Discovering Thiamine Transporters as Targets of Chloroquine Using a Novel Functional Genomics Strategy (original) (raw)
Abstract
Chloroquine (CQ) and other quinoline-containing antimalarials are important drugs with many therapeutic benefits as well as adverse effects. However, the molecular targets underlying most such effects are largely unknown. By taking a novel functional genomics strategy, which employs a unique combination of genome-wide drug-gene synthetic lethality (DGSL), gene-gene synthetic lethality (GGSL), and dosage suppression (DS) screens in the model organism Saccharomyces cerevisiae and is thus termed SL/DS for simplicity, we found that CQ inhibits the thiamine transporters Thi7, Nrt1, and Thi72 in yeast. We first discovered a thi3Δ mutant as hypersensitive to CQ using a genome-wide DGSL analysis. Using genome-wide GGSL and DS screens, we then found that a thi7Δ mutation confers severe growth defect in the thi3Δ mutant and that THI7 overexpression suppresses CQ-hypersensitivity of this mutant. We subsequently showed that CQ inhibits the functions of Thi7 and its homologues Nrt1 and Thi72. In particular, the transporter activity of wild-type Thi7 but not a CQ-resistant mutant (Thi7T287N) was completely inhibited by the drug. Similar effects were also observed with other quinoline-containing antimalarials. In addition, CQ completely inhibited a human thiamine transporter (SLC19A3) expressed in yeast and significantly inhibited thiamine uptake in cultured human cell lines. Therefore, inhibition of thiamine uptake is a conserved mechanism of action of CQ. This study also demonstrated SL/DS as a uniquely effective methodology for discovering drug targets.
Author Summary
By using a novel SL/DS methodology in the model organism yeast, we discovered that the antimalarial drug CQ inhibits thiamine transporters and consequently causes thiamine (vitamin B1) deficiency and growth defects. This mechanism of action (MOA) is conserved in human cells and possibly also in other organisms. Given that both thiamine deficiency and treatment with CQ cause retinal, neurological, and cardiovascular disorders in humans, our results suggest that thiamine deficiency might be a root cause of some of CQ's adverse effects, which might be preventable with concomitant dietary thiamine supplementation. Such a MOA by CQ could also be responsible for its therapeutic effects against malarial parasites, which need exogenous thiamine for survival. Such a possibility needs to be investigated before dietary thiamine supplementation can be used to prevent CQ's adverse effects.
Introduction
Chloroquine (CQ) and other quinoline-containing compounds have been major antimalarial drugs for many decades. They are also effective treatments for systematic lupus erythematosus, rheumatoid arthritis, and many other rheumatic and skin diseases [1]. In recent years, their effects in treating viral, bacterial, and fungal infections and cancer have also been explored [2], [3]. Despite being relatively safe, these drugs can cause severe adverse side effects, including retinopathy, myopathy, cardiopathy, peripheral neuropathy, and others [4], [5], [6]. In many cases, the underlying molecular mechanisms of the therapeutic and deleterious effects are not well understood.
The model organism yeast Saccharomyces cerevisiae is an excellent system for discovering conserved targets of bioactive compounds [7]. In this study, we took a novel functional genomics approach in yeast to explore the mechanism(s) of action (MOA) of CQ. By first performing a genome-wide drug-gene synthetic lethality (DGSL) screen, we identified 95 CQ-hypersensitive deletion mutants, including those involved in vacuole functions (e.g., mon2Δ, vma4Δ, and vma8Δ) [8], [9], iron homeostasis (e.g., fet3Δ) [10], and thiamine metabolism (e.g., thi3Δ) [11]. By centering on the thi3Δ mutation, we next performed genome-wide gene-gene synthetic lethality (GGSL) and dosage suppression (DS) screens and discovered the high affinity thiamine transporter Thi7 [12] as a candidate target of CQ. For simplicity, this unique combination of DGSL, GGSL, and DS screens was termed SL/DS. We subsequently showed that CQ inhibits Thi7-related functions, particularly Thi7-dependent uptake of thiamine. We also showed that CQ likely inhibits the low affinity thiamine transporters Nrt1 and Thi72 [13] in yeast. This MOA is also shared by other quinoline-containing antimalarials. Moreover, we demonstrated that CQ completely inactivates a human thiamine transporter (SLC19A3) [14], [15] expressed in yeast cells and significantly inhibited thiamine uptake in HeLa and HT1080 cells, suggesting that such a MOA is conserved across species. This study also demonstrated that SL/DS is an effective strategy for drug target identification, especially for discovering non-essential genes as drug targets.
Results
A genome-wide DGSL screen revealed multiple distinct functions affected by CQ
To discover the in vivo target(s) of CQ that might mediate its effects in a eukaryote, we first explored haploinsufficiency [16] by screening a yeast genome-wide heterozygous diploid deletion library for hypersensitive mutants. This identified six mutants as CQ-hypersensitive, with the NEO1/neo1Δ mutant exhibiting the highest sensitivity (Figure S1). The defect of this mutant was complemented with expressing NEO1 from a plasmid (Figure S1). NEO1 encodes an essential aminophospholipid flippase involved in endocytosis and vacuolar biogenesis [17]. It is also required for resistance to other compounds [18]. Possibly, Neo1 is generally involved in regulating the accumulation of many different compounds in vacuoles. It is thus not further explored as a target of CQ in this study.
With a genome-wide DGSL screen, we next identified and validated 95 CQ-hypersensitive haploid deletion mutants (Table S1). Gene Ontology enrichment analysis revealed that these mostly affected vacuole functions, steroid biosynthesis, endocytosis, iron homeostasis, and post-Golgi transport (Figure S2). However, instead of relying on such an enrichment analysis, we took a novel approach by emphasizing individual mutants exhibiting the highest levels of sensitivity to CQ. We reasoned that such a mutant would most likely affect a function closely related to a drug target. In support of this hypothesis, mutants defective in yeast vacuolar functions (e.g., vma4Δ and mon2Δ) [8], [9] and iron metabolism (e.g., fet3Δ) [10] were among those exhibiting the highest levels of sensitivity (Figure 1A), consistent with the general idea that CQ concentrates in vacuoles or lysosomes [19] and, as previously observed, inhibits iron uptake in budding yeast [20]. In addition, we found that a thi3Δ mutant affecting thiamine biosynthesis [11] also exhibited comparably high levels of CQ-hypersensitivity (Figure 1A), and this defect was complemented by expressing THI3 from a plasmid. Furthermore, a fet3Δ thi3Δ double mutant was not apparently more sensitive to CQ than either single mutant (Figure S3), suggesting that the two mutations affect either the same or completely unrelated pathways. Consistent with the latter possibility, CQ-hypersensitive phenotype of the fet3Δ mutant was suppressed by exogenously supplied excess amount of iron but not thiamine, whereas that of the thi3Δ mutant was suppressed by thiamine but not iron (Figure 1B). Similarly, a NEO1/neo1Δ thi3Δ/thi3Δ mutant was no more sensitive than the thi3Δ/thi3Δ mutant to CQ (Figure S4). These results together suggested that CQ likely inhibits at least three independent biological processes in yeast: vacuolar functions, iron homeostasis, and thiamine metabolism or thiamine-dependent functions.
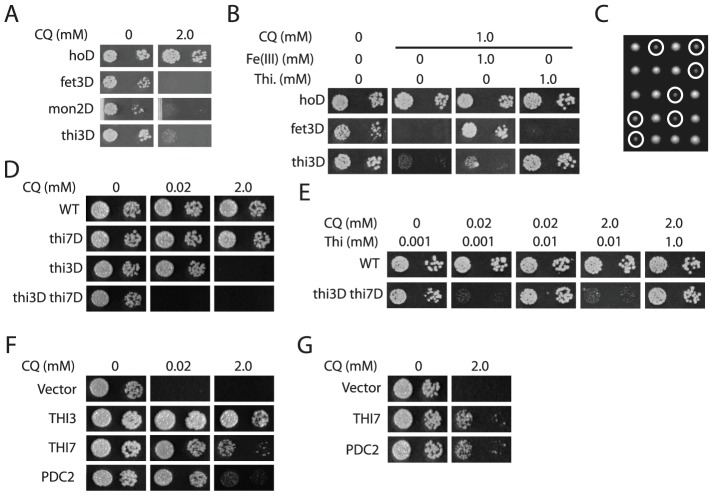
Figure 1. CQ possibly affects multiple biological functions.
A. Representative yeast haploid deletion mutants exhibiting the highest levels of sensitivity to CQ. B. Suppression of CQ-hypersensitivity of the fet3Δ and thi3Δ mutants by exogenously supplied iron [Fe(III)] and thiamine (Thi), respectively. C. Yeast tetrad analysis of the synthetically sick interaction between thi3Δ and thi7Δ. Colonies of the thi3Δ thi7Δ double mutant were all circled. D. Increased CQ-sensitivity of a thi3Δ thi7Δ double mutant as compared to either single mutant. E. Dosage-dependent suppression of CQ-hypersensitivity of the thi3Δ thi7Δ double mutant by exogenously supplied thiamine. F. Suppression of CQ-hypersensitivity of the thi3Δ thi7Δ double mutant by overexpressing THI3, THI7, and PDC2. G. Suppression of CQ-hypersensitivity of the thi3Δ single mutant by overexpressing THI7 and PDC2.
Genome-wide GGSL and DS screens identified Thi7 as a candidate target of CQ
To further elucidate the MOA(s) of CQ that underlie the hypersensitivity of the thi3Δ mutant, we performed a genome-wide GGSL screen. We reasoned that a thi3Δ mutant is hypersensitive to CQ because Thi3 is required to functionally compensate for inactivation of the drug target. A genome-wide GGSL screen with thi3Δ, which discovers functional compensation between genes in an unbiased manner, could reveal such a target or components of a target pathway. Among 5 genes discovered (Table S2), deleting the high affinity thiamine transporter Thi7 [12] caused severe growth defects, although not lethality, in the thi3Δ mutant (Figure 1C), possibly as a consequence of reduction in both thiamine synthesis and uptake in the double mutant. This double mutant was still viable, likely due to the expression of two low-affinity thiamine transporters Nrt1 and Thi72 [13]. Although a thi7Δ mutant was no more sensitive to CQ than a wild-type strain, a thi3Δ thi7Δ double mutant was much more sensitive than the thi3Δ single mutant (Figure 1D). In addition, thiamine suppressed CQ-hypersensitivity of this thi3Δ thi7Δ mutant, and the amount of thiamine needed for such suppression roughly correlated with the amount of CQ in the media (Figure 1E). Significantly, the CQ concentration (i.e., 20 µM) needed to completely inhibit growth of the thi3Δ thi7Δ double mutant was also achievable in human patients, animal studies or human cell culture experiments, suggesting that the underlying MOA, if conserved, are likely medically relevant.
In parallel, we performed a dosage suppression (DS) screen for genes that would suppress the CQ-hypersensitivity of the thi3Δ mutant. Such a screen could also discover a drug target or component of a target pathway. In order to increase specificity, we performed the screen first in the thi3Δ thi7Δ double mutant, which is much more sensitive to CQ than the thi3Δ single mutant, and subsequently tested candidate suppressors in the single mutant. We thought that screening in the double mutant would permit the use of a relatively low dose of CQ (i.e., 20 µM) and potentially minimize inhibition of other pathways. The screen identified THI3, THI7, THI20, and PDC2 (Figure 1F and data not shown). We subsequently showed that overexpression of THI7 and PDC2 also suppressed CQ-hypersensitivity of the thi3Δ single mutant at a higher CQ concentration (Figure 1G). However, the effect of PDC2 overexpression under this condition largely depended on THI7 (Figure 1F and 1G), consistent with a previous report that PDC2 controls expression of THI7 and thiamine biosynthesis genes [13]. Taken together, both the GGSL and DS screens in the thi3Δ mutant background discovered Thi7, suggesting that it might be a target of CQ.
CQ likely inhibits both high- and low-affinity thiamine transporters in yeast
To investigate such a possibility, we tested whether CQ affects other phenotypes controlled by Thi7. Thi7 was previously shown to be required for the uptake and toxicity of pyrithiamine in yeast [12], and as expected, a thi7Δ mutant was resistant to pyrithiamine (Figure 2A). Consistent with our model, CQ also partly suppressed the toxic effect of pyrithiamine in a wild-type strain (Figure 2A). Furthermore, thiamine deprivation due to thi7Δ mutation or the lack of thiamine in growth medium was previously shown to induce expression of thiamine biosynthesis genes in a Thi3-dependent manner [13]. We found that CQ treatment induces Thi3-dependent expression of thiamine biosynthesis genes THI6 and THI11 (Figure 2B and data not shown), indicating that it causes thiamine deficiency. These results were consistent with the model that CQ inhibits Thi7.
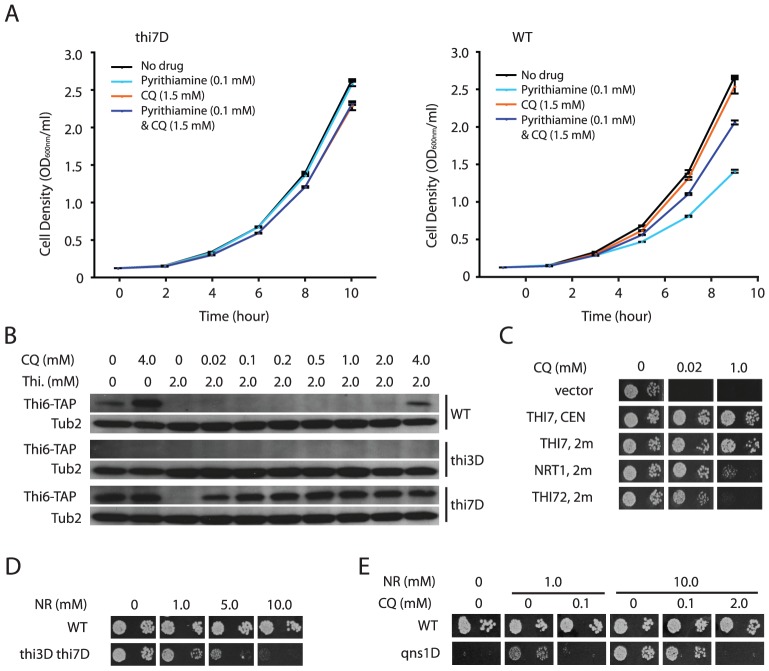
Figure 2. CQ-treatment mimics the effects of inactivating thiamine transporters.
A. CQ partially suppresses Thi7-dependent toxicity of pyrithiamine. Growth curves of a wild-type (WT) strain and a thi7Δ mutant under the indicated conditions were plotted. B. Thi3-dependent induction of expression of a Thi6-TAP fusion protein by CQ-treatment, a thi7Δ mutation, and thiamine deprivation. The fusion protein was detected with an anti-TAP antibody. Tub2 was used as a loading control. C. Suppression of CQ-hypersensitivity of the thi3Δ thi7Δ double mutant by expressing THI3, THI7, NRT1, or THI72 from a centromeric (CEN) or high copy (2µ) plasmid. D. Excess amount of nicotinamide riboside (NR) inhibits growth of the thi3Δ thi7Δ double mutant. E. CQ inhibits NR-dependent growth of a qns1Δ mutant.
However, the thi7Δ mutation further enhanced the effect of CQ treatment on THI6 expression (Figure 2B), suggesting that CQ likely also inhibit additional targets to augment thiamine deficiency. In addition to Thi7, the yeast genome encodes two low affinity thiamine transporters Nrt1 and Thi72, which share high sequence identity (>84%) with Thi7 [13]. Possibly, CQ also inhibits these two thiamine transporters, a model consistent with the observation that the thi3Δ thi7Δ double mutant is viable but much more sensitive to CQ than the thi3Δ single mutant (Figure 1D). Presumably, it takes much less CQ to inhibit thiamine uptake through these low affinity transporters. This model was further supported by the observation that both Nrt1 and Thi72 confer CQ-resistance in the thi3Δ thi7Δ double mutant when overexpressed from a high copy plasmid under control of the THI7 promoter (Figure 2C). To further corroborate this model, we took advantage of the fact that Nrt1 is also a high affinity transporter for nicotinamide riboside (NR) [21], and tested if growth of the thi3Δ thi7Δ mutant is also inhibited by NR. Similar to CQ, 10 µM of NR impaired growth of the thi3Δ thi7Δ double mutant in the presence of 1 µM of thiamine (Figure 2D), indicating that Nrt1 is at least partly responsible for thiamine uptake in this strain. We next tested whether CQ impairs the function of Nrt1. By taking advantage of the observation that a qns1Δ mutant requires Nrt1-dependent uptake of exogenously supplied NR for survival [21], [22], we found that CQ inhibited NR-dependent growth of such a qns1Δ mutant, and that the amount of CQ needed for growth inhibition roughly correlated with the amount of NR present in the medium (Figure 2E). These results together strongly suggested that CQ inhibits both the high- and low-affinity thiamine transporters in yeast.
CQ inhibits thiamine uptake via wild-type Thi7
We next directly tested the model that CQ inhibits thiamine transporters using a well-defined uptake assay [23]. As expected, thiamine uptake in the thi3Δ thi7Δ mutant was undetectable, but this was restored with expression of wild-type THI7 from a plasmid (Figure 3A). CQ blocked thiamine uptake mediated by the wild-type Thi7 transporter in a dose-dependent manner (Figure 3A and 3B). In contrast, it completely failed to inhibit thiamine uptake mediated by a CQ-resistant Thi7 allele (Thi7R9G T287N E573G) (Figure 3A) isolated from screening a THI7 random mutagenesis library expressed in the thi3Δ thi7Δ double mutant. This allele conferred higher levels of CQ-resistance as compared to wild-type THI7 when expressed in the thi3Δ thi7Δ double mutant (Figure S5). We subsequently found that the T287N substitution was largely responsible for the resistance phenotype of this mutant (Figure 3A and Figure S5). These results together indicated that CQ directly inhibits the thiamine transporter activity of yeast Thi7.
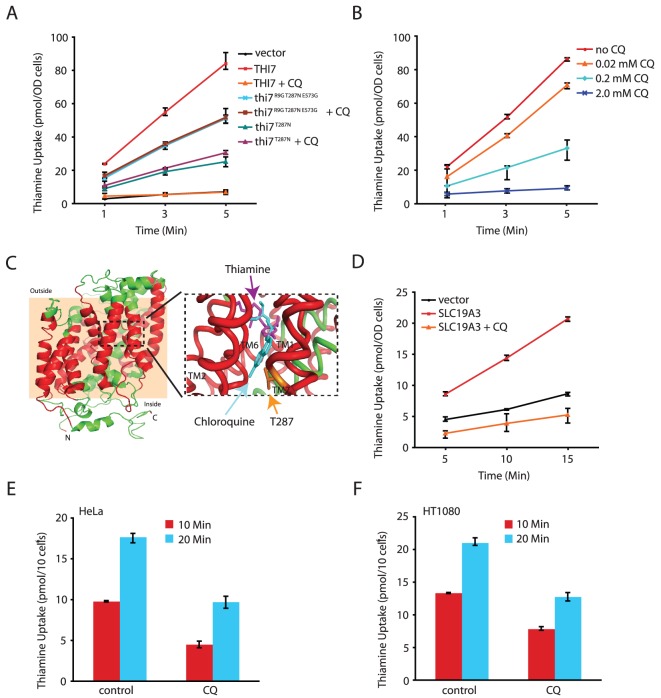
Figure 3. CQ inhibits thiamine transporters from yeast and human.
A. CQ (1.0 mM) completely inhibits thiamine uptake mediated by wild-type Thi7 but not CQ-resistant mutants of indicated genotypes. B. CQ dose-dependent inhibition of the transporter activity of wild-type Thi7. C. A homology model of the closed form of Thi7. The transmembrane (TM) helices are shown in red with the membrane region shown in a peach background. The black dashed rectangle is shown on the right in detail with docked substrates. The lowest energy docking results of thiamine (magenta) and chloroquine (cyan) are shown, with the side chain of T287 depicted in orange. D. CQ (1.0 mM) completely inhibits the human thiamine transporter SLC19A3 expressed in yeast cells. E and F. CQ (0.25 mM) inhibits thiamine uptake in human HeLa (E) and HT1080 (F) cell lines.
To gain further insights into how CQ might inhibit thiamine uptake through Thi7, we performed 3D- homology modeling of Thi7 using the crystal structure of the substrate bound benzyl-hydantoin transporter Mhp1 from Microbacterium liquefaciens [24] as a template. A model of correct topology and close structural homology was obtained as judged by confidence (C) and template modeling (TM) scores of 0.87 and 0.83, respectively. Analogous to the Mhp1 structure [24], the transmembrane (TM) helices 1, 2, 6, and 7 of the Thi7 model form a four-helix bundle that harbors a putative substrate-binding site. The CQ-resistance T287N mutation was mapped to TM7 (Figure 3C). Importantly, both thiamine and CQ could be docked into the substrate-binding site of Thi7 with high affinity (Figure 3C and Table S3). We thus tentatively conclude that CQ might compete with thiamine for binding to the transporter. Such a model is also consistent with the observation that excess amount of thiamine suppresses the inhibitory effect of CQ on cellular growth (Figure 1B and 1E). Based on the docking results, the CQ-resistant T287N mutation is located closer to CQ than to thiamine (Figure 3C). However, at a distance of about 6.1 Å, a close interaction between this residue and CQ does not seem possible. In addition, mutating this Thr287 residue to Ala, Asp, Gln, and Ile did not confer CQ-resistance (data not shown). It is possible that the T287N mutation affects the conformation of the substrate-binding site of the transporter, a hypothesis also consistent with the observation of partial reduction in the thiamine uptake activity of the mutant even in the absence of CQ (Figure 3A). However, understanding how the T287N mutation completely abolishes the inhibitory effect of CQ on thiamine uptake will likely require a crystal structure of Thi7.
CQ inhibits a human thiamine transporter
We next investigated whether CQ inhibition of thiamine transporters is conserved. Human cells express two thiamine transporters SLC19A2 and SLC19A3 that are ∼70% identical in amino acid sequences [14], [15]. We found that expression of human SLC19A3, which share ∼15% sequence identity with Thi7, partly restored thiamine uptake in the thi3Δ thi7Δ double mutant (Figure 3D). Importantly, CQ almost completely inactivated such an activity (Figure 3D). Moreover, CQ significantly inhibited thiamine uptake in two human cell lines tested (Figure 3E and 3F). These results together demonstrated that inhibition of thiamine transporters and reduction in thiamine uptake is a conserved MOA of CQ in both yeast and human.
Other quinoline-containing antimalarials also inhibit thiamine transporters
We next asked whether other quinoline-containing antimalarials also inhibit thiamine uptake through the transporters. When applied at 0.2 mM, amodiaquine, quinacrine, mefloquine, primaquine, quinine, and quinidine all inhibited growth of a thi3Δ thi7Δ mutant (Figure 4A and Figure S6). Some of them also inhibited growth of the thi3Δ single mutant (Figure 4A). Similar to CQ, the inhibitory effects of these other antimalarials on cellular growth were suppressed by excess amount of thiamine in the medium (Figure 4B and data not shown). Most of these other antimalarials also inhibited thiamine uptake mediated by the wild-type Thi7, with amodiaquine having the strongest effect (Figure 4C). Similar to CQ, amodiaquine completely failed to inhibit thiamine uptake mediated by the Thi7R9G T287N E573G mutant (Figure 4D). These results together suggested that inhibition of thiamine uptake is a conserved mechanism among quinoline-containing antimalarials.
Figure 4. Other antimalarials inhibit thiamine transporters.
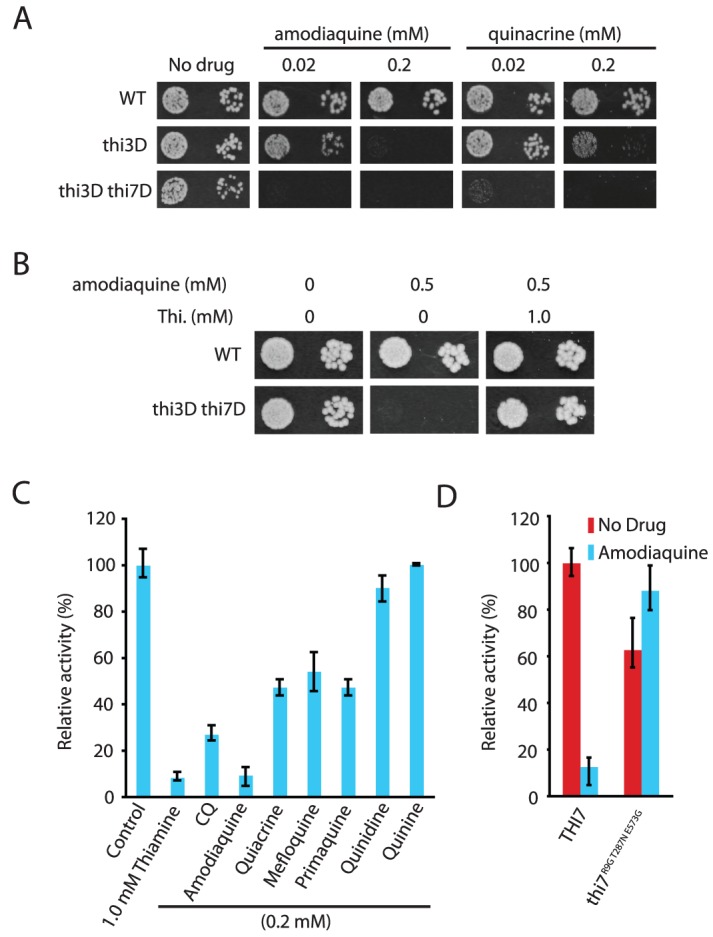
A. Hypersensitivity of the thi3Δ and thi3Δ thi7Δ mutants to amodiaquine and quinacrine. B. Suppression of amodiaquine-hypersensitivity of the thi3Δ and thi3Δ thi7Δ mutants by exogenously supplied thiamine. C. Inhibition of thiamine uptake by indicated compounds. D. Amodiaquine (0.25 mM) inhibits thiamine uptake mediated by wild-type Thi7 but not a CQ-resistant Thi7R9G T287N E573G mutant.
Discussion
In this study, we demonstrated that CQ and other quinoline-containing antimalarials inhibit thiamine transporters in yeast. We also showed that such a MOA is conserved between yeast and humans. In particular, the human thiamine transporter SLC19A3 was completely inhibited by CQ when expressed in yeast cells (Figure 3D). This MOA is likely medically relevant. First, much like the thi3Δ yeast mutant, human cells completely depend on exogenously supplied thiamine for survival, and the thiamine transporters play essential roles in this process. Second, at 20 µM, a concentration achievable in human patients, CQ completely inhibited growth of the thi3Δ thi7Δ double mutant (Figure 2B). The concentration need to significantly inhibit the thi3Δ single mutant was about 10 times higher, but yeast cells are generally known to be more resistant to many drugs than mammalian cells due to the presence of cell wall and potent drug pumps. Third, the concentration of thiamine in human serum is in the 10–20 nM range [25], [26], [27], more than two-magnitude lower than those used in this study. The putatively competitive relationship between CQ and thiamine suggests that inhibition of thiamine uptake in human body is achievable using CQ concentrations much lower than those used in this study. Fourth, CQ accumulates in certain tissues (e.g. the retina) at high concentrations, an observation particularly relevant to retinopathy caused by CQ-based medications [4], [28], [29]. In this regard, there is already a connection between thiamine deficiency and retinopathy in diabetic patients [30], and diabetic retinopathy can be prevented with thiamine supplementation in a rodent animal model [31]. In addition, thiamine deficiency and CQ treatment both lead to neurological and cardiovascular disorders [5], [6], [32], [33]. Based on these, it will be interesting to investigate whether thiamine deficiency might underlie some of the CQ-induced adverse effects and whether these can be prevented with concomitant thiamine supplementation.
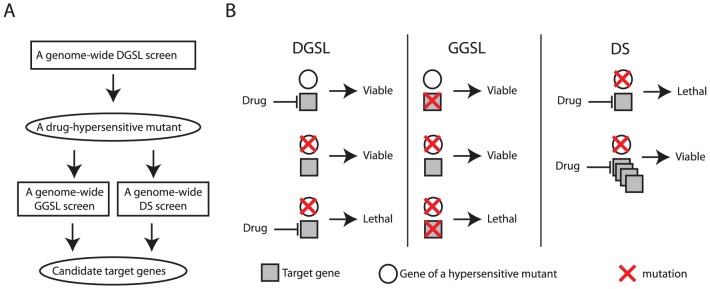
This study also demonstrated SL/DS as a novel and effective functional genomics strategy for discovering drug targets. This strategy starts with identifying mutants that are hypersensitive specifically to a drug treatment with a genome-wide DGSL screen (Figure 5A). Such a drug-hypersensitive mutant (e.g, thi3Δ) is then used as a key to directly discover drug target(s) with a genome-wide GGSL or DS screen, or both (Figure 5A). Discovering a drug target with a subsequent GGSL screen is based on the premise that genetic and pharmacological inactivation of a drug target produce similar effects (e.g., fitness defect in the hypersensitive mutant) (Figure 5B). Discovering a drug target with a subsequent DS screen is based on the principle that overexpressing a drug target confers drug resistance [34], in this case, in a hypersensitive mutant (Figure 5B). That both GGSL and DS screens identified Thi7 greatly simplified its selection as a high likelihood candidate CQ target for validation. We note that the particular DS screen reported in this study was performed in the thi3Δ thi7Δ double mutant, with an intention of using a low dose of CQ to potentially minimize inhibition of additional targets to increase pathway specificity. Such a DS screen would probably have also succeeded if a thi3Δ single mutant had been used.
Figure 5. A diagram and concept of discovering drug target using the SL/DS strategy.
A flow chart (A) and concept (B) of drug target identification with a combination of DGSL, GGSL, and DS screens.
Most existing in vivo target identification methods such as haploinsufficiency profiling [16], outright dosage suppression [35], and discovering resistance mutations with genome-sequencing or high throughput complementation [36], [37] typically rely on a drug's ability to completely or severely inhibit growth of wild-type cells. In contrast, SL/DS does not have such a requirement and thus can be used to discover non-essential genes as drug targets, as shown with Thi7 in this study. This feature is very significant considering that >80% of all proteins encoded by the yeast genome are non-essential. As a result, this method will offer much broader opportunity than the existing methods for discovering drug targets, especially with drugs that inhibit the growth of certain mutants but not wild-type cells.
SL/DS should also be useful in discovering essential proteins as drug targets. In this regard, it may not be as straightforward as the other methods. However, we have found that the existing methods fail to discover targets of many cytotoxic drugs (unpublished). A possible reason for that is that some drugs simultaneously inhibit multiple targets and that, consequentially, overexpressing or mutating any single target gene does not confer drug resistance in an otherwise wild-type strain background. In such a case, SL/DS could be effective because it is always possible to first discover drug-hypersensitive deletion mutants using a DGSL screen and subsequently identify the drug targets using GGSL and DS screens in these mutant backgrounds. The DS screen in a hypersensitive mutant could work because a lower drug dose can be used to minimize inhibition of other target pathways.
The SL/DS methodology seems to be similar to but is distinctly different from a previously described compendium approach, where targets of novel drugs are inferred from comparing a large compendium of genome-wide DGSL profiles of old drug treatments and GGSL profiles of genetic perturbation for similarities [38]. Like SL/DS, this compendium approach could identify both essential and non-essential proteins as drug targets using the DGSL and GGSL profiles [38]. However, it does not directly identify drug targets but instead infers candidate targets from profile similarity. A potential limitation is that perturbations in potentially many components of a given drug target pathway typically produce similar profiles, making it difficult to determine the actual drug target. Its discovery scope is also limited to the available DGSL or GGSL reference profiles, which are very difficult to generate at a large scale in higher eukaryotes. In contrast, SL/DS directly identifies a drug's target with only three genome-wide screens: DGSL followed with GGSL and DS. It does not rely on DGSL profiles of other drugs or GGSL profiles of other genetic perturbation as references. A similar SL/DS strategy will likely also be useful for drug target identification in human cells, where genome-wide DGSL, GGSL, and DS screens are now possible [39], [40], [41], [42].
Methods
Strains and plasmids
Yeast strains used in this study include the wild-type strains BY4741a (MAT a his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0) and BY4743a/α (MAT a/α his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 LYS2/lys2Δ0 met15Δ0/MET15 ura3Δ0/ura3Δ0) [43] and isogenic mutants derived from a genome-wide deletion library [44]. The haploid-convertible heterozygous diploid deletion library used in screening for CQ hypersensitive mutants was previously described [44]. The Thi6-TAP and Thi11-TAP strains were obtained from Open Biosystems and the derivative thi7Δ::kanMX and thi3Δ::natMX mutants were constructed by deleting the THI7 and THI3 genes, respectively in these strain backgrounds. Bacterial strain DH5α was used as the host during molecular cloning. Plasmids used in this study are listed in Table S4. The vectors used are pRS416 [43] and YEplac195 [45].
Yeast media and chemicals
Yeast media used in this study include a haploid selection synthetic complete medium SC−Leu−His−Arg+G418+canavanine [46] and a synthetic complete (SC) medium that either contained or lacked uracil (SC-Ura) or leucine (SC-Leu). Amodiaquine dihydrochloride, Chloroquine diphosphate, mefloquine hydrochloride, primaquine bisphosphate, pyrithiamine hydrobromide, quinacrine dihydrochloride, quinine, quinidine, thiamine hydrochloride, and ferric chloride were all purchased from Sigma. Nicotinamide riboside (NR) was freshly derived from enzymatic hydrolysis of NMN (Sigma) and quantified as previously described [22]. Stock solutions were made in ddH2O with the help of adjusting pH when necessary, filter sterilized, and stored at −20°C or directly used.
Genome-wide DGSL, GGSL, and DS screens
Genome-wide DGSL screens and subsequent individual validation were carried out as previously described [46], [47]. 5 mM of CQ was used in the screen. Concentrations of 0.5 mM, 1 mM, 2 mM, 3 mM, 4 mM, and 5 mM were used in validation assays to further distinguish among the sensitivities of different mutants. Genome-wide GGSL screen with a thi3Δ::URA3 query construct was carried out as previously described [48], [49]. Candidate hits were validated with tetrad dissection. Briefly, the heterozygous diploid deletion mutant of each candidate gene was transformed with a thi3Δ::URA3 query construct to disrupt one copy of THI3. The resultant heterozygous diploid double mutant was sporulated and the spores were dissected under a microscope on a YPD plate. The plate was incubated at 30°C for 2 days and photographed and the genotype of the dissected spores were determined by their growth on SC-Ura and YPD+G418 plates. Genome-wide DS screen in the thi3Δ thi7Δ double mutant was performed using a genome-wide tiling library containing ∼95% of the yeast genomic sequences [50]. This library was en masse transformed into XPY1263a (MAT a thi3Δ::natMX thi7Δ::kanMX). An aliquot of ∼105 cells of the transformed pool was subsequently plated on a solid synthetic complete medium that lacked leucine (SC-Leu) but contained 20 µM CQ to select for resistant colonies. Plasmids were recovered from 96 representative colonies and sequenced at one end to identify the responsible genes. Candidate genes were then individually validated on YEplac195 for their ability to confer CQ-resistance in the thi3Δ thi7Δ double and thi3Δ single mutants.
Western blotting
A yeast strain expressing Thi6-TAP or Thi11-TAP from the endogenous locus in a wild-type, thi3Δ, or thi7Δ background was grown in 5 ml of regular liquid SC at 30°C for an overnight. Cells were harvested, washed with 5 ml of sterile water, and inoculated into 5 ml of liquid SC that lacked thiamine at an starting cell density of ∼0.15 OD600 nm/ml. CQ and thiamine were added at the indicated final concentrations. The cultures were incubated at 30°C for 4 hr with shaking. About 1.0 OD600 nm cells were collected for each sample, directly lysed with boiling in 1× SDS buffer, and analyzed with western blot using an anti-TAP antibody (Open biosystems) and an anti-Tub2 antibody.
Thiamine uptake assays
Thiamine uptake in yeast cells was carried out as described [23] with minor modifications. Yeast cells of XPY1263a harboring an empty vector or expressing wild-type THI7, thi7R9G T287N E573G, thi7T287N, or SLC19A3 were grown in 3 ml liquid SC-Ura containing 100 uM at 30°C for overnight. 1 ml of each overnight culture was inoculated into 50 ml of fresh SC-Ura liquid and incubated at 30°C for 4.5 hrs. Cells were harvested, washed twice each with 10 ml of ddH2O, and suspended in citric acid/phosphate buffer (pH 4.5) containing 1% D-glucose at a density of 2.0–2.5 OD600 nm/ml. For each uptake experiment, 500 µl of cells were pre-warmed at 30°C for 3 min in a microcentrifuge tube in the presence or absence of CQ or another antimalarial at indicated concentrations. [H3]-Thiamine (American Radiochemical Company) was added at a final concentration of 2 µM and a specificity of 0.2 Ci/mmol and immediately mixed on a Mixmate at 30°C. 100 µl of each sample was taken at indicated time points (1 min, 3 min, and 5 min) and transferred to a microcentrifuge tube that contains 900 µl of ice-cold 1 mM thiamine in citric acid/phosphate buffer (pH 4.5) to terminate uptake of H3]-Thiamine. Cells were collected by filtering and washed with 10 ml of ddH2O. Radioactivity associated with each filter was measured with a Beckman scintillation counter and used to calculate thiamine uptake activity as pmol/OD600 nm cells. Three independent repeats were performed for each time point and the results were averaged.
Thiamine uptake in human cells was carried out as described in another previous study [51] with minor modifications. HeLa and HT1080 cells were grown in DMEM medium until confluent monolayers in 12-well plates, with ∼5.0×105 cells in each well. Medium was aspirated 4 days following confluence, and each culture was washed twice with the uptake buffer (NaCl, 125 mM; KCl, 4.8 mM; KH2PO4, 1.2 mM; MgSO4, 1.2 mM; CaCl2, 1.2 mM; Glucose, 5 mM; Glutamine, 5 mM; HEPES-NaOH, 12.5 mM; MES, 12.5 mM; pH 8.0) that had been pre-warmed at 37°C. Cell monolayers were then pre-incubated in 0.2 ml uptake buffer that either contained or lacked CQ at 0.25 mM at 37°C for 10 min. H3]-Thiamine was subsequently added at a final concentration of 5 µM and a specificity of 1 Ci/mmol. [H3]-Thiamine uptake was terminated at 10- or 20-min time point by addition of 1 ml of ice-cold buffer that contained 1 mM of unlabeled thiamine into each well. Buffer was immediately aspirated. Cells from each well were rinsed twice with 1 ml of ice-cold buffer containing unlabeled thiamine, digested with 0.25 ml of 1 N NaOH for 2 hours, and neutralized with 0.25 ml of 1 N HCl. Cell lysates (∼0.5 ml each) were transferred into scintillation vials. Residual lysate in each well was washed with 0.3 ml of stoppage buffer and also transferred to the same scintillation vials. Radioactivity of each sample was measured with a Beckman scintillation counter and used to calculate thiamine uptake activity as pmol/106 cells. Three independent repeats were performed for each time point and the results were averaged.
Homology modeling of Thi7
Coordinates of the substrate bound form of the benzyl-hydantoin transporter Mhp1 structure from Microbacterium liquefaciens, (PDB code :2JLO) [24]. was used as a starting template to obtain a structural model of the yeast Thi7 through the online server I-TASSER (http://zhanglab.ccmb.med.umich.edu/I-TASSER/) [52]. Thi7 has 21% sequence identity and 35% similarity with the benzyl-hydantoin transporter Mhp1 from Microbacterium liquefaciens. The Thi7 residues from A22 to E537 were used as the input sequence based on a BLAST sequence analysis with the Mhp1 sequence. Sequence template alignments were generated using the program MUSTER, which is built into I-TASSER. The quality of the generated model was assessed in I-TASSER based on two major criteria, the C- and the TM-scores.
Docking of thiamine and CQ into the Thi7 model
Thiamine (Pubchem ID: 1130) and chloroquine (Pubchem ID: 2719) were processed for docking using ADT tools. Addition of hydrogen atoms and setting of rotatable bonds for these substrates were carried out in ADT tools (Molecular graphics lab of the Scripps research institute). The docking of substrates to the Thi7 model was performed using the AutoDockVina software [53]. A grid box with a dimension of 15×15×15 points was used.
Supporting Information
Figure S1
CQ-hypersensitive heterozygous diploid deletion mutants identified from a genome-wide screen. A. Growth of mutants of indicated genotypes in the presence or absence of CQ of indicated concentrations. B. Complementation of CQ-hypersensitivity of a NEO1/neo1Δ mutant with expressing NEO1 from a high copy plasmid.
(EPS)
Figure S2
GO terms enriched for CQ-hypersensitive haploid deletion mutants. Gene lists identified from the genome-wide DGSL screen were subject to DAVID for functional analysis. Significantly enriched cellular functions were selected based on a cutoff Q value of 0.05 and plotted.
(EPS)
Figure S3
A non-additive relationship between the fet3Δ and thi3Δ mutations for their effects on CQ-sensitivity. Haploid strains of indicated genotypes were grown in the presence or absence of CQ (0.5 mM).
(EPS)
Figure S4
Non-additive effects of the NEO1/neo1Δ and thi3Δ/thi3Δ mutations on CQ-hypersensitivity. Isogenic diploid strains of indicated genotypes were grown in the presence of absence of CQ at indicated concentrations.
(EPS)
Figure S5
A T287N mutation on Thi7 confers CQ-resistance. Growth of a thi3Δ thi7Δ double mutant expressing centromeric plasmids of indicated genotypes in the presence or absence of CQ at indicated concentrations.
(EPS)
Figure S6
Other quinoline-containing antimalarials inhibit growth of a thi3_Δ_ thi7_Δ_ double mutant. Isogenic haploid strains of indicated genotype were grown in the presence or absence of 0.2 mM of indicated drugs.
(EPS)
Table S1
Chloroquine hypersensitive haploid deletion mutants. Haploid-convertible heterozygous diploid eletion mutants of the listed genes were sporulated on a solid sporulation medium and spotted as 10× serial dilution on a haploid selection medium that either contained or lacked CQ of indicated concentration. Growth of each haploid deletion mutant was inspected and scored, with its growth in the absence of the drug as a reference. S–sick; L–Lethal.
(DOC)
Table S2
Genes identified from a genome-wide thi3Δ synthetic lethality screen. Heterozygous diploid double mutants of between the listed genes and THI3 were constructed and subjected to tetrad analysis. Synthetic sick interactions were observed in all cases.
(DOC)
Table S3
Docking results of thiamine or chloroquine into a 3D-model of Thi7. Both thiamine and chloroquine were docked into a 3D-model of Thi7 using ADT tools. Results of top 9 docking modes were listed.
(DOC)
Table S4
Plasmids used in this study.
(DOC)
Acknowledgments
We thank Dr. Pamela Meluh for providing the anti-Tub2 antibody and Drs. Zhou Songyang and Dong Yang for providing HeLa and HT1080 cells. We are grateful to Drs. Jurgen Stolz, Dominik Mojzita, and Stefan Hohmann for helpful discussions concerning the yeast thiamine uptake assay and the yeast thiamine regulon, respectively.
Funding Statement
This work was supported by the National Institutes of Health grant HG004840 to XP and the Welch Foundation grant Q-581 to FAQ. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ben-Zvi I, Kivity S, Langevitz P, Shoenfeld Y (2012) Hydroxychloroquine: from malaria to autoimmunity. Clin Rev Allergy Immunol 42: 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rolain JM, Colson P, Raoult D (2007) Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int J Antimicrob Agents 30: 297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solomon VR, Lee H (2009) Chloroquine and its analogs: a new promise of an old drug for effective and safe cancer therapies. Eur J Pharmacol 625: 220–233. [DOI] [PubMed] [Google Scholar]
- 4.Tehrani R, Ostrowski RA, Hariman R, Jay WM (2008) Ocular toxicity of hydroxychloroquine. Semin Ophthalmol 23: 201–209. [DOI] [PubMed] [Google Scholar]
- 5.Costedoat-Chalumeau N, Hulot JS, Amoura Z, Delcourt A, Maisonobe T, et al. (2007) Cardiomyopathy related to antimalarial therapy with illustrative case report. Cardiology 107: 73–80. [DOI] [PubMed] [Google Scholar]
- 6.Kwon JB, Kleiner A, Ishida K, Godown J, Ciafaloni E, et al. (2010) Hydroxychloroquine-induced myopathy. J Clin Rheumatol 16: 28–31. [DOI] [PubMed] [Google Scholar]
- 7.Hughes TR (2002) Yeast and drug discovery. Funct Integr Genomics 2: 199–211. [DOI] [PubMed] [Google Scholar]
- 8.Jochum A, Jackson D, Schwarz H, Pipkorn R, Singer-Kruger B (2002) Yeast Ysl2p, homologous to Sec7 domain guanine nucleotide exchange factors, functions in endocytosis and maintenance of vacuole integrity and interacts with the Arf-Like small GTPase Arl1p. Mol Cell Biol 22: 4914–4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foury F (1990) The 31-kDa polypeptide is an essential subunit of the vacuolar ATPase in Saccharomyces cerevisiae. J Biol Chem 265: 18554–18560. [PubMed] [Google Scholar]
- 10.Askwith C, Eide D, Van Ho A, Bernard PS, Li L, et al. (1994) The FET3 gene of S. cerevisiae encodes a multicopper oxidase required for ferrous iron uptake. Cell 76: 403–410. [DOI] [PubMed] [Google Scholar]
- 11.Nishimura H, Kawasaki Y, Kaneko Y, Nosaka K, Iwashima A (1992) A positive regulatory gene, THI3, is required for thiamine metabolism in Saccharomyces cerevisiae. J Bacteriol 174: 4701–4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singleton CK (1997) Identification and characterization of the thiamine transporter gene of Saccharomyces cerevisiae. Gene 199: 111–121. [DOI] [PubMed] [Google Scholar]
- 13.Mojzita D, Hohmann S (2006) Pdc2 coordinates expression of the THI regulon in the yeast Saccharomyces cerevisiae. Mol Genet Genomics 276: 147–161. [DOI] [PubMed] [Google Scholar]
- 14.Eudy JD, Spiegelstein O, Barber RC, Wlodarczyk BJ, Talbot J, et al. (2000) Identification and characterization of the human and mouse SLC19A3 gene: a novel member of the reduced folate family of micronutrient transporter genes. Mol Genet Metab 71: 581–590. [DOI] [PubMed] [Google Scholar]
- 15.Rajgopal A, Edmondnson A, Goldman ID, Zhao R (2001) SLC19A3 encodes a second thiamine transporter ThTr2. Biochim Biophys Acta 1537: 175–178. [DOI] [PubMed] [Google Scholar]
- 16.Giaever G, Shoemaker DD, Jones TW, Liang H, Winzeler EA, et al. (1999) Genomic profiling of drug sensitivities via induced haploinsufficiency. Nat Genet 21: 278–283. [DOI] [PubMed] [Google Scholar]
- 17.Hua Z, Fatheddin P, Graham TR (2002) An essential subfamily of Drs2p-related P-type ATPases is required for protein trafficking between Golgi complex and endosomal/vacuolar system. Mol Biol Cell 13: 3162–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prezant TR, Chaltraw WE Jr, Fischel-Ghodsian N (1996) Identification of an overexpressed yeast gene which prevents aminoglycoside toxicity. Microbiology 142 Pt 12: 3407–3414. [DOI] [PubMed] [Google Scholar]
- 19.Homewood CA, Warhurst DC, Peters W, Baggaley VC (1972) Lysosomes, pH and the anti-malarial action of chloroquine. Nature 235: 50–52. [DOI] [PubMed] [Google Scholar]
- 20.Emerson LR, Nau ME, Martin RK, Kyle DE, Vahey M, et al. (2002) Relationship between chloroquine toxicity and iron acquisition in Saccharomyces cerevisiae. Antimicrob Agents Chemother 46: 787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belenky PA, Moga TG, Brenner C (2008) Saccharomyces cerevisiae YOR071C encodes the high affinity nicotinamide riboside transporter Nrt1. J Biol Chem 283: 8075–8079. [DOI] [PubMed] [Google Scholar]
- 22.Bieganowski P, Brenner C (2004) Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell 117: 495–502. [DOI] [PubMed] [Google Scholar]
- 23.Vogl C, Klein CM, Batke AF, Schweingruber ME, Stolz J (2008) Characterization of Thi9, a novel thiamine (Vitamin B1) transporter from Schizosaccharomyces pombe. J Biol Chem 283: 7379–7389. [DOI] [PubMed] [Google Scholar]
- 24.Weyand S, Shimamura T, Yajima S, Suzuki S, Mirza O, et al. (2008) Structure and molecular mechanism of a nucleobase-cation-symport-1 family transporter. Science 322: 709–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tallaksen CM, Bohmer T, Bell H, Karlsen J (1991) Concomitant determination of thiamin and its phosphate esters in human blood and serum by high-performance liquid chromatography. J Chromatogr 564: 127–136. [DOI] [PubMed] [Google Scholar]
- 26.Bettendorff L, Grandfils C, De Rycker C, Schoffeniels E (1986) Determination of thiamine and its phosphate esters in human blood serum at femtomole levels. J Chromatogr 382: 297–302. [DOI] [PubMed] [Google Scholar]
- 27.Weber W, Kewitz H (1985) Determination of thiamine in human plasma and its pharmacokinetics. Eur J Clin Pharmacol 28: 213–219. [DOI] [PubMed] [Google Scholar]
- 28.Kobak S, Deveci H (2010) Retinopathy due to antimalarial drugs in patients with connective tissue diseases: are they so innocent? A single center retrospective study. Int J Rheum Dis 13: e11–15. [DOI] [PubMed] [Google Scholar]
- 29.Okun E, Gouras P, Bernstein H, Sallmann LV (1963) Chloroquine Retinopathy. A report of eight cases with ERG and dark adaptation. Arch Ophthalmol 69: 93–105. [DOI] [PubMed] [Google Scholar]
- 30.Thornalley PJ (2005) The potential role of thiamine (vitamin B1) in diabetic complications. Curr Diabetes Rev 1: 287–298. [DOI] [PubMed] [Google Scholar]
- 31.Hammes HP, Du X, Edelstein D, Taguchi T, Matsumura T, et al. (2003) Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med 9: 294–299. [DOI] [PubMed] [Google Scholar]
- 32.Tanphaichitr V (1994) Thiamin. Modern Nutrition in Health and Disease, 8th ed Philadelphia: Lea and Febiger: 359–375.
- 33.Victor MG, Adams RD, Collins GH (1989) The Wernicke-Korsakoff Syndrome and Related Neurological Disorders Due to Alcoholism and Malnutrition. 2nd Edition Philadelphia: FA Davis Co.
- 34.Rine J, Hansen W, Hardeman E, Davis RW (1983) Targeted selection of recombinant clones through gene dosage effects. Proc Natl Acad Sci U S A 80: 6750–6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butcher RA, Bhullar BS, Perlstein EO, Marsischky G, LaBaer J, et al. (2006) Microarray-based method for monitoring yeast overexpression strains reveals small-molecule targets in TOR pathway. Nat Chem Biol 2: 103–109. [DOI] [PubMed] [Google Scholar]
- 36.Albert TJ, Dailidiene D, Dailide G, Norton JE, Kalia A, et al. (2005) Mutation discovery in bacterial genomes: metronidazole resistance in Helicobacter pylori. Nat Methods 2: 951–953. [DOI] [PubMed] [Google Scholar]
- 37.Ho CH, Magtanong L, Barker SL, Gresham D, Nishimura S, et al. (2009) A molecular barcoded yeast ORF library enables mode-of-action analysis of bioactive compounds. Nat Biotechnol 27: 369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parsons AB, Brost RL, Ding H, Li Z, Zhang C, et al. (2004) Integration of chemical-genetic and genetic interaction data links bioactive compounds to cellular target pathways. Nat Biotechnol 22: 62–69. [DOI] [PubMed] [Google Scholar]
- 39.Kiefer J, Yin HH, Que QQ, Mousses S (2009) High-throughput siRNA screening as a method of perturbation of biological systems and identification of targeted pathways coupled with compound screening. Methods Mol Biol 563: 275–287. [DOI] [PubMed] [Google Scholar]
- 40.Lin YY, Kiihl S, Suhail Y, Liu SY, Chou YH, et al. (2012) Functional dissection of lysine deacetylases reveals that HDAC1 and p300 regulate AMPK. Nature 482: 251–255. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Liu G, Wong-Staal F, Li Q (2006) Recent development of RNAi in drug target discovery and validation. Drug Discov Today Technol 293–300. [DOI] [PubMed] [Google Scholar]
- 42.Luesch H, Wu TY, Ren P, Gray NS, Schultz PG, et al. (2005) A genome-wide overexpression screen in yeast for small-molecule target identification. Chem Biol 12: 55–63. [DOI] [PubMed] [Google Scholar]
- 43.Brachmann CB, Davies A, Cost GJ, Caputo E, Li J, et al. (1998) Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14: 115–132. [DOI] [PubMed] [Google Scholar]
- 44.Pan X, Ye P, Yuan DS, Wang X, Bader JS, et al. (2006) A DNA integrity network in the yeast Saccharomyces cerevisiae. Cell 124: 1069–1081. [DOI] [PubMed] [Google Scholar]
- 45.Gietz RD, Sugino A (1988) New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74: 527–534. [DOI] [PubMed] [Google Scholar]
- 46.Huang Z, Chen K, Xu T, Zhang J, Li Y, et al. (2011) Sampangine inhibits heme biosynthesis in both yeast and human. Eukaryot Cell 10: 1536–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pan X, Reissman S, Douglas NR, Huang Z, Yuan DS, et al. (2010) Trivalent arsenic inhibits the functions of chaperonin complex. Genetics 186: 725–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pan X, Yuan DS, Ooi SL, Wang X, Sookhai-Mahadeo S, et al. (2007) dSLAM analysis of genome-wide genetic interactions in Saccharomyces cerevisiae. Methods 41: 206–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pan X, Yuan DS, Xiang D, Wang X, Sookhai-Mahadeo S, et al. (2004) A robust toolkit for functional profiling of the yeast genome. Mol Cell 16: 487–496. [DOI] [PubMed] [Google Scholar]
- 50.Jones GM, Stalker J, Humphray S, West A, Cox T, et al. (2008) A systematic library for comprehensive overexpression screens in Saccharomyces cerevisiae. Nat Methods 5: 239–241. [DOI] [PubMed] [Google Scholar]
- 51.Said HM, Ortiz A, Kumar CK, Chatterjee N, Dudeja PK, et al. (1999) Transport of thiamine in human intestine: mechanism and regulation in intestinal epithelial cell model Caco-2. Am J Physiol 277: C645–651. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Y (2008) I-TASSER server for protein 3D structure prediction. BMC Bioinformatics 9: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31: 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
CQ-hypersensitive heterozygous diploid deletion mutants identified from a genome-wide screen. A. Growth of mutants of indicated genotypes in the presence or absence of CQ of indicated concentrations. B. Complementation of CQ-hypersensitivity of a NEO1/neo1Δ mutant with expressing NEO1 from a high copy plasmid.
(EPS)
Figure S2
GO terms enriched for CQ-hypersensitive haploid deletion mutants. Gene lists identified from the genome-wide DGSL screen were subject to DAVID for functional analysis. Significantly enriched cellular functions were selected based on a cutoff Q value of 0.05 and plotted.
(EPS)
Figure S3
A non-additive relationship between the fet3Δ and thi3Δ mutations for their effects on CQ-sensitivity. Haploid strains of indicated genotypes were grown in the presence or absence of CQ (0.5 mM).
(EPS)
Figure S4
Non-additive effects of the NEO1/neo1Δ and thi3Δ/thi3Δ mutations on CQ-hypersensitivity. Isogenic diploid strains of indicated genotypes were grown in the presence of absence of CQ at indicated concentrations.
(EPS)
Figure S5
A T287N mutation on Thi7 confers CQ-resistance. Growth of a thi3Δ thi7Δ double mutant expressing centromeric plasmids of indicated genotypes in the presence or absence of CQ at indicated concentrations.
(EPS)
Figure S6
Other quinoline-containing antimalarials inhibit growth of a thi3_Δ_ thi7_Δ_ double mutant. Isogenic haploid strains of indicated genotype were grown in the presence or absence of 0.2 mM of indicated drugs.
(EPS)
Table S1
Chloroquine hypersensitive haploid deletion mutants. Haploid-convertible heterozygous diploid eletion mutants of the listed genes were sporulated on a solid sporulation medium and spotted as 10× serial dilution on a haploid selection medium that either contained or lacked CQ of indicated concentration. Growth of each haploid deletion mutant was inspected and scored, with its growth in the absence of the drug as a reference. S–sick; L–Lethal.
(DOC)
Table S2
Genes identified from a genome-wide thi3Δ synthetic lethality screen. Heterozygous diploid double mutants of between the listed genes and THI3 were constructed and subjected to tetrad analysis. Synthetic sick interactions were observed in all cases.
(DOC)
Table S3
Docking results of thiamine or chloroquine into a 3D-model of Thi7. Both thiamine and chloroquine were docked into a 3D-model of Thi7 using ADT tools. Results of top 9 docking modes were listed.
(DOC)
Table S4
Plasmids used in this study.
(DOC)