Association between Micronutrients and Heart Rate Variability: A Review of Human Studies (original) (raw)
ABSTRACT
Heart rate variability (HRV) is a measure of the variation between consecutive heartbeats. It provides a marker of the interplay between the parasympathetic and sympathetic nervous systems, and there is an increasing body of evidence confirming an increased HRV is associated with better mental and physical health. HRV may be a useful marker of stress as it represents the ability of the heart to respond to a variety of physiological and environmental stimuli. HRV tends to decrease as we age and is positively associated with physical activity, fitness, and healthier lifestyles. The relation between HRV and micronutrients (vitamins and minerals) has also received some attention in the research literature. In this review, cross-sectional and interventional studies on human populations examining the relation between HRV and micronutrients are appraised. Micronutrients identified and examined in this review include vitamins D, B-12, C, and E; the minerals magnesium, iron, zinc, and coenzyme Q10; and a multivitamin-mineral formula. Due to the paucity of research and significant heterogeneity in studies, definitive conclusions about the effects of these micronutrients on HRV cannot be made at this time. However, there is accumulating evidence suggesting deficiencies in vitamins D and B-12 are associated with reduced HRV, and zinc supplementation during pregnancy can have positive effects on HRV in offspring up until the age of 5 y. To further elucidate the relation between micronutrients and HRV, additional robustly designed and adequately powered studies are required.
Keywords: heart rate variability, HRV, micronutrients, vitamins, minerals
Introduction
Heart rate variability (HRV) is a measure of the variation between consecutive heartbeats. The time period between 2 consecutive heartbeats is called the RR (R wave to R wave) or NN (normal beat to normal beat) interval, and HRV is the variation between these successive intervals. HRV is a marker of the responsiveness of the autonomic nervous system, a component of the peripheral nervous system that regulates involuntary physiologic processes including heart rate, blood pressure, respiration, digestion, and sexual arousal (1). In particular, HRV is a measure of the interplay between 2 arms of the autonomic nervous system: the parasympathetic and sympathetic nervous systems (PNS and SNS, respectively). Activation of the SNS leads to a “fight or flight” response, a state of overall elevated activity and attention whereby physiological changes such as increases in blood pressure, heart rate, and glycogenolysis occur, as well as a slowing of gastrointestinal peristalsis. Activation of the PNS promotes the “rest and digest” process, whereby heart rate and blood pressure decrease and gastrointestinal peristalsis and digestive processes prevail (1).
A healthy heart is not a metronome because there are complex and nonlinear oscillations. This variability provides flexibility to cope with uncertain and changing environmental circumstances (2). Reduced HRV is a marker of increased SNS activity or decreased PNS activity, whereas increased HRV is indicative of decreased SNS activity or increased PNS activity. There is an increasing body of evidence confirming an association between HRV and mental and physical health. In fact, HRV is believed to be a psychophysiological marker of health and well-being (3). HRV represents the ability of the heart to respond to a variety of physiological and environmental stimuli. A high HRV is a sign of a healthy heart that responds flexibly to physiological or environmental stressors, whereas a low HRV is a marker of reduced health that is associated with impaired immune function and a poorer self-regulatory process (3). Through its relation with neural structures associated with the appraisal of threat and safety, HRV may also provide a useful index of stress (4).
Even though findings are inconsistent, HRV tends to decrease as we age (5), is positively associated with physical activity and fitness (6), and is higher in people with healthier lifestyles (7). HRV values have been shown to predict C-reactive protein concentrations 4 y later (8), and in a meta-analysis was confirmed to be negatively correlated with markers of inflammation (9). It is theorized that the autonomic nervous system via an inflammatory reflex of the vagus nerve (the primary nerve of the PNS), and involving the cholinergic anti-inflammatory pathway, is responsible for a quick reflexive response to inflammation (10). HRV is also inversely correlated with the risk of developing, and with a poorer prognosis of, cardiovascular disease (11). Low HRV has also been independently predictive of increased mortality in post–myocardial infarction and heart failure patients (12). In a meta-analysis, it was concluded that HRV decreases in response to stress (13), and in another meta-analysis of 21 studies it was concluded that depression is associated with lower HRV (14). A reduction in HRV has also been found across the spectrum of clinical and nonclinical populations of young people with depression or anxiety (15) and is lower in people with post-traumatic stress disorder (16). Higher HRV has also been shown to be positively associated with improved cancer progression and outcome (17).
Given its positive association with overall health, identifying risk factors and interventions to modify HRV has been investigated. Increasing physical exercise (18), stress reduction (19), meditation (20), HRV biofeedback (21), dietary changes (22), increased omega-3 PUFA intake (23), weight loss (24), moderate alcohol intake (25), and avoidance of nicotine (26) are all associated with increases in HRV and vagal tone. The relation between HRV and micronutrients (vitamins and minerals) has also received some attention in the research literature. However, a comprehensive review of these studies has not been undertaken. The aim of this review is to critically summarize the research investigating the relation between micronutrient status and HRV and to examine the impact of micronutrient supplementation on HRV parameters.
Heart rate measurements are usually examined through an electrocardiogram (ECG) with recordings typically lasting from several minutes to 24 h. HRV can also be measured during different postures (e.g., supine and sitting), following periods of rest, and during exposure to varying stressors (27). The analysis of HRV can be performed through time, frequency, and nonlinear calculations. Various mathematical algorithms are used to calculate HRV parameters. Time-domain analyses measure variation in heart rate over time, involving calculations of mean NN (or RR) intervals. Frequency-domain measurements estimate the distribution of absolute or relative power in frequency bands. Nonlinear analysis methods differ from the conventional HRV methods because they do not assess the magnitude of variability but rather the quality, scaling, and correlation properties of the signals (28). A description of HRV parameters measured in the studies cited in this article is detailed in Table 1. The measures detailed in Table 1 are often closely correlated, although levels vary based on the recording duration and the task(s) undertaken during the assessment period (29, 30). This makes a direct comparison of HRV measurements difficult. However, all measures detailed in Table 1 can be collected through Holter recordings and calculated using relevant software. This makes their measurement possible in both research and clinical settings.
TABLE 1.
A selection of HRV measurements and their descriptions1
| Measure | Definition | Description |
|---|---|---|
| Time-domain measurements | ||
| SDNN (ms) | SD of all NN intervals | Indicates total variability with a high value believed to be associated with better health; when measured over 24 h provides a measure of cardiac risk; provides an index of physiological resilience against stress (higher values indicate higher resiliency) (2, 27) |
| SDANN (ms) | SD of all the averages of NN intervals in all 5-min segments of the entire recording | Is highly correlated with SDNN and is generally considered redundant if SDNN is measured |
| RMSSD (ms) | The square root of the mean of the sum of the squares of differences between adjacent NN intervals | Higher values indicate greater vagal and parasympathetic nervous system activity; is positively correlated highly with HF (2, 27) |
| NN50 count | Number of pairs of adjacent NN intervals differing by >50 ms in the entire recording | Higher values indicative of greater parasympathetic activity |
| pNN50 (%) | NN50 count divided by the total number of all NN intervals | Higher values indicative of greater parasympathetic activity; is highly positively correlated with RMSSD and HF |
| Frequency domain measurements | ||
| Total power (ms2) | The variance of NN intervals over the temporal segment (≤0.4 Hz) | Provides a broad measure of autonomic activity; a high value is believed to be associated with better health; does not distinguish between sympathetic and parasympathetic contributions (2, 27) |
| VLF (ms2) | Power in very-low-frequency (VLF) range (≤0.04 Hz) | Reflects vasomotor changes, thermoregulatory, and possibly parasympathetic influences on heart rate; is negatively correlated with all-cause mortality; is negatively correlated with inflammation (2, 27) |
| LF (ms2) | Power in low-frequency (LF) range (0.04–0.15 Hz) | Mixture of vagal (parasympathetic) and sympathetic influences, with greater sympathetic sensitivity |
| HF (ms2) | Power in high-frequency (HF) range (0.15–0.4 Hz) | Reflects parasympathetic and vagal activity; correlates highly and positively with RMSSD |
| LF:HF (ratio) | Ratio of LF to HF | Used as an index of autonomic balance; previously believed to be a measure of sympathetic/parasympathetic dominance, however, this is strongly debated |
| Nonlinear measurements | ||
| HRV correlation dimension (HRV-CD) | Derived from chaos theory comprising the calculation of the fractal dimension of the NN interval sequence | Has been shown to more accurately differentiate between healthy and high-risk patients with 96% accuracy (28) |
Methods
Search strategy and eligibility criteria
Information for this review was compiled by searching PubMed (https://www.ncbi.nlm.nih.gov/pubmed), Google Scholar (https://scholar.google.com.au), PsycINFO (https://search.proquest.com/psycinfo), Scopus (https://www.scopus.com), and the Cochrane Library (http://www.cochranelibrary.com) databases. Additional studies were also identified during the review of relevant articles and by examining their reference lists. Databases were scanned from all years of study until October 2019 for human studies. A systematic search of databases with the use of the terms (“heart rate variability” or vagus) and (nutrient* or vitamin or mineral or diet* or zinc or magnesium or iron or calcium or copper or manganese or molybdenum or potassium or sodium) was completed. Specific inclusion criteria included the following: 1) published in English, 2) cross-sectional or interventional studies, 3) utilized ≥1 HRV measure, 4) assessed micronutrient status through dietary intake or a biological measure (e.g., blood, urine, or saliva), and 5) investigations were conducted in human participants with no limit on sample size.
Results
As detailed in Figure 1, 69 full-text articles were assessed for eligibility. After the screening of articles, 31 studies were identified as eligible for inclusion. Some of these studies comprised a combination of correlational analyses and interventional designs. Fifteen cross-sectional studies examining the relation between micronutrient concentrations (vitamin D, vitamin B-12, iron, magnesium, and zinc) were identified (Table 2), and a summary of levels of HRV parameters in low-micronutrient (or deficient) populations is detailed in Table 3. Only HRV parameters that were measured in >1 study are included in Table 3; however, there remain inconsistencies in the duration periods used to calculate these parameters. Eighteen studies investigating the effects of micronutrient administration [magnesium, vitamin C, vitamin B-12, zinc, vitamin D, vitamin E, coenzyme Q10 (CoQ10), and a multivitamin-mineral formula] on HRV values were identified and are summarized in Table 4. A summary of the effects of micronutrient administration on HRV parameters is detailed in Table 5.
FIGURE 1.
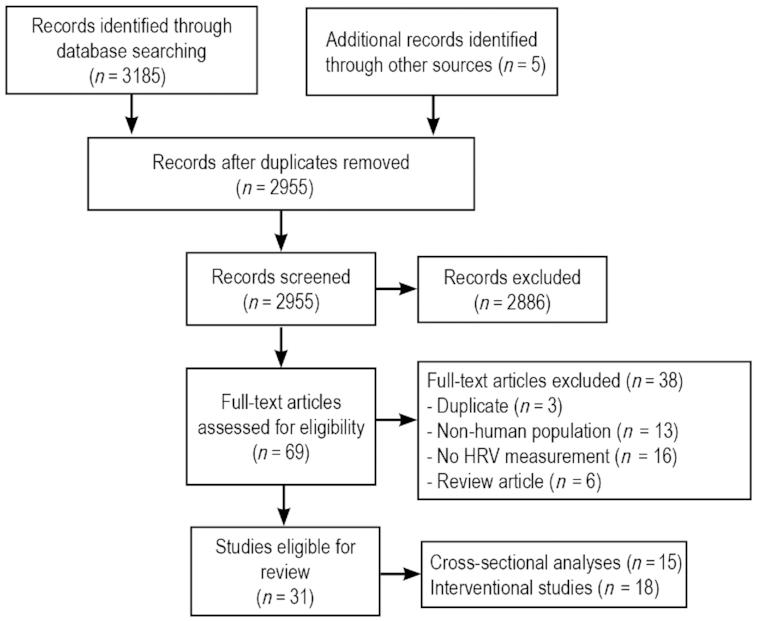
Flowchart of search strategy and selection of relevant studies. HRV, heart rate variability.
TABLE 2.
Human studies examining the association between micronutrient concentrations and HRV parameters1
| Study, year (reference) | Study population | Nutrient measured | HRV measurement | HRV indicators | Findings |
|---|---|---|---|---|---|
| Vitamin D | |||||
| Cetin et al., 2014 (36) | 71 hospitalized patients with chronic heart failure (36 with NIDCM and 35 with IDCM) (mean age of 60 y) | Serum 25(OH)D and calcitriol concentrations | 24-h Holter monitor | SDNN, SDANN, RMSSD | No differences in HRV parameters between vitamin D–deficient and –sufficient subjects In patients with NIDCM only, there was a significant positive correlation between vitamin D concentrations and the HRV parameters SDNN and SDANN |
| Tak et al., 2014 (37) | 103 healthy adults (mean age 55 y): vitamin D deficient, 25(OH)D <15 ng/mL vitamin D nondeficient, 25(OH)D ≥15 ng/mL | Serum 25(OH)D | 5-min ECG recording while sitting | SDNN, RMSSD, TP, VLF, LF, HF, LF:HF | Vitamin D significantly positively correlated with SDNN and LF SDNN significantly lower in vitamin D deficient compared with nondeficient |
| Canpolat et al., 2015 (38) | 24 healthy vitamin D–deficient adults (mean age: 38 y); 50 age-, gender-, and BMI-matched vitamin D–sufficient adults | Serum 25(OH)D | 24-h Holter monitor | SDNN, SDANN, RMSSD, pNN50, HF, LF, LF:HF | SDNN, SDANN, RMSDD, pNN50, HF lower in vitamin D–deficient group LF and LF:HF higher in vitamin D–deficient group |
| Nalbant et al., 2017 (39) | 54 patients with low vitamin D (mean age: 29 y); 51 healthy controls with sufficient vitamin D concentrations | Serum 25(OH)D | 1-h Holter monitor while sitting | SDNN, SDANN, RMSSD, and pNN50 | No differences in HRV parameters between the 2 groups |
| Jung et al., 2015 (40) | 163 patients with type 2 diabetes (mean age: 57 y): vitamin D sufficient, ≥20 ng/mL vitamin D insufficient, 11–19 ng/mL vitamin D deficiency, <10 ng/mL | Serum 25(OH)D | ECG in supine and upright position over short time period (duration not specified) | SDNN, RMSSD, VLF, LF, HF, LF:HF | SDNN in supine position significantly lower in vitamin D–deficient compared with –sufficient subjects RMSSD in supine position significantly lower in vitamin D–deficient compared with –insufficient subjects SDNN in upright position significantly lower in vitamin D–deficient compared with other groups LF:HF in upright position significantly higher in vitamin D–deficient group compared with other groups Vitamin D significantly positively correlated with SDNN in the supine position only |
| Hansen et al., 2017 (41) | 113 people with type 1 or type 2 diabetes (mean age: 56 y) | Serum 25(OH)D | 5-min supine, resting ECG | RMSSD, SDNN, HF, LF, TP, LF:HF | Significant inverted U-shaped association between serum vitamin D and SDNN, RMSSD, and HF HRV indices peaked at vitamin D concentrations of 92, 98, and 102 nmol/L for SDNN, RMSSD, and HF, respectively |
| Vitamin B-12 | |||||
| Sozen et al., 1998 (31) | 12 patients with pernicious anemia (mean age: 51 y); 12 age- and sex-matched healthy controls | Serum B-12 and hemoglobin | 24-h Holter monitor | SDNN, SDANN, RMSSD, pNN50, TP, LF, HF | SDNN, SDANN, RMSSD, TP, LF, and HF significantly lower in patients with pernicious anemia |
| Aytemir et al., 2000 (32) | 17 adults with vitamin B-12 deficiency (mean age: 58 y); 15 age- and sex-matched healthy volunteers | Serum B-12 and hemoglobin | 5-min ECG recordings at rest | LF, HF, LF:HF | Vitamin B-12–deficient patients had significantly lower LF, HF, and LF:HF than controls All HRV parameters positively correlated with vitamin B-12 concentration and negatively with the duration of the illness |
| Sucharita et al., 2012 (33) | 29 healthy elderly adults with vitamin B-12 deficiency (mean age: 67 y); 18 matched volunteers with sufficient vitamin B-12 concentrations | Serum B-12 | 10-min ECG measurements in supine position | HF, LF, TP, LF:HF | LF lower in B-12–deficient compared with B-12–replete patients |
| Sucharita et al., 2013 (34) | 14 healthy young adults (mean age not specified) | Plasma vitamin B-12 and MMA | Resting ECG recordings | TP, LF, HF | Plasma MMA negatively correlated with LF and TP Plasma MMA positively correlated with HF There was no correlation between vitamin B-12 and any HRV parameters |
| Sucharita et al., 2014 (35) | 79 children (mean age: 5 y); based on median vitamin B-12 concentration of mothers (114 pmol/L), children categorized into lower or higher vitamin B-12 status group | Mother's vitamin B-12 status during first trimester | 10-min ECG recording in supine position | LF, HF, TP, LF:HF | LF significantly lower in low B-12 status children Strong trends of lower HF and TP levels in low B-12 status children LF, TP, and HF positively correlated with cord blood B-12 concentrations measured at birth |
| Iron | |||||
| Yokusoglu et al., 2007 (42) | 43 community-based patients with iron deficiency anemia (mean age: 34 y); 39 age- and gender-matched healthy adults | Iron-deficient anemia (serum ferritin <12 ng/mL; hemoglobin <12 g/dL in women and <13 g/dL in men) | 24-h Holter monitor | SDNN, SDANN, RMSSD, SNN50 count, pNN50 | SDNN, SDANN, SNN50 count, and pNN50 lower in adults with iron deficiency |
| Tuncer et al., 2009 (43) | 23 hospitalized patients with iron deficiency anemia (mean age: 30 y); 10 age- and gender-matched healthy people | Iron-deficient anemia (low serum ferritin, hemoglobin <11.7 g/dL for women and <13 g/dL for men) | 24-h Holter monitor | SDNN, MSRSD, HRVM, SDHRV, SDANN | No differences in any HRV parameters |
| Magnesium | |||||
| Kim et al., 2012 (44) | 166 healthy women (mean age: 46 y) | Serum calcium and magnesium | 5 min at rest measured with photoplethysmogram sensor | SDNN, RMSSD, TP, LF, HF, LF:HF | Magnesium: SDDN and TP higher in women with higher magnesium concentrations; SDNN positively correlated with magnesium concentrations Calcium to magnesium ratio: SDNN and LF lower in women with higher ratios. SDNN, TP, and LF negatively associated with ratio |
| Zinc | |||||
| Spann et al., 2015 (45) | Pregnant adolescent mothers aged 14–19 y, 34–36 wk of gestation | 24-h dietary recall to estimate intake of zinc and folate | 20-min fetal heart rate placed on mother's abdomen | Mean difference in fetal heart rate between adjacent epochs | HRV lower in mothers consuming a zinc-deficient but not folate-deficient diet |
TABLE 3.
Levels of HRV parameters in low-micronutrient (or deficient) populations compared with micronutrient-sufficient controls1
| Study, year (reference) | SDNN | SDANN | RMSSD | pNN50 | TP | VLF | LF | HF | LF:HF |
|---|---|---|---|---|---|---|---|---|---|
| Vitamin D deficiency | |||||||||
| Cetin et al., 2014 (36) | → | → | → | ||||||
| Tak et al., 2014 (37) | ↓ | → | → | → | → | → | → | ||
| Canpolat et al., 2015 (38) | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↑ | ||
| Nalbant et al., 2017 (39) | → | → | → | → | |||||
| Jung et al., 2015 (40) | ↓ | ↓ | → | → | → | → | ↑ | ||
| Vitamin B-12 deficiency | |||||||||
| Sozen et al., 1998 (31) | ↓ | ↓ | ↓ | → | ↓ | ↓ | ↓ | ||
| Aytemir et al., 2000 (32) | ↓ | ↓ | ↓ | ||||||
| Sucharita et al., 2012 (33) | → | → | → | ||||||
| Sucharita et al., 2014 (35) | ↓ | ↓ | ↓ | ||||||
| Iron deficiency | |||||||||
| Yokusoglu et al., 2007 (42) | ↓ | ↓ | → | ↓ | |||||
| Tuncer et al., 2009 (43) | → | → | |||||||
| Low magnesium | |||||||||
| Kim et al., 2012 (44) | ↓ | ↓ |
TABLE 4.
Human studies examining the effects of micronutrient administration on HRV parameters1
| Study, year (reference) | Population | Treatments | Control | Design | Treatment duration | HRV measurement | HRV indicators | Statistically significant findings |
|---|---|---|---|---|---|---|---|---|
| Vitamin B-12 | ||||||||
| Aytemir et al., 2000 (32) | 17 adults with vitamin B-12 deficiency (mean age: 58 y); 15 age- and sex-matched healthy volunteers | Vitamin B-12 treatment (dosage and type not specified) | None | Open label | 3 mo | 5-min ECG recordings at rest | LF, HF, LF:HF | All HRV parameters increased significantly Baseline levels in B-12–deficient group were lower than controls but were equivalent after treatment |
| Sucharita et al., 2012 (33) | 29 healthy elderly adults with vitamin B-12 deficiency (mean age: 67 y); 18 matched volunteers with sufficient vitamin B-12 concentrations | Vitamin B-12 as cyanocobalamin (100 μg) daily | None | Open label | 3 mo | 10-min ECG measurements in supine position after a 30-min rest period | HF, LF, TP, LF:HF | After B-12 treatment LF and TP increased significantly In B-12–treated patients, LF levels were similar to B-12–replete concentrations at end of treatment Nonsignificant increases in HF after B-12 treatment |
| Vitamin D | ||||||||
| Mann et al., 2014 (46) | 13 healthy adults with vitamin D insufficiency (mean age not cited) | Vitamin D3 5000 IU (n = 11) or 10,000 IU (n = 2) daily | None | Open label | 28 d | ECG during exposure to a physiological stressor: a graded angiotensin II (AngII) infusion | HF, LF, LF:HF | At pretreatment, volunteers displayed unfavorable sympatho-vagal balance (LF:HF) in response to AngII Postsupplementation, volunteers maintained sympatho-vagal balance in response to AngII, largely driven by an increase in HF |
| Mann et al., 2016 (47) | 56 patients with end-stage kidney disease on hemodialysis (mean age: 66 y) | Intensive treatment: 0.25 μg alfacalcidol 3×/wk plus 50,000 IU ergocalciferol (vitamin D) once weekly | Conventional treatment: 0.25 μg alfacalcidol plus placebo 3×/wk | Randomized, double-blind, crossover trial | 6 wk | 4- to 24-h ambulatory ECG measure | LF:HF, SDNN, SDANN, pNN50 | No significant changes in HRV parameters after both treatments Conventional and intensive treatment: people who remained vitamin D deficient showed a trend towards increased LF:HF over time Intensive treatment: people who achieved vitamin D sufficiency demonstrated an increase in HF |
| Vitamin C | ||||||||
| Piccirillo et al., 2003 (48) | 33 chronic heart failure patients (mean age: 56 y); 11 healthy matched controls | Vitamin C infusion, 2.5 mg over 5 min | Placebo, saline solution | Randomized, double-blind, parallel-group trial | 5 min | ECG over 50-min period | RMSSD, TP, HF, LF | In chronic heart failure patients, vitamin C decreased LF, and increased HF and RMSSD; no change occurred in controls |
| Gomes et al., 2011 (49) | 11 patients with chronic heart failure (mean age: 56 y) | Vitamin C as ascorbic acid, 2 g/d | Placebo | Randomized, double-blind, parallel-group trial | 3 d | 10-min ECG recording after 30-min rest | TP, HF, LF, LF:HF | No differences between placebo and vitamin C on any HRV measures |
| Bruno et al., 2012 (50) | 32 untreated patients with essential hypertension (mean age: 46 y); 20 normotensive subjects | Vitamin C 3 g delivered intravenously in 5 min | Placebo, saline solution | Randomized, double-blind, parallel-group trial | 5-min infusion | ECG measures: 5-min baseline, during 5-min infusion, and 15 min thereafter | HF, LF, LF:HF | In patients with hypertension (but not normotensive patients) vitamin C infusion associated with significant reduction in LF and LF:HF |
| Vitamin E | ||||||||
| Manzella et al., 2001 (51) | 50 patients with type 2 diabetes (mean age: 65 y) | Vitamin E, 600 mg/d as ɑ-tocopherol acetate | Placebo | Randomized, double-blind, parallel-group trial | 4 mo | 60-min ECG recording | R-R interval, TP, LF, HF, LF:HF | Vitamin E treatment was associated with increases in R-R interval, TP, and HF, and decreases in LF and LF:HF; there were no significant changes with placebo |
| Magnesium | ||||||||
| Bashir et al., 1993 (52) | 21 patients with stable congestive heart failure secondary to coronary artery disease (mean age: 63 y) | Magnesium chloride (3204 mg/d) equivalent to 15.8 mmol of elemental magnesium | Placebo | Randomized, double-blind, crossover trial | 6 wk | 24-h Holter ECG | Mean RR interval, pNN50, RMSSD, LF, HF, LF:HF | No significant changes in the mean RR interval or in any of the temporal or spectral indexes of HRV |
| Frick et al., 1999 (53) | 30 patients with chronic atrial fibrillation with normal serum magnesium (mean age: 68 y) | Magnesium infusion (16 mmol) delivered over 1 h | Low-dose magnesium (10 mmol mg) delivered over 1 h | Randomized, double-blind, crossover trial | 1 h | 2-h Holter ECG | SDANN, maximal RR variability | Magnesium had no effect on HRV parameters |
| Parikka et al., 1999 (54) | 59 patients with acute myocardial infarction (mean age: 60 y) | Magnesium infusion (70 mmol over 24 h) | Placebo | Randomized, double-blind, crossover trial | 24 h | 24-h Holter ECG prior to treatment and 7–14 d after treatment | SDANN, LF:HF | No change in SDANN and LF:HF |
| Almoznino-Sarafian et al., 2009 (55) | 32 patients with systolic heart failure and normal serum magnesium (mean age: 71 y) | Magnesium (300 mg/d) as citrate | No treatment | Randomized, double-blind, parallel-group trial | 5 wk | 24-h Holter ECG | HRV-CD | HRV-CD increased significantly in magnesium-treated group only |
| Brilla et al., 2010 (56) | 36 healthy active individuals (mean age: 30 y) | Magnesium, (500 mg/d) as bisglycinate chelate (MgC) or oxide (MgO) | Placebo | Randomized, double-blind, parallel-group trial | 6 wk | Not specified | LF, HF, LF:HF | LF:HF decreased in both magnesium groups but only significantly in MgO group MgO intake significantly increased LF, HF, and LF:HF Significant postintervention differences in LF, HF, and LF:HF between MgC and placebo Postintervention differences in LF:HF between MgO and placebo |
| Zinc | ||||||||
| Merialdi et al., 1999 (57) | 55 fetuses whose mothers were supplemented with or without zinc at 10–24 wk of gestation | Zinc sulfate 15 mg/d plus 250 μg folic acid and 60 mg iron | 250 μg folic acid and 60 mg iron only (no zinc) | Randomized, double-blind, parallel-group trial | 10–24 wk of gestation until 4 wk postpartum | Fetal actocardiograph for 50 min while mother was at rest | Fetal HRV and fetal heart rate accelerations | From 36 wk of gestation, compared with non–zinc-supplemented fetuses, zinc-supplemented fetuses had fewer episodes of minimal HRV and more heart rate accelerations |
| Merialdi et al., 2004 (58) | 195 fetuses whose mothers were supplemented with or without zinc at 10–16 wk of gestation | Zinc sulfate 25 mg daily plus 250 μg folic acid and 60 mg iron | 250 μg folic acid and 60 mg iron only (no zinc) | Randomized, double-blind, parallel-group trial | 20–38 wk of gestation | Fetal actocardiograph for 50 min while mother was at rest | Fetal HRV and fetal heart rate accelerations | Compared with non–zinc-supplemented fetuses, zinc-supplemented fetuses had greater HRV and more heart rate accelerations which became pronounced from 28 wk |
| Caulfield et al., 2011 (59) | 165 mothers at 10–14 wk of gestation (mean age: 28 y) | Zinc sulfate 25 mg daily plus 250 μg folic acid and 60 mg iron | 250 μg folic acid and 60 mg iron only (no zinc) | Randomized, double-blind, parallel-group trial | 10–14 wk of gestation until 1 mo postpartum | 5 min of ECG at baseline and during cognitive testing | HP, HP range, HPV, SDHP, MSSD | At ∼4.5 y of age, children of zinc-consuming mothers had statistically significant greater HPV and MSSD at baseline and during cognitive tasks |
| CoQ10 | ||||||||
| Zheng and Moritani 2008 (60) | 11 nonsmoking healthy male students (mean age: 26 y) | CoQ10, 30 mg | Placebo | Randomized, double-blind, crossover trial | Single administration | ECG recordings at rest and during 10-min exercise on a stationary bike | TP, LF, HF | There were no significant differences in HRV parameters at rest for CoQ10 or placebo During exercise, CoQ10 associated with a significant increase in TP and a strong increased trend for LF and HF; no changes occurred on placebo |
| Multivitamins | ||||||||
| Fukuda et al., 2015 (61) | 202 hemodialysis patients (mean age: 56 y) | Multivitamin and mineral drink taken 3 d/wk after each dialysis treatment | Placebo | Randomized, double-blind, parallel-group trial | 12 wk | Acceleration plethysmography in a sitting position | HF, LF, LF:HF | LF:HF decreased in placebo condition from weeks 4 to 12 but increased in the treatment condition |
TABLE 5.
Changes in HRV parameters after micronutrient supplementation1
| Study, year (reference) | SDANN | RMSSD | TP | LF | HF | LF:HF |
|---|---|---|---|---|---|---|
| Vitamin B-12 | ||||||
| Aytemir et al., 2000 (32) | ↑ | ↑ | ↑ | |||
| Sucharita et al., 2012 (33) | ↑ | ↑ | → | → | ||
| Vitamin D | ||||||
| Mann et al., 2014 (46) | → | ↑ | → | |||
| Mann et al., 2016 (47) | ↑2 | |||||
| Vitamin C | ||||||
| Piccirillo et al., 2003 (48) | → | → | ↓ | ↑ | ||
| Gomes et al., 2011 (49) | → | → | → | → | ||
| Bruno et al., 2012 (50) | ↓ | → | ↓ | |||
| Vitamin E | ||||||
| Manzella et al., 2001 (51) | ↑ | ↓ | ↑ | ↓ | ||
| Magnesium | ||||||
| Bashir et al., 1993 (52) | → | → | → | → | ||
| Frick et al., 1999 (53) | → | |||||
| Parikka et al., 1999 (54) | → | → | ||||
| Brilla et al., 2010 (56) | ↑ | ↑ | ↓ | |||
| CoQ10 | ||||||
| Zheng and Moritani, 2008 (60) | ↑3 | ↑3 | ||||
| Multivitamins | ||||||
| Fukuda et al., 2015 (61) | ↑ |
Vitamin B-12 and HRV
Five cross-sectional and 2 interventional studies examined the relation between vitamin B-12 and HRV. In 3 cross-sectional studies, HRV values in adults with vitamin B-12 deficiency were compared with vitamin B-12–sufficient, age- and gender-matched controls. In all of these studies vitamin B-12 deficiency was associated with several differences in HRV parameters. In a study by Sozen et al. (31), levels of SD of all the RR intervals (SDNN), SD of the 5-min RR interval means (SDANN), root mean square of differences of successive RR interval (RMSSD), total power (TP), low-frequency power (LF), and high-frequency power (HF) were lower in patients with pernicious anemia compared with controls. Aytemir et al. (32) found that levels of LF, HF, and the ratio of LF to HF (LF:HF) were also lower in vitamin B-12–deficient patients compared with controls, and all HRV parameters were positively correlated with vitamin B-12 concentrations and negatively with the duration of deficiency. LF levels were also lower in vitamin B-12–deficient adults compared with controls; however, there were no differences in HF, TP, and LF:HF (33). In contrast to the previous 2 studies, the mean age of participants in this study was ∼10–15 y older. In a study in healthy young adults, vitamin B-12 concentrations were not associated with HRV values; however, methylmalonic acid (MMA) concentration (a marker of vitamin B-12 deficiency) was negatively associated with LF and TP but was positively correlated with HF (34). Finally, vitamin B-12 concentrations were measured in pregnant women during their first trimester and HRV was measured in their child at ∼5 y of age. LF was significantly lower in children born to mothers with lower vitamin B-12 concentrations compared with mothers with high vitamin B-12. There were also strong trends of lower HF and TP in children of mothers with low vitamin B-12. In all children, LF, TP, and HF positively correlated with cord blood vitamin B-12 concentrations measured at birth (35).
The effects of 3-mo administration of vitamin B-12 supplements on HRV in adults with vitamin B-12 deficiency have been investigated in 2 open-label studies. In 1 study, vitamin B-12 (dosage and type not specified) was associated with increases in levels of LF, HF, and LF:HF. Levels of the HRV parameters were lower at baseline compared with vitamin B-12–sufficient controls but were comparable at the end of treatment (32). In a study in older people (≥60 y; mean age: 67 y) with vitamin B-12 deficiency, vitamin B-12 supplementation (100 μg as cyanocobalamin) was associated with significant increases in LF and TP, and a strong trend that was suggestive of increases in HF (33).
As detailed in Tables 3 and 5, the bulk of evidence suggests that a deficiency in vitamin B-12 is associated with reductions in most HRV parameters and its administration leads to increased HRV values. In the only negative adult study, vitamin B-12 was not correlated with HRV in healthy adults but was associated with concentrations of MMA (22). In the only study in pregnant mothers, vitamin B-12 status during the first trimester was positively correlated with HRV values of their offspring at age 5 (35). Both the interventional studies conducted to date have demonstrated that vitamin B-12 supplementation for 3 mo can increase HRV parameters (32, 33). However, these were open-label studies so further controlled trials are required to validate these findings. Dosage, duration, and effects of supplementation in different populations also require further investigation.
Vitamin D and HRV
Six cross-sectional (36–41) and 2 interventional studies (46, 47) have examined the relation between vitamin D and HRV. In 3 cross-sectional studies, the relation between vitamin D and HRV was examined in healthy adults: 2 studies were conducted in adults with type 1 or type 2 diabetes and 1 study investigated the relation in hospitalized patients with chronic heart failure. In 1 study in healthy adults, vitamin D was positively correlated with SDNN and LF, and SDNN was significantly lower in vitamin D–deficient adults {25-hydroxyvitamin D [25(OH)D] <15 ng/mL} compared with nondeficient adults [mean 25(OH)D: ≥15 ng/mL] (37). However, there was no relation between vitamin D and RMSSD, TP, VLF, HF, and LF:HF, as measured via a 5-min ECG. Canpolat et al. (38) found that levels of SDNN, SDANN, RMSDD, percentage of the beats with consecutive RR interval difference of >50 ms (pNN50), and HF were lower in vitamin D–deficient adults [25(OH)D <20 ng/mL] compared with vitamin D–sufficient adults [25(OH)D ≥30 ng/mL]. LF and LF:HF were also higher in the vitamin D–deficient group. In this study, HRV measurements comprised a 24-h ECG. In another study, no differences in HRV parameters (SDNN, SDANN, RMSSD, and pNN50) were identified between vitamin D–deficient [mean 25(OH)D: ≤6.1 ng/mL] and –sufficient adults [mean 25(OH)D: ≥21 ng/mL], as measured via a 1-h ECG (39). In 2 studies in adults with diabetes, vitamin D deficiency was associated with several changes in HRV parameters. In 1 study, SDNN and RMSSD levels were lower and LF:HF was higher in the vitamin D–deficient group [25(OH)D <10 ng/mL] compared with the vitamin D–sufficient group [25(OH)D ≥20 ng/mL]. However, there was no significant difference in VLF, LF, and HF (40). In a study on type 1 and 2 diabetes, Hansen et al. (41) identified an inverted U-shaped association between serum vitamin D and SDNN, RMSSD, and HF. In contrast to these findings, no differences in SDNN, SDANN, and RMSSD were identified in vitamin D–deficient [25(OH)D ≤20 ng/mL] and –sufficient hospitalized patients with chronic heart failure. However, there was a significant positive correlation between vitamin D and SDNN and SDANN in patients with nonischemic dilated cardiomyopathy (NIDCM) but not ischemic dilated cardiomyopathy (IDCM) (36).
Two interventional studies were identified examining the effect of vitamin D supplementation on HRV (46, 47). In a study in healthy adults with vitamin D insufficiency, vitamin D3 supplementation for 28 d, at a dose of 5000–10,000 IUs improved cardiac autonomic tone, as measured by an increase in HF, during exposure to an acute physiological stressor (46). In another study, once-weekly intravenous administration of vitamin D (50,000 IU ergocalciferol) for 6 wk in patients with end-stage kidney disease on hemodialysis had no effect on HRV parameters. However, most patients continued to be vitamin D deficient at the end of the treatment and, in patients who achieved vitamin D sufficiency, HF levels increased after treatment. There was no effect on LF:HF, SDNN, SDANN, or pNN50 following vitamin D supplementation (47). It should be noted that supplementation with the nonactivated form of vitamin D, ergocalciferol, is often not recommended in people with kidney disease as activation of vitamin D occurs in the kidneys (62). Consequently, greater efficacy may have been obtained by supplementation with activated forms of vitamin D (e.g., calcitriol and paricalcitol).
In summary, in the 6 cross-sectional studies on vitamin D, a significant relation between ≥1 HRV parameter was identified in 4 studies. Two of these were conducted in healthy adults with vitamin D deficiency (37, 38) and 2 were conducted in adults with type 1 or 2 diabetes (40, 41). However, a null relation between HRV and vitamin D was identified in a study in healthy adults with vitamin D deficiency (39); and in another study in patients with chronic heart failure, there was a significant positive correlation between vitamin D and HRV in patients with NIDCM but not IDCM (36). Discrepancies between the studies in the criteria for vitamin D–deficient and –sufficient states may account for some of the inconsistencies in these findings. In the 2 interventional studies, oral vitamin D supplementation increased HF in healthy adults with vitamin D deficiency (46), but intravenous vitamin D had no effect on patients with kidney disease (47). Overall, the bulk of evidence suggests that there is a positive relation between vitamin D and HRV parameters; however, further research is required. There are significant differences across studies in populations recruited, vitamin D criteria used, and HRV measurements conducted. There also remains a paucity of studies on the effect of supplementation on HRV parameters.
Vitamin C and HRV
There have been 3 interventional studies examining the effect of vitamin C administration on HRV values in adults with cardiovascular disease. In 2 studies, vitamin C was delivered intravenously and its acute effects on HRV were investigated. In a randomized, double-blind, placebo-controlled study, vitamin C infusion acutely decreased LF and increased HF and RMSSD (48). No changes were observed after placebo (saline) administration. In a study by Bruno et al. (50), intravenous vitamin C also acutely reduced LF and LF:HF but had no significant effect on HF. However, the 3-d oral intake of 2 g of vitamin C in patients with chronic heart failure had no effect on TP, HF, LF, and LF:HF (49).
In summary, 2 studies using intravenous vitamin C have demonstrated acute effects on HRV parameters; however, no changes were observed after a 3-d oral administration. All studies were conducted in patients with cardiovascular disease, so the effects of vitamin C in other populations are unknown. Further research is required to examine the effects of intravenous and oral administrations of vitamin C at varying doses, over extended time periods, in differing populations and on differing HRV parameters.
Vitamin E and HRV
In a randomized, double-blind, placebo-controlled study, patients with type 2 diabetes were given either vitamin E (600 mg/d as α-tocopherol acetate) or placebo for 4 mo. Vitamin E treatment was associated with increases in R-R interval, TP, and HF and decreases in LF and LF:HF. However, there were no changes in the placebo-administered group (51). Although this single study is positive, further research is required to examine the effects of vitamin E supplementation on HRV.
Magnesium and HRV
One cross-sectional and 5 interventional studies were identified examining the relation between magnesium and HRV. In the cross-sectional study, serum magnesium concentrations in 166 healthy women with a mean age of 46 y were positively associated with 5-min HRV measurements of SDDN and TP, but not RMSSD, LF, HF, and LF:HF. SDNN and LF were also lower in women with a higher calcium to magnesium ratio (44). In the 5 interventional studies, 4 were conducted in adults with cardiovascular disease with a mean age of ≥59 y. In these studies, varying doses and forms of magnesium were administered either orally or intravenously, with treatments ranging from 1 h to 6 wk. In 2 studies, the acute, intravenous administration of magnesium had no effect on HRV parameters (53, 54) in adults with cardiovascular disease. Oral magnesium citrate at a dose of 300 mg of elemental magnesium for 5 wk administered to patients with systolic heart failure and normal pretreatment serum magnesium concentrations increased HRV correlation dimension (HRV-CD) (55). However, there was no change in several HRV parameters in adults with congestive heart failure after a 6-wk oral intake of magnesium chloride, delivering 15.8 mmol of elemental magnesium (52). In a randomized, double-blind, placebo-controlled study in 36 healthy adults with a mean age of 30 y, 500 mg of magnesium delivered as either bis-glycinate chelate or oxide for 6 wk was associated with increases in several HRV parameters, including LF, HF, and LF:HF, compared with placebo (56).
In summary, research on the relation between HRV and magnesium is inconsistent. The single, cross-sectional study conducted so far in healthy women has indicated that magnesium concentrations are positively correlated with HRV measures of SDNN and TP (44). As detailed in Table 5, the results from interventional studies are inconclusive. Interventional studies have primarily been conducted in adults with cardiovascular disease using varying doses, routes of administration (intravenous and oral), and treatment duration. No change in HRV parameters was found in 3 studies comprising 2 acute intravenous administrations (53, 54) and 1 oral intake for 6 wk (52). However, in another study in patients with cardiovascular disease, oral magnesium intake for 5 wk was associated with increases in HRV-CD (55). In this study, a nonlinear HRV method was used, which has been shown to more accurately differentiate between healthy and high-risk patients with 96% accuracy (63). This positive finding is supported by another study conducted in healthy adults where oral magnesium intake for 6 wk improved several HRV parameters (56). Given the variability in these findings, further studies are required to elucidate the relation between magnesium and HRV.
Zinc and HRV
Amounts of dietary intake of zinc (as measured by a 24-h dietary recall) were measured in pregnant, adolescent mothers at 34–36 wk of gestation. HRV values in the fetuses were lower in mothers consuming a zinc-deficient, but not folate-deficient, diet (45). Three randomized, double-blind, placebo-controlled studies were identified examining HRV changes in fetuses after mothers were supplemented with zinc. In 1 study, compared with non–zinc-supplemented mothers, fetuses of mothers who were supplemented with zinc (15 mg/d) commencing 10–24 wk of gestation until 4 wk postpartum had fewer episodes of minimal HRV and more heart rate accelerations from 36 wk of gestation (57). In another similarly designed study, compared with non–zinc-supplemented mothers, fetuses of mothers who were supplemented with zinc (25 mg) at 10–16 wk of gestation also had a higher HRV and more heart rate accelerations, which became pronounced from 28 wk of gestation (58). In a final study, compared with non–zinc-supplemented mothers, children (mean age of 4.5 y) of mothers who were supplemented with zinc (25 mg/d) commencing 10–15 wk of gestation until 4 wk postpartum had significantly greater levels of variability in heart period R waves (HPV) and mean square of successive differences (MSSD) measured at baseline and during a cognitive task compared with the non–zinc-supplemented group (59).
In summary, the relation between zinc and HRV has only been investigated in fetuses and/or children born of zinc-supplemented mothers. Even though the results so far have been positive, further research is required to examine the relation between zinc and HRV in adults. Whether zinc deficiency is associated with differences in HRV has not yet been investigated, and the effects of supplementation during childhood or adulthood have also not been studied.
Iron and HRV
There have been 2 studies examining the relation between iron-deficiency anemia and HRV. In 1 study in community-dwelling adults with iron deficiency, levels of SDDN, SDANN, number of R-R intervals >50 ms (SNN50) counts, and pNN50 were lower compared with matched controls (42). However, in a study in hospitalized patients with iron-deficiency anemia, there were no differences in HRV parameters [SDNN, mean square root of differences between adjacent normal-to-normal intervals (MSRSD), mean of the SD in all 5-min intervals (HRVM), SD in all 5-min intervals (SDHRV), and SDANN] compared with iron-sufficient matched controls (43). These inconsistent findings may result from differences in physical activity between the populations examined, as one was conducted in community-dwelling adults (42) while the other was conducted in hospitalized patients (43). Therefore, further research is required to understand the relation between iron and HRV. Interventional studies are also required to determine the effect of iron repletion on HRV parameters in iron-deficient individuals.
CoQ10 and HRV
In a randomized, double-blind, placebo-controlled, crossover study, the single administration of CoQ10 (30 mg) was not associated with changes in HRV parameters at rest. However, during exercise, CoQ10 was associated with a significant increase in TP and a strong trend suggestive of increases in LF and HF (60). Although this study is positive, further investigation into the effects of CoQ10 on HRV is required.
Multivitamins/minerals and HRV
The effects of a 12-wk intake of a multivitamin and mineral drink on HRV in renal-disease patients receiving hemodialysis was investigated in a randomized, double-blind, placebo-controlled trial. LF:HF increased in patients taking the multivitamin/mineral drink but decreased in those receiving the placebo (61). This preliminary study requires replication with studies using different populations and treatment periods. However, by utilizing multinutrient formulas it will be difficult to determine the ideal nutrient dose or most significant nutrient(s) affecting HRV.
Discussion
From a systematic search of the literature, 15 cross-sectional and 18 interventional studies were identified examining the relation between micronutrients and HRV. Micronutrients investigated included vitamin D (8 studies), vitamin B-12 (7 studies), vitamin C (3 studies), vitamin E (1 study), zinc (4 studies), magnesium (6 studies), iron (2 studies), CoQ10 (1 study), and a multivitamin/mineral formula (1 study). However, definitive conclusions about the effects of these micronutrients on HRV cannot be made due to the paucity of research, significant heterogeneity in populations examined, HRV measurements used, treatment dosages administered, treatment duration, routes of supplement administration, and often, the small recruited sample sizes. Overall, the strongest evidence is for vitamins D and B-12 where a deficiency was associated with reduced HRV. There is evidence that zinc administration during pregnancy is associated with increases in HRV in fetuses and children up to the age of 5. The research on magnesium is inconsistent and is likely due to differences in HRV measures used, supplement forms, treatment duration, and populations examined. To clarify the relation between micronutrients and HRV, further robustly designed and adequately powered studies are required.
The potential of micronutrients to influence HRV is based on sound theoretical premises as micronutrients can affect both heart and brain function. For example, vitamin D deficiency is associated with poor cardiovascular outcomes (64) and vitamin D affects cardiac contractility through its effects on vitamin D receptors and indirectly via its impact on calcium metabolism (65). Vitamin D is also often low in people with psychiatric (66) and neurological (67) conditions, and influences brain integrity via its effects on neuronal and glial tissue (68). Vitamin D also has anti-inflammatory effects, which may affect vagal activity (69). Vitamin B-12 deficiency is associated with coronary artery disease (70), diabetes (71), and neuropsychiatric disease (72), conditions also associated with low HRV. Vitamin B-12 can influence mitochondrial activity, neurotransmitter production, and overall brain activity (73). Magnesium deficiency is associated with increased negative cardiovascular events and has diverse effects on cardiovascular activity at both the biochemical and cellular level (74). Generally, when considering most micronutrients there are several plausible generic mechanisms that may be associated with their influence on HRV. For example, most micronutrients have anti-inflammatory or immune-modulating effects. Since HRV is negatively correlated with inflammation (9), reducing inflammation and altering vagal tone, via micronutrient supplementation, may have a positive impact on HRV parameters. Oxidative stress is also negatively correlated with HRV (75). As many micronutrients have antioxidant activity, a reduction in oxidative stress may also contribute to their positive effects on HRV. There is increasing research to suggest that intestinal microbiota influences the activity of the vagus nerve (76), and micronutrients such as magnesium (77), vitamin D (78), and iron (79) can alter the composition of the intestinal microbiota. Therefore, via their effects on the intestinal microbiome, micronutrients may alter vagal activity and HRV. In addition, micronutrients are important for the production of hormones, neurotransmitters, and neurotrophins (80), which can all alter neurological activity. Through their effect on brain activity, HRV may again be altered by micronutrients.
It is important to note that age, fitness level, medical diseases, and psychiatric conditions are associated with changes in HRV. Micronutrient concentrations present as potential confounding factors as many of these aforementioned states are associated with variations in micronutrient concentrations. For example, vitamin D and B-12 deficiency are common in the elderly (81, 82) and vitamin D concentrations are often lower in adults with depression (66). If micronutrients are found to influence HRV, studies controlling for micronutrient concentrations are therefore essential to elucidate the true impact of investigated factors on HRV. Therefore, to better understand the relation between micronutrients and HRV it is important that in future studies the following variables are considered:
- The effects of different dosages, routes of administration (e.g., oral and intravenous), and micronutrient forms (e.g., oxide versus glycinate). This will help to determine optimal dosages and forms of micronutrient administration and whether excess intake may have a deleterious effect on HRV.
- Control for comorbid medications that may affect HRV, as this may confound the findings.
- Control for respiratory patterns, which can influence HRV.
- The effect of acute and long-term administration of micronutrients on HRV.
- The types and time periods of HRV measurements. This is important for all HRV-related studies as inconsistencies in measured HRV parameters, periods of measurement, and measurement settings make interpretation difficult. Establishing greater consistency across studies will help clarify the relevance and robustness of findings.
- Influence of micronutrient concentrations and supplementation on HRV in diseased versus healthy populations. The studies identified in this review comprised the recruitment of a heterogeneous population of individuals, including healthy people, individuals with micronutrient deficiencies, and adults with varying diseases including cardiovascular disease, diabetes, and kidney disease. Differences in HRV parameters and the efficacy of micronutrient supplementation may vary significantly across these different populations. Therefore, the relation between micronutrients and HRV in these different populations requires further investigation as this could not be elucidated using the current body of literature.
- Influence of micronutrient status and supplementation in different patient characteristics such as gender and age.
- Influence of micronutrient supplementation on HRV in nutrient-deficient and -replete participants.
- The influence of baseline dietary patterns on HRV. Moreover, the effects of micronutrient repletion via dietary changes compared with single- or multinutrient supplementation on HRV will be important to investigate.
- Physiological mechanisms associated with micronutrient effects on HRV.
- Effects of single and combination micronutrient supplementation on HRV.
- Association of HRV changes with symptomatic, physiological, and health-related parameters.
- The relevance of HRV as an objective outcome measure in nutritional trials. Identifying validated and objective outcome measures is important to help elucidate the efficacy of an intervention. HRV has the potential to be used as an outcome measure in dietary and nutritional trials to support commonly used, symptom-based, or biological measures of efficacy. This also has relevance for other drug, lifestyle, and environmental interventions. However, as already discussed, developing consistent HRV measurement protocols will be essential.
Although the goal of this review was to summarize the research on micronutrients, herbal and dietary interventions also present as potential avenues to modify HRV. For example, there is preliminary evidence that a high-fat diet (83, 84), caloric restriction (85), fasting (86), and meal timing (87) can alter HRV. In animal studies, curcumin (88), saffron (89), and a garlic extract (90) increased vagal activity. Moreover, probiotics have been shown to increase vagal activity in animal studies (91–93). It is likely that anti-inflammatory and antioxidant mechanisms may be associated with the effect of nutrients, foods, and herbs on HRV. Their impact on intestinal microbiota that can alter vagal activity also presents as another mechanism of action (94, 95).
In conclusion, definitive conclusions about the effect of micronutrients on HRV cannot be made due to the paucity of available studies and heterogeneity of investigations. Given the increasing body of research confirming a relation between HRV and health, further research is essential to help elucidate the impact of micronutrients on HRV and the potential of supplementation or dietary repletion to manipulate HRV and overall health status. Given the increasing association between HRV and health, HRV also presents as an objective outcome measure to validate nutritional, herbal, and dietary interventions.
ACKNOWLEDGEMENTS
I thank Stephen J Smith for his assistance in editing this article. ALL: wrote the entire manuscript and read and approved the final manuscript.
Notes
The author reported no funding received for this study.
Author disclosures: The author reports no conflicts of interest.
Abbreviations used: CoQ10, coenzyme Q10; ECG, electrocardiogram; HF, high-frequency power; HRV, heart rate variability; HRV-CD, HRV correlation dimension; IDCM, ischemic dilated cardiomyopathy; LF, low-frequency power; MMA, methylmalonic acid; NIDCM, nonischemic dilated cardiomyopathy; NN, normal beat to normal beat; pNN50, percentage of the beats with consecutive RR interval difference of >50 ms; PNS, parasympathetic nervous system; RMSSD, root mean square of differences of successive RR interval; RR, R wave to R wave; SDANN, SD of the 5-min RR interval means; SDNN, SD of all the RR intervals; SNS, sympathetic nervous system; TP, total power; VLF, very-low-frequency power; 25(OH)D, 25-hydroxyvitamin D.
References
- 1.Waxenbaum JA, Varacallo M. Anatomy, autonomic nervous system. Treasure Island (FL): StatPearls; 2019. [PubMed] [Google Scholar]
- 2.Shaffer F, McCraty R, Zerr CL. A healthy heart is not a metronome: an integrative review of the heart's anatomy and heart rate variability. Front Psychol. 2014;5:1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kemp AH, Koenig J, Thayer JF. From psychological moments to mortality: a multidisciplinary synthesis on heart rate variability spanning the continuum of time. Neurosci Biobehav Rev. 2017;83:547–67. [DOI] [PubMed] [Google Scholar]
- 4.Thayer JF, Ahs F, Fredrikson M, Sollers JJ 3rd, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev. 2012;36(2):747–56. [DOI] [PubMed] [Google Scholar]
- 5.Jandackova VK, Scholes S, Britton A, Steptoe A. Are changes in heart rate variability in middle-aged and older people normative or caused by pathological conditions? Findings from a large population-based longitudinal cohort study. J Am Heart Assoc. 2016;5(2):e002365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong JG. The role of heart rate variability in sports physiology. Exp Ther Med. 2016;11(5):1531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jandackova VK, Scholes S, Britton A, Steptoe A. Healthy lifestyle and cardiac vagal modulation over 10 years: Whitehall II Cohort Study. J Am Heart Assoc. 2019;8(19):e012420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jarczok MN, Koenig J, Mauss D, Fischer JE, Thayer JF. Lower heart rate variability predicts increased level of C-reactive protein 4 years later in healthy, nonsmoking adults. J Intern Med. 2014;276(6):667–71. [DOI] [PubMed] [Google Scholar]
- 9.Williams DP, Koenig J, Carnevali L, Sgoifo A, Jarczok MN, Sternberg EM, Thayer JF. Heart rate variability and inflammation: a meta-analysis of human studies. Brain Behav Immun. 2019;80:219–26. [DOI] [PubMed] [Google Scholar]
- 10.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117(2):289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He L, Li C, Luo Y, Dong W, Yang H. Clinical prognostic significance of heart abnormality and heart rate variability in patients with stroke. Neurol Res. 2010;32(5):530–4. [DOI] [PubMed] [Google Scholar]
- 12.Sessa F, Anna V, Messina G, Cibelli G, Monda V, Marsala G, Ruberto M, Biondi A, Cascio O, Bertozzi G et al.. Heart rate variability as predictive factor for sudden cardiac death. Aging. 2018;10(2):166–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HG, Cheon EJ, Bai DS, Lee YH, Koo BH. Stress and heart rate variability: a meta-analysis and review of the literature. Psychiatry Investig. 2018;15(3):235–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koch C, Wilhelm M, Salzmann S, Rief W, Euteneuer F. A meta-analysis of heart rate variability in major depression. Psychol Med. 2019;49(12):1948–57. [DOI] [PubMed] [Google Scholar]
- 15.Paniccia M, Paniccia D, Thomas S, Taha T, Reed N. Clinical and non-clinical depression and anxiety in young people: a scoping review on heart rate variability. Auton Neurosci. 2017;208:1–14. [DOI] [PubMed] [Google Scholar]
- 16.Campbell AA, Wisco BE, Silvia PJ, Gay NG. Resting respiratory sinus arrhythmia and posttraumatic stress disorder: a meta-analysis. Biol Psychol. 2019;144:125–35. [DOI] [PubMed] [Google Scholar]
- 17.Kloter E, Barrueto K, Klein SD, Scholkmann F, Wolf U. Heart rate variability as a prognostic factor for cancer survival—a systematic review. Front Physiol. 2018;9:623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villafaina S, Collado-Mateo D, Fuentes JP, Merellano-Navarro E, Gusi N. Physical exercise improves heart rate variability in patients with type 2 diabetes: a systematic review. Curr Diab Rep. 2017;17(11):110. [DOI] [PubMed] [Google Scholar]
- 19.Pal GK, Ganesh V, Karthik S, Nanda N, Pal P. The effects of short-term relaxation therapy on indices of heart rate variability and blood pressure in young adults. Am J Health Promot. 2014;29(1):23–8. [DOI] [PubMed] [Google Scholar]
- 20.Blase KL, van Waning A. Heart rate variability, cortisol and attention focus during Shamatha quiescence meditation. Appl Psychophysiol Biofeedback. 2019;44(4):331–42. [DOI] [PubMed] [Google Scholar]
- 21.Goessl VC, Curtiss JE, Hofmann SG. The effect of heart rate variability biofeedback training on stress and anxiety: a meta-analysis. Psychol Med. 2017;47(15):2578–86. [DOI] [PubMed] [Google Scholar]
- 22.Young HA, Benton D.. Heart-rate variability: a biomarker to study the influence of nutrition on physiological and psychological health?. Behav Pharmacol. 2018;29(2 and 3; Special Issue):140–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christensen JH. Omega-3 polyunsaturated fatty acids and heart rate variability. Front Physiol. 2011;2:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mouridsen MR, Bendsen NT, Astrup A, Haugaard SB, Binici Z, Sajadieh A. Modest weight loss in moderately overweight postmenopausal women improves heart rate variability. Eur J Prev Cardiol. 2013;20(4):671–7. [DOI] [PubMed] [Google Scholar]
- 25.Cheng YC, Huang YC, Huang WL. Heart rate variability as a potential biomarker for alcohol use disorders: a systematic review and meta-analysis. Drug Alcohol Depend. 2019;204:107502. [DOI] [PubMed] [Google Scholar]
- 26.Sumartiningsih S, Lin HF, Lin JC. Cigarette smoking blunts exercise-induced heart rate response among young adult male smokers. Int J Environ Res Public Health. 2019;16(6):1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. 2017;5:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Godoy MF. Nonlinear analysis of heart rate variability: a comprehensive review. J Cardiol Ther. 2016;3(3):528–33. [Google Scholar]
- 29.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93(5):1043–65. [PubMed] [Google Scholar]
- 30.Allen JJ, Chambers AS, Towers DN. The many metrics of cardiac chronotropy: a pragmatic primer and a brief comparison of metrics. Biol Psychol. 2007;74(2):243–62. [DOI] [PubMed] [Google Scholar]
- 31.Sozen AB, Demirel S, Akkaya V, Kudat H, Tukek T, Yeneral M, Ozcan M, Guven O, Korkut F. Autonomic dysfunction in vitamin B12 deficiency: a heart rate variability study. J Auton Nerv Syst. 1998;71(1):25–7. [DOI] [PubMed] [Google Scholar]
- 32.Aytemir K, Aksoyek S, Buyukasik Y, Haznedaroglu I, Atalar E, Ozer N, Ovunc K, Ozmen F, Oto A. Assessment of autonomic nervous system functions in patients with vitamin B12 deficiency by power spectral analysis of heart rate variability. Pacing Clin Electrophysiol. 2000;23(6):975–8. [DOI] [PubMed] [Google Scholar]
- 33.Sucharita S, Thomas T, Antony B, Vaz M. Vitamin B12 supplementation improves heart rate variability in healthy elderly Indian subjects. Auton Neurosci. 2012;168(1–2):66–71. [DOI] [PubMed] [Google Scholar]
- 34.Sucharita S, Sowmya S, Thomas T, Kurpad AV, Vaz M. Plasma vitamin B12, methylmalonic acid and heart rate variability in healthy young Indian adults. Int J Vitam Nutr Res. 2013;83(3):147–53. [DOI] [PubMed] [Google Scholar]
- 35.Sucharita S, Dwarkanath P, Thomas T, Srinivasan K, Kurpad AV, Vaz M. Low maternal vitamin B12 status during pregnancy is associated with reduced heart rate variability indices in young children. Matern Child Nutr. 2014;10(2):226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cetin M, Kozdag G, Ural D, Kahraman G, Yilmaz I, Akay Y, Onuk R, Dursun N. Could decreased vitamin D levels be related with impaired cardiac autonomic functions in patients with chronic heart failure: an observational study. Anadolu Kardiyol Derg. 2014;14(5):434–41. [DOI] [PubMed] [Google Scholar]
- 37.Tak YJ, Lee JG, Kim YJ, Lee SY, Cho BM. 25-Hydroxyvitamin D and its relationship with autonomic dysfunction using time- and frequency-domain parameters of heart rate variability in Korean populations: a cross-sectional study. Nutrients. 2014;6(10):4373–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Canpolat U, Ozcan F, Ozeke O, Turak O, Yayla C, Acikgoz SK, Cay S, Topaloglu S, Aras D, Aydogdu S. Impaired cardiac autonomic functions in apparently healthy subjects with vitamin D deficiency. Ann Noninvasive Electrocardiol. 2015;20(4):378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nalbant A, Vatan MB, Varim P, Varim C, Kaya T, Tamer A. Does vitamin D deficiency effect heart rate variability in low cardiovascular risk population?. Open Access Maced J Med Sci. 2017;5(2):197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jung CH, Jung SH, Kim KJ, Kim BY, Kim CH, Kang SK, Mok JO. The relationship between vitamin D status and cardiac autonomic neuropathy in patients with type 2 diabetes mellitus. Diab Vasc Dis Res. 2015;12(5):342–51. [DOI] [PubMed] [Google Scholar]
- 41.Hansen CS, Fleischer J, Vistisen D, Ridderstrale M, Jensen JS, Jorgensen ME. High and low vitamin D level is associated with cardiovascular autonomic neuropathy in people with type 1 and type 2 diabetes. Diabet Med. 2017;34(3):364–71. [DOI] [PubMed] [Google Scholar]
- 42.Yokusoglu M, Nevruz O, Baysan O, Uzun M, Demirkol S, Avcu F, Koz C, Cetin T, Hasimi A, Ural AU et al.. The altered autonomic nervous system activity in iron deficiency anemia. Tohoku J Exp Med. 2007;212(4):397–402. [DOI] [PubMed] [Google Scholar]
- 43.Tuncer M, Gunes Y, Guntekin U, Gumrukcuoglu HA, Eryonucu B, Guler N, Dilek I, Demir C. Heart rate variability in patients with iron deficiency anemia. Arq Bras Cardiol. 2009;92(5):368–71. [DOI] [PubMed] [Google Scholar]
- 44.Kim YH, Jung KI, Song CH. Effects of serum calcium and magnesium on heart rate variability in adult women. Biol Trace Elem Res. 2012;150(1–3):116–22. [DOI] [PubMed] [Google Scholar]
- 45.Spann MN, Smerling J, Gustafsson H, Foss S, Altemus M, Monk C. Deficient maternal zinc intake—but not folate—is associated with lower fetal heart rate variability. Early Hum Dev. 2015;91(3):169–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mann MC, Exner DV, Hemmelgarn BR, Turin TC, Sola DY, Ellis L, Ahmed SB. Vitamin D supplementation is associated with improved modulation of cardiac autonomic tone in healthy humans. Int J Cardiol. 2014;172(2):506–8. [DOI] [PubMed] [Google Scholar]
- 47.Mann MC, Exner DV, Hemmelgarn BR, Hanley DA, Turin TC, MacRae JM, Wheeler DC, Sola DY, Ramesh S, Ahmed SB. The VITAH trial—vitamin D supplementation and cardiac autonomic tone in patients with end-stage kidney disease on hemodialysis: a blinded, randomized controlled trial. Nutrients. 2016;8(10):E608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Piccirillo G, Nocco M, Moise A, Lionetti M, Naso C, di Carlo S, Marigliano V. Influence of vitamin C on baroreflex sensitivity in chronic heart failure. Hypertension. 2003;41(6):1240–5. [DOI] [PubMed] [Google Scholar]
- 49.Gomes ME, El Messaoudi S, Lenders JW, Bellersen L, Verheugt FW, Smits P, Tack CJ. High dose ascorbic acid does not reverse central sympathetic overactivity in chronic heart failure. J Clin Pharm Ther. 2011;36(5):546–52. [DOI] [PubMed] [Google Scholar]
- 50.Bruno RM, Daghini E, Ghiadoni L, Sudano I, Rugani I, Varanini M, Passino C, Emdin M, Taddei S. Effect of acute administration of vitamin C on muscle sympathetic activity, cardiac sympathovagal balance, and baroreflex sensitivity in hypertensive patients. Am J Clin Nutr. 2012;96(2):302–8. [DOI] [PubMed] [Google Scholar]
- 51.Manzella D, Barbieri M, Ragno E, Paolisso G. Chronic administration of pharmacologic doses of vitamin E improves the cardiac autonomic nervous system in patients with type 2 diabetes. Am J Clin Nutr. 2001;73(6):1052–7. [DOI] [PubMed] [Google Scholar]
- 52.Bashir Y, Sneddon JF, Staunton HA, Haywood GA, Simpson IA, McKenna WJ, Camm AJ. Effects of long-term oral magnesium chloride replacement in congestive heart failure secondary to coronary artery disease. Am J Cardiol. 1993;72(15):1156–62. [DOI] [PubMed] [Google Scholar]
- 53.Frick M, Ostergren J, Rosenqvist M. Effect of intravenous magnesium on heart rate and heart rate variability in patients with chronic atrial fibrillation. Am J Cardiol. 1999;84(1):104–8., A9. [DOI] [PubMed] [Google Scholar]
- 54.Parikka H, Toivonen L, Naukkarinen V, Tierala I, Pohjola-Sintonen S, Heikkila J, Nieminen MS. Decreases by magnesium of QT dispersion and ventricular arrhythmias in patients with acute myocardial infarction. Eur Heart J. 1999;20(2):111–20. [DOI] [PubMed] [Google Scholar]
- 55.Almoznino-Sarafian D, Sarafian G, Berman S, Shteinshnaider M, Tzur I, Cohen N, Gorelik O. Magnesium administration may improve heart rate variability in patients with heart failure. Nutr Metab Cardiovasc Dis. 2009;19(9):641–5. [DOI] [PubMed] [Google Scholar]
- 56.Brilla L, Teichler L, Hahn T, Freeman J, Li Y. Effect of magnesium on heart rate variability in healthy subjects. FASEB J. 2010;24(Suppl 1):1.20047894 [Google Scholar]
- 57.Merialdi M, Caulfield LE, Zavaleta N, Figueroa A, DiPietro JA. Adding zinc to prenatal iron and folate tablets improves fetal neurobehavioral development. Am J Obstet Gynecol. 1999;180(2 Pt 1):483–90. [DOI] [PubMed] [Google Scholar]
- 58.Merialdi M, Caulfield LE, Zavaleta N, Figueroa A, Dominici F, Dipietro JA. Randomized controlled trial of prenatal zinc supplementation and the development of fetal heart rate. Am J Obstet Gynecol. 2004;190(4):1106–12. [DOI] [PubMed] [Google Scholar]
- 59.Caulfield LE, Zavaleta N, Chen P, Lazarte F, Albornoz C, Putnick DL, Bornstein MH, DiPietro JA. Maternal zinc supplementation during pregnancy affects autonomic function of Peruvian children assessed at 54 months of age. J Nutr. 2011;141(2):327–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zheng A, Moritani T. Influence of CoQ10 on autonomic nervous activity and energy metabolism during exercise in healthy subjects. J Nutr Sci Vitaminol (Tokyo). 2008;54(4):286–90. [DOI] [PubMed] [Google Scholar]
- 61.Fukuda S, Koyama H, Kondo K, Fujii H, Hirayama Y, Tabata T, Okamura M, Yamakawa T, Okada S, Hirata S et al.. Effects of nutritional supplementation on fatigue, and autonomic and immune dysfunction in patients with end-stage renal disease: a randomized, double-blind, placebo-controlled, multicenter trial. PLoS One. 2015;10(3):e0119578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Melamed ML, Thadhani RI. Vitamin D therapy in chronic kidney disease and end stage renal disease. Clin J Am Soc Nephrol. 2012;7(2):358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Voss A, Kurths J, Kleiner HJ, Witt A, Wessel N, Saparin P, Osterziel KJ, Schurath R, Dietz R. The application of methods of non-linear dynamics for the improved and predictive recognition of patients threatened by sudden cardiac death. Cardiovasc Res. 1996;31(3):419–33. [PubMed] [Google Scholar]
- 64.Deo R, Katz R, Shlipak MG, Sotoodehnia N, Psaty BM, Sarnak MJ, Fried LF, Chonchol M, de Boer IH, Enquobahrie D et al.. Vitamin D, parathyroid hormone, and sudden cardiac death: results from the Cardiovascular Health Study. Hypertension. 2011;58(6):1021–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tishkoff DX, Nibbelink KA, Holmberg KH, Dandu L, Simpson RU. Functional vitamin D receptor (VDR) in the t-tubules of cardiac myocytes: VDR knockout cardiomyocyte contractility. Endocrinology. 2008;149(2):558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anglin RE, Samaan Z, Walter SD, McDonald SD. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013;202:100–7. [DOI] [PubMed] [Google Scholar]
- 67.Di Somma C, Scarano E, Barrea L, Zhukouskaya VV, Savastano S, Mele C, Scacchi M, Aimaretti G, Colao A, Marzullo P. Vitamin D and neurological diseases: an endocrine view. Int J Mol Sci. 2017;18(11):E2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anjum I, Jaffery SS, Fayyaz M, Samoo Z, Anjum S. The role of vitamin D in brain health: a mini literature review. Cureus. 2018;10(7):e2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de Oliveira C, Biddulph JP, Hirani V, Schneider IJC. Vitamin D and inflammatory markers: cross-sectional analyses using data from the English Longitudinal Study of Ageing (ELSA). J Nutr Sci. 2017;6:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kumar J, Garg G, Sundaramoorthy E, Prasad PV, Karthikeyan G, Ramakrishnan L, Ghosh S, Sengupta S. Vitamin B12 deficiency is associated with coronary artery disease in an Indian population. Clin Chem Lab Med. 2009;47(3):334–8. [DOI] [PubMed] [Google Scholar]
- 71.Kibirige D, Mwebaze R. Vitamin B12 deficiency among patients with diabetes mellitus: is routine screening and supplementation justified?. J Diabetes Metab Disord. 2013;12(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stanger O, Fowler B, Piertzik K, Huemer M, Haschke-Becher E, Semmler A, Lorenzl S, Linnebank M. Homocysteine, folate and vitamin B12 in neuropsychiatric diseases: review and treatment recommendations. Expert Rev Neurother. 2009;9(9):1393–412. [DOI] [PubMed] [Google Scholar]
- 73.Kennedy DO. B vitamins and the brain: mechanisms, dose and efficacy—a review. Nutrients. 2016;8(2):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.DiNicolantonio JJ, Liu J, O'Keefe JH. Magnesium for the prevention and treatment of cardiovascular disease. Open Heart. 2018;5(2):e000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fadaee SB, Beetham KS, Howden EJ, Stanton T, Isbel NM, Coombes JS. Oxidative stress is associated with decreased heart rate variability in patients with chronic kidney disease. Redox Rep. 2017;22(5):197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bonaz B, Bazin T, Pellissier S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front Neurosci. 2018;12:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Winther G, Pyndt Jorgensen BM, Elfving B, Nielsen DS, Kihl P, Lund S, Sorensen DB, Wegener G. Dietary magnesium deficiency alters gut microbiota and leads to depressive-like behaviour. Acta Neuropsychiatr. 2015;27(3):168–76. [DOI] [PubMed] [Google Scholar]
- 78.Waterhouse M, Hope B, Krause L, Morrison M, Protani MM, Zakrzewski M, Neale RE. Vitamin D and the gut microbiome: a systematic review of in vivo studies. Eur J Nutr. 2019;58(7):2895–910. [DOI] [PubMed] [Google Scholar]
- 79.Parmanand BA, Kellingray L, Le Gall G, Basit AW, Fairweather-Tait S, Narbad A. A decrease in iron availability to human gut microbiome reduces the growth of potentially pathogenic gut bacteria; an in vitro colonic fermentation study. J Nutr Biochem. 2019;67:20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gomez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci. 2008;9(7):568–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boucher BJ. The problems of vitamin D insufficiency in older people. Aging Dis. 2012;3(4):313–29. [PMC free article] [PubMed] [Google Scholar]
- 82.Stover PJ. Vitamin B12 and older adults. Curr Opin Clin Nutr Metab Care. 2010;13(1):24–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Graff SK, Mario FM, Magalhaes JA, Moraes RS, Spritzer PM. Saturated fat intake is related to heart rate variability in women with polycystic ovary syndrome. Ann Nutr Metab. 2017;71(3–4):224–33. [DOI] [PubMed] [Google Scholar]
- 84.Pellizzer AM, Straznicky NE, Lim S, Kamen PW, Krum H. Reduced dietary fat intake increases parasympathetic activity in healthy premenopausal women. Clin Exp Pharmacol Physiol. 1999;26(8):656–60. [DOI] [PubMed] [Google Scholar]
- 85.Nicoll R, Henein MY. Caloric restriction and its effect on blood pressure, heart rate variability and arterial stiffness and dilatation: a review of the evidence. Int J Mol Sci. 2018;19(3):E751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mzoughi K, Zairi I, Jabeur M, Kraiem S. The effects of fasting on heart rate variability in hypertensive patients. Clin Exp Hypertens. 2018;40(8):793–6. [DOI] [PubMed] [Google Scholar]
- 87.Tada Y, Yoshizaki T, Tanaka I, Kanehara R, Kato M, Hatta N, Hida A, Kawano Y. Higher energy intake at dinner decreases parasympathetic activity during nighttime sleep in menstruating women: a randomized controlled trial. Physiol Behav. 2018;194:252–9. [DOI] [PubMed] [Google Scholar]
- 88.Thephinlap C, Phisalaphong C, Lailerd N, Chattipakorn N, Winichagoon P, Vadolas J, Fucharoen S, Porter JB, Srichairatanakool S. Reversal of cardiac iron loading and dysfunction in thalassemic mice by curcuminoids. Med Chem. 2011;7(1):62–9. [DOI] [PubMed] [Google Scholar]
- 89.Joukar S, Dehesh MM. The safety assessment of saffron (Crocus sativus L.) on sympathovagal balance and heart rate variability; a comparison with amiodarone. Auton Autacoid Pharmacol. 2015;35(4):46–50. [DOI] [PubMed] [Google Scholar]
- 90.Supakul L, Pintana H, Apaijai N, Chattipakorn S, Shinlapawittayatorn K, Chattipakorn N. Protective effects of garlic extract on cardiac function, heart rate variability, and cardiac mitochondria in obese insulin-resistant rats. Eur J Nutr. 2014;53(3):919–28. [DOI] [PubMed] [Google Scholar]
- 91.Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, Deng Y, Blennerhassett PA, Fahnestock M, Moine D et al.. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil. 2011;23(12):1132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Perez-Burgos A, Wang B, Mao YK, Mistry B, McVey Neufeld KA, Bienenstock J, Kunze W. Psychoactive bacteria Lactobacillus rhamnosus (JB-1) elicits rapid frequency facilitation in vagal afferents. Am J Physiol Gastrointest Liver Physiol. 2013;304(2):G211–20. [DOI] [PubMed] [Google Scholar]
- 93.Tunapong W, Apaijai N, Yasom S, Tanajak P, Wanchai K, Chunchai T, Kerdphoo S, Eaimworawuthikul S, Thiennimitr P, Pongchaidecha A et al.. Chronic treatment with prebiotics, probiotics and synbiotics attenuated cardiac dysfunction by improving cardiac mitochondrial dysfunction in male obese insulin-resistant rats. Eur J Nutr. 2018;57(6):2091–104. [DOI] [PubMed] [Google Scholar]
- 94.Cryan JF, O'Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV et al.. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877–2013. [DOI] [PubMed] [Google Scholar]
- 95.Fulling C, Dinan TG, Cryan JF. Gut microbe to brain signaling: what happens in vagus. Neuron. 2019;101(6):998–1002. [DOI] [PubMed] [Google Scholar]