Maresin-1 engages regulatory T cells to limit type 2 innate lymphoid cell activation and promote resolution of lung inflammation (original) (raw)
. Author manuscript; available in PMC: 2016 Jan 31.
Published in final edited form as: J Immunol. 2014 Dec 24;194(3):863–867. doi: 10.4049/jimmunol.1402534
Abstract
Asthma is a chronic inflammatory disease that fails to resolve. Recently, a key role for type 2 innate lymphoid cells (ILC2) was linked to asthma pathogenesis; however, mechanisms for ILC2 regulation remain to be determined. Here, metabololipidomics of murine lungs identified temporal changes in endogenous maresin 1 (MaR1) during self-limited allergic inflammation. Exogenous MaR1 reduced lung inflammation, ILC2 expression of interleukin-5 and 13, and increased amphiregulin. MaR1 augmented de novo generation of regulatory T cells (Tregs), which interacted with ILC2 to markedly suppress cytokine production in a TGF-β-dependent manner. Antibody-mediated depletion of Tregs interrupted MaR1 control of ILC2 expression of IL-13 in vivo. Together, the findings uncover Tregs as potent regulators of ILC2 activation; MaR1 targets Tregs and ILC2 to restrain allergic lung inflammation, suggesting MaR1 as the basis for a new pro-resolving therapeutic approach to asthma and other chronic inflammatory diseases.
Introduction
Asthma is a chronic inflammatory disease of the lower airways, classically considered to be mediated by an overzealous CD4+ Th2 immune response (1). The innate immune system, specifically type 2 innate lymphoid cells (ILC2), has been shown to have an important role in the initiation (2) and amplification of allergic inflammation (3-5). Although a number of molecular triggers of ILC development and function have been identified(6), there is a fundamental gap in our understanding of the mechanism(s) by which their effector function is regulated. Dysregulated cytokine production by ILC2 has been linked to the pathogenesis in asthma, and other autoimmune diseases (7), but mechanisms to mitigate unrestrained activation of ILC2 are not yet established (8).
Specialized pro-resolving mediators (SPM) are a family of natural autacoids enzymatically derived from essential fatty acids (9). Recently, the regulation of human ILC2 activation by the SPM lipoxin A4 was identified (10). Airway mucosal levels of the essential fatty acid docosahexaenoic acid (DHA) are reduced in human asthma (11) and select SPM derived from DHA decrease murine allergic inflammation (12,13), so the actions of DHA derived SPM on ILC2 responses in asthma are of interest.
Here, lipid mediator metabololipidomics of murine lungs identified increased levels of DHA-derived maresin 1 (MaR1) during the resolution phase of self-limited allergic inflammation. Exogenous MaR1 potently regulated ILC2 and lung inflammation, in part via de novo generation of Tregs that inhibited ILC2 in a TGF-β-dependent manner during cell-cell interactions. These results point to a pivotal role for Tregs in the control of ILC2 in a new pro-resolving mechanism engaged by the SPM MaR1.
Materials and Methods
Mice
Balb/c and FvB mice were purchased from Charles River Laboratories. EGFP-Foxp3 Knockin (006769) and DO11.10 (003303) mice were purchased from The Jackson Laboratory. Mice were used between 7 and 9 weeks of age. All animal experiments were approved by the Animal Care and Use Committee (IACUC) at the Harvard Medical School.
Antibody and flow cytometry
The following antibodies were used in flow cytometry:
Anti-CD3 (17A2; BD Biosciences), anti-CD19 (6D5 BioLegend), anti-CD11b Pacific Blue (M1/70; BioLegend), anti-CD49b(DX5; eBioscience), anti-CD25 APC (PC61; BD Biosciences) ; anti-CD90.2 (anti-Thy-1.2; 53-2.1; eBioscience); CD11c (N418; eBioscience); and anti-ST2(Biolegend), Anti-CD4 (RM4-5, BD Biosciences), Foxp3(FJK-16S, eBioscience,), anti-CD62L(MEL-14, eBioscience), anti-IL-13(ebio13A, eBioscience), and anti IL-5 (TRFK5, BioLegend).
Lipid mediator metabololipidomics
Lungs were harvested at the indicated time points, and metabolipidomics performed as described, and matched to authentic MaR1(7,14). MaR1 physical properties were validated prior to each experiment in accordance with published criteria.
Induction of allergic inflammation
Allergic inflammation was induced as described in Supplemental Fig.1A (12). In experiments involving MaR1, SPM (1 ng/mouse) or vehicle control was administered intravenously 20 minutes prior to aerosol challenge (Supplemental Fig.1B). To deplete Tregs, anti-CD25 antibody (clone PC-61.5.3) 350μg/ mouse or isotype control (rIgG) was administered days 13, 15, and 17.
Adoptive transfer of Treg cells from DO11.10 mice
DO11.10 splenic CD4+ T cells depleted of natural Tregs (CD4+ CD25+) were adoptively transferred intravenously (3*106 cells/recipient) just prior to aerosol challenge on d13. 24h following adoptive transfer, the mice were subjected to aerosol challenge.
Cell isolation and sorting
Lung cells were enriched via negative selection using CD4 isolation kit (Miltenyi Biotec), and sorted for naïve CD4 T cells (CD44loCD62LhiCD25−). Cells were stained with anti-CD4-APC and Tregs were sorted as CD4+ Foxp3+ (EGFP) from _EGFP-Foxp3_-knockin mice. ILC were sorted as shown in Supplemental Fig 2A. ILC were cultured overnight with IL-7 (BioLegend) followed by 4h of PMA/ionomycin stimulation in the presence of golgi stop to determine production of cytokines.
ILC:Treg suppression assay
ILC2s were sorted from lungs following allergic inflammation. ILC2 numbers were kept at 50,000 cells/well. Tregs sorted from lungs of naïve EGFP-Foxp3 knock-in mice were added in cell ratios from 50:1 to 1:1 (ILC2:Treg). Cells were cultured with anti-CD3ε and anti-CD28 (2 μg/ml). 72h following stimulation, supernatants were harvested and IL-13 analyzed. A similar set up was performed for ILC and Tregs isolated with and without MaR1 treatment.
Generation of induced Treg cells in vitro
Naïve CD4 T cells were cultured in the presence of anti-CD3 and CD28-specific antibodies (BD Biosciences, 1 μg/ml), TGF-β (3 ng/ml; R&D), MaR1 (1ng/ml) and alltrans retinoic acid, ATRA, (Sigma; 1 nM). Cultures were supplemented with ATRA, and MaR1 every alternate day for 5d.
ELISA
Murine IL-5, IL-13, amphiregulin and TGF- β purchased from and used according to R&D instruction. Sera from mice were used for IgE quantification.
Statistical analyses
Student's unpaired two-tailed t-test was used for all statistical analyses involving two groups, One-way ANOVA compared differences between groups, and Dunnett's post test for multiple comparisons. All statistical analyses were performed with GraphPad Prism. Results are mean ± SD. Differences between the groups were considered significant if p<0.05. * p<0.05, **p<0.01, ***p<0.001. All data are representative of at least two independent experiments.
Results
Maresin 1 is temporally regulated during allergic inflammation
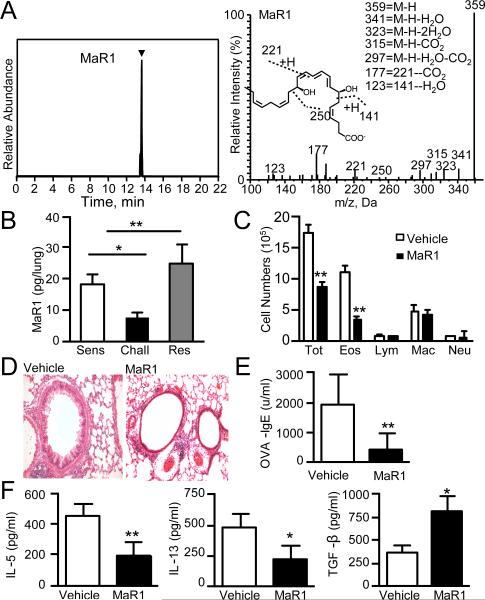
Targeted lipid mediator metabololipidomics was performed to identify endogenous molecules involved in the resolution of allergic inflammation. DHA-derived mediators were determined in lung extracts obtained during self-limited murine allergic responses to ovalbumin (OVA) (Supplemental Fig. 1A). Using reported criteria (7), MaR1 was identified in murine lungs (Fig. 1A). Quantitation employing multiple reaction monitoring demonstrated temporal regulation of lung MaR1, which decreased after allergen challenge and increased during resolution (Fig. 1B).
Figure 1. Maresin 1 is temporally regulated during allergic inflammation.
(A) (Upper Panel) Representative multiple reaction trace for MaR1 (m/z = 359-250) (Lower Panel) MS/MS spectrum employed in the identification of MaR1. (B) Lung MaR1 levels measured during allergic inflammation. (A) and (B) Results are mean ± SD and are representative of two independent experiments (n=3 mice per time point). (C) Cell differentials in BALF, (D) Histology of hematoxylin and eosin–stained lung tissue, 40X**. (E)** OVA-specific IgE measured in serum. (F) Cytokines in BALF measured by ELISA. Results are mean ± SD and are representative of four independent experiments (n>3 mice per group).
Based on MaR1's temporal regulation, exogenous administration of MaR1 (1ng/mouse) during the challenge phase was tested to determine its impact on allergen-triggered inflammation (Supplemental Fig. 1B). MaR1 blunted multiple parameters of allergic inflammation, including significant decreases in bronchoalveolar lavage fluid (BALF) eosinophils (Fig. 1C), tissue inflammation analyzed by histology, (Fig. 1D) and OVA-specific IgE levels (Fig. 1E). Type 2 cytokines IL-5 and IL-13 were significantly reduced in BALF with increased TGF-β levels (Fig 1F).
Maresin 1 promotes de novo generation of Tregs and regulates cytokine production from ILC2
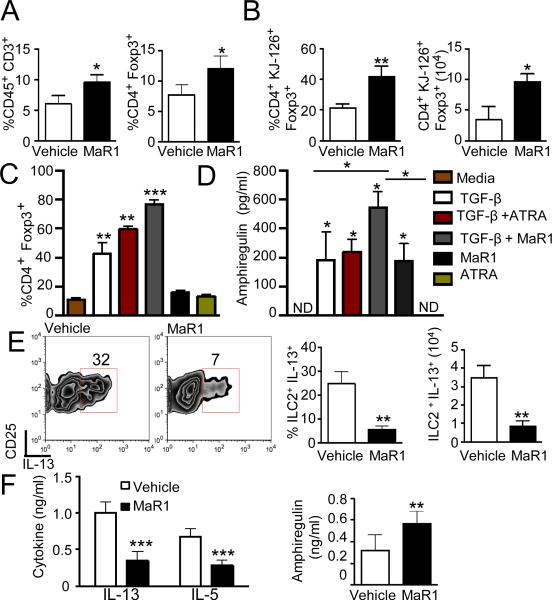
TGF-β plays an important role in skewing CD4+ T cell phenotype (15), so the MaR1-mediated increase in BALF TGF-β (Fig. 1F) suggested an altered T cell profile. Immunoprofiling of lungs following MaR1 relative to vehicle indicated that total leukocytes (CD45+) were decreased while the percentage of CD45+CD3+ cells were increased (Fig. 2A left; Supplemental Fig. 2B). In addition, amongst CD4+ cells, the percentage of Foxp3+ expression increased with MaR1 exposure (Fig. 2A right; Supplemental Fig. 2C). To determine if MaR1 increased antigen-specific Tregs, CD4+ T cells from naïve spleens of DO11.10 mice depleted of natural Tregs were adoptively transferred into sensitized mice (protocol d13). MaR1-exposed mice displayed increased Foxp3 expression in CD4+KJ-126+ cells (protocol d18, Fig. 2B), consistent with de novo generation of antigen-specific Foxp3+ Tregs in vivo by MaR1.
Figure 2. Maresin 1 promotes de novo generation of Foxp3-expressing Tregs.
(A)% CD3 and Tregs (CD4+F+) in lungs (B) DO11.10 CD4+ T (depleted of natural Tregs) cells were adoptively transferred into sensitized mice prior to aerosol challenge. Antigen-specific Tregs were analyzed by flow cytometry. (C) Tregs generation from naïve CD4+ T cells. (D) Amphiregulin production measured by ELISA (right panel). Results are mean ± SD and are representative of three independent experiments with duplicate determinations by ELISA. (E) Flow cytometry of cytokine production by ILC from allergic inflammation model. Plots are gated for ILC2 (Lin−CD45+Thy1.2+CD25+) (F) IL-13 and IL-5 (left panel) and amphiregulin concentrations (right panel) from supernatants of lungs cells. (A, B, E, F) Results are mean ± SD and are representative of three independent experiments (n>3 mice per group).
To determine if MaR1 could directly induce Treg generation, naive CD4+ T cells (spleen) were cultured under Treg inducing conditions (Fig. 2C). MaR1 alone did not promote Treg generation, but there was marked synergy with TGF-β in skewing Treg differentiation (Fig. 2C). Together with TGF-β, MaR1 triggered expression of Foxp3 was similar in amplitude to that induced by all-trans retinoic acid (ATRA)(Fig. 2C). The MFI for Foxp3 expression in these cells also increased with MaR1 (Supplemental Fig. 2D). In addition, MaR1 influenced Treg production of amphiregulin with additive increases when combined with TGF-β (Fig. 2D). In contrast, amphiregulin was undetectable in naive CD4+ T cell cultures. These findings support a pivotal role for MaR1 in promoting de novo Treg differentiation.
To determine if MaR1's regulation of allergic inflammation occurred via targeting ILC2, cytokine production by ILC2 was measured in lungs obtained from mice given MaR1 or vehicle following the last allergen challenge. ILC2 were identified as lineage negative (CD3−CD4−CD8−CD19−CD11b−CD11c−GR1−CD49b−TCRβ−TCRγ/δ−) and CD45+Thy1.2+ST2+CD25+ (See Methods). ILC2 from MaR1-exposed mice had significantly reduced IL-13 production (Fig. 2E). Release of type 2 cytokines from lung cells following overnight stimulation with IL-7 was also significantly decreased in MaR1-exposed mice (Fig. 2F, left panel). In contrast to type 2 cytokines, MaR1 increased levels of lung amphiregulin (Fig. 2F, right panel), a mediator of mucosal protection (16,17). CD4+ Th2 cells from MaR1-exposed mice also displayed markedly lower IL-13 production (Supplemental Fig. 2E). These results indicated that MaR1 selectively regulated lung cytokine levels, including decreased type 2 cytokine production by both ILC2 and CD4+ Th2 cells.
Maresin 1 and Tregs regulate cytokine production from ILC2
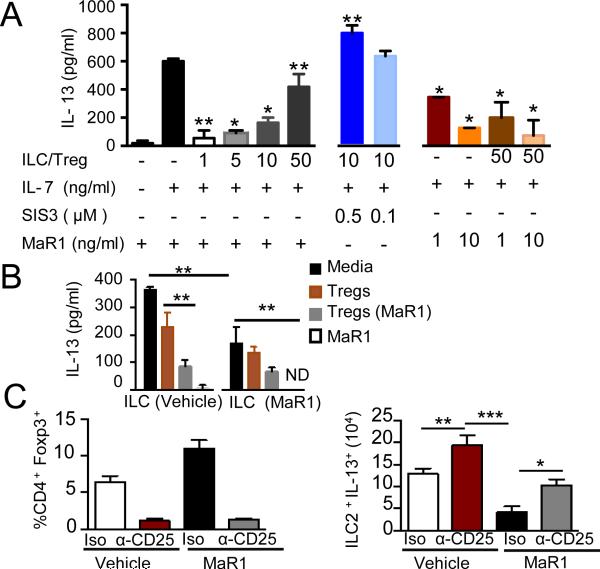
To assess if MaR1's regulation of ILC2 in vivo was via Tregs, ILC2 obtained from allergic murine lungs were co-cultured with Tregs isolated from naive lungs. IL-7 stimulation promoted IL-13 release by ILC2 (Fig. 3A left panel), and increasing ratios of Tregs:ILC2 led to dose dependent inhibition of IL-13 production by ILC2 (Fig. 3A). This Treg mediated inhibition of ILC2 was blocked by a SMAD3 inhibitor (SIS3), suggesting a dependence on TGF-β signaling (Fig. 3A middle panel). MaR1 alone led to concentration dependent inhibition of ILC2 IL-13 and exerted additive inhibition with Tregs (Fig. 3A right panel). Together, these findings were consistent with dual mechanisms for MaR1-mediated inhibition of ILC2, both direct and Treg-mediated, with an important role for TGF-β signaling.
Figure 3. Maresin 1 and Tregs regulates cytokine production from ILC2.
(A) Lung ILCs were sorted from mice following allergic inflammation. EGFP+Tregs from lungs of naïve mice were added in titrated ratios to specific wells. IL-13 levels were measured 72h by ELISA (B) Lung ILC and EGFP+ Tregs were sorted from sensitized mice exposed to vehicle or MaR1 just prior to allergen challenge. The ILCs were cultured in the absence or presence of Tregs for 72h. IL-13 production was measured by ELISA. ND (not detected). Results are mean ± SD and are representative of three independent experiments with duplicate determinations. (C) Foxp3 expression on CD4 T cells (left panel) and IL-13 (right panel) production in lungs ILC2 following administration of anti-CD25 or isotype control was analyzed by flow cytometry. Results are mean ± SD and are representative of two independent experiments (n>3 mice per group).
To determine whether in vivo MaR1 exposure altered Treg and ILC2 functional responses, ILC2 were sorted from lungs following allergic inflammation and exposed to IL-7 in the absence or presence of Tregs isolated from the two groups. The levels of IL-13 were significantly reduced in MaR1-exposed ILC2s compared to vehicle control (Fig. 3B). In addition, MaR1-exposed Tregs were more potent than Tregs from control mice in suppressing IL-13 production by ILC2.
To test the importance of ILC2 suppression by Tregs, an anti-CD25 depleting antibody for Tregs and CD4+ Th2 effector cells was administered prior to aerosol challenge. Foxp3-expressing Tregs were significantly depleted (Fig. 3C left panel). Anti-CD25-administration along in the absence of MaR1 resulted in higher IL-13 production than the isotype antibody, underscoring the role of Tregs in tempering type 2 allergic inflammation (Fig. 3C right panel). Potent inhibition of IL-13 levels was seen with administration of MaR1 and isotype antibody, wherein Tregs were preserved (Fig. 3C). Of note, MaR1-mediated suppression of ILC2 production of IL-13 was blunted in the absence of Tregs. These findings are consistent with ILC2 serving as a major source of IL-13, and indicate MaR1's ability to act directly on ILC2 and potentiate Treg suppressive actions.
Maresin 1 accelerates the resolution of allergic inflammation
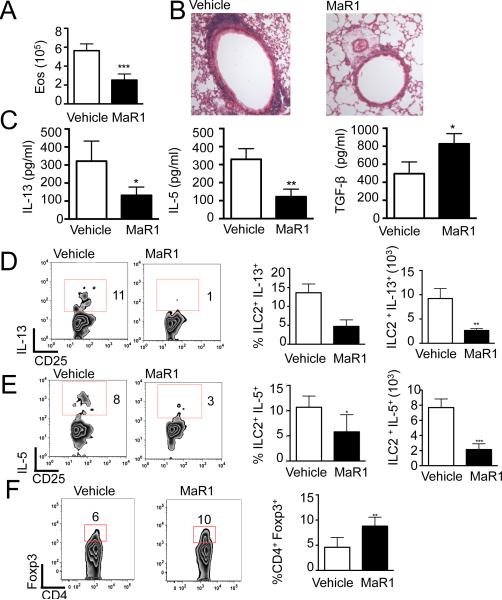
Given the temporal increase in MaR1 levels during resolution and its regulation of ILC2 and Tregs, the impact of MaR1 on resolution was next examined. Mice were allowed to develop maximal allergic lung inflammation, then MaR1 was given for 3 consecutive days (d18–20; Supplemental Fig 1A), followed by immunophenotyping on day 21. MaR1 administration significantly accelerated the decrease in BALF eosinophils (Fig. 4A) and lung tissue inflammation (Fig. 4B). BALF type 2 cytokines were significantly decreased in MaR1-treated mice and TGF-β levels were increased (Fig.4C). During the resolution phase, MaR1 administration resulted in significantly decreased expression of type 2 cytokines (IL-5 and IL-13) by ILC2 (Fig. 4D, E) and by CD4+ Th2 cells (Supplemental Fig. 2F). MaR1-treated mice also showed an increased percentage of Tregs during resolution (Fig. 4F). Together with the results in Figures 1-3, MaR1 prevented the development of allergic lung inflammation and accelerated its resolution by decreasing ILC2 and CD4+ Th2 production of type 2 cytokines and increasing Treg generation and suppressive actions.
Figure 4. Maresin 1 accelerates the resolution of allergic inflammation.
Inflammation analyzed on day 21 of protocol (A) Leukocyte differential counts of BALF cells were performed. (B) Histology of hematoxylin and eosin–stained lung tissue, 40x. (C) Type 2 cytokines and TGF-β levels in the lung BALF measured by ELISA. (D,E) IL-13 and IL-5 cytokine production by ILC2 analyzed by flow cytometry. (F) Lung Treg expression analyzed by flow cytometry. Results are mean ± SD and are representative of three independent experiments (n>3 mice per group).
Discussion
The paradigm of asthma being solely driven by the adaptive immune system has been transformed (18,19); its pathogenesis is significantly influenced by ILC2 serving as an antigen-independent source of type 2 cytokines (18,19). Here, targeted lipid mediator metabololipidomics revealed lung production of a natural pro-resolving mediator MaR1 with spatial and temporal regulation to limit allergic inflammation and ILC2 functional responses. MaR1 is a product of enzymatic conversion of DHA by 12-lipoxygenase, in particular by macrophages during resolution of acute inflammation (7). MaR1's action on murine ILC2 was notable for suppression of IL-5 and IL-13 and increased amphiregulin expression. Together these actions promoted lung catabasis for murine allergic lung inflammation.
MaR1 regulation of ILC2 was accompanied by the induction of Tregs from naïve CD4+ T cells. When isolated from MaR1-treated mice, the Tregs were more potent in suppressing IL-13 production from ILC2s. IL-13-producing ILC2 in the lung were far fewer in number than Tregs, even in allergic inflammation, suggesting that ILC2 effector function is a rational target for induced Tregs. Our in vitro data also indicated that MaR1 had a direct inhibitory effect on ILC2 effector function, suggesting that in the absence of an adaptive immune response (e.g., SCID or RAG deficient mice) MaR1 would still exert an inhibitory effect.
The suppressive mechanism mediated by Tregs involved TGF-β signaling, since addition of a SMAD3 inhibitor reversed Treg inhibition. It is possible that MaR1 enhanced the suppressive function of Tregs by inducing select transcription factors like Itch, which can modulate allergic responses (20). In conjunction with the results from in vivo depletion of Tregs disrupting MaR1-mediated suppression of ILC2 function, data presented here uncover interactions between regulatory immune effectors and a SPM (MaR1) to control lung inflammation, identifying a new pro-resolving cellular mechanism and suggesting a new small molecule or cell-based therapeutic strategy for allergic inflammation that engages MaR1-conditioned Tregs to control ILC2 and CD4+ T cell effector functions.
Supplementary Material
1
Acknowledgments
We thank GuangLi Zhu and Bonna Ith for technical assistance. We also thank Yiling Qui and Deneen Kozoriz for technical assistance in sorting of ILC and Tregs. This research was supported in part by P01-GM095467 (B.D.L., C.N.S) and HL122531 (B.D.L.).
Footnotes
Disclosures
C.N.S. is an inventor on patents [resolvins] assigned to BWH and licensed to Resolvyx Pharmaceuticals. C.N.S. was scientific founder of Resolvyx Pharmaceuticals and owns founder stock in the company. C.N.S.' interests were reviewed and are managed by the Brigham and Women's Hospital and Partners HealthCare according to conflict of interest policies.
B.D.L. is an inventor on patents [resolvins] assigned to BWH and licensed to Resolvyx Pharmaceuticals. B.D.L.'s interests were reviewed and are managed by the Brigham and Women's Hospital and Partners HealthCare according to conflict of interest policies.
The remaining authors declare that they have no competing interests.
References
- 1.Lloyd CM, Hessel EM. Nat Rev Immunol. 2010;10:838–848. doi: 10.1038/nri2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halim TY, Steer CA, Matha L, Gold MJ, Martinez-Gonzalez I, McNagny KM, McKenzie AN, Takei F. Immunity. 2014;40:425–435. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barlow JL, Bellosi A, Hardman CS, Drynan LF, Wong SH, Cruickshank JP, McKenzie AN. J Allergy Clin Immunol. 2012;129:191–198. e191–194. doi: 10.1016/j.jaci.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 4.Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. J Immunol. 2012;188:1503–1513. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spits H, Di Santo JP. Nature immunology. 2011;12:21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 6.Di Santo JP. Immunological reviews. 2014;261:169–176. doi: 10.1111/imr.12202. [DOI] [PubMed] [Google Scholar]
- 7.Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M. The Journal of experimental medicine. 2009;206:15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sonnenberg GF, Artis D. Immunity. 2012;37:601–610. doi: 10.1016/j.immuni.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckley CD, Gilroy DW, Serhan CN. Immunity. 2014;40:315–327. doi: 10.1016/j.immuni.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnig C, Cernadas M, Dutile S, Liu X, Perrella MA, Kazani S, Wechsler ME, Israel E, Levy BD. Science translational medicine. 2013;5:174ra126. doi: 10.1126/scitranslmed.3004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freedman SD, Blanco PG, Zaman MM, Shea JC, Ollero M, Hopper IK, Weed DA, Gelrud A, Regan MM, Laposata M, Alvarez JG, O'Sullivan BP. The New England journal of medicine. 2004;350:560–569. doi: 10.1056/NEJMoa021218. [DOI] [PubMed] [Google Scholar]
- 12.Rogerio AP, Haworth O, Croze R, Oh SF, Uddin M, Carlo T, Pfeffer MA, Priluck R, Serhan CN, Levy BD. J Immunol. 2012;189:1983–1991. doi: 10.4049/jimmunol.1101665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levy BD, Kohli P, Gotlinger K, Haworth O, Hong S, Kazani S, Israel E, Haley KJ, Serhan CN. J Immunol. 2007;178:496–502. doi: 10.4049/jimmunol.178.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalli J, Zhu M, Vlasenko NA, Deng B, Haeggstrom JZ, Petasis NA, Serhan CN. FASEB J. 2013;27:2573–2583. doi: 10.1096/fj.13-227728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 16.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, Kubota M, Turner D, Diamond JM, Goldrath AW, Farber DL, Collman RG, Wherry EJ, Artis D. Nature immunology. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner JE, Morrison PJ, Wilhelm C, Wilson M, Ahlfors H, Renauld JC, Panzer U, Helmby H, Stockinger B. The Journal of experimental medicine. 2013;210:2951–2965. doi: 10.1084/jem.20130071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, Krummel MF, Chawla A, Liang HE, Locksley RM. Nature. 2013;502:245–248. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu S, Kim HY, Chang YJ, Dekruyff RH, Umetsu DT. J Allergy Clin Immunol. 2014;133:943–950. doi: 10.1016/j.jaci.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 20.Chen W. J Clin Invest. 2013;123:4576–4578. doi: 10.1172/JCI72477. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1