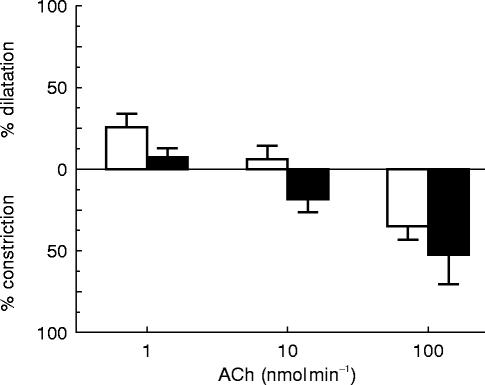
In the early 1980s endothelium-dependent relaxation was demonstrated in vitro in response to a variety of agonists—bradykinin, substance P, calcium ionophore—in blood vessels from birds, reptiles, rodents, dogs and humans [2]. In order to determine whether the phenomenon also occurred in vessels in humans in vivo experiments were undertaken in veins on the back of the hand. To remove the endothelium the vein was isolated from the circulation by means of two weighted occluding wedges and distilled water was circulated through the isolated segment. This manoeuvre causes osmotic lysis of the fragile endothelial cells whilst leaving the more robust smooth muscle cells intact [3]. These studies demonstrated that in the healthy hand vein, acetylcholine produces a biphasic response comprising dilatation at low doses and constriction at higher doses [4]. Once the endothelium is removed, the dilator response disappears and the constrictor response is enhanced (Figure 1). The dilatation to acetylcholine is entirely dependent upon the presence of an intact endothelium whereas the constriction is due to a direct effect on smooth muscle. Thus what Furchgott & Zawadzki had seen in the rabbit aorta in vitro occurs also in a human blood vessel in vivo.
Figure 1.
Adapted from ref 4. Acetylcholine (ACh) produces a biphasic response when infused into a preconstricted hand vein in vivo. At low doses dilatation is seen but as the dose is increased constriction occurs. Removal of the endothelium with distilled water abolishes the dilatation and enhances the constriction. l-NMMA produced similar effects to endothelial denudation. □ endothelium intact; ▪ endothelium denuded
Nitric oxide
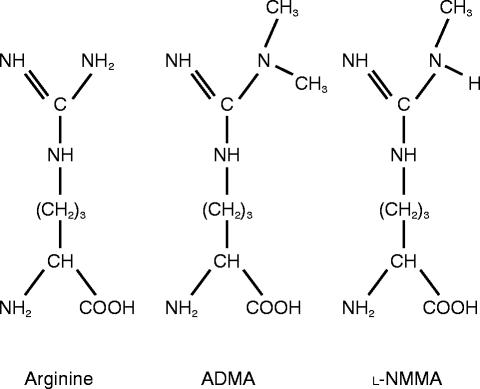
Six years after his initial discovery Furchgott suggested that EDRF might be nitric oxide and within a year of this suggestion two groups had confirmed that release of nitric oxide accounts for the biological activity of EDRF [5, 6]. Moncada's group demonstrated that endothelial cells synthesize nitric oxide from the amino acid l-arginine and that the synthetic process is inhibited by an analogue of l-arginine in which one of the guanidino nitrogen atoms is methylated (NG monomethyl-l-arginine; l-NMMA; Figure 2) [7].
Figure 2.
Structures of arginine (substrate for nitric oxide synthase) and two inhibitors, asymmetric dimethylarginine (ADMA) and NG monomethyl-arginine (l-NMMA). l-NMMA and ADMA are both naturally occurring compounds that compete with arginine and inhibit nitric oxide synthesis. Another endogenous compound, symmetric dimethylarginine (SDMA) in which methyl groups are present on each of the guanidino nitrogen atoms does not act as an inhibitor of nitric oxide synthesis
Infusion of l-NMMA into the hand veins of volunteers mimics endothelial denudation; the dilator response to acetylcholine is lost and the constrictor response enhanced, whereas the dilatation to glyceryl trinitrate remains unaffected [8]. The effect of l-NMMA is specific for the l-arginine:nitric oxide pathway since the inactive stereoisomer (d-NMMA) is without effect and the inhibitory actions of l-NMMA are reversed by l-arginine. Recently, electrochemical systems for detecting nitric oxide have been developed and it has been possible to record in the hand vein the burst of nitric oxide generated in response to acetylcholine, and demonstrate the inhibition of this process by l-NMMA [9]. It is clear that EDRF is generated in human hand veins in vivo, that it mediates the dilator effects of acetylcholine and that nitric oxide generated from l-arginine accounts for the action of EDRF in these vessels.
Basal generation of nitric oxide in the arterial system
To explore the l-arginine:nitric oxide pathway in the arterial system l-NMMA was infused directly into the brachial artery and forearm blood flow assessed by venous occlusion plethysmography [10]. Flow through the forearm is predominantly through skeletal muscle and changes in flow at constant perfusion pressure represent changes in the contractile state of resistance vessels supplying skeletal muscle [11]. Infusion of l-NMMA attenuated but did not block completely the dilator response to acetylcholine. Subsequent studies have confirmed this and at the level of resistance vessels it seems that only part of the response to acetylcholine is mediated by nitric oxide. Furthermore, some muscarinic agonists appear to induce vasodilatation which is largely independent of nitric oxide [12, 13]. Presumably acetylcholine acts on multiple muscarinic receptor subtypes in the forearm arterial bed, some of which are coupled to the l-arginine:nitric oxide pathway whilst others relax or contract smooth muscle through alternative mechanisms. It has been suggested that the forearm arterial vessels are supplied by cholinergic dilator nerves [14, 15] and certain other vessels innervated by cholinergic nerves show an endothelium-independent response to acetylcholine [16]. Thus the response to acetylcholine in the forearm bed is complex and should not be taken as a simple measure of nitric oxide generation.
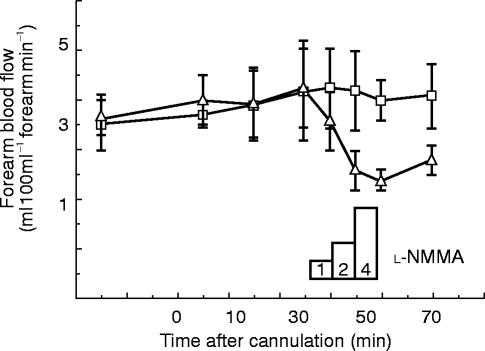
In addition to attenuating the response to acetylcholine, l-NMMA reduces basal (resting) blood flow (Figure 3) [10]. Indeed, blockade of nitric oxide synthesis in the forearm causes a halving of resting flow—a doubling of vascular resistance. Basal generation of nitric oxide is not unique to the forearm resistance vessels, it occurs in virtually every arterial bed that has been studied (including cerebral, renal, mesenteric and cardiac), and systemic blockade of nitric oxide generation causes an immediate hypertension [17–20], indicating that normal arterial pressure is kept down by the continuous generation of nitric oxide in resistance vessels [10]. The only other endogenous mediator that exerts such a profound and rapidly modulating local effect on vascular tone is noradrenaline released from sympathetic nerves (the constrictor peptide endothelin also appears to provide a more slowly modulating influence). Blocking sympathetic neurotransmission leads to a 2–3 fold reduction in vascular resistance whereas blockade of endothelium-derived nitric oxide doubles it.
Figure 3.
The effects of l-NMMA on resting forearm blood flow. l-NMMA was infused into one arm and the other arm acted as a control. l-NMMA produced a dose-dependent fall in blood flow as endogenous nitric oxide synthesis was blocked
Biochemical assessment of nitric oxide in vivo
Biochemical assessment of nitric oxide in vivo is not straightforward. Nitric oxide is a labile species with a half-life of only a few seconds in biological systems. In cell culture systems NO degrades rapidly to NO2− (nitrite) but in the presence of Fe2+haem, or certain other transition metals, NO2− is converted to the more stable product NO3− (nitrate). Thus in vivo, nitrite is unstable and has a short half-life and the major ion is nitrate. However, the metabolic fate of endogenous NO is surprisingly poorly understood. Putative intermediate metabolites include an array of low and high molecular weight thiols—nitrosoglutathione, nitrosoalbumin, S-nitrosohaemoglobin [21]—some of which might be present in sufficient quantities to exert biological effects in their own right. Furthermore, NO reacts with another endogenous radical, superoxide anion (O2−), to form peroxynitrite (ONOO−). Peroxynitrite may isomerase to yield nitrate ion or might nitrate tyrosine residues on proteins [22]. The extent to which this occurs in vivo, whether it is a major route of metabolism for endogenous NO, and the routes of metabolism of nitrated proteins remain largely unknown.
Measurement of nitrite or nitrosothiols provides a snapshot of NO in an intermediary state of metabolism and the concentration present will depend on rates of synthesis and destruction/elimination. Currently it is not known what proportion of NO goes through which particular pathway and the concentrations of these intermediary metabolites are unlikely to give an accurate reflection of overall NO generation. However, most intermediaries eventually yield nitrate. Nitrate is stable and is the usual choice as a measure of NO generation. In comparison with the other metabolites its concentration in biological fluids is less prone to rapid changes due to alterations in metabolism or decay during sample collection or storage, but it has the disadvantage that it is a constituent of the diet and about 50% of the nitrate present in blood comes from dietary sources. A more accurate assessment of nitric oxide generation may be made by measuring the formation of 15NO3− from 15NG-l-arginine [23–25]. However, even with this technique it is not possible to determine the cellular origins of the nitric oxide.
Cardiovascular disease
Due to the difficulties in accurately measuring biochemical generation of nitric oxide, most researchers have adopted a functional approach, assessing changes in flow in response to endothelium-dependent dilators such as acetylcholine [26–28], or in response to blockade of basal nitric oxide generation with l-NMMA [29–31]. Interpretation of the responses to acetylcholine and other muscarinic agonists is not straightforward (see above) and has been discussed in detail elsewhere [2]. The response to l-NMMA gives a measure of basal nitric oxide-mediated dilatation and has been used extensively to probe the pathway in disease states. In certain patients with essential hypertension, the constrictor response to l-NMMA appears to be diminished [29–32], suggesting that basal nitric oxide-mediated dilatation is reduced. The change is probably a reaction to the raised pressure rather than causal, since reduction of the pressure restores the response to l-NMMA towards normal [29, 30]. Recently, biochemical data have been reported that support the concept that nitric oxide generation is reduced in hypertensive individuals. The synthesis of 15NO3− from 15NG-arginine is significantly reduced in hypertensive patients when compared with normotensive controls [25]. Functional changes in nitric oxide-mediated dilatation have also been detected in patients with diabetes (type I+II) [2, 33], hypercholesterolaemia [34, 35], overt atheroma and even in smokers [36].
Reduced nitric oxide-mediated dilatation would lead to increased vascular tone and reactivity, and might result in a ‘stiffer’ vascular system, manifested by a widening of pulse pressure, and impaired ability to accommodate increases in intravascular volume. But nitric oxide is also released luminally where it inhibits platelet aggregation and adhesion [37] and prevents the adhesion of white cells to the endothelium. In experimental animals, inhibition of nitric oxide synthesis promotes atherogenesis [38, 39] and it is possible that changes in the generation or stability of nitric oxide, or alterations in the responsiveness of tissues to nitric oxide, provide a mechanism to link apparently disparate cardiovascular risk factors to the common end point of atheroma formation or vessel occlusion. Consistent with a link between nitric oxide and athrogenesis, certain polymorphisms of the gene encoding endothelial nitric oxide synthase appear to be associated with enhanced atheroma [40, 41]. One polymorphism of particular interest encodes an amino acid substitution in endothelial nitric oxide synthase (Figure 4) [41]. Although the effects of this variation on enzyme function are not yet known, epidemiological studies have identified an association with cardiovascular diseases, particularly enhanced atherogenesis.
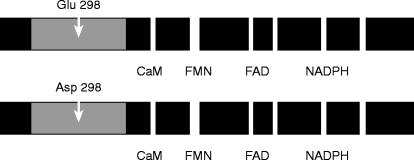
Figure 4.
A common polymorphism of the endothelial nitric oxide synthase (eNOS) gene has been identified (ref 41). About 10% of the population are homozygous for the Asp (298) variant, the remainder being either homozygous for the usual Glu (298) form or heterozygotes. The amino acid substitution occurs in the region of the protein thought to be responsible for binding arginine and the cofactor tetrahydrobiopterin. Although it is not yet known how the amino acid change affects enzyme function, individuals homozygous for the Asp variant appear to be at increased risk of coronary artery disease (ref 41). Other functional domains shown are the calmodulin binding site (CaM), the binding sites for flavins (FMN, FAD) and the NADPH site
Increasing nitric oxide delivery
Experimental data from animals and humans suggest that a reduction in functional activity of the l-arginine:nitric oxide pathway may contribute to vascular disease. Possible therapeutic strategies to restore nitric oxide-mediated effects include provision of excess l-arginine, stimulation of the endogenous pathway, prolongation of the half-life of nitric oxide by removing superoxide, the use of nitric oxide donors or the development of guanylyl cyclase activators. Full discussion of these options is outside the scope of this article but two areas are of particular relevance in relation to clinical studies—the use of l-arginine and nitric oxide donors.
l-arginine
The K m of nitric oxide synthases for l-arginine is in the order of 1–2 μm. The concentration of arginine in blood is in the order of 40–90 μm and the concentration within cells may reach 1 mm [42]. Theoretically the supply of arginine should not be rate-limiting for nitric oxide generation. Nonetheless a number of studies report cardiovascular effects of arginine which are assumed to be mediated by nitric oxide [43–45]. Many investigators have used doses of arginine in the order of 30 g. This increases the plasma concentration of arginine to about 10 mm. At these sorts of concentrations arginine causes vascular relaxation and stimulates the release of a variety of hormones including insulin, growth hormone and prolactin [42, 46]. However, these effects are not stereospecific and d-arginine is as effective as l-arginine [42, 46]. Since d-arginine is not a substrate for nitric oxide synthase it seems unlikely that the effects seen are due to provision of excess substrate for nitric oxide generation and they appear to represent some other action of arginine—possibly related to its antioxidant actions. Consistent with the idea that some of the actions of arginine are independent of its role as substrate for nitric oxide generation, other basic amino acids, including lysine also produce cardiovascular [47] and hormonal effects at high concentrations.
Despite these concerns and theoretical considerations, there is one situation in which provision of arginine does appear to increase nitric oxide-mediated dilatation. In patients with hypercholesterolaemia l-arginine restores the dilator response to acetylcholine and the effect is stereospecific [34, 35]. The mechanism remains to be determined but it is possible that the K m of nitric oxide synthase for arginine changes in hypercholesterolaemia or that endogenous inhibitors (see below) block or modify the arginine binding site of the enzyme. Whatever the mechanism, the action is potentially useful and clinical trials are underway to determine the effects of long-term arginine supplementation in patients with raised cholesterol levels.
Nitric oxide donors
Nitric oxide is the active agent formed when amyl nitrite, glyceryl trinitrate and other nitrovasodilators are metabolized within the body [48]. These drugs, which have been in clinical use for well over 100 years, are pro-drugs; donors of nitric oxide, that mimic the endogenous mediator [48]. These nitric oxide donors preferentially dilate veins, possibly because veins do not generate endogenous nitric oxide basally and therefore the guanylyl cyclase is up-regulated. The challenge is to design novel nitric oxide donors or activators of guanylyl cyclase that differ from existing drugs and show selectivity for individual cell types (platelets, white cells, etc.) or certain tissues.
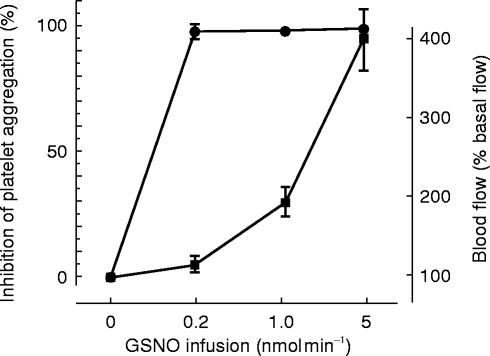
One class of experimental nitric oxide donor does appear to differ significantly from clinically used drugs. Nitrosothiols donate NO+- like species and the agent which has been studied in humans is nitrosoglutathione [49–51]. This compound is significantly less venoselective than existing drugs and has very marked antiplatelet effects. When infused into the forearm, it fully inhibits platelet aggregation at doses that barely alter blood flow (Figure 5). Similarly, the compound inhibits the activation of platelets that occurs in the coronary circulation following angioplasty [51]. In contrast, drugs like glyceryl trinitrate have minimal if any antiplatelet action in therapeutic doses. The nitrosothiols are useful pharmacological tools and might be developed into novel therapies to achieve arterial dilatation coupled with antiplatelet effects.
Figure 5.
Adapted from ref 49. Nitrosoglutathione (GSNO) was infused into the brachial artery of healthy volunteers. Blood flow (▪) was measured by plethysmography and platelet aggregation (•) was assessed in blood draining the arm. At low doses GSNO preferentially inhibited platelet aggregation. The antiplatelet effect of nitric oxide donors is prominent in nitrosothiols but largely absent in conventional drugs such as glyceryl trinitrate
Endogenous inhibitors of nitric oxide synthesis
l-NMMA and arginine are closely related compounds (Figure 2) and it seemed possible that l-NMMA might occur endogenously. With a novel assay small amounts of endogenous l-NMMA were detected in plasma and urine and two other compounds with similar physicochemical properties were found [52]. Using mass spectrometry and NMR they were identified as asymmetric and symmetric dimethylarginine (ADMA and SDMA) [52]. ADMA inhibits the activity of purified nitric oxide synthase, causes endothelium-dependent contractions of rat aorta (due to inhibition of basal nitric oxide), increases blood pressure in animals and reduces resting forearm blood flow in a manner similar to l-NMMA [52]. In summary, in human plasma and in urine there is a compound, ADMA, that is a specific and selective inhibitor of nitric oxide synthase in vitro and in vivo, in animals and humans. It is not yet known whether ADMA acts physiologically to reduce nitric oxide generation, but circulating levels of dimethylarginines are increased in certain disease states (most notably in uraemia [52] and hypercholesterolaemia [53, 54]) and might contribute to the pathophysiology of these conditions. Furthermore, accumulation of ADMA sufficient to inhibit nitric oxide synthase would also have the effect of making the generation of nitric oxide critically dependent on the prevailing concentration of arginine [55].
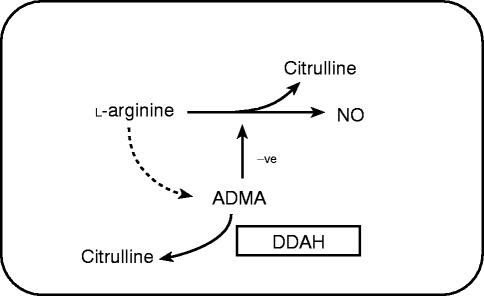
More recently, it has been demonstrated that endothelial cells synthesize ADMA from arginine and metabolize ADMA to citrulline [55, 56]. The enzyme responsible for the metabolism is dimethylarginine dimethylaminohydrolase (DDAH) (Figure 6). This enzyme has been sequenced and cloned [57] and an inhibitor has been identified [56], Inhibition of DDAH leads to accumulation of ADMA within the endothelial cell and thereby causes indirect inhibition of nitric oxide generation. It remains to be determined whether changes in activity or expression of DDAH contribute to the alterations of ADMA levels reported in disease states and how this might affect the output of nitric oxide. When interpreting the likely significance of plasma concentrations of endogenous methylarginines it is important to note that l-NMMA, ADMA and SDMA are actively transported into cells through the Y+ cationic amino acid transporter and the concentrations inside cells are higher than those outside [58]. This transport process is up-regulated by certain pro-inflammatory cytokines [58].
Figure 6.
l-arginine is metabolized to nitric oxide. However, after incorporation into protein it is methylated by the action of protein methylase I to form ADMA. ADMA acts as an inhibitor of nitric oxide synthase. The ADMA is metabolized to citrulline by the enzyme dimethylarginine dimethylaminohydrolase. The regulation of ADMA levels may provide a mechanism for altering nitric oxide synthesis in health or disease
Overproduction of nitric oxide
It is clear that underproduction of nitric oxide might contribute to disease, but what of overproduction? Three isoforms of nitric oxide synthase have been identified; these are an endothelial isoform (eNOS; gene located on chromosome 7), a neuronal isoform (nNOS; gene located on chromosome 12) and a macrophage or inducible isoform (iNOS; gene located on chromosome 17). eNOS and nNOS are normal constituents of healthy cells and both might contribute to physiological regulation of the cardiovascular system. It is assumed that the effects of l-NMMA in healthy individuals are due exclusively to inhibition of eNOS activity in endothelium. In the hand veins this is almost certainly the case, for no messenger RNA for any other isoform of NOS is present (our unpublished observations). However, it may not be true in the forearm or other vascular beds in which nitric oxide releasing (nitrergic) nerves may play a significant role. Certainly nitrergic nerves do control vascular tone in some situations; in the penis nitrergic nerves contribute to the process of erection [59] and in the brain nitrergic nerves may help couple neuronal activation to vasodilatation to increase blood flow to metabolically active areas [60]. To determine the role of nNOS in cardiovascular control in humans it will be necessary to develop selective inhibitors of this isoform.
In terms of over-production of nitric oxide it is iNOS that has received most attention [61]. This isoform is considered part of the host-defence reaction and is expressed in a wide variety of cell types once they have been exposed to bacterial endotoxin and/or inflammatory cytokines. If rat blood vessels are exposed to endotoxin, they gradually lose tone and become hyporesponsive to vasoconstrictors [62]. This is because iNOS is synthesized de novo and expressed throughout the vessel wall, in muscle layers as well as endothelium. Once expressed iNOS generates large quantities of nitric oxide. This mechanism has been implicated in the pathogenesis of septic shock in patients.
There is biochemical evidence of increased generation of nitric oxide during systemic inflammatory responses in humans. The circulating concentrations of nitrate or 24 h urinary excretion of nitrate are elevated in patients with fever, in septic shock and following immunotherapy with inflammatory cytokines [61]. Furthermore injection of l-NMMA restores blood pressure to normal in patients who are resistant to conventional pressor agents [63, 64]. Whilst these studies indicate that nitric oxide contributes to vasodilatation in septic shock, and suggest the possibility for novel treatments, they do not clearly identify iNOS as the source of the nitric oxide since l-NMMA can inhibit all three isoforms of NOS. Indeed human iNOS remains something of an enigma. It is easy to induce iNOS in rodents but difficult to do so in human cells and tissues at least in vitro [65]. eNOS and nNOS are highly conserved between species, yet iNOS is not and appears to be evolving more rapidly [65].
To try to explore the mechanisms by which sepsis induces nitric oxide generation in humans an experimental model has been developed in the hand vein. Endotoxin or various pro-inflammatory cytokines are instilled into the vein for 1h and dose-response curves to noradrenaline constructed at various times thereafter. These studies demonstrate that endotoxin alone is insufficient to induce nitric oxide generation [66], but the pro-inflammatory interleukin-1 (IL-1β) is effective. A 1h exposure to IL-1β produces a slowly developing hyporesponsiveness to constrictors that is prevented by prior treatment with glucocorticoids and reversed by l-NMMA. This picture is consistent with induction of nitric oxide generation. It should be possible to use this or similar models to explore the molecular basis of inflammatory and infective vasodilatation in a controlled and safe fashion in humans.
Conclusions
The discovery of nitric oxide has changed the way in which vascular control is perceived. The basal nitric oxide-mediated dilator tone exerts a powerful influence on vascular behaviour and changes in its magnitude appear to contribute to disease states. There are many novel targets for drug treatments—for example, nitric oxide donors or stimulants for the prevention or treatment of atheromatous, vasospastic or thrombotic disorders; and inhibitors of nitric oxide synthesis for septic shock or inflammatory vasodilatation. Over the next decade it will become clear whether the experimental promise translates into clinically efficacious new therapies.
Acknowledgments
The work described in the lecture was funded largely by the British Heart Foundation and Wellcome Trust. I owe a debt of gratitude to my teachers, Joe Collier and Salvador Moncada and to the many colleagues and students who contributed to the research. Parts of this article are based upon an early paper (Vallance P. Exploring vascular nitric oxide in health and disease; Goulstonian Lecture 1996. J R Coll Phys 1997; 13: 321–327.)
References
- 1.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 2.Calver A, Collier J, Vallance P. Nitric oxide cardiovascular control. Exp Phys. 1993;78:303–326. doi: 10.1113/expphysiol.1993.sp003687. [DOI] [PubMed] [Google Scholar]
- 3.Collier J, Vallance P. EDRF is an endogenous vasodilator in man. Br J Pharmacol. 1989;97:639–641. doi: 10.1111/j.1476-5381.1989.tb11998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vallance P, Collier J. Biphasic response to acetylcholine in human veins; the role of the endothelium. Clin Sci. 1990;78:101–104. doi: 10.1042/cs0780101. [DOI] [PubMed] [Google Scholar]
- 5.Palmer R, Ferrige A, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 6.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci (USA) 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmer R, Ashton D, Moncada S. Vascular endothelial cells synthesize nitric oxide from l-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 8.Vallance P, Collier J, Moncada S. Nitric oxide synthesised from l-arginine mediates endothelium-dependent dilatation in human veins in vivo. Cardiovasc Res. 1989;23:1053–1057. doi: 10.1093/cvr/23.12.1053. [DOI] [PubMed] [Google Scholar]
- 9.Vallance P, Patton S, Bhagat K, et al. Direct measurement of nitric oxide in humans. Lancet. 1995;346:153–154. doi: 10.1016/s0140-6736(95)91211-8. [DOI] [PubMed] [Google Scholar]
- 10.Vallance P, Collier J, Moncada S. Effects of endothelium-derived nitric oxide on peripheral arterial tone in man. Lancet. 1989;ii:997–1000. doi: 10.1016/s0140-6736(89)91013-1. [DOI] [PubMed] [Google Scholar]
- 11.Benjamin N, Calver A, Collier J, Robinson B, Vallance P, Webb D. Measuring forearm blood flow and interpreting the responses to drugs and mediators. Hypertension. 1995;25:918–923. doi: 10.1161/01.hyp.25.5.918. [DOI] [PubMed] [Google Scholar]
- 12.Chowienczyk PJ, Cockcroft JR, Ritter JM. Differential inhibition by NG-monomethyl-l-arginine of vasodilator effects of acetylcholine. Br J Pharmacol. 1993;110:736–738. doi: 10.1111/j.1476-5381.1993.tb13873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chowienczyk PJ, Cockcroft JR, Ritter JM. Inhibition of acetylcholinesterase selectively potentiates NG monomethyl-l-arginine-resistant actions of acetylcholine in human forearm. Clin Sci. 1995;88:111–117. doi: 10.1042/cs0880111. [DOI] [PubMed] [Google Scholar]
- 14.Blair DA, Glover WE, Greenfield ADM, Roddie IC. Excitation of cholinergic vasodilator nerves to human skeletal muscle during emotional stress. J Physiol. 1959;148:633–647. doi: 10.1113/jphysiol.1959.sp006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanders JS, Mark AL, Ferguson DW. Evidence for cholinergically-mediated vasodilation at the beginning of isometric exercise in humans. Circulation. 1989;79:815–824. doi: 10.1161/01.cir.79.4.815. [DOI] [PubMed] [Google Scholar]
- 16.Brayden JE, Large WA. Electrophysiological analysis of neurogenic vasodilation in the isolated lingual artery of the rabbit. Br J Pharmacol. 1986;89:163–171. doi: 10.1111/j.1476-5381.1986.tb11132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haynes W, Noon J, Walker B, Webb D. Inhibition of nitric oxide synthesis increases blood pressure in healthy humans. J Hypertens. 1993;11:1375–1380. doi: 10.1097/00004872-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Stamler JS, Loh E, Roddy MA, Currie KE, Creager MA. Nitric oxide regulates systemic and pulmonary vascular resistance in healthy humans. Circulation. 1994;89:2035–2040. doi: 10.1161/01.cir.89.5.2035. [DOI] [PubMed] [Google Scholar]
- 19.White RP, Vallance P, Deane C, Markus HS. Maitenance of human basal cerebral blood flow is nitric oxide dependent. In: Klingelhofer J, et al., editors. New Trends in Cerebral Hemodynamics. Elsevier (London): 1997. pp. 1–5. [Google Scholar]
- 20.Lefroy DC, Crake T, Uren NG, Davies GJ, Maseri A. Effect of inhibition of nitric oxide synthesis on epicardial coronary artery caliber and coronary blood flow in humans. Circulation. 1993;88:43–54. doi: 10.1161/01.cir.88.1.43. [DOI] [PubMed] [Google Scholar]
- 21.Jia L, Bonaventura J, Stamler JS. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature. 1996;380:221–226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- 22.Darley-Usmar V, Halliwell B. Blood radicals: reactive nitrogen species, reactive oxygen species, transition metal ions, and the vascular system. Pharm Res. 1996;13:649–662. doi: 10.1023/a:1016079012214. [DOI] [PubMed] [Google Scholar]
- 23.Castillo L, deRojas TC, Chapman TE, et al. Splanchnic metabolism of dietary arginine in relation to nitric oxide synthesis in normal adult man. Proc Natl Acad Sci (USA) 1993;90:193–197. doi: 10.1073/pnas.90.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hibbs JB, Jr, Westenfelder C, Taintor R, et al. Evidence for cytokine-inducible nitric oxide synthesis from l-arginine in patients receiving interleukin-2 therapy. J Clin Invest. 1992;89:867–877. doi: 10.1172/JCI115666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forte P, Copland M, Smith LM, Milne E, Sutherland J, Benjamin N. Basal nitric oxide synthesis in essential hypertension. Lancet. 1997;349:837–842. doi: 10.1016/S0140-6736(96)07631-3. [DOI] [PubMed] [Google Scholar]
- 26.Calver A, Collier J, Vallance P. Effects of local intra-arterial NG monomethyl-l-arginine in patients with hypertension: the nitric oxide dilator mechanism appears abnormal. J Hypertension. 1992;10:1025–1031. [PubMed] [Google Scholar]
- 27.Panza JA, Casino PR, Kilcoyne CM, Quyyumi AA. Role of endothelium-derived nitric oxide in the abnormal endothelium-dependent vascular relaxation of patients with essential hypertension. Circulation. 1993;87:1468–1474. doi: 10.1161/01.cir.87.5.1468. [DOI] [PubMed] [Google Scholar]
- 28.Lyons D, Webster J, Benjamin N. The effect of antihypertensive therapy on responsiveness to local intra-arterial NG-monomethyl-l-arginine in patients with essential hypertension. J Hypertension. 1994;12:1047–1052. [PubMed] [Google Scholar]
- 29.Panza JA, Quyyumi AA, Brush JE, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. New Engl J Med. 1990;323:22–27. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- 30.Cockcroft JR, Chowienczyk PJ, Benjamin N, Ritter JM. Preserved endothelium-dependent vasodilatation in patients with essential hypertension. New Engl J Med. 1994;330:1036–1040. doi: 10.1056/NEJM199404143301502. [DOI] [PubMed] [Google Scholar]
- 31.Taddei S, Virdis A, Ghiadoni L, Magagna A, Salvetti A. Cyclooxygenase inhibition restores nitric oxide activity in essential hypertension. Hypertension. 1997;29:274–279. doi: 10.1161/01.hyp.29.1.274. [DOI] [PubMed] [Google Scholar]
- 32.Calver A, Collier J, Vallance P. Effects of l-NMMA in patients with treated hypertension. Cardiovasc Res. 1994;28:1720–1725. doi: 10.1093/cvr/28.11.1720. [DOI] [PubMed] [Google Scholar]
- 33.Calver A, Collier J, Vallance P. Inhibition and stimulation of nitric oxide synthesis in the human forearm arterial bed of patients with insulin-dependent diabetes. J Clin Invest. 1992;90:2548–2554. doi: 10.1172/JCI116149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Creager MA, Cooke JP, Mendelsohn ME, et al. Impaired vasodilation of forearm resistance vessels in hypercholesterolemic humans. J Clin Invest. 1990;86:228–234. doi: 10.1172/JCI114688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chowienczyk PJ, Watts GF, Cockcroft JR, Ritter JM. Impaired endothelium-dependent vasodilation of forearm resistance vessels in hypercholesterolaemia. Lancet. 1992;340:1430–1432. doi: 10.1016/0140-6736(92)92621-l. [DOI] [PubMed] [Google Scholar]
- 36.Heitzer T, Yla-Herttuala S, Luoma J, et al. Cigarette smoking potentiates endothelial dysfunction of forearm resistance vessels in patients with hypercholesterolaemia: Role of oxidized LDL. Circulation. 1996;93:1346–1353. doi: 10.1161/01.cir.93.7.1346. [DOI] [PubMed] [Google Scholar]
- 37.Radomski M, Palmer R, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987;ii:1057–1058. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- 38.Cayatte A, Palacino J, Horten K, Cohen R. Chronic inhibition of nitric oxide production accelerates neointima formation and impairs endothelial function in hypercholesterolemic rabbits. Arterioscler Thromb. 1994;14:753–759. doi: 10.1161/01.atv.14.5.753. [DOI] [PubMed] [Google Scholar]
- 39.Naruse K, Shimizu K, Muramatsu M, et al. Long-term inhibition of NO synthesis promotes atherosclerosis in the hypercholesterolemic rabbit thoracic aorta. Arterioscler Thromb. 1994;14:746–752. doi: 10.1161/01.atv.14.5.746. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Sim A, Badenhop R, McCredie R, Wilcken D. A smoking-dependent risk of coronary artery disease associated with a polymorphism of the endothelial nitric oxide synthase gene. Nat Med. 1996;2:41–45. doi: 10.1038/nm0196-41. [DOI] [PubMed] [Google Scholar]
- 41.Hingorani AD, Liang C, Fatibene J, et al. A variant of the endothelial nitric oxide synthase gene is a risk factor for coronary atherosclerosis. Clin Sci. 1997;93 [Google Scholar]
- 42.MacAllister RJ, Edwards M, Herrerous B, et al. Vascular and hormonal responses to arginine: provision of susbtrate for nitric oxide or non-specific effect? Clin Sci. 1995;89:183–190. doi: 10.1042/cs0890183. [DOI] [PubMed] [Google Scholar]
- 43.Hishikawa K, Nakaki T, Suzuki H, Saruta T, Kato R. l-arginine induced hypotension. Lancet. 1991;337:683–684. doi: 10.1016/0140-6736(91)92510-9. [DOI] [PubMed] [Google Scholar]
- 44.Nakayama M, Nakano H, Yamada K, et al. Depressor effect of l-arginine in normotensive patient, on regular dialysis. Clin Nephrol. 1996;46:286. [PubMed] [Google Scholar]
- 45.Bode-Boger SM, Boger RH, Creutzig A, et al. l-arginine infusion decreases peripheral arterial resistance and inhibits platelet aggregation in healthy subjects. Clin Sci. 1994;87:303–310. doi: 10.1042/cs0870303. [DOI] [PubMed] [Google Scholar]
- 46.Calver A, Collier J, Vallance P. Dilator actions of arginine in humans peripheral vasculature. Clin Sci. 1991;81:695–700. doi: 10.1042/cs0810695. [DOI] [PubMed] [Google Scholar]
- 47.Rhodes P, Barr CS Struthers AD. Arginine, lysine and ornithine as vasodilators in the forearm of man. Eur J Clin Invest. 1996;26:325–331. doi: 10.1046/j.1365-2362.1996.144277.x. [DOI] [PubMed] [Google Scholar]
- 48.Feelisch M, Noack EA. Correlation between nitric oxide formation during degredation of organic nitrates and activation of guanylate cyclase. Eur J Pharmacol. 1987;139:19–30. doi: 10.1016/0014-2999(87)90493-6. [DOI] [PubMed] [Google Scholar]
- 49.de Belder A, MacAllister R, Radomski M, Moncada S, Vallance P. Selective inhibition of platelet aggregation by nitrosoglutathione. Cardiovasc Res. 1994;5:691–694. doi: 10.1093/cvr/28.5.691. [DOI] [PubMed] [Google Scholar]
- 50.MacAllister R, Calver A, Riezebos J, Collier J, Vallance P. Relative potency and arteriovenous selectivity of nitrovasodilators on humans blood vessels: an insight into the targeting of nitric oxide delivery. J Pharmacol Exp Ther. 1995;273:154–160. [PubMed] [Google Scholar]
- 51.Langford EJ, Brown AS, Wainwright RJ, et al. Inhibition of platelet activity by s-nitrosoglutathione during coronary angioplasty. Lancet. 1994;344:1458–1460. doi: 10.1016/s0140-6736(94)90287-9. [DOI] [PubMed] [Google Scholar]
- 52.Vallance P, Leone A, Calver A, Collier J, Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339:572–576. doi: 10.1016/0140-6736(92)90865-z. [DOI] [PubMed] [Google Scholar]
- 53.Yu XJ, Li YJ, Xiong Y. Increase of an endogenous inhibitor of nitric oxide synthesis in serum of high cholesterol fed rabbits. Life Sci. 1994;54:753–758. doi: 10.1016/0024-3205(94)00443-9. [DOI] [PubMed] [Google Scholar]
- 54.Bode-Boger SM, Boger RH, Kienke S, Junker W, Frolich JC. Eleevated l-arginine/dimethylarginine ratio contributes to enhanced systemic NO production by dietary l-arginine in hypercholesterolemic rabbits. Biochem Biophys Res Commun. 1996;219:598–603. doi: 10.1006/bbrc.1996.0279. [DOI] [PubMed] [Google Scholar]
- 55.MacAllister R, Whitley G, Parry H, et al. Regulation of nitric oxide synthase by endogenous dimethylarginine. Br J Pharmacol. 1996;119:1533–1540. doi: 10.1111/j.1476-5381.1996.tb16069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.MacAllister R, Whitley G, Vallance P. Metabolism of methylarginines by human vasculature: implications for the regulation of nitric oxide synthesis. Br J Pharmacol. 1994;112:43–48. doi: 10.1111/j.1476-5381.1994.tb13026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kimoto M, Sasakawa T, Tsuji H, et al. Cloning and sequencing of cDNA encoding NG,NG dimethylarginine dimethylaminohydrolase from rat kidney. Biochim Biophys Acta. 1997;1337:6–10. doi: 10.1016/s0167-4838(96)00196-3. [DOI] [PubMed] [Google Scholar]
- 58.Bogle RG, MacAllister RJ, Whitley G,StJ, Vallance P. Induction of NG monomethyl-l-arginine (l-NMMA) uptake: a mechanism for differential inhibition of NO synthases? Am J Physiol. 1995;269:C750–C756. doi: 10.1152/ajpcell.1995.269.3.C750. [DOI] [PubMed] [Google Scholar]
- 59.Lugg JA, Gonzalez-Cadavid NF, Rajfer J. The role of nitric oxide in erectile function. J Androl. 1995;16:2–4. [PubMed] [Google Scholar]
- 60.Toda N, Okamura T. Nitroxidergic nerve: regulation of vascular tone and blood flow in the brain. J Hypertension. 1996;14:423–434. [PubMed] [Google Scholar]
- 61.Vallance P, Moncada S. The role of endogenous nitric oxide in human septic shock. New Horizons. 1993;1:77–86. [PubMed] [Google Scholar]
- 62.Rees DD, Cellek S, Palmer RMJ, Moncada S. Dexamethasone prevents the induction by endotoxin of a nitric oxide synthase and the associated effects on vascular tone: an insight into endotoxin shock. Biochem Biophys Res Commun. 1990;173:541–547. doi: 10.1016/s0006-291x(05)80068-3. [DOI] [PubMed] [Google Scholar]
- 63.Petros A, Bennett D, Vallance P. Effect of nitric oxide synthase inhibitors on hypertension in patients with septic shock. Lancet. 1991;338:1557–1558. doi: 10.1016/0140-6736(91)92376-d. [DOI] [PubMed] [Google Scholar]
- 64.Petros A, Leone A, Moncada S, Bennett D, Vallance P. Effect of a nitric oxide synthase inhibitor in patients with septic shock: a randomised study. Cardiovasc Res. 1994;28:34–39. doi: 10.1093/cvr/28.1.34. [DOI] [PubMed] [Google Scholar]
- 65.Charles I, Vallance P. Inducible nitric oxide synthase in mice and men. Sepsis. (in press)
- 66.Bhagat K, Collier J, Vallance P. The local venous responses to endotoxin in humans. Circulation. 1996;94:490–497. doi: 10.1161/01.cir.94.3.490. [DOI] [PubMed] [Google Scholar]