Vaccines for preventing Japanese encephalitis (original) (raw)
Abstract
Background
Vaccination is recognized as the only practical measure for preventing Japanese encephalitis. Production shortage, costs, and issues of licensure impair vaccination programmes in many affected countries. Concerns over vaccine effectiveness and safety also have a negative impact on acceptance and uptake.
Objectives
To evaluate vaccines for preventing Japanese encephalitis in terms of effectiveness, adverse events, and immunogenicity.
Search methods
In March 2007, we searched the Cochrane Infectious Diseases Group Specialized Register, CENTRAL (The Cochrane Library 2007, Issue 1), MEDLINE, EMBASE, LILACS, BIOSIS, and reference lists. We also attempted to contact corresponding authors and vaccine companies.
Selection criteria
Randomized controlled trials (RCTs), including cluster‐RCTs, comparing Japanese encephalitis vaccines with placebo (inert agent or unrelated vaccine), no intervention, or alternative Japanese encephalitis vaccine.
Data collection and analysis
Authors independently extracted data and assessed methodological quality. Dichotomous data were compared with risk ratios and a 95% confidence interval (CI), and converted into percentage vaccine efficacy.
Main results
Eight RCTs involving 358,750 participants were included. These trials investigated two available and three pre‐licensure vaccines. Two RCTs assessing efficacy of the commercially available inactivated Nakayama vaccine were identified. A two‐dose schedule of the licensed vaccine provided significant protection of 95% (95% CI 10% to 100%) for one year only, while two doses of an unpurified precursor vaccine protected children by 81% (95% CI 45% to 94%) in year one and by 59% (95% CI 2% to 83%) in year two. Serious adverse events were not observed. Mild and moderate episodes of injection site soreness, fever, headache, and nausea were reported in less than 6% of children receiving inactivated vaccine compared to 0.6% of unvaccinated controls. One cluster‐RCT compared the live‐attenuated SA14‐14‐2 vaccine (widely used in China) with no intervention measuring adverse events. Fever was reported in 2.7% of vaccinees compared to 3.1% of controls, while 0.1% of both groups suffered diarrhoea or seizures. Four small pre‐licensure RCTs assessing a genetically engineered vaccine and two cell culture‐derived inactivated vaccines revealed high immunogenicity and relative safety.
Authors' conclusions
Only one of the three currently used vaccines has been assessed for efficacy in a RCT. Other RCTs have assessed their safety, however, and they appear to cause only occasional mild or moderate adverse events. Further trials of effectiveness and safety are needed for the currently used vaccines, especially concerning dose levels and schedules. Trials investigating several new vaccines are planned or in progress.
Keywords: Humans; Encephalitis, Japanese; Encephalitis, Japanese/prevention & control; Japanese Encephalitis Vaccines; Japanese Encephalitis Vaccines/adverse effects; Japanese Encephalitis Vaccines/immunology; Japanese Encephalitis Vaccines/therapeutic use; Randomized Controlled Trials as Topic; Vaccines, Attenuated; Vaccines, Attenuated/adverse effects; Vaccines, Attenuated/immunology; Vaccines, Attenuated/therapeutic use; Vaccines, Inactivated; Vaccines, Inactivated/adverse effects; Vaccines, Inactivated/immunology; Vaccines, Inactivated/therapeutic use
Two doses of an inactivated vaccine can help prevent Japanese encephalitis disease for at least one year; however, comparisons with other widely used vaccines are not available
Japanese encephalitis is a viral disease of the central nervous system with general symptoms of headache, fever, vomiting, and diarrhoea. Most people recover within a week without further complications, but approximately 1 in 300 suffers additional and severe symptoms such as disorientation, seizures, paralysis, and coma. Around thirty per cent of the severe cases are fatal and most survivors are left with serious and often chronic disabilities such as mental impairment, limb paralysis, and blindness. In this review of randomized controlled trials, a commercially available inactivated vaccine given in two doses was shown to provide disease protection for at least one year after vaccination, but with some adverse events. Disease protection by two vaccines, widely used in China but presently commercially unavailable, has not been investigated in randomized controlled trials. Further research is needed on all currently used as well as newly developed vaccines.
Background
Japanese encephalitis is a disease of the central nervous system caused by the Japanese encephalitis virus. This virus is a member of the Flavivirus genus in the Flaviviridae family, which includes several other important human pathogens such as yellow fever virus, West Nile virus, and the dengue viruses. Like most flaviviruses, Japanese encephalitis virus is transmitted by mosquitoes. The main vectors are mosquitoes of the genus Culex, in particular those of the C. vishnui subgroup (Anonymous 2000; Endy 2002). These Culex mosquitoes persist in temperate and tropical climate zones where they breed in stagnant or slow running vegetated waters. Their abundance throughout rural areas of the Asian continent is often linked to irrigation and rice cultivation practices (Lacey 1990; Takagi 1997; Sunish 2001). The Japanese encephalitis virus is transmitted between a wide range of vertebrate hosts including birds and mammals. Domestic animals, especially pigs, are generally implicated as reservoirs of the virus in terms of human infections (Reuben 1992; Peiris 1993; Anonymous 2000). Humans are not part of the natural transmission cycle, hence cannot pass the virus to other hosts (Endy 2002). The significance of livestock as reservoir hosts and the character of the vector habitats explain why Japanese encephalitis occurs almost exclusively in rural areas.
Japanese encephalitis is a considerable public health problem in most Asian regions but particularly in East, South, and South‐East Asia (Tsai 2000). Reduction or successful control of Japanese encephalitis has been reported from countries such as Japan, Thailand, and the Republic of Korea. This achievement has been attributed to vaccination and vector control programmes as well as changes in agricultural practices and general socioeconomic improvements (Endy 2002; Igarashi 2002; WHO 2003). However, in several other countries transmission is reported to be intensifying (Matsunaga 1999; Wu 1999; Sohn 2000; Tsai 2000). Continued expansion of the geographic range is also observed. Notably, the Japanese encephalitis virus was introduced into mainland Australia as recently as 1998 (Hanna 1999). The epidemiology of Japanese encephalitis varies between different regions. The disease incidence is mainly influenced by vector abundance, which again is determined by several factors including temperature, rainfall, and agricultural practices (Khan 1996; Sunish 2001). Transmission is generally epidemic during the summer months in temperate climates, whereas most subtropical and tropical zones maintain endemic transmission (Vaughn 1992). Approximately 50,000 cases of Japanese encephalitis are reported each year, yet the true incidence is suspected to be significantly higher as many countries lack adequate disease surveillance and reporting systems (WHO 1998; Tsai 2000). People of all ages are susceptible to Japanese encephalitis virus infection, yet most cases are observed for children under the age of 15 years (WHO 1998). Presumably, most adults living in endemic areas have acquired immunity from previous exposure to the Japanese encephalitis virus (Vaughn 1992). Lack of pre‐existing immunity explains why both children and adults are affected on entering endemic areas (eg as tourists) or when the virus first spreads into new regions. More than three thousand million people are currently living in areas affected by Japanese encephalitis; of those, one thousand million children are considered at risk (Kurane 2000; Tsai 2000).
Few infections by the Japanese encephalitis virus cause overt clinical disease in humans. It is estimated that on average 1 in 300 infections are symptomatic (Gajanana 1995; WHO 2003). Most people suffer a relatively mild non‐specific febrile illness, following 5 to 15 days of incubation. Apart from fever, common manifestations may include headache, runny nose, vomiting, and diarrhoea (Solomon 2002). Recovery in these cases occurs after one to six days and is generally uneventful (Gajanana 1995; Solomon 2000). Overt Japanese encephalitis, due to infection of the central nervous system, may become evident after a few days of febrile illness, as patients display confusion and reduced levels of consciousness. Severe disease progression is rapid and involves frequent episodes of seizure (especially in children) as well as paralysis and coma (Solomon 2002). Although severe Japanese encephalitis is relatively rare, reported mortality rates may be as high as 30% (Kumar 1993; Schneider 1974; Tsai 1994). Treatment of severe Japanese encephalitis is limited to intensive supportive care and fails to prevent subsequent disability in almost half of the survivors. Disabling deficits can be subtle and may resolve or diminish over time, but severe and permanent sequelae are commonly reported. These include various psychiatric and neurological conditions such as behavioural disorders, cognitive and language impairments, psychomotor deficits, limb paralysis, convulsions, and blindness (Schneider 1974; Kumar 1993; Huy 1994; Solomon 2002). Laboratory confirmation of Japanese encephalitis virus infection is required because it may be clinically confused with several other febrile diseases and other viral acute encephalitis as well as bacterial meningitis (Tsai 1994). Diagnostic tests include virus isolation from the central spinal fluid, virus isolation from brain tissue (post mortem), and detection of Japanese encephalitis antibodies in the blood or central spinal fluid.
Reduction of the disease burden caused by Japanese encephalitis is restricted by the lack of therapeutic treatment, the impracticality of animal immunization, and the absence of efficient vector control methods (IOM 1986; Hoke 1992; Igarashi 2002). As a consequence, human vaccination is considered the primary long‐term measure for preventing Japanese encephalitis (Tsai 2000). Different types of Japanese encephalitis vaccines have been developed and are currently produced in several countries. To date only one of the vaccines (inactivated mouse brain‐derived Nakayama strain) has obtained wider international licensure and commercial distribution. Japanese encephalitis vaccines are generally employed in national programmes for childhood vaccination or as vaccines for travellers visiting endemic areas (Barrett 1997; WHO 1998; Monath 2002a). However, many affected countries fail to maintain sufficient vaccine coverage or lack national vaccine policies. The absence of reliable surveillance data, difficulties of vaccine production, and shortage and high costs of the commercial vaccine are thought to restrict the implementation of vaccine programmes, especially in low‐income countries (WHO 1998; Monath 2002a). A number of recent reports on systemic and neurological adverse effects have also raised concerns over the safety of the commercially available vaccine (CDC 1993; Nothdurft 1996; Plesner 1996; Takahashi 2000; Shlim 2002; Okabe 2005). The risk of adverse events is particularly discouraging in countries where transmission rates are considered to be low or for travellers for whom the risk of disease exposure may be very limited. Efforts to develop alternative Japanese encephalitis vaccines are ongoing and include both conventional and genetically engineered vaccines (Chambers 1997; Barrett 2001; Srivastava 2001; Monath 2002a). The currently used vaccines and vaccine candidates identified by this review are detailed in Appendix 1 and described below.
Vaccines in current use
Inactivated vaccine (mouse brain‐derived Nakayama or Beijing‐1 strain)
A mouse brain‐derived vaccine based on the Nakayama or Beijing‐1 strain was originally developed in Japan. It has since been adapted for national production in a number of countries including Taiwan, India, Russia, The Republic of Korea, The Democratic People's Republic of Korea, Thailand, China, and Vietnam. Vaccination schedules in terms of dose volume and allotments, booster regimens, vaccination age, and coverage rate are determined nationally and vary considerably among different countries (Barrett 1997; Tsai 2000). The Nakayama vaccine is presently the only commercially available vaccine with wider international distribution (WHO 2003). However, the major manufacturers have recently stopped the production of this vaccine in expectation of new cell‐derived formulae (PATH 2006a).
Live‐attenuated vaccine (SA14‐14‐2 strain)
The attenuated vaccine clone, SA14‐14‐2, was generated by serial passage of the SA14 strain in primary hamster kidney cell cultures. It has been licensed in China since 1988 where currently it is administered to more than 20 million children each year. The vaccine regimen dictates one dose at age one year with subsequent boosters at ages two and six years (WHO 1998; Tsai 2000; Monath 2002a). The license to manufacture and distribute the SA14‐14‐2 vaccine was obtained from the Chinese Government in the mid‐1990s by a commercial company in the Republic of Korea. The vaccine has recently been approved in this country as well as in Nepal, Sri Lanka, and India (PATH 2006b). The live‐attenuated vaccine currently constitutes more than 50% of the global production of all Japanese encephalitis vaccines (WHO 2005a). Vaccine production and control standards have been upgraded in order to acquire wider international licensure (Glovax 2005; WHO 2005a). Further efficacy and safety studies are also planned (WHO 2005a).
Inactivated vaccine (Beijing‐3 strain)
A cell culture‐derived vaccine based on the Beijing‐3 strain (P‐3) was developed in primary hamster kidney cells in China in 1968. Although widely used in China and relatively inexpensive, this vaccine is being gradually replaced by the live‐attenuated SA14‐14‐2 vaccine that requires fewer doses (Barrett 1997; Monath 2002a).
Vaccine candidates
Inactivated vaccine (Vero cell‐derived Beijing‐1 strain)
A vaccine candidate based on the Beijing‐1 strain derived from Vero cell cultures is currently under late‐stage clinical assessment (WHO 2005b).
Inactivated vaccine (Vero cell SA14‐14‐2 strain)
A vaccine candidate derived from Vero cell cultures using the attenuated SA14‐14‐2 strain has been investigated in early clinical trials. Late‐stage assessment and initial manufacturing procedures were initiated in 2006 (Chang 2004; WHO 2005b).
Genetically engineered vaccine (SA14‐14‐2 and YF 17D chimera)
A genetically engineered vaccine that combines the attenuated SA14‐14‐2 strain and yellow fever vaccine strain 17D (YF 17D) has been tested in several clinical trials and is under continued assessment (Acambis 2007b).
Genetically engineered vaccine (Nakayama and poxvirus recombinant)
Two recombinant vaccines using the Nakayama strain with poxvirus vectors (vaccinia and canarypox viruses) have been tested in an early trial. Development of both candidates was discontinued following the initial assessment of safety and immunogenicity (Tsai 1994).
Objectives
To evaluate vaccines for preventing Japanese encephalitis in terms of effectiveness, adverse events, and immunogenicity.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs), including cluster‐RCTs.
Types of participants
People of all ages.
Types of interventions
Intervention
Japanese encephalitis vaccine (live‐attenuated, inactivated, subunit, or genetically engineered vaccine) administered by any route and in any dose.
Control
Placebo (inert agent or unrelated vaccine), no intervention, or alternative Japanese encephalitis vaccine.
Types of outcome measures
Effectiveness
- Primary: Laboratory‐confirmed Japanese encephalitis virus infections (clinical and asymptomatic) during the follow‐up period as detailed by trial authors.
- Secondary: Severe Japanese encephalitis (including confusion, reduced level of consciousness, seizures, paralysis and coma followed by death, partial or full recovery) confirmed by standard laboratory tests.
Immunogenicity
Immunological endpoints (as detailed by trial authors) presented in the form of seroconversion rates.
Adverse events
Any adverse event; where detailed, we categorized events as serious (fatal, life threatening, or required hospitalization), moderate (caused systemic effects), or mild (caused local effects).
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press, and in progress).
Databases
We searched the following databases using the search terms and strategy described in Appendix 2: Cochrane Infectious Diseases Group Specialized Register (March 2007); Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library (2007, Issue 1); MEDLINE (1966 to March 2007); EMBASE (1974 to March 2007); LILACS (1982 to March 2007); and BIOSIS (1985 to March 2007).
Organizations
We contacted the U.S. Army Medical Component, Armed Forces Research Institute of Medical Sciences (Dr Hoke) in October 2000 and the Chengdu Institute of Biological Products (Dr Lifeng) in February 2005.
Reference lists
We also checked the reference lists of all studies identified by the above methods.
Data collection and analysis
Selection of studies
KLS and MS screened the titles and abstracts of all potentially relevant trials identified by the search strategy. The corresponding full articles were retrieved, and we independently assessed the studies for inclusion using a standard eligibility form based on the inclusion criteria. We also scrutinized papers for evidence of duplicate reporting of the same trial data. We resolved differences in opinion through discussion and described the excluded studies in the 'Characteristics of excluded studies'.
Data extraction and management
All three authors independently extracted the outcome data and details of trial characteristics into a standard extraction form for each trial. The corresponding publication author was contacted in a single case of unclear or missing data. We resolved any differences through discussion.
We aimed to extract data in order to carry out a complete‐case analysis; however, we used the number randomized when the number lost to follow up was not available from the publication. If there was discrepancy in the number randomized and the numbers analysed in each treatment group, we calculated the percentage loss to follow up in each group and reported this information.
For trials that randomized individuals (individual‐RCTs), we recorded dichotomous outcomes as the number of participants experiencing the given event and the number analysed in each trial group. For trials randomized using clusters (cluster‐RCTs), we recorded the number of clusters. The unit of randomization and statistical methods used to analyse the trial were documented along with details describing whether these methods adjusted for clustering. When trials were randomized by cluster units, we extracted a cluster‐adjusted measure of effect, such as the risk ratio (RR) and the 95% confidence interval (CI).
Assessment of risk of bias in included studies
KLS and MS independently assessed the methodological quality using a standard quality assessment form. We classed the generation of allocation sequence and allocation concealment as adequate, inadequate, or unclear as described in Juni 2001. We also reported who was blinded in each trial, and classified inclusion of all randomized participants as adequate if at least 90% of participants were followed up to the trial's completion or inadequate if less than 90%. We did not contact the authors for trial reports with unclear description of validity components and resolved disagreements through discussion.
Data synthesis
KLS conducted all analyses using Review Manager 4.2. This review did not combine any trials in a meta‐analysis due to heterogeneity across the included studies. We stratified the results by vaccine type (inactivated, live‐attenuated, and genetically engineered) and reported the results in tables.
Dichotomous data from individual‐RCTs were compared using RRs and presented with a 95% CI. The RR of disease prevention was converted to an estimate of vaccine efficacy using: efficacy = (1‐RR) x 100%. The 95% CI for the vaccine efficacy was obtained by substituting the upper and lower 95% CI of the RR into the formula.
We extracted the adjusted RR and 95% CI of adverse events from one cluster‐RCT. The trial authors reported that the data were adjusted to account for intra‐cluster variation using the CSAMPLE component of EpiInfo (version 6.04). We also presented the type of adverse events and the calculated event rates for each trial.
Results
Description of studies
We retrieved reports of 43 potentially relevant vaccine trials and included eight RCTs (see 'Characteristics of included studies'). Reasons for study exclusion are detailed in the 'Characteristics of excluded studies'. Five studies are awaiting assessment, either pending translation or requests for further details ('Characteristics of studies awaiting classification').
Participants
The eight included trials involved 358,750 participants, mostly children aged less than 15 years.
Intervention
Three trials tested inactivated mouse brain vaccine − one tested an unpurified precursor (Hsu 1971), and two trials tested vaccines in current use (Hoke 1988; Rojanasuphot 1989). One trial assessed the live‐attenuated vaccine SA14‐14‐2 (Liu 1997), while four smaller trials reported preliminary findings for three vaccine candidates (Monath 2002b; Kuzuhara 2003; Monath 2003; Lyons 2004).
Outcome measures
Only two trials reported the efficacy of the investigated vaccine in preventing disease, while six trials reported the development of neutralizing antibodies in response to vaccination (immunogenicity). Each trial reported safety data for the tested vaccine.
Vaccines in current use
Inactivated vaccine (mouse brain‐derived Nakayama and/or Beijing‐1 strain)
Hsu 1971: An unpurified precursor to the now commercially available Nakayama strain vaccine was evaluated in this large trial conducted in northern Taiwan. The study area was recognized as having a high incidence of Japanese encephalitis. A total of 265,808 children, between ages three and seven years, were randomly allocated into four groups receiving one or two doses of either vaccine or placebo (unrelated tetanus toxoid vaccine). The two doses were administered 7 to 10 days apart. The trial compared the number of disease cases identified in each group for the duration of two epidemic seasons (equal to two years). Adverse events were monitored in a subgroup of children up to 48 hours following vaccination.
Hoke 1988: This trial was conducted in northern Thailand and involved 65,224 children between ages 1 and 14 years. The children were randomly assigned to three groups receiving placebo (unrelated tetanus toxoid vaccine), monovalent (Nakayama strain), or bivalent (Nakayama and Beijing‐1 strains) vaccines. Two doses were administered one week apart in each group. The disease incidence was examined for a period comprising two epidemic seasons, while adverse events were monitored for six weeks after vaccination in a subgroup of children.
Rojanasuphot 1989: This trial compared the safety and immunogenicity of a Nakayama strain vaccine produced in Thailand to that of the inactivated vaccine produced in Japan (Nakayama as well as Beijing‐1 formula). The study included 1160 children between ages five and nine years living in an area of central Thailand recognized as having low endemic transmission of Japanese encephalitis virus. The children were randomized into four groups receiving one of the three vaccines or no vaccine. Two doses of vaccine were administered two weeks apart in each of the intervention groups. Adverse effects were detected during an observation period of one month, while the mobilization of neutralizing antibodies was evaluated at one, six, and 12 months following vaccination.
Live‐attenuated vaccine (SA14‐14‐2 strain)
Liu 1997: The safety of the live‐attenuated SA14‐14‐2 vaccine produced in China was examined in this cluster‐RCT based on pair‐matched health centres. The health centres were located in the urban capital of a Chinese province. The study included 26,239 children at an average age of 1.9 years scheduled to receive their first or second dose of vaccine as part of the general vaccination campaign against Japanese encephalitis. Children attending treatment centres were vaccinated as scheduled, while those assigned to control centres were withheld from vaccination for one month to act as unvaccinated controls. The occurrence of mild and moderate adverse events was monitored in a subgroup of children during the first seven days, while moderate and serious events were registered throughout the study period of 30 days for all children.
Vaccine candidates
Genetically engineered vaccine (SA14‐14‐2 and YF 17D chimera)
Monath 2002b: This small trial of the ChimeriVax‐JE vaccine candidate was performed in a group of 36 adults aged between 18 and 59 years in the USA. Half of the participants demonstrated pre‐existing antibodies to yellow fever virus. The yellow fever immune and non‐immune participants were randomized, separately, into groups of three receiving either ChimeriVax‐JE (two different dose levels) or the yellow fever control vaccine (YF 17D). Adverse events and immunogenicity following a single dose were examined after 31 days.
Monath 2003: This study was conducted in the USA and included 99 adults between the ages of 18 and 59 years with no history of previous yellow fever or Japanese encephalitis immunization. The participants were randomly allocated to nine groups. Five groups received variable dose levels of ChimeriVax‐JE in a two‐dose schedule with a 30‐day interval. Sequential administration of ChimeriVax‐JE and YF 17D, or YF 17D then ChimeriVax‐JE was carried out in two groups with a 30‐day interval. One group received an unspecified placebo followed by ChimeriVax‐JE at day 30, while the reverse sequence was administered to the final group. Adverse events and immunogenicity were assessed during the first week and again on days 14, 21, 44, and 60.
Inactivated vaccine (Vero cell‐derived Beijing‐1)
Kuzuhara 2003: This trial was conducted in Japan on 60 male adults aged between 20 and 35 years. None of the participants had measurable levels of pre‐existing antibodies to Japanese encephalitis virus. The participants were randomly assigned into two groups receiving three doses of a Vero cell‐derived Beijing‐1 vaccine candidate or the licensed mouse brain‐derived Beijing‐1 vaccine. The sequential doses were administered between one and eight weeks after the first dose and four and eight weeks after the second dose. Adverse events were recorded up to three days after each dose, while immunogenicity was examined up to four weeks after the last dose.
Inactivated vaccine (Vero cell‐derived SA14‐14‐2 strain)
Lyons 2004: This trial was conducted in the USA and evaluated the vaccine candidate IC51 (JE‐PIV). A total of 94 adults between ages 18 and 49 years were randomly assigned into four groups. Three groups received IC51 (JE‐PIV) at different dose levels and/or different time intervals between doses, while a control group received a mouse brain‐derived inactivated vaccine. Adverse events were recorded during an unspecified period of time, while vaccine immunogenicity was measured after 24 and 56 days and again after 12 and 24 months.
Risk of bias in included studies
SeeAppendix 3for a summary of the assessment and the 'Characteristics of included studies' for details.
Vaccines in current use
Inactivated vaccine (mouse brain‐derived Nakayama and/or Beijing‐1 strain)
Hsu 1971: The method used to randomize the participants was unclear. Assignment into letter‐coded groups insured adequate concealment of participant allocation, while all of those involved in the trial were blinded to participant assignment (ie the participants (including parents), vaccine providers, and outcome assessors). It is unclear if any participants were lost to follow up.
Hoke 1988: Rotational allocation of participants into groups was considered an inadequate method of randomization given that the sequence of allocation was determined by the time of participant presentation. The allocation concealment by use of colour‐codes for the different groups was considered adequate. Blinding was reported for participants, providers, and outcome assessors; however, a difference in volume and appearance between vaccine and placebo prevented complete blinding of the vaccine providers. It is unclear how many participants may have been lost to follow up. It should be noted that the trial authors suggested potential selection bias of participants based on the socioeconomic status of the trial volunteers.
Rojanasuphot 1989: The methods of randomization and allocation concealment were unclear for this trial. Due to the study design (no intervention for controls) neither participants nor providers could be blinded. It is uncertain whether the outcome assessors were blinded. Any loss of participants to follow up was unclear.
Live‐attenuated vaccine (SA14‐14‐2 strain)
Liu 1997: In this cluster‐RCT, the allocation of participants was determined by a draw between pair‐matched health centres (to which participants were pre‐assigned). This method of randomization was considered adequate, but it is unclear if the trial included any form of allocation concealment. The outcome assessors were blinded, while the study design prevented blinding of participants and providers (absence of intervention in control group). Twenty‐two participants (0.1%) were lost to follow up and excluded by the trial authors in the final analysis. The overall inclusion of participants was considered as adequate given that more than 90% of all participants were included in the analysis.
Vaccine candidates
Genetically engineered vaccine (SA14‐14‐2 and YF 17D chimera)
Monath 2002b: A computer‐generated allocation sequence and coded‐group assignment were considered adequate methods for randomization and allocation concealment. Blinding was reported for participants and all trial personnel (pharmacists, vaccine providers, and outcome assessors). There were no losses to follow up and all participants were included in the final analysis.
Monath 2003: The methods of randomization and allocation concealment were unclear for this trial. Blinding was reported for the participants and all study personnel, as in Monath 2002b. A single participant withdrew from the trial, while all remaining participants were included in the final analysis.
Inactivated vaccine (Vero cell‐derived Beijing‐1)
Kuzuhara 2003: All the quality components of this trial were unclear, given a lack of reporting, with the exception of participant inclusion. Two of the randomized participants were reportedly lost to follow up and were excluded from the final analysis.
Inactivated vaccine (Vero cell‐derived SA14‐14‐2 strain)
Lyons 2004: This was an open‐label RCT with no clear description of the randomization method or of efforts made to ensure concealed assignment. One participant was lost to follow up, but included in the final analysis.
Effects of interventions
We did not prepare meta‐analyses for any of the included trials. First of all, a combined analysis of the two trials investigating vaccine efficacy did not seem appropriate given that one of the trials assessed a precursor formula rather than a fully purified and processed vaccine. Secondly, a meta‐analysis of the six trials reporting vaccine immunogenicity was considered inappropriate due to trial heterogeneity. The differences between trials included the vaccine type, type of challenge strains used in neutralization assay (where reported), length of follow up, and participant pre‐exposure to Japanese encephalitis virus and other flaviviruses (especially yellow fever virus). Finally, we did not cross‐analyse the safety data for any of the included trials for reasons of heterogeneity (as stated above) as well as unclear subgroup selection and lack of comparison to controls in some of the trials (see 'Characteristics of included studies' and Appendix 5 for details). We present a narrative summary of the individual results for each of the investigated outcome measures − effectiveness, immunogenicity, and safety.
Vaccines in current use
Inactivated mouse brain‐derived Nakayama strain versus placebo (tetanus toxoid vaccine):
A single dose of this precursor vaccine did not confer any statistically significant protection against disease during the two‐year follow‐up period (estimated efficacy 51%, 95% CI ‐95% to 88%; 43,893 participants, Analysis 1.1) Hsu 1971. However, the estimated efficacy of a two‐dose schedule was 81% (95% CI 45% to 94%; 221,915 participants) after the first year of vaccination. During the second year the efficacy of the same schedule dropped to 59% (95% CI 2% to 83%; 221,915 participants), thus the suggested vaccine protection for the entire follow‐up period was 71% (95% CI 44% to 85%; 221,915 participants). The data presented by trial authors for the second year of the trial represented the sum of events for participants receiving either one or two doses of placebo vaccine (ie 20 cases in 131,865 controls). In order to calculate the risk ratio for one‐ and two‐dose schedules separately, we assumed the same event rate for the two placebo groups giving: 3 cases in 21,699 participants receiving one dose of placebo and 17 cases in 110,166 participants receiving two doses.
Analysis 1.1.
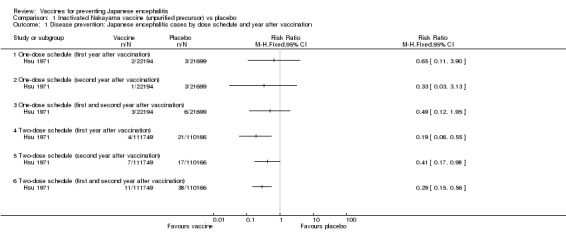
Comparison 1 Inactivated Nakayama vaccine (unpurified precursor) vs placebo, Outcome 1 Disease prevention: Japanese encephalitis cases by dose schedule and year after vaccination.
One death among the 21,699 children in the placebo group was serologically confirmed as Japanese encephalitis, while there were no deaths related to Japanese encephalitis among the 22,194 vaccinees (RR 0.33, 95% CI 0.01 to 8.00). Four other deaths were recorded in the placebo group; these were clinically diagnosed but not serologically confirmed as Japanese encephalitis. Deaths were only reported in the first year of follow up.
Immunogenicity was not reported.
Adverse events were monitored within a subgroup of 1376 children at 24 and 48 hours after each of two doses of the Nakayama or placebo vaccine (tetanus toxoid vaccine). No serious adverse events were reported during the follow‐up period (Appendix 5). The most frequent event was injection site redness and swelling, particularly after the second dose. This mild event occurred at similar rates for vaccinees and controls (3.9% versus 3.4%). The rate of moderate events, namely fever and diarrhoea, was 0.4% for vaccinees and 0.8% for controls.
Inactivated mouse brain‐derived Nakayama strain alone and combined with Beijing‐1 strains versus placebo (tetanus toxoid vaccine):
As shown in Analysis 2.1, two doses of the monovalent Nakayama vaccine provided an estimated protection of 95% (95% CI 10% to 100%; 43,144 participants) during the first year after vaccination, but was unable to confer any statistically significant protection during the second year (estimated efficacy 50%, 95% CI ‐449% to 95%; 43,144 participants) Hoke 1988. The bivalent Nakyama and Beijing‐1 vaccine showed an estimated efficacy of 89% (95% CI 15% to 99%) during the first year. As for the monovalent vaccine, the bivalent formula did not confer any statistically significant protection during the second year (efficacy 81%, 95% CI ‐306% to 99%; 43,596 participants). Both vaccine formulations presented an efficacy estimate of 91% for the entire follow‐up period (monovalent: 95% CI 30% to 99%, 43,144 participants; bivalent: 95% CI 31% to 99%, 43,596 participants). Hence, in this trial setting there was no statistical difference in the disease protection provided by the monovalent and bivalent formulations (see Analysis 3.1).
Analysis 2.1.
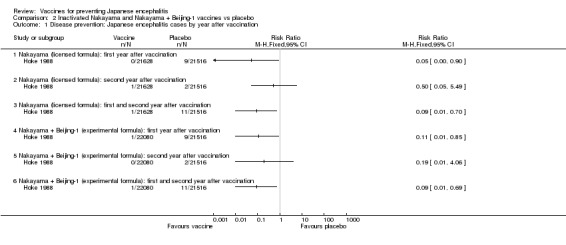
Comparison 2 Inactivated Nakayama and Nakayama + Beijing‐1 vaccines vs placebo, Outcome 1 Disease prevention: Japanese encephalitis cases by year after vaccination.
Analysis 3.1.
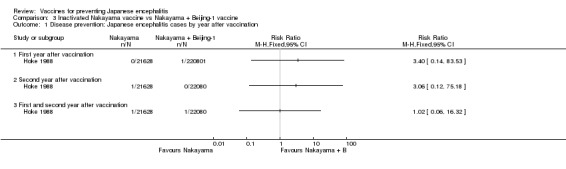
Comparison 3 Inactivated Nakayama vaccine vs Nakayama + Beijing‐1 vaccine, Outcome 1 Disease prevention: Japanese encephalitis cases by year after vaccination.
Japanese encephalitis was confirmed in 13 children, 11 children from the placebo group and one child from each vaccine group. One death was reported in the placebo group (n = 21,516), while no deaths were reported for children receiving either monovalent (n = 21,628) or bivalent (n = 22,080) vaccines (RR 0.33 95% CI 0.01 to 8.14 and RR 0.32 95% CI 0.01 to 7.97, respectively). Eleven children were examined two to three years after the diagnosis of which 10 were reported as suffering considerable impairments. It is unclear if either of the vaccinees were among these 10 cases.
The reported immunogenicity of the two vaccine formulations was not compared to the placebo group; hence the level of seroconversion attributable to natural infection is unknown. Development of neutralizing antibodies was tested seven days after the first dose and 30 days after the second dose. The method of testing was not detailed nor was the actual number of participants. A four‐fold (or larger) increase in the titre of antibodies to the Nakayama strain was reported in 96% of the vaccinees with pre‐existing antibodies to Japanese encephalitis (Appendix 4). In comparison, children without previous exposure were shown to seroconvert in 46% of the cases following vaccination. The titre of anti‐Beijing‐1 antibodies increased by four‐fold or more in 83% of the seronegative children receiving monovalent vaccine compared with 85% of the bivalent vaccinees.
Detection of adverse events, occurring within 30 days of vaccination, was conducted in a subgroup of 1517 children. Serious adverse events were not observed for any of the children in this trial (Appendix 5). Fever was the only adverse event for which the rate was specified as occurring in 2.9% of the monovalent vaccine group and 1.7% of the bivalent group against 0.8% of the placebo group. Events including headache, sore arm, injection site swelling, and rash were reported to occur in less than 1% of the cases and at similar rates to those in the placebo group for both monovalent and bivalent vaccinees; exact details were not provided (Appendix 5).
Inactivated mouse brain‐derived Nakayama strain (produced in Thailand), Nakayama or Beijing‐1 strain (produced by BIKEN in Japan) versus no intervention:
Vaccine effectiveness was not reported Rojanasuphot 1989.
The rate of seroconversion was reported 1 and 12 months after vaccination. Neutralizing antibody titres were determined using a plaque reduction neutralization test in LLC‐KK2 cells, with titres measured at 50% plaque reduction. Titres of 1:10 dilution or above were considered positive. Seroconversion (using Nakayama as the challenge strain) was demonstrated after one month in 99.4% of children receiving the vaccine produced in Thailand (Appendix 4). The Nakayama and Beijing‐1 formulations produced in Japan caused seroconversion in 97% and 79.9% of the vaccinees, respectively, while 2.5% of the unvaccinated controls were shown to seroconvert. After one year, the seroconversion rates were reported as 94.3% (Nakayama; Thailand), 78.8% (Nakayama; Japan), and 55.2% (Beijing‐1; Japan) for the different vaccine groups, and 6.6% for the unvaccinated controls. Using Beijing‐1 as the challenge strain in the neutralization assay, seroconversion was demonstrated after one month in 94.4% of the children receiving the Beijing‐1 formula as compared to 3.3% of the unvaccinated controls. The conversion rate remained high at 92.2% after one year as compared to 8.8% in the controls.
Adverse events were monitored for a week after the first dose and between one and three weeks after the second dose. No serious events were reported (Appendix 5). In each of the three vaccine groups, the occurrence of both mild events (arm soreness) and moderate events (fever, headache, and nausea) was reported at a higher rate after the first than the second dose (Appendix 5). Arm soreness was reported at a rate between 1.1% and 2.8% for the three vaccine groups. Moderate events occurred in approximately 3.5% of vaccinees in both of the two Nakayama groups and in 5.6% of the Beijing‐1 vaccinees. Only 0.6% of the controls experienced moderate events.
Live‐attenuated vaccine (SA14‐14‐2 strain) versus no intervention:
Effectiveness and immunogenicity were not reported Liu 1997.
A wide range of mild and moderate adverse events was observed during a 30‐day follow‐up period within a subgroup of children receiving the SA14‐14‐2 vaccine in this cluster‐RCT (Appendix 5). Unfortunately, a comparison to unvaccinated controls was not reported for this subgroup. Comparative data for the entire study group (using the pooled incidence for first and second dose recipients) were only detailed in terms of fever, diarrhoea, seizures, and all‐cause hospitalization. Fever was the most common event reported for the group of vaccinated (2.7%) as well as unvaccinated children (3.4%). Although the rate of fever was lower for vaccine recipients, this was not statistically significant when the data were adjusted for clustering (adjusted RR 0.79, 95% CI 0.56 to 1.11; provided in trial report). Seizures and diarrhoea were observed at a rate of 0.1% for both groups. The rate of all‐cause hospital admissions was lower for the group of vaccinated (0.6%) than unvaccinated children (0.9%), but not significantly when accounting for clustering (RR 0.70, 95% CI 0.43 to 1.15; provided in trial report).
Vaccine candidates
Genetically engineered vaccine (SA14‐14‐2/YF 17D chimera (ChimeriVax‐JE)) − two doses versus placebo:
Effectiveness was not reported Monath 2002b.
The rate of seropositive participants was reported at day 31 after vaccination (Appendix 4). Neutralizing antibody titres were determined using a plaque reduction neutralization test in LLC‐KK2 cells, with titres measured at 50% plaque reduction. Sera were tested for neutralizing antibodies against the vaccine strain as well as the three wild‐type strains − Beijing‐1, P3, and Nakayama. The trial showed 100% seroconversion to the ChimeriVax‐JE vaccine strain for all participants regardless of dosage (4 or 5 log10 plaque‐forming units (PFU)). Seroconversion to the wild‐type strains varied from 25% to 100% depending on dosage and challenge strain. Controls receiving the YF 17D vaccine developed neutralizing antibodies to the ChimeriVax‐JE virus (41.7%), Beijing‐1 strain (8.3%), and P3 strain (33.3%).
The group receiving the higher of the two vaccine doses experienced fewer total adverse events (10 events) than both the control (24 events) and lower dose vaccine groups (39 events), with each group consisting of 12 participants. The rate of mild adverse events including injection site redness and pain was much lower for the two vaccine groups combined (16.7%) than for the YF 17D control group (66.7%) (Appendix 5). The occurrence of moderate events, however, was higher in the combined vaccine groups than for the control group in which only headache was reported.
Genetically engineered vaccine (SA14‐14‐2/YF 17D chimera (ChimeriVax‐JE)) − various dosing regimens versus placebo:
Effectiveness was not reported Monath 2003.
The rate of seropositive participants was reported at day 30 after vaccination using a 50% plaque reduction neutralization test in LLC‐KK2 cells (Appendix 4). Sera were tested for neutralizing antibodies against the vaccine strain as well as the wild‐type Beijing‐1 and Nakayama strains. The seroconversion rates for each challenge strain were calculated from the sum of seropositive participants receiving graded doses of ChimeriVax‐JE between 1.8 and 5.8 log10 PFU (the seroconversion rate was not dose related). Seroconversion to the ChimeriVax‐JE vaccine strain was reported for 98.9% of participants. Seroconversion to wild‐types Beijing‐1 and Nakayama was 89.7% and 85.1%, respectively. Neutralizing antibodies to Beijing‐1 was confirmed for one participant (9%) in both control groups (YF 17D and unspecified placebo), while seroconversion to ChimeriVax‐JE occurred in one person in the YF 17D control group (9%). Administration of a second dose (same dose level) at day 30 was reported as having little effect on seroconversion rates and did not boost the antibody titre.
All groups reported moderate and mild adverse events for as many as 82% (9/11) to 100% (11/11) of the participants. The rate of reported events was not significantly different between participants receiving ChimeriVax‐JE (data pooled for graded doses) and unspecified placebo control. Comparison of ChimeriVax‐JE to the YF 17D control demonstrated much lower rates of injection site redness (erythema) for the vaccinees than the controls (18.2% versus 63.6%) (Appendix 5). Injection site pain was also markedly lower for ChimeriVax‐JE recipients than the YF 17D controls (12.7% versus 45.5%). Fever was noted in 14.5% of the ChimeriVax‐JE recipients compared to 9.1% of the controls. The occurrence of other moderate systemic events was comparable to or lower than those of the YF 17D control group. Notably, headache was reported for as many as 50.9% of the vaccinees and 54.5% of the controls. Serious events were not observed during the follow‐up period in any of the trial participants.
Inactivated vaccine (Vero cell‐derived Beijing‐1) versus alternative vaccine (inactivated mouse brain‐derived Beijing‐1 strain):
Effectiveness was not reported Kuzuhara 2003.
The seroconversion rate was determined using a 50% plaque reduction neutralization test in Vero cells with Beijing‐1 as the challenge strain (cut‐off titre 1:10). Neutralizing antibodies were measured after each dose showing similar conversion rates at 96.7% (candidate) and 92.9% (control) after the first dose, and 100% conversion for both vaccines after the second and third doses (Appendix 4).
Low numbers of mild and moderate adverse events were reported for both groups (Appendix 5). The most common event reported was local reactions at the injection site observed in 6.7% of the candidate recipients and 13.3% of the controls.
Inactivated vaccine (Vero cell‐derived SA14‐14‐2 strain) versus alternative vaccine (inactivated Nakayama vaccine):
Effectiveness was not reported Lyons 2004.
Seroconversion to the IC51 (JE‐PIV) vaccine was defined as titres greater than 1:10 in plaque reduction neutralization test (challenging strain not specified). The seroconversion rates of three different IC51 (JE‐PIV) allotments were determined with 56 days as the primary endpoint of the trial. Seroconversion was achieved for 95.5% to 100% of the vaccinees in each dose group, as compared to 73.7% of the controls receiving the commercial inactivated mouse brain‐derived vaccine (Appendix 4). After 12 months, seroconversion rates were reported as 100% for all three vaccine groups as compared to 54.5% of the controls.
Serious adverse events were not reported for any of the trial participants, but mild events (arm soreness, injection site redness, and swelling) and moderate events (fever, headache, and myalgia) were reported for all groups at average rates between 4.8% and 83.6% (Appendix 5). The average rate of each event was calculated for all vaccinees, given that the three vaccine groups showed little variance in reported numbers. Injection site redness and swelling were reported at a higher rate in the Nakayama controls (from 28.6% to 33.3%) than for the IC51 (JE‐PIV) vaccinees (15.1%). However, injection site soreness occurred in 83.6% of vaccinees compared to 57.1% of controls. Headache was the most frequently reported systemic event occurring at a similar rate for both vaccinees (54.8%) and controls (57.1%). Rates of myalgia were also prominent but comparable in both groups (45.2% of vaccinees versus 42.9% of controls).
Discussion
Overview of outcome measures
Effectiveness
RCTs of actual disease prevention were only identified for the mouse brain‐derived inactivated vaccine (based on Nakayama and/or Beijing‐1 strains). It is important to note that these included trials investigated vaccine schedules of one or two doses without subsequent boosters, which differ from the three‐dose schedule recommended by producers. The effects of a three‐dose schedule and various booster regimens as used in different countries today have not been investigated in RCTs.
Immunogenicity
Because the incidence of symptomatic Japanese encephalitis is relatively low in most endemic areas, it is necessary to enrol large numbers of participants to ensure that a statistically significant difference in disease activity can be detected between the vaccine and the control groups (power of study). The undertaking of such large‐scale trials is a logistical challenge rendered even more complicated by the difficulties in identifying and diagnosing the disease. A more feasible approach used in most new studies is to test the presence of vaccine‐induced neutralizing antibodies (immunogenicity) as a surrogate measure for vaccine efficacy (Markoff 2000). This approach allows for smaller trials and for trials to be conducted in non‐endemic settings where natural infection is unlikely. However, this approach is problematic as currently there is no direct evidence to support a relationship between immunogenicity and critical vaccine protection in humans (Markoff 2000). The generally accepted cut‐off titre for positive protection (1:10 dilution of neutralizing antibodies in a plaque reduction neutralization test) is based on animal studies rather than clinical evidence in humans. Furthermore, at present there is no standard protocol to measure neutralizing antibodies.
As shown in this review, the choice of challenge strain in neutralization assays may affect the measured immunogenicity of a given vaccine (as may the choice of cell lines and cell substrate). The issue of possible pre‐exposure of trial participants to Japanese encephalitis virus (and other flaviviruses) also needs to be addressed, as pre‐exposure may augment the antibody response to vaccination and thereby inflate the expectations of vaccine effectiveness in unexposed people. Finally, it may be erroneous to determine clinical protection based on antibody titres exclusively (particularly for live vaccines), given that the role of cellular immunity remains largely undisclosed in Japanese encephalitis. The World Health Organization has ongoing consultations to establish immunological endpoints as correlates for disease protection along with a standard protocol for the plaque reduction neutralization test to be used in vaccine trials (Hombach 2005). Although we included immunological endpoints as an outcome measure in this review (not stated in the protocol), we await the final recommendations by the World Health Organization and the supporting evidence of these before we draw any conclusions as to the effectiveness of assessed vaccines on the basis of reported immunogenicity data.
Adverse events
All of the Japanese encephalitis vaccines assessed in this review were associated with mild or moderate adverse events but not with any serious adverse events. The rates of reported events were generally higher for vaccines tested in small pre‐licensure investigations than in large trials. This may be due to reporting bias as a result of differences in trial design and size rather than true differences in vaccine reactogenicity. Notably, the rate of reported events for the inactivated mouse brain‐derived vaccine (Nakayama strain) was considerably higher when used as a control in a pre‐licensure trial (Lyons 2004) than when directly assessed in field trials (Hoke 1988; Rojanasuphot 1989).
Vaccines in current use
Inactivated vaccine (mouse brain‐derived Nakayama and Beijing‐1 strains)
The currently licensed monovalent Nakayama vaccine and bivalent experimental Nakayama and Beijing‐1 vaccine were effective in protecting against Japanese encephalitis, mainly during the first year, when administered in a two‐dose schedule (approximately one week apart). Disease protection by the licensed Nakayama vaccine was 95% (10% to 100%) one year after vaccination (Hoke 1988). The persistence of disease prevention beyond two years is uncertain, but the data do indicate a decline in vaccine effectiveness over time. Most countries using the inactivated vaccine today have added annual or tri‐annual booster doses to a three‐dose vaccination schedule. Epidemiological studies in countries with universal childhood vaccination programmes suggest that protection by the inactivated vaccine may be lasting under the given regimens (Wu 1999; Yang 2006). The effect of external factors on vaccine persistence, particularly boosting through natural exposure, and possible differences in protection against heterogenic strains is unclear.
The inactivated Nakayama vaccine was shown to be highly immunogenic in the Rojanasuphot 1989 trial in which seroconversion rates to the Nakayama strain virus ranged between 97% and 99.4% (depending on production site) as compared to 2.5% in the unvaccinated controls (one month post‐vaccination). The Beijing‐1 formula tested in the same trial was equally immunogenic with seroconversion rates of 94.4% compared to 3.3% of the controls. These rates were observed in children reported as having no detectable antibodies before vaccination. Noticeably, children who were not pre‐exposed displayed Nakayama seroconversion rates of just 46% in the Hoke 1988 trial, while pre‐exposed children converted at rates of 96%. These rates were not compared to that of the control group, but the data support the suggestion that an anamnestic response (immunological memory) can augment the vaccine effect in pre‐exposed people. Similarly, a third dose given one year after vaccination was shown to boost the waning immune response in a trial conducted in Thailand (Nimmannitya 1995). This trial did not include a comparison to a control group but reported seroconversion rates of 100% (one month post‐vaccination) in children receiving a booster dose of either Nakayama or Beijing‐1 inactivated vaccine.
The mouse brain‐derived vaccines were not associated with serious adverse events, while mild and moderate adverse events occurred at low rates. The rates were comparable to those of controls except for moderate events such as fever. It should be noted that several cases of hypersensitivity reactions have been reported following administration of the commercial Nakayama vaccine, mainly among North American, Australian, and European adults (Andersen 1991; Ruff 1991; Berg 1997; Plesner 1997; Plesner 2000; Takahashi 2000). The reports of allergic reactions, some of which lead to hospitalization of vaccine recipients, emerged in the late 1980s. The temporal occurrence of the adverse events has prompted speculations of batch‐related complications. Allergic disposition and potential differences in reactogenicity among children and adults have also been suggested, yet the phenomenon remains unexplained. In 2005, the Government of Japan suspended routine vaccination using the mouse brain‐derived vaccine (WHO 2005a). The suspension was primarily prompted by the detection of acute disseminated encephalomyelitis following vaccination (Kurane 2005; Okabe 2005). A causal link has not been demonstrated between vaccine administration and the case of acute disseminated encephalomyelitis; however, Japanese authorities now recommend limiting vaccination to residents in and visitors to high‐risk areas until alternative vaccines have been proven safe (Kurane 2005; WHO 2005a).
Live‐attenuated vaccine (SA14‐14‐2 strain)
No RCTs of effectiveness were identified for this vaccine, but disease protection was reported in three smaller case‐controlled studies (Hennessy 1996; Bista 2001; Ohrr 2005). Although case‐controlled studies fall outside the inclusion criteria of this review, we find that the result should be noted given the extensive use of the SA14‐14‐2 vaccine in China and its prospective international licensure (details of each study are presented in Appendix 6). The case‐control studies suggested that a single dose of SA14‐14‐2 may be up to 99% protective for the first year after vaccination. A cautious interpretation of the study results is needed because of the relatively small size of the studies and the general limitations of this study design in terms of potential selection and evaluation bias. However, the findings support epidemiological observations of significant disease reduction following the launch of vaccination programmes using the live‐attenuated vaccine (Zhou 1999; Zhou 2001).
The SA14‐14‐2 vaccine was not associated with any adverse events when compared to unvaccinated controls in a cluster‐RCT (Liu 1997). This safety trial observed a lower rate of fever in the vaccinated than unvaccinated participants, in addition to a lower rate of all‐cause hospitalizations. However, the rate of adverse events was not significantly different between the two groups when the trial data were adjusted for clustering. Seizures occurred in both groups at a rate of 0.1%. It was not specified whether the episodes of seizure were related to Japanese encephalitis virus infection, but there were no reports of encephalitis or meningitis among any of the trial participants. Recent media reports of serious events (including deaths) coinciding with a SA14‐14‐2 mass vaccination programme in India should be noted (Chaudhuri 2006). However, these events and their possible association with the vaccine have not been confirmed. The occurrence of any adverse events remains to be reported by the Indian authorities responsible for monitoring and investigation of the programme (Jacobson 2006).
Inactivated vaccine (Beijing‐3 strain)
No RCTs were identified for the inactivated Beijing‐3 vaccine. Case‐control data from a single study showed that two doses of inactivated Beijing‐3 vaccine offered 78% protection if followed by annual boosters (Luo 1994). Evidence of duration and adverse events has not been reported. The requirement for multiple doses and the low disease protection, as suggested by this non‐randomized study, may explain the Chinese decision to replace inactivated Beijing‐3 with the live‐attenuated SA14‐14‐2 in current vaccination programmes (see Appendix 6 for details).
Vaccine candidates
Genetically engineered vaccine (SA14‐14‐2/YF 17D chimera (ChimeriVax‐JE))
This vaccine was highly immunogenic after one month with conversion rates between 85.1% and 98.9% depending on the challenge strain. Seroconversion was observed for some participants in the control groups, particularly for those receiving the flavivirus YF 17D vaccine (Monath 2002b; Monath 2003). The persistence of neutralizing antibodies beyond two months was not reported.
The chimera did not provoke any serious adverse events in the preliminary assessment trials. The two trials suggested that the vaccine candidate caused lower rates of mild injection site events than the YF 17D vaccine but as many and as frequent moderate systemic events. The relatively high event rates, such as for headache (50.9% of vaccinees versus 54.5% of controls), may reflect more careful reporting in the small studies than in the large field trials included in this review. Yet, the frequency of adverse events is disconcerting.
Inactivated vaccine (Vero cell‐derived Beijing‐1)
The vaccine candidate displayed a high conversion rate (96.7%) among recipients within weeks of the first dose and complete conversion after administration of a second and third dose. The performance of the vaccine candidate was equal to that of the mouse brain‐derived Beijing‐1 vaccine (92.9%) used as the control (Kuzuhara 2003).
The occurrence of mild and moderate events was limited to injection site reactions, urticaria, and headache. The spectrum of mild and moderate adverse events was broader for the control group; no serious adverse events were observed.
Inactivated vaccine (Vero cell‐derived SA14‐14‐2 strain)
Seroconversion rates for the vaccine candidate ranged between 77.2% (day 28) and 100% (day 365). In comparison, controls receiving the commercial inactivated Nakayama vaccine (BIKEN) displayed rates between 84.2% (day 28) and 54.5% (day 365) (Lyons 2004). Both vaccine types (candidate and control) were thus shown to be highly immunogenic in people with no pre‐exposure to flaviviruses. The seroconversion rates observed after one year may indicate a more prolonged persistence for the vaccine candidate with less need for boosters.
Serious adverse events were not observed following administration of the inactivated vaccine candidate. The occurrence of systemic events such as myalgia and headache was high, but similar to that of the inactivated Nakayama vaccine used as control. Arm soreness was the only mild event reported at a higher rate in the vaccine group (83.6%) than for the controls (57.1%). While the high event rates may reflect more thorough reporting as compared to that of large field trials, they are none the less disconcerting, especially if the vaccine is intended for childhood programmes.
Authors' conclusions
Vaccines in current use
It is not possible to compare the effectiveness of currently used vaccines in preventing clinical disease as only one of three vaccines have been directly investigated for effectiveness in a RCT. Available evidence shows that two doses of the currently used inactivated Nakayama vaccine is over 95% protective for the first year with subsequent decline in the second year. Protection after two years has not been investigated. Mild and moderate events such as arm soreness, fever, and headache occurred in less than 6% of vaccinees receiving an inactivated vaccine. Safety data for the live‐attenuated SA14‐14‐2 vaccine show a range of mild and moderate adverse events of which fever is the most common (less than 3% of vaccinees) when compared to no intervention.
Vaccine candidates
Large‐scale investigations of effectiveness, duration, and safety are awaited for all vaccine candidates. The recombinant ChimeriVax‐JE vaccine has caused no serious adverse events at the investigated dose levels in early trials. Mild and moderate adverse events are frequent but mostly at lower or similar rates to that of control vaccines. A Vero cell‐derived Beijing‐1 vaccine has been associated with a few mild and moderate events but not with serious episodes. Cell culture‐derived inactivated IC51 (JE‐PIV) vaccine was not associated with serious adverse events at the investigated dose levels, but mild and moderate adverse events were frequent.
Data from RCTs that assess the long‐term effectiveness and safety of currently used and prospective vaccines are needed. The timing and effects of booster doses should also be analysed in these trials. Such trials should aim to demonstrate the superior performance (in terms of effectiveness and safety) of the tested vaccine to that of approved Japanese encephalitis vaccines. A comparison with placebo would be unethical in endemic settings given the demonstrated effectiveness of the current vaccines.
Vaccine immunogenicity may represent an effective surrogate measure for vaccine protection, but the protective level of neutralizing antibodies in humans has to be verified in clinical studies. The role of cellular immunity should also be evaluated as a potential component in a surrogate assay of vaccine effectiveness. Once proven, a standard immunogenicity assay should be developed. Standardized trial formats (eg length of follow‐up period) and standard methods for reporting of outcomes such as disease activity, immunogenicity, and adverse events are also recommended for future trials. Finally, differences in strain and genotype cross protection should be assessed in order to detail expected vaccine effectiveness in various geographic settings. Potential differences in the type and rate of outcomes between children and adults should also be investigated.
Acknowledgements
The editorial base for the Cochrane Infectious Diseases Group is funded by the UK Department for International Development (DFID) for the benefit of developing countries.
Appendices
Appendix 1. Identified Japanese encephalitis vaccines
| Vaccine type | Strain | Source | Type of use | Primary production | Licensure | Nominations | Primary company |
|---|---|---|---|---|---|---|---|
| In use | |||||||
| Inactivated | Nakayama | Mouse brain | Childhood and travellers vaccination | Japan and several Asian countries | International | JE‐VAX/JEVAC | BIKEN (Distribution: Sanofi Pasteur) |
| Beijing‐1 | Mouse brain | Childhood vaccination | Japan and several Asian countries | International | JE‐VAX/JEVAC | BIKEN | |
| Beijing‐3 | Primary hamster kidney cells | Childhood vaccination | China | China | P‐3 | — | |
| Live‐attenuated | SA14‐14‐2 | Primary hamster kidney cells | Childhood vaccination | China (Republic of Korea) | China, Republic of Korea, Nepal, Sri Lanka | SA14‐14‐2 | Chengdu Biological Products/Glovax |
| Candidates | |||||||
| Inactivated | SA14‐14‐2 | Vero cell culture | In clinical trials | USA | N/A | IC51 (JE‐PIV) | VaccGen, WRAIR, InterCell |
| Beijing‐1 | Vero cell culture | In clinical trials | Japan | N/A | — | BIKEN, KAKETSUKEN | |
| Genetically engineered | SA14‐14‐2 and YF 17D | Vero cell culture | In clinical trials | USA | N/A | ChimeriVax‐JE | Acambis (Distribution: Sanofi Pasteur and Bharat Biotech International Limited) |
| Nakayama and pox virus | Chick embryo fibroblasts | Development discontinued | USA | N/A | NYVAC/ALVAC | WRAIR |
BIKEN: the Japanese Research Foundation for Microbial Diseases; KAKETSUKEN: The Chemo‐Sero‐Therapeutic Research Institute; WRAIR: Walter Reed Army Institute of Research.
Appendix 2. Search methods: detailed search strategies
| Search set | CIDG SRa | CENTRAL | MEDLINEb | EMBASEb | LILACSb | BIOSISb |
|---|---|---|---|---|---|---|
| 1 | Japanese encephalitis | Japanese encephalitis | Japanese encephalitis | Japanese encephalitis | Japanese encephalitis | Japanese encephalitis |
| 2 | vaccin* | JE | ENCEPHALITIS, JAPANESE | JE | vaccin* | vaccin* |
| 3 | 1 and 2 | 1 and 2 | JE | 1 or 2 | 1 or 2 | 1 or 2 |
| 4 | — | vaccin* | 1 or 2 or 3 | 1 or 2 or 3 | — | — |
| 5 | — | 3 and 4 | vaccin* | 3 AND 4 | — | — |
| 6 | — | — | 4 and 5 | JAPANESE‐ENCEPHALITIS‐VACCINE | — | — |
| 7 | — | — | JAPANESE ENCEPHALITIS VACCINES | 5 OR 6 | — | — |
| 8 | — | — | 6 OR 7 | Limit 7 to human | — | — |
| 9 | — | — | Limit 7 to human | — | — | — |
aCochrane Infectious Diseases Group Specialized Register. bSearch terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration (Higgins 2006; upper case: MeSH or EMTREE heading; lower case: free text term.
Appendix 3. Risk of bias assessmenta
| Trial | Allocation sequence generation | Allocation concealment | Blinding | Inclusionb |
|---|---|---|---|---|
| Hsu 1971 | Unclear | Adequate | Participants (and parents), vaccine providers, and outcome assessors | Not described |
| Hoke 1988 | Inadequate | Adequate | Participants, vaccine providers, and outcome assessors | Not described |
| Rojanasuphot 1989 | Unclear | Unclear | Participants and providers not blinded; unclear for outcome assessors | Not described |
| Liu 1997 | Adequate | Unclear | Outcome assessors and study analysts | Adequate |
| Monath 2002b | Adequate | Adequate | Participant, study pharmacist (vaccine preparation), vaccine provider, outcome assessors, and all other personnel | Adequate |
| Monath 2003 | Unclear | Unclear | Participant, vaccine provider, and outcome assessors | Adequate |
| Kuzuhara 2003 | Unclear | Unclear | Unclear | Adequate |
| Lyons 2004 | Unclear | Unclear | None blinded | Adequate |
aSee 'Characteristics of included studies' for details. bInclusion of randomized participants in analysis.
Appendix 4. Immunogenicity
| Trial | Intervention | Vaccine strain | Challenge strain | Seroconversion | Seroconversion | Seroconversion | Seroconversion | Notes |
|---|---|---|---|---|---|---|---|---|
| Hoke 1988 | — | — | — | DAY 30 | — | — | — | Test method: PRNT, cell line not reported |
| Inactivated Nakayama (BIKEN) | Nakayama | Nakayama | 96% | — | — | — | Pre‐trial JE seropositive; number of tested not reported | |
| — | — | Nakayama | 46% | — | — | — | Pre‐trial JE seronegative; number of tested not reported | |
| — | — | Beijing‐1 | 83% | — | — | — | Pre‐trial JE seronegative; number of tested not reported | |
| Inactivated Nakayama + Beijing‐1 (BIKEN) | Nakayama + Beijing‐1 | Nakayama | Not reported | — | — | — | — | |
| — | — | Beijing‐1 | 85% | — | — | — | Pre‐trial JE seronegative; number of tested not reported | |
| Tetanus toxoid (placebo) | Not reported | Not reported | Not reported | — | — | — | — | |
| Rojanasuphot 1989 | — | — | — | DAY 30 | DAY 365 | — | — | Test method: 50% PRNT in LLC‐KK2 cells (1:10 cut‐off titre) |
| Inactivated Nakayama (Thailand) | Nakayama | Nakayama | 99.4% (318/320) | 94.3% 280/297) | — | — | Pre‐trial JE seronegative | |
| Inactivated Nakayama (BIKEN) | Nakayama | Nakayama | 97.0% (319/329) | 78.8% (245/311) | — | — | Pre‐trial JE seronegative | |
| Inactivated Beijing‐1 (BIKEN) | Beijing‐1 | Nakayama | 79.9% (47/59) | 55.2% (32/56) | — | — | Pre‐trial JE seronegative | |
| — | — | Beijing‐1 | 94.4% (51/54) | 92.2% (47/51) | — | — | Pre‐trial JE seronegative | |
| No intervention (control) | N/A | Nakayama | 2.5% (3/118) | 6.6% (7/106) | — | — | Pre‐trial JE seronegative | |
| — | — | Beijing‐1 | 3.3% (2/61) | 8.8% (5/57) | — | — | Pre‐trial JE seronegative | |
| Monath 2002b | — | — | — | DAY 31 | — | — | — | Test method: 50% PRNT in LLC‐KK2 cells (1:10 cut‐off titre) |
| ChimeriVax‐JE: 4 log10 PFU | SA14‐14‐2/YF 17D | SA14‐14‐2/YF 17D | 100% (12/12) | — | — | — | ChimeriVax‐JE: 4 log10 PFU: 6/12 participants seropositive for YF | |
| — | — | Beijing‐1 | 75% (9/12) | — | — | — | — | |
| — | — | P3 | 100% (12/12) | — | — | — | — | |
| — | — | Nakayama | 25% (3/12) | — | — | — | — | |
| ChimeriVax‐JE: 5 log10 PFU | SA14‐14‐2/YF 17D | SA14‐14‐2/YF 17D | 100% (12/12) | — | — | — | ChimeriVax‐JE: 5 log10 PFU: 6/12 participants seropositive for YF | |
| — | — | Beijing‐1 | 91.7% (11/12) | — | — | — | — | |
| — | — | P3 | 91.7% (11/12) | — | — | — | — | |
| — | Nakayama | 58.3% (7/12) | — | — | — | — | ||
| YF 17D (placebo) | YF 17D | SA14‐14‐2/YF 17D | 41.7% (5/12) | — | — | — | YF 17D (placebo): 6/12 participants seropositive for YF | |
| — | — | Beijing‐1 | 8.3% (1/12) | — | — | — | — | |
| — | — | P3 | 33.3% (4/12) | — | — | — | — | |
| — | — | Nakayama | 0% (0/12) | — | — | — | — | |
| Monath 2003 | — | — | — | DAY 30 | — | — | — | Test method: 50% PRNT in LLC‐KK2 cells (1:10 cut‐off titre) |
| ChimeriVax‐JE (1.8 to 5.8 log10 PFU) | SA14‐14‐2/YF 17D chimera | SA14‐14‐2/YF 17D | 98.9% (86/87) | — | — | — | ChimeriVax‐JE (1.8 to 5.8 log10 PFU): data compiled for vaccine groups receiving between 1.8 and 5.8 log10 PFU | |
| — | — | Beijing‐1 | 89.7% (78/87) | — | — | — | — | |
| — | — | Nakayama | 85.1% (74/87) | — | — | — | — | |
| YF 17D (placebo) | YF 17D | SA14‐14‐2/YF 17D | 9% (1/11) | — | — | — | — | |
| — | — | Beijing‐1 | 9% (1/11) | — | — | — | — | |
| — | — | Nakayama | 0% (0/11) | — | — | — | — | |
| Unspecified placebo | Not reported | SA14‐14‐2/YF 17D | 0% (0/11) | — | — | — | — | |
| — | — | Beijing‐1 | 0% (0/11) | — | — | — | — | |
| — | — | Nakayama | 0% (0/11) | — | — | — | — | |
| Kuzuhara 2003 | — | — | — | BETWEEN DAY 7 AND 28 | BETWEEN DAY 28 AND 56 | BETWEEN DAY 42 AND 84 | — | Test method: 50% PRNT in Vero cells (1:10 cut‐off titre) |
| Inactivated Vero cell‐derived Beijing‐1 strain (Kaketsuken): 3 x 0.5 mL | Beijing‐1 | Beijing‐1 | 96.7% (29/30) | 100% (30/30) | 100% (30/30) | — | — | |
| Inactivated mouse brain‐derived Beijing‐1 strain (Kaketsuken): 3 x 0.5 mL | Beijing‐1 | Beijing‐1 | 92.9% (26/28) | 100% (28/28) | 100% (28/28) | — | — | |
| Lyons 2004 | — | — | — | DAY 28 | DAY 56 | DAY 365 | DAY 730 | Test method: PRNT unspecified (1:10 cut‐off titre) |
| IC51 (JE‐PIV): 2 x 6 µg | SA14‐14‐2 | Not reported | 77.2% (17/22) | 95.5% (21/22) | 100% (11/11) | 87.5% (7/8) | — | |
| IC51 (JE‐PIV): 3 x 6 µg | SA14‐14‐2 | Not reported | 95.7% (22/23) | 100% (23/23) | 100% (16/16) | 83.3% (5/6) | — | |
| IC51 (JE‐PIV): 2 x 12 µg | SA14‐14‐2 | Not reported | 95.7% (22/23) | 100% (23/23) | 100% (11/11) | 100% (2/2) | ||
| Inactivated Nakayama (BIKEN) (control): 3 x 1 mL | Nakayama | Not reported | 84.2% (16/19) | 73.7% (14/19) | 54.5% (6/11) | 68.7% (4/6) | — |
JE: Japanese encephalitis; PRNT: plaque reduction neutralization test; YF: yellow fever.
Appendix 5. Adverse events
| Trial and comparison | Type of event | Vaccine n/N (rate %) | Control n/N(rate %) | Time of follow up | RR (95% CI) | Event description | Notes |
|---|---|---|---|---|---|---|---|
| Hsu 1971: inactivated Nakayama (precursor) vs tetanus toxoid vaccine (placebo)a | Mild | 29/728 (3.9) | 22/648 (3.4) | 24 and 48 h | — | Injection site: redness, swelling, eruption | — |
| Moderate | 3/728 (0.4) | 5/648 (0.8) | 24 and 48 h | — | Fever, diarrhoea | — | |
| Serious | 0/728 (0.0) | 0/648 (0.0) | 24 and 48 h | — | — | — | |
| Hoke 1988: inactivated Nakayama (BIKEN) vs tetanus toxoid vaccine (placebo)a | Mild | See Notes | — | Within 30 days | — | Injection site: Sore arm, swelling, rash | Mild events reported as less than 1% and as non‐significant |
| Moderate | 14/488 (2.9) | 4/490 (0.8) | Within 30 days | — | Fever | — | |
| Serious | 0/488 (0.0) | 0/490 (0.0) | Within 30 days | — | — | — | |
| Hoke 1988: inactivated Nakayama + Beijing‐1 (BIKEN) vs tetanus toxoid vaccine (placebo)a | Mild | See Notes | — | Within 30 days | — | Injection site: sore arm, swelling, rash | Mild events reported as less than 1% and as non‐significant |
| Moderate | 9/539 (1.7) | 4/490 (0.8) | Within 30 days | — | Fever | — | |
| Serious | 0/539 (0.0) | 0/490 (0.0) | Within 30 days | — | — | — | |
| Rojanasuphot 1989: inactivated Nakayama (Thailand) vs no intervention | Mild | 13/457 (2.8) | 0/161 (0.0) | 7 days (dose 1), 7 to 21 days (dose 2) | — | Sore arm | Sum of mild events after doses 1 and 2 |
| Moderate | 16/457 (3.5) | 1/161 (0.6) | 7 days (dose 1), 7 to 21 days (dose 2) | — | Fever, headache, nausea | Sum of moderate events after doses 1 and 2 | |
| Serious | 0/457 (0.0) | 0/161 (0.0) | 7 days (dose 1), 7 to 21 days (dose 2) | — | — | — | |
| Rojanasuphot 1989: inactivated Nakayama (BIKEN) vs no intervention | Mild | 11/448 (2.5) | 0/161 (0.0) | 7 days (dose 1), 7 to 21 days (dose 2) | — | Sore arm | Sum of mild events after doses 1 and 2 |
| Moderate | 16/448 (3.6) | 1/161 (0.6) | 7 days (dose 1), 7 to 21 days (dose 2) | — | Fever, headache, nausea | Sum of moderate events after doses 1 and 2 | |
| Serious | 0/448 (0.0) | 0/161 (0.0) | 7 days (dose 1), 7 to 21 days (dose 2) | — | — | — | |
| Rojanasuphot 1989: inactivated Beijing‐1 (BIKEN) vs no intervention | Mild | 1/90 (1.1) | 0/161 (0.0) | 7 days (dose 1), 7 to 21 days (dose 2) | — | Sore arm | Sum of mild events after doses 1 and 2 |
| Moderate | 5/90 (5.6) | 1/161 (0.6) | 7 days (dose 1), 7 to 21 days (dose 2) | — | Fever, headache, nausea | Sum of moderate events after doses 1 and 2 | |
| Serious | 0/90 (0.0) | 0/161 (0.0) | 7 days (dose 1), 7 to 21 days (dose 2) | — | — | — | |
| Liu 1997: lLive‐attenuated SA14‐14‐2 vs no intervention | Mild | 2/266 (0.8) | Not reported | 30 days | N/A | Injection site: hives, angioedema, tenderness | Mild events reported for subgroup of trial participants; selection method of subgroup is unclear |
| Moderate | 43/266 (16.1) | Not reported | 30 days | N/A | Rash, vomiting, cough, irritability | Moderate events reported for subgroup of trial participants; selection method of subgroup is unclear | |
| 357/13,266 (2.7) | 442/12,951 (3.4) | 30 days | 0.79 (0.56 to 1.11) | Fever | Calculation of RR and 95% CI adjusted for clustering by health centre (conducted by trial authors) | ||
| 12/13,266 (0.1) | 11/12,951 (0.1) | 30 days | 1.06 (0.46 to 2.49) | Diarrhoea | — | ||
| Serious | 82/13,266 (0.6) | 114/12,951 (0.9) | 30 days | 0.70 (0.43 to 1.15) | Hospital admission | Reason for hospital admission not stated | |
| — | 14/13,266 (0.1) | 15/12,951 (0.1) | 30 days | 0.91 (0.37 to 2.22) | Seizure | — | |
| Monath 2002b: ChimeriVax‐JE vs yellow fever vaccine YF 17D (placebo)b | Mild | 4/24 (16.7) | 8/12 (66.7) | 31 days | — | Injection site: erythema and pain | — |
| Moderate | 2/24 (8.3) | 0/12 (0.0) | 31 days | — | Fever | — | |
| 7/24 (29.2) | 2/12 (16.7) | 31 days | — | Headache | — | ||
| 4/24 (16.7) | 0/12 (0.0) | 31 days | — | Myalgia | — | ||
| 5/24 (20.8) | 0/12 (0.0) | 31 days | — | Diarrhoea | — | ||
| Monath 2003: ChimeriVax‐JE vs unspecified placeboc | Mild | 3/55 (5.4) | 2/11 (18.2) | 30 days | — | Injection site bruising | — |
| 10/55 (18.2) | 2/11 (18.2) | 30 days | — | Injection site erythema | — | ||
| 7/55 (12.7) | 2/11 (18.2) | 30 days | — | Injection site pain | — | ||
| 2/55 (3.6) | 0/11 (0.0) | 30 days | — | Injection site oedema | — | ||
| Moderate | 6/55 (10.9) | 2/11 (18.2) | 30 days | — | Skin (supposedly rash) | — | |
| 8/55 (14.5) | 0/11(0.0) | 30 days | — | Fever | — | ||
| 28/55 (50.9) | 5/11 (45.5) | 30 days— | — | Headache | — | ||
| 13/55 (23.6) | 3/11 (27.3) | 30 days | — | Myalgia | — | ||
| 7/55 (12.7) | 1/11 (9.1) | 30 days | — | Diarrhoea | — | ||
| Monath 2003: ChimeriVax‐JE vs Yellow fever vaccine YF 17D (placebo)c | Mild | 3/55 (5.5) | 0/11 (0.0) | 30 days | — | Injection site bruising | — |
| 10/55 (18.2) | 7/11 (63.6) | 30 days | — | Injection site erythema | — | ||
| 7/55 (12.7) | 5/11 (45.5) | 30 days | — | Injection site pain | — | ||
| 2/55 (3.6) | 0/11 (0.0) | 30 days | — | Injection site oedema | — | ||
| Moderate | 6/55 (10.9) | 4/11 (36.4) | 30 days | — | Skin (supposedly rash) | — | |
| 8/55 (14.5) | 1/11 (9.1) | 30 days | — | Fever | — | ||
| 28/55 (50.9) | 6/11 (54.5) | 30 days | — | Headache | — | ||
| 13/55 (23.6) | 3/11 (27.3) | 30 days | — | Myalgia | — | ||
| 7/55 (12.7) | 3/11 (27.3) | 30 days | — | Diarrhoea | — | ||
| Kuzuhara 2003: iInactivated Vero cell‐derived Beijing‐1 strain (Kaketsuken) vs inactivated mouse brain‐derived Beijing‐1 strain (Kaketsuken) | Mild | 2/30 (6.7) | 4/30 (13.3) | 3 days | — | Injection site reaction | — |
| Moderate | 1/30 (3.3) | 0/30 (0.0) | 3 days | — | Urticaria | — | |
| 0/30 (0.0) | 1/30 (3.3) | 3 days | — | Headache | — | ||
| 1/30 (3.3) | 1/30 (3.3) | 3 days | — | Malaise | — | ||
| 0/30 (0.0) | 1/30 (3.3) | 3 days | — | Pain in pharynx | — | ||
| 0/30 (0.0) | 1/30 (3.3) | 3 days | — | Nasal discharge | — | ||
| 0/30 (0.0) | 1/30 (3.3) | 3 days | — | Acute gastroenteritis | — | ||
| Lyons 2004: inactivated IC51 (JE‐PIV) vs inactivated Nakayama (BIKEN)d | Mild | 61/73 (83.6) | 12/21 (57.1) | Not reported | — | Injection site: sore arm | — |
| 11/73 (15.1) | 7/21 (33.3) | Not reported | — | Injection site: swelling | — | ||
| 11/73 (15.1) | 6/21 (28.6) | Not reported | — | Injection site: redness | — | ||
| Moderate | 12/73 (16.4) | 1/21 (4.8) | Not reported | — | Fever | — | |
| 40/73 (54.8) | 12/21 (57.1) | Not reported | — | Headache | — | ||
| 33/73 (45.2) | 9/21 (42.9) | Not reported | Myalgia | — |
aAll events reported for subgroup of trial participants; selection method of subgroup unclear. bSum of events for 2 dose levels. cSum of events for 5 dose levels. dSum of events for 3 vaccine schedules.
Appendix 6. Case‐control studies: live‐attenuated SA14‐14‐2 & inactivated Beijing‐3 vaccines
| Study details | Details | Vaccination status | Cases (%) | Controls (%) | Reported OR | 95% CI | Reported efficacy | 95% CI |
|---|---|---|---|---|---|---|---|---|
| Luo 1994 | Vaccine: inactivated Beijing‐3 (P3)Location: Gus County, Hen an Province, China (endemic JE transmission)Study conducted 2 to 6 months following administration of the last annual boosterCases: 50 children ages 6 months to 10 years with serologically confirmed JE infectionControls: 100 children matched for neighbourhood, gender, and age (within 1 year)Confounding factors: adjustment for bed‐net impregnation, housing, income, and parental educationComplete vaccination: 2 primary doses + annual booster Partial vaccination: incomplete primary doses and/or no annual booster | None | — | — | — | — | — | — |
| Partial | 39 (78) | 44 (44) | — | — | — | — | ||
| Partial | 7 (14) | 26 (26) | 0.32 | 0.08 tp 1.29 | 68% | ‐29% to 92% | ||
| Complete | 4 (8) | 30 (30) | 0.22 | 0.06 to 0.84 | 78% | 16% to 94% | ||
| Hennessy 1996 | Vaccine: live‐attenuated SA14‐14‐2 (1 to 3 doses, annual administration)Location: Sichuan Province, China (endemic JE transmission)Cases: 56 children ages less than 15 years of age with serologically confirmed JE infectionControls: 1299 children matched for village, gender, and age (born same year) | None | 38(68) | 615 (47) | — | — | — | — |
| 1‐dose | 11 (20) | 332 (26) | — | — | 80% | 44% to 93% | ||
| 2‐dose | 6 (11) | 308 (24) | — | — | 97.5% | 86% to 99.6% | ||
| 3‐dose | 1 (2) | 44 (3) | — | — | Insufficient data | — | ||
| Bista 2001 | Vaccine: live‐attenuated SA14‐14‐2 (single dose)Location: Bardiya and Banke districts, NepalStudy conducted within 7 to 28 days of vaccination programmeCases: 20 children ages 1 to 15 years with serologically confirmed JE infectionControls: 557 children matched for neighbourhood, gender, and age (within 1 year) | None | 20 (100) | 231 (41.5) | — | — | — | — |
| 1 dose | 0 (0) | 326 (58.5) | 0.007 | 0.0051 to 0.000 | 99.3% | 94.9% to 100% | ||
| Ohrr 2005 | Vaccine: live‐attenuated SA14‐14‐2 (single dose) Location: Bardiya and Banke districts, Nepal Study conducted 1 year after vaccination programme Cases: 35 children ages 2 to 16 years with serologically confirmed JE infection Controls: 430 children matched for neighbourhood, gender, and age (within 1 year) | None | 34 (97.1) | 196 (45.6) | — | — | — | — |
| 1 dose | 1 (2.9) | 234 (54.4) | 0.0155 | 0.0004 to 0.0986 | 98.5% | 1% to 99.2% (CI 90%) |
CI: confidence interval; JE: Japanese encephalitis; OR: odds ratio.
Data and analyses
Comparison 1.
Inactivated Nakayama vaccine (unpurified precursor) vs placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Disease prevention: Japanese encephalitis cases by dose schedule and year after vaccination | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 One‐dose schedule (first year after vaccination) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 One‐dose schedule (second year after vaccination) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 One‐dose schedule (first and second year after vaccination) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Two‐dose schedule (first year after vaccination) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Two‐dose schedule (second year after vaccination) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Two‐dose schedule (first and second year after vaccination) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2.
Inactivated Nakayama and Nakayama + Beijing‐1 vaccines vs placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Disease prevention: Japanese encephalitis cases by year after vaccination | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Nakayama (licensed formula): first year after vaccination | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Nakayama (licensed formula): second year after vaccination | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Nakayama (licensed formula): first and second year after vaccination | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Nakayama + Beijing‐1 (experimental formula): first year after vaccination | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Nakayama + Beijing‐1 (experimental formula): second year after vaccination | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Nakayama + Beijing‐1 (experimental formula): first and second year after vaccination | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3.
Inactivated Nakayama vaccine vs Nakayama + Beijing‐1 vaccine
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Disease prevention: Japanese encephalitis cases by year after vaccination | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 First year after vaccination | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Second year after vaccination | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 First and second year after vaccination | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
What's new
| Date | Event | Description |
|---|---|---|
| 14 October 2008 | Amended | Converted to new review format with minor editing. |
Differences between protocol and review
2007, Issue 3: Immunogenicity included as a primary outcome measure because most trials reported the use of immunological endpoints as a surrogate measure for disease protection.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomized controlled trial (3 groups)Generation of allocation sequence: rotational allocation on presentationAllocation concealment: colour‐coded group assignmentBlinding: participants (and parents), vaccine providers (incomplete blinding due to differences in dose volumes), and clinical assessorsInclusion of randomized participants in analysis: number lost to follow up not stated |
|---|---|
| Participants | Number: 65,224 enrolledAge and gender: 1 to 14 years, both gendersPrevious exposure to Japanese encephalitis (JE) or flavivirus: some, but number not reported |
| Interventions | 1. Vaccine: inactivated mouse brain‐derived Nakayama strain (BIKEN); 2 x 1.0 mL (0.5 mL < 3 years) 2. Vaccine: inactivated mouse brain‐derived Nakayama and Beijing‐1 strains (BIKEN); 2 x 1.0 mL (0.5 mL < 3 years) 3. Placebo: tetanus toxoid vaccine; 2 x 0.5 mLDose interval: 7 days for each group |
| Outcomes | 1. Clinical cases with laboratory confirmation (follow‐up period: 2 years) 2. Immunogenicity (follow‐up period: < 30 days) 3. Adverse events (follow‐up period: < 30 days) |
| Notes | Location: province of Kampangphet, northern Thailand; JE endemicDevelopment and/or production site of JE vaccine: The Japanese Research Foundation for Microbial Diseases (BIKEN) |
| Methods | Randomized controlled trial (4 groups)Generation of allocation sequence: unclearAllocation concealment: letter‐coded group assignmentBlinding: participants (and parents), vaccine providers, and outcome assessorsInclusion of randomized participants in analysis: number lost to follow up not stated |
|---|---|
| Participants | Number: 265,808 enrolledAge and gender: 3 to 7 years, both gendersPrevious exposure to Japanese encephalitis (JE) or flavivirus: not reported |
| Interventions | 1. Vaccine: inactivated mouse brain‐derived Nakayama strain (unpurified precursor); 1 x 1.0 mL 2. Vaccine: inactivated mouse brain‐derived Nakayama strain (unpurified precursor); 2 x 1.0 mL 3. Placebo: tetanus toxoid vaccine; 1 x 1.0 mL 4. Placebo: tetanus toxoid vaccine; 2 x 1.0 mLDose interval: 7 to 10 days for Groups 2 and 4 |
| Outcomes | 1. Clinical cases with laboratory confirmation (follow‐up period: 2 years) 2. Adverse events (follow‐up period: 24 h (1st dose) and 48 h (2nd dose)) |
| Notes | Location: counties of Miaoli, Hsinchu, Taoyuan, and Taipei, northern Taiwan; JE endemicDevelopment and/or production site of JE vaccine: The Japanese Association of Biological Products |
| Methods | Randomized controlled trial (2 groups)Generation of allocation sequence: unclearAllocation concealment: unclearBlinding: unclearInclusion of randomized participants in analysis: 97% (2 lost to follow up) |
|---|---|
| Participants | Number: 60 enrolledAge and gender: 20 to 35 years, malePrevious exposure to Japanese encephalitis (JE) or flavivirus: all JE seronegative at baseline |
| Interventions | 1. Vaccine: inactivated Vero cell‐derived Beijing‐1 strain (Kaketsuken); 3 x 0.5 mL 2. Alternative vaccine: inactivated mouse brain‐derived Beijing‐1 strain (Kaketsuken); 3 x 0.5 mLDose interval for groups 1 and 2: 1 to 4 weeks and 4 to 8 weeks |
| Outcomes | 1. Immunogenicity: detection of neutralizing antibodies (follow‐up period: 2 to 4 weeks after last dose) 2. Adverse events (follow‐up period: 3 days after each dose) |
| Notes | Location: Japan |
| Methods | Cluster randomized controlled trial (2 groups)Cluster randomization: health centres (units of randomization) were pair‐matched based on neighbourhood and number of children served by each centreGeneration of allocation sequence: drawing of lots (between pair matched units of randomization)Allocation concealment: unclearBlinding: outcome assessors and study analystsInclusion of randomized participants in analysis: 99.9% (22 lost to follow up) |
|---|---|
| Participants | Number: 26,239 enrolledAge and gender: 1.9 years (average), both gendersPrevious exposure to Japanese encephalitis (JE)/flavivirus: 45% received previous JE vaccination |
| Interventions | 1. Vaccine: live‐attenuated SA14‐14‐2 strain; 1 dose (volume not reported) 2. No intervention |
| Outcomes | 1. Immunogenicity: detection of neutralizing antibodies (follow‐up period: 2 years) 2. Adverse events (follow‐up period: 30 days) |
| Notes | Location: Chengdu, urban capital of the Sichuan province, ChinaDevelopment and/or production site of JE vaccine: Chengdu Biological Products Institute, China |
| Methods | Randomized controlled trial (4 groups)Generation of allocation sequence: unclearAllocation concealment: unclearBlinding: noInclusion of randomized participants in analysis: 98.9% (1 lost to follow up) |
|---|---|
| Participants | Number: 94 enrolledAge and gender: 18 to 49 years, both gendersPrevious exposure to Japanese encephalitis (JE)/flavivirus: JE antibodies not detected |
| Interventions | 1. Vaccine: inactivated SA14‐14‐2 strain (IC51 (JE‐PIV)); 2 x 6 µg 2. Vaccine: inactivated SA14‐14‐2 strain (IC51 (JE‐PIV)); 3 x 6 µg 3. Vaccine: inactivated SA14‐14‐2 strain (IC51 (JE‐PIV)); 2 x 12 µg 4. Alternative vaccine: inactivated Nakayama vaccine (BIKEN); 3 x 1 mLDose intervals: 28 days for group 1 and 2; 14 days for group 3; and 7 and 28 days for group 4 |
| Outcomes | 1. Immunogenicity: detection of neutralizing antibodies (follow‐up period: 24, 56, 365, and 730 days) 2. Adverse events (follow‐up period: 30 days) |
| Notes | Location: USADevelopment and/or production site of JE vaccine: Walter Reed Army Institute of Research, VaccGen LLC and Intercell AG, USA |
| Methods | Randomized controlled trial (3 groups)Generation of allocation sequence: computer‐generated random planAllocation concealment: number‐coded group assignmentBlinding: participant, study pharmacist (vaccine preparation), vaccine provider, outcome assessors, and all other personnelInclusion of randomized participants in analysis: 100% (0 lost to follow up) |
|---|---|
| Participants | Number: 36 enrolledAge and gender: 18 to 59 years, both gendersPrevious exposure to Japanese encephalitis (JE)/flavivirus: 50% received previous yellow fever (YF) vaccination |
| Interventions | 1. Vaccine: SA14‐14‐2/YF 17D chimera (ChimeriVax‐JE); 1 x 4 log10 plaque‐forming units (PFU) 2. Vaccine: SA14‐14‐2/YF 17D chimera (ChimeriVax‐JE); 1 x 5 log10 PFU 3. Placebo: YF 17D; 1 x 5 log10 PFU |
| Outcomes | 1. Immunogenicity: detection of neutralizing antibodies (follow‐up period: 31 days) 2. Adverse events (follow‐up period: 31 days) |
| Notes | Location: USADevelopment and/or production site of JE vaccine: Acambis, USA |
| Methods | Randomized controlled trial (9 groups)Generation of allocation sequence: unclearAllocation concealment: unclearBlinding: participant, vaccine provider, and outcome assessorInclusion of randomized participants in analysis: 99% (1 lost to follow up) |
|---|---|
| Participants | Number: 99 enrolledAge and gender: 18 to 59 years, both gendersPrevious exposure to Japanese encephalitis (JE) or flavivirus: all JE seronegative at baseline |
| Interventions | 1. Vaccine: SA14‐14‐2/yellow fever (YF) 17D chimera (ChimeriVax‐JE); 2 x 5.8 log10 plaque‐forming units (PFU) 2. Vaccine: SA14‐14‐2/YF 17D chimera (ChimeriVax‐JE); 2 x 4.8 log10 PFU 3. Vaccine: SA14‐14‐2/YF 17D chimera (ChimeriVax‐JE); 2 x 3.8 log10 PFU 4. Vaccine: SA14‐14‐2/YF 17D chimera (ChimeriVax‐JE); 2 x 2.8 log10 PFU 5. Vaccine: SA14‐14‐2/YF 17D chimera (ChimeriVax‐JE); 2 x 1.8 log10 PFU 6. Control: SA14‐14‐2/YF 17D chimera (ChimeriVax‐JE) (day 0) + 1 x log10 PFU YF 17D (placebo) (day 30); 1 x 4.8 log10 PFU 7. Control: YF 17D (placebo) (day 0) + 1 x 4.8 log10 PFU ChimeriVax‐JE (day 0); 1 x log10 PFU 8. Control: 1 x unspecified placebo (day 0) + 1 x 4.8 log10 PFU ChimeriVax‐JE (day 30); 1 x log10 PFU 9. Control: SA14‐14‐2/YF 17D chimera (ChimeriVax‐JE) (day 0) + 1 x unspecified placebo (day 30); 1 x 4.8 log10 PFUDose interval of 30 days for groups 1 to 4 |
| Outcomes | 1. Immunogenicity: detection of neutralizing antibodies (follow‐up period: 1 to 7, 14, 21, 44, and 60 days) 2. Adverse events (follow‐up period: 31 days) |
| Notes | Location: USADevelopment and/or production site of JE vaccine: Acambis, USA |
| Methods | Randomized controlled trial (4 groups)Generation of allocation sequence: unclearAllocation concealment: unclearBlinding: participants and providers not blinded; unclear for outcome assessors and all other personnelInclusion of randomized participants in analysis: numbers lost to follow up not stated |
|---|---|
| Participants | Number: 1160 enrolledAge and gender: 5 to 9 years, both gendersPrevious exposure to Japanese encephalitis (JE) or flavivirus: some, but number not reported |
| Interventions | 1. Vaccine: inactivated mouse brain‐derived Nakayama strain (Thailand); 2 x 1.0 mL 2. Vaccine: inactivated mouse brain‐derived Nakayama strain (BIKEN); 2 x 1.0 mL 3. Vaccine: inactivated mouse brain‐derived Beijing‐1 strain (BIKEN); 2 x 0.5 mL 4. No interventionDose interval of 14 days for groups 1 to 3 |
| Outcomes | 1. Immunogenicity: detection of neutralizing antibodies (follow‐up period: 30, 182, and 365 days) 2. Adverse events (follow‐up period: 7 days (1st dose), 7 to 21 days (2nd dose)) |
| Notes | Location: province of Ratchaburi, central Thailand |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ao 1983 | Study evaluating the safety and immunogenicity at different dose‐levels of live‐attenuated SA14‐14‐2 vaccine using 1‐dose schedule with booster vaccination. SA14‐14‐2 compared with alternative vaccines; randomization not reported |
| Bhau 1988 | Non‐randomized study assessing the immunogenicity of inactivated mouse brain‐derived Nakayama vaccine; no comparison to control group |
| Chen 1999 | Non‐randomized safety study comparing live‐attenuated SA14‐14‐2 vaccine with an inactivated vaccine |
| Defraites 1999 | Randomized safety and immunogenicity trial comparing different lots of inactivated mouse brain‐derived Nakayama vaccine (BIKEN); no comparison to placebo, control vaccine, or unvaccinated control group |
| Gowal 1995 | Non‐randomized study examining persistence of neutralizing antibodies in adults following vaccination with inactivated Nakayama vaccine; no comparison to control group |
| Guo 1998 | Study evaluating the safety and immunogenicity of unspecified live‐attenuated vaccine (presumably SA14‐14‐2) using a 1‐dose schedule with booster compared to unspecified inactivated vaccine; randomization not reported |
| Hanna 2005 | Non‐randomized study measuring the persistence of neutralizing antibodies following vaccination with inactivated mouse brain‐derived vaccine; no comparison to control group |
| Hazarika 1991 | Non‐randomized immunogenicity study of inactivated Nakayama vaccine following second and third dose administration; no comparison to control group |
| Henderson 1984 | Non‐randomized study assessing the safety and immunogenicity of the inactivated Nakayama vaccine (BIKEN); no comparison to control group |
| Intralawan 1993 | Randomized controlled trial assessing the dose level and route of allocation (intradermally or subcutaneously) for inactivated mouse brain‐derived Nakayama vaccine (BIKEN) in adults; no comparison to unvaccinated or placebo control group |
| Juang 1983 | Non‐randomized study assessing the level of neutralizing antibodies to Nakayama, JaGAr‐01, and E‐50 strains following vaccination with inactivated Nakayama vaccine (2‐dose schedule; no comparison to control group |
| Kanesa‐thasan 2000 | Randomized controlled trial assessing safety and immunogenicity of 2 discontinued vaccine candidates (NYVAC‐JE and ALVAC‐JE); excluded on the basis of low power (5 participants per subgroup) and lack of prospective data due to discontinued investigations |
| Kitchener 2006 | Randomized controlled trial assessing safety and immunogenicity of inactivated mouse brain‐derived Nakayama, comparing conventional subcutaneous vaccination to either single intradermal administration or 2 intradermal injections at 2 separate sites; no comparison to a placebo or unvaccinated control group |
| Ku 1994 | Non‐randomized study of immune response to each dose of inactivated mouse brain‐derived Nakayama or Beijing‐1 vaccine administered in a 3‐dose schedule; no comparison to a placebo or unvaccinated control group |
| Lyons 2007 | Randomized safety and immunogenicity study comparing different dose levels of inactivated JE‐PIV vaccine administered intramuscularly in a 2‐ or 3‐dose schedule; no comparison to a placebo or unvaccinated control group |
| Ma 1993 | Non‐randomized trial assessing the safety and immunogenicity of live‐attenuated SA14‐14‐2; no comparison to a control group |
| Ma 2003 | Study investigating the safety and immunogenicity of a vaccine schedule combining inactivated vaccine (not specified) with live‐attenuated (not specified) booster; randomization of trial participants was unclear |
| Mohan 1993 | Non‐randomized trial assessing the safety and immunogenicity of inactivated Nakayama vaccine (BIKEN); no comparison to control group |
| Nimmannitya 1995 | Randomized study comparing the immunogenicity of alternative strain formulas of the inactivated mouse brain‐derived vaccine; no comparison to a control group |
| Nothdurft 1996 | Non‐randomized study of adverse events in receiving inactivated Nakayama vaccine (BIKEN); no comparison to a control group |
| Poland 1990 | Non‐randomized study of safety and neutralizing antibody response to 2‐ and 3‐dose regimens of the inactivated Nakayama vaccine (BIKEN); no comparison to a control group |
| Rodrigues 1986 | Study comparing the immune response to the Nakayama and P20778 strains following vaccination with inactivated Nakayama vaccine (liquid or freeze‐dried); randomization of participants was not reported and there was no comparison to a control group |
| Sanchez 1990 | Non‐randomized study of safety and immunogenicity for the inactivated mouse brain‐derived Nakayama vaccine (BIKEN); no comparison to a control group |
| Sohn 1999 | Non‐randomized study assessing the safety and immunogenicity of live‐attenuated SA14‐14‐2 vaccine; no comparison to a control group |
| Tsai 1998 | Study of the live‐attenuated SA14‐14‐2 vaccine comparing immune responses between different vaccine dilutions and administration schedules; randomization of the different vaccine groups was not mentioned and no control group was included |
| Tseng 1999 | Study comparing immune response between simultaneous and sequential (6‐week interval) administration of measles‐mumps‐rubella and Japanese encephalitis; randomization between groups was uncertain and there was no comparison to control group |
| Xin 1988 | Study assessing the safety and immunogenicity of live‐attenuated SA14‐14‐2 vaccine at different dose levels in different age groups; randomization of participants was uncertain and there was no comparison to control group |
| Yamada 1986 | Non‐randomized study investigating the immunogenicity and safety of inactivated vaccines comparing participants with or without underlying disease; no comparison to placebo or unvaccinated control group |
| Zhou 1999 | Non‐randomized study assessing the effectiveness, safety and immunogenicity of live‐attenuated SA14‐14‐2 vaccine; no comparison to control group |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | — |
|---|---|
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | — |
| Methods | — |
|---|---|
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | — |
| Methods | — |
|---|---|
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | — |
| Methods | — |
|---|---|
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | — |
| Methods | — |
|---|---|
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | — |
Contributions of authors
KL Schiøler completed the protocol, extracted all data, conducted the analyses, and wrote the review. M Samuel initiated the protocol, extracted all data, and commented on the protocol and review manuscripts. KL Wai helped initiate the protocol, did partial data extraction, and commented on the final review manuscript.
Sources of support
Internal sources
- Aubrey Sheiham Public Health and Primary Care Scholarship, The Cochrane Collaboration, UK.
- Clinical Trials & Epidemiology Research Unit, National Medical Research Council, Singapore.
External sources
- Department of International Development, UK.
Declarations of interest
None known.
Unchanged
References
References to studies included in this review
- Hoke CH. Lessons from field testing of non‐HIV vaccines: A field trial of Japanese encephalitis vaccine in Thailand. AIDS Research and Human Retroviruses 1993;9 Suppl 1:161‐7. [Google Scholar]; Hoke CH, Nisalak A, Sangawhipa N, Jatanasen S, Laorakapongse T, Innis BL, et al. Protection against Japanese encephalitis by inactivated vaccines. New England Journal of Medicine 1988;319(10):608‐14. [DOI] [PubMed] [Google Scholar]
- Hsu TC, Chow LP, Wei HY, Chen CL, Hsu ST. A controlled field trial for an evaluation of effectiveness of mouse‐brain Japanese encephalitis vaccine. Taiwan Yi Xue Hui Za Zhi 1971;70(2):55‐62. [PubMed] [Google Scholar]; Hsu TC, Chow LP, Wei HY, Chen CL, Hsu ST, Huang CT, et al. A completed field trial for an evaluation of the effectiveness of mouse‐brain Japanese encephalitis vaccine. Conference on Japanese encephalitis virus vaccine. Tokyo: Igaku Shoin Ltd, 1971:258‐65. [Google Scholar]; Hsu TC, Hsu ST. Supplementary report. Effectiveness of Japanese encephalitis vaccine: Study in the second year following vaccination. Conference on Japanese encephalitis virus vaccine. Tokyo: Igaku Shoin Ltd, 1971:266‐7. [Google Scholar]
- Kuzuhara S, Nakamura H, Hayashida K, Obata J, Abe M, Sonoda K, et al. Non‐clinical and phase I clinical trials of a Vero cell‐derived inactivated Japanese encephalitis vaccine. Vaccine 2003;21(31):4519‐26. [DOI] [PubMed] [Google Scholar]
- Liu ZL, Hennessy S, Strom BL, Tsai TF, Wan CM, Tang S, C, et al. Short‐term safety of live attenuated Japanese encephalitis vaccine (SA14‐14‐2): results of a randomized trial with 26,239 subjects. Journal of Infectious Diseases 1997;176(5):1366‐9. [DOI] [PubMed] [Google Scholar]
- Lyons AG, Kuschner RA, Putnak JR, Eckels KH, Sun W, Towle AC, et al. Long term immunogenicity results of a phase 2 study of purified, inactivated vaccine against Japanese encephalitis virus (IC51, JE‐PIV). American Society of Tropical Medicine and Hygiene, 53rd Annual Meeting. 2004. [Google Scholar]
- Monath TP, McCarthy K, Bedford P, Johnson CT, Nichols R, Yoksan S, et al. Clinical proof of principle for ChimeriVax: recombinant live, attenuated vaccines against flavivirus infections. Vaccine 2002;20(7‐8):1004‐18. [DOI] [PubMed] [Google Scholar]
- Monath TP, Guirakhoo F, Nichols R, Yoksan S, Schrader R, Murphy C, et al. Chimeric live, attenuated vaccine against Japanese encephalitis (ChimeriVax‐JE): phase 2 clinical trials for safety and immunogenicity, effect of vaccine dose and schedule, and memory response to challenge with inactivated Japanese encephalitis antigen. Journal of Infectious Diseases 2003;188(8):1213‐30. [DOI] [PubMed] [Google Scholar]
- Rojanasuphot S, Charoensook O‐A, Ungchusak K, Srijaggrawalwong A, Panthumachinda B. A field trial of inactivated mouse brain Japanese encephalitis vaccines produced in Thailand. Mosquito Borne Diseases Bulletin 1991;8(1):11‐6. [Google Scholar]; Rojanasuphot S, Charoensuk O, Kitprayura D, Likityingvara C, Limpisthien S, Boonyindee S, et al. A field trial of Japanese encephalitis vaccine produced in Thailand. Southeast Asian Journal of Tropical Medicine and Public Health 1989;20(4):653‐4. [PubMed] [Google Scholar]
References to studies excluded from this review
- Ao J, Yu YX, Tang YS, Cui BC, Jia DJ, Li HM. Selection of a better immunogenic and highly attenuated live vaccine strain of Japanese encephalitis II. Safety and immunogenecity of live vaccine SA14‐14‐2 observed in inoculated children. Chinese Journal of Microbiology and Immunology 1983;3:245‐48. [Google Scholar]
- Rao Bhau LN, Singh G, Gowal D, Saxena SN, Kobayashi M, Oya A, et al. Safety & efficacy of Japanese encephalitis vaccine produced in India. Indian Journal of Medical Research 1988;88:301‐7. [PubMed] [Google Scholar]; Saxena SN, Bhau LN, Singh G, Gowal D, Sood YK, Misra CN, et al. Immune status of volunteers one year after administration of Japanese encephalitis vaccine produced in India. Indian Journal of Medical Research 1989;89:362‐7. [PubMed] [Google Scholar]
- Chen HY, Ma FB, Zhang JL. Observation on safety of the live attenuated SA14‐14‐2 Japanese encephalitis vaccine. Chinese Journal of Epidemiology 1999;5(3). [Google Scholar]
- Defraites RF, Gambel JM, Hoke CH Jr, Sanchez JL, Withers BG, Karabatsos N, et al. Japanese encephalitis vaccine (inactivated, BIKEN) in U.S. soldiers: immunogenicity and safety of vaccine administered in two dosing regimens. American Journal of Tropical Medicine and Hygiene 1999;61(2):288‐93. [DOI] [PubMed] [Google Scholar]
- Gowal D, Tahlan AK. Evaluation of effectiveness of mouse brain inactivated Japanese encephalitis vaccine produced in India. Indian Journal of Medical Research 1995;102:267‐71. [PubMed] [Google Scholar]
- Guo W, Li L, Wu Z. Analysis on the immuno‐effects of two different Japanese encephalitis virus vaccines. Zhonghua Liu Xing Bing Xue Za Zhi 1998;19(2):97‐9. [PubMed] [Google Scholar]
- Hanna JN, Smith GA, McCulloch BG, Taylor CT, Pyke A, Brookes DL. An assessment of the interval between booster doses of Japanese encephalitis vaccine in the Torres Strait. Australian and New Zealand Journal of Public Health 2005;29(1):44‐7. [DOI] [PubMed] [Google Scholar]
- Hazarika NC. Project study on Japanese encephalitis vaccination at Gogamukh, Assam. Indian Pediatrics 1991;28(9):1029‐34. [PubMed] [Google Scholar]
- Henderson A. Immunisation against Japanese encephalitis in Nepal: experience of 1152 subjects. Journal of the Royal Army Medical Corps 1984;130(3):188‐91. [DOI] [PubMed] [Google Scholar]
- Intralawan P, Paupunwatana S. Immunogenicity of low dose Japanese encephalitis vaccine (BIKEN) administered by the intradermal route: preliminary data. Asian Pacific Journal of Allergy and Immunology 1993;11(1):79‐83. [PubMed] [Google Scholar]
- Juang RF, Okuno Y, Fukunaga T, Tadano M, Fukai K, Baba K, et al. Neutralizing antibody responses to Japanese encephalitis vaccine in children. Biken Journal 1983;26(1):25‐34. [PubMed] [Google Scholar]
- Kanesa‐thasan N, Smucny JJ, Hoke CH, Marks DH, Konishi E, Kurane I, et al. Safety and immunogenicity of NYVAC‐JEV and ALVAC‐JEV attenuated recombinant Japanese encephalitis virus‐‐poxvirus vaccines in vaccinia‐nonimmune and vaccinia‐immune humans. Vaccine 2000;19(4‐5):483‐91. [DOI] [PubMed] [Google Scholar]
- Kitchener S, Nasveld P, Brennan L, Ward D. Comparative safety and efficacy of subcutaneous and intradermal administration of inactivated Japanese encephalitis vaccine during predeployment preparations in the Australian Defence Force. Military Medicine 2006;171(12):1190‐5. [DOI] [PubMed] [Google Scholar]
- Ku CC, King CC, Lin CY, Hsu HC, Chen LY, Yueh YY, et al. Homologous and heterologous neutralization antibody responses after immunization with Japanese encephalitis vaccine among Taiwan children. Journal of Medical Virology 1994;44(2):122‐31. [DOI] [PubMed] [Google Scholar]
- Lyons A, Kanesa‐Thasan N, Kuschner RA, Eckels KH, Putnak R, Sun W, et al. A Phase 2 study of a purified, inactivated virus vaccine to prevent Japanese encephalitis. Vaccine 2007;25(17):3445‐53. [DOI] [PubMed] [Google Scholar]
- Ma X, Yu YX, Wang SG. Observations on safety and serological efficacy from a large scale field trial of Japanese encephalitis vaccine. Chinese Journal of Biologicals 1993;6(4):188‐91. [Google Scholar]
- Ma FB, Zheng L, Bi C, Tao H, Zhou YL, Zhang JL, et al. Study on the strategy of Japanese encephalitis immunization using live attenuated vaccine combined with inactivated vaccine. Zhonghua Liu Xing Bing Xue Za Zhi 2003;24(2):113‐5. [PubMed] [Google Scholar]
- Mohan Rao CV, Risbud AR, Dandawate CN, Umarani UB, Ayachit VM, Rodrigues FM, et al. Serological response to Japanese encephalitis vaccine in a group of school children in South Arcot district of Tamil Nadu. Indian Journal of Medical Research 1993;97:53‐9. [PubMed] [Google Scholar]
- Nimmannitya S, Hutamai S, Kalayanarooj S, Rojanasuphot S. A field study on Nakayama and Beijing strains of Japanese encephalitis vaccines. Southeast Asian Journal of Tropical Medicine and Public Health 1995;26(4):689‐93. [PubMed] [Google Scholar]
- Nothdurft HD, Jelinek T, Marschang A, Maiwald H, Kapaun A, Loscher T. Adverse reactions to Japanese encephalitis vaccine in travellers. Journal of Infection 1996;32(2):119‐22. [DOI] [PubMed] [Google Scholar]
- Poland JD, Cropp CB, Craven RB, Monath TP. Evaluation of the potency and safety of inactivated Japanese encephalitis vaccine in US inhabitants. Journal of Infectious Diseases 1990;161(5):878‐82. [DOI] [PubMed] [Google Scholar]
- Rodrigues FM, Mohan Rao CV, Mandke VB, Pinto BD, Pavri K. Neutralizing antibody response to Japanese encephalitis inactivated mouse brain vaccine among laboratory personnel. Transactions of the Royal Society of Tropical Medicine and Hygiene 1986;80(2):301‐4. [DOI] [PubMed] [Google Scholar]
- Sanchez JL, Hoke CH, McCown J, DeFraites RF, Takafuji ET, Diniega BM, et al. Further experience with Japanese encephalitis vaccine. Lancet 1990;335(8695):972‐3. [DOI] [PubMed] [Google Scholar]
- Sohn YM, Park MS, Rho HO, Chandler LJ, Shope RE, Tsai TF. Primary and booster immune responses to SA14‐14‐2 Japanese encephalitis vaccine in Korean infants. Vaccine 1999;17(18):2259‐64. [DOI] [PubMed] [Google Scholar]
- Tsai TF, Yu YX, Jia LL, Putvatana R, Zhang R, Wang S, et al. Immunogenicity of live attenuated SA14‐14‐2 Japanese encephalitis vaccine‐‐a comparison of 1‐ and 3‐month immunization schedules. Journal of Infectious Diseases 1998;177(1):221‐3. [DOI] [PubMed] [Google Scholar]
- Tseng CY, Hwang KP, Lin KH, Chen HY, Lu CC, Chiang CH. Comparison of immunogenicity of simultaneous and nonsimultaneous vaccination with MMR and JE vaccine among 15‐month‐old children. Acta Paediatrica Taiwanica 1999;40(3):161‐5. [PubMed] [Google Scholar]
- Xin YY, Ming ZG, Peng GY, Jian A, Min LH. Safety of a live‐attenuated Japanese encephalitis virus vaccine (SA14‐14‐2) for children. American Journal of Tropical Medicine and Hygiene 1988;39(2):214‐7. [DOI] [PubMed] [Google Scholar]
- Yamada A, Imanishi J, Juang RF, Fukunaga T, Okuno Y, Tadano M, et al. Trial of inactivated Japanese encephalitis vaccine in children with underlying diseases. Vaccine 1986;4(1):32‐4. [DOI] [PubMed] [Google Scholar]
- Zhou B, Jia L, Xu X. A large‐scale study on the safety and epidemiological efficacy of Japanese encephalitis (JE) live vaccine (SA14‐14‐2) in the JE endemic areas. Zhonghua Liu Xing Bing Xue Za Zhi 1999;20(1):38‐41. [PubMed] [Google Scholar]
References to studies awaiting assessment
- Acambis. Positive results from pivotal Phase 3 trials of Acambis’ JE vaccine. www.acambis.co.uk/default.asp?id=1724 30 October 2006 (accessed March 2007).
- Acambis. Acambis’ JE vaccine meets and exceeds primary endpoint in pivotal Phase 3 efficacy trial. www.acambis.com/default.asp?id=1839 1 March 2007 (accessed March 2007). [Google Scholar]
- Grachev VP, Sumarokov AA, Kharazian EG, Shkol'nik RIa, Karinskaia GA. Determination of the optimal dose of a cultured inactivated vaccine for preventing Japanese encephalitis. Zhurnal Mikrobiologii Epidemiologii i Immunobiologii 1984;1:98‐103. [PubMed] [Google Scholar]
- Ishikawa T. Development of new Japanese encephalitis vaccine. Nippon Rinsho 2005;63(12):2133‐7. [PubMed] [Google Scholar]
- Sumarovkov AA, Grachev VP, Kharazian EG, Shkol'nik RIa, Vorob'eva MS. Comparative study of the reactogenicity and immunogenicity of new Soviet and commercial vaccines for preventing Japanese encephalitis. Zhurnal Mikrobiologii Epidemiologii i Immunobiologii 1983;9:74‐9. [PubMed] [Google Scholar]
Additional references
- Acambis. ChimeriVax™‐JE: A single‐dose JE vaccine for endemic markets and travellers. www.acambis.co.uk/default.asp?id=936(accessed March 2007).
- Andersen MM. Ronne T. Side‐effects with Japanese encephalitis vaccine. Lancet 1991;337(8748):1044. [DOI] [PubMed] [Google Scholar]
- Anonymous. Japanese Encephalitis virus infection in mosquitoes and its epidemiological implications. Indian Council of Medical Research (ICMR) Bulletin April, 2000;30(4):1‐7. [Google Scholar]
- Barrett AD. Japanese encephalitis and dengue vaccines. Biologicals 1997;25(1):27‐34. [DOI] [PubMed] [Google Scholar]
- Barrett AD. Current status of flavivirus vaccines. Annals of the New York Academy of Sciences 2001;951:262‐71. [DOI] [PubMed] [Google Scholar]
- Berg SW, Mitchell BS, Hanson RK, Olafson RP, Williams RP, Tueller JE, et al. Systemic reactions in U.S. Marine Corps personnel who received Japanese encephalitis vaccine. Clinical Infectious Diseases 1997;24(2):265‐6. [DOI] [PubMed] [Google Scholar]
- Bista MB, Banerjee MK, Shin SH, Tandan JB, Kim MH, Sohn YM, et al. Efficacy of single‐dose SA 14‐14‐2 vaccine against Japanese encephalitis: a case control study. Lancet 2001;358(9284):791‐5. [DOI] [PubMed] [Google Scholar]
- Anonymous. Inactivated Japanese encephalitis virus vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report 1993;42(RR‐1):1‐15. [PubMed] [Google Scholar]
- Chambers TJ, Tsai TF, Pervikov Y, Monath TP. Vaccine development against dengue and Japanese encephalitis: report of a World Health Organization meeting. Vaccine 1997;15(14):1494‐502. [DOI] [PubMed] [Google Scholar]
- Chang GJ, Kuno G, Purdy DE, Davis BS. Recent advancement in flavivirus vaccine development. Expert Review of Vaccines 2004;3(2):199‐220. [DOI] [PubMed] [Google Scholar]
- Chaudhuri M. Chinese vaccine kills 10 children in UP. www.ndtv.com/morenews/showmorestory.asp?id=90630&frmsrch=1&txtsrch=chinese+vaccine+kills+10+children+in+UP# 25 July 2006 (accessed March 2007).
- Endy TP, Nisalak A. Japanese encephalitis virus: ecology and epidemiology. Current Topics in Microbiology and Immunology 2002;267:11‐48. [DOI] [PubMed] [Google Scholar]
- Gajanana A, Thenmozhi V, Samuel PP, Reuben R. A community‐based study of subclinical flavivirus infections in children in an area of Tamil Nadu, India, where Japanese encephalitis is endemic. Bulletin of the World Health Organization 1995;73(2):237‐44. [PMC free article] [PubMed] [Google Scholar]
- Glovax. Live‐attenuated JE vaccine. www.glovax.com/99/default.asp?PageZoneNumber=3&GroupID=99‐2&PageID=37564u4887152778‐99&CompanyID=99&ParentPageID=99(accessed March 2007).
- Hanna JN, Ritchie SA, Phillips DA, Lee JM, Hills SL, Hurk AF, et al. Japanese encephalitis in north Queensland, Australia, 1998. Medical Journal of Australia 1999;170(11):533‐6. [DOI] [PubMed] [Google Scholar]
- Hennessy S, Liu Z, Tsai TF, Strom BL, Wan CM, Liu HL, et al. Effectiveness of live‐attenuated Japanese encephalitis vaccine (SA14‐14‐2): a case‐control study. Lancet 1996;347(9015):1583‐6. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S, editors. Highly sensitive search strategies for identifying reports of randomized controlled trials in MEDLINE. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006]; Appendix 5b (accessed 1 March 2007).
- Hoke CH Jr, Vaughn DW, Nisalak A, Intralawan P, Poolsuppasit S, Jongsawas V, et al. Effect of high‐dose dexamethasone on the outcome of acute encephalitis due to Japanese encephalitis virus. Journal of Infectious Diseases 1992;165(4):631‐7. [DOI] [PubMed] [Google Scholar]
- Hombach J, Solomon T, Kurane I, Jacobson J, Wood D. Report on a WHO consultation on immunological endpoints for evaluation of new Japanese encephalitis vaccines, WHO, Geneva, 2‐3 September, 2004. Vaccine 2005;23(45):5205‐11. [DOI] [PubMed] [Google Scholar]
- Huy BV, Tu HC, Luan TV, Lindqvist R. Early mental and neurological sequelae after Japanese B encephalitis. The Southeast Asian Journal of Tropical Medicine and Public Health 1994;25(3):549‐53. [PubMed] [Google Scholar]
- Igarashi A. Control of Japanese encephalitis in Japan: immunization of humans and animals, and vector control. Current Topics in Microbiology and Immunology 2002;267:139‐52. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. The prospects for immunizing against Japanese encephalitis virus. Diseases of importance in developing countries: Establishing priorities; Volume II, Diseases of importance in developing countries. Washington, DC: National Academy Press, 1986:223‐40. [Google Scholar]
- Jacobson J. Japanese encephalitis (JE) vaccine in India (Uttar Pradesh)(02): Vaccine Safety, RFI. ProMED‐mail: Archive number 20060727.2064. ProMED (International society for infectious diseases), 26 July 2006 (accessed March 2007).
- Juni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SA, Narain K, Handigue R, Dutta P, Mahanta J, Satyanarayana K, et al. Role of some environmental factors in modulating seasonal abundance of potential Japanese encephalitis vectors in Assam, India. The Southeast Asian Journal of Tropical Medicine and Public Health 1996;27(2):382‐91. [PubMed] [Google Scholar]
- Kumar R, Mathur A, Singh KB, Sitholey P, Prasad M, Shukla R, et al. Clinical sequelae of Japanese encephalitis in children. The Indian Journal of Medical Research 1993;97:9‐13. [PubMed] [Google Scholar]
- Kurane I, Takasaki T. Immunogenicity and protective efficacy of the current inactivated Japanese encephalitis vaccine against different Japanese encephalitis virus strains. Vaccine 2000;18(2):33‐5. [DOI] [PubMed] [Google Scholar]
- Kurane I. Evaluation of mouse brain‐derived, inactivated Japanese encephalitis vaccine. Uirusu 2005;55(2):307‐12. [DOI] [PubMed] [Google Scholar]
- Lacey LA, Lacey CM. The medical importance of riceland mosquitoes and their control using alternatives to chemical insecticides. Journal of the American Mosquito Control Association 1990;2:1‐93. [PubMed] [Google Scholar]
- Luo D, Yin H, Xili L, Song J, Wang Z. The efficacy of Japanese encephalitis vaccine in Henan, China: a case‐control study. Southeast Asian Journal of Tropical Medicine and Public Health 1994;25(4):643‐6. [PubMed] [Google Scholar]
- Markoff L. Points to consider in the development of a surrogate for efficacy of novel Japanese encephalitis virus vaccines. Vaccine 2000;18 Suppl 2:26‐32. [DOI] [PubMed] [Google Scholar]
- Matsunaga Y, Yabe S, Taniguchi K, Nakayama M, Kurane I. Current status of Japanese encephalitis in Japan. Kansenshogaku Zasshi 1999;73(2):97‐103. [DOI] [PubMed] [Google Scholar]
- Monath TP. Japanese encephalitis vaccines: current vaccines and future prospects. Current Topics in Microbiology and Immunology 2002;267:105‐38. [DOI] [PubMed] [Google Scholar]
- Nothdurft HD, Jelinek T, Marschang A, Maiwald H, Kapaun A, Loscher T. Adverse reactions to Japanese encephalitis vaccine in travellers. Journal of Infection 1996;32(2):119‐22. [DOI] [PubMed] [Google Scholar]
- Ohrr H, Tandan JB, Sohn YM, Shin SH, Pradhan DP, Halstead SB. Effect of single dose of SA 14‐14‐2 vaccine 1 year after immunisation in Nepalese children with Japanese encephalitis: a case‐control study. Lancet 2005;366(9494):1375‐8. [DOI] [PubMed] [Google Scholar]
- Okabe N. Background of recent JE vaccine issues. Uirusu 2005;55(2):303‐6. [DOI] [PubMed] [Google Scholar]
- PATH. Vaccine resource library: Japanese encephalitis vaccine. www.path.org/vaccineresources/japanese_encephalitis‐vaccine.php(accessed August 2006).
- PATH. Historic protection for Asia’s children: PATH and our partners take action to eliminate Japanese encephalitis. www.path.org/projects/japanese_encephalitis_project.php(accessed August 2006).
- Peiris JS, Amerasinghe FP, Arunagiri CK, Perera LP, Karunaratne SH, Ratnayake CB, et al. Japanese encephalitis in Sri Lanka: comparison of vector and virus ecology in different agro‐climatic areas. Transactions of the Royal Society of Tropical Medicine and Hygiene 1993;87(5):541‐8. [DOI] [PubMed] [Google Scholar]
- Plesner AM, Arlien‐Soborg P, Herning M. Neurological complications and Japanese encephalitis vaccination. Lancet 1996;348(9021):202‐3. [DOI] [PubMed] [Google Scholar]
- Plesner AM, Ronne T. Allergic mucocutaneous reactions to Japanese encephalitis vaccine. Vaccine 1997;15(11):1239‐43. [DOI] [PubMed] [Google Scholar]
- Plesner A, Ronne T, Wachmann H. Case‐control study of allergic reactions to Japanese encephalitis vaccine. Vaccine 2000;18(17):1830‐6. [DOI] [PubMed] [Google Scholar]
- Reuben R, Thenmozhi V, Samuel PP, Gajanana A, Mani TR. Mosquito blood feeding patterns as a factor in the epidemiology of Japanese encephalitis in southern India. American Journal of Tropical Medicine and Hygiene 1992;46(6):654‐63. [DOI] [PubMed] [Google Scholar]
- Ruff TA, Eisen D, Fuller A, Kass R. Adverse reactions to Japanese encephalitis vaccine. Lancet 1991;338(8771):881‐2. [DOI] [PubMed] [Google Scholar]
- Schneider R J, Firestone MH, Edelman R, Chieowanich P, Pornpibul R. Clinical sequelae after japanese encephalitis: a one year follow‐up study in Thailand. Southeast Asian Journal of Tropical Medicine and Public Health 1974;5(4):560‐8. [PubMed] [Google Scholar]
- Shlim DR, Solomon T. Japanese encephalitis vaccine for travelers: exploring the limits of risk. Clinical Infectious Diseases 2002;35(2):183‐8. [DOI] [PubMed] [Google Scholar]
- Sohn YM. Japanese encephalitis immunization in South Korea: past, present, and future. Emerging Infectious Dieseases 2000;6(1):17‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon T, Dung NM, Kneen R, Gainsborough M, Vaughn DW, Khanh VT. Japanese encephalitis. Journal of Neurology, Neurosurgery and Psychiatry 2000;68(4):405‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon T, Vaughn DW. Pathogenesis and clinical features of Japanese encephalitis and West Nile virus infections. Current Topics in Microbiology and Immunology 2002;267:171‐94. [DOI] [PubMed] [Google Scholar]
- Srivastava AK, Putnak JR, Lee SH, Hong SP, Moon SB, Barvir DA, et al. A purified inactivated Japanese encephalitis virus vaccine made in Vero cells. Vaccine 2001;19(31):4557‐65. [DOI] [PubMed] [Google Scholar]
- Sunish IP, Reuben R. Factors influencing the abundance of Japanese encephalitis vectors in ricefields in India‐‐I. Abiotic. Medical and Veterinary Entomology 2001;15(4):381‐92. [DOI] [PubMed] [Google Scholar]
- Takagi M, Suwonkerd W, Tsuda Y, Sugiyama A, Wada Y. Effects of rice culture practices on the abundance of Culex mosquitoes (Diptera:Culicidae) in northern Thailand. Journal of Medical Entomology 1997;34(3):272‐6. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Pool V, Tsai TF, Chen RT. Adverse events after Japanese encephalitis vaccination: review of post‐marketing surveillance data from Japan and the United States. The VAERS Working Group. Vaccine 2000;18(26):2963‐9. [DOI] [PubMed] [Google Scholar]
- Tsai TF, Chang GJ, Yu YX. Japanese encephalitis vaccines. Vaccines. 2nd Edition. Philadelphia: Saunders W.B., 1994:671‐713. [Google Scholar]
- Tsai TF. New initiatives for the control of Japanese encephalitis by vaccination: Minutes of a WHO/CVI meeting, Bangkok, Thailand, 13‐15 October 1998. Vaccine 2000;18 Suppl 2:1‐25. [DOI] [PubMed] [Google Scholar]
- Vaughn DW, Hoke CH Jr. The epidemiology of Japanese encephalitis: prospects for prevention. Epidemiologic Reviews 1992;14:197‐221. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Japanese encephalitis vaccines. WHO position paper. Weekly Epidemiological Record 1998;73(44):337‐44. 9817024 [Google Scholar]
- WHO Initiative for Vaccine Research. State of the art of new vaccines: research and development (www.who.int/vaccine\_research/documents/en/stateofart\_excler.pdf). Geneva: World Health Organization, 2003. [Google Scholar]
- World Health Organization. Global Advisory Committee on Vaccine Safety, 9‐10 June 2005. Weekly Epidemiological Record 2005;80(28):242‐3. 16047931 [Google Scholar]
- WHO Initiative for Vaccine Research. New vaccines against infectious diseases: research and development status; April 2005, updated February 2006. www.who.int/vaccine_research/documents/en/Status_Table.pdf(accessed August 2006).
- Wu YC, Huang YS, Chien LJ, Lin TL, Yueh YY, Tseng WL, et al. The epidemiology of Japanese encephalitis on Taiwan during 1966‐1997. American Journal of Tropical Medicine and Hygiene 1999;61(1):78‐84. [DOI] [PubMed] [Google Scholar]
- Yang SE, Pan MJ, Tseng HF, Liau MY. The efficacy of mouse‐brain inactivated Nakayama strain Japanese encephalitis vaccine‐‐results from 30 years experience in Taiwan. Vaccine 2006;24(14):2669‐73. [DOI] [PubMed] [Google Scholar]
- Zhou B, Zhang M, Chen P, et al. An 11 year follow‐up of epidemiological effect Japanese encephalitis vaccine, live (SA14‐14‐2). Chinese Journal of Biologicals2001; Vol. 14(3) (translated by Chengdu Institute of Biologic Products).