MCAK-Independent Functions of ch-Tog/XMAP215 in Microtubule Plus-End Dynamics (original) (raw)
Abstract
The formation of a functional bipolar mitotic spindle is essential for genetic integrity. In human cells, the microtubule polymerase XMAP215/ch-Tog ensures spindle bipolarity by counteracting the activity of the microtubule-depolymerizing kinesin XKCM1/MCAK. Their antagonistic effects on microtubule polymerization confer dynamic instability on microtubules assembled in cell-free systems. It is, however, unclear if a similar interplay governs microtubule behavior in mammalian cells in vivo. Using real-time analysis of spindle assembly, we found that ch-Tog is required to produce or maintain long centrosomal microtubules after nuclear-envelope breakdown. In the absence of ch-Tog, microtubule assembly at centrosomes was impaired and microtubules were nondynamic. Interkinetochore distances and the lengths of kinetochore fibers were also reduced in these cells. Codepleting MCAK with ch-Tog improved kinetochore fiber length and interkinetochore separation but, surprisingly, did not rescue centrosomal microtubule assembly and microtubule dynamics. Our data therefore suggest that ch-Tog has at least two distinct roles in spindle formation. First, it protects kinetochore microtubules from depolymerization by MCAK. Second, ch-Tog plays an essential role in centrosomal microtubule assembly, a function independent of MCAK activity. Thus, the notion that the antagonistic activities of MCAK and ch-Tog determine overall microtubule stability is too simplistic to apply to human cells.
Mitotic microtubules (MTs) are dynamic polymers that switch rapidly between states of polymerization and depolymerization (14, 41). This behavior is essential for building a functional mitotic spindle, a complex MT-based structure that is responsible for the proper segregation of chromosomes during cell division. In most animal cells, the centrosome organizes the poles of the bipolar mitotic spindle. Although it is clear that bipolar spindles can form in the absence of centrosomes through a chromatin-dependent spindle assembly pathway (8, 9, 24, 30, 35, 44), the centrosomal pathway of spindle assembly is dominant when centrosomes are present (25). Cooperation of these pathways improves the efficiency of mitosis in vivo (44).
ch-Tog belongs to the highly conserved XMAP215 family of centrosomal and MT-binding proteins (11). Work from several groups indicates that XMAP215/ch-Tog and the kinesin-13 family member Kif2C/XKCM1/MCAK (called MCAK hereafter) have opposing effects on MT dynamics in vitro; XMAP215/ch-Tog promotes polymerization and MT nucleation, and MCAK promotes depolymerization (19, 28, 49, 56, 65). Interestingly, XMAP215 family members have been identified as both MT stabilizers and destabilizers, suggesting that XMAP215 may be an important antipause factor that promotes overall MT dynamicity (52). More recently, XMAP215 has been shown to act as a processive MT polymerase (7).
A high incidence of multipolar spindle formation has been observed in cells where ch-Tog was depleted by small interfering RNA (siRNA), but spindle bipolarity was restored by codepleting MCAK with ch-Tog (10, 21, 26). In human cells, MCAK activity is dispensable for MT flux, bipolar spindle assembly, or chromosome movement but is required for proper chromosome attachment to spindle MTs, including the correction of erroneous attachments (10, 15, 26, 32, 66). Multiple pathways have evolved to suppress unwelcome MCAK activity within the cell; the calmodulin-dependent protein kinase CamKIIγ suppresses cytoplasmic MCAK activity and multipolar spindle formation, the chromosome passenger complex inhibits MCAK at the centromeres, and most recently, Aurora-A has been found to negatively regulate MCAK in spindle poles (2, 27, 33, 45, 67).
However, much less is understood of the roles of ch-Tog in mammalian mitosis. For instance, we still do not know how and when multipolar spindles arise in ch-Tog-depleted cells in vivo. Multiple poles could form by de novo assembly of MT asters or by centrosome fragmentation. Several laboratories have reported that centrosome integrity is perturbed in ch-Tog-depleted cells (10, 21, 26), but it remains unclear whether centrosome fragmentation involves the centrioles as well as the pericentriolar matrix (PCM).
ch-Tog interacts with TACC3, a member of the transforming acidic coiled-coil-containing protein family (20, 34). tacc3 is an essential gene in mice, and its prolonged depletion in human cells leads to apoptosis (47, 51). Similarly to ch-Tog, TACC3 localizes to the centrosome and the mitotic spindle (22). Depletion of TACC3 causes only a small increase in multipolarity, suggesting that TACC3 is not essential for maintenance of bipolarity by ch-Tog (21, 51). In Xenopus egg extracts, TACC3 enhances the ability of XMAP215/ch-Tog to counteract the activity of MCAK, but it is unclear if such a relationship exists in mammalian cells (46). It also remains to be seen if ch-Tog and TACC3 function in other aspects of mitotic spindle formation, such as MT stability, dynamics, or the formation of MT-kinetochore attachments, and whether any or all of these involve countering MCAK activity.
Here, we set out to establish the relative importance of ch-Tog and MCAK in mitotic MT behavior, centrosome integrity, and kinetochore function in synchronized mammalian cells in vivo and in vitro. Contrary to expectations, we found that while ch-Tog indeed counters MCAK activity on kinetochore MTs, it also plays essential, MCAK-independent roles in promoting centrosomal MT growth and maintaining a pool of dynamic centrosomal MTs that are independent of MCAK activity.
MATERIALS AND METHODS
Antibodies and immunofluorescence.
HeLa cells were cultured and stained with antibodies as described previously (22). tsBN2 cells were cultured in Dulbecco's modified Eagle's medium with 10% fetal bovine serum at 32°C in 5% CO2. Centrin-green fluorescent protein (GFP)-expressing HeLa cells (48) and GFP-tubulin-expressing cells were cultured in the presence of 400 μg/ml G418. GFP-tubulin was a kind gift from Peter Coopman. tsBN2 cells (a kind gift of Erich Nigg) were cultured as described previously (53). The cells were fixed either in cold methanol to detect ch-TOG, TACC3, and acetylated-α-, α-, β-, and γ-tubulin, centrin, and pericentrin or in 3.5% formaldehyde to detect CREST, Hec1, CDK5RAP2, MCAK, and β-tubulin staining. For kinetochore fiber staining, cells were treated with extraction buffer {1 mM CaCl2, 1 mM MgCl2, 100 mM PIPES [piperazine-N,_N_′-bis(2-ethanesulfonic acid)], pH 6.8, 0.1% Triton X-100} for 2 min prior to fixation with 3.5% formaldehyde. Monastrol (Tocris) was used at 100 μM.
All antibodies listed below were used at 0.5 to 1 μg/ml in both immunofluorescence and Western blot analyses. Centrosomes were detected by GTU-88 anti-γ-tubulin antibody (Sigma), by antipericentrin antibody (Covance), or by CDK5RAP2 (Bethyl), while centrioles were stained using mouse monoclonal anticentrin antibodies (kindly provided by J. Salisbury). MTs were detected using DM1 anti-α-tubulin antibody (Sigma) or Cy3-conjugated anti-β-tubulin antibody (Sigma). Other antibodies used in this study were rabbit anti-ch-TOG (a kind gift of Lynne Cassimeris), rabbit anti-MCAK (Cytoskeleton Inc.), rabbit anti-TACC3 (22), mouse anti-TACC3 (Abcam), mouse anti-acetylated tubulin (Sigma), mouse anti-Hec1 (GeneTex), and rabbit anti-phospho-histone H3 (Upstate) antibodies. Appropriate Alexa 488-, Alexa 555 (Molecular Probes)-, Cy5-, or Cy3 (Jackson ImmunoResearch)-coupled secondary antibodies were used at 1/1,000 dilution. DNA was detected with Hoechst (Sigma). Imaging was performed on Zeiss LSM 510 and Nikon Eclipse 90i scanning confocal microscopes. The images presented are three-dimensional projections of Z sections taken every 0.5 μm from across the cell to include two centrosomes. The images were imported into Volocity or Adobe Photoshop, and images in any individual figure were processed in exactly the same way.
siRNA preparation and transfection.
Target cDNA sequences employed in the RNA interference experiments are shown in Table S1 in the supplemental material. To establish an siRNA target sequence in hamster ch-Tog, genomic DNA was extracted from the golden hamster tsBN2 cell line using the Gentra Puregene Cell and Tissue kit (according to the manufacturer's instructions). Primers were designed using genomic sequence information from mouse and rat. Exons 17 and 44 of tsBN2 ch-TOG were amplified by PCR using Phusion Taq (NEB) according to the manufacturers' instructions and the following primers: Exon17F (5′-GTTGCTTTGATTGCCCAG), Exon17R (5′-GCTTCTTTTGCATTGTTC), Exon44F (5′-TGACCTCTTTGCTCTCCA), and Exon44R (5′-TACATACAACCAGTTTGT). The PCR-amplified exons were then subcloned and sequenced. E44 (see Table S1 in the supplemental material) is identical to the target sequence used previously (21). Other siRNA duplexes were synthesized and annealed by Ambion or Dharmacon (see Table S1 in the supplemental material for sequences). HeLa cells were seeded on metasilicate-coated coverslips to 60% confluence in 24-well plates. To ensure that we observed siRNA-treated cells in their first mitosis, we employed the following synchronization protocol. HeLa cells were incubated with 2 mM thymidine for 20 h. They were then released, and the siRNAs were introduced 6 h later by transfecting 0.1 nmol of RNA duplex per well using Oligofectamine reagent (Invitrogen). After 4 h, 2 mM thymidine was added to the cells for a further 24 h. The cells were then washed and incubated for 9 to 10 h before fixation or live-cell imaging. tsBN2 cells were processed in an identical manner but were subjected to only a single 20-h thymidine block. For kinetochore fiber measurements, monastrol was added to the cells 3 h after release from the second thymidine block. After 16 h in monastrol, the cells were washed and incubated for 1 min in the calcium-containing extraction buffer before being fixed in formaldehyde as before.
FRAP and time-lapse imaging.
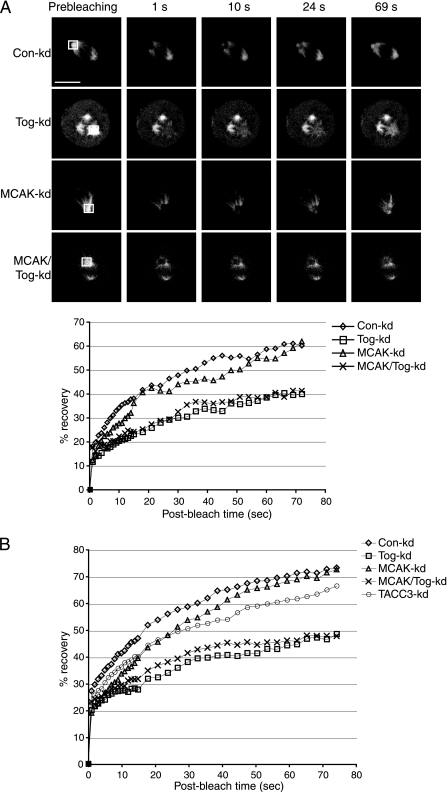
HeLa cells stably expressing GFP-tubulin were synchronized and treated with siRNA as described above. Nine hours after the second thymidine block, Dulbecco's modified Eagle's medium was replaced by prewarmed Leibowitz medium (Invitrogen) without serum or antibiotics. Fluorescence recovery after photobleaching (FRAP) was carried out using a Nikon inverted TE2000-E scanning confocal microscope. In Tog knockdown (Tog-kd) cells containing multiple spindle poles, the most prominent one was selected for FRAP. The setup was as follows: (i) a prebleaching image was acquired, (ii) 20 pulses (1 pulse/second) of 488-nm laser light (100% intensity) were applied in a selected bleach window of 4.5 μm2, and (iii) images were collected at a rate of 1 frame/second for the first 15 s, followed by 1 frame/3 s for another 59 s (74 s in total). Background intensity levels were determined for the same time course using a window of 4.5 μm2 in an area excluding the spindles. Nikon EZC1 software calculated signal intensities in the windows, and the data were exported to Microsoft Excel. For each time point, we subtracted the background intensity from prebleach and recovered signal intensities. This value was then normalized against the prebleaching signal intensity to eliminate clonal variability of GFP expression levels. We plotted these values as percentage recoveries against time (see Fig. 2; see Fig. S3 in the supplemental material).
FIG. 2.
ch-Tog is required for efficient recovery of GFP-tubulin signal at spindle poles following photobleaching. (A) Examples of GFP-tubulin recovery after photobleaching of the framed areas (shown in the prebleaching column). The graph shows mean signal intensities in the bleach window plotted against time for individual cells from the images. Tog-kd and MCAK/Tog-kd cells display similar recovery patterns that are clearly distinct from the recovery patterns of Con-kd and MCAK-kd cells. Scale bar = 10 μm. (B) Averaged signal intensities from 10 cells per siRNA treatment. The distribution of the data is summarized in a box plot in Fig. S3 in the supplemental material.
For time-lapse imaging, optical Z sections in 1-μm steps were collected every 40 s. Imaging was performed on Improvision's spinning-disk confocal microscope equipped with a Hamamatsu EM-charge-coupled-device camera.
Fluorescence intensity measurements and image analysis.
Cells were fixed and stained as described above. Images within the same experiment were acquired using identical settings on confocal microscopes. Optical Z sections of fields of cells typically containing two to four mitotic cells were imported into the image analysis software Volocity (Improvision), where the following procedure was followed. Using the Cy3-anti-β-tubulin stainings, pixels with intensities of 60% and over (Fig. 3B) or 25% and over (Fig. 3C) were selected in Volocity. The mean intensities of other fluorophores (corresponding to anti-ch-Tog and anti-EB1 [see Fig. 3B] and to anti-acetylated tubulin [see Fig. 3C]) were established across the selected volumes. Similarly, fluorophores corresponding to pericentrin and γ-tubulin levels were measured in centrin-GFP-positive volumes. To measure interkinetochore distances, we imported optical Z sections of cells (with a step size of 0.4 μm) stained with anti-Hec1 antibody into Volocity. Using anti-Hec1 as a kinetochore marker, separation distances between kinetochore pairs were established with the manual line measurement tool. Only kinetochore pairs present on the same focal plane were included in these measurements. Kinetochore MT length was determined with the manual line measurement tool. Anti-Hec1 and centrosomal CDK5RAP2 stainings marked the extremities of kinetochore MTs. The lengths of individual MTs were determined using these markers, together with Cy3-anti-β-tubulin staining. We included only the 10 longest MTs from each cell in our data set.
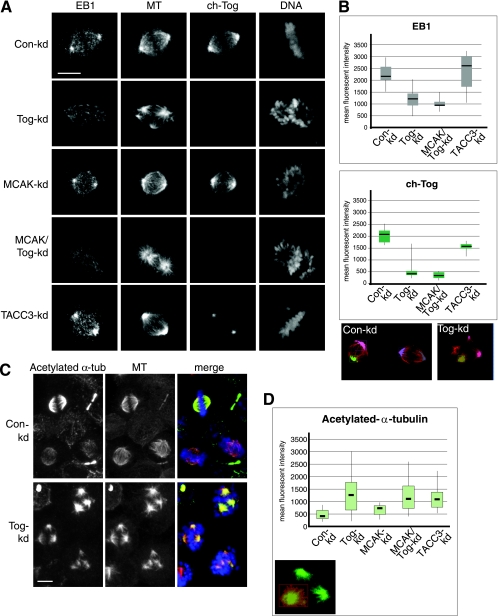
FIG. 3.
MTs in Tog-kd spindles are long lived, and their growing tips do not cluster around centrosomes. (A) EB1 signal is reduced in the spindle poles of Tog-kd and MCAK/Tog-kd cells. The cells were immunostained with anti-EB1, Cy3-anti-β-tubulin/MT (MT), and anti-ch-Tog antibodies. DNA was stained with Hoechst stain. Scale bar = 10 μm. (B) Box plots representing distributions of mean fluorescence intensities of anti-EB1 (top) and anti-ch-Tog (bottom) antibody staining at the spindle pole area (see Materials and Methods for details). Examples of spindle pole area selections in Con-kd and Tog-kd cells are highlighted in various colors on projected images in which MTs are stained with Cy3-anti-β-tubulin (red). Note that as these areas encompass the minus-end region of the spindle, TACC3-kd selections contain reduced mean fluorescence intensities of anti-ch-Tog, which is consistent with a decrease in ch-Tog levels on spindle MTs in these cells. The horizontal lines indicate median values. The data represent the spindle poles of 20 cells per siRNA treatment processed within a single experiment. Similar distributions were obtained in three independent experiments. (C) Acetylated-α-tubulin (α-tub) levels are elevated in Tog-kd spindles. In prometaphase, anti-acetylated-α-tubulin antibodies stain Tog-kd spindles more strongly than Con-kd spindles. Anti-acetylated α-tubulin is shown in green, Cy3-anti-β-tubulin/MT in red, and DNA in blue in the merged images. Note that a significant proportion of MTs are acetylated in Tog-kd cells. (D) Box plot representing the distribution of mean fluorescence intensities of anti-acetylated-α-tubulin staining in the spindles of 30 cells. Similar results were obtained in three independent experiments. An example of area selection in a Tog-kd cell is highlighted in red on the projected image, in which MTs are stained with Cy3-anti-β-tubulin (green). Note that examples of selections are shown on projected images, but all calculations were carried out in three-dimensional volumes built of Z stacks. Scale bar = 10 μm.
Western blotting.
Whole-cell extracts were made by pelleting HeLa cells and boiling the pellet in sodium dodecyl sulfate sample buffer. The extracts were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted to nitrocellulose as described previously (57). The blots were incubated with primary antibodies at a 1-μg/ml final concentration, and antibody binding was detected using the Supersignal kit (Pierce) according to the manufacturer's instructions.
RESULTS
Real-time analysis of mitotic spindle assembly in Tog-kd cells.
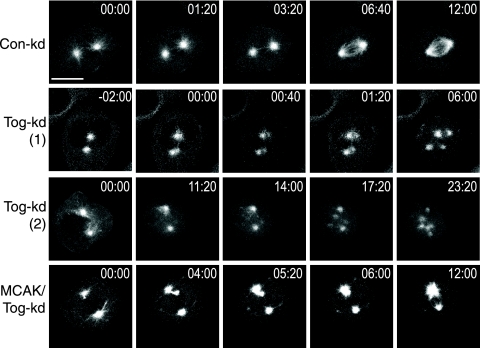
In the absence of ch-Tog, cells arrest in mitosis with multipolar spindles in a spindle assembly checkpoint-dependent manner (40). To establish how multipolar spindles form in ch-Tog-kd cells in real time, we used HeLa cells stably expressing GFP-tubulin (for the knockdown efficiencies, see Fig. S1 in the supplemental material). Cells transfected with a negative-control siRNA (Con-kd) assembled a bipolar spindle soon after nuclear-envelope breakdown (NEBD) (Fig. 1 and Table 1; see movie S1 in the supplemental material). Tog-kd cells looked similar to Con-kd cells until NEBD. However, only 35% of Tog-kd cells assembled bipolar spindles after NEBD (Table 1). Bipolar spindles also took longer to form in Tog-kd than in Con-kd cells, and they appeared shorter and less dense. The other 65% of Tog-kd cells developed supernumerary MT asters soon after NEBD. Extra asters either appeared near existing poles (the “splitting” phenotype) [Tog-kd (1) in Fig. 1; see movie S2 in the supplemental material] or they formed de novo at a distance from major poles [Tog-kd (2) in Fig. 1; see movie S3 in the supplemental material]. We noticed a tendency of these de novo asters to move toward larger asters (see movies S3 and S5 and Fig. S2 in the supplemental material). Out of 18 Tog-kd cells that displayed at least transient multipolarity, 6 showed the “splitting” phenotype and 12 displayed de novo pole formation. Cells with supernumerary asters exhibited one of two fates: either the extra asters were incorporated into a bipolar spindle (seen in 3 cells), or the multipolar spindle structure persisted (seen in 15 cells). Importantly, our data here describe events that take place within the first 20 to 30 min of mitotic spindle assembly. As Tog-kd cells exhibited prolonged mitotic arrest (4 to 12 h), we noted that spindle architecture and the number of spindle poles could both change over time in these cells (data not shown). Codepleting MCAK with ch-Tog (MCAK/Tog-kd) substantially reduced the number of multipolar spindles compared to ch-Tog-only depletions in fixed cells (see Fig. S4B in the supplemental material) (10, 26, 27). To distinguish between the possibilities of codepleted cells not forming or not maintaining multipolar spindles, we followed spindle formation in vivo. Out of 12 MCAK/Tog-kd cells, 2 displayed transiently multipolar spindles that coalesced into a bipolar spindle (Fig. 1; see movie S4 in the supplemental material) and only 1 developed a persistent multipolar spindle (Table 1). Our results suggest that codepleting MCAK with ch-Tog both reduces the frequency of multipolar spindle formation and promotes the coalescing of extra poles.
FIG. 1.
Tog-kd cells develop multipolar spindles after NEBD. Shown are still images from movies following spindle assembly in siRNA-treated synchronized HeLa cells expressing GFP-tubulin. Time 00:00 (minutes:seconds) indicates NEBD. The images in the top row follow a Con-kd cell from NEBD to assembly of the bipolar spindle. Two examples are shown for multipolar-spindle assembly in Tog-kd cells: row Tog-kd (1) depicts aster formation near existing poles (the “splitting” phenotype), while row Tog-kd (2) shows de novo aster formation at random sites. Note that spindle pole splitting occurs almost simultaneously with NEBD in Tog-kd (1). Supernumerary poles move toward and then cluster around the centrosomes in Tog-kd (2). The images in the bottom row show how a small aster appears and moves away from the spindle pole in MCAK/Tog-kd cells before being incorporated into a bipolar spindle. Scale bar = 10 μm.
TABLE 1.
Real-time analysis of spindle assemblya
| siRNA-treated cell | % (t [min ± SD]) | |||
|---|---|---|---|---|
| Bipolar spindle | Bipolar after multipolar intermediates | Tripolar | ≥4 poles | |
| Con-kd (n = 10) | 100 (8.7 ± 1.6) | 0 | 0 | 0 |
| Tog-kd (n = 28) | 35 (10.4 ± 3.6) | 11 (28.9 ± 6.99) | 32 (8.5 ± 3.4) | 21 (11.2 ± 6.7) |
| MCAK/Tog-kd (n = 12) | 75 (9.2 ± 3.2) | 17 (11.5 ± 3.5) | 0 | 8 (6) |
In summary, our in vivo analyses revealed that multipolar spindles appear soon after NEBD in Tog-kd cells. The observations that extra asters form at a distance from the centrosomes in the majority of Tog-kd cells and that supernumerary asters tend to move toward the centrosomes suggest that (i) unlike what we and others previously suggested, centrosome fragmentation cannot be the sole cause of supernumerary pole formation, and (ii) while some MT motor activity may drive apart the extra poles, other activity clearly promotes the coalescing of these poles (10, 26, 27).
ch-Tog promotes MT assembly at spindle poles.
ch-Tog in Xenopus egg extracts promotes MT polymerization, but it is unclear whether it regulates MT behavior in a similar manner during spindle assembly of mammalian cells. To assay centrosomal-MT assembly, we used FRAP in siRNA-treated HeLa cells stably expressing GFP-tubulin. While we cannot formally exclude the possibility that MT flux contributes to recovery in our experimental setup, we noted that in control cells the GFP signal recovered faster at the spindle poles than in the rest of the spindle area (Fig. 2A, Con-kd). This suggests a significant contribution to recovery by centrosomal MT assembly. Moreover, following bleaching, fluorescent GFP-tubulin molecules from the soluble pool are incorporated predominantly into the plus ends of growing MTs. Therefore, the rate of recovery in our FRAP experiments depended both on the number of MTs assembled at the centrosome and on their growth characteristics. Con-kd cells recovered 73.8% of their initial signal within 75 s after being bleached (see Materials and Methods for details), Tog-kd cells averaged only 48.9% recovery (individual cells are shown in Fig. 2A and averages in Fig. 2B; see Fig S3 in the supplemental material). We next asked whether the reduced FRAP rate in Tog-kd cells was a result of unbalanced MCAK activity. First, we examined signal recovery in MCAK-depleted (MCAK-kd) cells. Similarly to Con-kd cells, the spindles of MCAK-kd cells recovered 73.1% of their initial signal after being bleached. However, when we codepleted MCAK with ch-Tog (MCAK/Tog-kd), fluorescence recovery at spindle poles was 45.6%, a value almost identical to that seen in Tog-kd cells. Therefore, the role of ch-Tog in centrosomal MT assembly does not involve countering the activity of MCAK. Moreover, this function of ch-Tog appears to be essential and nonredundant.
The TACC proteins interact with ch-Tog/XMAP215 in an evolutionarily highly conserved manner, and TACC3 is required for targeting ch-Tog to spindle MTs, but not to centrosomes (Fig. 3A; see Fig. S4C in the supplemental material) (21). We therefore asked if TACC3 function, similarly to ch-Tog, is required for normal MT assembly at the spindle poles. To our surprise, TACC3 depletion impaired fluorescence recovery to a lesser extent, which suggests that (i) TACC3 is not essential for this aspect of ch-Tog function and (ii) centrosomal ch-Tog is sufficient for maintaining centrosomal MT assembly.
ch-Tog is required for efficient MT growth at the centrosome.
The reduction in GFP-tubulin recovery near the spindle poles of Tog-kd cells could suggest a defect in centrosomal MT assembly or growth. It has been shown that the distribution of EB1/MAPRE1, an antipause MT binding factor that associates with growing MT plus ends (60), is relatively normal in Tog-kd spindles (10). However, in light of our FRAP data, we decided to quantitate EB1 localization in the spindle pole regions of synchronized Tog-kd cells (see Materials and Methods) (Fig. 3). In Con-kd cells, anti-EB1 antibodies strongly stained the spindle poles and clearly marked MT ends within the spindle. In contrast, in Tog-kd cells, EB1 levels were reduced at the spindle poles (Fig. 3A and B; see Fig. S5A in the supplemental material). We also noted a reduction in the overall intensity of EB1 staining throughout the spindle, but MT ends were still detectable within the spindle (consistent with reference 10). A similar degree of reduction in EB1 levels was observed in cells codepleted of MCAK and ch-Tog (Fig. 3A and B), while levels were normal in MCAK-kd cells (Fig. 3A). Surprisingly, EB1 levels were slightly elevated at the spindle poles of TACC3-kd cells (Fig. 3A and B; see Fig. S5B in the supplemental material). This increase in EB1 levels coincided with stronger-than-normal anti-β-tubulin staining in the pericentrosomal region, suggesting an overall increase in short centrosomal MTs.
Importantly, overall EB1 protein levels were comparable in Con-kd, Tog-kd, and TACC3-kd cells (see Fig. S1 in the supplemental material), indicating that the differences seen in anti-EB1 staining are likely to result from differential protein recruitment. Since ch-Tog is still present in the centrosomes of TACC3-depleted cells (Fig. 3A) and EB1 is reduced in the spindle poles of Tog-kd cells, we wondered whether ch-Tog could be directly involved in recruiting EB1 to the poles. ch-Tog has been recently shown to interact with EB1, using a visual immunoprecipitation assay in Xenopus mitotic egg extracts (43). However, our coimmunoprecipitation assays using mitotic extracts of HeLa cells failed to reveal an interaction between endogenous or overexpressed ch-Tog and EB1 (data not shown). Moreover, weak centrosomal EB1 staining was present in control-, ch-Tog-, and TACC3-depleted cells when MTs were absent (data not shown). While we cannot exclude the possibility that ch-Tog binds EB1 and recruits it to the centrosomal region in the presence of MTs, there are at least two alternative explanations: (i) ch-Tog is required to initiate centrosomal-MT assembly, so the decrease of EB1 at the poles reflects a reduction of growing centrosomal MT, or (ii) ch-Tog binding to MTs facilitates the recruitment of EB1. Regardless of the precise mechanism, ch-Tog seems to be required for this function even in the absence of MCAK, since codepleting MCAK with ch-Tog does not restore EB1 levels in the spindle pole area.
Tog-kd cells contain a substantial long-lived MT population.
As we can generally detect MTs in the spindles poles of Tog-kd cells, the lack of EB1 in this area could also suggest that centrosomal MTs are less dynamic than normal in Tog-kd cells. The lower rate of GFP-tubulin recovery observed in these cells would be consistent with this, since plus ends of nondynamic MTs are expected to recruit fewer subunits. As α-tubulin becomes acetylated in a time-dependent manner in polymerized MTs (62), antibodies against acetylated α-tubulin serve as markers to assess the longevity of MTs within cells. Using these antibodies, we could therefore investigate if long-lived MTs were present in Tog-kd cells. In Con-kd cells, anti-acetylated-α-tubulin antibody staining was low in prometaphase cells but became more prominent by metaphase (Fig. 3C). This is consistent with the view that tubulin acetylation occurs in kinetochore fibers (64). To our surprise, anti-β-tubulin and anti-acetylated-α-tubulin stainings frequently overlapped in Tog-kd cells even in the absence of a metaphase plate, indicating that long-lived MTs constitute a substantial fraction of their MT network. Subsequent quantitation of the levels of acetylated tubulin within the mitotic spindles revealed a three-fold increase in Tog-kd compared to Con-kd cells (Fig. 3C and D) (see Materials and Methods for details). A similar effect was observed in TACC3-kd cells. To assess the relevance of these long-lived MTs to MCAK function and multipolarity, we measured acetylated-α-tubulin levels in MCAK-kd and MCAK/Tog-kd cells. MCAK-kd cells displayed a moderate increase in acetylated-α-tubulin levels, consistent with a role of MCAK in promoting MT dynamics (56, 66). The acetylated-α-tubulin levels of MCAK/Tog-kd spindles, however, were markedly higher, displaying values very similar to those of ch-Tog-only depletions.
Elevated acetylated-α-tubulin levels could point to the presence of stable kinetochore MTs in Tog-kd cells, but it is equally possible that these cells contain a population of long-lived nonkinetochore MTs. Cells lacking TACC3 also showed elevated levels of acetylated tubulin. While we cannot rule out the possibility that TACC3 plays a role independent of ch-Tog in regulating MT lifetime in the spindle, it is more likely that this is linked to the role of TACC3 in targeting ch-Tog to spindle MTs. Our data imply that this role of ch-Tog is again nonredundant and required even in the absence of MCAK activity, since tubulin acetylation levels are identical in Tog-kd and MCAK/Tog-kd cells. These findings suggest that ch-Tog may be necessary to convert a population of centrosomal MTs into dynamic MTs through its polymerase activity, a role that could involve TACC3.
ch-Tog promotes both long kinetochore MTs and tension at kinetochores by countering MCAK activity.
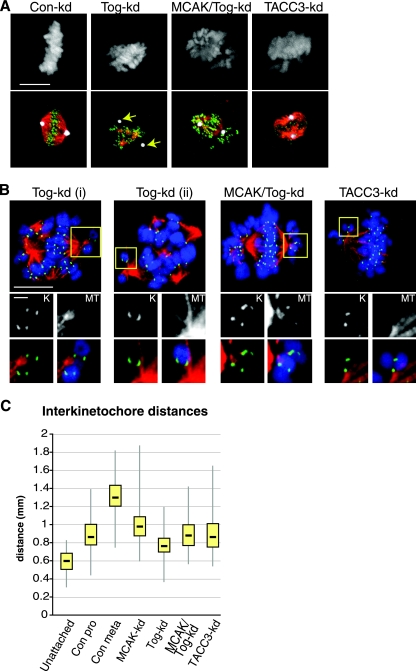
Since ch-Tog family members are found at kinetochores in yeast (17, 18, 42) and Tog-kd cells display a reduction in kinetochore occupancy and interkinetochore distances (40), we wondered whether the disruption of MT-kinetochore attachments is linked to MCAK activity. To assess the state of kinetochore MTs in the absence of ch-Tog, we treated the cells with a calcium-containing extraction buffer to depolymerize nonkinetochore MTs prior to fixation. The majority of Tog-kd cells contained some calcium-stable MTs, suggesting that kinetochore MTs form in these cells. Interestingly, in 22.6% ± 4% of Tog-kd cells, calcium-stable MTs did not associate with the centrosome (50 cells; n = 3) (Fig. 4A). This phenotype was rare in MCAK/Tog-kd cells (3.5% ± 2.4%). The lack of calcium-stable MTs in the centrosomes of Tog-kd cells is intriguing, since asters stained with anti-acetylated-α-tubulin antibodies mostly associated with centrin-GFP-labeled centrosomes (see Fig. S6 in the supplemental material). This argues that the acetylated-tubulin population is not necessarily calcium stable in Tog-kd cells and could include MT asters associated with chromatin but not attached to kinetochores.
FIG. 4.
Kinetochore MTs are abundant in Tog-kd cells, but they neither associate with the centrosome nor provide tension at kinetochores. (A) Kinetochore MTs were visualized in siRNA-treated cells following treatment with calcium extraction buffer. Centrosomes associate with few or no calcium-stable MTs in Tog-kd cells (arrows). Codepleting MCAK with ch-Tog rescued this phenotype. The panels on the left show DNA staining. In the merged images (bottom row), centrosomes are stained with anti-CDK5RAP2 antibodies (white), kinetochores with anti-Hec1 antibodies (green), and MTs with Cy3-anti-β-tubulin (red). Scale bar = 10 μm. (B) Close-ups of chromosome attachments in siRNA-treated cells. The cells were treated with calcium extraction buffer prior to fixation. Kinetochores are marked with anti-Hec1 (green) and MTs with Cy3-anti-β-tubulin (red) antibodies. DNA staining is in blue in the merged images. The large panels (scale bar = 10 μm) show individual cells, whereas the images (scale bar = 1 μm) arranged below depict magnifications of the areas framed in yellow. The black-and-white images are close-ups of either kinetochores (K) or MTs (MT), while the merged images below show the same areas without (left) or with (right) DNA staining. The images are projections of four confocal Z slices (0.3-μm steps). The two chromosomes shown in Tog-kd (i) have monopolar attachments, but kinetochore MTs are weak in both cases. The chromosome in Tog-kd (ii) shows syntelic-like attachment. Note that the kinetochores display side-on instead of end-on attachments to MTs, a common phenotype in these cells. Monopolar attachments remain frequent in MCAK/Tog-kd cells, but kinetochore MTs are more pronounced there than in Tog-kd cells. Monopolar attachments are also present in TACC3-kd cells. (C) Box plot representation of the distribution of interkinetochore distances in various siRNA-treated cells. Unattached chromosomes were scored in prophase Con-kd cells. Prometaphase (Con pro) and metaphase (Con meta) Con-kd cells were scored separately. In Tog-kd, TACC3-kd, and Tog/MCAK-kd cells, only kinetochores of chromosomes present on metaphase-like plates were included. The data represent 200 kinetochore pairs (10 pairs per cell) for each category.
We also noticed that kinetochore MTs appeared shorter in Tog-kd cells. As Tog-kd cells often have multiple metaphase-like plates, the lengths of kinetochore MTs within individual cells vary a lot, making quantification difficult. To circumvent this problem, we used the small molecule monastrol, which generates monopolar spindles by inhibiting the MT motor, Eg5 (38). Treating monopolar spindles with the calcium-containing extraction buffer allowed us to reproducibly measure the lengths of kinetochore fibers in cells (see Fig. S7A in the supplemental material). The average length of kinetochore MTs was 1.98 ± 0.18 μm in Tog-kd and 3.4 ± 0.38 μm in Con-kd cells (10 fibers/cell; 15 cells; n = 2). Codepleting MCAK with ch-Tog increased the average length to 2.52 ± 0.15 μm.
Next, we visualized kinetochore-MT attachments by staining cells with anti-β-tubulin and an antibody against Hec1, a resident kinetochore protein (Fig. 4A) (37). Chromosome alignment was severely compromised in Tog-kd cells (21). Unaligned chromosomes mostly displayed monotelic or syntelic attachments in Tog-kd cells (Fig. 4B), but unattached chromosomes were also frequent (data not shown). However, most Tog-kd cells contained at least some amphitelically attached chromosomes that aligned on one of several metaphase-like plates, and these were used exclusively to measure interkinetochore separation distances (Fig. 4C). Bioriented chromosomes in Tog-kd cells displayed reduced interkinetochore distances compared to Con-kd prometaphase cells (median values, 0.76 μm and 0.86 μm, respectively). Calcium-stable MTs were readily detectable in MCAK/Tog-kd cells, but despite the spindles being mostly bipolar, chromosomes did not fully align on a metaphase plate and monotelic attachments remained frequent in these cells (Fig. 4B). Still, compared to Tog-kd cells, bioriented chromosomes in MCAK/Tog-kd cells displayed an increase in interkinetochore distances (0.87 μm in MCAK/Tog-kd). While this is a significant rise, it is still comparable to interkinetochore distances of prometaphase Con-kd cells, suggesting that codepleting MCAK with ch-Tog improves tension across individual kinetochore pairs but does not restore chromosome congression.
Since codepleting MCAK with ch-Tog rescues multipolarity and increases tension at kinetochores, we asked if MT-kinetochore attachments in general could play a role in multipolar-spindle formation in Tog-kd cell. We therefore codepleted cells of ch-Tog and the essential kinetochore components Hec1 and Nuf2, members of the Ndc80 complex (12, 39, 63). Eliminating attachments, however, did not significantly affect multipolarity in Tog-kd cells (see Fig. S7B in the supplemental material). As MCAK depletion is also known to alter MT dynamics (56), we wondered if this could contribute to the multipolar phenotype of Tog-kd cells. We therefore suppressed MT dynamics using low doses of nocodazole (29). Despite entering mitosis in the presence of 100 nM nocodazole, Tog-kd cells still assembled multipolar spindles (data not shown).
In summary, our results indicate that kinetochore MTs are abnormally short in Tog-kd cells. Calcium-stable MTs are detectable in these cells but do not necessarily associate with centrosomes. Codepleting MCAK with ch-Tog improves these defects but does not fully restore normal chromosome alignment. It is possible that MCAK depletion causes elongation of MTs produced at the kinetochores that can more efficiently contact the short centrosomal aster and hence rescue multipolarity in Tog-kd cells. MCAK depletion may also allow efficient centrosomal-MT assembly through a ch-Tog-independent pathway (58). Crucially, MT/kinetochore attachments do not drive multipolar-spindle formation, since multipolarity is seen in Tog-kd cells in which attachments have been eliminated by Ndc80 depletion.
ch-Tog contributes to, but is not essential for, centrosome integrity.
If MT/kinetochore attachments do not drive multipolarity, we wondered if the formation of the extra poles is due to defects in centrosome integrity. Our in vivo data are consistent with this possibility, as some of the new spindle poles appeared in close proximity to existing poles. Several groups, including ours, proposed that centrosome structure is affected in Tog-kd cells, mostly relying on markers of the PCM (10, 21, 26). We decided to study the effects of ch-Tog depletion on centrioles in a HeLa cell line stably expressing centrin-GFP, which allowed the unambiguous identification of centriole-containing spindle poles. A higher proportion of Tog-kd cells (74%; 60 centrosomes counted) than of controls (60%; 60 centrosomes counted) displayed two clearly distinguishable centrioles within their centrosomes, indicating that the centriole separation distance was slightly elevated (average distance, 0.53 μm in control and 0.62 μm in Tog-kd cells). Importantly, codepleting MCAK with ch-Tog rescued multipolarity, but it had little impact on the extent of centriole separation (65% with clearly distinguishable centrioles; 87 centrosomes counted; average distance between centrioles, 0.64 μm), suggesting that the small increase in centriole separation in Tog-kd cells is unlikely to account for the generation of extra poles. Moreover, split centrioles were present in only 14.4% ± 4% of multipolar Tog-kd cells (30 multipolar cells; n = 3), indicating that centrioles stayed in pairs and contributed to a single spindle pole in the majority of multipolar Tog-kd cells (see Fig. S8A in the supplemental material).
We next examined PCM markers with respect to centrin-GFP signal within Tog-kd cells. Pericentrin, γ-tubulin, CDK5RAP2, and Aurora-A stainings overlapped with centrin-GFP in both the presence and absence of MTs (data not shown). However, we noted that pericentrin staining appeared weaker and more diffuse in Tog-kd centrosomes when MTs were present, but the staining was also highly variable between cells. Therefore, we used the centrin-GFP signal to quantitate pericentrin staining in the centrosomes (see Materials and Methods) (see Fig. S8B and C in the supplemental material). We observed a reduction of about 25% in the staining intensity of pericentrin. However, since γ-tubulin staining was present in all centrosomes of Tog-kd cells and γ-tubulin has been shown to be recruited to the mitotic-spindle poles by pericentrin (68), we believe that functional levels of pericentrin are still present in Tog-kd centrosomes. Moreover, a degree of reduction in PCM components associated with the centrioles is to be expected in Tog-kd cells, since the excess spindle poles could compete for soluble PCM components. Consistently, supernumerary poles have been reported to contain γ-tubulin, pericentrin, Aurora A, and TACC3 (10, 21, 26). We conclude, therefore, that centrosomes are not severely fragmented in the absence of ch-Tog, as centrioles remain associated with PCM components in these cells.
Spindle morphology and MT polymer levels are maintained by chromatin-dependent MT assembly when ch-Tog is absent.
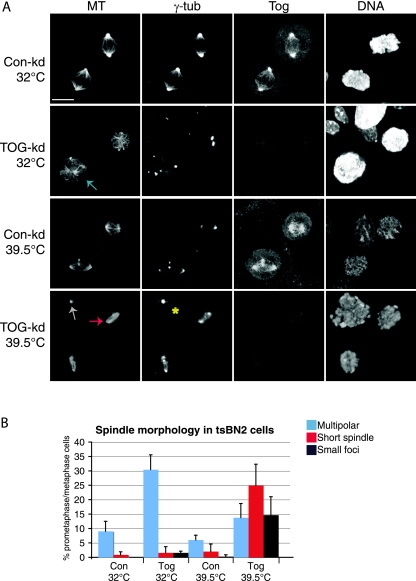
Our in vivo analysis suggested that the majority of excess spindle poles formed at sites away from centrosomes. Moreover, in the multipolar spindles of Tog-kd cells, aster sizes reflected the size of the chromatin they associated with (see Fig. S4A in the supplemental material). We therefore hypothesized that spindle formation in Tog-kd cells may be particularly reliant on chromatin-driven pathways, given the deficiencies of centrosomal-spindle assembly. To assess the importance of the chromatin-driven spindle assembly pathway, we used tsBN2 hamster cells, which carry a temperature-sensitive mutation in RCC1 (59), the chromatin-bound GTP exchange factor required for Ran activation (6). In Xenopus egg extracts, RCC1 activity produces a gradient of Ran-GTP that contributes to chromatin-dependent MT nucleation and enhances chromosome capture by astral MTs (24). In mammalian cells, however, Ran-GTP does not seem to be an essential factor for spindle assembly when centrosomes are present (5, 23). Consistently, we found that the majority of tsBN2 cells could assemble or maintain a bipolar spindle at the restrictive temperature (39.5°C). When ch-Tog in tsBN2 cells was depleted at the permissive temperature (32°C), we noted an increase in multipolar spindles (Fig. 5A and B). Interestingly, at 39.5°C, the spindle morphology dramatically changed in Tog-kd but not in Con-kd cells. In particular, multipolar spindles became less frequent in Tog-kd cells and the overall MT mass decreased. Barrel-shaped, short bipolar and occasionally tripolar spindles of uniform MT density were present in 24.9% ± 7% of Tog-kd cells (Fig. 5A) but in only 2% ± 2.5% of Con-kd cells (100 mitotic cells; n = 3). At 39.5°C, we found only a few mitotic cells with no detectable ch-Tog, and these usually contained highly disorganized chromatin and two centrosomes with no MT polymers (Fig. 5A). Importantly, spindle abnormalities, such as small extra spindle poles, became more common at the restrictive temperature even in Con-kd cells (Fig. 5A, lower Con-kd cells). Taken together, these observations suggest that the Ran-GTP pathway is important in maintaining overall MT polymer levels and spindle morphology in Tog-kd cells. Intriguingly, MCAK has been reported to suppress chromatin-dependent spindle assembly in Xenopus egg extracts and human cells (50). Therefore, it is possible that MCAK codepletion rescues the multipolarity of Tog-kd cells by promoting more efficient chromatin-dependent MT assembly. Clearly, further studies are needed to establish if this is indeed the case.
FIG. 5.
Chromatin-dependent MT assembly is important to maintain spindle morphology and MT polymer levels in Tog-kd cells. (A) tsBN2 cells treated with control or ch-Tog siRNAs are shown at permissive (32°C) and restrictive (39.5°C) temperatures. The cells were incubated for 4 h at the restrictive temperature before fixation. At 39.5°C, the spindle morphology radically changes in Tog-kd cells. The cell marked with an asterisk is devoid of ch-Tog and MT polymer. Note that extra poles are visible in the lower Con-kd cell at 39.5°C. MTs were detected using Cy3-anti-β-tubulin antibodies and centrosomes with anti-γ-tubulin antibodies. Scale bar = 10 μm. (B) Graph representing quantitation of mitotic-spindle phenotypes in tsBN2 cells at 32°C and 39.5°C. Specific examples for each category are highlighted in panel A with arrows of matching colors. The data were collected from 100 mitotic cells (n = 3). The error bars correspond to standard deviations.
DISCUSSION
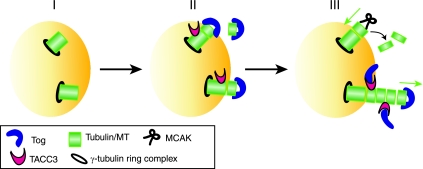
Our study provides novel insight into how MCAK, ch-Tog, and TACC3 control global MT dynamics in early mitosis. First, we showed that ch-Tog plays an essential role in promoting MT growth near the centrosomes in mammalian cells. This TACC3-independent function of ch-Tog is just as important when MCAK is absent, suggesting that MCAK activity is not responsible for impeding centrosomal MT growth in Tog-kd cells. Therefore, we identified a nonredundant and MCAK-independent role for ch-Tog in promoting centrosomal MT growth (steps I and II in the model in Fig. 6). Second, long-lived MTs are abundant in both Tog-kd and TACC3-kd cells. TACC3, therefore, functions together with ch-Tog to establish a dynamic MT population. Surprisingly, MCAK activity again does not seem to be responsible for the nondynamic MTs in Tog-kd cells. Third, Tog-kd cells have short calcium-stable kinetochore MTs that cluster in supernumerary poles, but kinetochore MTs are often absent from Tog-kd centrosomes. Chromosome alignment and tension at kinetochores are both severely compromised in Tog-kd cells. These defects are at least partially rescued by codepleting MCAK with ch-Tog, implying that MCAK activity compromises kinetochore MTs in Tog-kd cells. TACC3-kd cells also exhibit problems with chromosome alignment, but these are more subtle.
FIG. 6.
Schematic model explaining how TACC3 and ch-Tog function at the plus ends of nascent MTs. (I) Short MT polymers are nucleated off the γ-tubulin ring complex. (II) ch-Tog initiates MT growth by binding tubulin heterodimers (1, 55) and loading them onto the plus ends of short MTs (7). In the absence of ch-Tog, centrosomal MT plus ends do not grow. TACC3 can bind MTs independently of ch-Tog (21). (III) MT-bound TACC3 interacts with ch-Tog. This interaction could perform a dual role by (i) promoting the rate of MT elongation by maintaining ch-Tog in the proximity of plus ends and (ii) inducing a conformational change in ch-Tog that allows effective recruitment of heterodimers into the MT lattice. TACC3 and ch-Tog therefore cooperate to achieve rapid polymerization of plus ends. The plus ends of fast-growing MTs (with ch-Tog/TACC3) may be less amenable to depolymerization by MCAK than slowly growing ones (without ch-Tog/TACC3).
As codepleting MCAK with ch-Tog rescues multipolar-spindle formation and restores many of the kinetochore-related abnormalities, we explored the role of kinetochore-MT attachments in multipolarity. Surprisingly, the multipolar phenotype seen in Tog-kd cells could not be rescued by either the stabilization or the destabilization of MT-kinetochore attachments. This indicates that while MCAK activity may disrupt kinetochore-MT attachments, abnormal attachments are not responsible for multipolarity. Centrosome fragmentation could certainly contribute to multipolarity, but we also frequently observed de novo aster formation in the cytoplasm that led to multipolar spindle formation. One possibility is that in Tog-kd cells, centrosomal MTs are fewer and shorter, and therefore MTs that assemble spontaneously or in a chromatin-dependent manner in the cytoplasm would not get efficiently incorporated into a bipolar spindle, resulting in multipolar spindles. As supernumerary MT asters of Tog-kd cells appear as small poles and remain focused throughout their lifetimes, we do not see evidence for abnormal MT motor activities.
We believe that the difference between EB1 localization in Tog-kd and TACC3-kd cells is particularly interesting. While EB1 and ch-Tog bind to the MT lattice at different sites, EB1 could preferentially bind to ch-Tog-processed MT ends and sustain the growth phase of these MTs by promoting MT sheet closure (54, 61). It is equally possible that ch-Tog is required for the rapid elongation of short centrosomal MTs through its polymerase activity and that the reduction of EB1 in the vicinity of the centrosomes indicates only the lack of these MTs (7). The accumulation of EB1 around TACC3-kd poles, the increase in the density of short centrosomal MTs (Fig. 3A; see Fig. S5B in the supplemental material), and the overall decrease in spindle MT density (21) together argue that ch-Tog can initiate MT growth without TACC3, but it may not be very efficient in sustaining elongation. The milder spindle phenotype of TACC3-kd cells, together with the fact that tacc3 knockout mice die only in late embryogenesis, indicates that TACC3 cannot be essential for all aspects of ch-Tog function (47).
The C-terminal region of ch-Tog interacts with the TACC proteins (16), and this domain has also been implicated in binding tubulin heterodimers in vertebrate cells (55). More recent structural studies implicated the N-terminal region of ch-Tog in tubulin binding and suggested that an open-closed structural change occurs in ch-Tog upon binding tubulin (1, 7). In either case, it is tempting to speculate that TACC3 contributes to the processivity of ch-Tog at nascent MT ends by inducing a conformational change in ch-Tog to regulate its binding to tubulin heterodimers while simultaneously holding it in the proximity of plus ends (step III in Fig. 6). Consistent with this hypothesis, a role for TACC3 at MT-kinetochore attachments has been postulated, and we could detect TACC3 at the plus ends of MTs (see Fig. S9 in the supplemental material) (51). To gain insight into this molecular interplay at the plus ends of MTs, it will be crucial to elucidate whether tubulin heterodimers, TACC3, and ch-Tog exist in the same or distinct molecular complexes.
FRAP experiments, as well as our analysis of EB1 distribution, address the behavior of MT plus ends in the proximity of the spindle poles. These results prompted us to postulate a model in which rapid elongation of centrosomal MT plus ends by ch-Tog and TACC3 protects against MCAK activity (Fig. 6). TACC3, ch-Tog, and MCAK, however, are all present at the spindle poles (11, 22, 36). It is therefore also feasible that TACC3 cooperates with ch-Tog to prevent MCAK-dependent depolymerization of MT minus ends in the centrosomes. Importantly, this interpretation is still consistent with our conclusion that ch-Tog has an MCAK-independent function at the plus ends of MTs. Clearly, further work is needed to distinguish the effects of these proteins on minus- and plus-end MT dynamics.
Cumulatively, our data suggest that ch-Tog and TACC3 are both important regulators of bipolar-spindle assembly and that their roles go beyond countering MCAK activity. Aurora-A kinase may act as the mastermind of centrosomal-MT assembly (3), disabling MCAK in the centrosome during prometaphase/metaphase (33, 67) and targeting ch-Tog to the centrosome (13) while simultaneously enhancing the processivity of ch-Tog through the phosphorylation of TACC3 (4, 31, 46).
Supplementary Material
[Supplemental material]
Acknowledgments
We thank K. J. Patel and Deborah Zyss for critical reading of the manuscript and Jordan Raff and the Gergely laboratory for useful discussions.
F.G. is the recipient of a Royal Society University Research Fellowship. A.R.B. is supported by a Cancer Research UK Ph.D. studentship. Research in F.G.'s laboratory is funded by Cancer Research UK.
Footnotes
▿
Published ahead of print on 22 September 2008.
REFERENCES
- 1.Al-Bassam, J., N. A. Larsen, A. A. Hyman, and S. C. Harrison. 2007. Crystal structure of a TOG domain: conserved features of XMAP215/Dis1-family TOG domains and implications for tubulin binding. Structure 15355-362. [DOI] [PubMed] [Google Scholar]
- 2.Andrews, P. D., Y. Ovechkina, N. Morrice, M. Wagenbach, K. Duncan, L. Wordeman, and J. R. Swedlow. 2004. Aurora B regulates MCAK at the mitotic centromere. Dev. Cell 6253-268. [DOI] [PubMed] [Google Scholar]
- 3.Barr, A. R., and F. Gergely. 2007. Aurora-A: the maker and breaker of spindle poles. J. Cell Sci. 1202987-2996. [DOI] [PubMed] [Google Scholar]
- 4.Barros, T. P., K. Kinoshita, A. A. Hyman, and J. W. Raff. 2005. Aurora A activates D-TACC-Msps complexes exclusively at centrosomes to stabilize centrosomal microtubules. J. Cell Biol. 1701039-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bastiaens, P., M. Caudron, P. Niethammer, and E. Karsenti. 2006. Gradients in the self-organization of the mitotic spindle. Trends Cell Biol. 16125-134. [DOI] [PubMed] [Google Scholar]
- 6.Bischoff, F. R., and H. Ponstingl. 1991. Catalysis of guanine nucleotide exchange on Ran by the mitotic regulator RCC1. Nature 35480-82. [DOI] [PubMed] [Google Scholar]
- 7.Brouhard, G. J., J. H. Stear, T. L. Noetzel, J. Al-Bassam, K. Kinoshita, S. C. Harrison, J. Howard, and A. A. Hyman. 2008. XMAP215 is a processive microtubule polymerase. Cell 13279-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carazo-Salas, R. E., O. J. Gruss, I. W. Mattaj, and E. Karsenti. 2001. Ran-GTP coordinates regulation of microtubule nucleation and dynamics during mitotic-spindle assembly. Nat. Cell Biol. 3228-234. [DOI] [PubMed] [Google Scholar]
- 9.Carazo-Salas, R. E., G. Guarguaglini, O. J. Gruss, A. Segref, E. Karsenti, and I. W. Mattaj. 1999. Generation of GTP-bound Ran by RCC1 is required for chromatin-induced mitotic spindle formation. Nature 400178-181. [DOI] [PubMed] [Google Scholar]
- 10.Cassimeris, L., and J. Morabito. 2004. TOGp, the human homolog of XMAP215/Dis1, is required for centrosome integrity, spindle pole organization, and bipolar spindle assembly. Mol. Biol. Cell 151580-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charrasse, S., M. Schroeder, C. Gauthier-Rouviere, F. Ango, L. Cassimeris, D. L. Gard, and C. Larroque. 1998. The TOGp protein is a new human microtubule-associated protein homologous to the Xenopus XMAP215. J. Cell Sci. 1111371-1383. [DOI] [PubMed] [Google Scholar]
- 12.DeLuca, J. G., Y. Dong, P. Hergert, J. Strauss, J. M. Hickey, E. D. Salmon, and B. F. McEwen. 2005. Hec1 and nuf2 are core components of the kinetochore outer plate essential for organizing microtubule attachment sites. Mol. Biol. Cell 16519-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Luca, M., L. Brunetto, I. A. Asteriti, M. Giubettini, P. Lavia, and G. Guarguaglini. 28 July 2008. Aurora-A and ch-TOG act in a common pathway in control of spindle pole integrity. Oncogene. doi: 10.1038/onc.2008.252. [DOI] [PubMed]
- 14.Desai, A., and T. J. Mitchison. 1997. Microtubule polymerization dynamics. Annu. Rev. Cell Dev. Biol. 1383-117. [DOI] [PubMed] [Google Scholar]
- 15.Ganem, N. J., K. Upton, and D. A. Compton. 2005. Efficient mitosis in human cells lacking poleward microtubule flux. Curr. Biol. 151827-1832. [DOI] [PubMed] [Google Scholar]
- 16.Gangisetty, O., B. Lauffart, G. V. Sondarva, D. M. Chelsea, and I. H. Still. 2004. The transforming acidic coiled coil proteins interact with nuclear histone acetyltransferases. Oncogene 232559-2563. [DOI] [PubMed] [Google Scholar]
- 17.Garcia, M. A., N. Koonrugsa, and T. Toda. 2002. Spindle-kinetochore attachment requires the combined action of Kin I-like Klp5/6 and Alp14/Dis1-MAPs in fission yeast. EMBO J. 216015-6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia, M. A., L. Vardy, N. Koonrugsa, and T. Toda. 2001. Fission yeast ch-TOG/XMAP215 homologue Alp14 connects mitotic spindles with the kinetochore and is a component of the Mad2-dependent spindle checkpoint. EMBO J. 203389-3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gard, D. L., and M. W. Kirschner. 1987. A microtubule-associated protein from Xenopus eggs that specifically promotes assembly at the plus-end. J. Cell Biol. 1052203-2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gergely, F. 2002. Centrosomal TACCtics. Bioessays 24915-925. [DOI] [PubMed] [Google Scholar]
- 21.Gergely, F., V. M. Draviam, and J. W. Raff. 2003. The ch-TOG/XMAP215 protein is essential for spindle pole organization in human somatic cells. Genes Dev. 17336-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gergely, F., C. Karlsson, I. Still, J. Cowell, J. Kilmartin, and J. W. Raff. 2000. The TACC domain identifies a family of centrosomal proteins that can interact with microtubules. Proc. Natl. Acad. Sci. USA 9714352-14357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorlich, D., M. J. Seewald, and K. Ribbeck. 2003. Characterization of Ran-driven cargo transport and the RanGTPase system by kinetic measurements and computer simulation. EMBO J. 221088-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heald, R., R. Tournebize, T. Blank, R. Sandaltzopoulos, P. Becker, A. Hyman, and E. Karsenti. 1996. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature 382420-425. [DOI] [PubMed] [Google Scholar]
- 25.Heald, R., R. Tournebize, A. Habermann, E. Karsenti, and A. Hyman. 1997. Spindle assembly in Xenopus egg extracts: respective roles of centrosomes and microtubule self-organization. J. Cell Biol. 138615-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holmfeldt, P., S. Stenmark, and M. Gullberg. 2004. Differential functional interplay of TOGp/XMAP215 and the KinI kinesin MCAK during interphase and mitosis. EMBO J. 23627-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holmfeldt, P., X. Zhang, S. Stenmark, C. E. Walczak, and M. Gullberg. 2005. CaMKIIγ-mediated inactivation of the Kin I kinesin MCAK is essential for bipolar spindle formation. EMBO J. 241256-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howard, J., and A. A. Hyman. 2007. Microtubule polymerases and depolymerases. Curr. Opin. Cell Biol. 1931-35. [DOI] [PubMed] [Google Scholar]
- 29.Jordan, M. A., and L. Wilson. 2004. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer. 4253-265. [DOI] [PubMed] [Google Scholar]
- 30.Khodjakov, A., R. W. Cole, B. R. Oakley, and C. L. Rieder. 2000. Centrosome-independent mitotic spindle formation in vertebrates. Curr. Biol. 1059-67. [DOI] [PubMed] [Google Scholar]
- 31.Kinoshita, K., T. L. Noetzel, L. Pelletier, K. Mechtler, D. N. Drechsel, A. Schwager, M. Lee, J. W. Raff, and A. A. Hyman. 2005. Aurora A phosphorylation of TACC3/maskin is required for centrosome-dependent microtubule assembly in mitosis. J. Cell Biol. 1701047-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kline-Smith, S. L., A. Khodjakov, P. Hergert, and C. E. Walczak. 2004. Depletion of centromeric MCAK leads to chromosome congression and segregation defects due to improper kinetochore attachments. Mol. Biol. Cell 151146-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lan, W., X. Zhang, S. L. Kline-Smith, S. E. Rosasco, G. A. Barrett-Wilt, J. Shabanowitz, D. F. Hunt, C. E. Walczak, and P. T. Stukenberg. 2004. Aurora B phosphorylates centromeric MCAK and regulates its localization and microtubule depolymerization activity. Curr. Biol. 14273-286. [DOI] [PubMed] [Google Scholar]
- 34.Lee, M. J., F. Gergely, K. Jeffers, S. Y. Peak-Chew, and J. W. Raff. 2001. Msps/XMAP215 interacts with the centrosomal protein D-TACC to regulate microtubule behaviour. Nat. Cell Biol. 3643-649. [DOI] [PubMed] [Google Scholar]
- 35.Mahoney, N. M., G. Goshima, A. D. Douglass, and R. D. Vale. 2006. Making microtubules and mitotic spindles in cells without functional centrosomes. Curr. Biol. 16564-569. [DOI] [PubMed] [Google Scholar]
- 36.Maney, T., A. W. Hunter, M. Wagenbach, and L. Wordeman. 1998. Mitotic centromere-associated kinesin is important for anaphase chromosome segregation. J. Cell Biol. 142787-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin-Lluesma, S., V. M. Stucke, and E. A. Nigg. 2002. Role of Hec1 in spindle checkpoint signaling and kinetochore recruitment of Mad1/Mad2. Science 2972267-2270. [DOI] [PubMed] [Google Scholar]
- 38.Mayer, T. U., T. M. Kapoor, S. J. Haggarty, R. W. King, S. L. Schreiber, and T. J. Mitchison. 1999. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science 286971-974. [DOI] [PubMed] [Google Scholar]
- 39.McCleland, M. L., R. D. Gardner, M. J. Kallio, J. R. Daum, G. J. Gorbsky, D. J. Burke, and P. T. Stukenberg. 2003. The highly conserved Ndc80 complex is required for kinetochore assembly, chromosome congression, and spindle checkpoint activity. Genes Dev. 17101-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meraldi, P., V. M. Draviam, and P. K. Sorger. 2004. Timing and checkpoints in the regulation of mitotic progression. Dev. Cell 745-60. [DOI] [PubMed] [Google Scholar]
- 41.Mitchison, T., and M. Kirschner. 1984. Dynamic instability of microtubule growth. Nature 312237-242. [DOI] [PubMed] [Google Scholar]
- 42.Nakaseko, Y., G. Goshima, J. Morishita, and M. Yanagida. 2001. M phase-specific kinetochore proteins in fission yeast: microtubule-associating Dis1 and Mtc1 display rapid separation and segregation during anaphase. Curr. Biol. 11537-549. [DOI] [PubMed] [Google Scholar]
- 43.Niethammer, P., I. Kronja, S. Kandels-Lewis, S. Rybina, P. Bastiaens, and E. Karsenti. 2007. Discrete states of a protein interaction network govern interphase and mitotic microtubule dynamics. PLoS Biol. 5e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Connell, C. B., and A. L. Khodjakov. 2007. Cooperative mechanisms of mitotic spindle formation. J. Cell Sci. 1201717-1722. [DOI] [PubMed] [Google Scholar]
- 45.Ohi, R., T. Sapra, J. Howard, and T. J. Mitchison. 2004. Differentiation of cytoplasmic and meiotic spindle assembly MCAK functions by Aurora B-dependent phosphorylation. Mol. Biol. Cell 152895-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peset, I., J. Seiler, T. Sardon, L. A. Bejarano, S. Rybina, and I. Vernos. 2005. Function and regulation of Maskin, a TACC family protein, in microtubule growth during mitosis. J. Cell Biol. 1701057-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piekorz, R. P., A. Hoffmeyer, C. D. Duntsch, C. McKay, H. Nakajima, V. Sexl, L. Snyder, J. Rehg, and J. N. Ihle. 2002. The centrosomal protein TACC3 is essential for hematopoietic stem cell function and genetically interfaces with p53-regulated apoptosis. EMBO J. 21653-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Piel, M., and M. Bornens. 2001. Centrosome reproduction in vitro: mammalian centrosomes in Xenopus lysates. Methods Cell Biol. 67289-304. [DOI] [PubMed] [Google Scholar]
- 49.Popov, A. V., F. Severin, and E. Karsenti. 2002. XMAP215 is required for the microtubule-nucleating activity of centrosomes. Curr. Biol. 121326- 1330. [DOI] [PubMed] [Google Scholar]
- 50.Sampath, S. C., R. Ohi, O. Leismann, A. Salic, A. Pozniakovski, and H. Funabiki. 2004. The chromosomal passenger complex is required for chromatin-induced microtubule stabilization and spindle assembly. Cell 118187-202. [DOI] [PubMed] [Google Scholar]
- 51.Schneider, L., F. Essmann, A. Kletke, P. Rio, H. Hanenberg, W. Wetzel, K. Schulze-Osthoff, B. Nurnberg, and R. P. Piekorz. 2007. The transforming acidic coiled coil 3 protein is essential for spindle-dependent chromosome alignment and mitotic survival. J. Biol. Chem. 28229273-29283. [DOI] [PubMed] [Google Scholar]
- 52.Shirasu-Hiza, M., P. Coughlin, and T. Mitchison. 2003. Identification of XMAP215 as a microtubule-destabilizing factor in Xenopus egg extract by biochemical purification. J. Cell Biol. 161349-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sillje, H. H., S. Nagel, R. Korner, and E. A. Nigg. 2006. HURP is a Ran-importin beta-regulated protein that stabilizes kinetochore microtubules in the vicinity of chromosomes. Curr. Biol. 16731-742. [DOI] [PubMed] [Google Scholar]
- 54.Slep, K. C., and R. D. Vale. 2007. Structural basis of microtubule plus end tracking by XMAP215, CLIP-170, and EB1. Mol. Cell 27976-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spittle, C., S. Charrasse, C. Larroque, and L. Cassimeris. 2000. The interaction of TOGp with microtubules and tubulin. J. Biol. Chem. 27520748-20753. [DOI] [PubMed] [Google Scholar]
- 56.Tournebize, R., A. Popov, K. Kinoshita, A. J. Ashford, S. Rybina, A. Pozniakovsky, T. U. Mayer, C. E. Walczak, E. Karsenti, and A. A. Hyman. 2000. Control of microtubule dynamics by the antagonistic activities of XMAP215 and XKCM1 in Xenopus egg extracts. Nat. Cell Biol. 213-19. [DOI] [PubMed] [Google Scholar]
- 57.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 764350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tulu, U. S., C. Fagerstrom, N. P. Ferenz, and P. Wadsworth. 2006. Molecular requirements for kinetochore-associated microtubule formation in mammalian cells. Curr. Biol. 16536-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Uchida, S., T. Sekiguchi, H. Nishitani, K. Miyauchi, M. Ohtsubo, and T. Nishimoto. 1990. Premature chromosome condensation is induced by a point mutation in the hamster RCC1 gene. Mol. Cell. Biol. 10577-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vaughan, K. T. 2005. TIP maker and TIP marker; EB1 as a master controller of microtubule plus ends. J. Cell Biol. 171197-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vitre, B., F. M. Coquelle, C. Heichette, C. Garnier, D. Chretien, and I. Arnal. 2008. EB1 regulates microtubule dynamics and tubulin sheet closure in vitro. Nat. Cell Biol. 10415-421. [DOI] [PubMed] [Google Scholar]
- 62.Webster, D. R., and G. G. Borisy. 1989. Microtubules are acetylated in domains that turn over slowly. J. Cell Sci. 9257-65. [DOI] [PubMed] [Google Scholar]
- 63.Wigge, P. A., and J. V. Kilmartin. 2001. The Ndc80p complex from Saccharomyces cerevisiae contains conserved centromere components and has a function in chromosome segregation. J. Cell Biol. 152349-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilson, P. J., A. Forer, and C. Leggiadro. 1994. Evidence that kinetochore microtubules in crane-fly spermatocytes disassemble during anaphase primarily at the poleward end. J. Cell Sci. 1073015-3027. [DOI] [PubMed] [Google Scholar]
- 65.Wordeman, L., and T. J. Mitchison. 1995. Identification and partial characterization of mitotic centromere-associated kinesin, a kinesin-related protein that associates with centromeres during mitosis. J. Cell Biol. 12895-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wordeman, L., M. Wagenbach, and G. von Dassow. 2007. MCAK facilitates chromosome movement by promoting kinetochore microtubule turnover. J. Cell Biol. 179869-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang, X., S. C. Ems-McClung, and C. E. Walczak. 2008. Aurora A phosphorylates MCAK to control Ran-dependent spindle bipolarity. Mol. Biol. Cell 192752-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zimmerman, W. C., J. Sillibourne, J. Rosa, and S. J. Doxsey. 2004. Mitosis-specific anchoring of gamma tubulin complexes by pericentrin controls spindle organization and mitotic entry. Mol. Biol. Cell 153642-3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental material]