Is HPV-18 present in human breast cancer cell lines? (original) (raw)
Sir,
Although several studies have suggested an association between breast cancer and human papillomavirus (HPV) infection (de Villiers et al, 2005; Cazzaniga et al, 2009), two recent papers published in the British Journal of Cancer (Heng et al, 2009; Lawson et al, 2009) were particularly provocative. The authors claimed that both primary human breast cancers and two well-characterised breast cancer cell lines (MDA-MB-175VII and SK-Br-3) contained HPV-18, a type of HPV found with increased frequency in adenocarcinomas (Iwasawa et al, 1996; Burk et al, 2003).
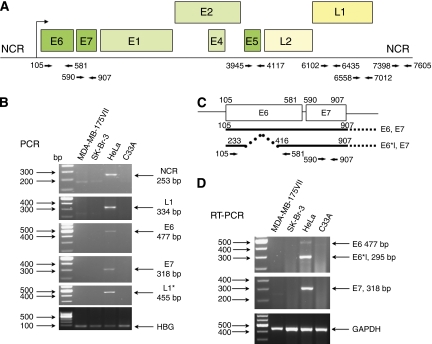
To further characterise the MDA-MB-175VII and SK-Br-3 cell lines for HPV-18 gene content and expression, we performed PCR and RT–PCR to detect viral DNA and mRNA. We also included two cell lines as controls. The HeLa cervical cancer cell line contains HPV-18 and was used as a positive control and the C33 cervical cancer cell line contains no detectable HPV genomes. All cell lines were grown in 10% FBS DMEM media to approximately 85% confluency and then harvested for DNA and RNA isolation, after which we performed standard PCR and RT–PCR reactions using the indicated primer sets for the early, late, and non-coding regions of HPV-18 (see Figure 1A). For reference, we also used the exact L1 primer sequence set that was used by Heng et al for detection of HPV-18 in MDA-MB-175VII and SK-Br-3 by in situ PCR.
Figure 1.
Absence of HPV-18 in the MDA-MB-175VII and SK-Br-3 breast cancer cell lines. (A) HPV-18 genome structure and PCR primer sets used in this study. (B) PCR for HPV-18 DNA in cell lines. The indicated primer sets were used to amplify the NCR, L1, E6 and E7 regions of HPV18 DNA. L1* indicates the primer set used in the article by Heng et al. (C) Early transcripts of HPV-18 and primer sets used for RT–PCR. (D) RT–PCR for mRNAs of HPV-18 early genes (E6 and E7).
In order to detect HPV-18 DNA, cellular DNA was isolated using a Qiagen DNA isolation kit. After performing PCR reactions (95°C for 5 min, 35 cycles: 95°C for 30 s, 55°C for 30 s and 72°C for 1 min, with a final extension at 72°C for 5 min) using different sets of primers, the PCR products were separated on a 2% agarose gel. Consistent with the studies of Schneider-Gadicke and Schwarz (1986) that mapped the genomic fragments of HPV-18 present in HeLa cells, our primer sets were able to amplify the L1, NCR, E6 and E7 regions of HPV-18 DNA in HeLa cells (panel B). C33A cells were uniformly negative (panel B). Surprisingly, the MDA-MB-175VII and SK-Br-3 breast cell lines were completely negative for HPV-18 DNA (panel B), indicating either the absence or very low abundance of HPV-18 DNA in these cell lines. As an internal control for verifying DNA quality isolated from the above cell lines, we performed PCR with primers specific for human β globin (HBG). All samples were uniformly positive for the presence of this gene (panel B, bottom).
To address the possibility that the copy number of HPV-18 genomes was extremely low in these cells and undetectable by PCR, we also performed more sensitive RT–PCR reactions to detect HPV-18 mRNA. Cellular RNA was isolated by the TRIzol method, followed by one-step RT–PCR (42°C for 60 min, 95°C for 2 min, 35 cycles: 95°C for 30 s, 55°C for 30 s and 72°C for 1 min, with a final extension at 72°C for 5 min). PCR products were separated on a 2% agarose gel. In HeLa cells we detected transcription products for the two major transforming genes of HPV-18, E6 and E7 (panel D). As expected, we also detected the long and short size variants of E6 mRNA that are generated by RNA splicing. Corresponding to our PCR data that indicated a lack of HPV DNA in the breast cells, we also found no evidence for expression of HPV-18 mRNA (panel D). To validate our RNA purification, we performed RT–PCR for GAPDH mRNA, which demonstrated that the RNA samples were of sufficient quality to detect the expression of a single copy gene.
To conclude, HPV-18 DNA and mRNA are not detectable in the MDA-MB-175VII and SK-Br-3 breast cancer cell lines, contradicting the study of Heng et al. As the E6 and E7 genes of the high-risk HPVs are retained and expressed in all HPV-induced cervical cancers (Androphy et al, 1987; Banks et al, 1987; Hawley-Nelson et al, 1989; Munger and Howley, 2002) and their cooperative interaction is required for efficient cell immortalisation and maintenance of the tumourigenic phenotype (Androphy et al, 1987; Banks et al, 1987; Hawley-Nelson et al, 1989; Munger and Howley, 2002), our results strongly indicate that HPV is not an aetiologic factor in the generation of these breast tumour cell lines. Although there may be a subset of breast cancers that are induced by HPV, the MDA-MB-175VII and SK-Br-3 cell lines clearly cannot be used to support this hypothesis and they are not valid cell lines for studying HPV-mediated transformation of breast cells.
References
- Androphy EJ, Hubbert NL, Schiller JT, Lowy DR (1987) Identification of the HPV-16 E6 protein from transformed mouse cells and human cervical carcinoma cell lines. EMBO J 6: 989–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks L, Spence P, Androphy E, Hubbert N, Matlashewski G, Murray A, Crawford L (1987) Identification of human papillomavirus type 18 E6 polypeptide in cells derived from human cervical carcinomas. J Gen Virol 68(Pt 5): 1351–1359 [DOI] [PubMed] [Google Scholar]
- Burk RD, Terai M, Gravitt PE, Brinton LA, Kurman RJ, Barnes WA, Greenberg MD, Hadjimichael OC, Fu L, McGowan L, Mortel R, Schwartz PE, Hildesheim A (2003) Distribution of human papillomavirus types 16 and 18 variants in squamous cell carcinomas and adenocarcinomas of the cervix. Cancer Res 63: 7215–7220 [PubMed] [Google Scholar]
- Cazzaniga M, Gheit T, Casadio C, Khan N, Macis D, Valenti F, Miller MJ, Sylla BS, Akiba S, Bonanni B, Decensi A, Veronesi U, Tommasino M (2009) Analysis of the presence of cutaneous and mucosal papillomavirus types in ductal lavage fluid, milk and colostrum to evaluate its role in breast carcinogenesis. Breast Cancer Res Treat 114: 599–605 [DOI] [PubMed] [Google Scholar]
- de Villiers EM, Sandstrom RE, zur Hausen H, Buck CE (2005) Presence of papillomavirus sequences in condylomatous lesions of the mamillae and in invasive carcinoma of the breast. Breast Cancer Res 7: R1–R11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley-Nelson P, Vousden KH, Hubbert NL, Lowy DR, Schiller JT (1989) HPV16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. EMBO J 8: 3905–3910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng B, Glenn WK, Ye Y, Tran B, Delprado W, Lutze-Mann L, Whitaker NJ, Lawson JS (2009) Human papilloma virus is associated with breast cancer. Br J Cancer 101: 1345–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasawa A, Nieminen P, Lehtinen M, Paavonen J (1996) Human papillomavirus DNA in uterine cervix squamous cell carcinoma and adenocarcinoma detected by polymerase chain reaction. Cancer 77: 2275–2279 [DOI] [PubMed] [Google Scholar]
- Lawson JS, Glenn WK, Heng B, Ye Y, Tran B, Lutze-Mann L, Whitaker NJ (2009) Koilocytes indicate a role for human papilloma virus in breast cancer. Br J Cancer 101: 1351–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger K, Howley PM (2002) Human papillomavirus immortalization and transformation functions. Virus Res 89: 213–228 [DOI] [PubMed] [Google Scholar]
- Schneider-Gadicke A, Schwarz E (1986) Different human cervical carcinoma cell lines show similar transcription patterns of human papillomavirus type 18 early genes. EMBO J 5: 2285–2292 [DOI] [PMC free article] [PubMed] [Google Scholar]