Meat Consumption and Its Association With C-Reactive Protein and Incident Type 2 Diabetes: The Rotterdam Study (original) (raw)
Abstract
OBJECTIVE
To investigate whether intake of different types of meat is associated with circulating C-reactive protein (CRP) and risk of type 2 diabetes in a prospective cohort study.
RESEARCH DESIGN AND METHODS
Our analysis included 4,366 Dutch participants who did not have diabetes at baseline. During a median follow-up period of 12.4 years, 456 diabetes cases were confirmed. Intake of red meat, processed meat, and poultry was derived from a food frequency questionnaire, and their association with serum high-sensitivity CRP was examined cross-sectionally using linear regression models. Their association with risk of type 2 diabetes was examined using multivariate Cox proportional hazards model, including age, sex, family history of diabetes, and lifestyle and dietary factors.
RESULTS
An increment of 50 g of processed meat was associated with increased CRP concentration (βprocessed meat = 0.12; P = 0.01), whereas intake of red meat and poultry was not. When comparing the highest to the lowest category of meat intake with respect to diabetes incidence, the adjusted relative risks were as follows: for red meat (1.42 [95% CI 1.06–1.91]), for processed meat (1.87 [1.26–2.78]), and for poultry (0.95 [0.74–1.22]). Additional analysis showed that the associations were not affected appreciably after inclusion of CRP into the model. After adjustment for BMI, however, the association for red meat attenuated to 1.18 (0.88–1.59).
CONCLUSIONS
Intake of processed meat is associated with higher risk of type 2 diabetes. It appears unlikely that CRP mediates this association.
Since the prevalence of type 2 diabetes has increased rapidly over the last decades, investigations into the effect of dietary and other lifestyle factors on type 2 diabetes have become important (1). One of the dietary factors of interest is meat. Three meta-analyses of prospective cohort studies showed that intake of processed meat is associated with a higher risk of type 2 diabetes (2–4). For red meat, two of these meta-analyses observed an adverse association (2,4), whereas one did not (3). For poultry, no data from meta-analyses were available. Results from six prospective studies on poultry, however, showed that it is not likely that poultry is associated with a higher risk of type 2 diabetes; three studies observed an inverse association (5–7), whereas three did not observe an association (8–10).
Intake of red meat and processed meat may increase risk of type 2 diabetes by mechanisms that increase circulating proinflammatory markers. Positive associations have been observed between red meat or processed meat and the proinflammatory blood marker C-reactive protein (CRP), which in turn has been associated with higher risk of type 2 diabetes (11,12,13). The positive association between intake of meat and CRP might be explained by several biological pathways. The binding capacity of iron in the body could be exceeded by the intake of meat, which contains high amounts of heme iron. Free iron can increase oxidative stress, thereby acting as proinflammatory agent (14). Advanced glycation end products (AGEs), which occur naturally in meat and are formed through heat processing (15), may also have proinflammatory actions (16). Thus, the observed positive associations between intake of red meat and processed meat and CRP, and CRP and risk of type 2 diabetes may indicate that CRP mediates the association between intake of meat, especially red and processed meat, and risk of type 2 diabetes. Therefore, we investigated whether intake of red meat, processed meat, and poultry was associated with CRP and risk of type 2 diabetes in a Dutch population.
RESEARCH DESIGN AND METHODS
Study population
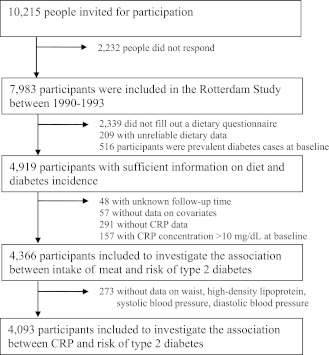
The current analysis was conducted within the Rotterdam Study. The Rotterdam Study is a population-based prospective cohort study among inhabitants of Ommoord, a district of the city of Rotterdam, the Netherlands (17). In 1990, all inhabitants of this district who were ≥55 years of age were invited for participation (n = 10,215). Of the 7,983 responders (78%), 2,339 did not fill out a dietary questionnaire, 209 did not provide sufficient dietary data, 516 had type 2 diabetes at baseline, 448 did not have sufficient data on CRP, and 105 did not have sufficient information on follow-up time or other covariates (Fig. 1). Hence, 4,366 participants were included in the current analysis. Compared with participants who were included in the analysis, people who were excluded tended to be older (74.6 [SD 10] vs. 67.3 [SD 8] years; P < 0.01), smoked less (22 vs. 23%; P = <0.01), and were less likely to be men (37 vs. 40%; P = 0.01), whereas BMI did not appear to be different (26.3 [SD 4] vs. 26.3 [SD 4] kg/m2; P = 0.51). The association between CRP and risk of type 2 diabetes was studied in 4,092 participants because we excluded participants with missing data on waist circumference (n = 253), systolic and diastolic blood pressure (n = 17), and HDL cholesterol (n = 4) (Fig. 1). The Medical Ethics Committee of Erasmus Medical Center approved the study. All participants gave informed consent.
Figure 1.
Flow diagram for inclusion of participants to investigate whether the intake of meat is associated with serum CRP and risk of type 2 diabetes.
Meat intake and other dietary covariates
Dietary assessment was performed at baseline (1990–1993) and comprised two steps: first, participants had to mark the foods and drinks they had consumed at least twice a month in the preceding year on a self-administered questionnaire at home; and second, at the research center, a trained dietitian obtained accurate information on the amount of foods and drinks indicated on the questionnaire using a semiquantitative food frequency questionnaire (18). This food frequency questionnaire comprised 170 food items in 13 food groups and additional questions about prescribed diets.
The food items included the intake of meat products, through which the intake of red meat, processed meat, and poultry could be calculated in grams per day. Total meat included red meat (e.g., beefsteak and pork fricandeau), processed meat (e.g., sausage and cold cuts), and poultry (e.g., chicken). Processed meat included meats that were preserved by smoking, curing, salting, or addition of preservatives. For the analysis, types of meat were adjusted for energy according to the residual method.
Intake of all food items was converted into total intake of energy and nutrients using the Dutch Food Composition Table 1993 (NEVO). Intake of fiber was derived from the next version of this table (NEVO 1996) because data on fiber were not sufficient in 1993.
CRP
Nonfasting serum samples were collected at the research center at baseline. These samples were immediately put on ice and processed within 30 min. High-sensitivity CRP was measured using a rate near-infrared particle immunoassay (IMMAGE Immunochemistry System; Beckman Coulter, Fullerton, CA). The procedure has been described in more detail elsewhere (19). CRP concentrations >10 mg/dL at baseline were excluded because these higher concentrations reflect acute rather than chronic inflammation.
Diabetes prevalence and incidence
Participants were considered a prevalent diabetic case subject when they used antidiabetes medication or had a nonfasting or postload glucose concentration ≥11.1 mmol/L.
During follow-up, information from general practitioners, pharmacies’ databases, and follow-up examinations in 1993–1995, 1997–1999, and 2002–2004 was used to identify cases of diabetes. Participants were considered an incident diabetes case when they were registered by a general practitioner as having type 2 diabetes and had at least one of the following four criteria: plasma glucose concentration ≥7.0 mmol/L, random plasma glucose concentration ≥11.1 mmol/L, antidiabetes medication, and/or treatment by diet. Diabetes cases were monitored until July 2005.
Nondietary covariates
General information (e.g., smoking status, education level, and family history of type 2 diabetes) was obtained with a questionnaire at baseline. A family history of type 2 diabetes was defined as having a parent, sibling, or both with type 2 diabetes. Information on energy expenditure (kcal/day) was obtained with a physical activity questionnaire (Longitudinal Aging Study Amsterdam Physical Activity Questionnaire) during follow-up from 1997 to 2000 for 3,244 participants of our study population. Consequently, energy expenditure could be used as a measure of physical activity in those participants.
Information on cardiovascular risk factors of each participant was obtained by clinical examinations during a visit at the research center at baseline. Height and weight were measured and BMI (kg/m2) was calculated. Waist circumference (cm) was measured at the level midway between the lower rib margin and the iliac crest with the participant in a standing position. Blood pressure was measured twice at the right brachial artery with a random-zero sphygmomanometer with the participant in a sitting position. The mean of two consecutive measurements was used. Hypertension was defined as systolic blood pressure ≥140 mmHg, and/or diastolic blood pressure ≥90 mmHg, and/or use of blood pressure–lowering medication.
Serum total cholesterol was determined in blood samples with an automated enzymatic procedure using Roche CHOD-PAP reagent agent. HDL cholesterol was measured with Roche HDL cholesterol essay using polyethylene glycol–modified enzymes and dextran sulfate.
Data analysis
Descriptive data were expressed as mean (SD), median (Q1–Q3), or percentage by the lowest and highest category of types of meat intake.
The association between intake of energy-adjusted types of meat per 50-g increase and ln(CRP) was investigated using linear regression models. CRP was transformed logarithmically to achieve a symmetric distribution. Adjustments were made for age (years), sex, family history of diabetes (yes or no), diet prescription (yes or no), smoking (current, former, or never), intake of energy (kcal/day), intake of energy-adjusted carbohydrates (g/day), intake of energy-adjusted polyunsaturated fatty acids (g/day), intake of alcohol (0, >0–10, >10–20, or >20 g/day), intake of energy-adjusted fiber (g/day), intake of energy-adjusted milk products (g/day), intake of energy-adjusted cheese (g/day), intake of soya (consumers or nonconsumers), intake of fish (nonconsumers and approximate tertiles), and intake of tea (g/day).
The association between CRP and risk of type 2 diabetes, as shown previously in the Rotterdam Study (n = 5,901) (19), was verified in our subpopulation of the Rotterdam Study (n = 4,093; _n_cases = 423; median follow-up time = 11.0 years). Adjustments were made for age (years), sex, BMI (kg/m2), waist circumference (cm), HDL cholesterol (mmol/L), systolic blood pressure (mmHg), and diastolic blood pressure (mmHg).
After obtaining the associations between intake of meat and CRP and CRP and risk of type 2 diabetes, we could further study the potential mediating effect of CRP on the association between energy-adjusted types of meat and risk of type 2 diabetes (n = 4,366; _n_cases = 456; median follow-up time = 12.4 years).
Intake of red meat was divided into quartiles based on the population distributions of intake. As processed meat and poultry were not used by a considerable number of participants, intake of processed meat and poultry were divided into four categories: nonconsumers and approximate tertiles based on the population distributions of intake. After testing the proportional hazards assumption, Cox proportional hazards models were used to calculate relative risks (RRs) and 95% CIs. The RR expressed the risk relative to the lowest category. The crude model included the intake of meat but was not adjusted for any covariate. In addition to intake of meat, model 1 included five other covariates as follows: age (years), sex, family history of diabetes (yes or no), diet prescription (yes or no), and smoking (current, former, or never). Model 2 was similar to model 1 with additional adjustment for intake of five dietary factors and five food products as follows: energy (kcal/day), energy-adjusted carbohydrates (g/day), energy-adjusted polyunsaturated fatty acids (g/day), alcohol (0, >0–10, >10–20, or >20 g/day), energy-adjusted fiber (g/day), energy-adjusted milk products (g/day), energy-adjusted cheese (g/day), soya (consumers or nonconsumers), fish (nonconsumers and approximate tertiles), and tea (g/day). Types of meat were adjusted for each other.
To investigate the potential mediating effect of CRP on the association between types of meat and type 2 diabetes, baseline ln(CRP) (mg/dL) was added to model 2 as an additional covariate. In addition to ln(CRP), BMI (kg/m2) was included in a second additional model.
To investigate potential effect measure modification by sex or BMI, an interaction term between types of meat and sex or BMI was included in model 2 with additional adjustment for ln(CRP) and BMI. Sensitivity analyses were performed by excluding vegetarians (n = 33), participants who developed type 2 diabetes within 2 years of follow-up (n = 437), or participants with coronary heart disease at baseline (n = 514), but none of them changed the interpretation of the results.
Tests for trend across categories were performed by assigning the median value for each category to each participant and modeling this variable as a continuous variable. All statistical analyses were performed in SAS (version 9.1; SAS Institute, Cary, NC). A two-sided P value ≤0.05 was considered as statistically significant for all analyses.
RESULTS
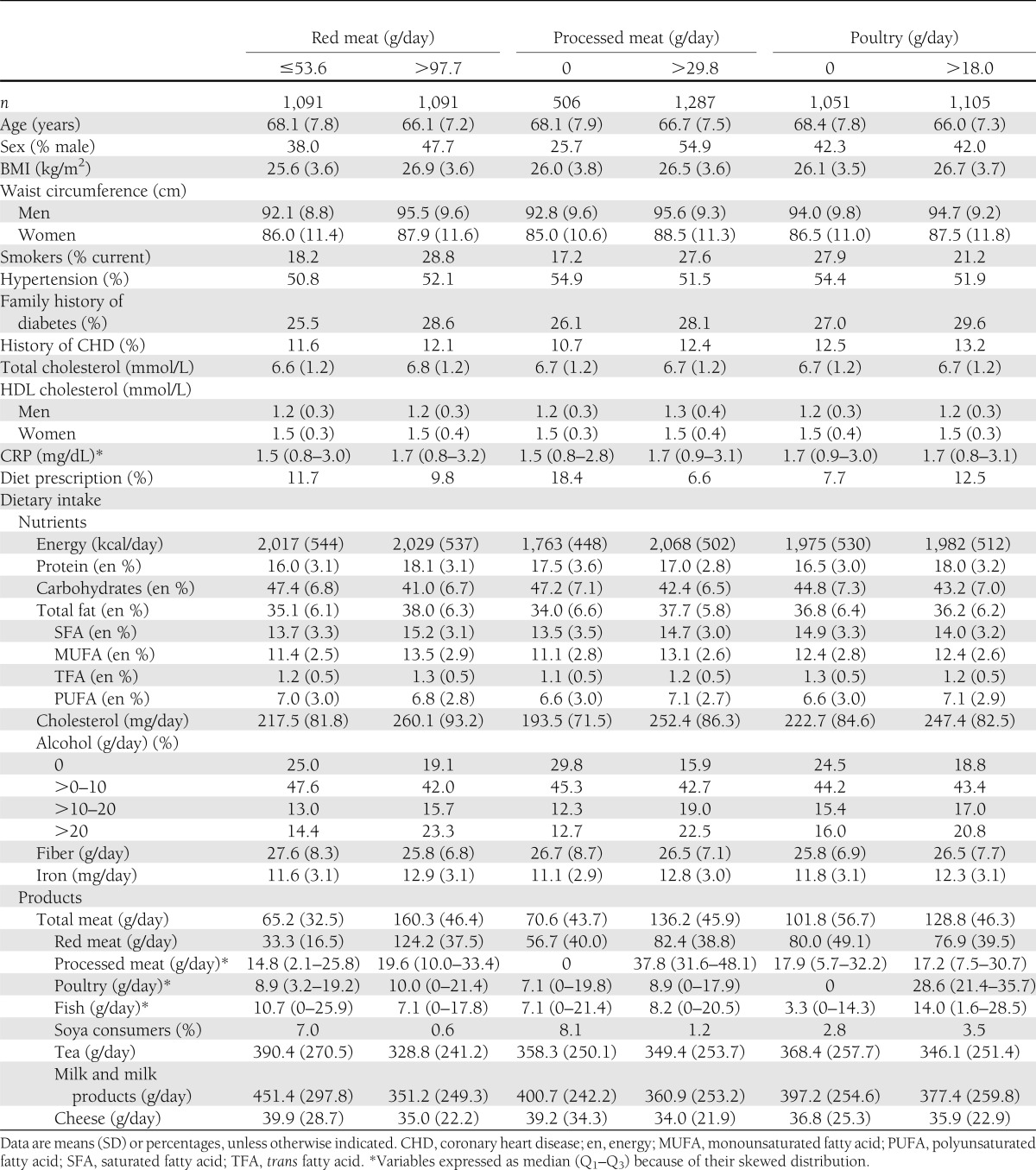
Meat was eaten by nearly all participants (99.2%). Red meat was the main type consumed (98.7%), followed by processed meat (88.2%) and poultry (75.6%). The relative contribution of red meat to total meat was 68%, of processed meat 19%, and of poultry 13%. Mean total meat intake was 112 g daily (SD 45). The highest category of red meat and processed meat included more men, smokers, participants with a larger BMI, and soya eaters than the lowest category (Table 1). In contrast, the highest category of poultry included fewer smokers and soya eaters than the lowest category, and the sex distribution did not differ between the highest and lowest category of poultry (Table 1).
Table 1.
Baseline characteristics of 4,366 Dutch adults aged ≥55 years by lowest and highest intake category of energy-adjusted types of meat
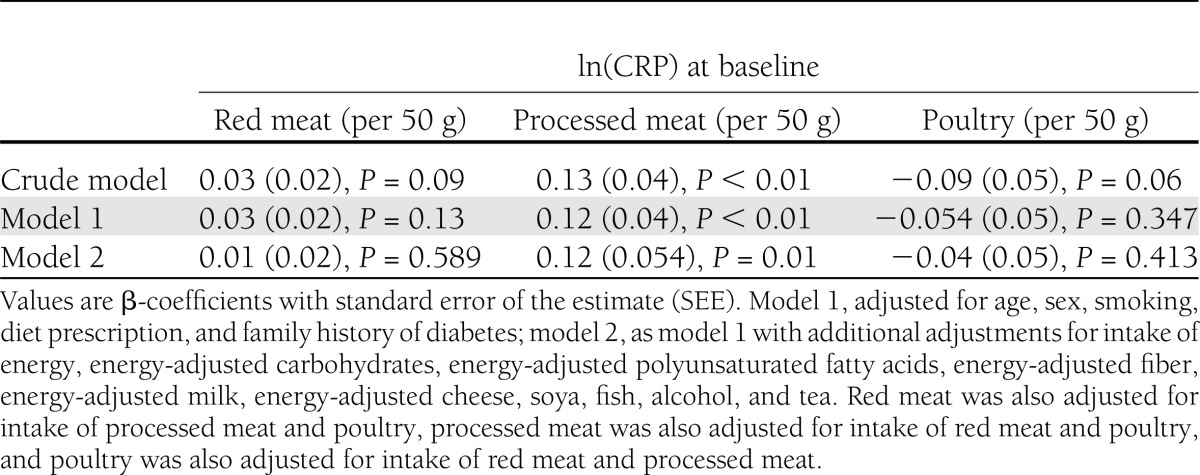
In line with the higher CRP concentration observed in the highest category of processed meat intake compared with the lowest (1.7 vs. 1.5 mg/dL) (Table 1), linear regression analysis showed that a 50-g higher intake of processed meat was associated with a higher CRP concentration after adjustment for dietary factors and other lifestyle factors (model 2) (Table 2). Intake of red meat and poultry was not associated with CRP. As intake of processed meat was associated with CRP and our analysis confirmed that CRP at baseline was associated with a higher risk of type 2 diabetes (RRCRP Q4 vs. Q1 1.76 [95% CI 1.27–2.45]; _P_trend < 0.01), we could further investigate a potential mediating effect of CRP on the association between intake of meat and risk of type 2 diabetes.
Table 2.
β-Coefficients (SEE) for the association of energy-adjusted type of meat intake with ln(CRP) in 4,366 Dutch adults aged ≥55 years
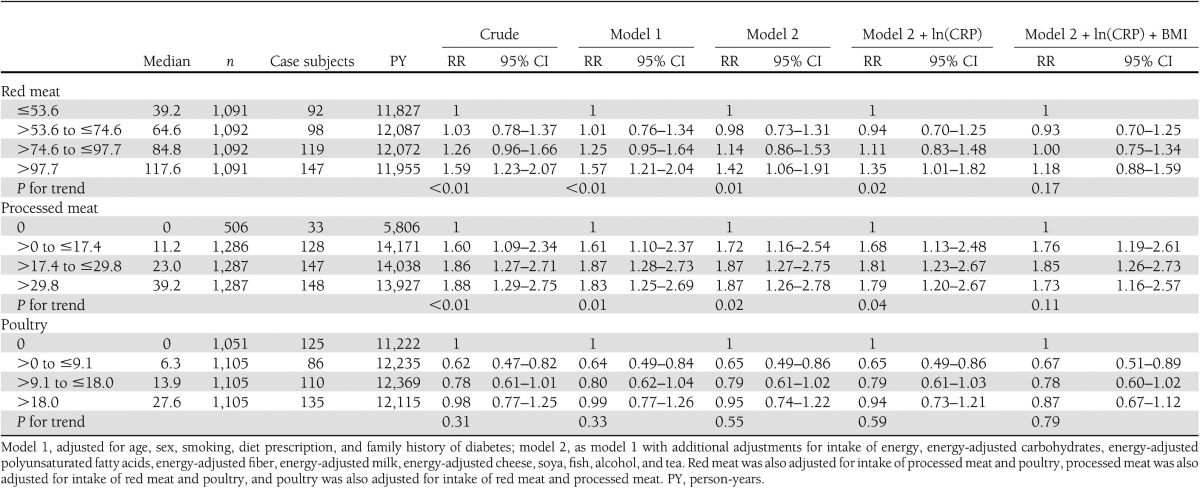
Initially, red meat (RR>97.7 vs. ≤53.6 1.42 [95% CI 1.06–1.91]; _P_trend = 0.01) and processed meat (RR>29.8 vs. 0 1.87 [1.26–2.78]; _P_trend = 0.02) were associated positively with risk of type 2 diabetes (model 2) (Table 3). Intake of poultry was not associated with risk of type 2 diabetes (RR>18.0 vs. 0 0.95 [0.74–1.22]; _P_trend = 0.55) (Table 3). Additional adjustment for CRP did not change RRs appreciably. Additional adjustment for BMI, however, attenuated the association for intake of red meat (RR>97.7 vs. ≤53.6 1.18 [0.88–1.59]; _P_trend = 0.17) (Table 3). The association between intake of total meat and risk of type 2 diabetes was also not statistically significant (model 2 + ln(CRP) + BMI RR≤85.2 vs. >139.1 1.21 [0.88–1.67]; _P_trend = 0.30). Additional adjustment by waist circumference (n = 4,113; _n_cases = 427) or physical activity (n = 3,244; _n_cases = 391) did not attenuate associations further. Furthermore, components of meat that may explain the observed associations (i.e., saturated fat, vitamin B12, and heme iron) were added to the model one by one, but inclusion of these components did not change the interpretation of the results.
Table 3.
RR and 95% CI for incident type 2 diabetes by intake categories of energy-adjusted types of meat in 4,366 Dutch adults aged ≥55 years
On the basis of P for interaction, the association between intake of red meat (_P_interaction sex = 0.29; _P_interaction BMI = 0.83), processed meat (_P_interaction sex = 0.26; _P_interaction BMI = 0.49), or poultry (_P_interaction sex = 0.07; _P_interaction BMI = 0.93) and risk of type 2 diabetes did not differ by sex and BMI.
CONCLUSIONS
In this prospective cohort study of Dutch adults, high intake of processed meat was associated with a higher risk of type 2 diabetes compared with no intake of processed meat, independently of CRP and BMI. Therefore, CRP does not appear to be an intermediate. Intake of red meat and poultry was not associated with risk of type 2 diabetes.
Strengths of our analyses included the prospective design, the inclusion of verified cases of diabetes, and the extensive information on potential confounders that minimized the presence of residual confounding.
The adverse association between intake of processed meat and risk of type 2 diabetes observed in the current study is in line with a meta-analysis conducted by Aune et al. (2) (RRhighest vs. lowest category 1.41 [95% CI 1.35–1.60]). The observed higher risk could be attributed to components present in processed meat, such as AGEs. AGEs, which are naturally present in meat and formed in meat through heating, have been associated with insulin resistance (20,21) and type 1 diabetes (22) in animal models. In addition, treatment with AGE inhibitor reduced risk of type 2 diabetes in mice (23). A 6-day, randomized, crossover trial, however, did not observe differences in changes in serum glucose concentration between a high- and low-AGE diet in participants with diabetes (24). AGEs might influence risk of type 2 diabetes by its proinflammatory properties (16). A crossover study showed that compared with a low-AGE diet, a high-AGE diet increased concentration of plasma CRP in participants with diabetes (25). In line with these findings, a randomized trial showed that circulating inflammation markers increased after eating a high-AGE diet for 6 weeks compared with eating a low-AGE diet (26). As intake of processed meat was not associated with CRP concentrations, however, our study did not find clear support for an inflammatory pathway through which processed meat increased risk of type 2 diabetes. In line with our findings, a cross-sectional study among children and adolescents living in the U.S. showed that the intake of processed meat was not higher in children with a CRP concentration of >3 mg/dL than those children with a CRP concentration of <1 mg/dL (27).
The higher risk observed for processed meat may also be explained by additives, e.g., nitrites, as processed meat contains more additives than other types of meat (28). Nitrites may be converted to nitrosamines within the food product or stomach by interaction with amines (28). These nitrosamines are of concern in the development of diabetes. Intake of nitrosamines was associated with type 1 diabetes in children (29–32) and decreased insulin secretion in animals (33). The role of nitrosamines in the etiology of type 2 diabetes is less clear. Low doses of streptozotocin, a nitrosamine-related compound, combined with dietary-induced insulin resistance, however, resulted in metabolic conditions in mice that are similar to type 2 diabetes in humans (34,35).
The higher risk may also be explained by the higher content of saturated fat (36), whereas it is not likely that the higher risk is explained by a higher iron intake associated with processed meat. Processed meat does not contain more iron compared with red meat for which we did not observe a higher risk after adjusting for BMI.
The point estimate for red meat (RR 1.18), however, was in line with the point estimate (RRhighest vs. lowest red meat category 1.21) observed in the meta-analysis conducted by Aune et al. (2). Without adjusting for BMI, however, a higher risk was observed for red meat as well. As additional adjustment for CRP or heme iron in our analysis did not appreciably attenuate the association for red meat, it is not likely that heme iron explained the observed association either by its direct effect on glucose metabolism or via an inflammatory pathway (14).
The small variation in intake of poultry may explain why an association between intake of poultry and risk of type 2 diabetes was not found in the total population. Our risk estimate in the highest category of poultry intake in the total population after inclusion of CRP and BMI, however, was comparable with RRs reported by studies showing inverse associations (5–7). A potential inverse association may reflect a “healthy diet” rather than a direct effect of consuming poultry. We were able to adjust for a range of lifestyle factors, including dietary factors, through which confounding due to a healthy diet was minimized.
Another explanation for the absence of an adverse association of poultry may be its effect on CRP. Dietary patterns, including high loadings on poultry, have been related to lower CRP concentrations (37–39). In the current study, however, it is not likely that CRP mediates the association between intake of poultry and risk of type 2 diabetes as additional adjustment for CRP did not affect the risk estimates.
Some limitations of the current study should be mentioned. First, information on dietary intake was obtained once. It could be that participants changed their diet through follow-up. However, exclusion of participants who are likely to change their diet during follow-up because of previous illness did not change the results. Second, misclassification of type and amount of meat could have occurred. As measurement error from dietary assessment was unlikely to be related to diabetes end point, it is likely that misclassification of meat intake was rather nondifferential than differential and would have attenuated observed associations overall, if present. Third, it is questionable whether BMI should be included in the model when investigating the association between red meat and risk of type 2 diabetes. As BMI may reflect an unhealthy lifestyle, BMI can be a confounder and, therefore, should be included in the model. If BMI is an intermediate, however, inclusion of BMI in the model will underestimate the association. BMI could be considered as intermediate because BMI is a major risk factor for diabetes and intake of red meat was associated with weight gain (40). Fourth, we may have missed undiagnosed cases of diabetes, because diabetes incidence was derived from general practitioner registration. Based on the descriptive tables, we can assume that undiagnosed diabetes may also have been higher in the high red meat and processed meat categories, and in this case, our results may even have been attenuated toward the null.
In conclusion, intake of processed meat was associated with higher risk of type 2 diabetes. It does not appear likely that CRP mediates this association. The underlying mechanism by which processed meat may increase risk of type 2 diabetes requires further investigation.
Acknowledgments
The Rotterdam Study is supported by the Erasmus Medical Center and Erasmus University Rotterdam; the Netherlands Organization for Scientific Research; the Netherlands Organization for Health Research and Development; the Research Institute for Diseases in the Elderly; the Ministry of Education, Culture, and Science; the Ministry of Health Welfare and Sports; the European Commission; and the Municipality of Rotterdam. G.J.v.W. and A.K. were supported, in part, by the InterAct project, funded by the European Union (Integrated Project LSHM-CT-2006-037197 in the Framework Programme 6 of the European Community).
No potential conflicts of interest relevant to this article were reported.
G.J.v.W. prepared the data for analysis, performed the analysis, and drafted the manuscript. A.K., B.T., and E.J.M.F. contributed to the interpretation of the results and revised the manuscript. E.J.G.S., F.J.A.v.R., and J.C.M.W. participated in the coordination of the study and revised the manuscript. A.H. participated in the design and coordination of the study. G.J.v.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors are greatly indebted to the many people that are involved in this longitudinal study, including all participants, general practitioners, and pharmacists of the Ommoord district in Rotterdam.
References
- 1.Green A, Christian Hirsch N, Pramming SK. The changing world demography of type 2 diabetes. Diabetes Metab Res Rev 2003;19:3–7 [DOI] [PubMed] [Google Scholar]
- 2.Aune D, Ursin G, Veierød MB. Meat consumption and the risk of type 2 diabetes: a systematic review and meta-analysis of cohort studies. Diabetologia 2009;52:2277–2287 [DOI] [PubMed] [Google Scholar]
- 3.Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation 2010;121:2271–2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan A, Sun Q, Bernstein AM, et al. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am J Clin Nutr 2011;94:1088–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montonen J, Järvinen R, Heliövaara M, Reunanen A, Aromaa A, Knekt P. Food consumption and the incidence of type II diabetes mellitus. Eur J Clin Nutr 2005;59:441–448 [DOI] [PubMed] [Google Scholar]
- 6.Schulze MB, Manson JE, Willett WC, Hu FB. Processed meat intake and incidence of type 2 diabetes in younger and middle-aged women. Diabetologia 2003;46:1465–1473 [DOI] [PubMed] [Google Scholar]
- 7.Villegas R, Shu XO, Gao YT, et al. The association of meat intake and the risk of type 2 diabetes may be modified by body weight. Int J Med Sci 2006;3:152–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinbrecher A, Erber E, Grandinetti A, Kolonel L, Maskarinec G. Meat consumption and risk of type 2 diabetes: the Multiethnic Cohort. Public Health Nutr 2011;14:568–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Männistö S, Kontto J, Kataja-Tuomola M, Albanes D, Virtamo J. High processed meat consumption is a risk factor of type 2 diabetes in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention study. Br J Nutr 2010;103:1817–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Dam RM, Willett WC, Rimm EB, Stampfer MJ, Hu FB. Dietary fat and meat intake in relation to risk of type 2 diabetes in men. Diabetes Care 2002;25:417–424 [DOI] [PubMed] [Google Scholar]
- 11.Lee CC, Adler AI, Sandhu MS, et al. Association of C-reactive protein with type 2 diabetes: prospective analysis and meta-analysis. Diabetologia 2009;52:1040–1047 [DOI] [PubMed] [Google Scholar]
- 12.Schulze MB, Hoffmann K, Manson JE, et al. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am J Clin Nutr 2005;82:675–684; quiz 714–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azadbakht L, Esmaillzadeh A. Red meat intake is associated with metabolic syndrome and plasma c-reactive protein concentrations in women. J Nutr 2009;139:335–339 [DOI] [PubMed] [Google Scholar]
- 14.Fernández-Real JM, López-Bermejo A, Ricart W. Cross-talk between iron metabolism and diabetes. Diabetes 2002;51:2348–2354 [DOI] [PubMed] [Google Scholar]
- 15.Uribarri J, Woodruff S, Goodman S, et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc 2010;110:911-16.e12. [DOI] [PMC free article] [PubMed]
- 16.Uribarri J, Cai W, Sandu O, Peppa M, Goldberg T, Vlassara H. Diet-derived advanced glycation end products are major contributors to the body’s AGE pool and induce inflammation in healthy subjects. Ann N Y Acad Sci 2005;1043:461–466 [DOI] [PubMed] [Google Scholar]
- 17.Hofman A, van Duijn CM, Franco OH, et al. The Rotterdam Study: 2012 objectives and design update. Eur J Epidemiol 2011;26:657–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klipstein-Grobusch K, den Breeijen JH, Goldbohm RA, et al. Dietary assessment in the elderly: validation of a semiquantitative food frequency questionnaire. Eur J Clin Nutr 1998;52:588–596 [DOI] [PubMed] [Google Scholar]
- 19.Dehghan A, Kardys I, de Maat MP, et al. Genetic variation, C-reactive protein levels, and incidence of diabetes. Diabetes 2007;56:872–878 [DOI] [PubMed] [Google Scholar]
- 20.Hofmann SM, Dong HJ, Li Z, et al. Improved insulin sensitivity is associated with restricted intake of dietary glycoxidation products in the db/db mouse. Diabetes 2002;51:2082–2089 [DOI] [PubMed] [Google Scholar]
- 21.Sandu O, Song K, Cai W, Zheng F, Uribarri J, Vlassara H. Insulin resistance and type 2 diabetes in high-fat-fed mice are linked to high glycotoxin intake. Diabetes 2005;54:2314–2319 [DOI] [PubMed] [Google Scholar]
- 22.Peppa M, He C, Hattori M, McEvoy R, Zheng F, Vlassara H. Fetal or neonatal low-glycotoxin environment prevents autoimmune diabetes in NOD mice. Diabetes 2003;52:1441–1448 [DOI] [PubMed] [Google Scholar]
- 23.Piercy V, Toseland CD, Turner NC. Potential benefit of inhibitors of advanced glycation end products in the progression of type II diabetes: a study with aminoguanidine in C57/BLKsJ diabetic mice. Metabolism 1998;47:1477–1480 [DOI] [PubMed] [Google Scholar]
- 24.Negrean M, Stirban A, Stratmann B, et al. Effects of low- and high-advanced glycation endproduct meals on macro- and microvascular endothelial function and oxidative stress in patients with type 2 diabetes mellitus. Am J Clin Nutr 2007;85:1236–1243 [DOI] [PubMed] [Google Scholar]
- 25.Vlassara H, Cai W, Crandall J, et al. Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc Natl Acad Sci USA 2002;99:15596–15601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peppa M, Goldberg T, Cai W, Rayfield E, Vlassara H. Glycotoxins: a missing link in the “relationship of dietary fat and meat intake in relation to risk of type 2 diabetes in men”. Diabetes Care 2002;25:1898–1899 [DOI] [PubMed] [Google Scholar]
- 27.Qureshi MM, Singer MR, Moore LL. A cross-sectional study of food group intake and C-reactive protein among children. Nutr Metab (Lond) 2009;6:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lijinsky W. N-nitroso compounds in the diet. Mutat Res 1999;443:129–138 [DOI] [PubMed] [Google Scholar]
- 29.Dahlquist GG, Blom LG, Persson LA, Sandström AI, Wall SG. Dietary factors and the risk of developing insulin dependent diabetes in childhood. BMJ 1990;300:1302–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parslow RC, McKinney PA, Law GR, Staines A, Williams R, Bodansky HJ. Incidence of childhood diabetes mellitus in Yorkshire, northern England, is associated with nitrate in drinking water: an ecological analysis. Diabetologia 1997;40:550–556 [DOI] [PubMed] [Google Scholar]
- 31.Sipetić SB, Vlajinac HD, Kocev NI, Marinković JM, Radmanović SZ, Bjekić MD. The Belgrade childhood diabetes study: a multivariate analysis of risk determinants for diabetes. Eur J Public Health 2005;15:117–122 [DOI] [PubMed] [Google Scholar]
- 32.Virtanen SM, Jaakkola L, Rasanen L, et al. Nitrate and nitrite intake and the risk for type 1 diabetes in Finnish children. Childhood Diabetes in Finland Study Group. Diabet Med 1994;11:656–662. [DOI] [PubMed]
- 33.Portha B, Giroix MH, Cros JC, Picon L. Diabetogenic effect of N-nitrosomethylurea and N-nitrosomethylurethane in the adult rat. Ann Nutr Aliment 1980;34:1143–1151 [PubMed] [Google Scholar]
- 34.Luo J, Quan J, Tsai J, et al. Nongenetic mouse models of non-insulin-dependent diabetes mellitus. Metabolism 1998;47:663–668 [DOI] [PubMed] [Google Scholar]
- 35.de la Monte SM, Neusner A, Chu J, Lawton M. Epidemilogical trends strongly suggest exposures as etiologic agents in the pathogenesis of sporadic Alzheimer’s disease, diabetes mellitus, and non-alcoholic steatohepatitis. J Alzheimers Dis 2009;17:519–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feskens EJ, Kromhout D. Habitual dietary intake and glucose tolerance in euglycaemic men: the Zutphen Study. Int J Epidemiol 1990;19:953–959 [DOI] [PubMed] [Google Scholar]
- 37.Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC. Dietary patterns and markers of systemic inflammation among Iranian women. J Nutr 2007;137:992–998 [DOI] [PubMed] [Google Scholar]
- 38.Fung TT, Rimm EB, Spiegelman D, et al. Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. Am J Clin Nutr 2001;73:61–67 [DOI] [PubMed] [Google Scholar]
- 39.Lopez-Garcia E, Schulze MB, Fung TT, et al. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr 2004;80:1029–1035 [DOI] [PubMed] [Google Scholar]
- 40.Vergnaud AC, Norat T, Romaguera D, et al. Meat consumption and prospective weight change in participants of the EPIC-PANACEA study. Am J Clin Nutr 2010;92:398–407 [DOI] [PubMed] [Google Scholar]