Genetic Fate Mapping Using Site-Specific Recombinases (original) (raw)
. Author manuscript; available in PMC: 2015 Dec 18.
Abstract
Understanding how cells are assembled in three dimensions to generate an organ, or a whole organism, is a pivotal question in developmental biology. Similarly, it is critical to understand how adult stem cells integrate into an existing organ during regeneration or in response to injury. Key to discovering the answers to these questions is being able to study the various behaviors of distinct cell types during development or regeneration. Fate mapping techniques are fundamental to studying cell behaviors such as proliferation, movement, and lineage segregation, as the techniques allow precursor cells to be marked and their descendants followed and characterized over time. The generation of transgenic mice, combined with the use of site-specific recombinases (SSR) in the mouse genome, has provided a means to develop powerful genetic fate mapping approaches. A key advantage of genetic fate mapping is that it allows cells to be genetically marked, and therefore the mark is transmitted to all the descendants of the initially marked cells. By making modifications to the SSRs that render their enzymatic activity inducible, and the development of an assortment of reporter alleles for marking cells, increasingly sophisticated genetic fate mapping studies can be performed. In this chapter, we review the four main genetic fate mapping methods that utilize intrachromosomal recombination to mark cells (cumulative, inducible, clonal, and intersectional) and one interchromosomal method, the tools required to carry out each approach, and the practical considerations that have to be taken into account before embarking on each type of genetic fate mapping study.
1. Principles Behind Genetic Fate Mapping
A critical question in developmental biology is how a cell acquires its ultimate differentiated state (or fate), which includes how it becomes assembled in three dimensions along with other cells to generate a functional organ. Equally important to understand is the potential of adult stem cells to replenish tissues during homeostasis and to repair injured tissues. Fate mapping is an essential technique for answering these questions. Fate mapping consists of marking a group of cells, or a single cell, in the embryo and then determining what the cells and all their descendents become after development is complete, or in the adult after a regenerative process has taken place. The final position at which the marked cells and/or their progeny settle is also revealed by fate mapping. By following the fate of the marked cells at different stages of development, the lineage of a cell, that is its genealogical series of ancestors (Fig. 10.1), can be uncovered to provide information on when and how different cell types become segregated from a common ancestor. In the mouse, fate mapping studies using traditional invasive methods, such as viral infection or dye marking, although they have provided invaluable knowledge (Fields-Berry et al., 1992; Golden et al., 1995; Lawson, 1999; Sanes et al., 1986), have been hampered by the inaccessibility of the embryo in the uterus and the limited time during which embryos can be cultured. The development of transgenic mice and site-specific recombinases (SSRs) that are effective in mice provided a new approach for genetically marking cells in the intact embryo or postnatal mouse, without a need for manipulation of the embryo or an organ itself. The application of these new fate mapping approaches is termed genetic fate mapping, since the marking of the cells involves a permanent change in their DNA that allows the cell to be identified from its unmarked neighbors. Furthermore, the genetic nature of the labeling ensures the transmission of the marker to all the progeny of the initially labeled cells, circumventing the problem of dilution of a dye marker during cell division. By choosing the appropriate promoter to drive expression of the SSR, a population of cells of interest can be precisely marked genetically at any time point during development or in the adult, and its progeny then followed over time. Thus, genetic fate mapping in mouse is a powerful method for studying the behavior of cell lineages in the intact mouse at any stage of development, or in the adult during homeostasis and organ regeneration.
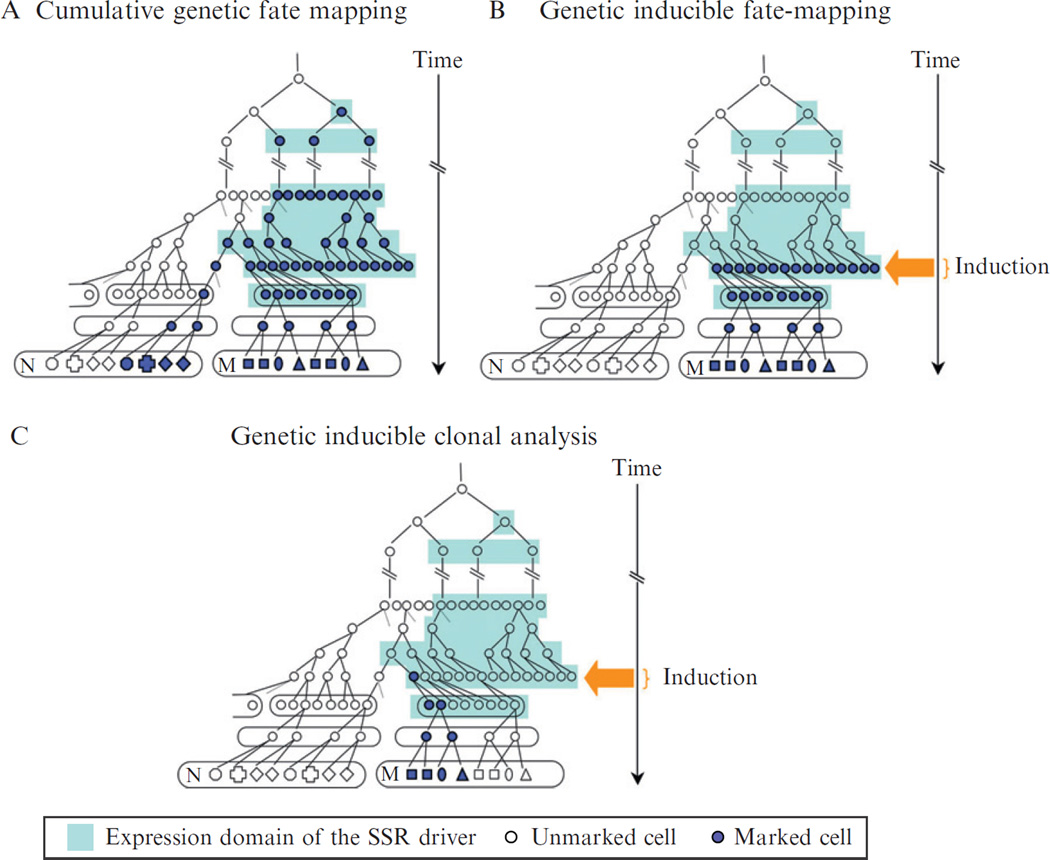
Figure 10.1.
Three types of genetic fate mapping lead to marking of different cell populations. A lineage tree is shown of the cells arising from a single ancestor that gives rise to two structures (ovals marked as M and N) in the mouse. The domain of expression of the promoter that drives the SSR is represented by light blue shading. It encompasses cells from several generations (aligned from top to bottom) and cells that are spatially distinct (aligned from left to right). The marked cells are represented with a bold outline and expression of a reporter protein by dark blue after recombination of the reporter allele. (A) In cumulative fate mapping, all the cells that express the SSR at some time point undergo recombination of the reporter allele, and if transcription of the reporter allele is driven by a ubiquitous promoter, all the cells that have ever expressed the SSR and their descendants are marked. (B) In genetic inducible fate mapping, only the cells that express the SSR at the time of induction (here represented over one cell generation by an orange arrow) can undergo recombination of the reporter allele and express the reporter allele, and transmit the recombined reporter allele to all their descendants. (C) In clonal analysis, the dose of ligand administered to the mouse is titrated down such that only one precursor undergoes recombination (is marked), and transmits the recombined allele to its descendants.
2. Genetic Fate Mapping Technique
Genetic fate mapping requires the generation of two transgenic mouse lines, and the two transgenes are then combined in one mouse by breeding. One transgene has a promoter that is expressed in the cell type of interest (i.e., the population of cells to be fate mapped) and drives expression of an SSR (SSR line). The second transgene typically contains a ubiquitous promoter, so that all the progeny of the population of interest can express the marker gene and be visualized, upstream of two copies of the DNA sequences recognized by the SSR flanking a cassette that inhibits expression of a downstream marker or reporter gene (reporter line). In a cell that expresses the SSR, the two SSR sites in the reporter allele will undergo recombination and the recombined DNA configuration will then be transmitted to all of its descendants, thus all cells expressing the SSR and all their descendents will be genetically marked (Fig. 10.1). If the promoter in the reporter allele is expressed ubiquitously, all the genetically marked cells will express the reporter protein, and thus can be visualized. In genetic fating mapping, the marking of cells is therefore heritable and permanent due to its genetic nature.
2.1. Tools for genetic fate mapping
2.1.1. The site-specific recombinases
Two SSRs have primarily been used for genetic fate mapping in mice; the Cre recombinase from the bacteriophage P1 (Austin et al., 1981) and the Flp recombinase from Saccharomyces cerevisiae (Farley et al., 2000). The Cre recombinase specifically acts on a 34-bp DNA sequence called a loxP site (Hoess et al., 1982), whereas the Flp recombinase acts on a 47-bp FRT site (Andrews et al., 1985). If a DNA sequence is flanked by two loxP or FRT sites in the same orientation, then the sequence will be efficiently excised by Cre or Flp recombination, respectively, leaving one loxP or FRT site behind (Sauer, 1993; Schaft et al., 2001). See Chapter 7 for more details.
2.1.2. Modifications to the sites-specific recombinases
A number of critical modifications have been made to the SSRs to maximize the activity of the recombinases (see Chapter 7 for details), as well as to gain temporal control over their activity. An optimized form of Flp that is more stable than the original yeast protein at mouse body temperature is referred to as Flpe (e = enhanced; Buchholz et al., 1998). More recently, new versions of Flpe have been generated in which codon usage was optimized for translation in mouse, and they are referred to as Flpo (o = optimized codon usage; Raymond and Soriano, 2007; Wu et al., 2009), these versions of Flp confer better efficiency to the SSR. Conversely, a thermolabile form of Flp exists that has a low recombinase efficiency (FlpL; Buchholz et al., 1996), and might be useful for clonal analysis (see below) (Dymecki and Kim, 2007).
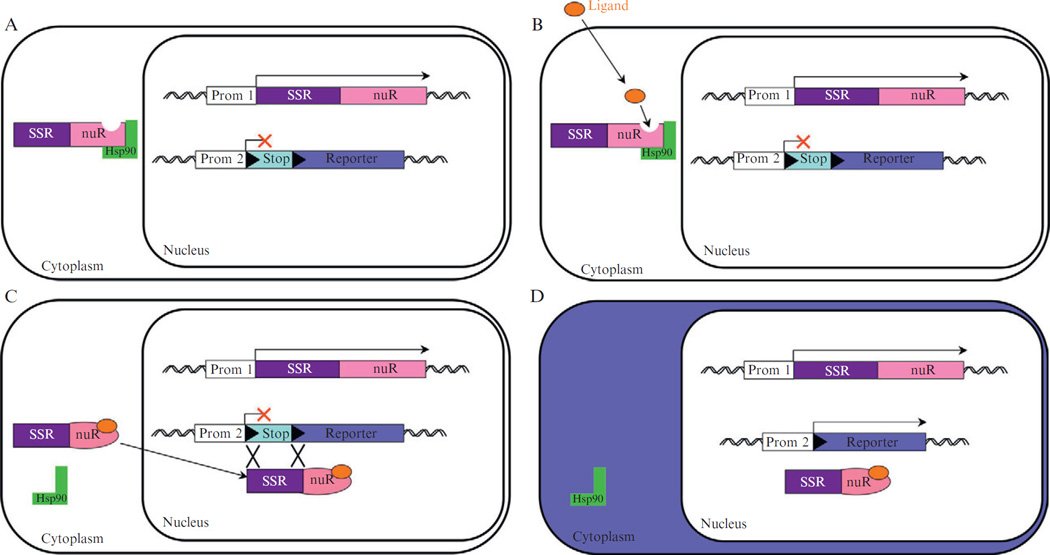
The most versatile modification of the SSRs in terms of genetic fate mapping studies has been the fusion of the SSRs with a protein domain that allows the activity of the SSRs to be temporally controlled. The hormone-binding domains from homodimeric nuclear receptors (nuR) have been used to control Cre and Flp activity. In the absence of the appropriate ligand, the hormone-binding domains ensure that the SSR-fusion protein is sequestered in the cytoplasm (Fig. 10.2A). Upon binding of the hormone to the domain, the cytoplasmic sequestration is relieved and the SSR-fusion protein is translocated into the nucleus (Fig. 10.2B and C). Thus, the fusion of an SSR to an nuR hormone-binding domain allows the subcellular localization of the SSR to be controlled, and therefore its accessibility to DNA. Site-specific recombination is therefore dependent on ligand administration. The hormone-binding domains from the human and mouse estrogen receptor (ER) or the human progesterone receptor (PR) have been used to generate CreER and FlpeER or CrePR fusions (Hunter et al., 2005; Metzger and Chambon, 2001; Tsujita et al., 1999). In order to avoid interaction of the hormone-binding domains with endogenous hormones, the ER or the PR binding domains have been modified such that their affinity for estrogen or progesterone is reduced, and their affinity for another ligand increased (Feil et al., 1996, 1997; Vegeto et al., 1992). A modified ER estrogen-binding domain that preferentially binds an antagonist of estrogen (4-hydroxy-tamoxifen) (Feil et al., 1996, 1997; Indra et al., 1999) or a truncated PR progesterone-binding domain that binds an antagonist of progesterone (RU-486) have been developed (Vegeto et al., 1992). The resulting modified ER hormone-binding domains are named ERT (T for tamoxifen, where a number following T indicates subsequent modifications of the domain). In the absence of ligand, the SSR-nuR hormone-binding domain fusion protein is sequestrated in the cytoplasm in a large protein aggregate that includes heat shock protein chaperones (Fig. 10.2A). Binding of the ligand to the modified nuR hormone-binding domain of the SSR-fusion protein (Fig. 10.2B) induces a change in conformation of the hormone-binding domain and its subsequent release from the chaperone. The SSR-fusion protein then translocates into the nucleus where the SSR can mediate site-specific recombination (Fig. 10.2C). For genetic fate mapping studies, a stop sequence flanked by two loxP or FRT sites is excised from a reporter allele leading to expression of a protein that marks the cell (Fig. 10.2D). Fate mapping approaches that utilize a temporally controlled SSR-fusion protein are referred to as genetic inducible fate mapping (GIFM; Joyner and Zervas, 2006), since the activity of the SSR-fusion protein can be induced at any time point (temporal control). GIFM is therefore a powerful approach since the marking of cells is controlled by both the precision of the promoter-driving expression of the SSR-fusion protein and a small time window when the SSR is active (see below).
Figure 10.2.
Genetic inducible fate mapping. (A) In the absence of ligand, the fusion protein between an SSR and nuclear receptor (nuR) is bound to Hsp90 and retained in the cytoplasm. (B) Binding of the ligand induces a conformational change, and Hsp90 is released. (C) The SSR-fusion protein then translocates into the nucleus where it induces recombination between the DNA target sites (▶) flanking a stop sequence in a reporter allele that inhibits transcription of the reporter gene. (D) Once the stop sequence is excised, the reporter protein is produced and the cell and its descendents are marked/labeled (e.g., cytoplasm expresses lacZ, indicated by blue).
2.1.3. The reporter alleles
2.1.3.1. Intrachromosomal recombination
In a reporter allele that undergoes intrachromosomal recombination, the recombination sites are placed in cis, and in the same orientation, flanking a stop sequence. The stop sequence typically includes a polyadenylation sequence that signals termination of transcription, and thus prevents transcription of a downstream reporter gene. Expression of the reporter protein is therefore dependent on the recombination and excision of the loxP/FRT flanked stop sequence by the SSR. The stop sequence often also contains a protein coding sequence such as neo. If the promoter driving the reporter gene is expressed in all cells of the mouse (ubiquitous promoter), then all cells that have undergone SSR-mediated recombination, as well as their descendants, will be permanently marked by expression of the reporter protein.
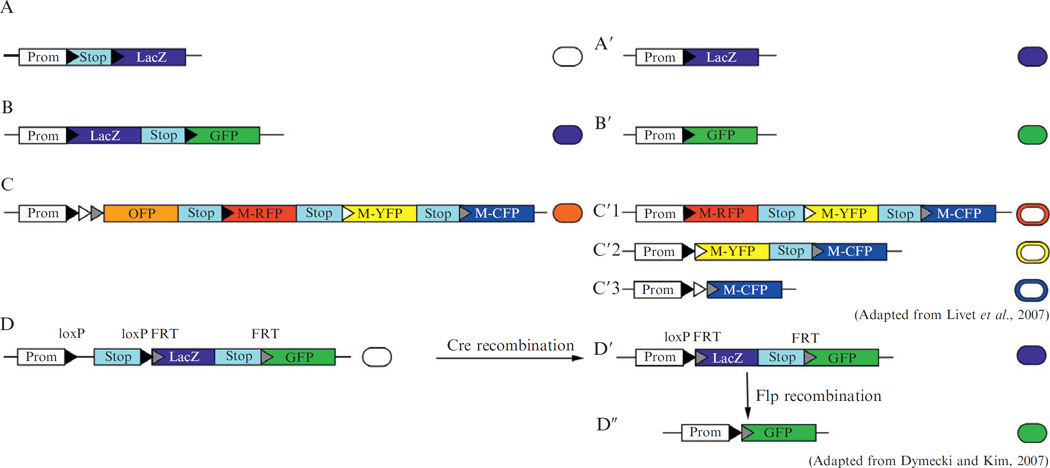
In the simplest reporter alleles, there is only one reporter gene, for instance, LacZ or GFP (Fig. 10.3A). Some reporter alleles contain an additional reporter gene as part of the stop sequence. For example, a LacZ gene has been incorporated into the stop sequence and an alkaline phosphatase (Z/AP reporter) or an eGFP (Z/EG reporter, Fig. 10.3B) gene placed downstream of the stop sequence (Lobe et al., 1999; Novak et al., 2000). The activity of the SSR thus switches expression (or marking) of cells from one reporter to the other—the reporter gene present in the stop sequence is expressed before site-specific recombination and the reporter gene downstream the stop sequence is expressed after recombination (Fig. 10.3B and B′).
Figure 10.3.
Examples of reporter alleles used for intrachromosomal recombination genetic fate mapping. Four major types of genetic fate mapping reporter alleles for marking cells using intrachromosomal recombination are shown, both before (left panel) and after recombination (right panel). The color in the circles to the right of each DNA configuration represents the protein that labels the cell. (A) The simplest reporter allele contains a stop sequence flanked by recombination sites upstream a reporter gene. (B) An example of a reporter gene (Z/EG; Novak et al., 2000) in which there is a switch from expression of one marker protein (color) to another. (C) The Brainbow reporter allele (Livet et al., 2007) contains three pairs of different loxP sites. It generates three products of recombination (C′1, C′2, and C′3) depending on which pair of loxP sites undergoes Cre-mediated recombination. (D) A reporter allele for intersectional genetic fate mapping is shown (Awatramani et al., 2003; Dymecki and Kim, 2007). Both loxP and FRT site-specific recombination sites are present in the reporter allele. Expression of both Cre and Flp is required for expression of the reporter protein GFP.
In an additional approach called intersectional (or combinatorial) genetic fate mapping, the reporter allele has both a pair of loxP and FRT sites and two reporters—the first reporter is preceded by a loxP (or FRT) flanked stop sequence and is also surrounded by FRT (or loxP) sites (Awatramani et al., 2003; Dymecki and Kim, 2007) (Fig. 10.3D) (see below and Chapter 11 for details). The power of intersectional fate mapping is that for the second reporter to be expressed, a cell must have expressed both Cre and Flp (be at the intersection of the two SSR expression domains) (Fig. 10.3D″), thus refining the domain or cell population that can be studied (marked).
More recently the production of spectral variants of GFP (Chudakov et al., 2005; Pakhomov and Martynov, 2008) and modified recombination sites for Cre (Kolb, 2001) have allowed for the generation of new reporter alleles that lead to random marking of cells with several different reporters within the same animal (Livet et al., 2007). Such reporters can be very useful for clonal analyses (see below). The reporter allele is engineered to comprise several reporter genes encoding fluorescent proteins with different spectral properties in tandem. Three different loxP sites are inserted upstream of the reporters genes, and in addition, each reporter gene is followed by a stop sequence and one of the modified loxP sites (Fig. 10.3C). SSR-mediated recombination leads to random deletion of the reporters situated between a pair of modified loxP sites, leading to expression of the remaining most 5 0 reporter (Fig. 10.3C′1, C′2, and C′3). If multiple reporter alleles are present in a cell, then multiple fluorescent proteins will be randomly coexpressed (one from each copy of the reporter allele). The various combinations of coexpressed fluorescent proteins result in an array of possible colors a cell and its descendants can be marked with (up to 10 to date; Livet et al., 2007). This additional complexity of marking thus provides a means for many distinct recombination events to be distinguished and studied within the same animal. To date, such reporter lines have been generated with a transgene that contains a Thy1 promoter fragment that is expressed in a variety of neurons, and have been named Brainbow (Livet et al., 2007). Finally, in the future it is likely that reporter alleles will be optimized for live imaging of marked cells by using proteins with enhanced fluorescence, minimal cell toxicity, and robust levels of protein expression (Luche et al., 2007 and see Chapter 20 in volume 476).
2.1.3.2. Interchromosomal recombination—Mosaic analysis with double markers
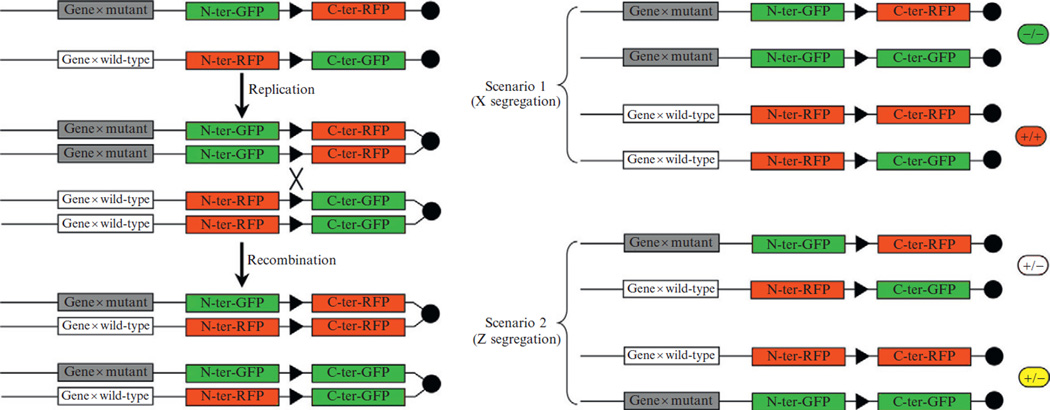
The Luo lab has generated a reporter system that is dependent on Cre activity and two reporter alleles, each carrying a complementary half of a reporter transgene inserted by gene targeting into the same locus on homologous chromosomes (Zong et al., 2005). One transgene contains the coding sequence for the N-terminal part of GFP and the C-terminal part of RFP separated by a loxP site that disrupts translation, while the other allele contains the N-terminal part of RFP and the C-terminal part of GFP separated by a loxP site (Fig. 10.4). When Cre is expressed in a cell, site-specific recombination between the two loxP sites on homologous chromosomes occurs leading to exchange of the C-terminal and N-terminal portions of the reporters, and production of GFP from one chromosome and RFP from the other (Fig. 10.4). A unique aspect of this system is that it allows for the generation of labeled cells that are also homozygous for a mutation on the chromosome harboring the reporter alleles. With this technique, named mosaic analysis with double markers (MADM; Zong et al., 2005), mutant cells can be induced that are genetically marked and therefore fate mapped in an otherwise wild-type environment.
Figure 10.4.
Schematic representation of a reporter allele for interchromosomal recombination (MADM; Zong et al., 2005). In MADM reporter allele, the loxP sites are on two homologous chromosomes. The sequences of the reporter genes are complementary, each containing either the N-terminal coding sequence of GFP and the C-terminal coding sequence of RFP or the N-terminal coding sequence of RFP and the C-terminal coding sequence of GFP. Recombination between the loxP sites restores the full coding sequences for both genes. The advantage of such a reporter allele is that they can be used to simultaneously mutate and mark cells, if a mutated gene is on the same chromosome as the reporter. Marking and mutating cells requires replication of DNA and depends on the segregation of the chromatids. The possible outcomes of chromatid segregation are represented on the right, with the genotype and labeling of the cells that are generated. In the X segregation scenario (the two recombined chromatids segregate to different cells), two labeled cells are generated: a mutant GFP-expressing cell and a wild-type RFP-expressing cell. In the Y segregation scenario, the two recombined chromatids segregate in the same cell and the cells generated are heterozygous for the mutation, one expresses both GFP and RFP and is represented in yellow and the other is not labeled.
2.2. Genetic fate mapping methods
With the modifications made in the SSRs and a variety of different reporter lines, the applications of genetic fate mapping have been broadened. In this section, we describe the basic uses of each type of genetic fate mapping.
2.2.1. Cumulative genetic fate mapping
Cumulative genetic fate mapping involves the use of an SSR (e.g., Cre) that is constitutively active. The approach is called “cumulative” because all the cells that at any point during development expressed a sufficient amount of Cre to induce recombination of the loxP sites in the reporter allele, as well as all their descendants, will be genetically marked. During development, any additional cell that begins to express Cre will add its contribution to the total number of cells that are ultimately marked (Fig. 10.1A). The specificity of potential cell labeling (spatial and temporal) in cumulative genetic fate mapping therefore comes entirely from the regulatory sequence chosen to drive SSR expression. Since promoter activity can change during development, it often is not possible to use this approach for definitive prospective fate mapping of a particular group of cells or cell lineage. All labeled cells therefore arise from the additive (cumulative) contribution of all the cells that at any previous time point expressed sufficient SSR to induce recombination of the reporter allele.
2.2.2. Genetic inducible fate mapping
In GIFM studies, the timing of the SSR-mediated recombination event (marking of cells) is controlled (i.e., induced by ligand), and limited to a discrete period of time (Fig. 10.1B). GIFM therefore utilizes one of the modified nuR hormone-binding domain SSR-fusion proteins that allow for temporal control over the site-specific recombination activity by administration of a specific ligand. Recombination occurs only over an approximately 24-h time period (see below for details), and recombination is less efficient than with constitutive SSRs. Although this leads to mosaic marking of the population of cells expressing the SSR, the general pattern of marking is reproducible between animals. This approach therefore allows for prospective fate mapping, since the initial population of marked cells can be definitively identified 24–48 h following administration of the ligand and then their fate determined. If the timing of marking is important for a study, and/or an ability to definitively identify the marked population, then GIFM is the approach of choice.
2.2.3. Genetic inducible clonal analysis
In clonal analysis, the goal is to achieve a level of recombination low enough such that the labeled cells observed can be identified as coming from a single recombined cell (a clone) (Fig. 10.1C). Genetic retrospective clonal analysis using spontaneous recombination provided new and extremely useful insights about cell behaviors. However, the approach does not allow for temporal control of the marking of the initial cell that produces the clone (Petit et al., 2005). Genetic inducible clonal analysis is an adaptation of the GIFM method, as clonality is achieved by decreasing the dose of ligand injected. In genetic inducible clonal analysis, the approach is partly prospective in the sense that the population from which, and the time when the initial cell is marked can be determined. However, it is also retrospective, since the exact position of the initially labeled cell within the population of cells that could potentially be marked is not known, and therefore must be inferred retrospectively based on analyzing a library of different clones induced at the same time point.
2.2.4. Intersectional genetic fate mapping
For intersectional genetic fate mapping, mice must carry three genetically modified alleles: one expressing Cre (or CreER), one expressing Flp (or FlpER), and a reporter allele with two tandem reporters in which the first is both preceded by a loxP (or FRT) flanked stop sequence and surrounded by FRT (or loxP) sites. Although the approach requires that an addition allele be bred onto the experimental mice, the advantage is that more precise marking can be achieved since expression of the second reporter protein depends on the expression of both Cre and Flp in a cell. The approach is also referred to as combinatorial fate mapping, since a combination of Cre and Flp is used for marking (Awatramani et al., 2003; Dymecki and Kim, 2007) (see Chapter 11 for details).
2.3. Important considerations in designing all types of genetic fate mapping studies
Initiating a genetic fate mapping study involves the use of at least two transgenic mouse lines. Since generation of transgenic lines is expensive and time-consuming, it is important to carefully choose or design the optimal mouse lines for each study. We first discuss general considerations relevant to all genetic fate mapping studies, and then describe ones specific to using GIFM and genetic inducible clonal analysis approaches, since more sophisticated tools are needed, and as a consequence, more parameters must be taken into account when designing an experiment.
2.3.1. Choice of promoters to drive the expression of a reporter allele
A key aspect of genetic fate mapping is knowing whether all cells that have undergone recombination, as well as all their descendants, can be visualized (i.e., expression a marker protein at sufficient levels). Depending on the question being addressed, the stages at which fate-mapped cells are observed, and the method of visualizing the marker protein, either a ubiquitous or restricted promoter can be used to drive the expression of the reporter allele.
The use of a ubiquitously expressed promoter, such as the endogenous ROSA promoter (Zambrowicz et al., 1997), allows for all marked cells to be visualized. It is important to note, however, that there may be a few cell types that do not express the ROSA gene, and that all cell types do not express the ROSA gene at the same level. An effective reporter allele for the neuroscience community was generated by targeting a reporter construct into the Tau gene that is expressed only in differentiated neurons (Hippenmeyer et al., 2005). The reporter has the advantage that it expresses both myristoylated GFP (Laux et al., 2000), which marks the axons and dendrites, and nuclear localized β-galactosidase that marks the location of the cell body. Since restricted promoters often have a dynamic pattern of expression and are not always expressed throughout development and into the adult, the ubiquitous promoters have the advantage of providing visualization of all labeled cells at any stage, during development and in the adult.
Instead of utilizing gene targeting to engineer reporter alleles that conditionally express a reporter from endogenous loci with known expression patterns, versions of the CAG promoter (Miyazaki et al., 1989; Niwa et al., 1991) have been used to make transgenic mice and multiple lines then screened for a line with broad expression (Akagi et al., 1997). The CAG promoter confers a high level of expression of the reporter gene in many cell types after SSR recombination. Since the complete expression of any of the transgene-based reporter alleles has not been published, it is necessary to test that the reporter expresses in the cells of interest. This can be achieved by deleting the stop sequence in the transgenic reporter in the germ line and observing expression of the reporter in offspring that have the stop sequence removed in all cells (Lallemand et al., 1998). An alternative is to use lines made by gene targeting in which a reporter construct with the CAG promoter has been targeted into the ROSA locus, as these reporter lines have widespread expression, that appears to be high in the postnatal nervous system(Zong et al., 2005).
For most studies, ubiquitous reporter alleles are the best, since all the progeny of the marked population of cells can be visualized, regardless of the organ or the cell type they contribute to. However, in some cases, for instance when a study is focused on one cell type, it can be advantageous to use a cell type specific reporter allele in order to limit marking to one cell type. For example, in some cases the marking of multiple cell types in an organ can confound the visualization of the cell type of interest.
Finally, the level of expression driven by the promoter in the reporter allele must be taken into account, since the ability to visualize a reporter protein depends on the sensitivity of the reporter detection technique. Different detection techniques are therefore chosen depending on the level of protein expressed. Relatively low levels of expression can be tolerated when fixed tissues are being analyzed, since detection techniques with high sensitivity can be used, such as immunohistochemical staining that involves an enzymatic reaction that allows for amplification of the signal. However, for live imaging of fate-mapped cells, since direct visualization of fluorescent reporters is used, it is crucial to use promoters that have a high level of expression.
2.3.2. Choice of promoters to drive SSR expression
The promoter that drives the SSR determines the population of cells that can be fate mapped. Spatial and/or cell lineage control of the SSR is achieved by choosing the appropriate promoter to drive its expression in the desired population of cells. Of great utility in designing new genetic fate mapping studies, many mouse lines have already been established and are freely available. In order to facilitate the search for existing Cre or CreERT lines, databases have been created:
- http://nagy.mshri.on.ca/cre/index.php
- http://www.informatics.jax.org/recombinase.shtml (mouse genome informatics)
More general databases can also be used to search for patterns of expression that match the desired population to be fate-mapped.
- http://www.ncbi.nlm.nih.gov/projects/gensat/ (brain)
- http://www.brain-map.org/ (brain)
- http://www.informatics.jax.org/expression.shtml (embryo)
- http://www.emouseatlas.org/emage/home.php (embryo)
Before starting a genetic fate mapping study, it is essential to characterize the pattern of expression of the SSR in the population of cells of interest, and the degree to which the population is marked with the reporter allele of choice (functional assay). To fate map a cell population that expresses a specific gene, it is important to use the line that most faithfully reproduces the pattern of the endogenous gene. Knock-in alleles are normally the best tools for achieving expression that is the same as an endogenous gene. However, for many other applications, transgenes can be an advantage if they express in a subset of the normal expression domain, or have ectopic expression in a cell population of interest. The use of a transgenic line rather than a targeted line requires a more thorough characterization of SSR expression and function to ensure the desired cell types are marked. A possible complication of targeted knock-in alleles, however, is that the allele is often a mutant allele and therefore could have a subtle phenotype in the heterozygous state, especially when combined with other heterozygous mutations, for example, in the reporter allele.
Another parameter to test is the level of expression of the SSR. A high level of expression ensures robust labeling of the cell population of interest (i.e., reproducible marking of a high percentage of the cell population). However, for some GIFM applications such as clonal analysis, low levels of expression of the SSR are preferred, since a low efficiency of recombination and no recombination in the absence of ligand is required (see below).
2.3.3. Kinetics of recombination
Three key components of the kinetics of site-specific recombination and marking of cells that must be taken into consideration are the time necessary for (1) the SSR to induce recombination, (2) the reporter gene to subsequently be transcribed and translated, and (3) the reporter protein to accumulate sufficiently to be detected. There is usually an approximately 12-h delay between the appearance of SSR transcripts and detection of reporter protein (Nakamura et al., 2006). However, this can vary depending on the level of expression of the SSR and reporter allele used, and the sensitivity of detection of the reporter protein. As a good approximation, in cumulative fate mapping studies, the first population of SSR-expressing cells is marked around 12 h after the onset of expression of the SSR. For studies using GIFM and clonal analysis, additional key components are added to the kinetics of recombination and are discussed in the following sections.
2.3.4. Cre toxicity
There are a number of publications documenting toxicity produced by the presence of Cre (or CreER) in the nucleus. Expression of nuclear-localized Cre in neural progenitors, under the control of Nestin regulatory elements, was found to cause hydrocephaly and microencephaly in mice carrying two copies of the transgene, and thus expressing high levels of Cre (Forni et al., 2006). Suggesting that the phenotype is due to toxic effects of Cre, rather than the site of insertion of the transgene, the group also found that two _Nestin_–CreER transgenics have a similar phenotype after tamoxifen administration. However, we have used a Nestin–Cre line (Tronche et al., 1999) and not found phenotypes in mice carrying one copy of the transgene (Blaess et al., 2006, 2008; Corrales et al., 2006). Another study documented that the CreER fusion protein expressed from the ubiquitous ROSA allele leads to widespread apoptosis and anemia when tamoxifen is administered during embryogenesis (Naiche and Papaioannou, 2007). These reports and others point to the importance of testing that the level of expression of Cre or CreER does not cause toxicity that can confound interpretation of a genetic fate mapping study, since toxicity appears to be dependent on the level of Cre/CreER protein present in the nucleus (Forni et al., 2006; Naiche and Papaioannou, 2007; Takebayashi et al., 2008). In vitro studies showed that the Cre cytotoxicity is dependent on the endonuclease activity of Cre (Loonstra et al., 2001). The presence of cryptic loxP sites in mammalian genomes are thought to trigger illegitimate Cre activity, leading to double-strand breaks or nicks that are converted into double-strand breaks after replication (Abremski et al., 1986), which results in reduced proliferation of the cells that carry damaged DNA (Loonstra et al., 2001).
2.3.5. Mouse breeding
At least two mouse lines are needed for genetic fate mapping experiments: the SSR line and the reporter line. An ideal breeding scheme is to use males homozygous for the reporter allele that also carry one copy of the SSR transgene and to cross them to Swiss Webster or other outbred female mice. Half of the litter will carry one copy of each transgene, and can be used for fate mapping analysis. Using outbred females that can be purchased when needed aids in generating the maximum number of transgenic mice for analysis, and in minimizing the size of the mouse colony needed for genetic fate mapping studies. Moreover, in the case of GIFM approaches, since many inbred strains of mice are more sensitive to ligand toxicity (see below), it is advantageous to use outbred females when ligand is administered to pregnant females.
2.3.6. Detection of marking
After recombination, the reporter gene will be expressed under the control of the promoter in the reporter allele. Marked cells can then be detected by a variety of methods. The technique chosen to reveal labeled cells depends on the reporter gene and aim of the study. If the reporter encodes a fluorescent protein, and the level of expression is high enough, it can be detected directly by using a fluorescence microscope with filters selecting the appropriate excitation and emission wavelengths. Fluorescent protein reporters thus have the advantage that they can be detected in living cells, making them ideal tools for live-imaging of whole embryos or slices of tissues in culture (see Chapter 20 in volume 476).
The other techniques for detecting reporter proteins involve fixation of the tissue. If the reporter gene encodes an enzyme (β-galactosidase or alkaline phosphatase), marked cells can be revealed by an enzymatic reaction. All reporters (enzymes and fluorescent proteins) can be detected by antibody staining. Both enzymatic reactions and antibody staining are sensitive techniques that allow for the detection of relatively low levels of protein. Enzymatic reactions have the advantage that the strength of the signal is directly related to the level of protein and can therefore be maximized by changing the length of staining, whereas antibody staining has the advantage that the signal can be amplified by adding additional steps, including enzymatic reactions.
2.3.6.1. Protocol for detection of β-galactosidase activity by X-gal staining
Whole embryos or dissected organs are fixed in 4% paraformaldehyde by immersion at 4 °C. The time of fixation depends on the size of the sample. A general guideline is that a 12.5-day embryo should be fixed for 30 min or a postnatal day 28 brain for 45 min. Alternatively, adult mice can be fixed by perfusion (see below). Note, over fixation inhibits enzyme activity, therefore the length of fixation is critical. However, some fixation is necessary to preserve the tissue for sectioning, thus the right balance must be found. The sample is then rinsed twice in PBS at 4 °C for 10 min. For whole-mount staining, the tissue is incubated in X-gal reaction solution (PBS, 0.1% NP40, 0.05% deoxycholate, 2 m_M_ MgCl2, 5 m_M_ K3Fe(CN)6, 5 mM K4Fe(CN)6, 0.5 mg/ml, 5-bromo-4-chloro-3-indolyl-b-d-galactoside) for 6–48 h at 30 °C. Incubation at 30 °C, rather than at 37 °C can reduce the background staining from endogenous enzyme (Bonnerot and Nicolas, 1993). The reaction is stopped when the staining is the desired level by rinsing the samples twice in PBS for 10 min. Samples can then be preserved in PBS with azide (0.05%), or 1% PFA. For staining of cryosections (10–20 µm), the tissue is immersed in sterile 30% sucrose in PBS at 4 °C overnight, and then briefly immersed in OCT compound (TissueTek) before freezing in cold 2-methylbutane (a beaker containing 2-methylbutane is placed in liquid nitrogen) for 2–4 min. After the tissue blocks are sectioned, the slides are placed in PBS at room temperature for 5 min, rinsed twice in X-gal washing solution (PBS, 0.1% NP40, 0.05% deoxycholate, 2m_M_ MgCl2) for 10 min at 4 °C and incubated at 37 °C (or 30 °C) in X-gal reaction solution for 2–48 h.
2.3.6.2. Protocol for antibody staining
For antibody staining, postnatal animals are fixed by intracardiac perfusion, before dissection of organs, with cold PBS first to flush out the blood and then cold 4% PFA in PBS to achieve efficient fixation. Equal volumes of PBS and 4% PFA are perfused. The volume used for perfusion depends on the size of the mouse. Approximately 10–15 ml is used for neonates and 50–60 ml for adults. After dissection of organs, the samples are postfixed in 4% PFA for at least 2 h at 4 °C and then prepared for cryosectioning as above. For antibody staining of embryos, they can be immersion fixed in 4% PFA in PBS at 4 °C. The length of time the tissue is left in fixation at 4 °C depends on the antibody. Also, some antibodies only detect the antigen in paraffin embedded tissue, or when the tissue is fixed with a different fixative such as alcohol. The best conditions must be determined for each antibody. After sectioning, the cryosections are rinsed in PBS, postfixed in 4% PFA for 5 min at room temperature and rinsed twice in PBS. Two types of antibody staining can be used: fluorescent staining for the reporter protein, which has the advantage of allowing for double staining with a second marker and/or DAPI staining to highlight the nuclei, whereas diaminobenzidine (DAB) stained sections have the advantage that they can be examined under bright field microscopy, therefore avoiding the problem of bleaching and/or dimming of the fluorescent signal, since the staining is permanent. For DAB staining that relies on the peroxidase enzymatic reaction, the endogenous peroxidases must be inhibited by incubating the sections in H2O2 (0.3% in PBS) for 20 min at room temperature, and then rinsing them twice for 10 min in PBS. For fluorescent and DAB staining, the sections are incubated in blocking solution (10% normal serum, 0.25% Triton) for 1 h at room temperature. They are then incubated in primary antibody solution (blocking solution + primary antibody). For example, a rabbit-anti-GFP antibody can be used (Invitrogen A11122, 1:2000 for DAB or fluorescent staining) or a rabbit-anti-β-galactosidase antibody (ICN 55976, 1:20,000 for DAB, 1:1000 for fluorescent staining) depending on the reporter gene present in the reporter allele and incubated overnight at 4 °C. The sections are then rinsed three times in PBS for 10 min at room temperature, and then incubated with the secondary antibody. For fluorescent staining, the secondary antibody is coupled to a fluorophore (Invitrogen) or coupled to biotin (Vector). For DAB staining, the secondary antibody can be directly coupled to the peroxidase (Dakocytomation) or coupled to biotin (Vector). The biotinylated secondary antibody adds an extra step of amplification of the signal. The secondary antibody is incubated for 2 h at room temperature, the concentration of the secondary antibody typically ranges from 1:300 to 1:500. The sections are rinsed three times in PBS. For fluorescent staining using secondary antibodies coupled to biotin, the sections are then incubated with Avidin coupled to a fluorophore for 1 h, and then three times in PBS. Fluorescent slides are coverslipped in Fluoro-Gel (Electron Microscopy Sciences). For DAB staining using a secondary antibody coupled to Biotin, the sections are incubated with Avidin and biotin coupled to peroxidase (Vectastain ABC kit, Vector) for 1 h and then rinsed three times 10 min in PBS. For DAB staining, the sections are then incubated at room temperature with the peroxidase substrate (DAB) for 3–10 min, until the staining is sufficiently strong. The reaction is stopped by rinsing the sections three times in PBS for 10 min at room temperature. DAB is prepared immediately before use (one tablet of 10 mg (Sigma-Aldrich, D5905) in 40 ml of PBS + 10 µl of 30% H2O2). DAB is highly carcinogenous, therefore gloves should be worn when handling DAB, and it must be disposed of according to safety guidelines: for example, DAB waste can be inactivated in permanganate solution (3% KMnO4, 2% Na2CO3 in H2O).
2.4. Special consideration when designing GIFM experiments
2.4.1. Choice of promoters for the inducible SSR and the reporter allele that influence recombination efficiency
Since the pattern of expression driven by a specific promoter and regulatory elements often varies during development, it is necessary to assess the expression and function of the inducible SSR at all the time points of ligand administration (induction). Importantly, the general pattern of expression for a given time point is usually consistent between mice, at least with robust regulatory elements driving expression of the SSR. However, the efficiency of recombination with inducible SSRs can vary between animals, even when the same dose of ligand is administered. Thus, for quantitative studies that attempt to compare the percentage of cells labeled at different time points the variability must be taken into consideration, and if possible, the numbers normalized to an internal standard at each time point. Alternatively, the relative proportions of different types of cells that are marked can be compared.
Moreover, the efficiency of recombination depends on both the SSR line and the reporter line. Not only is the level of expression of the SSR critical but also the accessibility of the DNA containing the SSR sites can differ between reporter alleles, and possibly in different cell types of a mouse or embryo. It is therefore important to assess the efficiency of recombination for each combination of SSR and reporter line, and at each time point analyzed, since the level of SSR and/or the accessibility of the recombination sites can change over time. For example, we find that with the same SSR line, the R26lox-stop-lacZ line (Soriano, 1999) recombines more efficiently than the R26lox-stop-YFP line (Srinivas et al., 2001), and the Taulox-stop-mGFP-IRES-nlacZ reporter (Hippenmeyer et al., 2005) recombines more efficiently than the R26lox-stop-lacZ line, at least in neurons (unpublished observations).
The dose and the number of administrations of ligand can be adjusted, in order to achieve an ideal (or maximal) level of recombination with a minimum of toxicity (see below). With a high dose of ligand, some combinations of alleles have been reported to achieve marking in 80–90% of the cells in the domain where the SSR is expressed (En2-CreER knock-in (Sato and Joyner, 2009; Sgaier et al., 2005); Hoxb6-CreER transgene (Nguyen et al., 2009; Zhu et al., 2008)), whereas other combinations of alleles can achieve as little as 1–5% (our unpublished results). The precise design of the transgene or knock-in allele can dramatically influence the level of SSR expression, and hence the efficiency of cell marking. For example, the presence of neo in a targeted allele can lower expression (Bai and Joyner, 2001; unpublished results), or the type of 3′ UTR sequences and polyadenylation sequence used (Kakoki et al., 2004; unpublished observations) can modify the expression level. Thus, it is important to carefully design the transgene or gene targeting construct for an inducible SSR prior to embarking on a GIFM study.
2.4.2. Ligand administration
The main technical difference between cumulative genetic fate mapping with a constitutive SSR and GIFM using an inducible SSR-fusion protein is that the latter requires ligand administration.
2.4.2.1. Preparation of the ligand
For CreER or FlpER, the ligand is 4-OHT; however, 4-OHT is expensive and very difficult to get into solution. The nonhydroxylated form of tamoxifen is metabolized by the liver into 4-OHT, and is easier to get into solution and much cheaper than 4-OHT. Tamoxifen is therefore used in most in vivo studies in mice. Tamoxifen should be diluted in fresh corn oil. The stock solution is typically 20 mg/ml. Tamoxifen powder (Sigma-Aldrich, T5648-1G) can be first partially dissolved in prewarmed corn oil (e.g., Sigma-Aldrich, C8267-500ML) by vortexing. To dissolve the tamoxifen completely, the solution can then either be sonicated at 60 Hz for approximately 30 min or heated at 37 °C overnight.
For CrePR the ligand is RU-486. It is suspended in a 0.25% carboxymethyl cellulose, 0.5% Tween 80 solution at a concentration of 50 mg/ml (Casper et al., 2007; Takeuchi et al., 2005). Alternatively, other antiprogestins such as Org 31376 or Org 31806 (N. V. Organon Scientific Development Group) can be used and dissolved in fresh sesame oil at a concentration of 6.5 µg/ml (Tsujita et al., 1999).
2.4.2.2. Routes of administration of ligand
Tamoxifen dissolved in corn oil can be administered orally by gavage, or by intraperitoneal (i.p.) or subcutaneous (s.c.) injection. The same range of concentrations is used in all three routes. Before administration, the animal should be weighed to adjust the dose administered. For adult inductions and embryonic inductions, gavage or i.p. injections are the most common routes of injection. In the case of embryonic inductions, the pregnant female receives the tamoxifen. For early postnatal stages (P0–P12), s.c. injections of a volume ranging from 30 to 50 µl or gavage with gavaging needles specific for mouse pups (UNO, the Netherlands) are used. S.c. injection is the easiest and safest way to administer tamoxifen at these early stages since the chance of unintentionally damaging essential organs is less than when using i.p. injection. Gavaging neonates is a delicate operation because of their small size and the fragility of their esophagus. Note, however, that the physical properties of the oil make it leak from the point of s.c. injection and thus the volume must be adjusted accordingly. Restraining the pup from moving by holding it still between two fingers and applying pressure on the point of injection helps for achieving a more accurate and reproducible injected volume. The use of a Hamilton syringes with a fine needle is also recommended.
2.4.2.3. Volumes injected
The volume injected depends on the desired dose and the weight of the animal. For reproducibility, we recommend that volumes of at least 100 µl are used for i.p. injections and gavage. Before administration, the animals should be weighed and the volume to be injected calculated. If the volume is too small, the concentration of the ligand should be adjusted and the volume recalculated accordingly. Up to 500µl can be used to gavage an adult animal and up to 1 ml for an i.p. injection. For s.c. injections in neonates, it is recommended that volumes of approximately 50 µl or less if using a Hamilton syringe are used.
2.4.3. Kinetics of labeling
In GIFM studies, it is important to identify the time window during which the recombination occurs. The kinetics of labeling for GIFM studies consist of several components in addition to the time it takes for (1) the SSR to induce recombination, (2) the reporter gene to subsequently be transcribed and translated, and (3) the reporter protein to accumulate sufficiently to be detected. These components are (4) the time for the ligand to be metabolized, in the case of tamoxifen, and to reach the cells and bind to the receptor part of the SSR-fusion protein, (5) the time for the fusion protein to be translocated into the nucleus, and (6) the time during which tamoxifen is present in the mouse tissue of interest and bound to the inducible SSR (in an active state).
The time between ligand administration and the first appearance of labeled cells has been described in several papers for CreER and FlpeER (Hayashi and McMahon, 2002; Hunter et al., 2005; Nakamura et al., 2006). Using CreER, the first labeled cells are detected approximately 8 h after tamoxifen administration, but there is a delay of about 12 h before recombination reaches a significant level (Hayashi and McMahon, 2002; Nakamura et al., 2006). With FlpeER, the first recombined cells appear around 12 h after tamoxifen administration (Hunter et al., 2005). The time during which tamoxifen is able to induce CreER to be active in cells initiating new expression of the inducible SSR is approximately 24 h (Kimmel et al., 2000; Nakamura et al., 2006) after administration.
All the lengths of time described above are approximate, and can vary depending on the dose of tamoxifen injected, the type of SSR-fusion protein used, and on the level of expression of the SSR. One study detected CreER in the nucleus up to 36 h after tamoxifen administration (Hayashi and McMahon, 2002). The higher the dose, the longer tamoxifen is likely to be present in mouse tissues and able to induce site-specific recombination. When performing embryonic inductions, whether or not the embryo is at a stage when feto-placental circulation has been established might also be a consideration (Nakamura et al., 2006), since drug delivery and clearance of tamoxifen could be different in embryos before and after the establishment of feto-placental circulation. Therefore, the kinetics of recombination might be different depending on the stage that the ligand is administered. The kinetics of labeling also differs depending on the route of administration. Reporter protein expression was found to be more rapid following i.p. injection compared to oral gavage (within 8–12 h compared to 12–18 h) and the peak of activity to occur earlier with i.p. injection (Nguyen et al., 2009).
2.4.4. Toxicity of ligands
The use of high doses of synthetic antagonists of steroids such as RU-486 and tamoxifen can induce toxic effects, especially in pregnant females. We have found a high rate of loss of pregnancy 4–6 days after administration of tamoxifen, and an even greater loss of whole litters around the time of birth. When a high dose of tamoxifen is administered (~125 µg/g of pregnant mouse) between E8.5 and E10.5, we have found in some experiments that as few as 5% of the litters survive past birth (unpublished results). The toxic effect of tamoxifen injection can be partially counteracted by coadministration of progesterone. However, progesterone administration interferes with birth, so it can only be used when embryonic time points are of interest for final analysis. Up to 200 µg of tamoxifen/g of mouse can be injected to P2 animals without an observable toxic effect. In adults, up to five daily repeated injections of 250 µg/g of mouse (our unpublished results), or up to 16 injections of ~145 µg/g of mouse every other day (Balordi and Fishell, 2007) can be tolerated. The genetic background of the mouse influences the severity of the toxicity. When possible, outbred mice should be used. Also, lowering the dose of tamoxifen to ~50 µg/g of mouse can increase survival of litters after birth to 30%. For clonal analysis, a dose of ~1 µg/g of pregnantmouse does not cause toxic effects (unpublished results).
2.4.5. Initial population
Since the expression pattern of the SSR-fusion protein often varies over time, the population of cells that is initially labeled must be determined for each time point of the study. The advantage of GIFM is that the initially marked and defined cells are the only cells that will undergo recombination by the action of the inducible SSR (Fig. 10.1B). To characterize the initial population, the tissue of interest should be collected 24–48 h after the last administration of the ligand. In order to characterize the type of cells within a tissue that are marked in a GIFM study, expression of the reporter gene in the initial population can be compared to cell type specific markers, or spatially restricted markers that provide spatial information. If the goal of the experiment is to fate map cells expressing a particular gene, then the initial population of labeled cells can be compared to the domain of expression of the endogenous gene from which the regulatory sequences were taken to express the inducible SSR. Finally, to test how faithfully the marked cells reflect expression of the inducible SSR, the initial population of labeled cells can be compared to Cre or Flp expression. However, we have found that most commercial antibodies are not effective at detecting Cre, and no Flp antibody is currently available, therefore RNA in situ hybridization analysis of sections or whole-mount embryos/organs must be performed. Comparison of SSR expression, to endogenous gene expression and reporter expression on adjacent sections is the ideal approach.
In some cases, the population of cells expressing the inducible SSR is so dynamic that the timing of active recombination can be after the time during which a cell transcribes the inducible SSR. In such a case, some of the labeled population of cells observed 24–36 h after ligand administration will no longer be expressing the SSR (Machold and Fishell, 2005). Finally, in experiments in which a cell-type-specific promoter is used instead of a ubiquitous promoter to drive expression of the reporter protein, it is important to be aware that the initially marked population might not express the reporter gene. It is therefore necessary to use a ubiquitous promoter to determine the initial population of cells that is labeled.
2.5. Special consideration when designing genetic inducible clonal analysis experiments
In clonal analysis studies, it is crucial to be able to demonstrate that the labeled cells are indeed clonally related and induced by the administration of the ligand. Two essential parameters have to be tested to validate the use of a particular combination of inducible SSR and reporter alleles for clonal analysis: the absence of labeling of cells in the absence of ligand, and a low enough level of recombination in the presence of ligand to ensure that the labeled groups of cells actually arose from a single cell (are clones).
2.5.1. Choice of SSR and reporter lines
Contrary to most genetic fate mapping studies, in clonal analysis, a low efficiency of recombination is an advantage since the labeling must be a rare event. Therefore, lines with low expression levels of an inducible SSR can be used. We and others have found FlpeER to be much less efficient than CreER, thus FlpeER can be very useful for clonal analysis. The first generation CreERT protein (Feil et al., 1996) also induces less recombination that later versions (e.g., CreERT2; Indra et al., 1999), and therefore might be useful for clonal analysis. Another factor that can limit recombination efficiency is the accessibility of the recombination sites in the reporter allele to the SSR. The reporter lines that are less efficiently recombined can therefore be an advantage. Another clonal analysis strategy is to use the interchromosomal reporter alleles (MADM system, see below). In this system, the recombination efficiency is extremely low, allowing for rare labeling events that generate clones (Espinosa and Luo, 2008; Zong et al., 2005). Finally, the use of the Brainbow reporter allele (Livet et al., 2007), and similar next-generation ubiquitous reporters that randomly express multiple different reporter proteins, should be a great advance to clonal analysis.
2.5.2. No recombination in the absence of ligand
The first step in developing a clonal analysis protocol is to test whether site-specific recombination leads to labeling of cells in the absence of ligand with the chosen inducible SSR and reporter lines. Most of the combinations between a CreER line and a reporter line we have tested display some labeled cells in the absence of tamoxifen, that is, in the absence of induction. We term such labeling events as ‘‘noninduced labeling.’’ If the level of noninduced labeling is too high, the allele combination cannot be used for a clonal analysis. To determinate the level of noninduced labeling, a detailed and systematic analysis of reporter expression in the adult organs of interest from several mice that carry both the inducible SSR and reporter alleles must be carried out after administration of oil alone, or in the absence of any treatment. Since the acceptable level of noninduced labeling can be higher for GIFM studies than clonal analysis, it is important to thoroughly test the inducible SSR and reporter allele combination before undertaking a clonal analysis, even with lines that have already been used for GIFM. When using a specific inducible SSR line, it is also important to test the level of noninduced labeling with all the reporter alleles that will be included in the study, since the combination of a given SSR line and different reporter alleles will not necessarily generate the same level of noninduced labeling (unpublished observation). Therefore, the choice of reporter allele is also dictated by the SSR line.
2.5.3. Titration of the dose of ligand administered
The optimal dose of ligand must be determined experimentally for each inducible SSR and reporter allele combination at each time point of ligand administration. This is achieved by decreasing the dose of ligand and observing the level of labeling at the time point the final analysis will be carried out. Serial dilutions of ligand should be prepared and tested. Again, it can be beneficial to use an inducible SSR line that is quite inefficient, or a reporter allele with loxP sites that are more difficult to recombine, or a combination of both that ultimately has an inefficient response to ligand administration. If too low a dose of ligand is needed, the dose injected could be close to the threshold necessary to bind to the nuR-modified binding domain and lead to variable labeling results, or no labeling at one dose and too much labeling at the next higher does (unpublished observations).
The optimal dose of ligand depends on the organ analyzed. If the pool of cells at the time of induction is small, the probability of hitting a single cell in the pool is low; therefore the appropriate dose for clonal labeling is probably higher than for a larger pool of cells. In addition, it depends on the shape of an organ and behavior of the cells (e.g., the degree of cell mixing) as to how spread out each clone must be to ensure each group of cells is a clone. For example, in order to study cell clones during hair follicle regeneration, since there are millions of hair follicles and no mixing between follicles, a dose that labels one in 10 hair follicles is ideal (Legué and Nicolas, 2005). However, there is no way to predict for a particular pair of alleles what the optimal dose will be. It must to be determined experimentally, first grossly and then refined to obtain the most promising dose. A good estimate of doses to first test are 100, 10, and 1 µg/g mouse.
3. Future Applications: Combining Genetic Fate Mapping with Mutant Analysis
Genetic fate mapping allows the fate of cell populations, or individual cells to be followed in wild-type mice. To understand how cell behaviors are regulated by genes, the next step is to follow the fate of mutant cells, either in a whole mutant mice or in a mosaic analysis in which a mutation is introduced into a subset of cells that are also genetically marked.
3.1. Intrachromosomal recombination: Using existing conditional loxP containing alleles
To induce mutant cells by intrachromosomal recombination, conditional mutant alleles made by gene targeting that contain critical exon sequences flanked by two loxP sites (floxed allele) could be used. To date, for such mosaic studies, an SSR line has been combined with a floxed allele (homozygous or over a null mutation at the same locus) and a reporter allele. However, recombination between the two loxP sites in the conditional allele as well as the two loxP sites in the reporter allele does not always occur in the same cell, especially when using inducible SSRs, and by definition in clonal analysis studies. Such an approach therefore leads to the production of three distinct mosaic populations of cells within a mouse: (i) labeled mutant cells, (ii) unlabeled mutant cells, and (iii) labeled wild-type cells. Thus, when using two floxed alleles one cannot assume that all labeled cells are mutant and conversely that all mutant cells are labeled. It is therefore essential to develop new tools to follow the fate of mutant cells created using the many available floxed conditional alleles.
3.2. Interchromosomal recombination: Mosaic analysis with double makers
The MADM system offers a way to circumvent the problem using floxed alleles and a separate marker allele, by ensuring that all mutant cells are labeled (Zong et al., 2005). A limitation, however, is that the mutant (often a null allele) allele of the gene to be studied (gene x) must be on the same chromosome as the MADM reporter alleles. In MADM studies the mutant mouse line for gene x is bred to one of the MADM reporter lines in order to generate a mouse with a chromosome containing the mutant allele of gene x and one MADM reporter line (Fig. 10.4). These mice are then bred to a mouse line that has the complementary MADM allele and a wild-type version of gene x to produce embryos that carry one MADM reporter allele and the mutant allele of gene x on one chromosome and the other MADM reporter allele and a wild-type allele of gene x on the homologous chromosome. After DNA replication in cells, each chromosome will have two copies (one on each chromatid) of its reporter allele and of either the mutant or the wild-type gene x allele. Upon Cre recombination, four chromosomal configurations are generated: the two parental configurations plus two recombined configurations, one with a full-length GFP and on the same chromosome as the mutant allele of gene x and one with a full-length RFP and a wild-type allele of gene x. Depending on the segregation of the chromatids during mitosis, two scenarios are possible. One scenario generates two cells that are labeled, one that expresses GFP and that is homozygous mutant for gene x and one that expresses RFP and is wild-type for gene x. The other scenario generates one cell that expresses both GFP and RFP, and a cell that is not labeled. Each cell in this scenario is heterozygous for gene x. By studying the products of recombination events that generate complementary GFP and RFP clones (mutant and wild type), the behaviors of mutant cells in an otherwise wild-type tissue can be analyzed. The approach also provides a direct comparison of cell behaviors between wild-type and mutant cells that are generated and labeled concomitantly. Two limiting factors of this system are that (1) cell division is needed to generate the recombination, and thus labeling and mutagenesis only can occur in dividing cells expressing active Cre, and (2) the mutated gene of interest must be on the same chromosome that carries the markers.
REFERENCES
- Abremski K, et al. Bacteriophage P1 Cre-loxP site-specific recombination. Site-specific DNA topoisomerase activity of the Cre recombination protein. J. Biol. Chem. 1986;261:391–396. [PubMed] [Google Scholar]
- Akagi K, et al. Cre-mediated somatic site-specific recombination in mice. Nucleic Acids Res. 1997;25:1766–1773. doi: 10.1093/nar/25.9.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews BJ, et al. The FLP recombinase of the 2 micron circle DNA of yeast: Interaction with its target sequences. Cell. 1985;40:795–803. doi: 10.1016/0092-8674(85)90339-3. [DOI] [PubMed] [Google Scholar]
- Austin S, et al. A novel role for site-specific recombination in maintenance of bacterial replicons. Cell. 1981;25:729–736. doi: 10.1016/0092-8674(81)90180-x. [DOI] [PubMed] [Google Scholar]
- Awatramani R, et al. Cryptic boundaries in roof plate and choroid plexus identified by intersectional gene activation. Nat. Genet. 2003;35:70–75. doi: 10.1038/ng1228. [DOI] [PubMed] [Google Scholar]
- Bai CB, Joyner AL. Gli1 can rescue the in vivo function of Gli2. Development. 2001;128:5161–5172. doi: 10.1242/dev.128.24.5161. [DOI] [PubMed] [Google Scholar]
- Balordi F, Fishell G. Mosaic removal of hedgehog signaling in the adult SVZ reveals that the residual wild-type stem cells have a limited capacity for self-renewal. J. Neurosci. 2007;27:14248–14259. doi: 10.1523/JNEUROSCI.4531-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaess S, et al. Sonic hedgehog regulates Gli activator and repressor functions with spatial and temporal precision in the mid/hindbrain region. Development. 2006;133:1799–1809. doi: 10.1242/dev.02339. [DOI] [PubMed] [Google Scholar]
- Blaess S, et al. Gli3 coordinates three-dimensional patterning and growth of the tectum and cerebellum by integrating Shh and Fgf8 signaling. Development. 2008;135:2093–2103. doi: 10.1242/dev.015990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnerot C, Nicolas JF. Application of LacZ gene fusions to postimplantation development. Methods Enzymol. 1993;225:451–469. doi: 10.1016/0076-6879(93)25031-v. [DOI] [PubMed] [Google Scholar]
- Buchholz F, et al. Different thermostabilities of FLP and Cre recombinases: Implications for applied site-specific recombination. Nucleic Acids Res. 1996;24:4256–4262. doi: 10.1093/nar/24.21.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz F, et al. Improved properties of FLP recombinase evolved by cycling mutagenesis. Nat. Biotechnol. 1998;16:657–662. doi: 10.1038/nbt0798-657. [DOI] [PubMed] [Google Scholar]
- Casper KB, et al. Characterization of astrocyte-specific conditional knockouts. Genesis. 2007;45:292–299. doi: 10.1002/dvg.20287. [DOI] [PubMed] [Google Scholar]
- Chudakov DM, et al. Fluorescent proteins as a toolkit for in vivo imaging. Trends Biotechnol. 2005;23:605–613. doi: 10.1016/j.tibtech.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Corrales JD, et al. The level of sonic hedgehog signaling regulates the complexity of cerebellar foliation. Development. 2006;133:1811–1821. doi: 10.1242/dev.02351. [DOI] [PubMed] [Google Scholar]
- Dymecki SM, Kim JC. Molecular neuroanatomy’s “Three Gs”: A primer. Neuron. 2007;54:17–34. doi: 10.1016/j.neuron.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa JS, Luo L. Timing neurogenesis and differentiation: Insights from quantitative clonal analyses of cerebellar granule cells. J. Neurosci. 2008;28:2301–2312. doi: 10.1523/JNEUROSCI.5157-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley FW, et al. Widespread recombinase expression using FLPeR (flipper) mice. Genesis. 2000;28:106–110. [PubMed] [Google Scholar]
- Feil R, et al. Ligand-activated site-specific recombination in mice. Proc. Natl. Acad. Sci. USA. 1996;93:10887–10890. doi: 10.1073/pnas.93.20.10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil R, et al. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem. Biophys. Res. Commun. 1997;237:752–757. doi: 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- Fields-Berry SC, et al. A recombinant retrovirus encoding alkaline phosphatase confirms clonal boundary assignment in lineage analysis of murine retina. Proc. Natl. Acad. Sci. USA. 1992;89:693–697. doi: 10.1073/pnas.89.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forni PE, et al. High levels of Cre expression in neuronal progenitors cause defects in brain development leading to microencephaly and hydrocephaly. J. Neurosci. 2006;26:9593–9602. doi: 10.1523/JNEUROSCI.2815-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden JA, et al. Construction and characterization of a highly complex retroviral library for lineage analysis. Proc. Natl. Acad. Sci. USA. 1995;92:5704–5708. doi: 10.1073/pnas.92.12.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: A tool for temporally regulated gene activation/inactivation in the mouse. Dev. Biol. 2002;244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- Hippenmeyer S, et al. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoess RH, et al. P1 site-specific recombination: Nucleotide sequence of the recombining sites. Proc. Natl. Acad. Sci. USA. 1982;79:3398–3402. doi: 10.1073/pnas.79.11.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter NL, et al. Ligand-activated Flpe for temporally regulated gene modifications. Genesis. 2005;41:99–109. doi: 10.1002/gene.20101. [DOI] [PubMed] [Google Scholar]
- Indra AK, et al. Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: Comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucleic Acids Res. 1999;27:4324–4327. doi: 10.1093/nar/27.22.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner AL, Zervas M. Genetic inducible fate mapping in mouse: Establishing genetic lineages and defining genetic neuroanatomy in the nervous system. Dev. Dyn. 2006;235:2376–2385. doi: 10.1002/dvdy.20884. [DOI] [PubMed] [Google Scholar]
- Kakoki M, et al. Altering the expression in mice of genes by modifying their 3′ regions. Dev. Cell. 2004;6:597–606. doi: 10.1016/s1534-5807(04)00094-2. [DOI] [PubMed] [Google Scholar]
- Kimmel RA, et al. Two lineage boundaries coordinate vertebrate apical ectodermal ridge formation. Genes Dev. 2000;14:1377–1389. [PMC free article] [PubMed] [Google Scholar]
- Kolb AF. Selection-marker-free modification of the murine beta-casein gene using a lox2272 [correction of lox2722] site. Anal. Biochem. 2001;290:260–271. doi: 10.1006/abio.2000.4984. [DOI] [PubMed] [Google Scholar]
- Lallemand Y, et al. Maternally expressed PGK-Cre transgene as a tool for early and uniform activation of the Cre site-specific recombinase. Transgenic Res. 1998;7:105–112. doi: 10.1023/a:1008868325009. [DOI] [PubMed] [Google Scholar]
- Laux T, et al. GAP43, MARCKS, and CAP23 modulate PI(4, 5)P(2) at plasma-lemmal rafts, and regulate cell cortex actin dynamics through a common mechanism. J. Cell Biol. 2000;149:1455–1472. doi: 10.1083/jcb.149.7.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson KA. Fate mapping the mouse embryo. Int. J. Dev. Biol. 1999;43:773–775. [PubMed] [Google Scholar]
- Legué E, Nicolas JF. Hair follicle renewal: Organization of stem cells in the matrix and the role of stereotyped lineages and behaviors. Development. 2005;132:4143–4154. doi: 10.1242/dev.01975. [DOI] [PubMed] [Google Scholar]
- Livet J, et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature. 2007;450:56–62. doi: 10.1038/nature06293. [DOI] [PubMed] [Google Scholar]
- Lobe CG, et al. Z/AP, a double reporter for cre-mediated recombination. Dev. Biol. 1999;208:281–292. doi: 10.1006/dbio.1999.9209. [DOI] [PubMed] [Google Scholar]
- Loonstra A, et al. Growth inhibition and DNA damage induced by Cre recombinase in mammalian cells. Proc. Natl. Acad. Sci. USA. 2001;98:9209–9214. doi: 10.1073/pnas.161269798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luche H, et al. Faithful activation of an extra-bright red fluorescent protein in “knock-in” Cre-reporter mice ideally suited for lineage tracing studies. Eur. J. Immunol. 2007;37:43–53. doi: 10.1002/eji.200636745. [DOI] [PubMed] [Google Scholar]
- Machold R, Fishell G. Math1 is expressed in temporally discrete pools of cerebellar rhombic-lip neural progenitors. Neuron. 2005;48:17–24. doi: 10.1016/j.neuron.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Metzger D, Chambon P. Site- and time-specific gene targeting in the mouse. Methods. 2001;24:71–80. doi: 10.1006/meth.2001.1159. [DOI] [PubMed] [Google Scholar]
- Miyazaki J, et al. Expression vector system based on the chicken beta-actin promoter directs efficient production of interleukin-5. Gene. 1989;79:269–277. doi: 10.1016/0378-1119(89)90209-6. [DOI] [PubMed] [Google Scholar]
- Naiche LA, Papaioannou VE. Cre activity causes widespread apoptosis and lethal anemia during embryonic development. Genesis. 2007;45:768–775. doi: 10.1002/dvg.20353. [DOI] [PubMed] [Google Scholar]
- Nakamura E, et al. Kinetics of tamoxifen-regulated Cre activity in mice using a cartilage-specific CreERT) to assay temporal activity windows along the proximodistal limb skeleton. Dev. Dyn. 2006;235:2603–2612. doi: 10.1002/dvdy.20892. [DOI] [PubMed] [Google Scholar]
- Nguyen MT, et al. Tamoxifen-dependent, inducible Hoxb6CreERT recombinase function in lateral plate and limb mesoderm, CNS isthmic organizer, posterior trunk neural crest, hindgut, and tailbud. Dev. Dyn. 2009;238:467–474. doi: 10.1002/dvdy.21846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H, et al. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Novak A, et al. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- Pakhomov AA, Martynov VI. GFP family: Structural insights into spectral tuning. Chem. Biol. 2008;15:755–764. doi: 10.1016/j.chembiol.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Petit AC, Legué E, Nicolas JF. Methods in clonal analysis and applications. Reprod. Nutr. Dev. 2005;45:321–339. doi: 10.1051/rnd:2005024. [DOI] [PubMed] [Google Scholar]
- Raymond CS, Soriano P. High-efficiency FLP and PhiC31 site-specific recombination in mammalian cells. PLoS One. 2007;2:e162. doi: 10.1371/journal.pone.0000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, et al. Use of a recombinant retrovirus to study post-implantation cell lineage in mouse embryos. EMBO J. 1986;5:3133–3142. doi: 10.1002/j.1460-2075.1986.tb04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Joyner AL. The duration of Fgf8 isthmic organizer expression is key to patterning different tectal-isthmo-cerebellum structures. Development. 2009;136:3617–3626. doi: 10.1242/dev.041210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer B. Manipulation of transgenes by site-specific recombination: Use of Cre recombinase. Methods Enzymol. 1993;225:890–900. doi: 10.1016/0076-6879(93)25056-8. [DOI] [PubMed] [Google Scholar]
- Schaft J, et al. Efficient FLP recombination in mouse ES cells and oocytes. Genesis. 2001;31:6–10. doi: 10.1002/gene.1076. [DOI] [PubMed] [Google Scholar]
- Sgaier SK, et al. Morphogenetic and cellular movements that shape the mouse cerebellum; insights from genetic fate mapping. Neuron. 2005;45:27–40. doi: 10.1016/j.neuron.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebayashi H, et al. Tamoxifen modulates apoptosis in multiple modes of action in CreER mice. Genesis. 2008;46:775–781. doi: 10.1002/dvg.20461. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, et al. Control of synaptic connection by glutamate receptor delta2 in the adult cerebellum. J. Neurosci. 2005;25:2146–2156. doi: 10.1523/JNEUROSCI.4740-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronche F, et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat. Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Tsujita M, et al. Cerebellar granule cell-specific and inducible expression of Cre recombinase in the mouse. J. Neurosci. 1999;19:10318–10323. doi: 10.1523/JNEUROSCI.19-23-10318.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegeto E, et al. The mechanism of RU486 antagonism is dependent on the conformation of the carboxy-terminal tail of the human progesterone receptor. Cell. 1992;69:703–713. doi: 10.1016/0092-8674(92)90234-4. [DOI] [PubMed] [Google Scholar]
- Wu Y. High-efficient FLPo deleter mice in C57BL/6J background. PLoS One. 2009;4:e8054. doi: 10.1371/journal.pone.0008054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrowicz BP, et al. Disruption of overlapping transcripts in the ROSA beta geo 26 gene trap strain leads to widespread expression of beta-galactosidase in mouse embryos and hematopoietic cells. Proc. Natl. Acad. Sci. USA. 1997;94:3789–3794. doi: 10.1073/pnas.94.8.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J. Uncoupling Sonic hedgehog control of pattern and expansion of the developing limb bud. Dev. Cell. 2008;14:624–632. doi: 10.1016/j.devcel.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong H, et al. Mosaic analysis with double markers in mice. Cell. 2005;121:479–492. doi: 10.1016/j.cell.2005.02.012. [DOI] [PubMed] [Google Scholar]