Molecular Profiling of Breast Cancer Cell Lines Defines Relevant Tumor Models and Provides a Resource for Cancer Gene Discovery (original) (raw)
Abstract
Background
Breast cancer cell lines have been used widely to investigate breast cancer pathobiology and new therapies. Breast cancer is a molecularly heterogeneous disease, and it is important to understand how well and which cell lines best model that diversity. In particular, microarray studies have identified molecular subtypes–luminal A, luminal B, ERBB2-associated, basal-like and normal-like–with characteristic gene-expression patterns and underlying DNA copy number alterations (CNAs). Here, we studied a collection of breast cancer cell lines to catalog molecular profiles and to assess their relation to breast cancer subtypes.
Methods
Whole-genome DNA microarrays were used to profile gene expression and CNAs in a collection of 52 widely-used breast cancer cell lines, and comparisons were made to existing profiles of primary breast tumors. Hierarchical clustering was used to identify gene-expression subtypes, and Gene Set Enrichment Analysis (GSEA) to discover biological features of those subtypes. Genomic and transcriptional profiles were integrated to discover within high-amplitude CNAs candidate cancer genes with coordinately altered gene copy number and expression.
Findings
Transcriptional profiling of breast cancer cell lines identified one luminal and two basal-like (A and B) subtypes. Luminal lines displayed an estrogen receptor (ER) signature and resembled luminal-A/B tumors, basal-A lines were associated with ETS-pathway and BRCA1 signatures and resembled basal-like tumors, and basal-B lines displayed mesenchymal and stem/progenitor-cell characteristics. Compared to tumors, cell lines exhibited similar patterns of CNA, but an overall higher complexity of CNA (genetically simple luminal-A tumors were not represented), and only partial conservation of subtype-specific CNAs. We identified 80 high-level DNA amplifications and 13 multi-copy deletions, and the resident genes with concomitantly altered gene-expression, highlighting known and novel candidate breast cancer genes.
Conclusions
Overall, breast cancer cell lines were genetically more complex than tumors, but retained expression patterns with relevance to the luminal-basal subtype distinction. The compendium of molecular profiles defines cell lines suitable for investigations of subtype-specific pathobiology, cancer stem cell biology, biomarkers and therapies, and provides a resource for discovery of new breast cancer genes.
Introduction
Breast cancer, a leading cause of cancer death in women, is recognized to be a molecularly heterogeneous disease. Markers such as estrogen receptor (ER), progesterone receptor (PR) and ERBB2/HER2 are used for prognostication, and to stratify patients for appropriately targeted therapies [1].
More recently, DNA microarray studies have suggested a refined classification of breast cancer, distinguishing five major subtypes based on different patterns of gene expression, underlying DNA copy number alterations (CNAs), and associated clinical outcomes [2]–[5]. Luminal subtypes A and B are ER positive and share expression markers with the luminal epithelial layer of cells lining normal breast ducts. Luminal-A tumors are genetically simple (1q/16p gain) and are associated with favorable outcome, while luminal-B tumors exhibit high proliferation rates, frequent DNA amplification (e.g. 8q24/MYC), and less favorable prognosis. Basal-like tumors share expression markers with the underlying basal (myoepithelial) layer of normal breast ducts, are ER negative, exhibit frequent chromosome segmental gains/losses, and are associated with poor outcome in most studies. The ERBB2 subtype is associated with expression of genes co-amplified with ERBB2 (encoding HER2) on chromosome cytoband 17q12, and the normal-like subtype shares expression patterns with normal breast tissue.
Breast cancer cell lines have been used widely to investigate breast cancer pathobiology, and to screen and characterize new therapeutics [6], [7]. Advantages of cell lines include the relative ease of pharmacologic and genetic manipulation, the variety of available functional assays, and, for some studies, the purity of the cancerous epithelial population (and absence of stromal cell contamination). However, while some investigators choose particular cell lines based on the known ER or HER2 status, many others rely on standard “workhorses” like MCF7 without regard to the particular tumor subtypes being modeled. The recent recognition of microarray molecular subtypes points to the need for additional consideration in cell line selection.
The goal of our study was to profile gene expression and CNAs genome-wide in a collection of 52 publicly-available and commonly-used breast cancer cell lines, in order to assess the relation of these cell lines to the recognized molecular subtypes of breast cancer, and to discover new candidate breast cancer genes and pathways.
Materials and Methods
Breast Cancer Cell Lines
184A1, BT20, BT474, BT483, BT549, Hs578T, hTERT-HME1, MCF7, MCF10A, MDA-MB134, MDA-MB157, MDA-MB175, MDA-MB231, MDA-MB361, MDA-MB436, MDA-MB453, MDA-MB468, SKBR3, T47D, UACC812, UACC893, ZR75-1 and ZR75-30 were obtained from ATCC (Manassas, VA, USA). EFM19 and EFM192A were obtained from DSMZ (Braunschweig, Germany). HCC38, HCC70, HCC202, HCC712, HCC1007, HCC1143, HCC1395, HCC1419, HCC1428, HCC1500, HCC1569, HCC1599, HCC1806, HCC1937, HCC1954, HCC2157, HCC2185, HCC2218, HCC2688 and HCC3153 were obtained from the cell repository of the Hamon Center for Therapeutic Oncology Research, UT Southwestern Medical Center (many are now available from ATCC). CAL51 was a kind gift from J. Gioanni from the Centre Antoine-Lacassagne, Nice, France. SUM44PE, SUM52PE, SUM102PT, SUM149PT and SUM190PT were kind gifts from Dr. Stephen P. Ethier (now available from Asterand, Detroit, MI). MCF10A was grown in MEGM media (Cambrex, East Rutherford, NJ). SUM52PE and SUM149PT were grown in Ham's F12 media with 5% FBS, supplemented with 5 µg/ml insulin and 1 µg/ml hydrocortisone. SUM44PE, SUM102PT and SUM190PT were grown in Ham's F12 with 0.1% BSA, supplemented with 5 µg/ml insulin, 1 µg/ml of hydrocortisone, 5 mM ethanolamine, 10 mM HEPES, 5 µg/ml transferrin, 10 nM of Triiodo Thyronin (T3) and 50 nM sodium selenite (10 ng/ml EGF was also included for SUM102PT). All other cell lines were grown in RPMI-1640 with 10% FBS and 1% Pen/Strep. Clinicopathological characteristics of cell lines are summarized in Table 1. A subset of cell lines (focused on the HCC series) was subjected to a more detailed molecular pathological characterization of ESR1, PGR, ERBB2, EGFR and BRCA1, as summarized in Table 2.
Table 1. Clinicopathological features of breast cancer cell lines.
| Cell line | Subtype# | ER* | PR* | ERBB2/HER2* | Source€ | Tumor type€ |
|---|---|---|---|---|---|---|
| 184A1 | B | − | NA | − | RM | NA |
| BT20 | A | − | − | − | PT | AC |
| BT474 | L | + | + | + | PT | IDC |
| BT483 | L | + | + | − | PT | IDC |
| BT549 | B | − | − | − | PT | IDC |
| CAL51 | B | − | NA | − | PE | AC |
| EFM19 | L | + | + | − | PE | IDC |
| EFM192A | L | + | + | + | PE | AC |
| HCC38 | B | − | − | − | PT | DC |
| HCC70 | A | − | − | − | PT | DC |
| HCC202 | L | − | − | + | PT | DC |
| HCC712 | L | + | − | − | PT | DC |
| HCC1007 | L | + | − | + | PT | DC |
| HCC1143 | A | − | − | − | PT | DC |
| HCC1187 | A | − | − | − | PT | DC |
| HCC1395 | B | − | − | − | PT | DC |
| HCC1419 | L | − | − | + | PT | DC |
| HCC1428 | L | + | + | − | PE | Met AC |
| HCC1500 | L | + | + | − | PT | DC |
| HCC1569 | A | − | − | + | PT | Met C |
| HCC1599 | A | − | − | − | PT | DC |
| HCC1806 | NA | − | − | − | PT | Sq C |
| HCC1937 | A | − | − | − | PT | DC |
| HCC1954 | A | − | − | + | PT | DC |
| HCC2157 | A | − | − | − | PT | NA |
| HCC2185 | L | − | − | − | PE | Met LC |
| HCC2218 | L | − | − | + | PT | DC |
| HCC2688 | L | − | NA | − | PT | DC |
| HCC3153 | A | − | − | − | PT | DC |
| HS578T | B | − | − | − | PT | C Sar |
| hTERT-HME1 | B | − | NA | − | RM | NA |
| MCF7 | L | + | + | − | PE | Met AC |
| MCF10A | B | − | − | − | RM | F |
| MDA134 | L | + | − | − | PE | IDC |
| MDA157 | B | − | − | − | PE | Med C |
| MDA175 | L | + | − | − | PE | IDC |
| MDA231 | B | − | − | − | PE | Met AC |
| MDA361 | L | + | + | + | BR | Met AC |
| MDA436 | B | − | − | − | PE | AC |
| MDA453 | L | − | − | +¶ | PE | Met C |
| MDA468 | A | − | − | − | PE | Met AC |
| SKBR3 | L | − | − | + | PE | AC |
| SUM44 | NA | + | + | + | PE | ILC |
| SUM52 | L | + | − | + | PE | Met C |
| SUM102 | B | − | − | − | PE | IDC, apocrine |
| SUM149 | B | − | − | − | PE | Inf |
| SUM190 | L | − | − | + | PT | Inf |
| T47D | L | + | + | − | PE | IDC |
| UACC812 | L | + | − | + | PT | IDC |
| UACC893 | L | − | − | + | PT | IDC |
| ZR75-1 | L | + | − | − | AF | IDC |
| ZR75-30 | L | + | − | + | AF | IDC |
Table 2. Molecular pathological analysis of breast cancer cell line subset.
| Cell line | Phenotype | BRCA1 | Q-PCR# ERBB2 | Q-RT-PCR* | IHC | Western | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ESR1 | PGR | ERBB2 | EGFR | ESR1 | PGR | ERBB2 | ESR1 | PGR | ERBB2 | EGFR | ||||
| HCC38 | Triple neg | 1.18 | − | − | − | − | − | − | − | − | − | − | − | |
| HCC70 | Triple neg | 0.37 | − | − | − | + | − | − | − | − | − | − | + | |
| HCC202 | ERBB2 amp | 28.88 | − | − | + | + | − | − | + | − | − | + | + | |
| HCC712 | Hormone+ | 0.95 | + | − | − | − | + | + | + | − | − | − | ||
| HCC1143 | Triple neg | 1.08 | − | − | − | + | − | − | − | − | − | − | + | |
| HCC1187 | Triple neg | 0.42 | − | − | − | − | − | − | − | − | − | + | ||
| HCC1395 | Triple neg | 0.36 | − | − | − | − | − | − | − | − | − | − | − | |
| HCC1419 | ERBB2 amp | 8.39 | − | − | + | − | − | + | − | − | + | − | ||
| HCC1428 | Hormone+ | 0.20 | + | + | − | − | + | + | − | + | + | − | − | |
| HCC1500 | Hormone+ | 0.38 | + | + | − | − | + | + | − | + | − | − | − | |
| HCC1569 | ERBB2 amp | 33.75 | − | − | + | + | − | − | + | − | − | + | + | |
| HCC1806 | Triple neg | 0.08 | − | − | − | + | − | − | − | − | − | − | + | |
| HCC1937 | Triple neg | INS C 5382 | 0.33 | − | − | − | + | − | − | − | − | − | − | + |
| HCC1954 | ERBB2 amp | 45.01 | − | − | + | + | − | − | + | − | − | + | + | |
| HCC2185 | Triple neg | 0.63 | − | − | − | + | − | − | − | − | − | + | ||
| HCC3153 | Triple neg | 943 ins 10 | 0.64 | − | − | − | + | − | − | − | − | − | − | + |
| MCF7 | Hormone+ | 0.56 | + | − | − | − | + | − | − | − | ||||
| BT483 | Hormone+ | 0.19 | + | + | − | − | + | + | − | − | ||||
| BT549 | Triple neg | 0.63 | − | − | − | + | − | − | − | + | ||||
| MDA157 | Triple neg | 0.76 | − | − | − | + | − | − | − | − | ||||
| MDA231 | Triple neg | 0.90 | − | − | − | + | − | − | − | + | ||||
| MDA453 | Triple neg | 3.88 | − | − | + | − | − | − | − | − | ||||
| MDA134 | Hormone+ | 0.76 | + | − | − | − | + | − | − | − | ||||
| MDA175 | Triple neg | 0.57 | − | − | − | − | − | − | − | − | ||||
| HMEC1585 | Control | 0.54 | − | − | − | + | − | − | − | + | ||||
| CALU3 | Control | 12.59 | − | − | + | + | − | − | + | + | ||||
| NC11 | Control | 1.75 | − | − | − | − | − | − | − | − | ||||
| DNA20 | Control | 2.00 |
RNA and DNA isolation
Cells were grown to 70–80% confluence, then harvested for total RNA and genomic DNA. For HCC lines, RNA was prepared using the Qiagen RNeasy Midi Kit (Qiagen, Valencia, CA) and DNA by phenol/chloroform extraction. For all other lines, RNA was isolated using Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol, and DNA using the Blood Cell Maxi Kit (Qiagen).
ERBB2 copy number assessment by quantitative PCR
ERBB2 copy number was quantified by real-time quantitative PCR (Q-OCR), using the Chromo4 PCR System (Bio-Rad Laboratories, Hercules, CA). GAST, located at 17q21 (on the same chromosomal arm as ERBB2) was used as a reference control. PCR primer sequences for ERBB2 and GAST are as follows (forward and reverse, respectively): ERBB2( 5′-TTGGGAGCCTGGCATTTCT-3′ and 5′-AGGTCATCGTGCCCACTCTT-3′); GAST (5′-GTAGGCATCCTTCCCCCATT-3′ and 5′-AGCCATGGTCCCTGCTTCTT-3′), with PCR product lengths of 59 and 70 base pairs, respectively. Primers were chosen by TaqMan Primer Express™ 1.5 (Applied Biosystem, Foster City, CA) and purchased from Invitrogen. PCR reactions were carried out in a final volume of 20 µl containing 20 ng genomic DNA, 300 nM each primer (for both ERBB2 and GAST, in independent reactions) and 1× Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). PCR conditions were as follows: one cycle at 95°C for 10 minutes, followed by 40 cycles each at 95°C for 15 seconds and 60°C for 1 minute. Samples were analyzed in triplicate. Each amplification reaction was checked for the absence of nonspecific PCR products by melting curve analysis. ERBB2 copy number calculation was carried out using the comparative Ct method [8] after validating that the efficiencies of PCR reactions of both ERBB2 and GAST were equal. Human Genomic DNA (DNA20) (EMD Biosciences, Darmstadt, Germany), a mixture of pooled human whole blood from 6–8 individual male and female donors, was run in every assay as a calibrator sample. ERBB2 gene copy number in normal human genomic DNA was set as 2 and copy number more than 4 in cell lines was considered to be increased.
mRNA levels of ESR1, PGR, ERBB2 and EGFR
Transcript levels of ESR1, PGR, ERBB2 and EGFR were analyzed as a part of RT2 Profiler Custom PCR Array (SuperArray Bioscience, Frederick, MD). After making cDNA from 1.0 µg total RNA using RT2 PCR Array First Strand Kit (SuperArray Bioscience), quantitative PCR was performed with the Chromo4 PCR System (Bio-Rad Laboratories) using RT2 Real-Time SYBR Green PCR Master Mix (SuperArray Bioscience) according to the manufacturer's protocol. We chose two different housekeeping genes, β-actin (ACTB) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as internal controls, using the average of their Ct values. Primers were chosen by Taqman Primer Express™ 1.5 and purchased from Invitrogen, as follows: (forward and reverse, respectively): ESR1 (5′-ATCTCGGTTCCGCATGATGAATCTGC-3′ and 5′-TGCTGGACAGAAATGTGTACACTCCAGA-3′); PGR (5′-CCTGTGGGAGCTGTAAGGTCTT-3′ and 5′-GCAGTCATTTCTTCCAGCACATA-3′), ERBB2 (5′-TGACCTGCTGGAAAAGGGGGAGCG-3′ and 5′-TCCCTGGCCATGCGGGAGAATTCAG-3′); EGFR (5′-ATAGTCGCCCAAAGTTCCGTGAGT-3′ and 5′-ACCACGTCGTCCATGTCTTCTTCA-3′); ACTB (5′ GGCTGTGCTGTGGAAGCTAAG-3′ and 5′-ATGATGGAGTTGAAGGTAGTTTCGT-3′) [9]. We also analyzed the values of NC11 (normal lymphocyte) cell line for ESR1, PGR, ERBB2 and EGFR mRNA expression, and the tumor cell values were reported relative to NC11. For data analysis, the comparative Ct method [8] was used.
Western blot analysis and immunohistochemistry (IHC)
Preparation of total cell lysates and Western blotting were done as described previously [10]. Primary antibodies used were mouse monoclonal anti-ER-α (Cell Signaling, Beverly, MA), mouse monoclonal PR (6A1) (Cell Signaling), mouse monoclonal anti-HER2 (Cell Signaling), rabbit monoclonal anti-EGFR (Cell Signaling) and mouse monoclonal anti-actin (Sigma-Aldrich). Actin levels were used as a control for protein loading. Peroxidase-labeled anti-mouse or anti-rabbit antibodies (Amersham Pharmacia, Piscataway, NJ) were used as secondary antibody. IHC on breast cancer cell lines was described previously [11].
BRCA1 mutation analysis
DNA sequence analysis was performed on the entire BRCA1 gene in available lymphocyte DNA matched to breast cancer cell lines. In the lymphocyte DNA matching HCC3153, a heterozygous duplication of 10 base pairs was detected at position 943 in exon 11 of BRCA1 (943ins10). The region of BRCA1 exon 11 containing the 943ins10 mutation was amplified from genomic DNA in the tumor cell line (HCC3153) using standard PCR conditions. Sequence analysis revealed only the mutant sequence. Absence of the normal allele was also confirmed by single strand conformation analysis as well as gel electrophoresis of the amplified fragment on 5% acrylamide denaturing gels.
Gene expression profiling
Gene expression profiling was performed on Human Exonic Evidence Based oligonucleotide (HEEBO) arrays obtained from the Stanford Functional Genomics Facility and containing 36,192 oligonucleotides representing 18,141 mapped human genes. 40 µg of sample RNA and 40 µg of “universal” reference RNA (derived from 11 different established human cell lines) were differentially labeled with Cy5 and Cy3, respectively, using an amino-allyl coupling protocol, then cohybridized onto the microarray in a high volume mixing hybridization at 65°C for 40 hrs. Details of the array processing and sample labeling/hybridization methods have been described [12]. Following hybridization, arrays were washed and scanned using a GenePix 4000B Axon scanner (Axon Instruments, Union City, CA). Fluorescence ratios were extracted using Spot Reader software (Niles Scientific, Portola Valley, CA) and uploaded to the Stanford Microarray Database [13] for storage, retrieval, and analysis. For two lines, HCC1806 and SUM44PE, expression profiling array hybridizations did not meet quality-control inspection and were excluded from analysis. The complete microarray expression data are available at the Stanford Microarray Database (SMD) (http://smd.stanford.edu) and at the Gene Expression Omnibus (GEO) (accession GSE15376); all microarray data reported in the manuscript are described in accordance with MIAME guidelines.
Gene expression profiling analysis
Background-subtracted fluorescence log2 ratios were globally normalized for each array, and then mean-centered for each gene (i.e. reporting relative to the average log ratio across all samples). Unless otherwise specified, we included for subsequent analysis only well-measured genes defined as those with fluorescence intensities in the Cy5 or Cy3 channel at least 1.5-fold above background in at least 60% of samples. For unsupervised hierarchical clustering, we included only the 8,750 well-measured genes whose expression varied at least 3-fold from the mean in at least 5 samples (Table S1). Hierarchical clustering was performed and displayed using Cluster and TreeView software (http://rana.lbl.gov/EisenSoftware.htm). Enrichment for functionally related genes was tested across a collection of 1,687 curated gene sets (C2) using Gene Set Enrichment analysis (GSEA; Release 2.0) [14]. Cell lines were classified according to breast tumor subtype (luminal-A, luminal-B, ERBB2, basal-like and normal-like) using the nearest centroid method applied to the set of “intrinsic genes” (i.e. genes with small within-specimen compared to between-specimen expression variance), as done previously [15], here using Euclidean distance. To classify breast tumors (from the Sorlie et al. dataset [3]) according to cell line subtype (luminal, basal A, or basal B), we first built a classifier by combining the top 100 genes positively and negatively correlating with each of the three “one vs. others” cell line subtype distinctions, using Significance Analysis of Microarrays (SAM) [16]. The cell line subtype classifier, comprising 484 genes, was then applied to classify primary tumors using the nearest centroid method (with Euclidean distance). We also classified each cell line as being associated with a good or bad prognosis signature (70-gene prognostic signature [17]), the presence or absence of a wound healing signature (512-gene wound signature [18]), and the presence or absence of an hypoxia signature (123-gene hypoxia signature [19]). For each signature, we calculated the gene expression centroid of the two groups of breast tumors (as determined in the original publications), and then correlated each centroid with cell line expression of the respective signature genes. Membership was assigned to the group with the highest correlation (Pearson correlation).
Array-based comparative genomic hybridization (aCGH)
Arrays for CGH were obtained from the Stanford Functional Genomics Facility. aCGH was performed using cDNA arrays containing 39,632 cDNAs, representing 22,279 mapped human genes (18,049 UniGene clusters [20], together with 4,230 additional mapped ESTs not assigned to UniGene IDs), according to previously published protocols [21], [22]. Briefly, 4 µg of genomic DNA from cell lines was random-primer labeled with Cy5 and co-hybridized onto a microarray along with 4 µg of Cy3 labeled normal leukocyte female reference DNA. Following overnight hybridization, the arrays were washed and scanned as above. The complete aCGH data are available at SMD and at GEO (accession GSE15376).
aCGH analysis
Background-subtracted log2 fluorescence ratios were normalized for each array by mean centering. Well-measured genes used for subsequent analysis were those with fluorescence intensities in the Cy3 reference channel at least 1.4 fold above background. Map positions for arrayed cDNA clones were assigned using the NCBI genome assembly, accessed through the UCSC genome browser database (NCBI Build 36.1). For genes represented by multiple arrayed cDNAs, the average log2 ratio was used. The complete processed aCGH dataset is available as Table S2. DNA gains and losses were identified using the cghFLasso (R package for Fused Lasso) method [23], which controls the false discovery rate (FDR) by using normal-normal hybridization arrays to approximate the null distribution of the test statistics (see [23] for more details). A FDR<1% was used to call gains and losses. The fraction of the genome altered was determined by calculating the fraction of genes with fluorescence ratios ≥3 (for amplifications) or with significant non-zero fused lasso calls (for gains and losses). Some analyses (where indicated) were carried out on cytobands (boundaries defined by NCBI Build 36.1) rather than individual genes. For each cell line, cytobands exhibiting CNA were defined as those with at least two genes called by cghFLasso, and the magnitude of the CNA defined as the average log2 ratio of genes within the cytoband. We defined high-level DNA amplifications and multi-copy deletions as continuous regions identified by cghFLasso with at least 50% of genes having fluorescence ratios ≥3 or ≤0.25 respectively. These sites were also checked against known copy number variants (CNVs) reported in the Database of Genomic Variants (http://projects.tcag.ca/variation). Significant associations between cytobands and gene-expression subtypes were identified using SAM with a FDR<5%.
Integrating genomic and transcriptional profiles
To integrate DNA copy number data (generated using cDNA microarrays) and gene-expression data (HEEBO oligonucleotide arrays), each gene expression measurement was first assigned a DNA copy number from either a probe interrogating the same named gene, or the average copy number of the nearest 5′ and 3′ probes (NCBI Build 36.1). Identification of genes with correlated copy number and expression was carried out using the DR-Correlate application of DR-Integrator (K. Salari, manuscript in preparation). Briefly, for each gene a modified Student's _t_-test was performed comparing gene expression levels in cell lines from the lowest and the highest deciles of all cell lines' copy number for the same gene; random permutations of sample labels were used to estimate a FDR.
Results
Transcriptional profiling identifies three breast cancer cell line subtypes
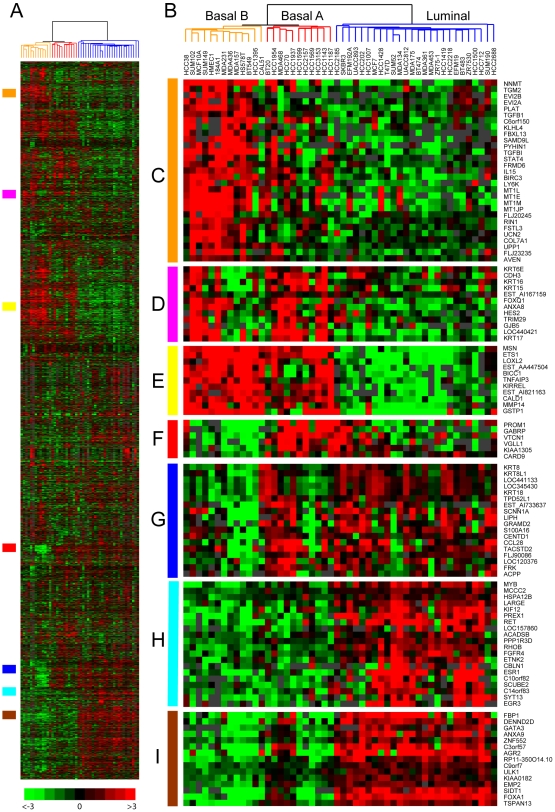
To catalog molecular variation in a collection of 52 widely-used breast cancer cell lines, we first profiled gene expression using whole genome oligonucleotide microarrays. Unsupervised hierarchical clustering of the 8,750 most variably expressed genes stratified cell lines into two main groups (see dendrogram, Fig. 1B). One group, designated “luminal” (blue dendrogram branches), contained all the ER-positive cell lines (Fig. 2A), and was characterized by the expression of ERα-regulated genes (e.g. MYB, RET, EGR3, TFF1; Fig. 1H, and not shown) [24]–[27], as well as genes associated with luminal epithelial differentiation (e.g. GATA3 and FOXA1, Fig. 1I) [28].
Figure 1. Clustering of expression profiles defines breast cancer cell line subtypes.
(A) Thumbnail “heatmap” of two-way hierarchical clustering of 50 breast cancer cell lines (columns) and 8,750 variably expressed genes (rows) (data available as Table S1). Gene expression ratios are depicted by log2 pseudocolor scale shown; gray represents poorly measured data. (B) Enlarged view of the sample dendrogram. Clustering stratifies cell lines into two main groups, luminal (blue dendrogram branches) and basal, the latter further subdivided into two subgroups, basal A (red) and basal B (orange). (C–I) Selected gene expression patterns extracted from the cluster; corresponding locations in the thumbnail are indicated by the vertical colored bars. (C) Basal-B; (D) Basal cytokeratins; (E) Basal; (F) Basal-A; (G) Luminal cytokeratins; (H) ER-associated; (I) Luminal differentiation.
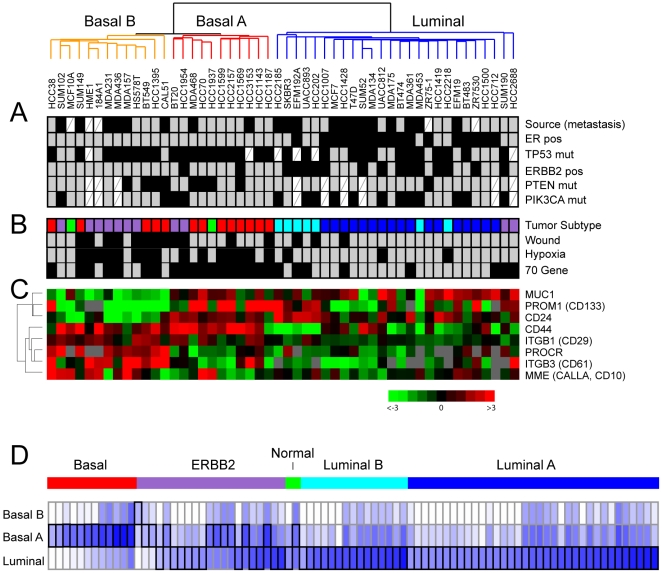
Figure 2. Subtype-specific expression and molecular characteristics.
(A) Clinical, pathological and molecular characteristics of cell line expression subtypes. Black boxes indicate metastasis derivation, ER-positivity, TP53 mutation, ERBB2/HER2 positivity, PTEN mutation, PIK3CA mutation. Mutation data compiled from the Sanger (http://www.sanger.ac.uk) and IARC (http://www-p53.iarc.fr) websites, and from refs. [94], [95]. White cross-hatched boxes indicate missing data. (B) Classification of cell lines by nearest resemblance to tumor gene-expression subtype: luminal A (dark blue), luminal B (light blue), ERBB2-associated (purple), basal-like (red) or normal-like (green); and by positivity (black boxes) for 70-gene, wound and hypoxia signature. (C) Expression levels of selected stem/progenitor cell relevant markers; log2 ratios are depicted by pseudocolor scale shown (gray represents poorly measured data). (D) Relation of tumor subtypes to cell line subtypes. Subtype of 86 tumors [3] is shown color-coded as above. Resemblance to each cell line subtype is depicted by Euclidian distance, indicated by blue intensity (representing shorter distances); best match is bracketed in black.
The other group, designated “basal”, contained only ER-negative cell lines (Fig. 2A) and was characterized by the expression of basal epithelial gene markers including MSN, ETS1, CAV1 and EGFR (Fig. 1E, and not shown) [29]–[32]. Basal cell lines were further stratified into two subgroups, designated A and B (in line with Neve et al. [33], discussed further below). The basal-A subtype (red dendrogram branches) contained many of the “HCC” lines established at UT Southwestern, including two known BRCA1 mutant lines (HCC1937, HCC3153) ([34], and this study). Basal-A lines were characterized by expression of PROM1 (aka CD133), a marker of various cancer stem cells [35], as well as other genes like GABRP and VTCN1 (Fig. 1F and 2C). Some of the basal-A lines also shared expression of luminal epithelial markers like KRT8 and KRT18 (Fig. 1G).
The basal-B subtype (orange dendrogram branches) included non-tumorigenic lines (MCF10A, hTERT-HME1, 184A1) as well as several highly invasive lines exhibiting features of epithelial-mesenchymal transition (EMT) (MDA-MB231, MDA-MB436, MDA-MB157, Hs578t) [36]. Basal-B lines were characterized by markers associated with aggressive tumor features, including PLAT (plasminogen activator) [37] and TGFB1 [38] (Fig. 1C), as well as marker phenotypes associated with normal breast and breast cancer progenitor/stem cells (MUC−/CALLA+; CD44+/CD24−/low; and ITGB3(CD61)+) (Fig. 2C) [39]–[41]. In contrast to other basal lines, the subset of mesenchymal-like basal-B lines lacked expression of basal cytokeratin markers KRT5 and KRT17 (Fig. 1D, and not shown).
Subtype-specific differences in gene expression could also be identified by pathway analysis, using Gene Set Enrichment Analysis (GSEA) [14]. Included among the top signature associations (Table 3), the luminal cell line subtype was characterized by enriched expression of ER and good prognosis signatures, basal-A by ETS pathway and BRCA1 signatures, and basal-B by EMT and epidermal growth factor (EGF) signatures.
Table 3. GSEA of breast cancer cell line subtypes.
| Subtype | Gene Set | Description | Source | FDR* |
|---|---|---|---|---|
| Luminal | BRCA_ER_POS | Correlated with ER+ in breast cancer | [17] | 0.017 |
| BRCA_PROGNOSIS_POS | Correlated with good prognosis in breast cancer | 0.094 | ||
| Basal-A | ETSPATHWAY | ETS transcription factor pathway | BioCarta | 0.063 |
| BRCA_BRCA1_POS | Correlated with BRCA1 (germline) in breast cancer | [17] | 0.063 | |
| IFN_ALL_UP | Upregulated with interferon-α,β,γ treatment | [96] | 0.071 | |
| IFNALPHA_HCC_UP | Upregulated with interferon-α treatment | [97] | 0.076 | |
| GLYCOGEN | Glycogen processing | Broad Institute | 0.078 | |
| Basal-B | JECHLINGER_EMT_UP | Upregulated in EMT | [98] | 0.040 |
| EGF_HDMEC_UP | Upregulated with EGF treatment | [99] | 0.042 | |
| DORSEY_DOXYCYCLINE_UP | Upregulated with GAB2 expression | [100] | 0.047 | |
| HTERT_DN | Downregulated with hTERT-immortalization | [101] | 0.048 | |
| HINATA_NFKB_UP | Upregulated by NF-κB | [102] | 0.049 |
In regard to molecular markers and gene mutations (Fig. 2A), the luminal subtype included all the ER-positive cancer lines (P<0.001, 2-tailed Fisher's exact test), and all but two of the ERBB2-positive lines (P = 0.002), half of which were also ER-positive. PTEN inactivating mutations and PIK3CA activating mutations, functioning on the same pathway, were mutually exclusive in all but one sample. Interestingly, PTEN mutations were more common in the combined basal-like cell lines (P = 0.020), while PIK3CA mutations were more frequent in luminal lines (P = 0.022). TP53 mutations occurred more often in basal-like lines (P = 0.038).
Relationship of breast cancer cell line and tumor subtypes
To determine the relation between breast cancer cell line subtypes (luminal, basal-A, basal-B) and breast tumor subtypes (luminal-A, luminal-B, ERBB2, basal-like, and normal-like), we first classified cell lines according to tumor subtype using a nearest centroid approach applied to the set of “intrinsic genes” used originally to define the tumor subtypes [2], [3] (see Methods) (Fig. 2B). By expression patterns, most of the luminal lines most closely resembled either luminal-A or luminal-B tumors. Most basal-A lines resembled basal-like tumors, and most basal-B lines resembled either basal-like or ERBB2 tumors (despite that none were ERBB2-positive).
We also carried out the reverse analysis, building a cell line subtype classifier to classify 86 breast tumors (from the original Stanford/Norway study defining the five tumor subtypes [3]) according to cell line subtype (see Methods) (Fig. 2D). Notably, all basal-like tumors most resembled basal-A cell lines. Luminal-A and -B tumors most resembled luminal cell lines, while ERBB2 subgroup tumors most resembled either luminal or basal-A cell lines. A similar analysis of breast tumors arising in carriers of BRCA1 mutation, analyzed from a different dataset (The Netherlands Cancer Institute) [17], revealed highest resemblance in 17 of 18 cases to basal-A lines (not shown), while two BRCA2 mutation associated cases most resembled luminal cell lines.
In addition to the above cluster-derived luminal/basal tumor subtypes, alternative breast tumor subtype classifiers have been proposed, including a 70-gene prognostic signature supervised on the metastatic/non-metastatic distinction [17], a “wound” signature trained on the serum response of cultured fibroblasts [18], and a hypoxia signature derived from the hypoxic response of cultured mammary and renal tubular epithelial cells [19]. Each of the three signatures predicts unfavorable clinical outcome. Interestingly, the basal-like lines (considered together) were those predominantly expressing the 70-gene (P = 0.001, Fisher's exact test) wound (P = 0.004), and hypoxia (P<0.001) signatures (Fig. 2B).
Genomic profiles of breast cancer cell lines
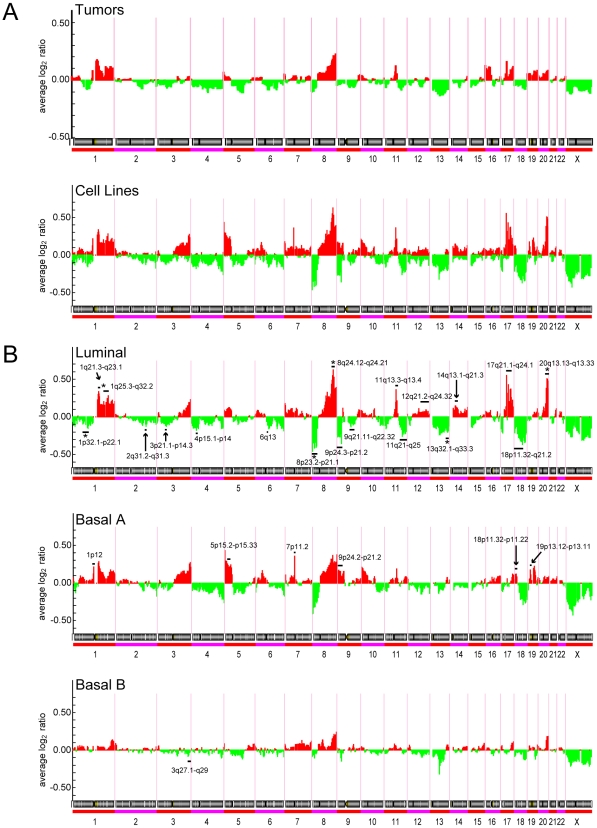
To survey DNA copy number alterations in the panel of 52 breast cancer cell lines, we carried out CGH on cDNA microarrays with validated performance characteristics [21] and covering 22,000 genes with an average mapping resolution (inter-probe distance) of <70 Kb. Across the sample set, the most frequent CNAs (called by cghFLasso–see Methods) were gains on 1q, 3q, 5p, 7p, 8q, 11q, 17q, and 20q, and losses on 3p, 4, 8p, 9p, 11q, 13q, 18p, and Xq.
Overall, the spectrum of cytoband gains and losses was similar in the cell lines compared to primary tumors (Fig. 3A), though the frequency of those CNAs was generally higher with the cell lines. Cell line subtype-specific CNAs could be identified by SAM analysis (Fig. 3B). Luminal cell lines were characterized by more frequent gains on 1q, 8q, 11q, 12q, 14q, 17q and 20q, and losses on 8p, 9p, 11q, 13q, and 18p. Of these, gains on 1q, 8q, and 20q, and losses on 1p, 8p and 13q (asterisked in Fig. 3B) also characterize luminal-B breast tumors, while 17q gain characterizes ERBB2-associated tumors [4], [5]. Notably, simple patterns characteristic of luminal-A tumors (1q+, 16p+, 16q−) were not well-represented among the luminal cell lines. Basal-A and basal-B cell lines also exhibited characteristic gains/losses (Fig. 2B), but none also selectively characteristic of basal-like tumors.
Figure 3. Genomic profiles define spectra of CNAs in cell line subtypes.
(A) Spectra of gains (red) and losses (green) across the genome, plotted as average log2 ratio, for 89 breast tumors [4], above, compared to the set of 50 cell lines (profiled for both expression and CNAs), below. (B) Spectra of gains and losses for the cell line subtypes: luminal (above), basal A (middle) and basal B (below). Statistically significant subtype-specific CNAs, called by SAM (FDR<5%), are marked by a black bar. The subset of those loci that also characterize the corresponding primary breast tumor subtype is marked by an asterisk.
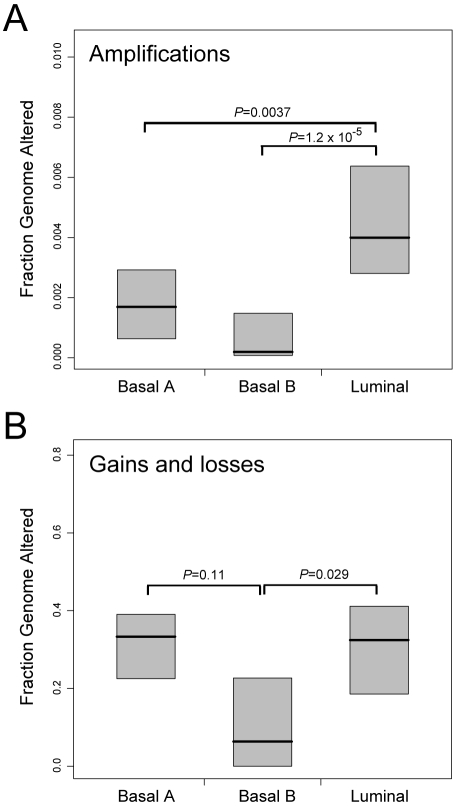
Luminal cell lines displayed overall higher frequencies of high-level DNA amplification (i.e. fluorescence ratios ≥3, corresponding to at least 5-fold amplification [21]) (Fig. 4A), a characteristic shared with luminal-B tumors [4]. Luminal and basal-A lines both exhibited overall higher frequencies of gain/loss (a characteristic feature of basal-like tumors [4]), compared to basal-B lines (Fig. 4B).
Figure 4. Cell line subtypes exhibit distinct genomic instabilities.
Fraction of genome comprising (A) high-level DNA amplification; or (B) low-level gain/loss, stratified by cell line subtype (luminal, basal-A, basal-B). Box plots show 25th, 50th and 75th percentiles; _P_-values (Students _t_-test) for pairwise comparisons are shown.
Integrated analysis for cancer gene discovery
The molecular profiles generated provide opportunities to identify breast cancer cell lines with an altered copy number and expression of known cancer genes, useful to model pathogenesis and therapy, and to discovery new breast cancer genes. For the latter, high-amplitude CNAs, i.e. high-level DNA amplifications and homozygous deletions, are particularly informative in pinpointing new cancer genes. Within the aCGH dataset we identified 80 loci of high-level amplification in 35 different cell lines, each spanning 49–49,014 Kb (median 1,115 Kb). We also identified 13 multi-copy (possibly homozygous) deletions (fluorescence ratios ≤0.25) in 8 cell lines spanning 132–7,825 Kb (median 1,477 Kb). The boundaries of amplicons/deletions did not correspond to known germline CNVs (reported in the Database of Genomic Variants), and, for the subset of recurrent alterations, finding distinct boundaries in different cell lines was more consistent with somatic alteration. Several regions of high-level amplification contained known oncogenes, like 8q24 (MYC), 11q13 (CCND1) and 17q12 (ERBB2). Other amplicons did not correspond to known oncogenes and presumably harbor novel breast cancer genes.
Gains and losses contribute to breast cancer by the increased and decreased expression of oncogenes and tumor suppressors, respectively. Using DR-Correlate (see Methods), we identified 3,511 genes (∼18% of all well-measured genes) whose altered expression correlated significantly (FDR<0.05) with altered gene copy number (Table S3). Of these, 487 resided within loci of high-amplitude CNA (Table 4). This subset included known breast cancer genes, like EGFR (7p11), FGFR1 (8p12), ERBB2 (17q12), PPM1D (17q23) and ZNF217 (20q13). This subset is likely also enriched for novel breast cancer genes, and as such represents a rich source for cancer gene discovery. Notably, among the larger group of amplified/overexpressed genes are several with known functions relevant to oncogenesis, like cell proliferation (e.g. EIF3H, HEY1, MELK, GAB2, CDC6, GRB2) [42]–[47], survival (e.g. HIPK1, MCL1, MAPKAPK2, VCP, VDAC2, APIP, MAP3K3) [48]–[54], migration/invasion (e.g. MUC1, ADAM9, SH3PXD2A, CD44, PAK1, GIT1, PTPN1 ) [55]–[61], ER-signaling (e.g. BCAS2, MUC1, NCOA3, TFAP2C ) [62]–[65], and maintenance of genome integrity (e.g. NBN, RAD21, FANCG, BUB3, RAD9A, TAOK1, RAD51C, RAE1) [66]–[73]. Also represented are several “druggable” classes [74], like kinases (e.g. HIPK1, MAPKAPK2, MELK, RPS6KB2, PAK1, TAOK1, PIP4K2B, RPS6KB1, TLK2, MAP3K3), phosphatases (e.g. PTPN1), proteases (e.g. ADAM9), G protein-coupled receptors (e.g. GPRC5C) and ion channels (e.g. VDAC2).
Table 4. High-amplitude amplifications and deletions.
| Cytoband | P-Border (nt) | Q-Border (nt) | Size (kB) | Cell Lines€ | Significant DNA-RNA Correlations# | Other notable genes≠ |
|---|---|---|---|---|---|---|
| AMPLIFICATION | ||||||
| 1p32.2 | 56946690 | 57156366 | 210 | EFM192A | ||
| 1p22.1-1p21.3 | 93549298 | 97052934 | 3504 | SUM44* | DR1, FNBP1L, ARHGAP29, ALG14 | |
| 1p13.3 | 107738670 | 109306637 | 1568 | HCC2688 | C1orf59, PRPF38B, STXBP3, GPSM2, CLCC1 | VAV3 |
| 1p13.2 | 114220960 | 115183599 | 963 | MCF7, UACC812 | AP4B1, DCLRE1B, HIPK1, TRIM33, BCAS2, CSDE1, NRAS | |
| 1q21.2 | 148738080 | 148885763 | 148 | HCC1143 | TARS2, MCL1, ENSA, GOLPH3L | |
| 1q21.2-q21.3 | 149460307 | 150130540 | 670 | HCC712, UACC812 | PIP5K1A, PSMD4, ZNF687, PI4KB, PSMB4, POGZ, SNX27, MRPL9 | |
| 1q21.3 | 151000411 | 151885402 | 885 | HCC712 | ||
| 1q22 | 153424958 | 153999982 | 575 | UACC812 | MUC1, C1orf2, CLK2, HCN3, PKLR, C1orf104, RUSC1, ASH1L, YY1AP1 | |
| 1q23.3 | 159283361 | 159357995 | 75 | SUM190 | KLHDC9 | |
| 1q32.1 | 204736293 | 205144756 | 408 | UACC812 | MAPKAPK2 | IKBKE |
| 3p14.2-p14.1 | 61765808 | 64574645 | 2809 | MCF7 | ||
| 3q26.32 | 178223920 | 180535525 | 2312 | HCC2185 | TBL1XR1, ZNF639 | PIK3CA |
| 3q29 | 194971434 | 195513283 | 542 | HCC1937 | ||
| 3q29 | 196883266 | 196931777 | 49 | HCC1937 | ||
| 4q12 | 53304442 | 54084198 | 780 | HCC1007 | SCFD2, FIP1L1 | |
| 5p15.33 | 712977 | 2811691 | 2099 | HCC1954 | ZDHHC11, PDCD6, MRPL36, NDUFS6 | TERT |
| 6p12.1 | 55358212 | 57236103 | 1878 | HCC1007 | KIAA11586, ZNF451, BAG2 | |
| 6q16.3-q21 | 104858272 | 109112665 | 4254 | HCC2185 | HACE1, ATG5, C6orf203, PDSS2, SEC63, OSTM1, SNX3, FOXO3A | |
| 6q21-q22.31 | 111961945 | 123089199 | 11127 | HCC2185 | C6orf225, HDAC2, DSE, GOPC, NUS1, ASF1A, HSF2, SERINC1 | |
| 7p15.2 | 26557965 | 27107611 | 550 | HCC1007 | ||
| 7p11.2 | 54595526 | 55931398 | 1336 | BT20, MDA468 | EGFR | |
| 7q21.13-q21.2 | 90779687 | 91868629 | 1089 | SUM52 | MTERF, AKAP9, CYP51A1, KRIT1, ANKIB1 | |
| 7q21.3 | 95239813 | 96489919 | 1250 | SUM52 | SLC25A13, SHFM1 | |
| 7q22.1 | 100294293 | 100421513 | 127 | SUM52 | SLC12A9 | |
| 8p21.3 | 21593811 | 21966432 | 373 | MDA134 | XPO7 | |
| 8p12-p11.21 | 32328805 | 41907423 | 9579 | BT483, HCC1500, HCC1599, MDA134, SUM44*, SUM52 | FUT10, C8orf41, MAK16, ZNF703, ERLIN2, PROSC, BRF2, RAB11FIP1, EIF4EBP1, ASH2L, LSM1, BAG4, DDHD2, WHSC1L1, LETM2, FGFR1, TACC1, PLEKHA2, TM2D2, ADAM9, GOLGA7, AGPAT6 | IKBKB |
| 8q12.2-q12.3 | 61817956 | 62960675 | 1143 | SUM190 | CHD7 | |
| 8q13.3 | 71707355 | 72999610 | 1292 | SKBR3 | ||
| 8q21.11-q21.13 | 79781799 | 85260376 | 5479 | EFM192A, HCC1419, HCC1599, SKBR3 | HEY1, TPD52, ZBTB10 | |
| 8q21.3-q22.1 | 89113344 | 95233478 | 6120 | EFM192A, HCC1419, SKBR3 | OSGIN2, NBN, DECR1, OTUD6B, RBM12B, TMEM67 | |
| 8q22.2-q22.3 | 100879473 | 101995283 | 1116 | HCC1419, HCC2185 | COX6C, POLR2K | |
| 8q22.3 | 104311423 | 104550566 | 239 | HCC1419 | FZD6 | |
| 8q23.1-q24.21 | 108267427 | 131134620 | 22867 | EFM192A, HCC1419, HCC1599, HCC2185, SKBR3, ZR75-30 | EIF3E, TRPS1, EIF3H, C8orf53, RAD21, TAF2, DSCC1, MRPL13, MTBP, DERL1, WDR67, C8orf76, ZHX1, ATAD2, C8orf32, FAM91A1, TMEM65, TRMT12, RNF139, TATDN1, NDUFB9, SQLE, KIAA0196, NSMCE2, FAM84B | MYC |
| 8q24.22 | 133917771 | 134337653 | 420 | ZR75-30 | PHF20L1 | |
| 8q24.3 | 141658961 | 143348731 | 1690 | HCC1419, MDA436, ZR75-30 | GPR20, FLJ43860 | |
| 8q24.3 | 144310706 | 144753628 | 443 | MDA436, ZR75-30 | ZFP41, GLI4, ZNF696, C8orf51, RHPN1, MAFA | |
| 8q24.3 | 145137850 | 146252219 | 1114 | BT483, HCC1419, MDA436, ZR75-30 | GRINA, OPLAH, SHARPIN, KIAA1833, FBXL6, CPSF1, VPS28, KIFC2, ZNF252 | |
| 9p13.3-p13.2 | 33876876 | 38058023 | 4181 | HCC2185 | UBE2R2, UBAP2, WDR40A, KIF24, KIAA1161, DCTN3, GALT, IL11RA, VCP, FANCG, PIGO, STOML2, RUSC2, TESK1, CD72, C9orf100, TLN1, CREB3, RGP1, HINT2, CLTA, RNF38, MELK, ZCCHC7, GRHPR, ZBTB5, POLR1E, FBXO10, RG9MTD3, WDR32, MCART1 | |
| 9q33.3 | 128307884 | 129195638 | 888 | SUM44* | RALGPS1 | |
| 10q21.1-q21.2 | 72507196 | 73797267 | 1290 | HCC2157 | DNAJB12 | |
| 10q22.2-q22.3 | 76461776 | 82106491 | 5645 | EFM19, HCC2157 | SAMD8, VDAC2, DLG5, POLR3A, RPS24, LOC283050, ZMIZ1, PPIF, SFTPA1, FAM22E, C10orf57, ANXA11 | |
| 10q24.33-q25.1 | 105307581 | 106054698 | 747 | EFM19 | SH3PXD2A | |
| 10q26.13 | 124598599 | 124962466 | 364 | SUM52 | IKZF5, BUB3 | |
| 11p13 | 33062705 | 35600197 | 2537 | HCC1806* | HIPK3, FBXO3, CAPRIN1, NAT10, ABTB2, CAT, APIP, PDHX, CD44 | |
| 11q13.2 | 66874536 | 67198753 | 324 | MDA134, ZR75-1 | RAD9A, RPS6KB2, CORO1B, TMEM134 | |
| 11q13.3-q13.4 | 68427956 | 70812048 | 2384 | HCC1143, HCC1500, HCC1954, MDA134, MDA175, MDA361, SUM44*, SUM190, | IGHMBP2, FADD, PPFIA1, CTTN, SHANK2 | CCND1 |
| 11q13.4 | 73316198 | 73649077 | 333 | BT474, MDA134, SUM190 | UCP2, C2CD3, PPME1 | |
| 11q13.4-q14.1 | 74648813 | 77963474 | 3315 | MDA134, SUM44*, SUM52, SUM190 | ARRB1, PRKRIR, EMSY, PHCA, PAK1, AQP11, CLNS1A, C11orf67, INTS4, NDUFC2, ALG8, GAB2, NARS2 | |
| 12p12.3 | 18727378 | 19246201 | 519 | HCC1500 | ||
| 12q21.31-q21.33 | 88265969 | 88443930 | 178 | SUM52 | WDR51B, GALNT4 | |
| 13q22.2-q31.1 | 74756931 | 78096263 | 3339 | UACC812 | UCHL3 | |
| 13q31.3-q32.1 | 90798074 | 93942902 | 3145 | UACC812 | ||
| 16q12.2 | 51800892 | 53524601 | 1724 | EFM19, SUM44* | CHD9, FTO | |
| 17p12 | 12611513 | 13636592 | 1025 | EFM192A | ELAC2 | |
| 17q11.2 | 23686912 | 24013273 | 326 | ZR75-30 | POLDIP2, TREM199, SLC46A1, PIGS, SPAG5, FLJ25006, KIAA0100, SDF2 | |
| 17q11.2 | 24894649 | 25818484 | 924 | HCC202 | TAOK1, LOC116236, GIT1, ANKRD13B, CPD | |
| 17q11.2 | 27727543 | 28293356 | 566 | SUM190 | ZNF207 | |
| 17q12 | 31206068 | 31649844 | 444 | MDA361 | FLJ12120 | |
| 17q12-q21.2 | 32627885 | 36209712 | 3582 | BT474, EFM192A, HCC202, HCC1419, HCC1569, HCC1954, HCC2218, MDA361, SKBR3, SUM190, UACC812, UACC893, ZR75-30 | ACACA, TADA2L, DDX52, SOCS7, MLLT6, CISD3, PCGF2, PSMB3, PIP4K2B, CCDC49, RPL23, LASP1, CACNB1, FAM153C, RPL19, LOC90110, FBXL20, MED1, PPP1R1B, STARD3, TCAP, PERLD1, ERBB2, C17orf37, GRB7, IKZF3, GSDML, ORMDL3, PSMD3, MED24, MSL-1, CASC3, CDC6, RARA, SMARCE1 | |
| 17q21.31 | 38419019 | 38738864 | 320 | SUM190 | RND2 | |
| 17q21.32-q25.1 | 43329972 | 50826668 | 7497 | BT474, EFM192A, HCC202, HCC712, HCC1419, HCC2218, ZR75-30 | SP2, PNPO, CDK5RAP3, SNX11, HOXB13, CALCOCO2, ATP5G1, UBE2Z, SNF8, ZNF652, PHB, SPOP, SLC35B1, FAM117A, MYST2, PDK2, XYLT2, MRPL27, LRRC59, EME1, ACSF2, RSAD1, EPN3, SPATA20, ABCC3, ANKRD40, CROP, TOB1, NME1, TOM1L1, COX11, STXBP4 | |
| 17q23.2-q24.2 | 53282667 | 63106134 | 9823 | BT474, HCC712, HCC2218, MCF7, MDA361, ZR75-30 | SFRS1, DYNLL2, MKS1, SUPT4H1, MTMR4, RAD51C, TRIM37, FAM33A, C17orf71, YPEL2, DHX40, CLTC, PTRH2, TMEM49, TUBD1, RPS6KB1, RNFT1, HEATR6, USP32, APPBP2, PPM1D, BRIP1, INTS2, MED13, METTL2A, TLK2, TANC2, CYB561, WDR68, CCDC44, MAP3K3, LYK5, CCDC47, DDX42, PSMC5, SMARCD2, DDX5, CCDC45, SMURF2, GNA13, HELZ | |
| 17q25.1 | 69755691 | 71418122 | 1662 | HCC2218, MDA361, MDA453, UACC893 | GPRC5C, SLC9A3R1, NAT9, TMEM104, FDXR, C17orf28, CDR2L, ICT1, KCTD2, SUMO2, NUP85, GGA3, MRPS7, MIF4GD, SLC25A19, GRB2, CASKIN2, TSEN54, MYO15B, SAP30BP, H3F3B, UNK, WBP2 | |
| 18q21.32-q21.33 | 55178911 | 57628085 | 2449 | HCC1500 | ||
| 19p13.2 | 14932742 | 15602448 | 670 | HCC1143 | ILVBL, BRD4, AKAP8L | |
| 19q12-q13.11 | 33966349 | 38052482 | 4086 | HCC1569, HCC1599 | UQCRFS1, POP4, PLEKHF1, C19orf2, DPY19L3, ANKRD27 | |
| 19q13.11 | 39866832 | 40146793 | 280 | HCC1599 | ||
| 19q13.42 | 60551045 | 60898029 | 347 | EFM19 | FIZ1, ZNF784, CCDC106 | |
| 19q13.43 | 63208125 | 63774724 | 567 | HCC1806* | ZNF329, ZNF274, ZNF8, ZSCAN22, ZNF324, TRIM28, CHMP2A, UBE2M | |
| 20p12.2 | 10224083 | 10433564 | 209 | HCC2185 | MKKS | |
| 20q11.22 | 32363269 | 33563203 | 1200 | BT474 | DYNLRB1, NCOA6, UQCC | |
| 20q13.12 | 42493067 | 43286511 | 793 | BT474, SUM52 | SERINC3 | |
| 20q13.12-q13.13 | 45234836 | 48636574 | 3402 | BT474, HCC1419, MCF7 | NCOA3, PREX1, ARFGEF2, STAU1, DDX27, ZNFX1, SLC9A8, SPATA2, PTPN1 | |
| 20q13.13-q13.32 | 49139330 | 57334442 | 8195 | BT474, HCC1419, MCF7, SKBR3 | ZFP64, ZNF217, BCAS1, PFDN4, C20orf108, CSTF1, C20orf43, TFAP2C, BMP7, RAE1, RBM38, RAB22A, VAPB, STX16, NPEPL1, GNAS, TH1L, ATP5E, SLMO2 | AURKA |
| 20q13.33 | 61801252 | 62370522 | 569 | HCC1419 | PRR17, OPRL1 | |
| 22q11.21 | 18256420 | 19686015 | 1430 | SUM190 | COMT, HTF9C, PI4KA | |
| 22q12.1 | 24895479 | 25885840 | 990 | HCC202 | HPS4 | |
| Xp11.23-p11.22 | 48635684 | 51225253 | 2590 | HCC712 | ||
| Xp11.22 | 52255712 | 54236019 | 1980 | HCC202 | TMEM29, PHF8 | |
| Xq28 | 148368959 | 149592006 | 1223 | HCC202 | ||
| DELETION | ||||||
| 6q16.3-q21 | 102493055 | 105832848 | 3340 | HCC1395 | HACE1 | |
| 7q11.23-q21.11 | 77246720 | 77484743 | 238 | HCC1806* | TMEM60, PHTF2 | |
| 8p23.3 | 604200 | 2080787 | 1477 | HCC2688 | ERICH1 | |
| 9p24.3-p24.2 | 958704 | 3213008 | 2254 | HCC2185 | VLDLR, KIAA0020 | |
| 9p21.2-p21.1 | 26894518 | 29207861 | 2313 | BT474, EFM19 | PLAA, IFT74 | CDKN2A |
| 13q14.3-13q21.2 | 52175620 | 60001053 | 7825 | HCC1395 | ||
| 15q24.3 | 74984799 | 75116728 | 132 | HCC1806* | RCN2 | |
| 17p12 | 11405197 | 11987872 | 583 | EFM19 | MAP2K4 | |
| 17q21.31 | 38252285 | 38419019 | 167 | HCC1806* | BRCA1 | |
| 18q11.2-q12.1 | 22256956 | 23913060 | 1656 | HCC2185 | ||
| 21q21.1 | 18342236 | 21590772 | 3249 | ZR75-30 | ||
| Xp11.3 | 46208136 | 46345060 | 137 | HCC2157 | ||
| Xq25 | 122657657 | 123338533 | 681 | HCC1806* |
Discussion
Using whole-genome DNA microarrays, we collected transcriptional and genomic profiles across a set of 52 widely used breast cancer cell lines, with the primary goals to establish their suitability in modeling known breast tumor heterogeneity, and to create a resource for cancer gene discovery. Cluster analysis of transcriptional profiles defined three cell line subtypes, one luminal and two basal (A and B), consistent with other recent studies of breast cancer cell lines [31], [33], [75]. The luminal subtype included all ER-positive cell lines, and associated gene expression patterns reflected both ER and luminal differentiation pathways, the latter including GATA3 and FOXA1, key transcriptional mediators of luminal differentiation [28], [76]. The basal-like cell lines were ER-negative and exhibited more frequent mutations of TP53 and PTEN, consistent with findings in basal-like tumors [3], [77]. The basal-A subtype exhibited enriched expression of ETS pathway genes, a pathway linked to diverse tumor phenotypes including invasion and metastasis [78]. The basal-B subtype, which included the three non-tumorigenic lines (consistent with prior studies [75]), as well as five highly invasive/metastatic lines with features of EMT, exhibited enriched expression of EMT and EGF regulated genes, the latter pathway also previously linked to basal-like tumors [79].
Recently, Neve et al. [33] profiled 51 breast cancer cell lines (though using a lower-resolution (∼1 Mb) CGH platform), 38 of which (∼3/4th) overlapped with the 52 we profiled. All the overlapping lines except for one clustered into the same corresponding gene-expression subtype in both their and our study. The exception was HCC1500, which we classified as luminal while Neve et al. labeled it as basal B. The discrepancy may reflect a cell line identification error. We note that ATCC describes the line as ER-positive, more consistent with a luminal classification.
Our comparisons of expression profiles between breast cancer cell line subtypes and breast tumor subtypes provided valuable information relevant to the suitability of cell lines in modeling known breast tumor heterogeneity. Luminal-A/B tumors best matched luminal cell lines. Notably, basal-like tumors most corresponded to basal-A cell lines. Consistent with this finding, two breast cancer cell lines from BRCA1 mutation carriers also clustered in basal-A (and basal-A lines exhibited enrichment of a BRCA1 signature), where it has been established that _BRCA1_-associated tumors share many features with sporadic basal-like tumors [80]. Interestingly, ERBB2-associated tumors matched both luminal and basal-A lines. While ERBB2 represents a distinct expression tumor subtype in multiple independent cohorts [3], [15], [81], it is noteworthy that most ERBB2 (HER2+) cell lines clustered in the luminal subtype. The basis for the discrepant ERBB2 grouping in cell lines and tumors is unclear but warrants further investigation.
It has been suggested that the origin of the luminal vs. basal breast cancer distinction reflects the transformation of different breast epithelial progenitor cell compartments [82], [83]. Breast epithelial stem/progenitor cells support mammary gland development during puberty and subsequent growth and remodeling during pregnancy [84]. A prevailing view is that breast epithelial stem cells give rise to bipotent basal/luminal progenitors, which then give rise to basal and luminal restricted progenitors, and from there to differentiated basal/myoepithelial and luminal epithelial cells [84], [85]. Bipotent human breast epithelial stem/progenitors have been characterized with the cell surface phenotype MUC−/low/CALLAlow/+ [39]. Separately, breast cancer stem cells, identified prospectively as tumor initiating cells when transplanted into immunodeficient mice, have been characterized by the surface expression phenotype CD44+/CD24−/low [40], also a presumed phenotype of normal breast epithelial stem or early progenitor cells [84].
Our transcriptional profiles of breast cancer cell lines are consistent with an origin in (or at least a likeness of the bulk cell population to) the various stem/progenitor cell compartments. Basal-B lines predominantly express CD44+/CD24−/low and MUC−/CALLA+ phenotypes characteristic of stem or bipotent progenitor cells, as well as ITGB3 (CD61), also recently characterized as a cancer stem cell marker in MMTV-wnt-1 induced murine breast cancer [41]. In contrast, basal-A lines appear mainly CD44+/CD24+, but express PROM1 (aka CD133), a marker of luminal progenitors in mice [86] also more recently characterized as a stem cell marker in BRCA1-associated breast cancer [87], while luminal lines express markers of luminal lineage restriction like GATA3 and FOXA1 [28]. Conspicuously absent from our analysis is a breast tumor subtype corresponding to the stem-cell like (and sometimes mesenchymal-like) basal-B lines. Whether basal-B lines reflect an uncommon tumor subtype not yet characterized, or else a stem/progenitor subpopulation of tumor cells enriched in culture, or even an artifact of cell culture, remains to be determined. Regardless, breast cancer cell lines are likely to prove useful for discovering new stem cell markers, and for studying stem/progenitor cell biology.
Our genomic profiles of breast cancer cell lines indicate that overall the spectra of CNAs is reflective of breast tumors, consistent with prior findings from loss of heterozygosity (LOH) analysis [11]. Overall, however, cell lines exhibited higher frequencies and greater complexities of CNAs, and seemingly more than might be explained by a higher sensitivity of detecting CNAs in stromal-free tumor cell populations. Notably absent among the luminal subtype were the “simple” karyotypes characteristic of luminal-A tumors (i.e. 1q+, 16p+/16q−). By genomic profiles, luminal cell lines shared features characteristic of luminal-B tumors, including certain subtype-specific CNAs and overall higher levels of DNA amplification. Likewise, basal-A cell lines and basal-like tumors shared the feature of high levels of chromosome segment gain/loss. However, overall only a subset of subtype-specific CNAs was preserved. Therefore, at the genomic level it is uncertain how well cell line subtypes faithfully represent tumor subtype counterparts.
Taken together, the transcriptional and genomic profiles support the conclusion that luminal and basal-A cell lines are the most appropriate cell line models of luminal-B and basal-like tumors, respectively. Further, the basal lines are likely useful models for biological studies of the 70-gene, wound and hypoxia signatures. Despite incongruent expression results, luminal lines with amplification/overexpression of ERBB2 are likely appropriate models of ERBB2-associated tumors. Our findings indicate that new cell lines are needed to more faithfully model luminal-A tumors. Currently available cell lines likely reflect certain biases in the specimen source of cell line, and/or in the culturing methods, as suggested by the predominance of HCC lines (from UT Southwestern) among the basal-A group. Different culturing methods (e.g. ref. [88]) might support the establishment of cell lines from luminal-A tumors.
Our genomic profiles also identified numerous high-level DNA amplifications and multi-copy deletions, pinpointing known and novel cancer genes. Further, by integrating the genomic and transcriptional datasets, we could define a set of candidate cancer genes residing at these loci and exhibiting both altered copy number and expression. The larger set of amplified/overexpressed genes included several known breast cancer oncogenes, as well as many plausible candidates including genes with known functions relevant to carcinogenesis, like cell proliferation, survival and motility/invasion, and genome integrity (e.g. DNA damage response). Though genes maintaining genome integrity are more typically considered candidate tumor suppressors, the overexpression of such genes has been linked to genome instability [67], [89]. The set of amplified/overexpressed genes also included many druggable targets [74], most notably several kinases. Importantly, the same cell lines used for discovery can also be used to functionally examine cancer gene candidates, for example using RNA interference to knockdown the expression of amplified oncogene candidates, and then assaying loss of tumorigenic phenotypes in cultured cells or in vivo (e.g. refs.[90], [91]). Indeed, high-throughput RNA interference approaches [92], [93] might be used to evaluate many or all of the candidate cancer genes simultaneously.
In summary, transcriptional and genomic profiling of 52 commonly used breast cancer cell lines identifies cell line subtypes, and defines the cell line subtypes that most faithfully capture the known heterogeneity of breast tumors. Specifically, luminal and basal-A lines appear to best model the features of luminal-B and basal-like tumors, while basal-B lines might inform stem cell biology. In addition, our integrated analysis of genomic and transcriptional profiles pinpoints loci and genes with altered copy number and expression, providing a rich source for discovery and future characterization of new breast cancer genes.
Supporting Information
Table S1
8,750 variably expressed genes (log2 ratios)
(1.11 MB ZIP)
Table S2
Processed aCGH data (log2 ratios)
(2.82 MB ZIP)
Table S3
Genes with significantly correlated copy number and expression
(0.13 MB TXT)
Acknowledgments
We wish to thank the SFGF for microarray manufacture, SMD for database support, and members of the Pollack lab for helpful discussion.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by grants from the NIH (CA97139; J.R.P.), the California Breast Cancer Research Program, (8KB-0135; J.R.P.), and the Longenbaugh Foundation (J.D.M.). K.S. is a Paul & Daisy Soros Fellow and fellow of the Medical Scientist Training Program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Subramaniam DS, Isaacs C. Utilizing prognostic and predictive factors in breast cancer. Curr Treat Options Oncol. 2005;6:147–159. doi: 10.1007/s11864-005-0022-1. [DOI] [PubMed] [Google Scholar]
- 2.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 3.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergamaschi A, Kim YH, Wang P, Sorlie T, Hernandez-Boussard T, et al. Distinct patterns of DNA copy number alteration are associated with different clinicopathological features and gene-expression subtypes of breast cancer. Genes Chromosomes Cancer. 2006;45:1033–1040. doi: 10.1002/gcc.20366. [DOI] [PubMed] [Google Scholar]
- 5.Chin K, DeVries S, Fridlyand J, Spellman PT, Roydasgupta R, et al. Genomic and transcriptional aberrations linked to breast cancer pathophysiologies. Cancer Cell. 2006;10:529–541. doi: 10.1016/j.ccr.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Lacroix M, Leclercq G. Relevance of breast cancer cell lines as models for breast tumours: an update. Breast Cancer Res Treat. 2004;83:249–289. doi: 10.1023/B:BREA.0000014042.54925.cc. [DOI] [PubMed] [Google Scholar]
- 7.Vargo-Gogola T, Rosen JM. Modelling breast cancer: one size does not fit all. Nat Rev Cancer. 2007;7:659–672. doi: 10.1038/nrc2193. [DOI] [PubMed] [Google Scholar]
- 8.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 9.Potemski P, Pluciennik E, Bednarek AK, Kusinska R, Kubiak R, et al. Evaluation of oestrogen receptor expression in breast cancer by quantification of mRNA. Histopathology. 2007;51:829–836. doi: 10.1111/j.1365-2559.2007.02886.x. [DOI] [PubMed] [Google Scholar]
- 10.Sato M, Vaughan MB, Girard L, Peyton M, Lee W, et al. Multiple oncogenic changes (K-RAS(V12), p53 knockdown, mutant EGFRs, p16 bypass, telomerase) are not sufficient to confer a full malignant phenotype on human bronchial epithelial cells. Cancer Res. 2006;66:2116–2128. doi: 10.1158/0008-5472.CAN-05-2521. [DOI] [PubMed] [Google Scholar]
- 11.Wistuba II, Behrens C, Milchgrub S, Syed S, Ahmadian M, et al. Comparison of features of human breast cancer cell lines and their corresponding tumors. Clin Cancer Res. 1998;4:2931–2938. [PubMed] [Google Scholar]
- 12.Bergamaschi A, Kim YH, Kwei KA, Choi Y-L, Bocanegra M, et al. CAMK1D amplification implicated in epithelial–mesenchymal transition in basal-like breast cancer. Molecular Oncology. 2008;2:327–339. doi: 10.1016/j.molonc.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demeter J, Beauheim C, Gollub J, Hernandez-Boussard T, Jin H, et al. The Stanford Microarray Database: implementation of new analysis tools and open source release of software. Nucleic Acids Res. 2007;35:D766–770. doi: 10.1093/nar/gkl1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 18.Chang HY, Sneddon JB, Alizadeh AA, Sood R, West RB, et al. Gene Expression Signature of Fibroblast Serum Response Predicts Human Cancer Progression: Similarities between Tumors and Wounds. PLoS Biol. 2004;2:E7. doi: 10.1371/journal.pbio.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chi JT, Wang Z, Nuyten DS, Rodriguez EH, Schaner ME, et al. Gene expression programs in response to hypoxia: cell type specificity and prognostic significance in human cancers. PLoS Med. 2006;3:e47. doi: 10.1371/journal.pmed.0030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuler GD. Pieces of the puzzle: expressed sequence tags and the catalog of human genes. J Mol Med. 1997;75:694–698. doi: 10.1007/s001090050155. [DOI] [PubMed] [Google Scholar]
- 21.Pollack JR, Perou CM, Alizadeh AA, Eisen MB, Pergamenschikov A, et al. Genome-wide analysis of DNA copy-number changes using cDNA microarrays. Nat Genet. 1999;23:41–46. doi: 10.1038/12640. [DOI] [PubMed] [Google Scholar]
- 22.Pollack JR, Sorlie T, Perou CM, Rees CA, Jeffrey SS, et al. Microarray analysis reveals a major direct role of DNA copy number alteration in the transcriptional program of human breast tumors. Proc Natl Acad Sci U S A. 2002;99:12963–12968. doi: 10.1073/pnas.162471999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tibshirani R, Wang P. Spatial smoothing and hot spot detection for CGH data using the fused lasso. Biostatistics. 2008;9:18–29. doi: 10.1093/biostatistics/kxm013. [DOI] [PubMed] [Google Scholar]
- 24.Gudas JM, Klein RC, Oka M, Cowan KH. Posttranscriptional regulation of the c-myb proto-oncogene in estrogen receptor-positive breast cancer cells. Clin Cancer Res. 1995;1:235–243. [PubMed] [Google Scholar]
- 25.Boulay A, Breuleux M, Stephan C, Fux C, Brisken C, et al. The Ret receptor tyrosine kinase pathway functionally interacts with the ERalpha pathway in breast cancer. Cancer Res. 2008;68:3743–3751. doi: 10.1158/0008-5472.CAN-07-5100. [DOI] [PubMed] [Google Scholar]
- 26.Inoue A, Omoto Y, Yamaguchi Y, Kiyama R, Hayashi SI. Transcription factor EGR3 is involved in the estrogen-signaling pathway in breast cancer cells. J Mol Endocrinol. 2004;32:649–661. doi: 10.1677/jme.0.0320649. [DOI] [PubMed] [Google Scholar]
- 27.Jeltsch JM, Roberts M, Schatz C, Garnier JM, Brown AM, et al. Structure of the human oestrogen-responsive gene pS2. Nucleic Acids Res. 1987;15:1401–1414. doi: 10.1093/nar/15.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kouros-Mehr H, Slorach EM, Sternlicht MD, Werb Z. GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell. 2006;127:1041–1055. doi: 10.1016/j.cell.2006.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones C, Mackay A, Grigoriadis A, Cossu A, Reis-Filho JS, et al. Expression profiling of purified normal human luminal and myoepithelial breast cells: identification of novel prognostic markers for breast cancer. Cancer Res. 2004;64:3037–3045. doi: 10.1158/0008-5472.can-03-2028. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 31.Charafe-Jauffret E, Ginestier C, Monville F, Finetti P, Adelaide J, et al. Gene expression profiling of breast cell lines identifies potential new basal markers. Oncogene. 2006;25:2273–2284. doi: 10.1038/sj.onc.1209254. [DOI] [PubMed] [Google Scholar]
- 32.Charafe-Jauffret E, Monville F, Bertucci F, Esterni B, Ginestier C, et al. Moesin expression is a marker of basal breast carcinomas. Int J Cancer. 2007;121:1779–1785. doi: 10.1002/ijc.22923. [DOI] [PubMed] [Google Scholar]
- 33.Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomlinson GE, Chen TT, Stastny VA, Virmani AK, Spillman MA, et al. Characterization of a breast cancer cell line derived from a germ-line BRCA1 mutation carrier. Cancer Res. 1998;58:3237–3242. [PubMed] [Google Scholar]
- 35.Neuzil J, Stantic M, Zobalova R, Chladova J, Wang X, et al. Tumour-initiating cells vs. cancer ‘stem’ cells and CD133: what's in the name? Biochem Biophys Res Commun. 2007;355:855–859. doi: 10.1016/j.bbrc.2007.01.159. [DOI] [PubMed] [Google Scholar]
- 36.Bae SN, Arand G, Azzam H, Pavasant P, Torri J, et al. Molecular and cellular analysis of basement membrane invasion by human breast cancer cells in Matrigel-based in vitro assays. Breast Cancer Res Treat. 1993;24:241–255. doi: 10.1007/BF01833264. [DOI] [PubMed] [Google Scholar]
- 37.Holst-Hansen C, Johannessen B, Hoyer-Hansen G, Romer J, Ellis V, et al. Urokinase-type plasminogen activation in three human breast cancer cell lines correlates with their in vitro invasiveness. Clin Exp Metastasis. 1996;14:297–307. doi: 10.1007/BF00053903. [DOI] [PubMed] [Google Scholar]
- 38.Dumont N, Bakin AV, Arteaga CL. Autocrine transforming growth factor-beta signaling mediates Smad-independent motility in human cancer cells. J Biol Chem. 2003;278:3275–3285. doi: 10.1074/jbc.M204623200. [DOI] [PubMed] [Google Scholar]
- 39.Stingl J, Eaves CJ, Zandieh I, Emerman JT. Characterization of bipotent mammary epithelial progenitor cells in normal adult human breast tissue. Breast Cancer Res Treat. 2001;67:93–109. doi: 10.1023/a:1010615124301. [DOI] [PubMed] [Google Scholar]
- 40.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vaillant F, Asselin-Labat ML, Shackleton M, Forrest NC, Lindeman GJ, et al. The mammary progenitor marker CD61/beta3 integrin identifies cancer stem cells in mouse models of mammary tumorigenesis. Cancer Res. 2008;68:7711–7717. doi: 10.1158/0008-5472.CAN-08-1949. [DOI] [PubMed] [Google Scholar]
- 42.Zhang L, Smit-McBride Z, Pan X, Rheinhardt J, Hershey JW. An oncogenic role for the phosphorylated h-subunit of human translation initiation factor eIF3. J Biol Chem. 2008 doi: 10.1074/jbc.M800956200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hulleman E, Quarto M, Vernell R, Masserdotti G, Colli E, et al. A role for the transcription factor HEY1 in glioblastoma. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gray D, Jubb AM, Hogue D, Dowd P, Kljavin N, et al. Maternal embryonic leucine zipper kinase/murine protein serine-threonine kinase 38 is a promising therapeutic target for multiple cancers. Cancer Res. 2005;65:9751–9761. doi: 10.1158/0008-5472.CAN-04-4531. [DOI] [PubMed] [Google Scholar]
- 45.Tari AM, Hung MC, Li K, Lopez-Berestein G. Growth inhibition of breast cancer cells by Grb2 downregulation is correlated with inactivation of mitogen-activated protein kinase in EGFR, but not in ErbB2, cells. Oncogene. 1999;18:1325–1332. doi: 10.1038/sj.onc.1202422. [DOI] [PubMed] [Google Scholar]
- 46.Borlado LR, Mendez J. CDC6: from DNA replication to cell cycle checkpoints and oncogenesis. Carcinogenesis. 2008;29:237–243. doi: 10.1093/carcin/bgm268. [DOI] [PubMed] [Google Scholar]
- 47.Bentires-Alj M, Gil SG, Chan R, Wang ZC, Wang Y, et al. A role for the scaffolding adapter GAB2 in breast cancer. Nat Med. 2006;12:114–121. doi: 10.1038/nm1341. [DOI] [PubMed] [Google Scholar]
- 48.Kondo S, Lu Y, Debbas M, Lin AW, Sarosi I, et al. Characterization of cells and gene-targeted mice deficient for the p53-binding kinase homeodomain-interacting protein kinase 1 (HIPK1). Proc Natl Acad Sci U S A. 2003;100:5431–5436. doi: 10.1073/pnas.0530308100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reynolds JE, Yang T, Qian L, Jenkinson JD, Zhou P, et al. Mcl-1, a member of the Bcl-2 family, delays apoptosis induced by c-Myc overexpression in Chinese hamster ovary cells. Cancer Res. 1994;54:6348–6352. [PubMed] [Google Scholar]
- 50.Reinhardt HC, Aslanian AS, Lees JA, Yaffe MB. p53-deficient cells rely on ATM- and ATR-mediated checkpoint signaling through the p38MAPK/MK2 pathway for survival after DNA damage. Cancer Cell. 2007;11:175–189. doi: 10.1016/j.ccr.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vandermoere F, El Yazidi-Belkoura I, Slomianny C, Demont Y, Bidaux G, et al. The valosin-containing protein (VCP) is a target of Akt signaling required for cell survival. J Biol Chem. 2006;281:14307–14313. doi: 10.1074/jbc.M510003200. [DOI] [PubMed] [Google Scholar]
- 52.Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science. 2003;301:513–517. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- 53.Cho DH, Lee HJ, Kim HJ, Hong SH, Pyo JO, et al. Suppression of hypoxic cell death by APIP-induced sustained activation of AKT and ERK1/2. Oncogene. 2007;26:2809–2814. doi: 10.1038/sj.onc.1210080. [DOI] [PubMed] [Google Scholar]
- 54.Samanta AK, Huang HJ, Bast RC, Jr, Liao WS. Overexpression of MEKK3 confers resistance to apoptosis through activation of NFkappaB. J Biol Chem. 2004;279:7576–7583. doi: 10.1074/jbc.M311659200. [DOI] [PubMed] [Google Scholar]
- 55.Schroeder JA, Adriance MC, Thompson MC, Camenisch TD, Gendler SJ. MUC1 alters beta-catenin-dependent tumor formation and promotes cellular invasion. Oncogene. 2003;22:1324–1332. doi: 10.1038/sj.onc.1206291. [DOI] [PubMed] [Google Scholar]
- 56.Mazzocca A, Coppari R, De Franco R, Cho JY, Libermann TA, et al. A secreted form of ADAM9 promotes carcinoma invasion through tumor-stromal interactions. Cancer Res. 2005;65:4728–4738. doi: 10.1158/0008-5472.CAN-04-4449. [DOI] [PubMed] [Google Scholar]
- 57.Seals DF, Azucena EF, Jr, Pass I, Tesfay L, Gordon R, et al. The adaptor protein Tks5/Fish is required for podosome formation and function, and for the protease-driven invasion of cancer cells. Cancer Cell. 2005;7:155–165. doi: 10.1016/j.ccr.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 58.Marhaba R, Zoller M. CD44 in cancer progression: adhesion, migration and growth regulation. J Mol Histol. 2004;35:211–231. doi: 10.1023/b:hijo.0000032354.94213.69. [DOI] [PubMed] [Google Scholar]
- 59.Adam L, Vadlamudi R, Mandal M, Chernoff J, Kumar R. Regulation of microfilament reorganization and invasiveness of breast cancer cells by kinase dead p21-activated kinase-1. J Biol Chem. 2000;275:12041–12050. doi: 10.1074/jbc.275.16.12041. [DOI] [PubMed] [Google Scholar]
- 60.Manabe R, Kovalenko M, Webb DJ, Horwitz AR. GIT1 functions in a motile, multi-molecular signaling complex that regulates protrusive activity and cell migration. J Cell Sci. 2002;115:1497–1510. doi: 10.1242/jcs.115.7.1497. [DOI] [PubMed] [Google Scholar]
- 61.Cheng A, Bal GS, Kennedy BP, Tremblay ML. Attenuation of adhesion-dependent signaling and cell spreading in transformed fibroblasts lacking protein tyrosine phosphatase-1B. J Biol Chem. 2001;276:25848–25855. doi: 10.1074/jbc.M009734200. [DOI] [PubMed] [Google Scholar]
- 62.Qi C, Zhu YT, Chang J, Yeldandi AV, Rao MS, et al. Potentiation of estrogen receptor transcriptional activity by breast cancer amplified sequence 2. Biochem Biophys Res Commun. 2005;328:393–398. doi: 10.1016/j.bbrc.2004.12.187. [DOI] [PubMed] [Google Scholar]
- 63.Wei X, Xu H, Kufe D. MUC1 oncoprotein stabilizes and activates estrogen receptor alpha. Mol Cell. 2006;21:295–305. doi: 10.1016/j.molcel.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 64.Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, et al. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277:965–968. doi: 10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- 65.Woodfield GW, Horan AD, Chen Y, Weigel RJ. TFAP2C controls hormone response in breast cancer cells through multiple pathways of estrogen signaling. Cancer Res. 2007;67:8439–8443. doi: 10.1158/0008-5472.CAN-07-2293. [DOI] [PubMed] [Google Scholar]
- 66.Varon R, Vissinga C, Platzer M, Cerosaletti KM, Chrzanowska KH, et al. Nibrin, a novel DNA double-strand break repair protein, is mutated in Nijmegen breakage syndrome. Cell. 1998;93:467–476. doi: 10.1016/s0092-8674(00)81174-5. [DOI] [PubMed] [Google Scholar]
- 67.Hauf S, Waizenegger IC, Peters JM. Cohesin cleavage by separase required for anaphase and cytokinesis in human cells. Science. 2001;293:1320–1323. doi: 10.1126/science.1061376. [DOI] [PubMed] [Google Scholar]
- 68.Kawabe T, Tsuyama N, Kitao S, Nishikawa K, Shimamoto A, et al. Differential regulation of human RecQ family helicases in cell transformation and cell cycle. Oncogene. 2000;19:4764–4772. doi: 10.1038/sj.onc.1203841. [DOI] [PubMed] [Google Scholar]
- 69.Yamamoto K, Ishiai M, Matsushita N, Arakawa H, Lamerdin JE, et al. Fanconi anemia FANCG protein in mitigating radiation- and enzyme-induced DNA double-strand breaks by homologous recombination in vertebrate cells. Mol Cell Biol. 2003;23:5421–5430. doi: 10.1128/MCB.23.15.5421-5430.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Babu JR, Jeganathan KB, Baker DJ, Wu X, Kang-Decker N, et al. Rae1 is an essential mitotic checkpoint regulator that cooperates with Bub3 to prevent chromosome missegregation. J Cell Biol. 2003;160:341–353. doi: 10.1083/jcb.200211048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Volkmer E, Karnitz LM. Human homologs of Schizosaccharomyces pombe rad1, hus1, and rad9 form a DNA damage-responsive protein complex. J Biol Chem. 1999;274:567–570. doi: 10.1074/jbc.274.2.567. [DOI] [PubMed] [Google Scholar]
- 72.Draviam VM, Stegmeier F, Nalepa G, Sowa ME, Chen J, et al. A functional genomic screen identifies a role for TAO1 kinase in spindle-checkpoint signalling. Nat Cell Biol. 2007;9:556–564. doi: 10.1038/ncb1569. [DOI] [PubMed] [Google Scholar]
- 73.French CA, Masson JY, Griffin CS, O'Regan P, West SC, et al. Role of mammalian RAD51L2 (RAD51C) in recombination and genetic stability. J Biol Chem. 2002;277:19322–19330. doi: 10.1074/jbc.M201402200. [DOI] [PubMed] [Google Scholar]
- 74.Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1:727–730. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- 75.Ross DT, Perou CM. A comparison of gene expression signatures from breast tumors and breast tissue derived cell lines. Dis Markers. 2001;17:99–109. doi: 10.1155/2001/850531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9:201–209. doi: 10.1038/ncb1530. [DOI] [PubMed] [Google Scholar]
- 77.Saal LH, Gruvberger-Saal SK, Persson C, Lovgren K, Jumppanen M, et al. Recurrent gross mutations of the PTEN tumor suppressor gene in breast cancers with deficient DSB repair. Nat Genet. 2008;40:102–107. doi: 10.1038/ng.2007.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oikawa T. ETS transcription factors: possible targets for cancer therapy. Cancer Sci. 2004;95:626–633. doi: 10.1111/j.1349-7006.2004.tb03320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Livasy CA, Karaca G, Nanda R, Tretiakova MS, Olopade OI, et al. Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Mod Pathol. 2006;19:264–271. doi: 10.1038/modpathol.3800528. [DOI] [PubMed] [Google Scholar]
- 80.Turner NC, Reis-Filho JS. Basal-like breast cancer and the BRCA1 phenotype. Oncogene. 2006;25:5846–5853. doi: 10.1038/sj.onc.1209876. [DOI] [PubMed] [Google Scholar]
- 81.Sotiriou C, Neo SY, McShane LM, Korn EL, Long PM, et al. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci U S A. 2003;100:10393–10398. doi: 10.1073/pnas.1732912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dontu G, El-Ashry D, Wicha MS. Breast cancer, stem/progenitor cells and the estrogen receptor. Trends Endocrinol Metab. 2004;15:193–197. doi: 10.1016/j.tem.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 83.Sims AH, Howell A, Howell SJ, Clarke RB. Origins of breast cancer subtypes and therapeutic implications. Nat Clin Pract Oncol. 2007;4:516–525. doi: 10.1038/ncponc0908. [DOI] [PubMed] [Google Scholar]
- 84.Molyneux G, Regan J, Smalley MJ. Mammary stem cells and breast cancer. Cell Mol Life Sci. 2007;64:3248–3260. doi: 10.1007/s00018-007-7391-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Polyak K. Breast cancer: origins and evolution. J Clin Invest. 2007;117:3155–3163. doi: 10.1172/JCI33295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sleeman KE, Kendrick H, Robertson D, Isacke CM, Ashworth A, et al. Dissociation of estrogen receptor expression and in vivo stem cell activity in the mammary gland. J Cell Biol. 2007;176:19–26. doi: 10.1083/jcb.200604065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wright MH, Calcagno AM, Salcido CD, Carlson MD, Ambudkar SV, et al. Brca1 breast tumors contain distinct CD44+/CD24- and CD133+ cells with cancer stem cell characteristics. Breast Cancer Res. 2008;10:R10. doi: 10.1186/bcr1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ince TA, Richardson AL, Bell GW, Saitoh M, Godar S, et al. Transformation of different human breast epithelial cell types leads to distinct tumor phenotypes. Cancer Cell. 2007;12:160–170. doi: 10.1016/j.ccr.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 89.Richardson C, Stark JM, Ommundsen M, Jasin M. Rad51 overexpression promotes alternative double-strand break repair pathways and genome instability. Oncogene. 2004;23:546–553. doi: 10.1038/sj.onc.1207098. [DOI] [PubMed] [Google Scholar]
- 90.Kao J, Pollack JR. RNA interference-based functional dissection of the 17q12 amplicon in breast cancer reveals contribution of coamplified genes. Genes Chromosomes Cancer. 2006;45:761–769. doi: 10.1002/gcc.20339. [DOI] [PubMed] [Google Scholar]
- 91.Streicher KL, Yang ZQ, Draghici S, Ethier SP. Transforming function of the LSM1 oncogene in human breast cancers with the 8p11–12 amplicon. Oncogene. 2007;26:2104–2114. doi: 10.1038/sj.onc.1210002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hannon GJ, Rossi JJ. Unlocking the potential of the human genome with RNA interference. Nature. 2004;431:371–378. doi: 10.1038/nature02870. [DOI] [PubMed] [Google Scholar]
- 93.Silva JM, Marran K, Parker JS, Silva J, Golding M, et al. Profiling essential genes in human mammary cells by multiplex RNAi screening. Science. 2008;319:617–620. doi: 10.1126/science.1149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hollestelle A, Elstrodt F, Nagel JH, Kallemeijn WW, Schutte M. Phosphatidylinositol-3-OH kinase or RAS pathway mutations in human breast cancer cell lines. Mol Cancer Res. 2007;5:195–201. doi: 10.1158/1541-7786.MCR-06-0263. [DOI] [PubMed] [Google Scholar]
- 95.Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, Neve RM, Kuo WL, et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008;68:6084–6091. doi: 10.1158/0008-5472.CAN-07-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Der SD, Zhou A, Williams BR, Silverman RH. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc Natl Acad Sci U S A. 1998;95:15623–15628. doi: 10.1073/pnas.95.26.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Radaeva S, Jaruga B, Hong F, Kim WH, Fan S, et al. Interferon-alpha activates multiple STAT signals and down-regulates c-Met in primary human hepatocytes. Gastroenterology. 2002;122:1020–1034. doi: 10.1053/gast.2002.32388. [DOI] [PubMed] [Google Scholar]
- 98.Jechlinger M, Grunert S, Tamir IH, Janda E, Ludemann S, et al. Expression profiling of epithelial plasticity in tumor progression. Oncogene. 2003;22:7155–7169. doi: 10.1038/sj.onc.1206887. [DOI] [PubMed] [Google Scholar]
- 99.Zhang HT, Gorn M, Smith K, Graham AP, Lau KK, et al. Transcriptional profiling of human microvascular endothelial cells in the proliferative and quiescent state using cDNA arrays. Angiogenesis. 1999;3:211–219. doi: 10.1023/a:1009085422384. [DOI] [PubMed] [Google Scholar]
- 100.Dorsey JF, Cunnick JM, Mane SM, Wu J. Regulation of the Erk2-Elk1 signaling pathway and megakaryocytic differentiation of Bcr-Abl(+) K562 leukemic cells by Gab2. Blood. 2002;99:1388–1397. doi: 10.1182/blood.v99.4.1388. [DOI] [PubMed] [Google Scholar]
- 101.Lindvall C, Hou M, Komurasaki T, Zheng C, Henriksson M, et al. Molecular characterization of human telomerase reverse transcriptase-immortalized human fibroblasts by gene expression profiling: activation of the epiregulin gene. Cancer Res. 2003;63:1743–1747. [PubMed] [Google Scholar]
- 102.Hinata K, Gervin AM, Jennifer Zhang Y, Khavari PA. Divergent gene regulation and growth effects by NF-kappa B in epithelial and mesenchymal cells of human skin. Oncogene. 2003;22:1955–1964. doi: 10.1038/sj.onc.1206198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
8,750 variably expressed genes (log2 ratios)
(1.11 MB ZIP)
Table S2
Processed aCGH data (log2 ratios)
(2.82 MB ZIP)
Table S3
Genes with significantly correlated copy number and expression
(0.13 MB TXT)