Magnetoreception in an avian brain in part mediated by inner ear lagena (original) (raw)
. Author manuscript; available in PMC: 2012 Mar 8.
Published in final edited form as: Curr Biol. 2011 Feb 25;21(5):418–423. doi: 10.1016/j.cub.2011.01.058
Summary
Many animals use the Earth’s geomagnetic field for orientation and navigation, but the neural mechanisms underlying that ability remain enigmatic[1, 2]. Support for at least two avian magnetoreceptors exists, including magnetically activated photo-chemicals in the retina[3, 4] and ferrimagnetic particles in the beak[5, 6]. The possibility for a third magnetoreceptor in the inner ear lagena organs has been suggested[7]. The brain must process magnetic receptor information to derive constructs representing directional heading and geosurface location. Here, we used the c-Fos transcription factor, a marker for activated neurons[8] to discover where in the brain computations related to a specific set of magnetic field stimulations occur. We found that neural activations in discrete brain loci known to be involved in orientation, spatial memory, and navigation may constitute a major magnetoreception pathway in birds. We also found, through ablation studies, that much of the observed pathway appears to receive magnetic information from the pigeon lagena receptor organs.
Results and Discussion
Birds appear to use magnetic cues to determine heading direction and location [2, 9]. Two avian magnetoreception mechanisms have been proposed. A photoreceptor where cryptochrome molecules form radical pairs in certain wavelengths of light within a magnetic field[3, 4] and a ferrimagnetic receptor in the beak that is innervated by the trigeminal nerve[5, 6]. In addition, the vestibular lagena of the inner ear contains possible ferrimagnetic compounds that could stimulate directionally selective receptor cells[7]. Indeed, lagena ablation has been shown to disrupt bird’s homing ability[10]. Consistent with a role for retinal and beak magnetoreceptors, the visual pallium[11], accessory optic system[12], and trigeminal brainstem complex[13] have all demonstrated magnetosensitivity. Yet, the primary neural pathways for magnetoreception remain largely unknown. Here, we used the c-Fos immediate early gene as a neuroanatomical marker[8] to delineate where in the pigeon brain geomagnetic information is processed. We also tested the hypothesis that lagena receptors function as magnetoreceptors, through lesion studies. Experiments were conducted using awake head-fixed pigeons in total darkness to minimize the response from retinal photopigments and an artificial rotating angle magnetic field was used to maximize responses to field inclination[14], as well as to minimize intensity responses[6]. The rotating magnetic field was delivered along differing elevations for 72 minutes (Fig. 1), then the animals were immediately euthanized and the brain processed for c-Fos labeled neurons.
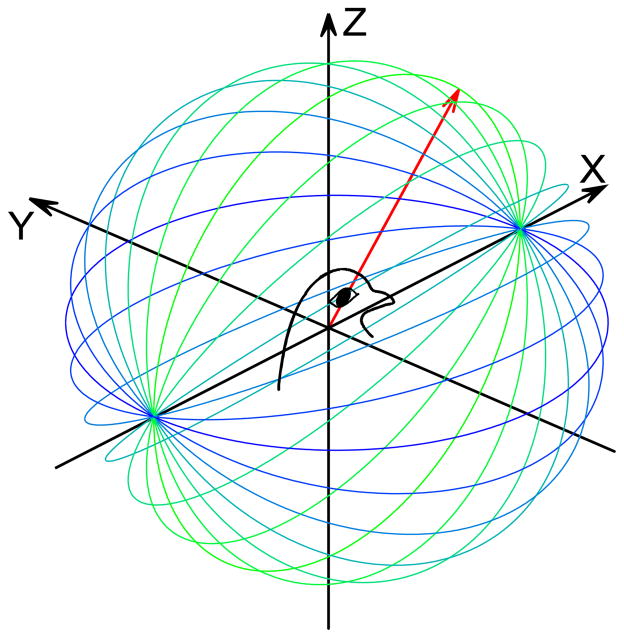
Figure 1.
Magnetic field stimulation. a) Schematic illustration of rotating (360° azimuth plane) magnetic field vector (red arrow) for 12 different elevations (blue-green lines). Thirty-six different vector planes were used for each stimulation, 12 directed along each of the X (shown), Y, and Z axes, referenced to the head-centered pigeon.
Neural pathway for magnetoreception
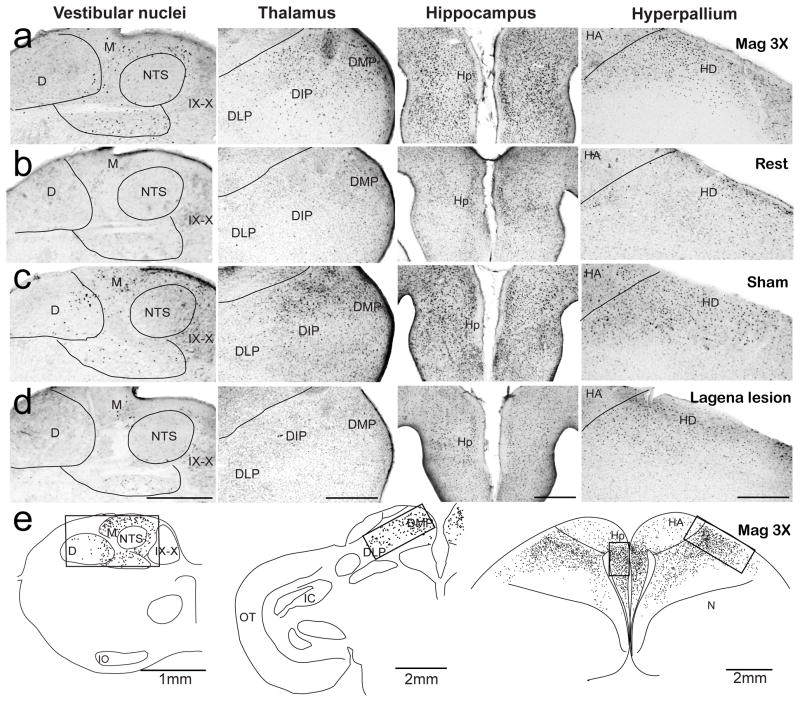
Serial brain sections of each animal were examined for c-Fos expression. In 10 pigeons either 1.5 (Mag3x; n = 7) or 0.5 (Mag1x, n = 3) Gauss magnetic field stimulation was found to elicit consistent neural activation in only four brain loci; including the posterior vestibular nuclei, dorsal thalamus, hippocampus, and visual hyperpallium (Fig. 2a). Since these activated neurons may also have included cells involved in homeostasis, arousal, or other neural tasks, c-Fos expression in control Rest birds (n=5) receiving no artificial magnetic field stimulation was also examined. In Rest animals, fewer c-Fos neurons were observed in all brain regions (Fig. 2b; Suppl. Fig. 2). To quantify neural activation, we counted c-Fos labeled cells in three alternate unilateral sections for each brain region (Fig. 2e). Left and right side counts were averaged separately and statistical comparisons showed that there were no differences between side counts for any region (p=0.91, ANOVA), thus the bilateral measures were pooled. Brainstem magnetic field activated neurons were primarily located in the posterior medial and descending vestibular nuclei (Fig. 2a). Significantly more activated cells in both the Mag3x (171 ±5, mean ±SEM) and Mag1x (62±7) stimulated birds were observed as compared to the Rest condition (14±5) (Mag3x: F(1,36)=493, Mag1x: F(1,36)=29.8, p<0.001; Fig. 3a). Further, there were nearly triple the mean number of activated neurons in the Mag3x as compared to the Mag1x pigeons, suggesting a proportional response to field intensity. In the thalamus, activated neurons were clustered in the posterior dorsomedial, dorsointermedial, and dorsolateral thalamic regions (Fig. 2a). On average, more thalamic cells were activated in Mag3x (158 ±6) than in Mag1x (78±9) birds, both being significantly higher as compared to 31±7 cells in Rest control birds (P<.001, ANOVA; Fig. 4a). Mag3x and Mag1x activated hippocampal neurons (Fig. 2a) averaged 388 ±12 and 233±18, respectively, both significantly higher than the 119 ±13 cells observed in the Rest birds (P<0.001, ANOVA; Figure 3a). In the dorsal hyperpallium (Fig. 2a), a mean of 477 ±10 Mag3x and 228±15 Mag1x activated neurons were observed, as compared to 109±12 for control Rest (Fig. 2b) birds (P<.001, ANOVA; Fig. 3a). There was a striking absence of cells in the hyperpallium apicale (primary visual Wulst). The mean density of neural activation in the hippocampus (431 ±13 cells/mm2) was significantly higher than either the vestibular nuclei (119 ±3 cells/mm2), thalamus (76 ±3 cells/mm2), or hyperpallial (239±5 cells/mm2) regions (p<.001, ANOVA).
Figure 2.
Photomicrographs and anatomical tracings for c-Fos positive neurons. a–d) Images arranged in columns for the vestibular nuclei, dorsal thalamus, hippocampus, and hyperpallium (left – right), for four stimulus conditions including Mag3x (a), Rest (b), Sham surgery (c), and lagena Lesion (d). C-Fos positive neurons identified by dark stained nuclei (immunolabel bound to the expressed c-Fos protein) are clearly visible, with the highest activation patterns exhibited in the Mag3x and Sham condition birds. Reconstructions of transverse sections with activated neurons (black dots) in the posterior vestibular nuclei (left column), dorsal thalamus (middle column), and hippocampus and dorsal hyperpallium (right column). Counting frames (boxes) used for quantification of activated cell counts are indicated. D, descending vestibular nucleus; DLP, dorsolateral posterior thalamic n.; DIP, dorsointermediate posterior thalamic n.; DMP, dorsomedial posterior thalamic n.; HA, hyperpallium apicale; HD, hyperpallium densocellulare; Hp, hippocampus; IO, inferior olivary n.; M, medial vestibular nucleus; MD; dorsal mesopallium; N, nidopallium; OT, optic tectum; ST, nucleus of the solitary tract; XII, hypoglossal n.; IX–X, glossopharyngeal and vagal motor nuclei.. Scale bar = 500 μm for (a–d). Scale bars in (e) as indicated.
Figure 3.
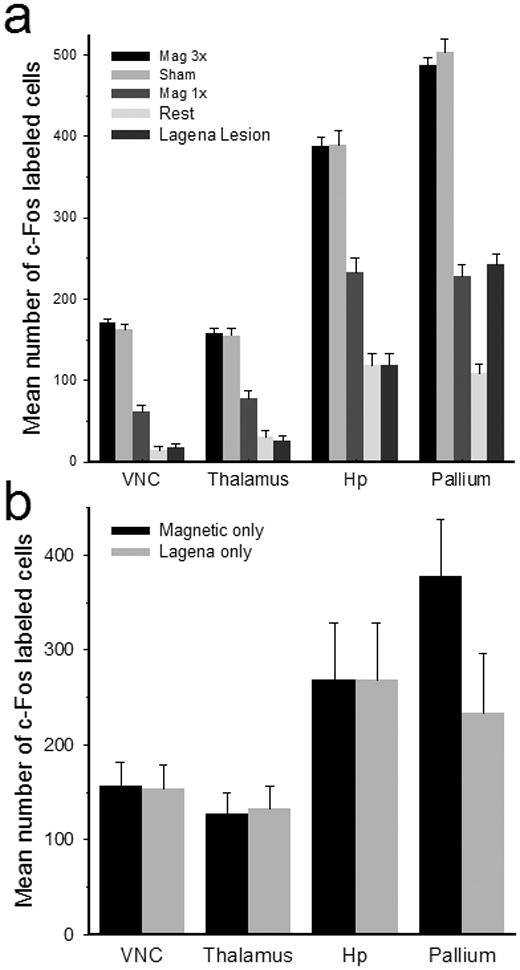
Number of activated cells for different brain regions and stimulus conditions. a) Mean number of activated neurons for all birds in the vestibular nuclei, dorsal thalamus, hippocampus, and hyperpallium for Mag3x (black), Sham (gray), Mag1x (dark gray), Rest (light gray), and lagena Lesion (light black) conditions. b) Mean values (propagation error formula) for Magnetic field only (Mag3x – Rest) and Lagena only (Mag3x – lagena Lesion) mean number of cells. Error bars = ±SEM.
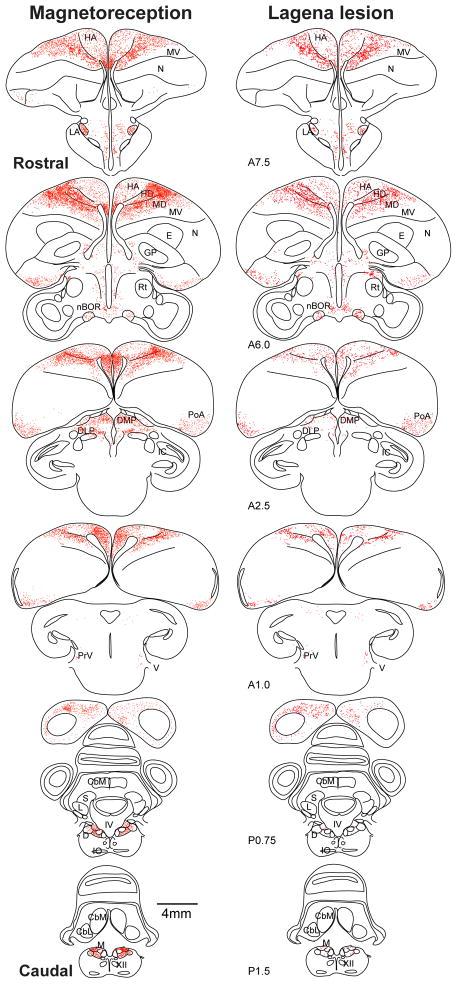
Figure 4.
Mag3x and lagena Lesion anatomical tracings. Transverse sections with all activated cells plotted (red dots) as to location to yield the neural distributions in representative birds for Mag3x (left column) and lagena Lesion (right column) conditions. Sections are arranged in rostral-to-caudal order, distances relative to AP0 (interaural axis). CbL, lateral cerebellar n.; CbM, medial cerebellar n.; GP, globus pallidus; IC, inferior colliculus; L, lateral vestibular nucleus; LA, lateral anterior thalamic n.; nBOR, n. of the basal optic root; PoA, amygdaloid complex; PrV, principal trigeminal n.; IV, forth ventricle; S, superior vestibular n. Other abbreviations as in Fig 2.
We next plotted the locations of all c-Fos positive cells throughout the brain onto anatomical tracings for each bird, as shown for one representative Mag3x animal in Figure 4. Outside the four brain loci described above, magnetic field activated neurons were less consistently observed in several additional brain areas (Supp. Fig. 1). For example, in 5/7 birds, we observed c-Fos labeled cells in the principal trigeminal nucleus (PrV), with a mean of 24±5 cells in the Mag3x birds as compared to 3±1 cells in Rest birds (p<.001, ANOVA). These findings are consistent with Heyers et. al.[13] who found a reduction in PrV activation after sectioning the ophthalmic branch of the trigeminal nerve. Avian trigeminal neural responses to magnetic field stimulation have been observed [15], as well as a dependence upon trigeminal nerve function to behaviorally detect local magnetic anomalies [16]. Taken together, these studies all support the existence of beak magnetoreceptors. We also observed magnetic field activation (Supp. Fig. 1) in the nucleus of the basal optic root (n=3), optic tectum (n=3), amygdaloid complex (n=3), ventral tegmental area (n=3), lateral anterior thalamic nucleus (n=3), and superficial parvocellular nucleus (n=6).
Lagena lesions
In 5 birds, the lagena organs were extirpated bilaterally and a 3 day survival period provided to allow lesion activation to subside [17]. Then, a Mag3x stimulation was applied. Figure 3a shows that lagena ablation significantly reduced the number of activated neurons in the vestibular nuclei, dorsal thalamus, and hippocampus, as compared to both the Mag3x and Mag1x birds ((p<.001, ANOVA; Fig. 2a). In the hyperpallium, lagena lesion reduced the number of activated neurons significantly for the Mag3x birds (p<.001, ANOVA), but was equivalent to that observed for the Mag1x condition. Next, we subtracted the Rest condition means from the Mag3x means (Propagation Error Formula) to obtain cell count estimates due to magnetic field stimulation only (Fig. 3b). Similar mean subtractions for lagena lesion values from Mag3x values provided an estimate of activated neurons due to lagena only stimulation. We found that Mag3x and Lagena responsive mean activations were similar for the vestibular nuclei, thalamus, and hippocampus regions (p>.94, Standard Equivalency Test (SET), Fig. 3B). Only the hyperpallium was significantly higher (p<.01, SET). This suggests that the magnetic field activation we observed in the vestibular nuclei, thalamus, and hippocampus was primarily due to lagena receptors, given the current test conditions, while the additional activated neurons in the hyperpallium likely arose from retina and/or beak magnetoreceptors (Supp. Fig. 3).
As a control, Sham lesion surgeries were performed in three birds, where the lagena organs were not extracted, and the birds were then exposed to the Mag3x stimulation following three days recovery. No significant differences in the number of activated neurons in any the four major brain regions were observed between Mag3x and Sham operated birds (p>.05, ANOVA, Fig. 3a).
Neural pathway for magnetoreception
Here, we describe a magnetoreception neural pathway in homing pigeons that includes loci known to be involved with orientation, spatial memory, and navigation functions. In the brainstem, vestibular nuclei neurons receive lagena afferent terminations[18], project to the avian dorsal thalamus[19], and have been reported to respond to both motion and magnetic field stimulation[20]. Thalamic regions receiving vestibular afferents in turn, project to the hippocampus[21] and to the lateral hyperpallium[22]. The dorsal lateral thalamus also receives projections from visual motion cells in the nucleus of the basal optic root[23] and optic tectum[24]. Tectal neurons respond to light-dependent magnetic field stimulation[12]. The hippocampus is involved in spatial navigation[25] that appears to depend upon functional vestibular input[26]. Hippocampal neurons also respond to magnetic field stimulation[27] and receive indirect projections from the vestibular nuclei through the thalamus[24]. Lastly, we observed magnetic field activated neurons in the hyperpallium, including Cluster N, a region that is involved in light dependent magnetoreception[11,28]. Lesions of cluster N have been shown to disrupt compass orientation in birds, while disruption of the ophthalmic branch of the trigeminal nerve did not[29]. Here, our data support findings in the garden warbler [13] suggesting that retina mediated magnetoreception projects through the thalamofugal visual pathway, as we observed magnetic field activated neurons in the nBOR, lateral dorsal thalamus, and dorsal hyperpallium[30]. Interestingly, in pigeons, neither hippocampal nor hyperpallium apicale (visual Wulst) lesions affected stored navigational map information, as initial homeward orientation from distant release sites was not significantly challenged[31]. However, hippocampal loss does affect landmark navigation, homing performance, and formation of new navigational maps [32, 33].
Lagena function as a magnetoreceptor
Geomagnetic field lines systematically vary in both intensity and direction dependent upon Earth surface location[34]. An inclination compass measures the angle (elevation) of the geomagnetic field which varies between 0° at the equator and ±90° (opposite polarity) at the North/South magnetic poles. The lagena is the third otolith organ found in fish, amphibians, reptiles, and birds, but is not present in mammals. In pigeons, the lagena lies at the base of the basilar papilla with receptor cells oriented in a parasagital plane[7, 35]. Lagena receptors are directionally tuned to changes in head tilt relative to gravity and translational motion[36]. We propose that lagena ferrimagnetic particles (not found in utricle or saccule)[7] stimulate lagena receptors whose afferents encode a “geomagnetic vector”, where direction and magnitude are referenced to gravity. The vector direction would encode the difference angle between the geomagnetic field inclination and gravity; independent of head orientation. Vector magnitude would encode field intensity and could aid in location determination due to specific local variations in geomagnetic intensities[2,34]. Through convergence of multisensory cues, the brain could use lagena information to help determine heading direction and location relative a geomagnetic map, as needed for accurate navigation[9, 26, 37, 38]. Since many vertebrate species possess lagenas, understanding their role in magnetoreception may be key to learning how these animals know where they are and where they’re headed.
Experimental procedures
Subjects and stimulation
Twenty three homing pigeons (Columba livia) were used in accordance with the National Institutes of Health Guidelines and the Animal Care and Use Committee at Washington University. In order to eliminate vestibular responses to head motion[39, 40] and to reduce potential contributions from retinal and beak magnetoreceptors[3,4,41], each bird was awake and placed head-fixed (implanted head-stud)[39] in the dark in the center of a 3D magnetic coil frame (Fig. 1a). The ambient geomagnetic field was measured at the bird’s head using a 3-axis magnetometer (HMC2003, Honywell), then was actively canceled. An artificial magnetic field was generated through three pairs of Helmholtz coils (61cm cube) using a direct current source and consisted of either a 1.5 Gauss (150,000nT; ~3x intensity of home laboratory) or 0.5 Gauss (50,000nT) vector that rotated through the 360° azimuth along each of 36 different elevation angles (12 each for X, Y, and Z axes; 15° increments; Fig. 1a). Each magnetic field vector rotation required 2 minutes duration, for a total stimulation period of 72 (2 × 36) minutes. No confounding radio frequency, acoustic noise, or temperature changes were observed during the magnetic field stimulation.
In the first magnetic stimulation group (Mag3x, n=7) the 1.5 Gauss intensity field was delivered. In a second group (Rest, n=5), animals were placed head-fixed in the coil frame in the dark, but no artificial magnetic field was applied and the ambient geomagnetic field (inclination 67.193°, mean intensity: 0.5314 G) was not canceled. In a third group (Lesion, n=5), the vestibular lagena receptors were bilaterally removed. A three day recovery period followed to allow decline of lesion induced c-Fos expression[17], then the birds were exposed to Mag3x stimulation. In a fourth group (Sham, n = 3), identical surgical lesion procedures were performed except that the lagenas were not removed; then after 3 days recovery birds were exposed to the Mag3x stimulation. In a fifth group (Mag1x, n=3), a rotating magnetic field vector of 0.5 Gauss intensity was delivered.
Immunohistochemistry
Each pigeon was immediately perfused following stimulation (4% paraformaldehyde). The brain was blocked 3mm anterior to the interaural axis (AP0), removed, then post-fixed for 12 hours. Serial brain sections (50 μm) were cut, incubated with 0.5% H2O2 in 90% methanol[18], followed by 0.5% NaBH4 and permeabilized in 0.05% Tween-20. Sections were then treated in 5% Normal Goat Serum (Vector Laboratories Inc.), rinsed and incubated in anti-c-Fos primary (1:2000, rabbit c-Fos Ab = K-25, sc-253, Santa Cruz, 48 hours). The secondary antibody (Biotin-SP-AffiniPure Goat Anti-Rabbit IgG, Jackson ImmunoResearch Laboratories, 1:400) was applied for 90 min, followed by AB solution (Vector ABC kit, Vector Laboratories) diluted 1:280, then incubated in 0.5 mg/ml diaminobenzidene peroxidase solution to visualize the c-Fos-antibody complexes. Sections were mounted onto slides and counterstained. For 9 of the birds, 3 brain sets (one magnetic field, one lagena lesion, and one sham bird) were processed simultaneously using the same chemistry. Cell counts from these animals were no different from those of similar group conditions processed individually or in pairs, as determined from the 95% confidence intervals.
Analyses
All c-Fos positive cells in the brain were plotted relative to AP0 in alternate traced sections (60 – 65/bird) for each animal, using video microscopy and an anatomical reconstruction program[18]. For quantification, c-Fos positive cells were counted in box frames in the vestibular nuclei (1.6 × 0.9mm), dorsal thalamus (2.3 × 0.9mm), medial hippocampus (0.9 × 1.0mm), dorsal hyperpallium (2.3 × 0.9mm), and trigeminal nucleus (1.5 × 0.8mm). Photo-micrographs at equal light intensity for all sections (4×) were converted to gray scale and cropped to the counting frame size for each region. The number of c-Fos positive cells were quantified using an automated analysis program written for the Python environment (v2.7.1), by an experimenter blind to stimulation condition. The program compared adjacent pixel contrast within a specified marker size (<5μm), using a filter threshold (set at 0.3, range from 0 (pure black) to 1.0 (pure white)), with markers placed on nuclei that passed criterion threshold. For each counting frame, the measurements were repeated and identical values were obtained each time, thus validating the quantification procedure.
Statistical comparisons were made using a multifactor repeated measures analysis of variance, with planned follow-up comparisons (ANOVA - Statistica). Factors included stimulus condition and left/right sides, while brain region was treated as a repeated measure (4 levels). Comparisons for the subtraction distribution means were performed using the propagation error formula (A−B =√((SDA)2 + (SDB)2) and significance was determined using the Standard Equivalency Test (|A−B| ≤2√((SDA)2 + (SDB)2).
Supplementary Material
01
Acknowledgments
We thank Sandra Aamodt, Dora Angelaki, Steve Lisberger, and Kim McArthur, for comments; as well as Isabel Acevedo, Katy Reed, Candice Ward, Casey Carrol, and Jian Chen for technical assistance. This work was supported by a National Institute for Deafness and Other Communication Disorders grant (DC007618).
Footnotes
Supplemental information: Supplemental information includes three figures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lohmann KL. Magnetic-field perception. Nature. 2010;464:1140–1142. doi: 10.1038/4641140a. [DOI] [PubMed] [Google Scholar]
- 2.Wiltschko R, Wiltschko W. Magnetic orientation and magnetoreception in birds and other animals. J Comp Physiol. 2005;191:675–693. doi: 10.1007/s00359-005-0627-7. [DOI] [PubMed] [Google Scholar]
- 3.Möller A, Sagasser S, Wiltschko W, Schierwater B. Retinal cryptochrome in a migratory passerine bird: a possible transducer for the avian magnetic compass. Naturwissenschaften. 2004;91:585–588. doi: 10.1007/s00114-004-0578-9. [DOI] [PubMed] [Google Scholar]
- 4.Ritz T, Wiltschko R, Hore PJ, Rogers CT, Stapput K, Thalau P, Timmel CR, Wiltschko W. Magnetic compass of birds is based on a molecule with optimal directional sensitivity. Biophys J. 2009;96:3451–3457. doi: 10.1016/j.bpj.2008.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleissner G, Stahl B, Thalau P, Falkenberg G, Fleissner G. A novel concept of Fe-mineral-based magnetoreception: histological and physicochemical data from the upper beak of homing pigeons. Naturwissenschaften. 2007;94:631–642. doi: 10.1007/s00114-007-0236-0. [DOI] [PubMed] [Google Scholar]
- 6.Wiltschko R, Schiffner I, Fuhrmann P, Wiltschko W. The role of the magnetite-based receptors in the beak in pigeon homing. Current Biol. 2010;20:1–5. doi: 10.1016/j.cub.2010.06.073. [DOI] [PubMed] [Google Scholar]
- 7.Harada Y, Taniguchi M, Namatame H, Iida A. Magnetic materials in otoliths of bird and fish lagena and their function. Acta Otolaryngol. 2001;121:590–595. [PubMed] [Google Scholar]
- 8.Heimovics SA, Riters LV. Breeding-context-dependent relationships between song and cFOS labeling within social behavior brain regions in male European starlings (Sturnus vulgaris) Horm Behav. 2006;50:726–735. doi: 10.1016/j.yhbeh.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lohmann KJ, Lohmann CMF, Putman NF. Magnetic maps in animals: nature’s GPS. J Exp Biol. 2007;210:3697–3705. doi: 10.1242/jeb.001313. [DOI] [PubMed] [Google Scholar]
- 10.Harada Y. Experimental analysis of behavior of homing pigeons as a result of functional disorders of their lagena. Acta Otolaryngol. 2002;122:132–137. doi: 10.1080/00016480252814126. [DOI] [PubMed] [Google Scholar]
- 11.Mouritsen H, Feenders G, Liedvogel M, Wada K, Jarvis E. Night-vision brain area in migratory song birds. PNAS. 2005;102:8339–8344. doi: 10.1073/pnas.0409575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Semm P, Demaine C. Neurophysiological properties of magnetic cells in the pigeon’s visual system. J Comp Physiol. 1986;159:619–625. doi: 10.1007/BF00612035. [DOI] [PubMed] [Google Scholar]
- 13.Heyers D, Zapka M, Hoffmeister M, Wild JM, Mouritsen H. Magnetic field changes activate the trigeminal brainstem complex in a migratory bird. PNAS. 2010;107:9394–9399. doi: 10.1073/pnas.0907068107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiltschko W, Wiltschko R. Magnetic compass of European robins. Science. 1972;176:62–64. doi: 10.1126/science.176.4030.62. [DOI] [PubMed] [Google Scholar]
- 15.Semm P, Beason RC. Responses to small magnetic variations by the trigeminal system of the bobolink. Brain Res Bull. 1990;25:735–740. doi: 10.1016/0361-9230(90)90051-z. [DOI] [PubMed] [Google Scholar]
- 16.Mora CV, Davison M, Wild JM, Walker MM. Magnetoreception and its trigeminal mediation in the homing pigeon. Nature. 2004;432:508–511. doi: 10.1038/nature03077. [DOI] [PubMed] [Google Scholar]
- 17.Kim MS, Kim JH, Jin Y, Kry D, Park BR. Temporal changes of cFos-like protein expression in medial vestibular nuclei following arsanilate-induced unilateral labyrinthectomy in rats. Neurosci Lett. 2002;319:9–12. doi: 10.1016/s0304-3940(01)02422-3. [DOI] [PubMed] [Google Scholar]
- 18.Dickman JD, Fang Q. Differential central projections of vestibular afferents in pigeons. J Comp Neurol. 1996;367:110–131. doi: 10.1002/(SICI)1096-9861(19960325)367:1<110::AID-CNE8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Vollrath FW, Delius JD. Vestibular projections to the thalamus of the pigeon. Brain Behav Evol. 1976;13:56–68. [PubMed] [Google Scholar]
- 20.Semm P, Nohr D, Demaine C, Wiltschko W. Neural basis of the magnetic compass: interactions of visual, magnetic and vestibular inputs in the pigeon’s brain. J Comp Physiol. 1984;155:283–288. [Google Scholar]
- 21.Montagnese CM, Mezey SE, Csillag A. Efferent connections of the dorsomedial thalamic nuclei in the domestic chick (Gallus domesitcus) J Comp Neurol. 2003;459:301–326. doi: 10.1002/cne.10612. [DOI] [PubMed] [Google Scholar]
- 22.Heyers D, Manns M, Luksch H, Güntürkün O, Mouritsen H. A visual pathway links brain structures active during magnetic compass orientation in migratory birds. PLOS One. 2007;2:e927–e937. doi: 10.1371/journal.pone.0000937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wylie DRW, Linkenhoker B, Lau KL. Projections of the nucleus of the basal optic root in pigeons (Columba livia) revealed with biotinylated dextran amine. J Comp Neurol. 1997;384:517–536. doi: 10.1002/(sici)1096-9861(19970811)384:4<517::aid-cne3>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 24.Korzeniewska E, Güntürkün O. Sensory properties and afferents of the N. dorsolateralis posterior thalami of the pigeon. J Comp Neurol. 1990;292:457–479. doi: 10.1002/cne.902920311. [DOI] [PubMed] [Google Scholar]
- 25.Bingman VP, Hough GE, Kahn MC, Siegel JJ. The homing pigeon hippocampus and space: in search of adaptive specialization. Brain Behav Evol. 2003;62:117–127. doi: 10.1159/000072442. [DOI] [PubMed] [Google Scholar]
- 26.Stackman RW, Clark AS, Taube JS. Hippocampal spatial representations require vestibular input. Hippocampus. 2002;12:291–303. doi: 10.1002/hipo.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vargas JP, Siegel J, Bingman VP. The effects of a changing ambient magnetic field on single-unit activity in the homing pigeon hippocampus. Brain Res Bull. 2006;70:158–164. doi: 10.1016/j.brainresbull.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 28.Liedvogel M, Feenders G, Wada K, Troje NF, Jarvis ED, Mouritsen H. Lateralized activation of Cluster N in the brains of migratory songbirds. Eur J Neurosci. 2007;25:1166–1177. doi: 10.1111/j.1460-9568.2007.05350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zapka M, Heyers D, Hein CM, Engels S, Schneider N, Hans J, Weiler S, Dreyer D, Kishkinev D, Wild JM, Mouritsen H. Visual but not trigeminal medication of magnetic compass information in a migratory bird. Nature. 2009;461:1274–1277. doi: 10.1038/nature08528. [DOI] [PubMed] [Google Scholar]
- 30.Reiner A, Yamamoto K, Karten HJ. Organization and evolution of the avian forebrain. Anat Rec. 2005;287:1080–1102. doi: 10.1002/ar.a.20253. [DOI] [PubMed] [Google Scholar]
- 31.Bingman VP, Bagnoli P, Ioale P, Casini G. Homing behavior of pigeons after telencephalic ablations. Brain Behav Evol. 1984;24:94–108. doi: 10.1159/000121308. [DOI] [PubMed] [Google Scholar]
- 32.Bingman VP, Yates G. Hippocampal lesions impair navigational learning in experienced homing pigeons. Beh Neurosci. 1992;106:229–232. doi: 10.1037//0735-7044.106.1.229. [DOI] [PubMed] [Google Scholar]
- 33.Bingman VP, Ioale P, Casini G, Bagnoli P. The avian hippocampus: evidence for a role in the development of the homing pigeon navigational map. Beh Neurosci. 1990;104:906–911. doi: 10.1037//0735-7044.104.6.906. [DOI] [PubMed] [Google Scholar]
- 34.Johnsen S, Lohmann KJ. The physics and neurobiology of magnetoreception. Nature Neuroscience. 2005;6:703–712. doi: 10.1038/nrn1745. [DOI] [PubMed] [Google Scholar]
- 35.Jorgensen JM, Andersen T. On the structure of the avian maculae. Acta Zool. 1973;54:121–130. [Google Scholar]
- 36.Baird RA, Lewis ER. Correspondences between afferent innervation patterns and response dynamics in the bullfrog utricle and lagena. Brain Res. 1986;369:48–64. doi: 10.1016/0006-8993(86)90512-3. [DOI] [PubMed] [Google Scholar]
- 37.Mouritsen H, Ritz T. Magnetoreception and its use in bird navigation. Curr Opin Neurobiol. 2005;15:406–414. doi: 10.1016/j.conb.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 38.Green AM, Angelaki DE. Coordinate transformations and sensory integration in the detection of spatial orientation and self motion: from models to experiments. Prog Brain Res. 2007;165:155–180. doi: 10.1016/S0079-6123(06)65010-3. [DOI] [PubMed] [Google Scholar]
- 39.Dickman JD, Correia MJ. Responses of pigeon horizontal semicircular canal afferent fibers. I. Step, trapezoid, and low frequency sinusoid mechanical and rotational stimulation. J Neurophysiol. 1989;62:1090–1101. doi: 10.1152/jn.1989.62.5.1090. [DOI] [PubMed] [Google Scholar]
- 40.Si X, Angelaki DE, Dickman JD. Response properties of pigeon otolith afferents to linear acceleration. Exp Brain Res. 1997;117:242–250. doi: 10.1007/s002210050219. [DOI] [PubMed] [Google Scholar]
- 41.Mouritsen H, Feenders G, Liedvogel M, Kropp W. Migratory birds use head scans to detect the direction of the Earth’s magnetic field. Current Biology. 2004;14:1946–1949. doi: 10.1016/j.cub.2004.10.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01