Biological underpinnings of breastfeeding challenges: the role of genetics, diet, and environment on lactation physiology (original) (raw)
Abstract
Lactation is a dynamic process that has evolved to produce a complex biological fluid that provides nutritive and nonnutritive factors to the nursing offspring. It has long been assumed that once lactation is successfully initiated, the primary factor regulating milk production is infant demand. Thus, most interventions have focused on improving breastfeeding education and early lactation support. However, in addition to infant demand, increasing evidence from studies conducted in experimental animal models, production animals, and breastfeeding women suggests that a diverse array of maternal factors may also affect milk production and composition. In this review, we provide an overview of our current understanding of the role of maternal genetics and modifiable factors, such as diet and environmental exposures, on reproductive endocrinology, lactation physiology, and the ability to successfully produce milk. To identify factors that may affect lactation in women, we highlight some information gleaned from studies in experimental animal models and production animals. Finally, we highlight the gaps in current knowledge and provide commentary on future research opportunities aimed at improving lactation outcomes in breastfeeding women to improve the health of mothers and their infants.
Keywords: lactation, mammary gland, genetics, diet, environment
breast milk is a complex biological fluid that provides for optimal infant growth and development, but only if lactation functions properly to produce an adequate volume of milk that contains optimal amounts of nutritive and nonnutritive factors. Successful lactation requires extensive breast tissue expansion and differentiation during pregnancy, followed by the ability to produce sufficient amounts of milk after birth. Although ∼75% of new mothers intend to breastfeed, not all women are able to breastfeed their infants exclusively for the first 6 mo of life, as recommended by the American Academy of Pediatrics and the World Health Organization. In fact, it has been estimated that the prevalence of women who overtly fail to produce enough milk may be as high as ∼10–15% (15, 64, 206) and can quickly lead to hypernatremia (28, 231, 293, 311), nutritional deficiencies (16, 139, 287), or failure to thrive (141, 164, 207). Moreover, the prevalence of lactation “insufficiency” may be much higher, as ∼40–50% of women in the US (1, 162) and 60–90% of women internationally (76, 99, 113, 135, 266) cite “not producing enough milk” or “baby not satisfied with breast milk” as the primary reasons for weaning prior to 6 mo. The etiology of lactation insufficiency is clearly multifactorial and complex. Breast hypoplasia or other abnormal breast conditions and previous breast surgeries (206) are certainly factors that contribute to lactation insufficiency. In addition, numerous social, psychological, and behavioral factors associated with early breastfeeding cessation have been described, and the reader is referred to excellent reviews on these topics (81, 90, 192, 280). What is much less appreciated and poorly understood is the role that maternal genetics and modifiable factors such as energy balance, diet, and environmental exposures may have on reproductive endocrinology, lactation physiology, and the ability to successfully breastfeed. The major unsolved questions regarding these maternal factors have been outlined in a recent review (209). In this review, we provide a broad overview on our current understanding of the molecular etiology behind these factors that play a critical role in lactation physiology and the ability to optimally nourish the nursing infant. Because studies in breastfeeding women are profoundly lacking, some aspects and comparisons will be drawn from experimental animal models and studies in production animals where relevant; however, it is important to note that lactation physiology between humans and other mammals can vary, and where appropriate these differences will be discussed in context.
Mammary Gland Biology and the Production of Milk
The mammary gland is a highly complex exocrine gland that functions to produce, secrete, and deliver milk to the infant. Mammary glands are unique in that most development occurs postnatal. From birth to puberty, the rudimentary branches elongate through the mammary fat pad and form terminal end buds in preparation for functional activation during pregnancy and lactation. Successful lactation requires coordination of mechanisms responsible for nutrient transport, milk production, and secretion from the mammary gland and is driven by molecular, biochemical, and cellular events that are regulated largely by reproductive hormones, which has been described thoroughly by Anderson et al. (7). During early pregnancy, primary hormones, including estrogen, progesterone, prolactin, and placental lactogen, induce the physiological transition of the mammary gland from a nonsecreting branched tissue into a highly active secreting organ comprised of a vast network of ducts and alveoli that are grouped into seven to 10 lobes in humans (243). Following this morphological change, the alveolar cells increase the expression of lactogenic genes, which differentiates them into secretory mammary epithelial cells (MECs) or lactocytes, and this process is termed secretory differentiation (225). In response to progesterone and estrogen withdrawal, concomitant with prolactin release following parturition, the differentiated epithelium gains a remarkable capacity to finely coordinate the synthesis and transport of various milk constituents for the onset of milk secretion, termed secretory activation (225), which usually occurs after full-term birth in humans (210). During this time, tight junctions between lactocytes close and limit the passage of ions (e.g., sodium and chloride) and small molecules, which is important for the establishment of lactation and motivating milk secretion (147, 272). Milk ejection is regulated by oxytocin, which acts on myoepithelial cells to generate contractile force required for milk ejection during lactation (103). In contrast, milk secretion from lactocytes is regulated by a complex hormonal milieu that includes numerous reproductive hormones (e.g., prolactin) and metabolic hormones [e.g., glucocorticoids, insulin, insulin-like growth factor 1 (IGF-I), growth hormone, and thyroid hormone]. These hormones can act directly on the lactocyte or indirectly by altering endocrine response and nutrient delivery to the mammary gland for milk production (34). The main stimulus for the initiation of lactation is infant suckling and milk removal, which activates the milk ejection reflex through the release of oxytocin from the posterior pituitary gland, leading to the contraction of the myoepithelial cells around the alveoli and ducts, ultimately forcing milk into the alveolar lumen and out the nipple, providing milk to the developing infant (225). Once lactation has been established, prolactin secretion from the pituitary gland decreases, but milk removal from the mammary gland promotes continuous milk production or galactopoesis (5, 19, 86, 138, 221). However, as discussed below, both genetic and modifiable factors may play important roles in the ability of the breast to respond to infant demand and provide optimal nutrition for growth and development.
Breast milk is considered the gold standard for infant feeding, as it provides a perfect combination of nutrient and nonnutritive factors that are essential for infant growth and development. Breast milk contains a diverse array of proteins, lipids, and carbohydrates that provide energy, bioactive proteins (e.g., bile salt stimulated lipase, lactoferrin, lysozyme, secretory IgA, and α-lactalbumin), and both group I (vitamins A, E, B1, B2, B6, B12, and D as well as choline, selenium, and iodine) and group II (calcium, iron, copper, zinc, and folate) micronutrients (6). In addition, breast milk contains human milk oligosaccharides (reviewed in Ref. 269) and numerous growth factors, hormones, chemokines, and cytokines (reviewed in Ref. 259) as well as DNA, RNA, microRNAs, white blood cells, and live bacteria (reviewed in Ref. 153) and stem cells (reviewed in Ref. 111). Breast milk composition changes over the course of lactation. In women, secretory activation occurs after parturition (30–40 h after birth), and the first fluid produced is colostrum (225). Only a small volume (∼30 ml/24 h) of colostrum is available for the first few days, but contains high concentrations of immunological components such as secretory IgA, lactoferrin, and leukocytes as well as developmental factors such as epidermal growth factor (49, 147, 225). The concentration of lactose, the most osmotically active component in breast milk, is low in colostrum, thus explaining the low volume of colostrum that is secreted and also indicating that the primary function of colostrum is immunological rather than nutritional (17). Compared with mature milk, colostrum contains higher levels of sodium, chloride, and magnesium and lower levels of potassium and calcium (17, 147, 225). As the mammary gland transitions from pregnancy to lactation, tight junctions between MECs close, and consequently the sodium/potassium ratio declines and the lactose concentration increases (147). This reflects secretory activation and the synthesis of transitional milk, which marks the period of copious milk production and the secretion of nutritive and nonnutritive factors to support nutritional and developmental needs of the growing infant. After the first month, breast milk is considered fully mature and contains ∼0.9–1.2 g protein/dl, 3.2–3.6 g fat/dl, and 6.7–7.8 g lactose/dl (17). Although there is a dramatic shift in composition during the first month, the concentration of macronutrients in breast milk remains relatively constant over the course of lactation, with gradual increases in milk fat content over the course of one feeding (17). However, it is important to note that milk composition, as well as mammary gland number, location, and architecture, is different between humans and animals, which is a limitation to extrapolating information on human lactation from animal studies (2). For example, cattle have a total of four inguinal mammary glands producing milk that is higher in protein compared with humans, and mice have four thoracic, two abdominal, and four inguinal mammary glands producing milk that is higher in protein, fat, and energy compared with humans (2, 128).
As a result of this complex mixture of nutritive and nonnutritive factors, numerous studies have shown that breastfed infants have a lower risk for adverse health consequences, including gastroenteritis, pneumonia, childhood obesity, leukemia, and sudden infant death syndrome (124). Therefore, the American Academy of Pediatrics recommends exclusive breastfeeding for the first 6 mo of life. Although breastfeeding initiation rates have increased to ∼75% in the US, the duration of exclusive breastfeeding continues to fall below national goals; only 47% of infants are breastfed at all by 6 mo of age, and of those, only 14% are exclusively breastfed as recommended (89). For the most part, it is assumed that once lactation is initiated, a breastfeeding woman can produce enough breast milk that meets the nutritional needs of her infant, unless she is severely malnourished. Yet, as noted above, ∼50% of mothers cite concerns about insufficient milk volume or poor milk quality as motivation to wean their infants early. Addressing this issue of lactation insufficiency is hampered by a lack of reliable tools to both accurately measure milk volume and diagnose mothers with molecular defects that impair lactation physiology and performance. Many studies use the validated approach of maternal report of breast fullness to assess the onset of secretory activation (52, 176, 255), but this approach may be somewhat imprecise due to variability in perception and the dependency upon neonate sucking and milk removal for robust secretory activation. The most widely accepted method for measuring milk production is test-weighing, in which the infant is weighed before and after each feeding using an electronic scale (71, 183). However, this approach can be cumbersome, requires a sensitive scale, and also depends upon neonate suckling and milk removal. An alternative is to identify factors in or missing from milk to assess lactation performance. Studies using preterm milk suggest that factors such as elevated sodium, citrate, and lactoferrin (63, 253) may predict suboptimal lactation performance (i.e., delayed onset of secretory activation, suboptimal milk production). However, much research is needed to identify and validate factors in milk that may eventually be useful in assessing lactation performance in breastfeeding women.
Increasing evidence supports a role for maternal factors such as genetic variation, diet composition, and environmental exposures in modulating lactation performance, milk volume, and composition. Identifying these factors and understanding their molecular and physiological role in lactation performance are important in successfully addressing the biological underpinnings of breastfeeding challenges, which has a substantial impact on human health and disease.
Lactation Endocrinology, Physiology, and Potential Genetic Modifiers
Prolactin-Jak2/STAT5 signaling.
As noted above, prolactin is the principal lactogenic hormone that regulates mammary gland differentiation, milk production, and active secretory mechanisms during lactation (116). When secreted into circulation, prolactin binds to its cognate prolactin receptor (PRLR). Upon prolactin binding, the nonreceptor tyrosine kinase Jak2 is activated and phosphorylates the transcription factor STAT5 to allow dimerization and translocation into the nucleus, where it binds to the promoters of prolactin-responsive genes and mediates mammary gland differentiation and milk synthesis. The critical importance of this pathway is illustrated in studies in which prolactin, PRLR, Jak2, or STAT5 genes are deleted in mice, leading to severe defects in mammary differentiation, lactogenesis, and lactation outcomes (53, 66, 118, 220). Moreover, disruption of stimulatory and inhibitory molecules that are directly involved in the prolactin signaling pathway [e.g., the tyrosine kinase ErbB4, negative regulators SOCS2 and SOCS3 (109, 120), transcription factors Elf5 (120), Miz1 (258), and SnoN (126), cytosolic protein-tyrosine phosphatases Shp2 and PTP1B (8), and NHERF1/EBP50, which forms a multimeric complex with PRLR (200)] all compromise differentiation and lactogenesis or lead to suboptimal lactation outcomes in experimental animal models. The complexity of this key lactogenic pathway provides numerous opportunities for (de)activation resulting from genetic variation in the genes that encode these molecules in humans.
Surprisingly, little is currently known about the genes that govern lactation physiology in humans. Recent studies using transcriptomic analysis of isolated milk fat globules (199) and milk fat (159) have begun to reveal changes in the patterns of gene expression that occur during secretory activation and across the course of lactation, respectively. In addition, Daniels et al. (70), Lemay et al. (160), and Rijnkels et al. (250) have shown that epigenetic regulation is important in lactation physiology, using the mouse as a model. Further transcriptomic and epigenetic studies will be critical to identify and understand the regulation of genes that are vital to human lactation. Studies in production animals suggest that genetic variation may also play an important role in modifying lactation success in humans. The role of genetics as a key modifier of milk production in the dairy industry has been recognized for many years (93), as the execution of large genome-wide association studies (GWAS) and linkage studies has been driven by the economics of the dairy industry (59, 112, 182, 246). Over the years, genomic evaluations of dairy cattle have discovered more than 43,000 single nucleotide polymorphisms (SNPs), which have profoundly improved breeding decisions and milk production traits (51) and have led to the development of SNP genotyping chips to predict lactation efficiency (302). Several different approaches can be used to identify genes involved in lactation physiology in women. One approach is to conduct GWAS and select the most abundant or significantly different genes between two different phenotypes (e.g., low-volume producers vs. high-volume producers) and then determine the functional consequences on mammary gland biology and lactation performance (60). Another approach is to use targeted exome sequencing to select genes that are involved in specific biological processes and pathways that are important for mammary development and/or milk production and determine whether variation in these genes is associated with milk production or composition (246). A third approach is to use quantitative trait locus analysis to identify polymorphisms at a specific chromosomal locus (e.g., chromosome 22) that is correlated with phenotype variation (e.g., milk fatty acid composition) (88).
Using a variety of these approaches, recent advancements have identified numerous genetic variants associated with milk production traits in production animals. A recent study using GWAS data collected from 16,812 Holstein and Jersey dairy cattle identified SNPs in key pathways that are critical for mammary gland development, prolactin signaling, and involution that explained variation in milk production and milk composition (246). For example, ∼50% of SNPs found in genes critical for prolactin signaling, including SOCS2, STAT3, STAT5A, STAT5B, PRLR, and β-casein, were associated with three or more milk production traits (246). Additionally, a large number of SNPs in genes that are critical for mammary gland involution, including ATF4, IGFBP4, IRF1, LIFR, OSMR, PTK2, and STAT3, were also associated with milk production traits (246). It is important to note that there are limited data in production animals that actually address the effect of genetic variation on mammary gland function or lactation physiology; these data have been used strictly for genetic association studies and breeding for production traits, and therefore, further research is required to better understand the physiological implications.
We propose that similar variation may govern lactation physiology, milk production, and composition in breastfeeding women; however, to date, few studies have explored this possibility. As proof of concept, several mutations in prolactin and PRLR have been identified in humans (155, 215). In addition, several studies have found that SNPs in PRLR are associated with elevated serum prolactin levels (rs62355518, rs10941235, rs1610218, rs34024951, and rs9292575) and increased risk for breast cancer (rs249537, rs7718468 and rs13436213) and gestational diabetes (rs10068521 and rs9292578). Collectively, this suggests that SNPs in PRLR may affect breast function; however, to our knowledge, the effects of SNPs in PRLR on lactation outcomes have not been explored. Recently, we identified a woman with a “gain-of-function” variant in PRLR (I170L, also known as I146L) who had elevated milk zinc concentration (2.82 mg/l; normal = ∼1 mg/l) (4). Subsequent analysis of her milk composition determined that the ratio of sodium to potassium was also elevated (∼0.9; Kelleher SL, unpublished data), also suggesting subclinical breast dysfunction (263). Moreover, a “loss-of-function” variant in PRLR (H188R) results in secondary hyperprolactinemia and is associated with persistent galactorrhea (212). Genetic variation in downstream molecules that elicit prolactin signaling may also be important candidates. Gain-of-function mutations in Jak2 (V617F) and STAT5B have been identified in humans (18, 239). Constitutive activation of Jak2 through expression of V617F in mice elevates STAT5 signaling and promotes alveologenesis, although lactation outcomes in women expressing V617F have not been investigated. Clearly, a profound lack of understanding exists regarding the consequences of genetic variation in the prolactin-Jak2/STAT5 signaling pathway on lactation outcomes. Collectively, these observations suggest that alterations in prolactin signaling pathways may affect breast function and lactation outcomes and warrant further investigation. The fact that recombinant prolactin has successfully been used to increase milk volume and lactose, calcium, and oligosaccharide concentrations in some women, but with limited success in others (235), suggests that pharmacogenomics targeted at this pathway may be an approach to improve lactation outcomes in some women in the future.
Other cell signaling pathways.
Apart from prolactin signaling, the mammary gland possesses a network of signaling pathways that detect metabolic status, regulate gene transcription and milk protein synthesis, and maintain many cellular processes during lactation. One such pathway is the mammalian target of rapamycin (mTOR) pathway, which has a well-established role in regulating protein synthesis by phosphorylating eukarytoric translation factor 4E binding protein 1 (4E-BP1), thereby releasing eukaryotic translation initiation factor 4E (eiF4E) to regulate translation (296). The mTOR pathway is a well-known therapeutic target for breast cancer (154), and emerging studies also illustrate the importance of mTOR in modulating lactation outcomes. Inhibition of mTOR signaling using RAD001 during lactation markedly impairs lactation (87). Moreover, a role for mTOR signaling in milk protein synthesis has been demonstrated recently (10, 11, 38, 39, 294, 313), as synthesis of proteins like β-lactoglobulin in bovine mammary glands (201) or α-s1 casein in bovine mammary cells (309) is upregulated through the phosphorylation of 4E-BP1. The mTOR pathway has also been shown to be the missing link between factors involved in milk synthesis and MEC proliferation, such as glycogen synthase kinase 3 (GSK-3) and leucyl-tRNA synthetase (LeuRS) (294, 313). In contrast, AMP-activated protein kinase (AMPK) activation by energy depletion in bovine MECs inhibits mTOR signaling and decreases milk protein synthesis rate (39). Numerous mutations in genes encoding mTOR components have been identified, and the reader is referred to an excellent review by Saxena and Sampson (260) on this topic. Another potential target is the peroxisome proliferator-activated receptor-γ (PPARγ) pathway, which is critical for the maintenance of the secreting mammary epithelium (9) and milk quality by suppressing the production of inflammatory lipids (292). Moreover, PPARγ regulates triglycerol synthesis and secretion in cultured breast cells (284) and in dairy goats (265). Although SNPs in PPARγ have been identified, are associated with enhanced breast density, and are modified by diet (157), no clear relationship with lactation outcomes (226) has yet been identified. Going forward, it will be important to differentiate between problems resulting from inadequate mammary gland development during puberty or inadequate secretory development in pregnancy from those that impact secretory activation or secretion of milk components.
Other hormones and endocrine factors/inhibitors.
In addition to prolactin, a complex combination of hormones works together to maintain the differentiated epithelium and milk secretion during lactation, including insulin, glucocorticoids, growth hormone, oxytocin, and thyroid hormone. Secretory activation and milk ejection require insulin and glucocorticoids to synergistically regulate the formation of tight junctions in the mammary gland (223, 312), stimulate mammary differentiation (48), and induce milk protein expression (73). Insulin levels rapidly decrease during early lactation and steadily increase over time (301). Recent work shows that insulin in the presence of prolactin and hydrocortisone plays a pivotal role in regulating milk protein synthesis in mammary explants from animals by increasing the expression of transcription factors Elf5 and STAT5 (193, 194). Studies in dairy cows show conflicting effects of exogenous insulin on milk fat levels, where long-lasting insulin treatment tends to increase milk fat content and milk yield (305), whereas hyperglycemic insulin clamp reduces milk fat and milk yield (61, 173). Similarly, women with gestational diabetes who are on insulin therapy have a delay in the onset of secretory activation (179), which suggests that insulin treatment may have adverse effects on milk production or composition in humans.
In contrast, growth hormone deficiency compromises lactation outcomes in rats, as it reduces milk yield by ∼24% (83). Moreover, artificial growth hormone or recombinant bovine somatotropin administration to ewes (56, 256) and dairy cows (91, 188, 230) increases milk protein yield and milk volume, and growth hormone has been used as a pharmacological agent to augment milk production in women (100, 198). It is speculated that this occurs through the activation of cell proliferation (174) and through increased STAT5 expression (33). Genetic variation in growth hormone receptors has recently been associated with milk production traits in dairy cattle (51, 240), sheep (175), and water buffalo (264). Although numerous SNPs in growth hormone, its cognate receptor, placental lactogen, and placental growth hormone have been identified in humans, to our knowledge, genetic variation in this pathway has yet to be explored as a modulator of lactation outcomes in women (3).
Several studies have shown that SNPs in the oxytocin gene are associated with shorter breastfeeding duration, which may be due to impaired myoepithelial contraction and milk ejection (131, 233). Finally, thyroid hormones are also galactopoietic and help to establish the metabolic priority of the mammary gland during lactation (43). Hypothyroidism has deleterious effects on milk composition (108) and milk synthesis and ejection in rats (107). However, there is little information on the effects of hypo- or hyperthyroidism on lactation outcomes in women.
In contrast to the factors that promote lactation, feedback inhibitor of lactation (FIL) is a polypeptide that controls lactation by reversibly blocking milk synthesis and secretion when the breast is full (229, 249, 303). Specifically, FIL is endogenously synthesized in lactocytes and secreted into the milk as whey protein, which allows for local regulation of milk secretion through an autocrine feedback inhibition (229, 303), and is critical for matching milk supply to infant demand (304). However, there is very little understanding of the precise mechanisms through which FIL may exert its actions. Finally, serotonin has been proposed to be an additional feedback inhibitor of lactation that reduces milk synthesis and milk yield (117). However, recent studies demonstrate that serotonin is also critical for numerous cellular pathways and biological processes regulating lactation (151). Further studies are needed to understand how factors secreted into milk assist in regulating milk volume and the onset of mammary gland remodeling during involution.
Milk Composition and Potential Genetic Modifiers
The discovery of variants in specific milk proteins dates back to 1955 by Aschaffenburg and Drewry (12), in which two variants of β-lactoglobulin were detected in bovine milk. In this section, we will review our current understanding the role of genetic variation of milk components on milk composition. Many studies have identified genetic variation in the major milk proteins, including α-lactalbumin (27, 57, 178), α-caseins [α-S1 and α-S2, (45, 46)], β-casein (134, 189), and κ-casein (140, 238). Variation in specific milk proteins appears to affect mammary gland function, as they are associated with differences in milk production and milk composition. A study in Holstein dairy cows found that α-S1 and β-casein variants were associated with higher milk volume, milk fat, and protein yield (213). Consequently, such cows with variants in caseins, as well as α-lactalbumin and β-lactoglobulin, are economically favored for selective breeding (29, 30, 119). α-Lactalbumin-deficient mice produce abnormally viscous milk and fail to expel milk to suckling pups (274); however, it is important to note that the concentration of α-lactalbumin is quite high in human milk and is likely not a limiting factor. Although one might speculate that since in humans genetic variation in α-lactalbumin is quite common (57), perhaps variation in α-lactalbumin may alter lactose production and thus affect milk quality and milk ejection in women. Because these major milk proteins also have biological functions beyond providing energy (195), genetic variation may have an effect on the growth and development of offspring; however, to our knowledge there is currently no information in this regard. In addition, the contribution of several candidate genes on lactation outcomes has been explored directly. For example, diacylglycerol _O_-acyltransferase 1 (DGAT1), which catalyzes the formation of triglycerides, is the most prominent gene affecting milk fat composition in cows (172, 261), and a K232A substitution very likely represents the causal mutation (282). DGAT1 is an example of a quantitative trait locus candidate gene in the centromeric region of chromosome 14 that was demonstrated to have a major effect on the fat content of cow milk associated specifically with higher saturated fat (i.e., C14:0) compared with unsaturated fat (i.e., C18:1) (261, 282). Studies of DGAT1-deficient mice demonstrate that these defects in lipid metabolism impair mammary development (47). Moreover, several variants of fibroblast growth factor 2 (FGF2) in Holstein cattle are associated with fat yield and milk quality, as measured by somatic cell scores (295). FGF2 is important for mammary gland development and also regulates the expression of interferon-tau, a key factor in STAT activation for milk production (295), which suggests that variation in FGF2 could be another potential candidate as a modifier of milk production in women.
Genetic variation in a few nutrient-specific genes has been associated with alterations in milk composition in humans. Polymorphisms in the vitamin D receptor are common and are associated with altered breast function and breast cancer in women (102, 241). Moreover, Bezerra et al. (21) found that SNPs in vitamin D receptor are associated with milk calcium concentration. Recently, there has been much interest in better understanding the mechanisms through which zinc is secreted into milk, as there are numerous reports in the literature of exclusively breastfed infants being diagnosed with severe zinc deficiency. We were the first to identify a mutation in the gene SLC30A2 that encodes the zinc transporter ZnT2 (58). This mutation leads to an amino acid substitution in the NH2 terminus of the protein and the secretion of ∼75% less zinc into breast milk than normal, and since then, several other mutations in SLC30A2 that lead to substitutions in ZnT2 (G87R, W152R, S296L, R340C) have subsequently been associated with this disorder (125, 152, 196). More recent studies indicate that defects in ZnT2 may have much broader implications for mammary gland biology, as ZnT2-null mice have severe defects in lactation outcomes, including profound impairments in mammary gland differentiation, milk production, composition, and secretion (158). Moreover, studies in mice and cultured lactocytes indicate that ZnT2 imports zinc into lysosomes and plays a critical role in activating cell death and mammary gland involution (114). This may be of importance to subpopulations of women because ∼35% of otherwise healthy breastfeeding women had nonsynonymous genetic variation in ZnT2 (4), which was associated with both low and high milk zinc concentrations. In addition, two particular variants (D103E and T288S) were found with high frequency (∼12%) and were associated with high milk sodium levels, a known a hallmark of breast dysfunction (4). Exciting opportunities exist to better understand the role that nutrigenetics plays in modulating lactation physiology and breast milk composition so that personalized strategies to optimize lactation outcomes can be developed.
Role of Energy Balance
The nutritional requirements during lactation increase considerably to maintain maximal mammary gland secretory capacity and milk production and also to maintain maternal energy balance for optimal milk production (234). Energy requirements increase during lactation (40), and the energy cost of lactation is determined by the amount of milk production and secretion, milk energy content, and the efficiency of converting dietary energy to milk energy (41), which usually increases over time in women who breastfeed exclusively (234). As a result, early investigations have focused on the role of diet in maintaining energy balance and milk production and composition during lactation (reviewed in Ref. 92). Because of the epidemic of obesity in Western countries, more recently the consequences of dietary excess on mammary gland function have garnered much interest. In this section, we will focus on recent advances in understanding the consequences of obesity and physical activity on mammary gland function and lactation outcomes.
Maternal metabolism and obesity.
The World Health Organization has declared that obesity is one of the most significant human health problems, with the expectation that 80% of women will be overweight (BMI of 25–29) or obese (BMI >30) by 2020 (297). This could have a profound consequence on the number of women who breastfeed successfully, and thus the health of infants and the US population at large, because mothers who are overweight or obese are less likely to initiate lactation, have delayed secretory activation, and are prone to early breastfeeding cessation (15, 130, 161, 244, 245). Biological complications include prolactin resistance and reduced STAT5 activation (37), which is linked to delayed initiation and premature cessation of lactation (245), and decreased insulin sensitivity (50), which may result in insufficient glandular development and reduced milk volume (202, 279). Recently, many researchers have used dietary-induced obese animal models to further investigate the effects of obesity on the lactating mammary gland and milk composition that in turn affect lactation outcomes. As described by multiple studies, the consequences of obesity in rodents include increased adipocyte size (115, 133) and disturbances in the mammary gland microenvironment and impaired development during pubertal stages (133). In addition, the growth hormone/IGF-I axis, somatostatin/cortistatin, and ghrelin systems are increased in the mammary fat pads of diet-induced obese mice (288), which may be involved in the pathophysiology of the mammary gland (77, 148). Although growth hormone is known to improve milk volume (197, 316), obese mice also have increased plasma leptin (288), which inhibits the effect of oxytocin on myoepithelial contraction for milk ejection (20). Moreover, during pregnancy and lactation, obese rodents have significant defects in alveolar development (84, 115), abnormal collagen deposition, and incomplete myoepithelial lining around ductal structures (133). During lactation, the mammary glands of obese mice accumulate lipid in the MECs, consistent with secretory inactivation (84, 156). Growing evidence suggests that in addition to systemic inflammation, obesity is also associated with an inflammatory microenvironment in the mammary gland (22, 36, 277), which has recently been associated with premature involution in murine models (115). A few studies provide insight into the possible mechanism underlying obesity-induced inflammation in the mammary gland. Inflammation in the mammary gland is marked by elevated levels of proinflammatory factors like TNFα, IL-1B, and Cox2 (277), increased macrophage infiltration (163), perturbed zinc metabolism (115), and increased NF-kB signaling (42). Moreover, there is some suggestion that obesity increases local estrogen production (36), which in turn may function to downregulate prolactin signaling, and suppress lactation (reviewed in Ref. 218). In addition, lipid synthesis is impaired, which can result from 1) interference with dietary lipid transport into and out of the mammary gland and 2) suppression of de novo lipogenesis in the mammary gland, resulting in lipid accumulation and low milk triglycerides (254, 291). Fundamentally, the altered mammary gland microenvironment that occurs in obesity can lead to failed secretory activation or suboptimal lactation, whereby the mammary gland is incapable of secreting copious milk to nourish the newborn. Therefore, understanding the lactogenic mechanisms that are compromised by obesity will assist clinicians in designing strategies to better support these mothers.
Physical activity and fasting.
A major concern of women postpartum is the desire to lose excess weight gained during pregnancy. The American College of Obstetricians and Gynecologists recommends that women resume physical activity after delivery as soon as it is physically and medically safe. However, intensity and duration appear to have important implications on the effects of exercise on lactation. Moderate levels of exercise do not affect milk production, milk composition, or infant growth (68, 166, 167, 276); rather, exercise improves the mother's overall health and sense of well-being (72, 168, 236). Moreover, short-term weight loss has no effect on lactation performance, milk volume, or milk protein concentration (187). However, several other reports examining the effects of more intense exercise and starvation (i.e., religious fasting) have observed significant changes in specific milk proteins and other essential factors in milk, such as immunoglobulins (94), lactose (315), and micronutrients (zinc, magnesium, and potassium) (242). This is consistent with studies in rats, where Matsuno et al. (181) found that chronic low-intensity exercise during lactation decreases milk lactose, increases milk protein and milk fat, and compromises offspring growth. However, it is important to note that lactation is much more energy demanding in rodents than humans. For example, rats lactate at their maximum physiological capacity and require ≥100–300% of their energy to produce milk (237), and therefore, changes in maternal diet and metabolism are more robustly reflected in their milk composition. However, this concept does not apply to breastfeeding women, who need only ∼20% of their total energy (237). For women, intense exercise or “exhaustive exercise” may have short-term effects on milk composition, such as increased lactic acid and decreased secretory IgA concentration; however, this appears to return to normal levels within 1 h (44, 94). It is important to note that increased milk lactic acid secretion after moderate- or high-intensity exercise is common and does not impede infant acceptance of breast milk (307). Likewise, short-term starvation decreases amino acid supply to the mammary gland and lowers free amino acid concentration in the milk of fasted rats (289) and also decreases expression and activity of essential enzymes such as fatty acid synthase, glucose-6-dehydrogenase, and lipoprotein lipase, all of which are critical for fatty acid production (95, 129). However, these effects are easily reversed with refeeding or resumption of normal activity. An additional concern is that with severe calorie restriction, fad diets and rapid weight loss, fat-soluble environmental contaminants, and toxins, including polychlorinated biphenyls (PCBs) and pesticides that are stored in body fat, can be secreted into milk (149). Moreover, it is hard to make generalized recommendations regarding diet and exercise during lactation due to differences in individual genetics and the changes in gene expression influenced by modulation of energy balance (224). Nowadays, much interest in understanding the effects of physical activity on breastfeeding has shifted toward studies on the effects of physical activity on breast cancer progression (205). Multiple studies have explored the effects of exercise on the normal mammary gland in an attempt to identify tumor-associated/inhibiting markers. Hopefully, these studies will indirectly provide information regarding the effects of exercise on breast development (298) and function (205) that impact lactation outcomes.
Role of Diet
Nutrient intake: deficiency and supplementation.
In recent years, increasing evidence shows not only that adequate nutrient intake and appropriate nutrient homeostasis are important for maintaining maternal energy balance but that suboptimal nutrition has significant effects on breast physiology and milk production, secretion, and composition. Nutrient deficiencies can result in failed secretory activation from several perspectives, such as inefficient hormone responsiveness, and defects in cellular processes involved in morphogenesis and secretory pathways. Moreover, energy/nutrient imbalance may cause more perverse effects on immune response and increased risk of mastitis (127, 203, 204, 216). This next section highlights our limited understanding of the role of diet and specific nutrients and dietary factors on lactation physiology. Because the ethics of manipulating maternal diet in humans preclude randomized clinical studies, much of our current understanding comes from studies conducted in animal models.
Vitamins.
Vitamin A deficiency remains a major worldwide health problem that leads to blindness, growth retardation, and death, particularly in developing countries. Its prevalence is estimated at ∼29%, with Sub-Saharan African and Southeast Asian countries most affected (273), and affects largely preschool children, pregnant and lactating mothers, and the rural poor (101). Whereas vitamin A intake is positively correlated to milk vitamin A concentration (97), vitamin A deficiency did not appear to have major effects on mammary gland biology in a rat model (257). However, vitamin A deficiency in rodent models decreases expression of mammary gland iron transporters and milk iron levels (136), alters zinc transporter expression (137), and leads to pathological damage to the mammary gland after intramammary challenge with staphylococcus (54). Similarly, vitamin D deficiency is presumed to be very common in pregnant women (177). Studies in rodent models found that vitamin D deficiency significantly reduces milk protein, calcium, and lactose levels and impairs milk protein synthesis, suggesting a role in hormone-induced functional differentiation of the mammary gland (23). However, studies on the effects of vitamin D deficiency on mammary gland function have not been conducted since the 1980s and necessitate more robust molecular investigation. Finally, B vitamins like thiamin (B1), riboflavin (B2), and niacin (B3) are critical for energy metabolism, and folate and B12 are essential for DNA synthesis. Although intake of B vitamins (particularly B1, folate, and B12) varies widely, few studies exploring the effects of B vitamin deficiency on mammary gland biology have been described. Dangat et al. (69) found that B12 deficiency may reduce milk volume; however, no studies toward understanding the molecular underpinnings have since been described. Given the importance of B vitamins in energy metabolism, DNA synthesis, and cell proliferation, understanding the effects of suboptimal B vitamin nutrition on lactation outcomes warrants further consideration.
Minerals.
Minerals play diverse roles in biology ranging from cofactors for protein stability or activity to the transfer of electrons in numerous biological pathways. The mammary gland has a remarkable capacity to homeostatically regulate milk concentrations of minerals such as iron and zinc in times of maternal deficiency in these minerals (165). Iron deficiency is one of the most common nutrient deficiencies in the world. Iron bound to transferrin in serum is taken into the mammary gland by transferrin receptors (267) and may be imported into the endoplasmic reticulum by ferroportin for incorporation of iron into proteins such as lactoferrin in humans and mice or transferrin in rats (165). However, there remains an incomplete understanding of the molecular regulation of iron transport in the mammary gland for secretion into milk. However, due to the role of iron in energy generation (306), it is reasonable to assume that iron deficiency may have an impact on mammary gland biology and lactation outcomes. Although Grill et al. (96) found that iron deficiency has minimal effects on pubertal mammary expansion, to our knowledge, no studies have explored effects of iron deficiency on mammary gland function during lactation. In contrast, the potential impact of zinc deficiency on lactation outcomes has been better documented. Zinc is a catalytic, structural, or regulatory cofactor for >10% of the proteome (184). Marginal zinc deficiency is common in women of reproductive age (262). Studies in mice show that marginal zinc deficiency leads to a toxic mammary gland microenvironment that impairs mammary development and subsequently affects lactation outcomes, including mammary gland expansion, milk composition, and milk volume (32). Most studies have not found a relationship between maternal zinc status and milk zinc levels (55, 145). However, a recent report in Thai mother-infant pairs found that exclusively breast-fed infants had the highest prevalence of zinc deficiency and that maternal zinc status was indeed positively correlated to milk zinc concentration (75). Our current understanding in lactocytes is that a complex network of zinc transporters may regulate zinc uptake and export into milk (184). It is not yet understood how the lactocyte takes up zinc from the systemic circulation or how this process is regulated; however, we previously proposed roles for ZIP5, ZIP8, and ZIP10 in this process. Much more is known about how zinc is secreted into milk. The important role of ZnT2 in zinc secretion was discussed above. In addition to ZnT2, several studies have shown that ZnT4 may also play a role in zinc secretion into milk; however, there is far less understanding of its relevance. We have shown previously that ZnT4 transports zinc into the _trans_-Golgi apparatus and across the apical cell surface (185). Presumably, insufficient zinc in the _trans_-Golgi apparatus impairing galactosyltransferase activity and the production of lactose may underlie the phenotype of reduced milk secretion and milk zinc levels in ZnT4-null mice (186). However, a much more impressive phenotype in ZnT4-null mice is the dramatic loss in alveolar structure and activation of precocious involution and early lactation failure (186); thus more research is required to understand the role of ZnT4 in lactation physiology and how the lactocyte regulates zinc transport to meet its unique physiological needs. Given the role of genetic variation in ZnT2 (and perhaps other zinc transporters) in modifying milk zinc levels, further studies are needed to determine the contribution of maternal genetics to regulating zinc homeostasis during times of zinc deficiency or excess. Finally, the other mineral that is of global concern is iodine. In the mammary gland, iodide transport is mediated by the sodium/iodide symporter (NIS) (251, 281), and the concentration of milk iodine varies widely, ranging from 5.4 to 2,170 μg/l, reflecting the lack of homeostasis (74). This not only affects infant iodine nutrition but can also have profound effects on breast physiology, resulting in mammary dysplasia and atypia due to atrophy and necrosis in the alveolar cells (78, 275), most likely through alterations in the production, secretion, and activity of thyroid hormones. As noted above, further studies are needed to better understand effects of hypo- and hyperthyroidism on lactation outcomes and milk composition. In contrast to iodine, maternal intake of calcium does not directly affect calcium levels in breast milk (123) because a large amount of calcium is drawn from the bone (144) to transfer ∼300–400 mg calcium/day into milk (142). Therefore, dietary calcium intake prior to pregnancy may be most critical for providing calcium for milk secretion during lactation and has been reviewed recently by Kovacs (143). This large amount of calcium is secreted by forming a complex with citrate, casein, or phosphates that are packaged into secretory vesicles for exocytosis (208) or by direct transport across the apical membrane through calcium ATPase type 2 (248). For an excellent review on calcium transport in the breast during lactation, the reader is referred to Cross et al. (65).
Protein and amino acids.
In addition to vitamins and minerals, protein deficiencies have been reported to reduce milk production (14). Rats fed an arginine-deficient diet had impaired mammary gland growth (228). Moreover, excess protein intake in lactating mice decreases mammary gland mass, reduces expression of major milk constituents, and lowers milk lactose concentration (146). Milk lactose and milk fat are essential components in milk that reflect the capacity for secretory activation, milk synthesis, and milk quality; therefore, when dietary glucose (a precursor for lactose synthesis) or fatty acids are restricted, substantial impacts on milk composition (protein, fat, and glucose) and mammary gland growth occur in lactating rat dams (150). However, it is not clear whether dietary glucose restriction will affect lactation in women, because studies in developing countries of women with poor diets have shown no effect on milk output (62).
Fatty acids.
Milk fat is a major source of energy and fat-soluble vitamins as well as essential fatty acids (31, 82) Fatty acid composition in milk is species specific and is extremely sensitive to maternal nutrition and dietary alterations (82, 227). In particular, long-chain polyunsaturated fatty acids (LCPUFAs) such as docosahexaenoic acid (DHA), ecosopentanoic acid (EPA), and arachidonic acid in breast milk are obtained from maternal diet and are implicated in neurological development and susceptibility to allergies during infancy (169, 283, 286). Maternal fatty acid intake not only affects the fatty acid profile of human milk but has also been shown to be an important dietary determinant of mammary gland function. For example, MECs of rats fed a LCPUFA-deficient diet are unresponsive to prolactin stimulation and have impaired secretion resulting from low phospholipid levels in the mammary gland and accumulation of secretory vesicles in the cytoplasm (219). This suggests that low intake of LCPUFAs may have profound effects on milk volume and composition. Moreover, medium-chain fatty acids (C:8–C14:0) are found in breast milk, and the concentration increases over the duration of lactation (13, 82, 104). Medium-chain fatty acids are synthesized in the mammary gland through de novo fatty acid synthesis, which is catalyzed by the enzyme fatty acid synthase (FASN) (278, 314). In addition to reduced milk fatty acids, FASN-deficient mice showed defects in mammary development and underwent precocious involution (278). Recent studies reveal genomic variability and functional expression of the FASN gene in buffalo and found the highest expression of FASN1 in mammary gland during lactation when fat turnover is the greatest (214), which implicates its importance in the active stage of milk production (190). Genetic variations in other genes that govern fatty acid metabolism have been shown to have a profound impact on milk composition and infant health outcomes. A T347S variant of apolipoprotein A4, which is involved in dietary fat absorption, is present in about one-third of the US population, and women with this variant have a greater level of DHA in their breast milk (98, 222). Moreover, 20% of the population carries one or two copies of the E4 variant of the apoliprotein E gene, which regulates circulating fat metabolism, and women with the E4 variant have significantly less milk fat than women without this variant (132, 268). The most well-studied genetic variants are SNPs in fatty acid desaturase 1 and 2 (FADS1 and FADS2), which encode the enzymes Δ5 and Δ6 desaturases, respectively, and are rate-limiting enzymes in the synthesis of polyunsaturated ω-3 and ω-6 fatty acids (arachidonic acid, eicosapentaenoic acid, and DHA). Several SNPs in FADS1 and FADS2 in Canadian Holstein cows (121) and breastfeeding women (308) are associated with alterations in milk fatty acid composition. Importantly, these alterations have critical implications for infant health and development, as an interaction between exclusive breastfeeding and asthma modified by FADS genotypes in children has been noted (271). Furthermore, breastfeeding, FADS genotype, and fish intake are important determinants of DHA status in late infancy (110). This may be particularly important given the role of EPA and DHA in neurological development during infancy, which has been elegantly reviewed by Innis (122).
Role of Environmental Factors on Mammary Gland Physiology
In addition to genetics and diet, environmental factors may contribute to suboptimal lactation (26, 35, 79, 211). Many of these environmental chemicals have become a part of our daily diet and exposures, especially since they are often found at high concentrations in fat-containing food such as red meat and dairy products (191). Constant exposure to various chemicals in the environment can put a toxic burden upon the body, and many such chemicals are stored in the mother's adipose tissue and then found in breast milk, which is then transferred to the nursing infant (191). This suggests that analysis of milk fat from breastfeeding women might be useful in assessing environmental contaminants in populations. For a more in-depth review of the transfer of toxins into breast milk and effects on child health, the reader is referred to several recent reviews (171, 285). However, in this section we will review the evidence that exposure to environmental toxins may also have important effects on mammary gland physiology. In particular, several studies have shown that exposure to chemicals or endocrine-disrupting compounds (EDCs) during gestation can cause adverse effects on mammary gland development and lactation (80). Numerous chemicals such as atrazine and dioxin (in herbicides), bisphenol A (BPA), and dibutylphthalate (in plastics), nonylphenol (in laundry and dish detergent), polybrominated diphenyl ethers (a flame retardant), and perfluorooctanoic acid (PFOA; in cleaning products and pesticides) have been observed to disrupt mammary gland function. Dioxins or dioxin-like compounds are comprised of members of three structurally related families of chemicals: polychlorinated dibenzo-_p_-dioxins, polychlorinated dibenzofurans, and polychlorinated biphenyls (PCBs). Dioxins activate the aryl hydrocarbon receptor, which increases expression of numerous genes, including ABCG2, which is the main xenobiotic transporter in the mammary gland (106). PFOA is a known agonist of PPARα, which profoundly impairs normal differentiation of lobular-alveolar units during lactation (310). Studies in rodents show that gestational exposure to dioxins (290), PFOA (299, 300), atrazine (247), and BPA (180) leads to changes in mammary gland development that include mammary hypoplasia, reduced apoptosis in terminal end buds, altered gene or protein expression, and accelerated alveolar differentiation. In addition, mice exposed to dioxins during gestation have activated aryl hydrocarbon receptor, severe defects in mammary gland differentiation during lactation, and reduced milk protein expression that leads to offspring death within 24 h postpartum (290). PFOA exposure in mice during early gestation or lactation permanently impairs mammary gland differentiation, reduces milk protein expression, and decreases pup survival over the lactation period (300). Finally, exposure to BPA during pregnancy in mice leads to lactation insufficiency and offspring death (180). Thus far, only a few studies in women have shown an association between toxins like PCBs and dichlorodiphenyl dichloroethene and lactation defects such that exposure to these toxins is associated with shorter breastfeeding duration (67, 252). Given the burden of these often hidden environmental toxins in daily life, understanding the consequences of these environmental toxins on mammary gland physiology in humans is an important area for future research.
The toxins mentioned above are man-made environmental contaminants, but even natural components such as heavy metals exposure can affect the mammary gland during lactation. Some heavy metals (copper, zinc, and manganese) are biologically essential; however, the most pollutant heavy metals are lead, cadmium, and mercury that bioaccumulate following absorption, causing adverse health effects. Human, ruminant, and rodent studies have all demonstrated that cadmium levels remain low in breast milk, suggesting that the mammary gland is capable of protecting the growing infant from heavy metal exposure (24, 25, 85, 105, 170, 232, 270). As a result, cadmium sequestration in the mammary glands of lactating rodents has been observed (232), which disturbs alveoli morphology and reduces β-casein production and secretory activation (217). Although these studies illustrate profound lactation defects resulting from these environmental factors, there remains conflicting information regarding the mechanistic explanation(s) (180, 290). Hence, the mechanism of action(s) of heavy metals on breast development and lactation outcomes still remains to be explored. More importantly, because exposures to heavy metals as well as environmental toxins have become inevitably common, it is of much greater significance to investigate potential ways to reduce transfer into milk or to buffer these harmful factors in infants (191).
Conclusion
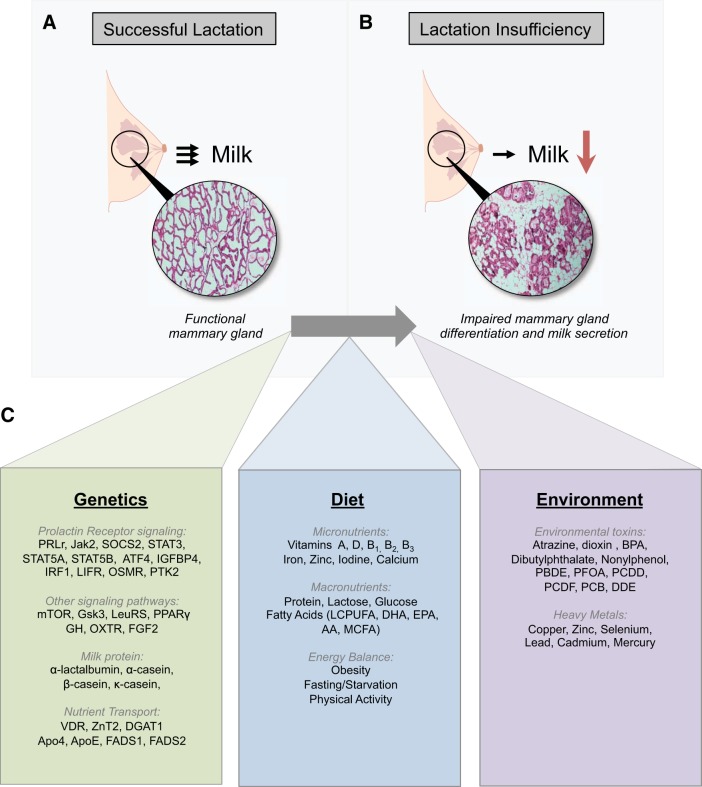
Breast milk clearly provides optimal nutrition for the growing infant as long as the mother is capable of producing and secreting an adequate volume of high-quality milk. Epidemiological studies suggest that many breastfeeding women may suffer from suboptimal lactation, and increasing evidence implicates maternal genetics, diet, and environmental toxins as modifiers of reproductive endocrinology, mammary gland physiology, lactation, and milk composition (Fig. 1). Although infant formula meets the nutritional needs of infants, there remain clear differences in short- and long-term health outcomes between breast-fed and formula-fed infants. Clearly, understanding factors that impact lactation and developing methods to accurately assess lactation outcomes before a breast-fed infant becomes ill will directly inform the development of therapeutic strategies to improve poor lactation performance. Importantly, these advancements will have a major impact on increasing the number of exclusively breastfed infants and decreasing the burden of noncommunicable disease. Moreover, understanding these factors and improving lactation success will be important to develop novel nutrigenomic or pharmacogenomic approaches for reducing breast disease and cancer.
Fig. 1.
Maternal factors that can lead to lactation insufficiency. Lactation insufficiency is a condition in which lactation is insufficient or unsuccessful due to inadequate milk production. A and B: images of hematoxylin and eosin-stained mouse mammary gland tissues represent a functional mammary gland undergoing successful lactation (A) and a mammary gland with impaired mammary differentiation and milk secretion that results in lactation insufficiency (B). C: list summarizing genetic, dietary, and environmental factors that have been shown to affect lactation outcomes. PRLR, prolactin receptor; Jak2, Janus kinase 2; SOCS2, suppressor of cytokine signaling 2; STAT, signal transducer and activator of transcription; ATF4, activating transcription factor 4; IGFBP-4, insulin-like growth factor-binding protein 4; IRF1, interferon regulatory factor 1; LIFR, leukemia inhibitory factor receptor; OSMR, oncostatin M receptor; PTK2, protein tyrosine kinase 2; mTOR, mammalian target of rapamycin; Gsk3, glycogen synthase kinase 3; LeuRS, leucyl-tRNA synthetase; PPARγ, peroxisome proliferator-activated receptor-γ; GH, growth hormone; OXTR, oxytocin receptor; FGF2, fibroblast growth factor 2; VDR, vitamin D receptor; ZnT2, zinc transporter 2; DGAT1, diacylglycerol _O_-acyltransferase 1; apo4, apolipoprotein 4; apoE, apolipoprotein E; FADS, fatty acid desaturase; LCPUFA, long-chain polyunsaturated fatty acids; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; MCFA, medium-chain fatty acid; AA, arachidonic Acid; BPA, bisphenol A; PBDE, polybrominated diphenyl ethers (PBDE); PFOA, perfluorooctanoic acid; PCDD, polychlorinated dibenzo-_p_-dioxins; PCDF, polychlorinated dibenzofurans; PCB, polychlorinated biphenyls; DDE, dichlorodiphenyl dichloroethene.
GRANTS
This work was supported in part by NIHD058614 to S. L. Kelleher, intramural support from the Penn State Hershey Department of Surgery to S. L. Kelleher, and the Huck Dissertation Research Award from the Huck Institute of Life Sciences at Penn State University to S. Lee.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.L. and S.L.K. prepared figures; S.L. and S.L.K. drafted manuscript; S.L. and S.L.K. edited and revised manuscript; S.L. and S.L.K. approved final version of manuscript.
REFERENCES
- 1.Ahluwalia IB, Morrow B, Hsia J. Why do women stop breastfeeding? Findings from the Pregnancy Risk Assessment and Monitoring System. Pediatrics 116: 1408–1412, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Akers RM. Lactation and the Mammary Gland. Hoboken, NJ: Wiley-Blackwell, 2002. [Google Scholar]
- 3.Akinci A, Kanaka C, Eble A, Akar N, Vidinlisan S, Mullis PE. Isolated growth hormone (GH) deficiency type IA associated with a 45-kilobase gene deletion within the human GH gene cluster. J Clin Endocrinol Metab 75: 437–441, 1992. [DOI] [PubMed] [Google Scholar]
- 4.Alam S, Hennigar SR, Gallagher C, Soybel DI, Kelleher SL. Exome Sequencing of SLC30A2 Identifies Novel Loss- and Gain-of-Function Variants Associated with Breast Cell Dysfunction. J Mammary Gland Biol Neoplasia 20: 159–172, 2015. [DOI] [PubMed] [Google Scholar]
- 5.Aljazaf K, Hale TW, Ilett KF, Hartmann PE, Mitoulas LR, Kristensen JH, Hackett LP. Pseudoephedrine: effects on milk production in women and estimation of infant exposure via breastmilk. Br J Clin Pharmacol 56: 18–24, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen LH. B vitamins in breast milk: relative importance of maternal status and intake, and effects on infant status and function. Adv Nutr 3: 362–369, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson SM, MacLean PS, McManaman JL, Neville MC. Lactation and its hormonal control. In: Knobil and Neill's Physiology of Reproduction, edited by Plant TM and Zelenick AJ. Atlanta, GA: Elsevier, 2014, p. 2055–2105. [Google Scholar]
- 8.Aoki N, Matsuda T. A cytosolic protein-tyrosine phosphatase PTP1B specifically dephosphorylates and deactivates prolactin-activated STAT5a and STAT5b. J Biol Chem 275: 39718–39726, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Apostoli AJ, Skelhorne-Gross GE, Rubino RE, Peterson NT, Di Lena MA, Schneider MM, SenGupta SK, Nicol CJ. Loss of PPARgamma expression in mammary secretory epithelial cells creates a pro-breast tumorigenic environment. Int J Cancer 134: 1055–1066, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Appuhamy JA, Nayananjalie WA, England EM, Gerrard DE, Akers RM, Hanigan MD. Effects of AMP-activated protein kinase (AMPK) signaling and essential amino acids on mammalian target of rapamycin (mTOR) signaling and protein synthesis rates in mammary cells. J Dairy Sci 97: 419–429, 2014. [DOI] [PubMed] [Google Scholar]
- 11.Arriola Apelo SI, Singer LM, Lin XY, McGilliard ML, St-Pierre NR, Hanigan MD. Isoleucine, leucine, methionine, and threonine effects on mammalian target of rapamycin signaling in mammary tissue. J Dairy Sci 97: 1047–1056, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Aschaffenburg R, Drewry J. Occurence of different beta-lactaglobulin in cow's milk. Nature 176: 218–219, 1955. [DOI] [PubMed] [Google Scholar]
- 13.Bahrami G, Rahimi Z. Fatty acid composition of human milk in Western Iran. Eur J Clin Nutr 59: 494–497, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Baker DH, Becker DE, Norton HW, Sasse CE, Jensen AH, Harmon BG. Reproductive performance and progeny development in swine as influenced by feed intake during pregnancy. J Nutr 97: 489–495, 1969. [DOI] [PubMed] [Google Scholar]
- 15.Baker JL, Michaelsen KF, Sorensen TI, Rasmussen KM. High prepregnant body mass index is associated with early termination of full and any breastfeeding in Danish women. Am J Clin Nutr 86: 404–411, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Balasubramanian S. Vitamin D deficiency in breastfed infants & the need for routine vitamin D supplementation. Indian J Med Res 133: 250–252, 2011. [PMC free article] [PubMed] [Google Scholar]
- 17.Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am 60: 49–74, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bandapalli OR, Schuessele S, Kunz JB, Rausch T, Stutz AM, Tal N, Geron I, Gershman N, Izraeli S, Eilers J, Vaezipour N, Kirschner-Schwabe R, Hof J, von Stackelberg A, Schrappe M, Stanulla M, Zimmermann M, Koehler R, Avigad S, Handgretinger R, Frismantas V, Bourquin JP, Bornhauser B, Korbel JO, Muckenthaler MU, Kulozik AE. The activating STAT5B N642H mutation is a common abnormality in pediatric T-cell acute lymphoblastic leukemia and confers a higher risk of relapse. Haematologica 99: e188–e192, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ben-Jonathan N, Hnasko R. Dopamine as a prolactin (PRL) inhibitor. Endocr Rev 22: 724–763, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Bever Babendure J, Reifsnider E, Mendias E, Moramarco MW, Davila YR. Reduced breastfeeding rates among obese mothers: a review of contributing factors, clinical considerations and future directions. Int Breastfeed J 10: 21, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bezerra FF, Cabello GM, Mendonca LM, Donangelo CM. Bone mass and breast milk calcium concentration are associated with vitamin D receptor gene polymorphisms in adolescent mothers. J Nutr 138: 277–281, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Bhardwaj P, Du B, Zhou XK, Sue E, Harbus MD, Falcone DJ, Giri D, Hudis CA, Kopelovich L, Subbaramaiah K, Dannenberg AJ. Caloric restriction reverses obesity-induced mammary gland inflammation in mice. Cancer Prev Res (Phila) 6: 282–289, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Bhattacharjee M, Wientroub S, Vonderhaar BK. Milk protein synthesis by mammary glands of vitamin D-deficient mice. Endocrinology 121: 865–874, 1987. [DOI] [PubMed] [Google Scholar]
- 24.Bhattacharyya MH. Bioavailability of orally administered cadmium and lead to the mother, fetus, and neonate during pregnancy and lactation: an overview. Sci Total Environ 28: 327–342, 1983. [DOI] [PubMed] [Google Scholar]
- 25.Bhattacharyya MH, Whelton BD, Peterson DP. Gastrointestinal absorption of cadmium in mice during gestation and lactation. II. Continuous exposure studies. Toxicol Appl Pharmacol 66: 368–375, 1982. [DOI] [PubMed] [Google Scholar]
- 26.Birnbaum LS, Fenton SE. Cancer and developmental exposure to endocrine disruptors. Environ Health Perspect 111: 389–394, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bleck GT, Bremel RD. Variation in expression of a bovine alpha-lactalbumin transgene in milk of transgenic mice. J Dairy Sci 77: 1897–1904, 1994. [DOI] [PubMed] [Google Scholar]
- 28.Boensch M, Oberthuer A, Eifinger F, Roth B. Life-threatening hypernatremic dehydration in a 7-week-old exclusively breastfed infant as a cause of a decline in breastmilk volume and parental language barriers in a North African family. Klin Padiatr 223: 40–42, 2011. [DOI] [PubMed] [Google Scholar]
- 29.Bonfatti V, Cecchinato A, Gallo L, Blasco A, Carnier P. Genetic analysis of detailed milk protein composition and coagulation properties in Simmental cattle. J Dairy Sci 94: 5183–5193, 2011. [DOI] [PubMed] [Google Scholar]
- 30.Bonfatti V, Di Martino G, Cecchinato A, Vicario D, Carnier P. Effects of beta-kappa-casein (CSN2-CSN3) haplotypes and beta-lactoglobulin (BLG) genotypes on milk production traits and detailed protein composition of individual milk of Simmental cows. J Dairy Sci 93: 3797–3808, 2010. [DOI] [PubMed] [Google Scholar]
- 31.Borschel MW, Elkin RG, Kirksey A, Story JA, Galal O, Harrison GG, Jerome NW. Fatty acid composition of mature human milk of Egyptian and American women. Am J Clin Nutr 44: 330–335, 1986. [DOI] [PubMed] [Google Scholar]
- 32.Bostanci Z, Mack RP Jr, Lee S, Soybel DI, Kelleher SL. Paradoxical zinc toxicity and oxidative stress in the mammary gland during marginal dietary zinc deficiency. Reprod Toxicol 54: 84–92, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boutinaud M, Jammes H. Growth hormone increases Stat5 and Stat1 expression in lactating goat mammary gland: a specific effect compared to milking frequency. Domest Anim Endocrinol 27: 363–378, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Brisken C, O'Malley B. Hormone action in the mammary gland. Cold Spring Harb Perspect Biol 2: a003178, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brody JG, Rudel RA, Michels KB, Moysich KB, Bernstein L, Attfield KR, Gray S. Environmental pollutants, diet, physical activity, body size, and breast cancer: where do we stand in research to identify opportunities for prevention? Cancer 109: 2627–2634, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Brown KA. Impact of obesity on mammary gland inflammation and local estrogen production. J Mammary Gland Biol Neoplasia 19: 183–189, 2014. [DOI] [PubMed] [Google Scholar]
- 37.Buonfiglio DC, Ramos-Lobo AM, Freitas VM, Zampieri TT, Nagaishi VS, Magalhaes M, Cipolla-Neto J, Cella N, Donato J Jr. Obesity impairs lactation performance in mice by inducing prolactin resistance. Sci Rep 6: 22421, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burgos SA, Cant JP. IGF-1 stimulates protein synthesis by enhanced signaling through mTORC1 in bovine mammary epithelial cells. Domest Anim Endocrinol 38: 211–221, 2010. [DOI] [PubMed] [Google Scholar]
- 39.Burgos SA, Kim JJ, Dai M, Cant JP. Energy depletion of bovine mammary epithelial cells activates AMPK and suppresses protein synthesis through inhibition of mTORC1 signaling. Horm Metab Res 45: 183–189, 2013. [DOI] [PubMed] [Google Scholar]
- 40.Butte NF, King JC. Energy requirements during pregnancy and lactation. Public Health Nutr 8: 1010–1027, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Butte NF, Lopez-Alarcon MG, Garza C. Nutrient Adequacy of Exclusive Breast Feeding for the Term Infant During the First Six Months of Life. Geneva: World Health Organization, 2002. [Google Scholar]
- 42.Cao Y, Karin M. NF-kappaB in mammary gland development and breast cancer. J Mammary Gland Biol Neoplasia 8: 215–223, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Capuco AV, Connor EE, Wood DL. Regulation of mammary gland sensitivity to thyroid hormones during the transition from pregnancy to lactation. Exp Biol Med (Maywood) 233: 1309–1314, 2008. [DOI] [PubMed] [Google Scholar]
- 44.Carey GB, Quinn TJ, Goodwin SE. Breast milk composition after exercise of different intensities. J Hum Lact 13: 115–120, 1997. [DOI] [PubMed] [Google Scholar]
- 45.Caroli A, Chessa S, Chiatti F, Rignanese D, Melendez B, Rizzi R, Ceriotti G. Short communication: Carora cattle show high variability in alpha(s1)-casein. J Dairy Sci 91: 354–359, 2008. [DOI] [PubMed] [Google Scholar]
- 46.Caroli A, Rizzi R, Luhken G, Erhardt G. Short communication: milk protein genetic variation and casein haplotype structure in the Original Pinzgauer cattle. J Dairy Sci 93: 1260–1265, 2010. [DOI] [PubMed] [Google Scholar]
- 47.Cases S, Zhou P, Shillingford JM, Wiseman BS, Fish JD, Angle CS, Hennighausen L, Werb Z, Farese RV Jr. Development of the mammary gland requires DGAT1 expression in stromal and epithelial tissues. Development 131: 3047–3055, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Casey TM, Boecker A, Chiu JF, Plaut K. Glucocorticoids maintain the extracellular matrix of differentiated mammary tissue during explant and whole organ culture. Proc Soc Exp Biol Med 224: 76–86, 2000. [DOI] [PubMed] [Google Scholar]
- 49.Castellote C, Casillas R, Ramírez-Santana C, Pérez-Cano FJ, Castell M, Moretones MG, López-Sabater MC, Franch A. Premature delivery influences the immunological composition of colostrum and transitional and mature human milk. J Nutr 141: 1181–1187, 2011. [DOI] [PubMed] [Google Scholar]
- 50.Catalano PM. Obesity, insulin resistance, and pregnancy outcome. Reproduction 140: 365–371, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chamberlain AJ, Hayes BJ, Savin K, Bolormaa S, McPartlan HC, Bowman PJ, Van der Jagt C, MacEachern S, Goddard ME. Validation of single nucleotide polymorphisms associated with milk production traits in dairy cattle. J Dairy Sci 95: 864–875, 2012. [DOI] [PubMed] [Google Scholar]
- 52.Chapman DJ, Pérez-Escamilla R. Does delayed perception of the onset of lactation shorten breastfeeding duration? J Hum Lact 15: 107–111; quiz 137–139, 1999. [DOI] [PubMed] [Google Scholar]
- 53.Chen CC, Stairs DB, Boxer RB, Belka GK, Horseman ND, Alvarez JV, Chodosh LA. Autocrine prolactin induced by the Pten-Akt pathway is required for lactation initiation and provides a direct link between the Akt and Stat5 pathways. Genes Dev 26: 2154–2168, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chew BP, Zamora CS, Luedecke LO. Effect of vitamin A deficiency on mammary gland development and susceptibility to mastitis through intramammary infusion with Staphylococcus aureus in mice. Am J Vet Res 46: 287–293, 1985. [PubMed] [Google Scholar]
- 55.Chierici R, Saccomandi D, Vigi V. Dietary supplements for the lactating mother: influence on the trace element content of milkarticle-title>. Acta Paediatr Suppl 88: 7–13, 1999. [DOI] [PubMed] [Google Scholar]
- 56.Chiofalo VB, Savoini G, Polidori F, Dell'Orto V, Politis I. Response of dairy ewes in late lactation to recombinant bovine somatotropin. Small Rumin Res 34: 119–125, 1999. [Google Scholar]
- 57.Chowanadisai W, Kelleher SL, Nemeth JF, Yachetti S, Kuhlman CF, Jackson JG, Davis AM, Lien EL, Lonnerdal B. Detection of a single nucleotide polymorphism in the human alpha-lactalbumin gene: implications for human milk proteins. J Nutr Biochem 16: 272–278, 2005. [DOI] [PubMed] [Google Scholar]
- 58.Chowanadisai W, Lonnerdal B, Kelleher SL. Identification of a mutation in SLC30A2 (ZnT-2) in women with low milk zinc concentration that results in transient neonatal zinc deficiency. J Biol Chem 281: 39699–39707, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Cole JB, VanRaden PM, O'Connell JR, Van Tassell CP, Sonstegard TS, Schnabel RD, Taylor JF, Wiggans GR. Distribution and location of genetic effects for dairy traits. J Dairy Sci 92: 2931–2946, 2009. [DOI] [PubMed] [Google Scholar]
- 60.Colodro-Conde L, Zhu G, Power RA, Henders A, Heath AC, Madden PA, Montgomery GW, Medland S, Ordonana JR, Martin NG. A twin study of breastfeeding with a preliminary genome-wide association scan. Twin Res Hum Genet 18: 61–72, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Corl BA, Butler ST, Butler WR, Bauman DE. Short communication: Regulation of milk fat yield and fatty acid composition by insulin. J Dairy Sci 89: 4172–4175, 2006. [DOI] [PubMed] [Google Scholar]
- 62.Coward WA, Paul AA, Prentice AM. The impact of malnutrition on human lactation: observations from community studies. Fed Proc 43: 2432–2437, 1984. [PubMed] [Google Scholar]
- 63.Cregan MD, De Mello TR, Kershaw D, McDougall K, Hartmann PE. Initiation of lactation in women after preterm delivery. Acta Obstet Gynecol Scand 81: 870–877, 2002. [DOI] [PubMed] [Google Scholar]
- 64.Cromi A, Serati M, Candeloro I, Uccella S, Scandroglio S, Agosti M, Ghezzi F. Assisted reproductive technology and breastfeeding outcomes: a case-control study. Fertil Steril 103: 89–94, 2015. [DOI] [PubMed] [Google Scholar]
- 65.Cross BM, Breitwieser GE, Reinhardt TA, Rao R. Cellular calcium dynamics in lactation and breast cancer: from physiology to pathology. Am J Physiol Cell Physiol 306: C515–C526, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cui Y, Riedlinger G, Miyoshi K, Tang W, Li C, Deng CX, Robinson GW, Hennighausen L. Inactivation of Stat5 in mouse mammary epithelium during pregnancy reveals distinct functions in cell proliferation, survival, and differentiation. Mol Cell Biol 24: 8037–8047, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cupul-Uicab LA, Gladen BC, Hernandez-Avila M, Weber JP, Longnecker MP. DDE, a degradation product of DDT, and duration of lactation in a highly exposed area of Mexico. Environ Health Perspect 116: 179–183, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Daley AJ, Thomas A, Cooper H, Fitzpatrick H, McDonald C, Moore H, Rooney R, Deeks JJ. Maternal exercise and growth in breastfed infants: a meta-analysis of randomized controlled trials. Pediatrics 130: 108–114, 2012. [DOI] [PubMed] [Google Scholar]
- 69.Dangat KD, Kale AA, Joshi SR. Maternal supplementation of omega 3 fatty acids to micronutrient-imbalanced diet improves lactation in rat. Metabolism 60: 1318–1324, 2011. [DOI] [PubMed] [Google Scholar]
- 70.Daniels KM, Farmer C, Jimenez-Flores R, Rijnkels M. Lactation Biology Symposium: the long-term impact of epigenetics and maternal influence on the neonate through milk-borne factors and nutrient status. J Anim Sci 91: 673–675, 2013. [DOI] [PubMed] [Google Scholar]
- 71.Dewey KG, Lonnerdal B. Breast milk intake: variations in breast-feeding practices. Am J Clin Nutr 38: 152–153, 1983. [DOI] [PubMed] [Google Scholar]
- 72.Dewey KG, Lovelady CA, Nommsen-Rivers LA, McCrory MA, Lonnerdal B. A randomized study of the effects of aerobic exercise by lactating women on breast-milk volume and composition. N Engl J Med 330: 449–453, 1994. [DOI] [PubMed] [Google Scholar]
- 73.Doppler W, Groner B, Ball RK. Prolactin and glucocorticoid hormones synergistically induce expression of transfected rat beta-casein gene promoter constructs in a mammary epithelial cell line. Proc Natl Acad Sci USA 86: 104–108, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dorea JG. Iodine nutrition and breast feeding. J Trace Elem Med Biol 16: 207–220, 2002. [DOI] [PubMed] [Google Scholar]
- 75.Dumrongwongsiri O, Suthutvoravut U, Chatvutinun S, Phoonlabdacha P, Sangcakul A, Siripinyanond A, Thiengmanee U, Chongviriyaphan N. Maternal zinc status is associated with breast milk zinc concentration and zinc status in breastfed infants aged 4–6 months. Asia Pac J Clin Nutr 24: 273–280, 2015. [DOI] [PubMed] [Google Scholar]
- 76.El Shafei AM, Labib JR. Determinants of exclusive breastfeeding and introduction of complementary foods in rural Egyptian communities. Glob J Health Sci 6: 236–244, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Emerman JT, Leahy M, Gout PW, Bruchovsky N. Elevated growth hormone levels in sera from breast cancer patients. Horm Metab Res 17: 421–424, 1985. [DOI] [PubMed] [Google Scholar]
- 78.Eskin BA, Bartuska DG, Dunn MR, Jacob G, Dratman MB. Mammary gland dysplasia in iodine deficiency. Studies in rats. JAMA 200: 691–695, 1967. [PubMed] [Google Scholar]
- 79.Euling SY, Selevan SG, Pescovitz OH, Skakkebaek NE. Role of environmental factors in the timing of puberty. Pediatrics 121, Suppl 3: S167–S171, 2008. [DOI] [PubMed] [Google Scholar]
- 80.Fenton SE. Endocrine-disrupting compounds and mammary gland development: early exposure and later life consequences. Endocrinology 147: S18–S24, 2006. [DOI] [PubMed] [Google Scholar]
- 81.Figueiredo B, Dias CC, Brandão S, Canário C, Nunes-Costa R. Breastfeeding and postpartum depression: state of the art review. J Pediatr (Rio J) 89: 332–338, 2013. [DOI] [PubMed] [Google Scholar]
- 82.Finley DA, Lonnerdal B, Dewey KG, Grivetti LE. Breast milk composition: fat content and fatty acid composition in vegetarians and non-vegetarians. Am J Clin Nutr 41: 787–800, 1985. [DOI] [PubMed] [Google Scholar]
- 83.Flint DJ, Gardner M. Evidence that growth hormone stimulates milk synthesis by direct action on the mammary gland and that prolactin exerts effects on milk secretion by maintenance of mammary deoxyribonucleic acid content and tight junction status. Endocrinology 135: 1119–1124, 1994. [DOI] [PubMed] [Google Scholar]
- 84.Flint DJ, Travers MT, Barber MC, Binart N, Kelly PA. Diet-induced obesity impairs mammary development and lactogenesis in murine mammary gland. Am J Physiol Endocrinol Metab 288: E1179–E1187, 2005. [DOI] [PubMed] [Google Scholar]
- 85.Floris B, Bomboi G, Sechi P, Pirino S, Marongiu ML. Cadmium chronic administration to lactating ewes: reproductive performance, cadmium tissue accumulation and placental transfer. Ann Chim 90: 703–708, 2000. [PubMed] [Google Scholar]
- 86.Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev 80: 1523–1631, 2000. [DOI] [PubMed] [Google Scholar]
- 87.Fu NY, Rios AC, Pal B, Soetanto R, Lun AT, Liu K, Beck T, Best SA, Vaillant F, Bouillet P, Strasser A, Preiss T, Smyth GK, Lindeman GJ, Visvader JE. EGF-mediated induction of Mcl-1 at the switch to lactation is essential for alveolar cell survival. Nat Cell Biol 17: 365–375, 2015. [DOI] [PubMed] [Google Scholar]
- 88.Garcia-Fernandez M, Gutierrez-Gil B, Garcia-Gamez E, Sanchez JP, Arranz JJ. Detection of quantitative trait loci affecting the milk fatty acid profile on sheep chromosome 22: role of the stearoyl-CoA desaturase gene in Spanish Churra sheep. J Dairy Sci 93: 348–357, 2010. [DOI] [PubMed] [Google Scholar]
- 89.Gartner LM, Morton J, Lawrence RA, Naylor AJ, O'Hare D, Schanler RJ, Eidelman AI; American Academy of Pediatrics Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics 115: 496–506, 2005. [DOI] [PubMed] [Google Scholar]
- 90.Gilmour C, Hall H, McIntyre M, Gillies L, Harrison B. Factors associated with early breastfeeding cessation in Frankston, Victoria: a descriptive study. Breastfeed Rev 17: 13–19, 2009. [PubMed] [Google Scholar]
- 91.Gohary K, LeBlanc SJ, Lissemore KD, Overton MW, Von Massow M, Duffield TF. Effect of prepartum administration of recombinant bovine somatotropin on health and performance of lactating dairy cows. J Dairy Sci 97: 6231–6241, 2014. [DOI] [PubMed] [Google Scholar]
- 92.Gopalan C. Effect of nutrition on pregnancy and lactation. Bull World Health Organ 26: 203–211, 1962. [PMC free article] [PubMed] [Google Scholar]
- 93.Gorski J. Endocrine factors in genetic improvement of milk production. J Dairy Sci 62: 814–817, 1979. [DOI] [PubMed] [Google Scholar]
- 94.Gregory RL, Wallace JP, Gfell LE, Marks J, King BA. Effect of exercise on milk immunoglobulin A. Med Sci Sports Exerc 29: 1596–1601, 1997. [DOI] [PubMed] [Google Scholar]
- 95.Grigor MR, Gain KR. The effect of starvation and refeeding on lipogenic enzymes in mammary glands and livers of lactating rats. Biochem J 216: 515–518, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Grill CJ, Cohick WS, Sherman AR. Postpubertal development of the rat mammary gland is preserved during iron deficiency. J Nutr 131: 1444–1448, 2001. [DOI] [PubMed] [Google Scholar]
- 97.Grilo EC, Lima MS, Cunha LR, Gurgel CS, Clemente HA, Dimenstein R. Effect of maternal vitamin A supplementation on retinol concentration in colostrum. J Pediatr (Rio J) 91: 81–86, 2015. [DOI] [PubMed] [Google Scholar]
- 98.Guclu-Geyik F, Onat A, Coban N, Komurcu-Bayrak E, Sansoy V, Can G, Erginel-Unaltuna N. Minor allele of the APOA4 gene T347S polymorphism predisposes to obesity in postmenopausal Turkish women. Mol Biol Rep 39: 10907–10914, 2012. [DOI] [PubMed] [Google Scholar]
- 99.Guerrero ML, Morrow RC, Calva JJ, Ortega-Gallegos H, Weller SC, Ruiz-Palacios GM, Morrow AL. Rapid ethnographic assessment of breastfeeding practices in periurban Mexico City. Bull World Health Organ 77: 323–330, 1999. [PMC free article] [PubMed] [Google Scholar]
- 100.Gunn AJ, Gunn TR, Rabone DL, Breier BH, Blum WF, Gluckman PD. Growth hormone increases breast milk volumes in mothers of preterm infants. Pediatrics 98: 279–282, 1996. [PubMed] [Google Scholar]
- 101.Gurmu F, Hussein S, Laing M. The potential of orange-fleshed sweet potato to prevent vitamin A deficiency in Africa. Int J Vitam Nutr Res 84: 65–78, 2014. [DOI] [PubMed] [Google Scholar]
- 102.Guy M, Lowe LC, Bretherton-Watt D, Mansi JL, Peckitt C, Bliss J, Wilson RG, Thomas V, Colston KW. Vitamin D receptor gene polymorphisms and breast cancer risk. Clin Cancer Res 10: 5472–5481, 2004. [DOI] [PubMed] [Google Scholar]
- 103.Haaksma CJ, Schwartz RJ, Tomasek JJ. Myoepithelial cell contraction and milk ejection are impaired in mammary glands of mice lacking smooth muscle alpha-actin. Biol Reprod 85: 13–21, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hachey DL, Silber GH, Wong WW, Garza C. Human lactation. II: Endogenous fatty acid synthesis by the mammary gland. Pediatr Res 25: 63–68, 1989. [DOI] [PubMed] [Google Scholar]
- 105.Hallen IP, Jorhem L, Lagerkvist BJ, Oskarsson A. Lead and cadmium levels in human milk and blood. Sci Total Environ 166: 149–155, 1995. [DOI] [PubMed] [Google Scholar]
- 106.Halwachs S, Wassermann L, Lindner S, Zizzadoro C, Honscha W. Fungicide prochloraz and environmental pollutant dioxin induce the ABCG2 transporter in bovine mammary epithelial cells by the arylhydrocarbon receptor signaling pathway. Toxicol Sci 131: 491–501, 2013. [DOI] [PubMed] [Google Scholar]
- 107.Hapon MB, Simoncini M, Via G, Jahn GA. Effect of hypothyroidism on hormone profiles in virgin, pregnant and lactating rats, and on lactation. Reproduction 126: 371–382, 2003. [DOI] [PubMed] [Google Scholar]
- 108.Hapon MB, Varas SM, Gimenez MS, Jahn GA. Reduction of mammary and liver lipogenesis and alteration of milk composition during lactation in rats by hypothyroidism. Thyroid 17: 11–18, 2007. [DOI] [PubMed] [Google Scholar]
- 109.Harris J, Stanford PM, Sutherland K, Oakes SR, Naylor MJ, Robertson FG, Blazek KD, Kazlauskas M, Hilton HN, Wittlin S, Alexander WS, Lindeman GJ, Visvader JE, Ormandy CJ. Socs2 and elf5 mediate prolactin-induced mammary gland development. Mol Endocrinol 20: 1177–1187, 2006. [DOI] [PubMed] [Google Scholar]
- 110.Harslof LB, Larsen LH, Ritz C, Hellgren LI, Michaelsen KF, Vogel U, Lauritzen L. FADS genotype and diet are important determinants of DHA status: a cross-sectional study in Danish infants. Am J Clin Nutr 97: 1403–1410, 2013. [DOI] [PubMed] [Google Scholar]
- 111.Hassiotou F, Hartmann PE. At the dawn of a new discovery: the potential of breast milk stem cells. Adv Nutr 5: 770–778, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hayes BJ, Pryce J, Chamberlain AJ, Bowman PJ, Goddard ME. Genetic architecture of complex traits and accuracy of genomic prediction: coat colour, milk-fat percentage, and type in Holstein cattle as contrasting model traits. PLoS Genet 6: e1001139, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Heath AL, Tuttle CR, Simons MS, Cleghorn CL, Parnell WR. A longitudinal study of breastfeeding and weaning practices during the first year of life in Dunedin, New Zealand. J Am Diet Assoc 102: 937–943, 2002. [DOI] [PubMed] [Google Scholar]
- 114.Hennigar SR, Seo YA, Sharma S, Soybel DI, Kelleher SL. ZnT2 is a critical mediator of lysosomal-mediated cell death during early mammary gland involution. Sci Rep 5: 8033, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hennigar SR, Velasquez V, Kelleher SL. Obesity-Induced Inflammation Is Associated with Alterations in Subcellular Zinc Pools and Premature Mammary Gland Involution in Lactating Mice. J Nutr 145: 1999–2005, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hennighausen L, Robinson GW. Information networks in the mammary gland. Nat Rev Mol Cell Biol 6: 715–725, 2005. [DOI] [PubMed] [Google Scholar]
- 117.Hernandez LL, Stiening CM, Wheelock JB, Baumgard LH, Parkhurst AM, Collier RJ. Evaluation of serotonin as a feedback inhibitor of lactation in the bovine. J Dairy Sci 91: 1834–1844, 2008. [DOI] [PubMed] [Google Scholar]
- 118.Horseman ND, Zhao W, Montecino-Rodriguez E, Tanaka M, Nakashima K, Engle SJ, Smith F, Markoff E, Dorshkind K. Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J 16: 6926–6935, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huang W, Penagaricano F, Ahmad KR, Lucey JA, Weigel KA, Khatib H. Association between milk protein gene variants and protein composition traits in dairy cattle. J Dairy Sci 95: 440–449, 2012. [DOI] [PubMed] [Google Scholar]
- 120.Huang YL, Zhao F, Luo CC, Zhang X, Si Y, Sun Z, Zhang L, Li QZ, Gao XJ. SOCS3-mediated blockade reveals major contribution of JAK2/STAT5 signaling pathway to lactation and proliferation of dairy cow mammary epithelial cells in vitro. Molecules 18: 12987–13002, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ibeagha-Awemu EM, Akwanji KA, Beaudoin F, Zhao X. Associations between variants of FADS genes and omega-3 and omega-6 milk fatty acids of Canadian Holstein cows. BMC Genet 15: 25, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Innis SM. Impact of maternal diet on human milk composition and neurological development of infants. Am J Clin Nutr 99: 734S–741S, 2014. [DOI] [PubMed] [Google Scholar]
- 123.Institute of Medicine (US); Subcommittee on Nutrition During Lactation; US Health Resources and Services Administration. Nutrition During Lactation. Washington, DC: National Academy, 1991, p. xiii, 309 p. [Google Scholar]
- 124.Ip S, Chung M, Raman G, Chew P, Magula N, DeVine D, Trikalinos T, Lau J. Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess (Full Rep) 1–186, 2007. [PMC free article] [PubMed]
- 125.Itsumura N, Inamo Y, Okazaki F, Teranishi F, Narita H, Kambe T, Kodama H. Compound heterozygous mutations in SLC30A2/ZnT2 results in low milk zinc concentrations: a novel mechanism for zinc deficiency in a breast-fed infant. PLoS One 8: e64045, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jahchan NS, Wang D, Bissell MJ, Luo K. SnoN regulates mammary gland alveologenesis and onset of lactation by promoting prolactin/Stat5 signaling. Development 139: 3147–3156, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Janosi S, Kulcsar M, Korodi P, Katai L, Reiczigel J, Dieleman SJ, Nikolic JA, Salyi G, Ribiczey-Szabo P, Huszenicza G. Energy imbalance related predisposition to mastitis in group-fed high-producing postpartum dairy cows. Acta Vet Hung 51: 409–424, 2003. [DOI] [PubMed] [Google Scholar]
- 128.Jenness R, Wong NP, Marth EH, Keeney M. Fundamentals of Dairy Chemistry. New York: Springer US, 1988. [Google Scholar]
- 129.Jensen DR, Gavigan S, Sawicki V, Witsell DL, Eckel RH, Neville MC. Regulation of lipoprotein lipase activity and mRNA in the mammary gland of the lactating mouse. Biochem J 298: 321–327, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jevitt C, Hernandez I, Groer M. Lactation complicated by overweight and obesity: supporting the mother and newborn. J Midwifery Womens Health 52: 606–613, 2007. [DOI] [PubMed] [Google Scholar]
- 131.Jonas W, Mileva-Seitz V, Girard AW, Bisceglia R, Kennedy JL, Sokolowski M, Meaney MJ, Fleming AS, Steiner M, Team MR. Genetic variation in oxytocin rs2740210 and early adversity associated with postpartum depression and breastfeeding duration. Genes Brain Behav 12: 681–694, 2013. [DOI] [PubMed] [Google Scholar]
- 132.Kallio MJ, Salmenpera L, Siimes MA, Perheentupa J, Gylling H, Miettinen TA. Apoprotein E phenotype determines serum cholesterol in infants during both high-cholesterol breast feeding and low-cholesterol formula feeding. J Lipid Res 38: 759–764, 1997. [PubMed] [Google Scholar]
- 133.Kamikawa A, Ichii O, Yamaji D, Imao T, Suzuki C, Okamatsu-Ogura Y, Terao A, Kon Y, Kimura K. Diet-induced obesity disrupts ductal development in the mammary glands of nonpregnant mice. Dev Dyn 238: 1092–1099, 2009. [DOI] [PubMed] [Google Scholar]
- 134.Kaminski S, Cieslinska A, Kostyra E. Polymorphism of bovine beta-casein and its potential effect on human health. J Appl Genet 48: 189–198, 2007. [DOI] [PubMed] [Google Scholar]
- 135.Karall D, Ndayisaba JP, Heichlinger A, Kiechl-Kohlendorfer U, Stojakovic S, Leitner H, Scholl-Burgi S. Breast-feeding Duration: Early Weaning—Do We Sufficiently Consider the Risk Factors? J Pediatr Gastroenterol Nutr 61: 577–582, 2015. [DOI] [PubMed] [Google Scholar]
- 136.Kelleher SL, Lonnerdal B. Low vitamin a intake affects milk iron level and iron transporters in rat mammary gland and liver. J Nutr 135: 27–32, 2005. [DOI] [PubMed] [Google Scholar]
- 137.Kelleher SL, Lonnerdal B. Zinc transporters in the rat mammary gland respond to marginal zinc and vitamin A intakes during lactation. J Nutr 132: 3280–3285, 2002. [DOI] [PubMed] [Google Scholar]
- 138.Kennett JE, McKee DT. Oxytocin: an emerging regulator of prolactin secretion in the female rat. J Neuroendocrinol 24: 403–412, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kienast A, Roth B, Bossier C, Hojabri C, Hoeger PH. Zinc-deficiency dermatitis in breast-fed infants. Eur J Pediatr 166: 189–194, 2007. [DOI] [PubMed] [Google Scholar]
- 140.Kishore A, Mukesh M, Sobti RC, Kataria RS, Mishra BP, Sodhi M. Analysis of genetic variations across regulatory and coding regions of kappa-casein gene of Indian native cattle (Bos indicus) and buffalo (Bubalus bubalis). Meta Gene 2: 769–781, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Koletzko B, Aggett PJ, Bindels JG, Bung P, Ferre P, Gil A, Lentze MJ, Roberfroid M, Strobel S. Growth, development and differentiation: a functional food science approach. Br J Nutr 80, Suppl 1: S5–S45, 1998. [DOI] [PubMed] [Google Scholar]
- 142.Kovacs CS. Calcium and bone metabolism during pregnancy and lactation. J Mammary Gland Biol Neoplasia 10: 105–118, 2005. [DOI] [PubMed] [Google Scholar]
- 143.Kovacs CS. Maternal Mineral and Bone Metabolism During Pregnancy, Lactation, and Post-Weaning Recovery. Physiol Rev 96: 449–547, 2016. [DOI] [PubMed] [Google Scholar]
- 144.Kovacs CS, Kronenberg HM. Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr Rev 18: 832–872, 1997. [DOI] [PubMed] [Google Scholar]
- 145.Krebs NF, Reidinger CJ, Hartley S, Robertson AD, Hambidge KM. Zinc supplementation during lactation: effects on maternal status and milk zinc concentrations. Am J Clin Nutr 61: 1030–1036, 1995. [DOI] [PubMed] [Google Scholar]
- 146.Kucia M, Langhammer M, Gors S, Albrecht E, Hammon HM, Nurnberg G, Metges CC. High-protein diet during gestation and lactation affects mammary gland mRNA abundance, milk composition and pre-weaning litter growth in mice. Animal 5: 268–277, 2011. [DOI] [PubMed] [Google Scholar]
- 147.Kulski JK, Hartmann PE. Changes in human milk composition during the initiation of lactation. Aust J Exp Biol Med Sci 59: 101–114, 1981. [DOI] [PubMed] [Google Scholar]
- 148.La Vecchia C, Giordano SH, Hortobagyi GN, Chabner B. Overweight, obesity, diabetes, and risk of breast cancer: interlocking pieces of the puzzle. Oncologist 16: 726–729, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.LaKind JS, Berlin CM Jr, Sjodin A, Turner W, Wang RY, Needham LL, Paul IM, Stokes JL, Naiman DQ, Patterson DG Jr. Do human milk concentrations of persistent organic chemicals really decline during lactation? Chemical concentrations during lactation and milk/serum partitioning Environ Health Perspect 117: 1625–1631, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lanoue L, Koski KG. Glucose-restricted diets alter milk composition and mammary gland development in lactating rat dams. J Nutr 124: 94–102, 1994. [DOI] [PubMed] [Google Scholar]
- 151.Laporta J, Penagaricano F, Hernandez LL. Transcriptomic Analysis of the Mouse Mammary Gland Reveals New Insights for the Role of Serotonin in Lactation. PLoS One 10: e0140425, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lasry I, Golan Y, Berman B, Amram N, Glaser F, Assaraf YG. In situ dimerization of multiple wild type and mutant zinc transporters in live cells using bimolecular fluorescence complementation. J Biol Chem 289: 7275–7292, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Latuga MS, Stuebe A, Seed PC. A review of the source and function of microbiota in breast milk. Semin Reprod Med 32: 68–73, 2014. [DOI] [PubMed] [Google Scholar]
- 154.Lauring J, Park BH, Wolff AC. The phosphoinositide-3-kinase-Akt-mTOR pathway as a therapeutic target in breast cancer. J Natl Compr Canc Netw 11: 670–678, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Le TN, Elsea SH, Romero R, Chaiworapongsa T, Francis GL. Prolactin receptor gene polymorphisms are associated with gestational diabetes. Genet Test Mol Biomarkers 17: 567–571, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Le TT, Rehrer CW, Huff TB, Nichols MB, Camarillo IG, Cheng JX. Nonlinear optical imaging to evaluate the impact of obesity on mammary gland and tumor stroma. Mol Imaging 6: 205–211, 2007. [PMC free article] [PubMed] [Google Scholar]
- 157.Lee E, Hsu C, Van den Berg D, Ursin G, Koh WP, Yuan JM, Stram DO, Yu MC, Wu AH. Genetic variation in peroxisome proliferator-activated receptor gamma, soy, and mammographic density in Singapore Chinese women. Cancer Epidemiol Biomarkers Prev 21: 635–644, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lee S, Hennigar SR, Alam S, Nishida K, Kelleher SL. Essential Role for Zinc Transporter 2 (ZnT2)-mediated Zinc Transport in Mammary Gland Development and Function during Lactation. J Biol Chem 290: 13064–13078, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lemay DG, Ballard OA, Hughes MA, Morrow AL, Horseman ND, Nommsen-Rivers LA. RNA sequencing of the human milk fat layer transcriptome reveals distinct gene expression profiles at three stages of lactation. PLoS One 8: e67531, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lemay DG, Pollard KS, Martin WF, Freeman Zadrowski C, Hernandez J, Korf I, German JB, Rijnkels M. From genes to milk: genomic organization and epigenetic regulation of the mammary transcriptome. PLoS One 8: e75030, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lepe M, Bacardí Gascón M, Castañeda-González LM, Pérez Morales ME, Jiménez Cruz A. Effect of maternal obesity on lactation: systematic review. Nutr Hosp 26: 1266–1269, 2011. [DOI] [PubMed] [Google Scholar]
- 162.Li R, Fein SB, Chen J, Grummer-Strawn LM. Why mothers stop breastfeeding: mothers' self-reported reasons for stopping during the first year. Pediatrics 122, Suppl 2: S69–S76, 2008. [DOI] [PubMed] [Google Scholar]
- 163.Lim HY, Im KS, Kim NH, Kim HW, Shin JI, Sur JH. Obesity, expression of adipocytokines, and macrophage infiltration in canine mammary tumors. Vet J 203: 326–331, 2015. [DOI] [PubMed] [Google Scholar]
- 164.Livingstone VH. Problem-Solving Formula for Failure to Thrive in Breast-fed Infants. Can Fam Physician 36: 1541–1545, 1990. [PMC free article] [PubMed] [Google Scholar]
- 165.Lonnerdal B. Trace element transport in the mammary gland. Annu Rev Nutr 27: 165–177, 2007. [DOI] [PubMed] [Google Scholar]
- 166.Lovelady C. Balancing exercise and food intake with lactation to promote post-partum weight loss. Proc Nutr Soc 70: 181–184, 2011. [DOI] [PubMed] [Google Scholar]
- 167.Lovelady CA, Fuller CJ, Geigerman CM, Hunter CP, Kinsella TC. Immune status of physically active women during lactation. Med Sci Sports Exerc 36: 1001–1007, 2004. [DOI] [PubMed] [Google Scholar]
- 168.Lovelady CA, Lonnerdal B, Dewey KG. Lactation performance of exercising women. Am J Clin Nutr 52: 103–109, 1990. [DOI] [PubMed] [Google Scholar]
- 169.Lowe AJ, Thien FC, Stoney RM, Bennett CM, Hosking CS, Hill DJ, Carlin JB, Abramson MJ, Dharmage SC. Associations between fatty acids in colostrum and breast milk and risk of allergic disease. Clin Exp Allergy 38: 1745–1751, 2008. [DOI] [PubMed] [Google Scholar]
- 170.Lucis OJ, Lucis R, Shaikh ZA. Cadmium and zinc in pregnancy and lactation. Arch Environ Health 25: 14–22, 1972. [DOI] [PubMed] [Google Scholar]
- 171.Lundqvist C, Zuurbier M, Leijs M, Johansson C, Ceccatelli S, Saunders M, Schoeters G, ten Tusscher G, Koppe JG. The effects of PCBs and dioxins on child health. Acta Paediatr Suppl 95: 55–64, 2006. [DOI] [PubMed] [Google Scholar]
- 172.Mach N, Blum Y, Bannink A, Causeur D, Houee-Bigot M, Lagarrigue S, Smits MA. Pleiotropic effects of polymorphism of the gene diacylglycerol-O-transferase 1 (DGAT1) in the mammary gland tissue of dairy cows. J Dairy Sci 95: 4989–5000, 2012. [DOI] [PubMed] [Google Scholar]
- 173.Mackle TR, Dwyer DA, Bauman DE. Intramammary infusion of insulin or long R3 insulin-like growth factor-I did not increase milk protein yield in dairy cows. J Dairy Sci 83: 1740–1749, 2000. [DOI] [PubMed] [Google Scholar]
- 174.Maningat PD, Sen P, Rijnkels M, Hadsell DL, Bray MS, Haymond MW. Short-term administration of rhGH increases markers of cellular proliferation but not milk protein gene expression in normal lactating women. Physiol Genomics 43: 381–391, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Marques Mdo R, Santos IC, Carolino N, Belo CC, Renaville R, Cravador A. Effects of genetic polymorphisms at the growth hormone gene on milk yield in Serra da Estrela sheep. J Dairy Res 73: 394–405, 2006. [DOI] [PubMed] [Google Scholar]
- 176.Marshall AM, Nommsen-Rivers LA, Hernandez LL, Dewey KG, Chantry CJ, Gregerson KA, Horseman ND. Serotonin transport and metabolism in the mammary gland modulates secretory activation and involution. J Clin Endocrinol Metab 95: 837–846, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Marshall I, Mehta R, Petrova A. Vitamin D in the maternal-fetal-neonatal interface: clinical implications and requirements for supplementation. J Matern Fetal Neonatal Med 26: 633–638, 2013. [DOI] [PubMed] [Google Scholar]
- 178.Martins LF, Milazzotto MP, Feitosa WB, Coutinho AR, Simoes R, Marques MG, Assumpcao ME, Visintin JA. Sequence variation of the alpha-lactalbumin gene in Holstein and Nellore cows. Anim Biotechnol 19: 194–198, 2008. [DOI] [PubMed] [Google Scholar]
- 179.Matias SL, Dewey KG, Quesenberry CP Jr, Gunderson EP. Maternal prepregnancy obesity and insulin treatment during pregnancy are independently associated with delayed lactogenesis in women with recent gestational diabetes mellitus. Am J Clin Nutr 99: 115–121, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Matsumoto C, Miyaura C, Ito A. Dietary Bisphenol A Suppresses the Growth of Newborn Pups by Insufficient Supply of Maternal Milk in Mice. J Health Sci 50: 315–318, 2004. [Google Scholar]
- 181.Matsuno AY, Esrey KL, Perrault H, Koski KG. Low intensity exercise and varying proportions of dietary glucose and fat modify milk and mammary gland compositions and pup growth. J Nutr 129: 1167–1175, 1999. [DOI] [PubMed] [Google Scholar]
- 182.Maxa J, Neuditschko M, Russ I, Forster M, Medugorac I. Genome-wide association mapping of milk production traits in Braunvieh cattle. J Dairy Sci 95: 5357–5364, 2012. [DOI] [PubMed] [Google Scholar]
- 183.McClellan HL, Hepworth AR, Kent JC, Garbin CP, Williams TM, Hartmann PE, Geddes DT. Breastfeeding frequency, milk volume, and duration in mother-infant dyads with persistent nipple pain. Breastfeed Med 7: 275–281, 2012. [DOI] [PubMed] [Google Scholar]
- 184.McCormick NH, Hennigar SR, Kiselyov K, Kelleher SL. The biology of zinc transport in mammary epithelial cells: implications for mammary gland development, lactation, and involution. J Mammary Gland Biol Neoplasia 19: 59–71, 2014. [DOI] [PubMed] [Google Scholar]
- 185.McCormick NH, Kelleher SL. ZnT4 provides zinc to zinc-dependent proteins in the _trans_-Golgi network critical for cell function and Zn export in mammary epithelial cells. Am J Physiol Cell Physiol 303: C291–C297, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.McCormick NH, Lee S, Hennigar SR, Kelleher SL. ZnT4 (SLC30A4)-null (“lethal milk”) mice have defects in mammary gland secretion and hallmarks of precocious involution during lactation. Am J Physiol Regul Integr Comp Physiol 310: R33–R40, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.McCrory MA, Nommsen-Rivers LA, Mole PA, Lonnerdal B, Dewey KG. Randomized trial of the short-term effects of dieting compared with dieting plus aerobic exercise on lactation performance. Am J Clin Nutr 69: 959–967, 1999. [DOI] [PubMed] [Google Scholar]
- 188.McDowell GH, Gooden JM, Leenanuruksa D, Jois M, English AW. Effects of exogenous growth hormone on milk production and nutrient uptake by muscle and mammary tissues of dairy cows in mid-lactation. Aust J Biol Sci 40: 295–306, 1987. [DOI] [PubMed] [Google Scholar]
- 189.McLean DM, Graham ER, Ponzoni RW, McKenzie HA. Effects of milk protein genetic variants on milk yield and composition. J Dairy Res 51: 531–546, 1984. [DOI] [PubMed] [Google Scholar]
- 190.McNamara JP, Murray CE. Sympathetic nervous system activity in adipose tissues during pregnancy and lactation of the rat. J Dairy Sci 84: 1382–1389, 2001. [DOI] [PubMed] [Google Scholar]
- 191.Mead MN. Contaminants in human milk: weighing the risks against the benefits of breastfeeding. Environ Health Perspect 116: A427–A434, 2008. [PMC free article] [PubMed] [Google Scholar]
- 192.Meier P, Patel AL, Wright K, Engstrom JL. Management of breastfeeding during and after the maternity hospitalization for late preterm infants. Clin Perinatol 40: 689–705, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Menzies KK, Lee HJ, Lefevre C, Ormandy CJ, Macmillan KL, Nicholas KR. Insulin, a key regulator of hormone responsive milk protein synthesis during lactogenesis in murine mammary explants. Funct Integr Genomics 10: 87–95, 2010. [DOI] [PubMed] [Google Scholar]
- 194.Menzies KK, Lefevre C, Macmillan KL, Nicholas KR. Insulin regulates milk protein synthesis at multiple levels in the bovine mammary gland. Funct Integr Genomics 9: 197–217, 2009. [DOI] [PubMed] [Google Scholar]
- 195.Mercier JC, Vilotte JL. Structure and function of milk protein genes. J Dairy Sci 76: 3079–3098, 1993. [DOI] [PubMed] [Google Scholar]
- 196.Miletta MC, Bieri A, Kernland K, Schöni MH, Petkovic V, Flück CE, Eblé A, Mullis PE. Transient Neonatal Zinc Deficiency Caused by a Heterozygous G87R Mutation in the Zinc Transporter ZnT-2 (SLC30A2) Gene in the Mother Highlighting the Importance of Zn (2+) for Normal Growth and Development. Int J Endocrinol 2013: 259189, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Milsom SR, Breier BH, Gallaher BW, Cox VA, Gunn AJ, Gluckman PD. Growth hormone stimulates galactopoiesis in healthy lactating women. Acta Endocrinol (Copenh) 127: 337–343, 1992. [DOI] [PubMed] [Google Scholar]
- 198.Milsom SR, Rabone DL, Gunn AJ, Gluckman PD. Potential role for growth hormone in human lactation insufficiency. Horm Res 50: 147–150, 1998. [DOI] [PubMed] [Google Scholar]
- 199.Mohammad MA, Haymond MW. Regulation of lipid synthesis genes and milk fat production in human mammary epithelial cells during secretory activation. Am J Physiol Endocrinol Metab 305: E700–E716, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Morales FC, Hayashi Y, van Pelt CS, Georgescu MM. NHERF1/EBP50 controls lactation by establishing basal membrane polarity complexes with prolactin receptor. Cell Death Dis 3: e391, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Moshel Y, Rhoads RE, Barash I. Role of amino acids in translational mechanisms governing milk protein synthesis in murine and ruminant mammary epithelial cells. J Cell Biochem 98: 685–700, 2006. [DOI] [PubMed] [Google Scholar]
- 202.Motil KJ, Thotathuchery M, Montandon CM, Hachey DL, Boutton TW, Klein PD, Garza C. Insulin, cortisol and thyroid hormones modulate maternal protein status and milk production and composition in humans. J Nutr 124: 1248–1257, 1994. [DOI] [PubMed] [Google Scholar]
- 203.Moyes KM, Drackley JK, Morin DE, Rodriguez-Zas SL, Everts RE, Lewin HA, Loor JJ. Predisposition of cows to mastitis in non-infected mammary glands: effects of dietary-induced negative energy balance during mid-lactation on immune-related genes. Funct Integr Genomics 11: 151–156, 2011. [DOI] [PubMed] [Google Scholar]
- 204.Moyes KM, Drackley JK, Salak-Johnson JL, Morin DE, Hope JC, Loor JJ. Dietary-induced negative energy balance has minimal effects on innate immunity during a Streptococcus uberis mastitis challenge in dairy cows during midlactation. J Dairy Sci 92: 4301–4316, 2009. [DOI] [PubMed] [Google Scholar]
- 205.Murphy EA, Davis JM, Barrilleaux TL, McClellan JL, Steiner JL, Carmichael MD, Pena MM, Hebert JR, Green JE. Benefits of exercise training on breast cancer progression and inflammation in C3(1)SV40Tag mice. Cytokine 55: 274–279, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Neifert M, DeMarzo S, Seacat J, Young D, Leff M, Orleans M. The influence of breast surgery, breast appearance, and pregnancy-induced breast changes on lactation sufficiency as measured by infant weight gain. Birth 17: 31–38, 1990. [DOI] [PubMed] [Google Scholar]
- 207.Neifert MR. Prevention of breastfeeding tragedies. Pediatr Clin North Am 48: 273–297, 2001. [DOI] [PubMed] [Google Scholar]
- 208.Neville MC. Calcium secretion into milk. J Mammary Gland Biol Neoplasia 10: 119–128, 2005. [DOI] [PubMed] [Google Scholar]
- 209.Neville MC, Anderson SM, McManaman JL, Badger TM, Bunik M, Contractor N, Crume T, Dabelea D, Donovan SM, Forman N, Frank DN, Friedman JE, German JB, Goldman A, Hadsell D, Hambidge M, Hinde K, Horseman ND, Hovey RC, Janoff E, Krebs NF, Lebrilla CB, Lemay DG, MacLean PS, Meier P, Morrow AL, Neu J, Nommsen-Rivers LA, Raiten DJ, Rijnkels M, Seewaldt V, Shur BD, VanHouten J, Williamson P. Lactation and neonatal nutrition: defining and refining the critical questions. J Mammary Gland Biol Neoplasia 17: 167–188, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Neville MC, Morton J. Physiology and endocrine changes underlying human lactogenesis II. J Nutr 131: 3005S–3008S, 2001. [DOI] [PubMed] [Google Scholar]
- 211.Neville MC, Walsh CT. Effects of xenobiotics on milk secretion and composition. Am J Clin Nutr 61: 687S–694S, 1995. [DOI] [PubMed] [Google Scholar]
- 212.Newey PJ, Gorvin CM, Cleland SJ, Willberg CB, Bridge M, Azharuddin M, Drummond RS, van der Merwe PA, Klenerman P, Bountra C, Thakker RV. Mutant prolactin receptor and familial hyperprolactinemia. N Engl J Med 369: 2012–2020, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ng-Kwai-Hang KF, Hayes JF, Moxley JE, Monardes HG. Association of genetic variants of casein and milk serum proteins with milk, fat, and protein production by dairy cattle. J Dairy Sci 67: 835–840, 1984. [DOI] [PubMed] [Google Scholar]
- 214.Niranjan SK, Goyal S, Dubey PK, Kumari N, Mishra SK, Mukesh M, Kataria RS. Genetic diversity analysis of buffalo fatty acid synthase (FASN) gene and its differential expression among bovines. Gene 575: 506–512, 2016. [DOI] [PubMed] [Google Scholar]
- 215.Nyante SJ, Faupel-Badger JM, Sherman ME, Pfeiffer RM, Gaudet MM, Falk RT, Andaya AA, Lissowska J, Brinton LA, Peplonska B, Vonderhaar BK, Chanock S, Garcia-Closas M, Figueroa JD. Genetic variation in PRL and PRLR, and relationships with serum prolactin levels and breast cancer risk: results from a population-based case-control study in Poland. Breast Cancer Res 13: R42, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Nyman AK, Emanuelson U, Holtenius K, Ingvartsen KL, Larsen T, Waller KP. Metabolites and immune variables associated with somatic cell counts of primiparous dairy cows. J Dairy Sci 91: 2996–3009, 2008. [DOI] [PubMed] [Google Scholar]
- 217.Ohrvik H, Yoshioka M, Oskarsson A, Tallkvist J. Cadmium-induced disturbances in lactating mammary glands of mice. Toxicol Lett 164: 207–213, 2006. [DOI] [PubMed] [Google Scholar]
- 218.Oladapo OT, Fawole B. Treatments for suppression of lactation. Cochrane Database Syst Rev 9: CD005937, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ollivier-Bousquet M, Guesnet P, Seddiki T, Durand G. Deficiency of (n-6) but not (n-3) polyunsaturated fatty acids inhibits the secretagogue effect of prolactin in lactating rat mammary epithelial cells. J Nutr 123: 2090–2100, 1993. [DOI] [PubMed] [Google Scholar]
- 220.Ormandy CJ, Camus A, Barra J, Damotte D, Lucas B, Buteau H, Edery M, Brousse N, Babinet C, Binart N, Kelly PA. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev 11: 167–178, 1997. [DOI] [PubMed] [Google Scholar]
- 221.Ostrom KM. A review of the hormone prolactin during lactation. Prog Food Nutr Sci 14: 1–43, 1990. [PubMed] [Google Scholar]
- 222.Ota VK, Chen ES, Ejchel TF, Furuya TK, Mazzotti DR, Cendoroglo MS, Ramos LR, Araujo LQ, Burbano RR, Smith Mde A. APOA4 polymorphism as a risk factor for unfavorable lipid serum profile and depression: a cross-sectional study. J Investig Med 59: 966–970, 2011. [DOI] [PubMed] [Google Scholar]
- 223.Owens MB, Hill AD, Hopkins AM. Ductal barriers in mammary epithelium. Tissue Barriers 1: e25933, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Padovani M, Lavigne JA, Chandramouli GV, Perkins SN, Barrett JC, Hursting SD, Bennett LM, Berrigan D. Distinct effects of calorie restriction and exercise on mammary gland gene expression in C57BL/6 mice. Cancer Prev Res (Phila) 2: 1076–1087, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Pang WW, Hartmann PE. Initiation of human lactation: secretory differentiation and secretory activation. J Mammary Gland Biol Neoplasia 12: 211–221, 2007. [DOI] [PubMed] [Google Scholar]
- 226.Park B, Shin A, Kim KZ, Lee YS, Hwang JA, Kim Y, Sung J, Yoo KY, Lee ES. Lack of effects of peroxisome proliferator-activated receptor gamma genetic polymorphisms on breast cancer risk: a case-control study and pooled analysis. Asian Pac J Cancer Prev 15: 9093–9099, 2014. [DOI] [PubMed] [Google Scholar]
- 227.Patton S, Jensen RS. Biomedical Aspects of Lactation: With Special Reference to Lipid Metabolism and Membrane Functions of the Mammary Gland. Oxford, UK: Pergamon, 1976. [Google Scholar]
- 228.Pau MY, Milner JA. Effect of arginine deficiency on mammary gland development in the rat. J Nutr 112: 1827–1833, 1982. [DOI] [PubMed] [Google Scholar]
- 229.Peaker M, Wilde CJ. Feedback control of milk secretion from milk. J Mammary Gland Biol Neoplasia 1: 307–315, 1996. [DOI] [PubMed] [Google Scholar]
- 230.Peel CJ, Fronk TJ, Bauman DE, Gorewit RC. Effect of exogenous growth hormone in early and late lactation on lactational performance of dairy cows. J Dairy Sci 66: 776–782, 1983. [DOI] [PubMed] [Google Scholar]
- 231.Peters JM. Hypernatremia in breast-fed infants due to elevated breast milk sodium. J Am Osteopath Assoc 89: 1165–1170, 1989. [PubMed] [Google Scholar]
- 232.Petersson Grawé K, Oskarsson A. Cadmium in milk and mammary gland in rats and mice. Arch Toxicol 73: 519–527, 2000. [DOI] [PubMed] [Google Scholar]
- 233.Pettibone DJ, Woyden CJ, Totaro JA. Identification of functional oxytocin receptors in lactating rat mammary gland in vitro. Eur J Pharmacol 188: 235–241, 1990. [DOI] [PubMed] [Google Scholar]
- 234.Picciano MF. Pregnancy and lactation: physiological adjustments, nutritional requirements and the role of dietary supplements. J Nutr 133: 1997S–2002S, 2003. [DOI] [PubMed] [Google Scholar]
- 235.Powe CE, Puopolo KM, Newburg DS, Lonnerdal B, Chen C, Allen M, Merewood A, Worden S, Welt CK. Effects of recombinant human prolactin on breast milk composition. Pediatrics 127: e359–e366, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Prentice A. Should lactating women exercise? Nutr Rev 52: 358–360, 1994. [DOI] [PubMed] [Google Scholar]
- 237.Prentice AM, Prentice A. Energy costs of lactation. Annu Rev Nutr 8: 63–79, 1988. [DOI] [PubMed] [Google Scholar]
- 238.Prinzenberg EM, Jianlin H, Erhardt G. Genetic variation in the kappa-casein gene (CSN3) of Chinese yak (Bos grunniens) and phylogenetic analysis of CSN3 sequences in the genus Bos. J Dairy Sci 91: 1198–1203, 2008. [DOI] [PubMed] [Google Scholar]
- 239.Quintas-Cardama A, Verstovsek S. Molecular pathways: Jak/STAT pathway: mutations, inhibitors, and resistance. Clin Cancer Res 19: 1933–1940, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Rahmatalla SA, Muller U, Strucken EM, Reissmann M, Brockmann GA. The F279Y polymorphism of the GHR gene and its relation to milk production and somatic cell score in German Holstein dairy cattle. J Appl Genet 52: 459–465, 2011. [DOI] [PubMed] [Google Scholar]
- 241.Raimondi S, Johansson H, Maisonneuve P, Gandini S. Review and meta-analysis on vitamin D receptor polymorphisms and cancer risk. Carcinogenesis 30: 1170–1180, 2009. [DOI] [PubMed] [Google Scholar]
- 242.Rakicioglu N, Samur G, Topcu A, Topcu AA. The effect of Ramadan on maternal nutrition and composition of breast milk. Pediatr Int 48: 278–283, 2006. [DOI] [PubMed] [Google Scholar]
- 243.Ramsay DT, Kent JC, Hartmann RA, Hartmann PE. Anatomy of the lactating human breast redefined with ultrasound imaging. J Anat 206: 525–534, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Rasmussen KM. Association of maternal obesity before conception with poor lactation performance. Annu Rev Nutr 27: 103–121, 2007. [DOI] [PubMed] [Google Scholar]
- 245.Rasmussen KM, Kjolhede CL. Prepregnant overweight and obesity diminish the prolactin response to suckling in the first week postpartum. Pediatrics 113: e465–e471, 2004. [DOI] [PubMed] [Google Scholar]
- 246.Raven LA, Cocks BG, Goddard ME, Pryce JE, Hayes BJ. Genetic variants in mammary development, prolactin signalling and involution pathways explain considerable variation in bovine milk production and milk composition. Genet Sel Evol 46: 29, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Rayner JL, Enoch RR, Fenton SE. Adverse effects of prenatal exposure to atrazine during a critical period of mammary gland growth. Toxicol Sci 87: 255–266, 2005. [DOI] [PubMed] [Google Scholar]
- 248.Reinhardt TA, Lippolis JD, Shull GE, Horst RL. Null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2 impairs calcium transport into milk. J Biol Chem 279: 42369–42373, 2004. [DOI] [PubMed] [Google Scholar]
- 249.Rennison ME, Kerr M, Addey CV, Handel SE, Turner MD, Wilde CJ, Burgoyne RD. Inhibition of constitutive protein secretion from lactating mouse mammary epithelial cells by FIL (feedback inhibitor of lactation), a secreted milk protein. J Cell Sci 106: 641–648, 1993. [DOI] [PubMed] [Google Scholar]
- 250.Rijnkels M, Freeman-Zadrowski C, Hernandez J, Potluri V, Wang L, Li W, Lemay DG. Epigenetic modifications unlock the milk protein gene loci during mouse mammary gland development and differentiation. PLoS One 8: e53270, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Rillema JA, Yu TX, Jhiang SM. Effect of prolactin on sodium iodide symporter expression in mouse mammary gland explants. Am J Physiol Endocrinol Metab 279: E769–E772, 2000. [DOI] [PubMed] [Google Scholar]
- 252.Rogan WJ, Gladen BC, McKinney JD, Carreras N, Hardy P, Thullen J, Tingelstad J, Tully M. Polychlorinated biphenyls (PCBs) and dichlorodiphenyl dichloroethene (DDE) in human milk: effects on growth, morbidity, and duration of lactation. Am J Public Health 77: 1294–1297, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Ronayne de Ferrer PA, Baroni A, Sambucetti ME, López NE, Ceriani Cernadas JM. Lactoferrin levels in term and preterm milk. J Am Coll Nutr 19: 370–373, 2000. [DOI] [PubMed] [Google Scholar]
- 254.Saben JL, Bales ES, Jackman MR, Orlicky D, MacLean PS, McManaman JL. Maternal obesity reduces milk lipid production in lactating mice by inhibiting acetyl-CoA carboxylase and impairing fatty acid synthesis. PLoS One 9: e98066, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Sakalidis VS, Williams TM, Hepworth AR, Garbin CP, Hartmann PE, Paech MJ, Al-Tamimi Y, Geddes DT. A comparison of early sucking dynamics during breastfeeding after cesarean section and vaginal birth. Breastfeed Med 8: 79–85, 2013. [DOI] [PubMed] [Google Scholar]
- 256.Sallam SM, Nasser ME, Yousef MI. Effect of recombinant bovine somatotropin on sheep milk production, composition and some hemato-biochemical components. Small Rumin Res 56: 165–171, 2005. [Google Scholar]
- 257.Sankaran L, Topper YJ. Effect of vitamin A deprivation on maintenance of rat mammary tissue and on the potential of the epithelium for hormone-dependent milk protein synthesis. Endocrinology 111: 1061–1067, 1982. [DOI] [PubMed] [Google Scholar]
- 258.Sanz-Moreno A, Fuhrmann D, Wolf E, von Eyss B, Eilers M, Elsasser HP. Miz1 deficiency in the mammary gland causes a lactation defect by attenuated Stat5 expression and phosphorylation. PLoS One 9: e89187, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Savino F, Benetti S, Liguori SA, Sorrenti M, Cordero Di Montezemolo L. Advances on human milk hormones and protection against obesity. Cell Mol Biol (Noisy-le-grand) 59: 89–98, 2013. [PubMed] [Google Scholar]
- 260.Saxena A, Sampson JR. Phenotypes associated with inherited and developmental somatic mutations in genes encoding mTOR pathway components. Semin Cell Dev Biol 36: 140–146, 2014. [DOI] [PubMed] [Google Scholar]
- 261.Schennink A, Stoop WM, Visker MH, Heck JM, Bovenhuis H, van der Poel JJ, van Valenberg HJ, van Arendonk JA. DGAT1 underlies large genetic variation in milk-fat composition of dairy cows. Anim Genet 38: 467–473, 2007. [DOI] [PubMed] [Google Scholar]
- 262.Scheplyagina LA. Impact of the mother's zinc deficiency on the woman's and newborn's health status. J Trace Elem Med Biol 19: 29–35, 2005. [DOI] [PubMed] [Google Scholar]
- 263.Semba RD, Kumwenda N, Taha TE, Hoover DR, Lan Y, Eisinger W, Mtimavalye L, Broadhead R, Miotti PG, Van Der Hoeven L, Chiphangwi JD. Mastitis and immunological factors in breast milk of lactating women in Malawi. Clin Diagn Lab Immunol 6: 671–674, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Shi DS, Wang J, Yang Y, Lu FH, Li XP, Liu QY. DGAT1, GH, GHR, PRL and PRLR polymorphism in water buffalo (Bubalus bubalis). Reprod Domest Anim 47: 328–334, 2012. [DOI] [PubMed] [Google Scholar]
- 265.Shi H, Luo J, Zhu J, Li J, Sun Y, Lin X, Zhang L, Yao D, Shi H. PPAR γ Regulates Genes Involved in Triacylglycerol Synthesis and Secretion in Mammary Gland Epithelial Cells of Dairy Goats. PPAR Res 2013: 310948, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Sibeko L, Dhansay MA, Charlton KE, Johns T, Gray-Donald K. Beliefs, attitudes, and practices of breastfeeding mothers from a periurban community in South Africa. J Hum Lact 21: 31–38, 2005. [DOI] [PubMed] [Google Scholar]
- 267.Sigman M, Lonnerdal B. Response of rat mammary gland transferrin receptors to maternal dietary iron during pregnancy and lactation. Am J Clin Nutr 52: 446–450, 1990. [DOI] [PubMed] [Google Scholar]
- 268.Singh PP, Singh M, Mastana SS. APOE distribution in world populations with new data from India and the UK. Ann Hum Biol 33: 279–308, 2006. [DOI] [PubMed] [Google Scholar]
- 269.Smilowitz JT, Lebrilla CB, Mills DA, German JB, Freeman SL. Breast milk oligosaccharides: structure-function relationships in the neonate. Annu Rev Nutr 34: 143–169, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Smith RM, Leach RM, Muller LD, Griel LC Jr, Baker DE. Effects of long-term dietary cadmium chloride on tissue, milk, and urine mineral concentrations of lactating dairy cows. J Anim Sci 69: 4088–4096, 1991. [DOI] [PubMed] [Google Scholar]
- 271.Standl M, Sausenthaler S, Lattka E, Koletzko S, Bauer CP, Wichmann HE, von Berg A, Berdel D, Kramer U, Schaaf B, Lehmann I, Herbarth O, Klopp N, Koletzko B, Heinrich J, Giniplus. Group LIS. FADS gene cluster modulates the effect of breastfeeding on asthma. Results from the GINIplus and LISAplus studies. Allergy 67: 83–90, 2012. [DOI] [PubMed] [Google Scholar]
- 272.Stelwagen K, Singh K. The role of tight junctions in mammary gland function. J Mammary Gland Biol Neoplasia 19: 131–138, 2014. [DOI] [PubMed] [Google Scholar]
- 273.Stevens GA, Bennett JE, Hennocq Q, Lu Y, De-Regil LM, Rogers L, Danaei G, Li G, White RA, Flaxman SR, Oehrle SP, Finucane MM, Guerrero R, Bhutta ZA, Then-Paulino A, Fawzi W, Black RE, Ezzati M. Trends and mortality effects of vitamin A deficiency in children in 138 low-income and middle-income countries between 1991 and 2013: a pooled analysis of population-based surveys. Lancet Glob Health 3: e528–e536, 2015. [DOI] [PubMed] [Google Scholar]
- 274.Stinnakre MG, Vilotte JL, Soulier S, Mercier JC. Creation and phenotypic analysis of alpha-lactalbumin-deficient mice. Proc Natl Acad Sci USA 91: 6544–6548, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Strum JM. Effect of iodide-deficiency on rat mammary gland. Virchows Arch B Cell Pathol Incl Mol Pathol 30: 209–220, 1979. [DOI] [PubMed] [Google Scholar]
- 276.Su D, Zhao Y, Binns C, Scott J, Oddy W. Breast-feeding mothers can exercise: results of a cohort study. Public Health Nutr 10: 1089–1093, 2007. [DOI] [PubMed] [Google Scholar]
- 277.Subbaramaiah K, Howe LR, Bhardwaj P, Du B, Gravaghi C, Yantiss RK, Zhou XK, Blaho VA, Hla T, Yang P, Kopelovich L, Hudis CA, Dannenberg AJ. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila) 4: 329–346, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 278.Suburu J, Shi L, Wu J, Wang S, Samuel M, Thomas MJ, Kock ND, Yang G, Kridel S, Chen YQ. Fatty acid synthase is required for mammary gland development and milk production during lactation. Am J Physiol Endocrinol Metab 306: E1132–E1143, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Sun Z, Shushanov S, LeRoith D, Wood TL. Decreased IGF type 1 receptor signaling in mammary epithelium during pregnancy leads to reduced proliferation, alveolar differentiation, and expression of insulin receptor substrate (IRS)-1 and IRS-2. Endocrinology 152: 3233–3245, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Szabo AL. Review article: Intrapartum neuraxial analgesia and breastfeeding outcomes: limitations of current knowledge. Anesth Analg 116: 399–405, 2013. [DOI] [PubMed] [Google Scholar]
- 281.Tazebay UH, Wapnir IL, Levy O, Dohan O, Zuckier LS, Zhao QH, Deng HF, Amenta PS, Fineberg S, Pestell RG, Carrasco N. The mammary gland iodide transporter is expressed during lactation and in breast cancer. Nat Med 6: 871–878, 2000. [DOI] [PubMed] [Google Scholar]
- 282.Thaller G, Kramer W, Winter A, Kaupe B, Erhardt G, Fries R. Effects of DGAT1 variants on milk production traits in German cattle breeds. J Anim Sci 81: 1911–1918, 2003. [DOI] [PubMed] [Google Scholar]
- 283.Thijs C, Muller A, Rist L, Kummeling I, Snijders BE, Huber M, van Ree R, Simoes-Wust AP, Dagnelie PC, van den Brandt PA. Fatty acids in breast milk and development of atopic eczema and allergic sensitisation in infancy. Allergy 66: 58–67, 2011. [DOI] [PubMed] [Google Scholar]
- 284.Tian L, Wang C, Hagen FK, Gormley M, Addya S, Soccio R, Casimiro MC, Zhou J, Powell MJ, Xu P, Deng H, Sauve AA, Pestell RG. Acetylation-defective mutant of Ppargamma is associated with decreased lipid synthesis in breast cancer cells. Oncotarget 5: 7303–7315, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Tsakiris IN, Kokkinakis E, Dumanov JM, Tzatzarakis MN, Flouris AD, Vlachou M, Tsatsakis AM. Comparative evaluation of xenobiotics in human and dietary milk: persistent organic pollutants and mycotoxins. Cell Mol Biol (Noisy-le-grand) 59: 58–66, 2013. [PubMed] [Google Scholar]
- 286.Uauy R, Dangour AD. Nutrition in brain development and aging: role of essential fatty acids. Nutr Rev 64: S24–S33; discussion S72–S91, 2006. [DOI] [PubMed] [Google Scholar]
- 287.van Hasselt PM, de Koning TJ, Kvist N, de Vries E, Lundin CR, Berger R, Kimpen JL, Houwen RH, Jorgensen MH, Verkade HJ; Netherlands Study Group for Biliary Atresia Registry. Prevention of vitamin K deficiency bleeding in breastfed infants: lessons from the Dutch and Danish biliary atresia registries. Pediatrics 121: e857–863, 2008. [DOI] [PubMed] [Google Scholar]
- 288.Villa-Osaba A, Gahete MD, Córdoba-Chacón J, de Lecea L, Pozo-Salas AI, Delgado-Lista FJ, Álvarez-Benito M, López-Miranda J, Luque RM, Castaño JP. Obesity alters gene expression for GH/IGF-I axis in mouse mammary fat pads: differential role of cortistatin and somatostatin. PLoS One 10: e0120955, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Vina JR, Puertes IR, Rodriguez A, Saez GT, Vina J. Effect of fasting on amino acid metabolism by lactating mammary gland: studies in women and rats. J Nutr 117: 533–538, 1987. [DOI] [PubMed] [Google Scholar]
- 290.Vorderstrasse BA, Fenton SE, Bohn AA, Cundiff JA, Lawrence BP. A novel effect of dioxin: exposure during pregnancy severely impairs mammary gland differentiation. Toxicol Sci 78: 248–257, 2004. [DOI] [PubMed] [Google Scholar]
- 291.Wahlig JL, Bales ES, Jackman MR, Johnson GC, McManaman JL, Maclean PS. Impact of high-fat diet and obesity on energy balance and fuel utilization during the metabolic challenge of lactation. Obesity (Silver Spring) 20: 65–75, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Wan Y, Saghatelian A, Chong LW, Zhang CL, Cravatt BF, Evans RM. Maternal PPAR gamma protects nursing neonates by suppressing the production of inflammatory milk. Genes Dev 21: 1895–1908, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Wang AC, Chen SJ, Yuh YS, Hua YM, Lu TJ, Lee CM. Breastfeeding-associated neonatal hypernatremic dehydration in a medical center: a clinical investigation. Acta Paediatr Taiwan 48: 186–190, 2007. [PubMed] [Google Scholar]
- 294.Wang L, Lin Y, Bian Y, Liu L, Shao L, Lin L, Qu B, Zhao F, Gao X, Li Q. Leucyl-tRNA synthetase regulates lactation and cell proliferation via mTOR signaling in dairy cow mammary epithelial cells. Int J Mol Sci 15: 5952–5969, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Wang X, Maltecca C, Tal-Stein R, Lipkin E, Khatib H. Association of bovine fibroblast growth factor 2 (FGF2) gene with milk fat and productive life: an example of the ability of the candidate pathway strategy to identify quantitative trait genes. J Dairy Sci 91: 2475–2480, 2008. [DOI] [PubMed] [Google Scholar]
- 296.Wang X, Proud CG. The mTOR pathway in the control of protein synthesis. Physiology (Bethesda) 21: 362–369, 2006. [DOI] [PubMed] [Google Scholar]
- 297.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 378: 815–825, 2011. [DOI] [PubMed] [Google Scholar]
- 298.Westerlind KC, McCarty HL, Gibson KJ, Strange R. Effect of exercise on the rat mammary gland: implications for carcinogenesis. Acta Physiol Scand 175: 147–156, 2002. [DOI] [PubMed] [Google Scholar]
- 299.White SS, Calafat AM, Kuklenyik Z, Villanueva L, Zehr RD, Helfant L, Strynar MJ, Lindstrom AB, Thibodeaux JR, Wood C, Fenton SE. Gestational PFOA exposure of mice is associated with altered mammary gland development in dams and female offspring. Toxicol Sci 96: 133–144, 2007. [DOI] [PubMed] [Google Scholar]
- 300.White SS, Kato K, Jia LT, Basden BJ, Calafat AM, Hines EP, Stanko JP, Wolf CJ, Abbott BD, Fenton SE. Effects of perfluorooctanoic acid on mouse mammary gland development and differentiation resulting from cross-foster and restricted gestational exposures. Reprod Toxicol 27: 289–298, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Whitmore TJ, Trengove N, Graham DF, Hartmann P. Analysis of insulin in human breast milk in mothers with type 1 and type 2 diabetes mellitus. Int J Endocrinol 2012: 296368, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Wiggans GR, Vanraden PM, Cooper TA. The genomic evaluation system in the United States: past, present, future. J Dairy Sci 94: 3202–3211, 2011. [DOI] [PubMed] [Google Scholar]
- 303.Wilde CJ, Addey CV, Boddy LM, Peaker M. Autocrine regulation of milk secretion by a protein in milk. Biochem J 305: 51–58, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Wilde CJ, Prentice A, Peaker M. Breast-feeding: matching supply with demand in human lactation. Proc Nutr Soc 54: 401–406, 1995. [DOI] [PubMed] [Google Scholar]
- 305.Winkelman LA, Overton TR. Long-acting insulins alter milk composition and metabolism of lactating dairy cows. J Dairy Sci 96: 7565–7577, 2013. [DOI] [PubMed] [Google Scholar]
- 306.Winter WE, Bazydlo LA, Harris NS. The molecular biology of human iron metabolism. Lab Med 45: 92–102, 2014. [DOI] [PubMed] [Google Scholar]
- 307.Wright KS, Quinn TJ, Carey GB. Infant acceptance of breast milk after maternal exercise. Pediatrics 109: 585–589, 2002. [DOI] [PubMed] [Google Scholar]
- 308.Xie L, Innis SM. Genetic variants of the FADS1 FADS2 gene cluster are associated with altered (n-6) and (n-3) essential fatty acids in plasma and erythrocyte phospholipids in women during pregnancy and in breast milk during lactation. J Nutr 138: 2222–2228, 2008. [DOI] [PubMed] [Google Scholar]
- 309.Yang JX, Wang CH, Xu QB, Zhao FQ, Liu JX, Liu HY. Methionyl-Methionine Promotes α-s1 Casein Synthesis in Bovine Mammary Gland Explants by Enhancing Intracellular Substrate Availability and Activating JAK2-STAT5 and mTOR-Mediated Signaling Pathways. J Nutr 145: 1748–1753, 2015. [DOI] [PubMed] [Google Scholar]
- 310.Yang Q, Kurotani R, Yamada A, Kimura S, Gonzalez FJ. Peroxisome proliferator-activated receptor alpha activation during pregnancy severely impairs mammary lobuloalveolar development in mice. Endocrinology 147: 4772–4780, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Zaki SA, Mondkar J, Shanbag P, Verma R. Hypernatremic dehydration due to lactation failure in an exclusively breastfed neonate. Saudi J Kidney Dis Transpl 23: 125–128, 2012. [PubMed] [Google Scholar]
- 312.Zettl KS, Sjaastad MD, Riskin PM, Parry G, Machen TE, Firestone GL. Glucocorticoid-induced formation of tight junctions in mouse mammary epithelial cells in vitro. Proc Natl Acad Sci USA 89: 9069–9073, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Zhang X, Zhao F, Si Y, Huang Y, Yu C, Luo C, Zhang N, Li Q, Gao X. GSK3beta regulates milk synthesis in and proliferation of dairy cow mammary epithelial cells via the mTOR/S6K1 signaling pathway. Molecules 19: 9435–9452, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Zhu JJ, Luo J, Wang W, Yu K, Wang HB, Shi HB, Sun YT, Lin XZ, Li J. Inhibition of FASN reduces the synthesis of medium-chain fatty acids in goat mammary gland. Animal 8: 1469–1478, 2014. [DOI] [PubMed] [Google Scholar]
- 315.Zimmerman DR, Goldstein L, Lahat E, Braunstein R, Stahi D, Bar-Haim A, Berkovitch M. Effect of a 24+ hour fast on breast milk composition. J Hum Lact 25: 194–198, 2009. [DOI] [PubMed] [Google Scholar]
- 316.Zuppa AA, Sindico P, Orchi C, Carducci C, Cardiello V, Romagnoli C. Safety and efficacy of galactogogues: substances that induce, maintain and increase breast milk production. J Pharm Pharm Sci 13: 162–174, 2010. [DOI] [PubMed] [Google Scholar]