Emerging Roles of Nodal and Cripto-1: From Embryogenesis to Breast Cancer Progression (original) (raw)
. Author manuscript; available in PMC: 2011 Sep 19.
Published in final edited form as: Breast Dis. 2008;29:91–103. doi: 10.3233/bd-2008-29110
Abstract
Breast carcinoma cells and embryonic progenitors similarly implement stem cell-associated signaling pathways to sustain continued growth and plasticity. Indeed, recent studies have implicated signaling pathways, including those associated with the Notch, and Transforming Growth Factor-Beta (TGF-β) superfamilies, as instrumental to both embryological development and breast cancer progression. In particular, Nodal, an embryonic morphogen belonging to the TGF-β superfamily, and its co-receptor, Cripto-1, are requisite to both embryogenesis and mammary gland maturation. Moreover, these developmental proteins have been shown to promote breast cancer progression. Here, we review the role of Nodal and its co-receptor Cripto-1 during development and we describe how this signaling pathway may be involved in breast cancer tumorigenesis. Moreover, we emphasize the potential utility of this signaling pathway as a novel target for the treatment and diagnosis of breast cancer.
Keywords: Nodal, Cripto-1, embryogenesis, mammary development, breast cancer
INTRODUCTION
Numerous genes have been shown to be expressed in embryonic stem cells and considered important during early embryonic development. These include, but are not limited to, Notch and its associated signaling transcription factors, such as HES [1,2], the GATA family of transcription factors [3]; the developmental pluripotency associated-3 (DPPA3) gene also known as STEL-LA [4]; stem cell related transcription factors like the POU domain transcription factor Oct4 [5]; members of the homeobox gene family, such as HOX [6] and zinc-finger transcription factors like snail and twist [7, 8]. Members of the Transforming Growth Factor-β (TGF-β) superfamily, including Nodal [9], Activin and GDF-1 and -3 [10] have also been documented as potent mediators of cell fate in both embryological and adult systems. Furthermore, changes in expression of some of these and other genes (Fig. 1) have been suggested to play a role in the formation of tumors such as breast cancer [11,12].
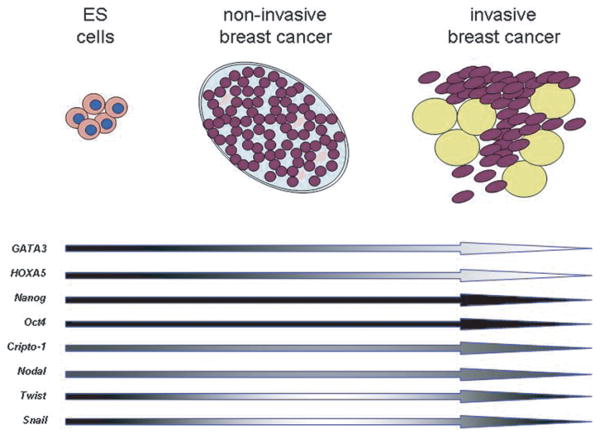
Fig. 1.
Hypothetical Evolution of Stem Cell Biomarkers. Changes in expression of common embryonic stem (ES) cell related molecules in mammary epithelial cells as they transform from normal cells to non-invasive and invasive cancer cells. (Dark shade = high expression; light shade = low expression).
The establishment of human embryonic stem cell (hESC) lines has facilitated the identification of other stem cell-related genes. A recent study of almost 60 hES cell lines from several different laboratories worldwide has established the expression of associated genes illuminating a more complex molecular basis for pluripotency [13]. These included Nanog, Oct4, Nodal and Nodal’s co-receptor teratocarcinoma-derived growth factor-1 (TDGF-1), also referred to as Cripto-1. Together with Nanog and Oct4, Nodal and Cripto-1 have been suggested to play a role in self-renewal and maintenance of pluripotency and are considered by some as markers for hESCs [14]. For example, Cripto-1 has been shown to be a direct target gene in embryonic stem (ES) cells for Nanog and Oct4 suggesting that many of the functions of Nanog and/or Oct4 may be mediated in part through expression of Cripto-1 [15]. In addition, Nodal maintains the pluripotency of ES cells and is one of the first genes to be down-regulated as totipotent ES cells differentiate during embryoid body formation. Moreover, inhibition of the Nodal signaling pathway, through pharmacological inhibition of its receptor, results in hESC differentiation [16]. This review will highlight the potential role of Nodal and its co-receptor Cripto-1 during mammary gland development and how this signaling pathway may be involved in breast cancer tumorigenesis and progression.
BIOCHEMICAL AND SIGNALING CHARACTERISTICS OF NODAL AND CRIPTO-1
The Nodal signaling pathway is tightly regulated by a myriad of transcriptional regulators, post-translational modifications and extracellular factors. The human Nodal gene, containing 3 exons, is located on Chromosome 10q22.1. In mice, Nodal expression is enhanced by at least 3 separate transcriptional regulatory regions, the Node Specific Enhancer (NDE), approximately 10 kb upstream of the gene locus, the Left Side specific Enhancer (LSE), approximately 4 kb upstream of the translational start site; and the ASymmetric Enhancer (ASE), located in the first intron [17–19]. Studies have determined that the LSE and the ASE are regulated in the mouse by Nodal via a positive feedback loop that culminates in the activation of FoxH1. In contrast, the NDE has been shown to induce Nodal expression in the mouse in response to Notch signaling through CBF1 binding elements in the promoter region [20,21]. Gene alignments indicate that the human Nodal locus contains similar enhancer elements, so it is likely that human Nodal expression is regulated in a similar manner. Indeed, our preliminary studies indicate that like mouse Nodal, human Nodal is up-regulated by Notch4 signaling in melanoma cells [22]. Moreover, a positive feedback loop, similar to that described for the LSE and ASE in mice, has been documented to sustain Nodal expression in human ES and melanoma cell types [23, 24]. As a complement to these canonical regulators of transcription, Nodal expression may also be governed by gene methylation and miRNA-directed degradation. For example, we have determined that there is a sizable CpG island (_>_1300 base pairs) near the transcription start site (TSS) of the Nodal gene, and this site may regulate Nodal expression [22]. Moreover, a novel miRNA (miR-430) has been shown to block the translation of a Nodal homolog, squint, in zebrafish [25]. MiR-430 target sites are also present in the mammalian Nodal gene; and so it is likely that Nodal expression is similarly affected by miRNA-mediated degradation in humans [26].
Nodal is also regulated post-translationally by subtilisin-like proprotein convertases, including PACE-4 and Furin [27], and by glycosylation. In a manner similar to most TGF-β family members, Nodal is synthesized as a pro-protein that is activated following proteolytic processing by covertases at R-X-(K/R)-R and R-X-X-R consensus sequences [26]. Removal of the pro domain reduces Nodal stability and signaling range, thereby promoting autocrine signaling [28]. Conversely, glycosylation of mature Nodal provides the protein with increased stability so that it can potentiate paracrine signaling events [28]. Hence, post-translational modifications of Nodal are important mediators of Nodal signaling outcomes.
Nodal propagates its signal by binding to heterodimeric complexes between type I (ALK 4/7) and type II (ActRIIB) activin-like kinase receptors. Assembly of this complex, which often includes Cripto-1, results in the phosphorylation and activation of ALK 4/7 by ActRIIB, followed by the ALK 4/7 mediated phosphorylation of Smad-2 and possibly Smad-3 (Fig. 2). Phosphorylated Smad 2/3 subsequently associates with Smad-4 and then translocates to the nucleus where it regulates gene expression through an association with transcription factors such as FoxH1 and Mixer [26]. Nodal up-regulates its own transcription via a positive feedback loop. In order to regulate the levels of this potent morphogen, embryological systems employ Nodal inhibitors such as Lefty A, Lefty B, Cerberus and Tomoregulin-1 [26,29]. Extracellular Nodal inhibitors control Nodal signaling by spatially and temporally restricting the Nodal-mediated activation of ALK 4/7. For example, Lefty-A and B, highly divergent members of the TGF_β_ superfamily, specifically antagonize the Nodal signaling pathway by binding to and interacting with Nodal and/or with Cripto-1 in a manner that blocks ALK activation [26,30]. This restriction of Nodal signaling can occur in the extracellular microenvironment, where Nodal and Cripto-1 are present, as well as at the cell surface. Of note, the Lefty proteins have not been found to bind ALK4 or ActRIIB; hence these Lefty proteins are not competitive inhibitors of the ALK receptor complex. Furthermore, in embryological systems, the Lefty genes are often downstream targets of Nodal signaling, thereby providing a powerful negative-feedback loop for this pathway [26,30].
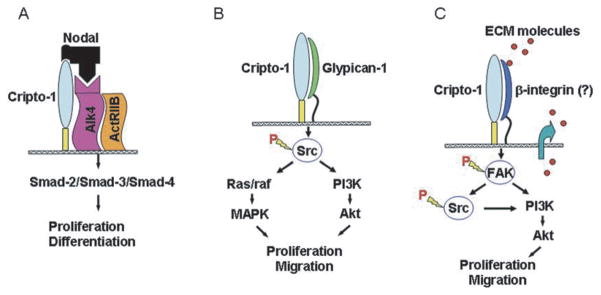
Fig. 2.
Schematic Overview of Nodal and Cripto-1 Signaling Pathways. A) Canonical Nodal signaling using Cripto-1 as a co-receptor. Nodal can also activate this pathway, albeit less efficiently, independently of Cripto-1. B) Cripto-1 Nodal-independent signaling via binding with Glypican-1 and activating src/Ras/raf/PI3K downstream signaling. C) Possible signaling of Cripto-1 through binding to beta-integrins and signaling via FAK-dependent signaling pathway.
Cripto-1 is a member of the EGF-CFC protein family [31]. EGF-CFC family members contain an NH2-terminal signal peptide, a modified EGF-like region, a conserved cysteine-rich domain (CFC motif) and a short hydrophobic COOH-terminus that contains additional sequences for glycosyl-phosphatidylinositol (GPI) cleavage and attachment [32] (Fig. 3). Full activity requires the presence of a peptide containing only an intact EGF domain and CFC domain [33,34]. All EGF-CFC proteins contain a consensus O-linked fucosylation site within the EGF-like motif that was considered necessary for their ability to function as co-receptors for the TGF_β_-related proteins, Nodal or Vg1/GDF-1 [34, 35]. However, a recent report shows that it is not fucose which is necessary for the coreceptor function but rather the amino acid threonine to which the fucose is attached [36]. Although Cripto-1 can be found both as a cell-associated form (cis) and extracellular soluble form (trans), new findings have demonstrated that the GPI-attachment of Cripto-1 is required for the paracrine activity as a Nodal co-receptor [37].
Fig. 3.
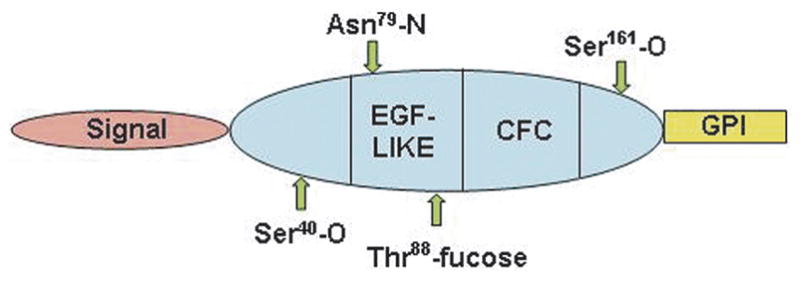
Schematic Representation of Cripto-1 Protein Sequence. Note positions of the EGF-like and CFC domains which bind Nodal and ALK4, respectively.
During embryogenesis, EGF-CFC proteins function, as mentioned previously, as cell-surface co-receptors for Nodal through activation of the serine threonine kinase type I (ALK4) and type II (ActRIIB) receptor complex in Xenopus [26] (Fig. 2). Biochemical evidence has demonstrated a direct interaction between mouse Cripto-1 and ALK4 in Xenopus oocytes and this interaction is necessary for Nodal to bind to the ALK4/ActRIIB receptor complex and to stimulate Smad-2 phosphorylation and activation [38,39]. Cripto-1 can also directly interact with serine threonine kinase receptor ALK7 enhancing the ability of ALK7 to respond to Nodal [40]. These phenomena are perhaps best exemplified in Cripto-1 null mice which die at day 7.5 due to their inability to gastrulate [41,42]. Studies have determined that Nodal may also signal in a Cripto-1-independent fashion. For example, Reissmann and colleagues revealed that Nodal can bind to activate ALK 7 in the absence of Cripto-1, but that Cripto-1 markedly enhances this process [40]. Another study showed that the Nodal precursor can bind to ALK 4 in the extraembryonic ectoderm of the developing mouse embryo in a Cripto-1-independent manner, and that this binding results in the expression of Nodal-responsive genes [43]. Finally, using murine knock out models, Liguori and colleagues recently demonstrated that Nodal can signal extensively and control axis specification in the absence of Cripto, if its inhibitor Cerberus is also inhibited [44]. More recent evidence also supports a role for Cripto-1 during anterior-posterior axis specification independently of canonical Nodal signaling pathway [45].
In addition to functioning as a coreceptor for Nodal, Cripto-1 has been shown to mediate signaling of other TGF-β ligands, such as Activin and Xenopus Vg1 and its ortholog in mouse GDF1 [46]. Similar to Nodal, Vg1 and GDF1 can bind to ALK4/ActRIIB receptor complex only in the presence of EGF-CFC co-receptors. In contrast, binding of Cripto-1 to Activin and TGF-β_1 can inhibit Activin and TGF_β_-1 signaling in mammalian cells [47,48]. Cripto-1 can also activate the ras/raf/MAPK and PI3-K/AKT/GSK-3_β signaling pathways [49,50]. Activation of these two intracellular pathways is independent of Nodal and ALK4, since Cripto-1 can stimulate MAPK and AKT phosphorylation in EpH4 mouse mammary epithelial cells and MC3T3-C1 osteoblast cells that lack ALK4 and Nodal expression, respectively [39]. Activation of these signaling pathways can be achieved with a soluble GPI-truncated Cripto-1 recombinant protein and can occur through direct binding of Cripto-1 to Glypican-1, a membrane-associated heparan sulfate proteoglycan [49,51]. Binding of Cripto-1 to Glypican-1 activates MAPK and AKT signaling pathways via phosphorylation of the cytoplasmic tyrosine kinase c-Src [51] (Fig. 2).
The interactions between integrins, extracellular matrix and signaling molecules like fibronectin and FAK, respectively, are capable of regulating cellular processes like growth and development and have been shown to be involved in tumor proliferation, thus representing attractive targets for potential cancer therapy [52–55]. Overexpression of Cripto-1 in vitro and in vivo has been associated with increased expression of fibronectin and various integrins and with increased activation of focal adhesion kinase [56]. Moreover, Cripto-1 was found to bind to _β_-4 integrin in an immunoprecipitation assay (unpublished data). These data suggest that Cripto-1 may be involved in regulating integrin signaling either directly by binding to integrins and subsequently activating integrin signaling or indirectly by regulating the expression of extracellular matrix proteins which are also capable of binding integrins and activating integrin signaling (Fig. 2).
FUNCTION AND EXPRESSION OF CRIPTO-1 AND NODAL DURING EMBRYONIC DEVELOPMENT
Cripto-1 and Nodal converge during embryogenesis to regulate axis formation, mesendoderm induction, axial patterning, and left-right asymmetry. This signaling axis also plays a role in the maintenance of pluripotency in the epiblast and trophectoderm compartments [27, 57]. During early mouse embryogenesis, Nodal exhibits a dynamic expression pattern that lasts until mid gestation. Nodal mRNA is first detected at approximately 5 days post coitus (dpc) in the visceral endoderm and throughout the epiblast. At about the time of gastrulation, Nodal is restricted to the proximal posterior regions of the embryonic ectoderm and the primitive endoderm overlying the primitive streak. Shortly after, Nodal becomes intensely localized to the periphery of the node in the anterior primitive streak [58,59]. This node is equivalent to the dorsal lip, or Spemann’s organizer in Xenopus, which initiates embryonic axis formation [26,60,61]. During somitogenesis, Nodal expression is increasingly restricted to the Left side of the Node, and by the 3–4 somite stage (8 dpc), Nodal is confined to a subpopulation of mesoderm cells on what will become the left side of the embryo [59]. This asymmetric Nodal expression precedes heart looping and axial rotation, and persists to the 12–14 somite stage (8–9.25 dpc), after which Nodal expression is not detected [59,62].
Nodal plays an essential role in embryogenesis by acting as an organizing signal before gastrulation, to initiate axis formation [26,60,63]. Studies in zebrafish and mice have demonstrated that Nodal is required for mesoderm and endoderm formation. In mice, _nodal_-null embryos fail to form a primitive streak and are deficient in endoderm and mesoderm. Moreover, these mice die shortly after gastrulation. Zebrafish similarly require Nodal-like proteins (cyclops and squint) for mesoderm and endoderm induction. Indeed double mutants for squint (sqt) and cyclops (cyc) lack head and trunk mesoderm and fail to form a germ ring, which is similar to the primitive streak [58,62,64,65]. In addition, Nodal has been shown to induce secondary axis formation when ectopically engrafted into zebrafish blastulas [66]. Studies in zebrafish have also determined that Nodal specifies anterior-posterior patterning such that specification of the anterior axial mesoderm is induced by higher Nodal levels than are required for more posterior fates [67].
During early mouse embryogenesis, Cripto-1 mRNA expression is found in the embryonic ectoderm following implantation of the blastocyst. On day 6.5 of gestation, Cripto-1 is detected at increasing levels in the epiblast cells undergoing epithelial to mesenchymal transition (EMT) as they migrate through the nascent primitive streak and in the developing mesoderm cells [42, 68–70]. By day 7, Cripto-1 is detected mostly in the truncus arterious of the developing heart. With the exception of the developing heart, little if any expression of Cripto-1 mRNA can be detected in the remainder of the embryo after day 8 [32,68]. As previously mentioned, Cripto-1 null mice (Cripto-1−/−) succumb at day 7.5 due to their inability to gastrulate and form appropriate germ layers [42]. Fibroblasts that were derived from Cripto-1−/− embryos were impaired in their ability to migrate towards either fibronectin or type 1 collagen as compared to embryonic fibroblasts from wild type embryos [70]. In zebrafish, the Cripto-1 ortholog, one-eyed pinhead (oep), with the two Nodal-related genes sqt and cyc, is necessary for initiating mesoderm, endoderm and A/P axis formation [9,71, 72]. Mutations in oep result in cyclopia, absence of head and trunk mesoderm, loss of prechordal plate and ventral neuroectoderm, impairment of gastrulation movements, loss of A/P axis patterning and positioning and L/R laterality defects [71,72]. Rescue of the oep mutant phenotype can be achieved by expression of either full-length or secreted COOH-terminal truncated forms of the oep protein suggesting that oep can function under certain conditions as a paracrine effector. Ectopic expression of Xenopus FRL-1 or mouse Cripto-1 or overexpression of Activin or activation of downstream components in an Activin-like signaling pathway such as the ALK4 receptor (TARAM-A) or Smad-2 can also rescue oep inactivating mutations [72]. Oep, like mouse Cripto-1, is absolutely required for the migration of mesendoderm cells through the primitive streak [73].
The Nodal/cryptic signaling pathway is also involved in the establishment of the L/R embryonic axis, demonstrating that the same signaling molecules are utilized in multiple developmental pathways [9]. However, at this developmental stage with the exception of oep, cryptic replaces the function of Cripto-1 as the co-receptor for Nodal. In mice, Nodal is expressed symmetrically before and during gastrulation but is restricted to the left side of the node and lateral plate mesoderm during early segmentation. Nodal proteins are similarly localized to the left side in the chick, frog and zebrafish. This restriction is essential for L/R asymmetry as ectopic Nodal expression can cause defects in L/R asymmetry of the heart and gut in chick, Xenopus and zebrafish [62]. Moreover, in mice hypomorphic mutants lacking nodal expression in the left lateral plate, mesoderm exhibit defects in left-right body patterning resulting in organ anomalies including random heart looping [62].
Nodal signaling in zebrafish during L/R asymmetry development also requires a functional oep gene [61, 74,75]. Mutation and partial rescue of oep in the zebrafish or targeted disruption of cryptic in mice (Cfc1) and humans (CFC1) results in laterality defects including atrial-ventricular septal defects, pulmonary right isomerization, inverted positioning of abdominal organs (situs inversus) and holoprosencephaly [76,77]. Germline deletion of cryptic eventually leads to post-natal death at approximately 2 weeks because of severe cardiac malfunction [61,75].
CRIPTO-1 AND NODAL DURING MAMMARY GLAND DEVELOPMENT AND TUMORIGENESIS
Cripto-1 mRNA can be detected in 4 to 12 week old virgin, mid-pregnant and lactating FVB/N mouse mammary glands [78,79]. Biologically active Cripto-1 protein has also been detected as a secretory component in human milk suggesting Cripto-1 secretion may be involved in regulating proliferation and differentiation of milk producing cells [80]. Further support for Cripto-1 regulation of mammary epithelial function comes from data showing decreased ability of mouse mammary epithelial cells to respond to the lactogenic hormones, dexamethasone, insulin and prolactin (DIP) when pre-treated with Cripto-1 and inhibition of _β_-casein expression, via p21_ras_- and phosphatidylinositol 3′-kinase (PI3K)-dependent pathways, when these cells were simultaneously treated with both Cripto-1 and DIP [81].
Transgenic mice models that overexpress the human Cripto-1 transgene (CR-1) have given some insight into the possible role of Cripto-1 during mammary gland development. In the MMTV-CR-1 transgenic mouse model, overexpression of Cripto-1 was associated with significantly increased lateral side branching of the developing mammary ducts [82]. In another transgenic model where Cripto-1 expression was driven by the Whey acidic protein promoter, the mammary glands of the transgenic mice overexpressing Cripto-1 were characterized by delayed lobuloalveolar development [83] (Fig. 4). In those studies, both transgenic models also showed increased incidence of mammary gland tumors with the predominant formation of papillary adenocarcinomas in the mammary glands of the MMTV-Cripto-1 transgenic mice and a mixture of papillary, microglandular, solid and myoepithelial histotypes containing multiple areas of squamous metaplasia in the mammary gland tumors of the WAP-Cripto-1 transgenic mice.
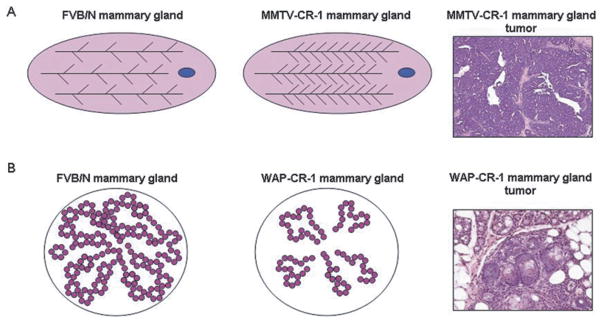
Fig. 4.
Effects of Cripto-1 Transgene Expression in the Mouse Mammary Gland. A) Overexpression of human Cripto-1 in MMTV-CR-1 transgenic mice is associated with increased lateral side branching of mammary ducts and increased incidence of mammary papillary adenocarcinoma in multiparous mice. B) Overexpression of Cripto-1 in WAP-Cripto-1 transgenic mice is associated with delayed lobuloalveolar development and increased incidence of mammary gland tumors with variable histotypes including multiple areas of squamous metaplasia.
Overexpression of Cripto-1 cDNA in normal mouse fibroblasts induce these cells to grow in soft agar and increase growth rates in several human breast cancer cell lines [84,85]. Insertion of Elvax pellets containing the EGF-like motif of Cripto-1 protein into the mammary gland of ovariectomized virgin mice also caused increases in DNA synthesis in mammary epithelial cells in proximity to the pellets [86]. Human MCF-7 breast cancer cells that overexpress Cripto-1 proliferate at higher rates in serum-free medium, form increased numbers of colonies in soft agar, are more resistant to apoptosis when grown under anchorage independent conditions, and show increased propensity to invade and migrate in vitro [87]. Overexpression of Cripto-1 in the mouse mammary epithelial cell line EpH4 also caused increased cell proliferation and anchorage-independent growth in soft agar and increased chemotaxsis and migration when these cells were cultured on plastic or on porous, matrix-coated filters [88]. Biochemical changes, such as reduced expression of E-cadherin or increased expression of vimentin that characterize EMT [89] were investigated in mammary gland hyperplasias and tumors from MMTV-CR-1 transgenic mice, and in the mouse mammary epithelial cell line, HC-11, overexpressing Cripto-1 (HC-11/Cr-1) [90]. E-cadherin expression was significantly decreased in tissue extracts from the mammary tumor lesions that express the human Cripto-1 transgene and in extracts from HC-11/Cr-1 cells. Those extracts also showed significant increases in the expression of N-cadherin, vimentin and integrins such as, _β_-4 integrin as well as increases in activated c-src, FAK and AKT, all known to be activated during EMT and likely to play a role in increasing tumor cell invasion [89]. Also, in the Cripto-1 transgenic mammary gland tumors and HC-11/Cr-1 cells, the zinc-finger repressor transcription factor, Snail, known to down-regulate or interfere with the normal expression of E-cadherin, was detected at significantly higher levels as compared to normal control mammary tissue, thus suggesting a novel link between Cripto-1 expression and Snail activity [56].
In contrast to Cripto-1, which has been studied extensively as a mediator of breast development and tumorigenesis, evidence for a direct role for Nodal in these processes is lacking. It cannot be excluded that Nodal can play a role during mammary gland development. In fact, Nodal mRNA expression in the mouse mammary gland parallels that of Cripto-1 during different stages of development [79]. Studies analyzing mammary glands from Nodal hypomorphic mice are currently in progress and preliminary findings seem to suggest the importance of Nodal for normal mammary gland development (D.S. personal communication). These observations suggest that Nodal may play a synergistic role with Cripto-1 during the development and functional differentiation of the mammary gland.
While Nodal has not yet been overexpressed in normal or neoplastic breast cell lines, the non-tumorigenic, poorly invasive, and well differentiated human melanoma cells (C81-61) has been transfected with a vector encoding mature murine Nodal. In this cell line, overexpression of Nodal resulted in the acquisition of a tumorigenic phenotype (unpublished work). Indeed, in contrast to control C81-61 cells, which are unable to form tumors, 100% of the animals injected with Nodal-expressing C81-61 cells formed palpable tumors within 5 weeks. It will be interesting to examine if ectopic Nodal expression in poorly aggressive breast cancer cells will similarly promote tumor formation. In addition, future studies will involve the creation of transgenic animals that overexpress the Nodal transgene driven by a mammary-specific promoter, so that the role of Nodal in breast development and tumor progression may be deciphered.
CRIPTO-1 EXPRESSION IN BREAST CANCER AND POTENTIAL DIAGNOSTIC AND THERAPEUTIC APPLICATION
Cripto-1 mRNA and protein have been detected in several human breast cancer cell lines in ductal carcinoma in situ (DCIS) and in infiltrating breast carcinomas analyzed. In comparison, a very low percentage of normal breast specimen and adjacent non-involved breast tissue show Cripto-1 expression [47, 91–93] (Fig. 5A). In addition, significantly higher levels of Cripto-1 can be detected by immunoassay in the plasma of breast cancer patients as compared to Cripto-1 levels detected in healthy controls suggesting that Cripto-1 might be useful in the early diagnosis of this disease [94] (Fig. 5A). More recently, Cripto-1 was detected in almost half of the breast cancer tissue samples analyzed and found to correlate with advanced stage disease [95]. In addition, Cripto-1 is frequently coexpressed with other EGF-related peptides, such as TGF_α_, amphiregulin (AR), and heregulin in human primary breast carcinomas suggesting that Cripto-1 might cooperate with these different growth factors in supporting the autonomous proliferation of breast cancer cells. A positive correlation between nuclear HER-4 expression and Cripto-1 expression in primary human breast carcinomas has also been described [96] further supporting the finding that Cripto-1 can indirectly enhance the tyrosine phosphorylation of HER-4 [97].
Fig. 5.
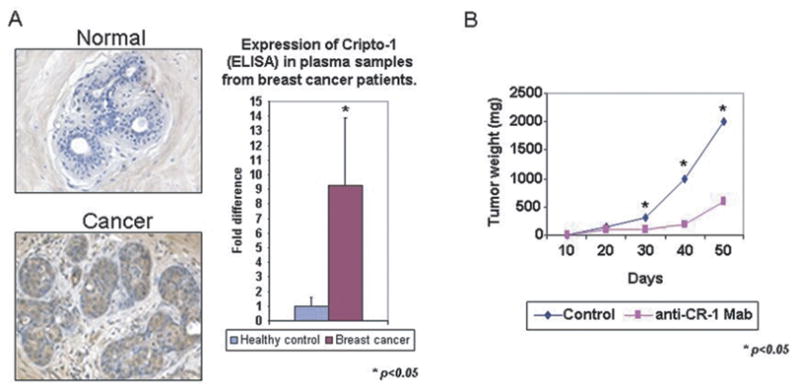
Potential for Cripto-1 as a Biomarker for Cancer and Therapeutic Target. A) Cripto-1 is expressed in breast cancer tissue and in plasma samples from breast cancer patients. B) Effect of anti-Cripto-1 monoclonal blocking antibody on growth of NCCIT human teratocarcinoma cells in vivo. Anti-Cripto-1 blocking antibody negatively affects growth of NCCIT in a xenograft model.
We have recently begun to examine if Nodal expression is similarly associated with breast carcinoma progression. Like Cripto-1, Nodal is expressed in breast carcinoma cell lines but is absent in normal breast derived cells, including human mammary epithelial and myoepithelial cells (Fig. 6A & B) [98]. Using a tissue microarray we have determined that Nodal correlates with clinical breast carcinoma progression [98]. Similar to Cripto-1, Nodal expression was absent in normal breast tissue. However, in contrast to Cripto-1, Nodal was not detected in DCIS. Rather, Nodal expression was observed later in more advanced invasive (IDC) and metastatic lesions (Figure 6C-F Nodal) [98]. This finding is comparable to that observed in melanoma specimens [23]. For example, Nodal was not detected in normal skin or in normal melanocytes, and was also absent in non-invasive radial growth phase (RGP) melanomas. Nodal was, however, detected in the invasive vertical growth phase (VGP) lesions and was present in over 60% of melanoma metastases [23]. Collectively, these results suggest that the acquisition of Nodal expression is associated with metastatic competence, which is obtained during the transition of a non-invasive cancer, such as DCIS or RGP melanoma, to an invasive lesion, such as IDC or VGP melanoma. Data generated to date with aggressive melanoma cells indicate less than 2% Cripto-1 positivity within the heterogenous cell lines [98]. Future studies will examine whether this subpopulation of Cripto-1 positive cells can potentiate Nodal expression, and if these two molecules, independently or in co-operation, can facilitate metastatic disease.
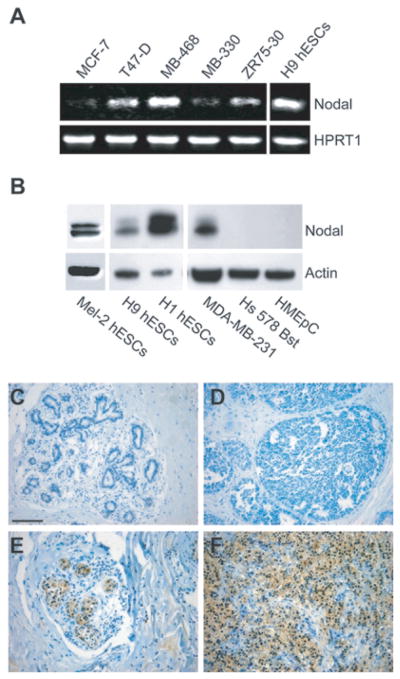
Fig. 6.
Nodal Expression Correlates with Breast Carcinoma Progression. A) Semiquantitative RT-PCR analysis of Nodal, mRNA expression in: MCF7, T47D, MDA-MB-468, MDA-MB-330, and ZR75-30 (human breast carcinoma cells). H9 hESC mRNA is used as a positive control for gene expression and HPRT1 is used as a loading control. B) Western blot analysis of Nodal in: MEL-2, H1 and H9 human embryonic stem cells (hESCs); MDA-MB-231, human metastatic breast carcinoma cells; Hs 578 Bst normal human myoepithelial cells; and HMEpC normal human mammary epithelial cells. Actin is used as a loading control. C) Immunohistochemical analysis of Nodal staining (dark color) in normal breast tissue, ductal carcinoma in situ (DCIS), nonspecific invasive ductal carcinoma (IDC) and metastatic IDC (to lymph nodes). The expression and prevalence of Nodal staining in breast tissue was designated as none, weak (_<_25%), moderate (25–75%) or strong (_>_75%). Spearman’s rank correlation showed a significant positive correlation between breast cancer progression and Nodal expression (p < 0.05). Bar equals 100 _μ_m for all representative images shown.
Since high expression of Cripto-1 can be detected in human cancers, as compared to normal tissues, this signaling pathway might represent a target for cancer therapy. This is supported by findings describing the use of antisense oligonucleotides that reduce Cripto-1 expression and cause significant reduction of cell proliferation in vitro [99]. In addition neutralizing antibodies against Cripto-1 [47] were able to significantly inhibit tumor cell growth in two xenograft models with testicular (NCCIT) and colon (GEO) cancer cells that express very high levels of Cripto-1 (Fig. 5B). Moreover, rat monoclonal antibodies directed against the EGF-like domain of the Cripto-1 peptide also produced a significant inhibition of in vitro and in vivo growth of colon cancer and leukemia cells [99]. These results suggest that similar approaches for inhibiting cancer growth by interfering with expression or function of Cripto-1 may also have beneficial effects in Cripto-1 expressing breast cancer.
Recent studies have demonstrated that Nodal may also be an excellent therapeutic target for breast cancer treatment. Similar to Cripto-1, Nodal expression is largely restricted to embryonic stem cells and reproductive tissues including the testes, ovary and placenta [22,98]. Studies in our laboratory have shown that Nodal is also essential for tumorigenesis [22,23, 98]. For example, using an orthotopic mouse model, we determined that knocking down Nodal expression in human breast carcinoma cells (MDA-MB-231) with Nodal targeting Morpholinos (MONodal) significantly mitigates the ability of these cells to form tumors (Fig. 7A). In order to establish a mechanism for the reduction in tumorigenicity, we have since examined the effects of this treatment on in vivo tumor cell proliferation and apoptosis [98]. Using immunohistochemical staining for Ki67 as a measure of proliferation, and terminal deoxynucleotidyl transferase biotin-dUTP nick-end labeling (TUNEL) as a measure of apoptosis, we determined that inhibition of Nodal expression with MONodal decreases proliferation and increases apoptosis in orthotopic breast tumors (Fig. 7B). These in vivo data support a role for Nodal in the maintenance of breast cancer tumorigenicity and implicate the potential involvement of apoptotic pathways.
Fig. 7.
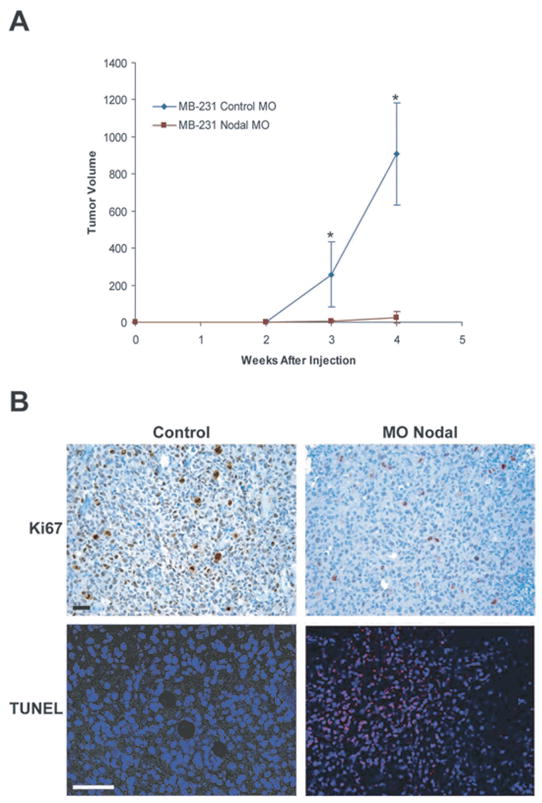
Nodal Inhibition Diminishes Breast Carcinoma Tumorigenicity in an Orthotopic Model. A) In vivo tumor formation in a mouse injected orthotopically with MDA-MB-231 human breast carcinoma cells treated with either a control morpholino (MO Control) or a morpholino targeting Nodal (MONodal) (n = 10). Values represent the mean tumor volume (mm3)± standard deviation, and tumor volumes were significantly different at the time points indicated by an asterisk (*) (P < 0.05). (B) Immunohistochemical analysis of Ki67 expression (dark) and TUNEL (light spots) staining in orthotopic breast carcinoma (MDA-MB-231) tumors. Prior to injection into a mouse, cells were treated with MO Nodal or left untreated (control). Proliferation is indicated by Ki67 staining and apoptotic nuclei were detected with confocal microscopy as light staining localized to the nuclei of apoptotic MDA-MB-231 cells. Bar equals 25 _μ_m.
Acknowledgments
The authors wish to gratefully acknowledge helpful scientific collaboration with Drs. Daniel Constam, Dawn Kirschmann and M. Bento Soares for Nodal gene expression. The research was funded by NIH CA59702, CA121205 and CA75681. Research by David S. Salomon was supported by NIH Intramural funding.
References
- 1.Kageyama R, Ohtsuka T, Kobayashi T. The Hes gene family: repressors and oscillators that orchestrate embryogenesis. Development. 2007;134:1243–1251. doi: 10.1242/dev.000786. [DOI] [PubMed] [Google Scholar]
- 2.Chiba S. Notch signaling in stem cell systems. Stem Cells. 2006;24:2437–2447. doi: 10.1634/stemcells.2005-0661. [DOI] [PubMed] [Google Scholar]
- 3.Technau U, Scholz CB. Origin and evolution of endoderm and mesoderm. Intl J Dev Biol. 2003;47:531–539. [PubMed] [Google Scholar]
- 4.Maldonado-Saldivia J, van den Bergen J, Krouskos M, Gilchrist M, Lee C, Li R, Sinclair AH, Surani MA, Western PS. Dppa2 and Dppa4 are closely linked SAP motif genes restricted to pluripotent cells and the germ line. Stem Cells. 2007;25:19–28. doi: 10.1634/stemcells.2006-0269. [DOI] [PubMed] [Google Scholar]
- 5.Babaie Y, Herwig R, Greber B, Brink TC, Wruck W, Groth D, Lehrach H, Burdon T, Adjaye J. Analysis of Oct4-dependent transcriptional networks regulating self-renewal and pluripotency in human embryonic stem cells. Stem Cells. 2007;25:500–510. doi: 10.1634/stemcells.2006-0426. [DOI] [PubMed] [Google Scholar]
- 6.Moens CB, Selleri L. Hox cofactors in vertebrate development. Dev Biol. 2006;291:193–206. doi: 10.1016/j.ydbio.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 7.Morales AV, Acloque H, Ocana OH, de Frutos CA, Gold V, Nieto MA. Snail genes at the crossroads of symmetric and asymmetric processes in the developing mesoderm. EMBO Rep. 2007;8:104–109. doi: 10.1038/sj.embor.7400867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Germanguz I, Lev D, Waisman T, Kim CH, Gitelman I. Four twist genes in zebrafish, four expression patterns. Dev Dyn. 2007;236:2615–2626. doi: 10.1002/dvdy.21267. [DOI] [PubMed] [Google Scholar]
- 9.Schier AF, Shen MM. Nodal signalling in vertebrate development. Nature. 2000;403:385–389. doi: 10.1038/35000126. [DOI] [PubMed] [Google Scholar]
- 10.Andersson O, Bertolino P, Ibanez CF. Distinct and cooperative roles of mammalian Vg1 homologs GDF1 and GDF3 during early embryonic development. Dev Biol. 2007;311:500–511. doi: 10.1016/j.ydbio.2007.08.060. [DOI] [PubMed] [Google Scholar]
- 11.Gupta V, Harkin DP, Kawakubo H, Maheswaran S. Transforming Growth Factor-beta superfamily: evaluation as breast cancer biomarkers and preventive agents. Curr Cancer Drug Targets. 2004;4:165–182. doi: 10.2174/1568009043481542. [DOI] [PubMed] [Google Scholar]
- 12.Ezeh UI, Turek PJ, Reijo RA, Clark AT. Human embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are expressed in both seminoma and breast carcinoma. Cancer. 2005;104:2255–2265. doi: 10.1002/cncr.21432. [DOI] [PubMed] [Google Scholar]
- 13.Adewumi O, Aflatoonian B, Ahrlund-Richter L, Amit M, Andrews PW, Beighton G, Bello PA, Benvenisty N, Berry LS, Bevan S, et al. Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat Biotech. 2007;25:803–816. doi: 10.1038/nbt1318. [DOI] [PubMed] [Google Scholar]
- 14.Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, Bourque G, George J, Leong B, Liu J, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–440. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- 15.Assou S, Lecarrour T, Tondeur S, Strom S, Gabelle A, Marty S, Nadal L, Pantesco V, Reme T, Hugnot JP, et al. A meta-analysis of human embryonic stem cells transcriptome integrated into a web-based expression atlas. Stem Cells. 2007;25:961–973. doi: 10.1634/stemcells.2006-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vallier L, Reynolds D, Pedersen RA. Nodal inhibits differentiation of human embryonic stem cells along the neuroectodermal default pathway. Dev Biol. 2004;275:403–421. doi: 10.1016/j.ydbio.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 17.Norris DP, Robertson EJ. Asymmetric and node-specific nodal expression patterns are controlled by two distinct cis-acting regulatory elements. Genes Dev. 1999;13:1575–1588. doi: 10.1101/gad.13.12.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saijoh Y, Oki S, Tanaka C, Nakamura T, Adachi H, Yan YT, Shen MM, Hamada H. Two nodal-responsive enhancers control left-right asymmetric expression of Nodal. Dev Dyn. 2005;232:1031–1036. doi: 10.1002/dvdy.20192. [DOI] [PubMed] [Google Scholar]
- 19.Vincent SD, Norris DP, Le Good JA, Constam DB, Robertson EJ. Asymmetric Nodal expression in the mouse is governed by the combinatorial activities of two distinct regulatory elements. Mech Dev. 2004;121:1403–1415. doi: 10.1016/j.mod.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Raya A, Kawakami Y, Rodriguez-Esteban C, Buscher D, Koth CM, Itoh T, Morita M, Raya RM, Dubova I, Bessa JG, et al. Notch activity induces Nodal expression and mediates the establishment of left-right asymmetry in vertebrate embryos. Genes Dev. 2003;17:1213–1218. doi: 10.1101/gad.1084403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krebs LT, Iwai N, Nonaka S, Welsh IC, Lan Y, Jiang R, Saijoh Y, O’Brien TP, Hamada H, Gridley T. Notch signaling regulates left-right asymmetry determination by inducing Nodal expression. Genes Dev. 2003;17:1207–1212. doi: 10.1101/gad.1084703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Postovit LM, Seftor EA, Seftor RE, Hendrix MJ. Targeting Nodal in malignant melanoma cells. Expert Opin Ther Targets. 2007;11:497–505. doi: 10.1517/14728222.11.4.497. [DOI] [PubMed] [Google Scholar]
- 23.Topczewska JM, Postovit LM, Margaryan NV, Sam A, Hess AR, Wheaton WW, Nickoloff BJ, Topczewski J, Hendrix MJ. Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nat Med. 2006;12:925–932. doi: 10.1038/nm1448. [DOI] [PubMed] [Google Scholar]
- 24.Hendrix MJ, Seftor EA, Seftor RE, Kasemeier-Kulesa J, Kulesa PM, Postovit LM. Reprogramming metastatic tumour cells with embryonic microenvironments. Nat Rev. 2007;7:246–255. doi: 10.1038/nrc2108. [DOI] [PubMed] [Google Scholar]
- 25.Choi WY, Giraldez AJ, Schier AF. Target protectors reveal dampening and balancing of Nodal agonist and antagonist by miR-430. Science. 2007;318:271–274. doi: 10.1126/science.1147535. [DOI] [PubMed] [Google Scholar]
- 26.Schier AF. Nodal signaling in vertebrate development. Annu Rev Cell Dev Biol. 2003;19:589–621. doi: 10.1146/annurev.cellbio.19.041603.094522. [DOI] [PubMed] [Google Scholar]
- 27.Beck S, Le Good JA, Guzman M, Ben Haim N, Roy K, Beermann F, Constam DB. Extraembryonic proteases regulate Nodal signalling during gastrulation. Nat Cell Biol. 2002;4:981–985. doi: 10.1038/ncb890. [DOI] [PubMed] [Google Scholar]
- 28.Le Good JA, Joubin K, Giraldez AJ, Ben-Haim N, Beck S, Chen Y, Schier AF, Constam DB. Nodal stability determines signaling range. Curr Biol. 2005;15:31–36. doi: 10.1016/j.cub.2004.12.062. [DOI] [PubMed] [Google Scholar]
- 29.Tabibzadeh S, Hemmati-Brivanlou A. Lefty at the crossroads of stemness and differentiative events. Stem cells (Dayton, Ohio) 2006;24:1998–2006. doi: 10.1634/stemcells.2006-0075. [DOI] [PubMed] [Google Scholar]
- 30.Shen MM. Nodal signaling: developmental roles and regulation. Dev. 2007;134:1023–1034. doi: 10.1242/dev.000166. [DOI] [PubMed] [Google Scholar]
- 31.Salomon DS, Bianco C, Ebert AD, Khan NI, De Santis M, Normanno N, Wechselberger C, Seno M, Williams K, Sanicola M, et al. The EGF-CFC family: novel epidermal growth factor-related proteins in development and cancer. Endocr Relat Cancer. 2000;7:199–226. doi: 10.1677/erc.0.0070199. [DOI] [PubMed] [Google Scholar]
- 32.Minchiotti G, Parisi S, Liguori G, Signore M, Lania G, Adamson ED, Lago CT, Persico MG. Membrane-anchorage of Cripto protein by glycosylphosphatidylinositol and its distribution during early mouse development. Mechanisms of development. 2000;90:133–142. doi: 10.1016/s0925-4773(99)00235-x. [DOI] [PubMed] [Google Scholar]
- 33.Minchiotti G, Manco G, Parisi S, Lago CT, Rosa F, Persico MG. Structure-function analysis of the EGF-CFC family member Cripto identifies residues essential for nodal signalling. Development. 2001;128:4501–4510. doi: 10.1242/dev.128.22.4501. [DOI] [PubMed] [Google Scholar]
- 34.Yan YT, Liu JJ, Luo YEC, Haltiwanger RS, Abate-Shen C, Shen MM. Dual roles of Cripto as a ligand and coreceptor in the nodal signaling pathway. Mol Cell Biol. 2002;22:4439–4449. doi: 10.1128/MCB.22.13.4439-4449.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schiffer SG, Foley S, Kaffashan A, Hronowski X, Zichittella AE, Yeo CY, Miatkowski K, Adkins HB, Damon B, Whitman M, et al. Fucosylation of Cripto is required for its ability to facilitate nodal signaling. J Biol Chem. 2001;276:37769–37778. doi: 10.1074/jbc.M104774200. [DOI] [PubMed] [Google Scholar]
- 36.Shi S, Ge C, Luo Y, Hou X, Haltiwanger RS, Stanley P. The threonine that carries fucose, but not fucose, is required for cripto to facilitate nodal signaling. J Biol Chem. 2007 doi: 10.1074/jbc.M702593200. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe K, Hamada S, Bianco C, Mancino M, Nagaoka T, Gonzales M, Bailly V, Strizzi L, Salomon DS. Requirement of glycosylphosphatidylinositol anchor of cripto-1 for ’trans’ activity as a nodal co-receptor. J Biol Chem. 2007 doi: 10.1074/jbc.M707351200. [DOI] [PubMed] [Google Scholar]
- 38.Yeo C, Whitman M. Nodal signals to Smads through Cripto-dependent and Cripto-independent mechanisms. Mol Cell. 2001;7:949–957. doi: 10.1016/s1097-2765(01)00249-0. [DOI] [PubMed] [Google Scholar]
- 39.Bianco C, Adkins HB, Wechselberger C, Seno M, Normanno N, De Luca A, Sun Y, Khan N, Kenney N, Ebert A, et al. Cripto-1 activates nodal- and ALK4-dependent and -independent signaling pathways in mammary epithelial Cells. Mol Cell Biol. 2002;22:2586–2597. doi: 10.1128/MCB.22.8.2586-2597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reissmann E, Jornvall H, Blokzijl A, Andersson O, Chang C, Minchiotti G, Persico MG, Ibanez CF, Brivanlou AH. The orphan receptor ALK7 and the Activin receptor ALK4 mediate signaling by Nodal proteins during vertebrate development. Genes Dev. 2001;15:2010–2022. doi: 10.1101/gad.201801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liguori G, Tucci M, Montuori N, Dono R, Lago CT, Pacifico F, Armenante F, Persico MG. Characterization of the mouse Tdgf1 gene and Tdgf pseudogenes. Mamm Genome. 1996;7:344–348. doi: 10.1007/s003359900100. [DOI] [PubMed] [Google Scholar]
- 42.Ding J, Yang L, Yan YT, Chen A, Desai N, Wynshaw-Boris A, Shen MM. Cripto is required for correct orientation of the anterior-posterior axis in the mouse embryo. Nature. 1998;395:702–707. doi: 10.1038/27215. [DOI] [PubMed] [Google Scholar]
- 43.Ben-Haim N, Lu C, Guzman-Ayala M, Pescatore L, Mesnard D, Bischofberger M, Naef F, Robertson EJ, Constam DB. The nodal precursor acting via activin receptors induces mesoderm by maintaining a source of its convertases and BMP4. Dev Cell. 2006;11:313–323. doi: 10.1016/j.devcel.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 44.Liguori GL, Borges AC, D’Andrea D, Liguoro A, Goncalves L, Salgueiro AM, Persico MG, Belo JA. Cripto-independent Nodal signaling promotes positioning of the A-P axis in the early mouse embryo. Dev Biol. 2007 doi: 10.1016/j.ydbio.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 45.D’Andrea D, Liguori G, Le Good A, Lonardo E, Amndersson O, Constam D, Persico M, Minchiotti G. Cripto promotes A-P axis specification independently of its stimulatory effect on Nodal autoinduction. J Cell Biol. 2008 doi: 10.1083/jcb.200709090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng SK, Olale F, Bennett JT, Brivanlou AH, Schier AF. EGF-CFC proteins are essential coreceptors for the TGF-beta signals Vg1 and GDF1. Genes Dev. 2003;17:31–36. doi: 10.1101/gad.1041203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adkins HB, Bianco C, Schiffer SG, Rayhorn P, Zafari M, Cheung AE, Orozco O, Olson D, De Luca A, Chen LL, et al. Antibody blockade of the Cripto CFC domain suppresses tumor cell growth in vivo. J Clin Invest. 2003;112:575–587. doi: 10.1172/JCI17788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gray PC, Harrison CA, Vale W. Cripto forms a complex with activin and type II activin receptors and can block activin signaling. Proc Natl Acad Sci USA. 2003;100:5193–5198. doi: 10.1073/pnas.0531290100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kannan S, De Santis M, Lohmeyer M, Riese DJ, 2nd, Smith GH, Hynes N, Seno M, Brandt R, Bianco C, Persico G, et al. Cripto enhances the tyrosine phosphorylation of Shc and activates mitogen-activated protein kinase (MAPK) in mammary epithelial cells. J Biol Chem. 2007;272:3330–3335. doi: 10.1074/jbc.272.6.3330. [DOI] [PubMed] [Google Scholar]
- 50.Ebert AD, Wechselberger C, Frank S, Wallace-Jones B, Seno M, Martinez-Lacaci I, Bianco C, De Santis M, Weitzel HK, Salomon DS. Cripto-1 induces phosphatidylinositol 3′-kinase-dependent phosphorylation of AKT and glycogen synthase kinase 3beta in human cervical carcinoma cells. Cancer Res. 1999;59:4502–4505. [PubMed] [Google Scholar]
- 51.Bianco C, Strizzi L, Rehman A, Normanno N, Wechselberger C, Sun Y, Khan N, Hirota M, Adkins H, Williams K, et al. A Nodal- and ALK4-independent signaling pathway activated by Cripto-1 through Glypican-1 and c-Src. Cancer Res. 2003;63:1192–1197. [PubMed] [Google Scholar]
- 52.Ruoslahti E. Fibronectin and its integrin receptors in cancer. Adv Cancer Res. 1999;76:1–20. doi: 10.1016/s0065-230x(08)60772-1. [DOI] [PubMed] [Google Scholar]
- 53.Ridyard MS, Sanders EJ. Potential roles for focal adhesion kinase in development. Anatomy and embryology. 1999;199:1–7. doi: 10.1007/s004290050203. [DOI] [PubMed] [Google Scholar]
- 54.Kornberg LJ. Focal adhesion kinase expression in oral cancers. Head Neck. 1998;20:634–639. doi: 10.1002/(sici)1097-0347(199810)20:7<634::aid-hed10>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 55.McLean GW, Carragher NO, Avizienyte E, Evans J, Brunton VG, Frame MC. The role of focal-adhesion kinase in cancer – a new therapeutic opportunity. Nat Rev Cancer. 2005;5:505–515. doi: 10.1038/nrc1647. [DOI] [PubMed] [Google Scholar]
- 56.Strizzi L, Bianco C, Normanno N, Seno M, Wechselberger C, Wallace-Jones B, Khan NI, Hirota M, Sun Y, Sanicola M, et al. Epithelial mesenchymal transition is a characteristic of hyperplasias and tumors in mammary gland from MMTV-Cripto-1 transgenic mice. J Cell Physiol. 2004;201:266–276. doi: 10.1002/jcp.20062. [DOI] [PubMed] [Google Scholar]
- 57.Guzman-Ayala M, Ben-Haim N, Beck S, Constam DB. Nodal protein processing and fibroblast growth factor 4 synergize to maintain a trophoblast stem cell microenvironment. Proc Natl Acad Sci USA. 2004;101:15656–15660. doi: 10.1073/pnas.0405429101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou X, Sasaki H, Lowe L, Hogan BL, Kuehn MR. Nodal is a novel TGF-beta-like gene expressed in the mouse node during gastrulation. Nature. 1993;361:543–547. doi: 10.1038/361543a0. [DOI] [PubMed] [Google Scholar]
- 59.Collignon J, Varlet I, Robertson EJ. Relationship between asymmetric nodal expression and the direction of embryonic turning. Nature. 1996;381:155–158. doi: 10.1038/381155a0. [DOI] [PubMed] [Google Scholar]
- 60.Smith WC, McKendry R, Ribisi S, Jr, Harland RM. A nodal-related gene defines a physical and functional domain within the Spemann organizer. Cell. 1995;82:37–46. doi: 10.1016/0092-8674(95)90050-0. [DOI] [PubMed] [Google Scholar]
- 61.Yan YT, Gritsman K, Ding J, Burdine RD, Corrales JD, Price SM, Talbot WS, Schier AF, Shen MM. Conserved requirement for EGF-CFC genes in vertebrate left-right axis formation. Genes Dev. 1999;13:2527–2537. doi: 10.1101/gad.13.19.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tian T, Meng AM. Nodal signals pattern vertebrate embryos. Cell Mol Life Sci. 2006;63:672–685. doi: 10.1007/s00018-005-5503-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tian T, Burrage K. Stochastic models for regulatory networks of the genetic toggle switch. Proc Natl Acad Sci USA. 2006;103:8372–8377. doi: 10.1073/pnas.0507818103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iannaccone PM, Zhou X, Khokha M, Boucher D, Kuehn MR. Insertional mutation of a gene involved in growth regulation of the early mouse embryo. Dev Dyn. 1992;194:198–208. doi: 10.1002/aja.1001940305. [DOI] [PubMed] [Google Scholar]
- 65.Conlon FL, Lyons KM, Takaesu N, Barth KS, Kispert A, Herrmann B, Robertson EJ. A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development. 1994;120:1919–1928. doi: 10.1242/dev.120.7.1919. [DOI] [PubMed] [Google Scholar]
- 66.Toyama R, O’Connell ML, Wright CV, Kuehn MR, Dawid IB. Nodal induces ectopic goosecoid and lim1 expression and axis duplication in zebrafish. Development. 1995;121:383–391. doi: 10.1242/dev.121.2.383. [DOI] [PubMed] [Google Scholar]
- 67.Gritsman K, Talbot WS, Schier AF. Nodal signaling patterns the organizer. Development. 2000;127:921–932. doi: 10.1242/dev.127.5.921. [DOI] [PubMed] [Google Scholar]
- 68.Dono R, Scalera L, Pacifico F, Acampora D, Persico MG, Simeone A. The murine cripto gene: expression during mesoderm induction and early heart morphogenesis. Development. 1993;118:1157–1168. doi: 10.1242/dev.118.4.1157. [DOI] [PubMed] [Google Scholar]
- 69.Johnson SE, Rothstein JL, Knowles BB. Expression of epidermal growth factor family gene members in early mouse development. Dev Dyn. 1994;201:216–226. doi: 10.1002/aja.1002010305. [DOI] [PubMed] [Google Scholar]
- 70.Xu C, Liguori G, Persico MG, Adamson ED. Abrogation of the Cripto gene in mouse leads to failure of postgastrulation morphogenesis and lack of differentiation of cardiomyocytes. Development. 1999;126:483–494. doi: 10.1242/dev.126.3.483. [DOI] [PubMed] [Google Scholar]
- 71.Zhang J, Talbot WS, Schier AF. Positional cloning identifies zebrafish one-eyed pinhead as a permissive EGF-related ligand required during gastrulation. Cell. 1998;92:241–251. doi: 10.1016/s0092-8674(00)80918-6. [DOI] [PubMed] [Google Scholar]
- 72.Gritsman K, Zhang J, Cheng S, Heckscher E, Talbot WS, Schier AF. The EGF-CFC protein one-eyed pinhead is essential for nodal signaling. Cell. 1999;97:121–132. doi: 10.1016/s0092-8674(00)80720-5. [DOI] [PubMed] [Google Scholar]
- 73.Warga RM, Kane DA. One-eyed pinhead regulates cell motility independent of Squint/Cyclops signaling. Dev Biol. 2003;261:391–411. doi: 10.1016/s0012-1606(03)00328-2. [DOI] [PubMed] [Google Scholar]
- 74.Saijoh Y, Adachi H, Sakuma R, Yeo CY, Yashiro K, Watanabe M, Hashiguchi H, Mochida K, Ohishi S, Kawabata M, et al. Left-right asymmetric expression of lefty2 and nodal is induced by a signaling pathway that includes the transcription factor FAST2. Mol Cell. 2000;5:35–47. doi: 10.1016/s1097-2765(00)80401-3. [DOI] [PubMed] [Google Scholar]
- 75.Gaio U, Schweickert A, Fischer A, Garratt AN, Muller T, Ozcelik C, Lankes W, Strehle M, Britsch S, Blum M, et al. A role of the cryptic gene in the correct establishment of the left-right axis. Curr Biol. 1999;9:1339–1342. doi: 10.1016/s0960-9822(00)80059-7. [DOI] [PubMed] [Google Scholar]
- 76.Schier AF, Talbot WS. Nodal signaling and the zebrafish organizer. Int J Dev Biol. 2001;45:289–297. [PubMed] [Google Scholar]
- 77.de la Cruz JM, Bamford RN, Burdine RD, Roessler E, Barkovich AJ, Donnai D, Schier AF, Muenke M. A loss-of-function mutation in the CFC domain of TDGF1 is associated with human forebrain defects. Hum Genet. 2002;110:422–428. doi: 10.1007/s00439-002-0709-3. [DOI] [PubMed] [Google Scholar]
- 78.Kenney NJ, Huang RP, Johnson GR, Wu JX, Okamura D, Matheny W, Kordon E, Gullick WJ, Plowman G, Smith GH, et al. Detection and location of amphiregulin and Cripto-1 expression in the developing postnatal mouse mammary gland. Mol Reprod Dev. 1995;41:277–286. doi: 10.1002/mrd.1080410302. [DOI] [PubMed] [Google Scholar]
- 79.Kenney NJ, Adkins HB, Sanicola M. Nodal and cripto-1: embryonic pattern formation genes involved in mammary gland development and tumorigenesis. J Mammary Gland Biol Neoplasia. 2004;9:133–144. doi: 10.1023/B:JOMG.0000037158.91940.1c. [DOI] [PubMed] [Google Scholar]
- 80.Bianco C, Wechselberger C, Ebert A, Khan NI, Sun Y, Salomon DS. Identification of Cripto-1 in human milk. Breast Cancer Res Treat. 2001;66:1–7. doi: 10.1023/a:1010648923432. [DOI] [PubMed] [Google Scholar]
- 81.De Santis ML, Kannan S, Smith GH, Seno M, Bianco C, Kim N, Martinez-Lacaci I, Wallace-Jones B, Salomon DS. Cripto-1 inhibits beta-casein expression in mammary epithelial cells through a p21ras-and phosphatidylinositol 3′-kinase-dependent pathway. Cell Growth Differ. 1997;8:1257–1266. [PubMed] [Google Scholar]
- 82.Wechselberger C, Strizzi L, Kenney N, Hirota M, Sun Y, Ebert A, Orozco O, Bianco C, Khan NI, Wallace-Jones B, et al. Human Cripto-1 overexpression in the mouse mammary gland results in the development of hyperplasia and adenocarcinoma. Oncogene. 2005;24:4094–4105. doi: 10.1038/sj.onc.1208417. [DOI] [PubMed] [Google Scholar]
- 83.Sun Y, Strizzi L, Raafat A, Hirota M, Bianco C, Feigenbaum L, Kenney N, Wechselberger C, Callahan R, Salomon DS. Overexpression of human Cripto-1 in transgenic mice delays mammary gland development and differentiation and induces mammary tumorigenesis. Am J Pathol. 2005;167:585–597. doi: 10.1016/S0002-9440(10)63000-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brandt R, Normanno N, Gullick WJ, Lin JH, Harkins R, Schneider D, Jones BW, Ciardiello F, Persico MG, Armenante F, et al. Identification and biological characterization of an epidermal growth factor-related protein: cripto-1. J Biol Chem. 1994;269:17320–17328. [PubMed] [Google Scholar]
- 85.Ciccodicola A, Dono R, Obici S, Simeone A, Zollo M, Persico MG. Molecular characterization of a gene of the ’EGF family’ expressed in undifferentiated human NTERA2 teratocarcinoma cells. Embo J. 1989;8:1987–1991. doi: 10.1002/j.1460-2075.1989.tb03605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kenney N, Smith G, Johnson M, Rosemberg K, Salomon DS, Dickson R. Cripto-1 activity in the intact and ovariectomized virgin mouse mammary gland. Pathogenesis. 1997;1:57–71. [Google Scholar]
- 87.Normanno N, De Luca A, Bianco C, Maiello MR, Carriero MV, Rehman A, Wechselberger C, Arra C, Strizzi L, Sanicola M, et al. Cripto-1 overexpression leads to enhanced invasiveness and resistance to anoikis in human MCF-7 breast cancer cells. J Cell Physiol. 2004;198:31–39. doi: 10.1002/jcp.10375. [DOI] [PubMed] [Google Scholar]
- 88.Wechselberger C, Ebert AD, Bianco C, Khan NI, Sun Y, Wallace-Jones B, Montesano R, Salomon DS. Cripto-1 enhances migration and branching morphogenesis of mouse mammary epithelial cells. Exp Cell Res. 2001;266:95–105. doi: 10.1006/excr.2001.5195. [DOI] [PubMed] [Google Scholar]
- 89.Thiery JP, Chopin D. Epithelial cell plasticity in development and tumor progression. Cancer Metastasis Rev. 1999;18:31–42. doi: 10.1023/a:1006256219004. [DOI] [PubMed] [Google Scholar]
- 90.Strizzi L, Bianco C, Normanno N, Seno M, Wecheselberger C, Wallace-Jones B, Khan NI, Hirota M, Sun Y, Sanicola M, Salomon DS. Epithelial mesenchymal transition is a characteristic of hyperplasias and tumors in mammary glands from MMTV-Cripto-1 transgenic mice. J Cell Physiol. 2004;201:266–276. doi: 10.1002/jcp.20062. [DOI] [PubMed] [Google Scholar]
- 91.Qi CF, Liscia DS, Normanno N, Merlo G, Johnson GR, Gullick WJ, Ciardiello F, Saeki T, Brandt R, Kim N, et al. Expression of transforming growth factor alpha, amphiregulin and cripto-1 in human breast carcinomas. Br J Cancer. 1994;69:903–910. doi: 10.1038/bjc.1994.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Normanno N, Kim N, Wen D, Smith K, Harris AL, Plowman G, Colletta G, Ciardiello F, Salomon DS. Expression of messenger RNA for amphiregulin, heregulin, and cripto-1, three new members of the epidermal growth factor family, in human breast carcinomas. Breast Cancer Res Treat. 1995;35:293–297. doi: 10.1007/BF00665981. [DOI] [PubMed] [Google Scholar]
- 93.Panico L, D’Antonio A, Salvatore G, Mezza E, Tortora G, De Laurentiis M, De Placido S, Giordano T, Merino M, Salomon DS, et al. Differential immunohistochemical detection of transforming growth factor alpha, amphiregulin and CRIPTO in human normal and malignant breast tissues. Int J Cancer. 1996;65:51–56. doi: 10.1002/(SICI)1097-0215(19960103)65:1<51::AID-IJC9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 94.Bianco C, Strizzi L, Mancino M, Rehman A, Hamada S, Watanabe K, De Luca A, Jones B, Balogh G, Russo J, et al. Identification of cripto-1 as a novel serologic marker for breast and colon cancer. Clin Cancer Res. 2006;12:5158–5164. doi: 10.1158/1078-0432.CCR-06-0274. [DOI] [PubMed] [Google Scholar]
- 95.Gong YP, Yarrow PM, Carmalt HL, Kwun SY, Kennedy CW, Lin BP, Xing PX, Gillett DJ. Overexpression of Cripto and its prognostic significance in breast cancer: a study with long-term survival. Eur J Surg Oncol. 2007;33:438–443. doi: 10.1016/j.ejso.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 96.Srinivasan R, Gillett CE, Barnes DM, Gullick WJ. Nuclear expression of the c-erbB-4/HER-4 growth factor receptor in invasive breast cancers. Cancer Res. 2000;60:1483–1487. [PubMed] [Google Scholar]
- 97.Bianco C, Kannan S, De Santis M, Seno M, Tang CK, Martinez-Lacaci I, Kim N, Wallace-Jones B, Lippman ME, Ebert AD, et al. Cripto-1 indirectly stimulates the tyrosine phosphorylation of erb B-4 through a novel receptor. J Biol Chem. 1999;274:8624–8629. doi: 10.1074/jbc.274.13.8624. [DOI] [PubMed] [Google Scholar]
- 98.Postovit LM, Margaryan NV, Seftor EA, Kirschmann DA, Lipavsky A, Wheaton WW, Abbott DE, Seftor REB, Hendrix MJ. Human embryonic stem cell microenvironment suppresses the tumorigenic phenotype of aggressive cancer cells. Proc Natl Acad Sci USA. 2008 doi: 10.1073/pnas.0800467105. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Normanno N, Tortora G, De Luca A, Pomatico G, Casamassimi A, Agrawal S, Mendelsohn J, Bianco AR, Ciardiello F. Synergistic growth inhibition and induction of apoptosis by a novel mixed backbone antisense oligonucleotide targeting CRIPTO in combination with C225 anti-EGFR monoclonal antibody and 8-Cl-cAMP in human GEO colon cancer cells. Oncol Rep. 1999;6:1105–1109. doi: 10.3892/or.6.5.1105. [DOI] [PubMed] [Google Scholar]