Positive modulation of NMDA receptors by AGN-241751 exerts rapid antidepressant-like effects via excitatory neurons (original) (raw)
Abstract
Dysregulation of the glutamatergic system and its receptors in medial prefrontal cortex (mPFC) has been implicated in major depressive disorder. Recent preclinical studies have shown that enhancing NMDA receptor (NMDAR) activity can exert rapid antidepressant-like effects. AGN-241751, an NMDAR positive allosteric modulator (PAM), is currently being tested as an antidepressant in clinical trials, but the mechanism and NMDAR subunit(s) mediating its antidepressant-like effects are unknown. We therefore used molecular, biochemical, and electrophysiological approaches to examine the cell-type-specific role of GluN2B-containing NMDAR in mediating antidepressant-like behavioral effects of AGN-241751. We demonstrate that AGN-241751 exerts antidepressant-like effects and reverses behavioral deficits induced by chronic unpredictable stress in mice. AGN-241751 treatment enhances NMDAR activity of excitatory and parvalbumin-inhibitory neurons in mPFC, activates Akt/mTOR signaling, and increases levels of synaptic proteins crucial for synaptic plasticity in the prefrontal cortex. Furthermore, cell-type-specific knockdown of GluN2B-containing NMDARs in mPFC demonstrates that GluN2B subunits on excitatory, but not inhibitory, neurons are necessary for antidepressant-like effects of AGN-241751. Together, these results demonstrate antidepressant-like actions of the NMDAR PAM AGN-241751 and identify GluN2B on excitatory neurons of mPFC as initial cellular trigger underlying these behavioral effects.
Subject terms: Cellular neuroscience, Prefrontal cortex
Introduction
Major depressive disorder (MDD) is a debilitating psychiatric disorder that affects more than 264 million people of all ages, imposing enormous socio economic burden [1]. Depression is estimated to be the leading cause of disability worldwide by 2030 [2]. Conventional antidepressants have significant limitations including therapeutic lag, limited efficacy, and treatment resistance, and inadequate treatment increases the risk for suicide in depressed patients [3–5], highlighting an unmet need for safer and efficacious rapid antidepressants.
Recent studies have identified deficits in glutamatergic transmission are related to depression pathophysiology. Consistent with these findings, targeting the glutamatergic system using NMDA receptor (NMDAR) or AMPA receptor (AMPAR) modulators has shown antidepressant-like effects in clinical and preclinical studies [6–8]. The discovery of ketamine as a rapid antidepressant has generated tremendous interest in the utility of NMDAR modulators for treating depression. A single dose of NMDAR antagonist ketamine produces rapid antidepressant effects that are sustained for 1–2 weeks [9], indicating a need for repeated administration of ketamine to sustain these effects in treatment resistant depressed patients. However, repeated ketamine use has been associated with risks like abuse potential and psychotomimetic effects [10, 11] limiting its widespread clinical use.
Interestingly, drugs like rapastinel, sarcosine, and D-serine which enhance NMDAR function also exert rapid antidepressant effects without ketamine-like psychotomimetic side effects [12–17]. However, mixed results in clinical trials with NMDAR enhancers highlight the need for development of medications to target this system, and in particular, the development of potent and efficacious NMDAR positive allosteric modulators (PAMs) is a promising approach. AGN-241751 is a rapastinel mimetic developed from the spirocyclic β-lactam chemical platform. AGN-241751 is a more potent NMDAR PAM than rapastinel, can be administered orally [18], and is currently in phase II clinical trials for treatment of MDD.
An important unanswered question is how both activation and blockade of NMDARs can exert antidepressant effects. Recent studies have shown that both the NMDAR antagonist ketamine and NMDAR enhancers activate similar downstream signaling cascades, resulting in enhanced AMPAR-mediated synaptic transmission and synaptic plasticity [8, 19]; however, the cellular basis for activation of convergent downstream signaling by these drugs remains unclear. NMDARs are widely expressed in both excitatory and inhibitory neurons in most brain areas, including regions implicated in depression such as prefrontal cortex (PFC), hippocampus and amygdala, etc. [20]. NMDARs are composed of two GluN1 and two GluN2 subunits (GluN2A-D), with predominant heterotrimeric complexes containing two GluN1 and two GluN2A and/or GluN2B subunits [20]. GluN2B is highly expressed in the PFC and has been shown to be critical for cellular and behavioral effects of ketamine relevant to its antidepressant efficacy [21, 22]. Importantly, GluN2B blockade in inhibitory neurons in medial PFC (mPFC) is essential for disinhibition mediated glutamate release and subsequent activation of glutamatergic neurons [22]. Furthermore, direct activation of glutamatergic neurotransmission in mPFC is necessary and sufficient to induce antidepressant-like effects in rodents, whereas blockade of glutamatergic transmission prevents these effects of both NMDAR antagonists and enhancers [12, 16, 23, 24]. These findings suggest that either direct or indirect activation of glutamatergic neurons in mPFC induces antidepressant-like effects.
Based on these findings, we hypothesized that GluN2B-containing NMDARs on excitatory neurons in mPFC mediate the antidepressant-like effects of AGN-241751. In the current study, we evaluated the dose-dependent effects of AGN-241751 on behaviors relevant to antidepressant efficacy, expression of synaptic proteins implicated in neuronal plasticity, and its ability to reverse the behavioral deficits induced by chronic unpredictable stress (CUS), a model of depressive-like behaviors in mice. Finally, we tested the direct activation hypothesis by determining whether AGN-241751 mediates its effects via GluN2B signaling in excitatory or inhibitory neurons in mPFC using viral-mediated delivery of cell-type-specific small hairpin RNAs (shRNA) to knockdown (KD) GluN2B selectively in each of these neuronal subtypes.
Materials and methods
Animals
Adult (8–12 weeks old) male or female mice were used for these studies. Wild-type C57BL/6 male mice (Jackson Laboratories) were used for electrophysiology (dose–response and pyramidal neurons) and biochemical/behavioral studies (dose–response and CUS experiments). C57BL/6 male and female mice were used to evaluate the acute behavioral effect (1 h) after AGN-241751 treatment. Camk2a-cre (male; obtained from Dr. Günter Schütz, German Cancer Research Center, Heidelberg, Germany), Gad1-cre (male/female; originally obtained from Dr. Liliana Minichiello, Oxford University, UK) mice, and WT littermates bred on a C57BL/6J background (Jackson Laboratories, Bar Harbor ME) were used for viral-mediated KD experiments. Parvalbumin (PV)-tdTomato male mice were used to evaluate NMDA- or AMPA-inward currents from PV-inhibitory neurons in mPFC slice electrophysiology experiments. Animals were housed under a 12 h light–dark cycle with ad libitum access to water and rodent chow. Animals were group housed until surgery and then single housed throughout the postoperative and behavioral testing period. Animal use and procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Yale University Animal Care and Use Committees.
Drugs
AGN-241751 was procured from Allergan Inc., USA. AGN-241751 was dissolved in vehicle (distilled water) and either vehicle or AGN-241751 (10, 50, 100, 1000 µg/Kg doses) were administered orally (p.o.). Animals were randomly assigned to drug treatment groups.
Chronic unpredictable stress
Animals were subjected to a random combination of two stressors per day for 4 weeks as previously described [25]. The following stressors were included: restraint (2 h), cage rotation, food or water deprivation, overnight isolation, crowding, overnight lighting, light-off during day (4 h), cage tilting (45°), wet bedding, and no bedding.
Behavioral tests
Behavioral experiments were performed 3 weeks after virus infusion and social isolation. Animals were habituated at least 30–45 min to experimental conditions prior to any behavioral tests.
Sucrose preference test (SPT)
The SPT was performed as previously described [26]. Briefly, animals were habituated to two bottles of water and subsequently two bottles of 1% sucrose for 48 h each. After CUS and drug administration, animals were water deprived (8 h) and presented with two identical pre-weighed bottles of water and 1% sucrose. Overnight consumption of water and 1% sucrose was quantified on day 1. The bottle positions were counterbalanced and the test was repeated the next day. Mean sucrose consumption relative to total fluid consumption was determined.
Forced swim test (FST)
Animals were placed in clear cylinders filled with water (24 ± 1 °C, 18 cm depth) and were video recorded for 6 min. Immobility time, defined as a lack of activity, except the minimal effort required to keep head above water, and was scored for minutes 2–6 by an experimenter blinded to treatment groups.
Female urine sniffing test (FUST)
The FUST was performed as previously described [27]. Briefly, each animal was placed in a novel cage for acclimatization (30 min) and habituated to a cotton tip for 1 h under dim lighting. Subsequently, a fresh water-soaked cotton tip was placed on the inner wall of the cage, and the animal was video recorded for 5 min. After 45 min, a cotton tip with fresh female urine was placed in the cage, and the animal was video recorded for 5 min. Time spent sniffing water- or female urine-soaked cotton tip was scored.
Novelty-suppressed feeding test (NSFT)
As previously described [27], animals were food deprived for 18–20 h prior to exposure to a novel open arena (40 × 40 × 25 cm) with chow pellet in the center and the latency to feed was recorded. Later, animals were allowed ad libitum access to a pre-weighed chow pellet in their home cages and food consumption was recorded.
Sucrose splash test (SST)
The SST was performed as previously described [28]. Animals were placed in clean cages and habituated for 30–45 min under red light. A 10% sucrose solution was squirted on the animal’s dorsal coat and grooming behavior was recorded for 5 min. The duration of total grooming time was determined.
Elevated plus maze (EPM)
The EPM test was performed as previously described [29]. Each animal was placed in the center of the maze facing an open arm, and allowed to explore the maze while being video recorded for 5 min. The time spent in each arm and number of entries into each were analyzed using an automated analysis software (ANY-maze, Stoelting).
Western blotting
PFC was dissected from coronal sections of brain (rostral to the corpus callosum) and homogenized in a solution containing 0.32 M sucrose, 20 mM HEPES (pH 7.4), 1 mM EDTA, 1× protease inhibitor cocktail, 5 mM NaF, and 1 mM sodium vanadate. The homogenate was centrifuged for 10 min at 2800 rpm at 4 °C and the supernatant was centrifuged at 12,000 rpm for 10 min. The supernatant (cytosolic fraction) was removed and the pellet (crude synaptosomal fraction) was resuspended by sonication in protein lysis buffer (50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 2 mM EDTA, 1 mM NaVO3, 5 mM NaF, and 1× protease inhibitor cocktail) and protein concentration was determined by BCA assay. Equal amounts of protein (20 μg) for each sample were loaded into 4–20% SDS PAGE gel for electrophoresis. Polyvinylidene difluoride membranes with transferred proteins were blocked with 5% skim milk in TBST (TBS + 0.1% Tween-20) for 1 h and incubated with primary antibodies [PSD95 (Cell Signaling #2507, 1:1000), GluA1 (Cell Signaling #13185, 1:1000), synaptic vesicle protein 2A (SV2A) (EMD Millipore#AB15224, 1:1000), pp70s6k (Cell Signaling #9204S, 1:1000), p70s6k (Cell Signaling #2708S, 1:1000), pAkt (Cell Signaling #4058S, 1:1000), Akt (Cell Signaling #9272S, 1:1000), pERK1/2 (Cell Signaling #4376S, 1:1000), ERK1/2 (Cell Signaling #9102S, 1:1000), peEF2 (Cell Signaling #2331S, 1:1000), and b-actin (Cell Signaling #3700S, 1:3000)] overnight at 4 °C. Blots were then washed three times in TBST and incubated with horseradish peroxidase conjugated anti-rabbit secondary antibody (1:10,000) for 1 h. After final three washes with TBST, bands were detected using enhanced chemiluminescence. Densitometric analysis of immunoreactivity for each protein was conducted using Image Lab (Bio-Rad). Immunoreactivity was normalized to GAPDH and then to control group values for each protein.
Viral constructs
The following shRNA sequence was designed to target GluN2B subunit 5′-TgtaccaacaggtctcaccttaaacTTCAAGAGAgtttaaggtgagacctgttggtacTTTTTTC-3′ (Integrated DNA Technologies) and was ligated into a TATAloxP-flanked CMV-EGFP cassette containing pSico plasmid, designed to restrict shRNA expression to cells that express Cre recombinase. The resulting pGluN2BshRNA constructs was subcloned into pAAV-mCherry (Virovek) to generate the plasmid which allows ubiquitous mCherry expression and conditional EGFP expression driven by floxed CMV cassette. These constructs were packaged into adeno-associated virus 2 (AAV2).
Surgical procedures for viral infusions
A cocktail of ketamine/xylazine (100/10 mg/Kg, i.p.) was injected to anesthetize mice, head fixed in a stereotaxic apparatus (David Kopf, Tujunga, CA) and ophthalmic ointment was applied on eyes. After incision and sterilization of incision site, a pair of craniotomies was made at following coordinates from bregma: +1.9 mm AP, ±0.4 mm ML, and –2.8 mm DV, and bilateral viral infusions into the mPFC (0.5 μL per side; 0.1 μL/min) were performed using Hamilton syringe (Reno, NV) fitted with a 30 gauge needle. Incision sites were closed with sutures, an antibiotic was applied, and carprofen (5 mg/Kg, i.p.) was injected immediately after surgery and daily for the next 2 days. Mice were individually housed (social isolation period) after viral infusions.
Immunohistology
Brains were collected from all virus-infused mice after transcardiac perfusion with sterile PBS and 4% paraformaldehyde (PFA). Brains were postfixed in 4% PFA for 24 h and incubated in 30% sucrose for an additional 24 h. 30-µm-thick sections were made from postfixed frozen brains using a Microm HM550 cryostat and mounted on slides for further immunofluorescence visualization. Images were acquired using confocal laser scanning microscope (Olympus FV1000).
Electrophysiology
Coronal slices of 300-μm thickness containing the mPFC were prepared from male C57BL/6J or PV-tdTomato mice and placed in artificial cerebrospinal fluid (ACSF) (pH 7.35–7.38) equilibrated with 95% O2/5% CO2 for 1 h before transferring them to recording chamber fixed with an Olympus BX50WI scope for electrophysiology recordings. The chamber was continuously perfused with normal ACSF (2–3 ml/min) and its temperature maintained at 33 ± 0.5 °C. The pipette solution contained the following: 115 mM K gluconate, 5 mM KCl, 2 mM MgCl2, 2 mM Mg-ATP, 2 mM Na2ATP, 10 mM Na2-phosphocreatine, 0.4 mM Na2GTP, and 10 mM Hepes, pH 7.33. Pyramidal neurons and _Pvalb_-inhibitory neurons in mPFC were visualized by video microscopy using microscope (40x IR lens) with infrared differential interference contrast (IR/DIC). Whole-cell recordings were performed using an Axoclamp-2B amplifier. NMDA- and AMPA-induced inward currents were measured in magnesium free ACSF upon bath application of 10 µM NMDA, or 5 µM AMPA alone, before and after application of vehicle or AGN-241751.
Statistics
Data are expressed as the mean ± standard error of mean (SEM) and analyzed using Graphpad prism v8.3. For multiple group comparisons and to determine treatment or genotype effects or interaction, one-way ANOVA or two-way ANOVA post hoc Tukey’s multiple comparison tests were used to determine statistical significance. For comparisons between two groups, either unpaired or paired Student’s t test (two tailed) was performed to determine statistical significance. p < 0.05 was considered as statistically significant. Any value > 2 standard deviation from the group mean was considered as an outlier and excluded from the analysis. The details of statistical tests and results were presented in the figure legends.
Results
AGN-241751 exerts dose-dependent antidepressant-like effects and increases levels of synaptic proteins in prefrontal cortex
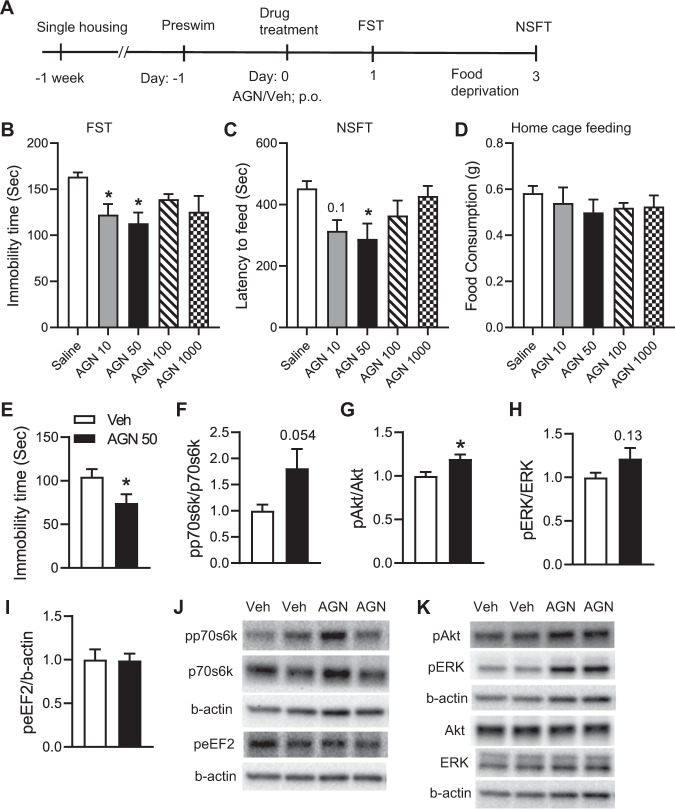
C57BL/6J mice were orally administered either vehicle or AGN-241751 (10, 50, 100, and 1000 µg/Kg doses) and antidepressant-like behavioral effects were evaluated in FST and NSFT 24 and 72 h post vehicle or drug treatments, respectively. Lower doses of AGN-241751 (10 and 50 µg/Kg) significantly reduced immobility time (a measure of behavioral despair) in the FST (Fig. 1B). Furthermore, the 50 µg/Kg dose significantly reduced the latency to feed in NSFT (Fig. 1C). However, higher doses of AGN-241751 (100 and 1000 µg/Kg) had no effect on either immobility time or latency to feed in FST and NSFT, respectively. There were no significant differences observed in home cage feeding with any of the tested doses of AGN-241751 (Fig. 1D). To evaluate the rapid antidepressant-like effect of AGN-241751, immobility time was measured in FST 1 h after drug treatment. AGN-241751 (50 µg/Kg) significantly decreased immobility time in FST (Fig. 1E). Furthermore, biochemical studies were performed to evaluate the activation of Akt/mTOR signaling in PFC and hippocampus, 1 h after treatment with AGN-241751 (50 µg/Kg). AGN-241751 significantly increased phosphorylation of Akt and induced a trend for an increase in phosphorylation of p70s6k and ERK1/2 without any change in phosphorylation of eEF2 (Fig. 1F–K) in PFC suggesting activation of Akt/mTOR signaling. However, no significant differences in phosphorylation of p70s6k, Akt, ERK1/2, and eEF2 were observed in hippocampus (Supplementary Fig. 1A–E).
Fig. 1. AGN-241751 treatment produces dose-dependent antidepressant-like effects and activates Akt/mTOR signaling in the PFC.
A Time line of drug administration and behavioral testing. Behavioral studies were performed 24 h after vehicle or AGN-241751 (10, 50, 100, and 1000 µg/Kg; p.o.) administration to single housed mice. Dose-dependent effects of AGN-241751 on immobility time (B) in the FST (_F_4,20 = 4.20, p = 0.01), latency to feed (C) in the NSFT (_F_4,20 = 3.33, p = 0.03), and food consumption (D) in home cage feeding test (_F_4,20 = 0.50, p = 0.73). Rapid antidepressant-like effect in the FST and biochemical studies on PFC tissue samples for activation of Akt/mTOR signaling were performed 1 h after vehicle or AGN-241751 (50 µg/Kg; p.o.) treatment. AGN-241751 (50 µg/Kg; p.o.) significantly decreased immobility time in the FST (E), 1 h after drug treatment. AGN-241751 (50 µg/Kg; p.o.) significantly increased phosphorylation of Akt (G), and showed a strong tendency for increase in phosphorylation of p70s6k (F) and ERK1/2 (H), but no change in phosphorylation of eEF2 (I) in the PFC. J, K Representative western blot images for quantified proteins. Data are expressed as mean ± SEM, n = 4–6 male (B–D); 4–5 male and female (E); 8 male (F–I) mice/group, *p < 0.05, one-way ANOVA post hoc Tukey’s multiple comparison test (B–D) or Student’s (unpaired) t test (E–I).
Previous studies demonstrated that rapid acting antidepressants like ketamine and rapastinel enhance synaptic function by increasing expression levels of synaptic proteins in mPFC [24, 30]. Since, the 50 µg/Kg dose of AGN-241751 produced significant antidepressant-like behavioral effects in both FST and NSFT, we used this dose for our biochemical experiments. Mice were treated with either vehicle or AGN-241751 (50 µg/Kg) and expression levels of synaptic proteins in PFC and hippocampus were evaluated 24 h after treatment using immunoblotting. AGN-241751 significantly increased levels of GluA1 subunit of AMPAR and PSD95 in mPFC. A trend for AGN-241751 to increase SV2A was observed, but this did not reach significance (Supplementary Fig. 2A–D). No significant differences in the expression of these proteins were observed in hippocampus (Supplementary Fig. 2E–H). These results suggest that AGN-241751 induces rapid and sustained antidepressant-like effects, activates Akt/mTOR signaling, and increases synaptic protein levels in PFC.
AGN-241751 reverses CUS-induced depressive-like behaviors
The CUS model has been used extensively to induce an anhedonic depression-like phenotype in rodents [31]. We therefore tested the effect of AGN-241751 (50 µg/Kg) on CUS-induced anhedonia using the SPT and depressive-like behaviors using the SST, the FUST, as well as in the FST and NSFT 1–4 days after drug treatment. As expected, CUS-exposed mice showed increased anhedonia, despair, and anxiogenic-like behaviors, including a significant reduction in sucrose preference in SPT (Fig. 2B), grooming time and female urine sniffing time in SST and FUST, respectively (Fig. 2C, E), and a significant increase in immobility time and latency to feed in FST and NSFT (Fig. 2D, F), respectively, compared to the saline-treated non-stressed group. However, AGN-241751 treatment significantly increased sucrose preference in SPT, grooming time and female urine sniffing time in SST and FUST, and decreased immobility time in FST in the CUS-exposed group when compared to saline-treated CUS-exposed mice. A trend for reduction in latency to feed (Fig. 2F) was also observed in NSFT after AGN-241751 treatment in CUS-exposed mice, but this did not reach significance. These results suggest that a single dose of AGN-241751 can reverse CUS-induced depressive-like behaviors.
Fig. 2. A single dose of AGN-241751 reverses CUS-induced depressive-like behaviors.
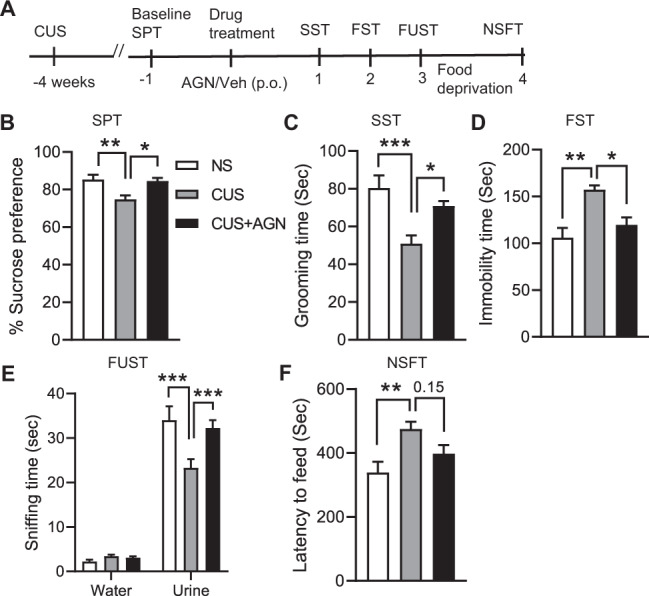
A Time line of CUS, drug administration, and behavioral testing. Behavioral studies were performed 24 h after vehicle or AGN-241751 (50 µg/Kg; p.o.) administration. Effects of stress and AGN-241751 on sucrose preference (B) in the SPT (_F_2,19 = 7.52, p = 0.004), grooming time (C) in the SST (_F_2,19 = 9.92, p = 0.001), immobility time (D) in the FST (_F_2,18 = 9.07, p = 0.002), female urine sniffing time (E) in the FUST (treatment: _F_2,36 = 5.41, p = 0.009; urine: _F_1,36 = 442, p < 0.001; interaction: _F_2,36 = 7.49, p = 0.002), and latency to feed (F) in the NSFT (_F_2,19 = 5.53, p = 0.01). Data are expressed as mean ± SEM, n = 7–8/group, *p < 0.05, **p < 0.01, ***p < 0.001, one-way (B–D, F) or two-way (E) ANOVA post hoc Tukey’s multiple comparison test.
AGN-241751 increases NMDAR-mediated currents in mPFC pyramidal neurons
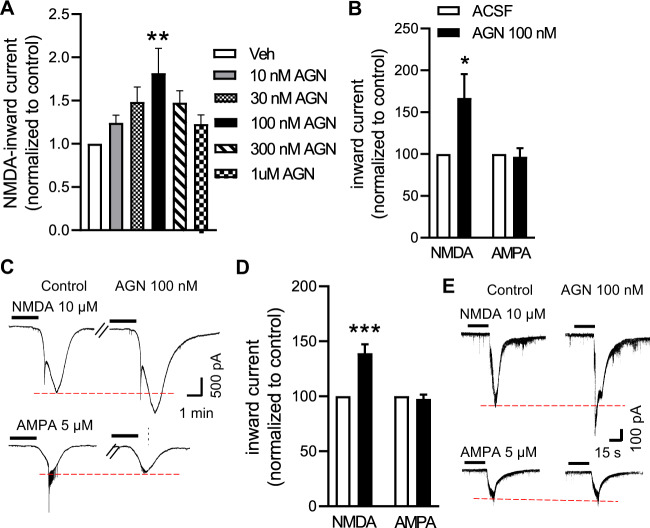
We evaluated the dose-dependent effects of AGN-241751 on NMDAR activity by patch clamp recording of layer V pyramidal neurons in mPFC mouse brain slices. Since AGN-241751 is an NMDAR PAM, we first bath applied a low dose of NMDA (10 µM) to mPFC slices to produce a distinct inward current, and then evaluated the effects of different concentrations of AGN-241751. A trend for an increase in NMDAR-mediated inward currents in layer V neurons was observed with 10 and 30 nM concentrations of AGN-241751, but this did not reach significance. A 100 nM concentration of AGN-241751 significantly enhanced the NMDAR-mediated inward currents. However, higher concentrations of AGN-241751 (300 nM and 1 µM) had no effect on NMDAR-mediated inward currents (Fig. 3A).
Fig. 3. AGN-241751 enhances NMDA-, but not AMPA-mediated, inward currents in excitatory and inhibitory neurons.
NMDAR-mediated inward currents were recorded from layer V pyramidal or Pvalb-inhibitory neurons of mPFC before and after bath application of either NMDA (10 µM) or AMPA (5 µM) alone or in combination with AGN-241751 (10, 30, 100, 300 nM and 1 µM). A AGN-241751 significantly increased NMDAR-mediated inward currents in layer V pyramidal neurons at 100 nM. A trend for an increase was observed at low nanomolar concentrations, but this did not reach significance. There was no effect of the highest (1 µM) concentration of AGN-241751. Data are expressed as mean ± SEM, n = 8–12 neurons from six mice (one-way ANOVA and post hoc Sidak’s multiple comparisons test, _F_5,60 = 3.06, p = 0.016). AGN-241751 (100 nM) significantly increased NMDAR-mediated inward currents in both excitatory (B) and Pvalb-expressing inhibitory neurons (D) with no effects on AMPAR-mediated inward currents. Representative traces of NMDAR- and AMPAR-mediated inward currents before and after application of AGN-241751 in excitatory neurons (C) and Pvalb-expressing inhibitory neurons (E). n = 9 (B) or 12–15 (D) neurons from five to six mice for each cell type (paired _t_-test, *p < 0.05, ***p < 0.001).
AGN-241751 enhances NMDAR-mediated inward currents in excitatory and PV-inhibitory neurons in mPFC
We next evaluated the effects of AGN-241751 on NMDA- and AMPA-induced inward currents of both excitatory and PV-inhibitory neurons in mPFC brain slices. Excitatory neurons were identified by their shape and electrophysiological properties, whereas a class of inhibitory neurons was marked by conditional _cis_-expression of the fluorescent marker tdTomato in transgenic mice expressing Cre recombinase in PV-expressing GABA interneurons (Pvalb-tdTomato transgenic mice). Bath application of AGN-241751 (100 nM) significantly enhanced NMDA-induced NMDAR-mediated inward currents in excitatory neurons (Fig. 3B, C) as well as in inhibitory PV-expressing interneurons (Fig. 3D, E). Consistent with a selective effect on NMDARs, there was no effect of AGN-241751 (100 nM) on AMPA-inward currents in either excitatory or PV-inhibitory neurons when AMPA (5 µM) was bath applied to produce a distinct AMPA-inward current (Fig. 3B–E).
GluN2B-containing NMDARs on excitatory, but not inhibitory, neurons block the antidepressant-like behavioral effects of AGN-241751
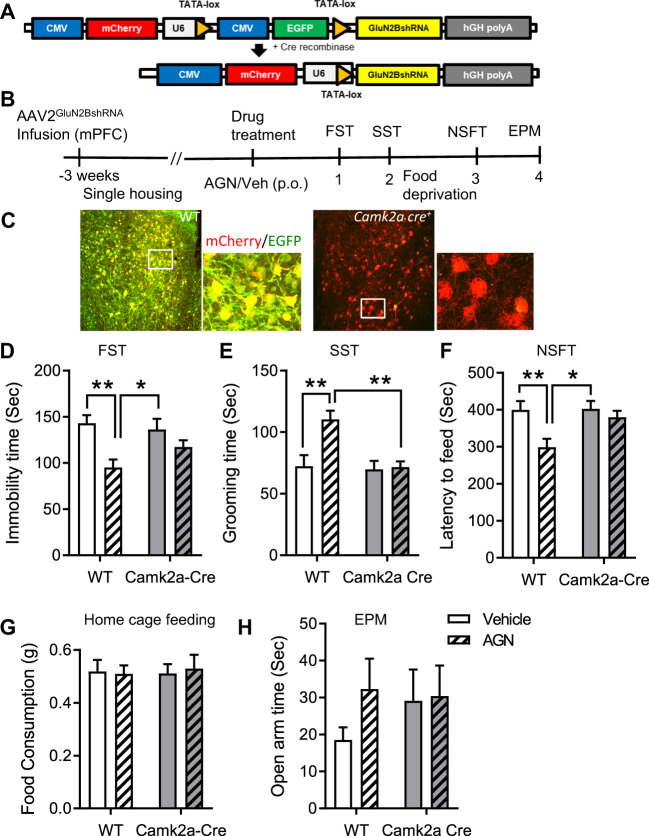
Based on previous studies demonstrating the crucial role of GluN2B subunit in mediating the actions of rapid antidepressants [22, 32–35], we used an AAV2 to deliver a Cre-dependent shRNA targeting GluN2B into the mPFC of _Camk2a_- or _Gad1_-Cre transgenic mice to generate cell-type-specific KD of GluN2B in excitatory or inhibitory neurons, and then tested the behavioral effects of AGN-241751. The conditional AAV2GluN2BshRNA construct drives expression of both mCherry and EGFP, but not the shRNA, in the absence of Cre expression. However, in cells expressing Cre recombinase, EGFP is excised resulting in expression of only mCherry along with the shRNA (Fig. 4A). We have validated the efficacy of this approach for selective KD of GluN2B using both electrophysiology and immunoblotting [22, 36].
Fig. 4. Knockdown of GluN2B in the mPFC of Camk2a-Cre mice prevents antidepressant-like effects of AGN-241751.
A Schematic showing pGluN2BshRNA construct and EGFP excision after Cre-mediated recombination. B Time line of viral infusion, single housing, drug administration, and behavioral testing. C Representative images of AAV2GluN2BshRNA virus expression showing fluorescent labeling of WT neurons (yellow: colocalization of EGFP and mCherry fluorescence) and a subset of mCherry-alone expressing pyramidal neurons in the mPFC of WTCre-/AAV2B and Camk2a Cre+/AAV2B mice, respectively. AGN-241751 (50 µg/Kg; p.o.) significantly reduced immobility time (D) in the FST (treatment: _F_1,36 = 13.5, p < 0.001, genotype: _F_1,36 = 0.72, p = 0.4, interaction: _F_1,36 = 2.5, p = 0.12), increased grooming time (E) in the SST (treatment: _F_1,36 = 7.55, p = 0.009, genotype: _F_1,36 = 8.14, p = 0.007, interaction: _F_1,36 = 6.23, p = 0.02) and reduced latency to feed (F) in the NSFT (treatment: _F_1,36 = 8.03, p = 0.007, genotype: _F_1,36 = 3.67, p = 0.06, interaction: _F_1,36 = 3.21, p = 0.08) in WTCre-/AAV2B but these effects were absent in Camk2a Cre+/AAV2B mice. G There were no significant differences observed in home cage feeding across groups. H No significant effects were observed in open arm time (treatment: _F_1,35 = 1.08, p = 0.31). Data expressed as the mean ± SEM and analyzed by two-way ANOVA with Tukey’s multiple comparisons (n = 8–11 per group; *p < 0.05, **p < 0.01).
AAV2GluN2BshRNA was bilaterally infused into the mPFC of adult Camk2a Cre+ mice (Camk2a Cre+/AAV2B) or Gad1 Cre+ mice (Gad1 Cre+/AAV2B) and littermate wild-type control mice (WTCre-/AAV2B) and behavioral testing was performed 3 weeks after virus infusions to allow for Cre-mediated recombination and shRNA expression. Immunohistochemistry revealed cells expressing both mCherry and EGFP (labeled in yellow color) in mPFC slices of WTCre-/AAV2B mice, demonstrating efficient infection and virus-mediated expression, and subsets of cells expressing only mCherry in mPFC slices of Camk2aCre+/AAV2B mice (Fig. 4C), demonstrating Cre-mediated recombination of the shRNA construct in the mPFC of Camk2a Cre+/AAV2B mice. Three weeks after AAV2GluN2BshRNA viral infusions, mice were treated with either vehicle or AGN-241751 (50 µg/Kg) and behavioral testing (SST, FST, NSFT, and EPM) was performed 1–4 days after drug treatment. In WTCre-/AAV2B mice, AGN-241751 significantly increased grooming time in SST, and decreased the immobility time and latency to feed in FST and NSFT, respectively (Fig. 4D–F). However, these effects of AGN-241751 were absent in Camk2a Cre+/AAV2B mice in FST, SST, and NSFT. There were no differences observed in home cage feeding (Fig. 4G). Effects of AGN-241751 in the EPM were not significant (Fig. 4H).
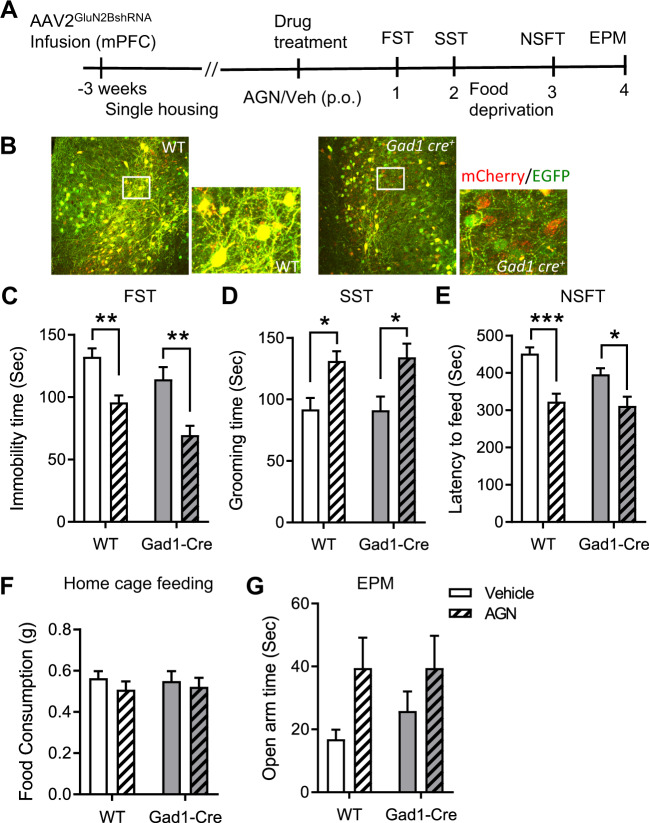
As in the previous experiment targeting excitatory neurons, immunohistochemistry revealed cells expressing both mCherry and EGFP (labeled in yellow color) in mPFC slices of WTCre-/AAV2B mice, demonstrating efficient infection and virus-mediated expression, and subsets of cells expressing only mCherry in mPFC slices of Gad1Cre+/AAV2B mice (Fig. 5B), demonstrating Cre-mediated recombination of the shRNA construct in the mPFC of Gad1Cre+/AAV2B mice. Drug treatment and behavioral studies were performed as described above. In contrast to what was observed in Camk2a Cre+/AAV2B mice, a single dose of AGN-241751 treatment significantly increased grooming time in SST and significantly decreased immobility time and latency to feed in FST and NSFT, respectively, in both WTCre-/AAV2B and Gad1 Cre+/AAV2B mice (Fig. 5C–E). There were no differences observed in home cage feeding (Fig. 5F). In EPM, a significant treatment effect was observed in open arm time of AGN-241751 treated WTCre-/AAV2B and Gad1 Cre+/AAV2B mice (Fig. 5G). Together, these results demonstrate that GluN2B-containing NMDARs on glutamatergic, but not GABAergic, neurons mediate the antidepressant-like actions of AGN-241751.
Fig. 5. Knockdown of GluN2B in the mPFC of Gad1-Cre mice does not block antidepressant-like effects of AGN-241751.
A Time line of viral infusion, single housing, drug administration, and behavioral testing. B Representative images of AAV2GluN2BshRNA virus expression showing fluorescent labeling of WT neurons (yellow: colocalization of EGFP and mCherry fluorescence) and a subset of mCherry-alone expressing inhibitory neurons in the mPFC of WTCre-/AAV2B and Gad1 Cre+/AAV2B mice, respectively. AGN-241751 (50 µg/Kg; p.o.) significantly reduced immobility time (C) in the FST (treatment: _F_1,38 = 30.4, p < 0.001, genotype: _F_1,38 = 8.91, p = 0.005, interaction: _F_1,38 = 0.3, p = 0.59), increased grooming time (D) in the SST (treatment: _F_1,35 = 17.8, p < 0.001, genotype: _F_1,35 = 0.0126, p = 0.91, interaction: _F_1,35 = 0.033, p = 0.86) and decreased latency to feed (E) in the NSFT (treatment: _F_1,38 = 28, p = 0.001, genotype: _F_1,38 = 2.77, p = 0.1, interaction: _F_1,38 = 1.18, p = 0.28) in both WTCre-/AAV2B and Gad1 Cre+/AAV2B mice. F There were no significant differences observed in home cage feeding across groups. G An increase in open arm time was observed after AGN-241751 in both WTCre-/AAV2B and Gad1 Cre+/AAV2B mice (n = 4–8 male or female mice/group for EPM; treatment: _F_1,20 = 6.49, p = 0.02). Data expressed as the Mean ± SEM and analyzed by two-way ANOVA with Tukey’s multiple comparisons (n = 8–12/group (C–F); *p < 0.05, **p < 0.01).
Discussion
Enhancers of NMDAR activity have recently emerged as promising candidates for next generation of rapid antidepressants without ketamine-like side effects. AGN-241751 is an NMDAR PAM, which is currently in phase II clinical trials for treatment of depression [18, 37], but the cellular trigger mediating the actions of NMDAR PAM AGN-241751 still remains elusive. In these studies, we demonstrate dose-dependent, rapid, and sustained antidepressant-like effects of AGN-241751, activation of Akt/mTOR signaling and increased expression of synaptic proteins in PFC, and reversal of CUS-induced behavioral deficits after a single low dose of AGN-241751, which lasts over 4 days, suggesting that the compound exerts rapid and sustained antidepressant-like effects. Furthermore, we show that low doses of AGN-241751 can increase NMDA-induced NMDAR-mediated inward currents in both excitatory and PV-inhibitory neurons in mPFC, however, GluN2B-containing NMDARs on excitatory, but not GABAergic, neurons in mPFC were essential for the antidepressant-like behavioral effects of AGN-241751. Together, these findings suggest that direct positive modulation of NMDARs on excitatory neurons is sufficient to induce rapid and sustained antidepressant-like effects.
NMDARs play a key role in activity-dependent synaptic plasticity, whereas deficits in structural and functional plasticity have been reported after chronic stress in animals [38–44]. NMDAR binding is reduced in the frontal cortex of suicide victims and NMDAR expression is decreased in postmortem PFC brain samples from depressed patients [45, 46]. These findings suggest that NMDAR-dependent synaptic function is impaired in individuals with depression and in rodent stress models. In contrast, enhancing NMDAR function with drugs like rapastinel, sarcosine, and D-serine exerts rapid antidepressant effects in both clinical and preclinical studies [12–17] potentially by inducing synaptic plasticity. Consistent with this hypothesis, we show here that enhancing NMDAR function using a novel NMDAR PAM, AGN-241751, produces rapid and sustained antidepressant-like effects. These rapid antidepressant-like behavioral effects of AGN-241751 were observed only at lower doses. Similarly, in PFC brain slices, only lower concentrations of AGN-241751 enhanced NMDA-inward currents, suggesting that AGN-241751 has an inverted-U shaped dose–response effect on both NMDAR function and behavior in mice. Consistent with these findings, other NMDAR-PAMs were also shown to exert an inverted-U shaped dose–response [14, 47] and only lower doses of NMDAR-PAMs were shown to enhance synaptic plasticity. Interestingly, higher doses of these NMDAR-PAMs were shown to weakly inhibit NMDAR activity [14, 47, 48]. Although, we did not observe an inhibition of NMDAR activity at the highest concentration (1 µM) tested in our study, higher micro molar concentrations could potentially inhibit NMDAR activity leading to different effects on synaptic transmission. For instance, rapastinel at high micro molar concentration was reported to inhibit NMDAR activity to cause disinhibition of glutamatergic transmission in hippocampal brain slices [48]. However, previous studies with NMDAR PAM rapastinel reported that low nano molar concentrations are associated with its antidepressant-like effect and metaplasticity [14]. These findings suggest that an optimal and subtle positive modulation of NMDAR activity could lead to antidepressant-like effects and higher doses of NMDAR-PAMs could potentially have different behavioral effects. AGN-241751 is more potent at NMDARs and has a longer plasma half-life than rapastinel, suggesting that AGN-241751 has a longer target engagement at NMDARs resulting into a longer duration of action than rapastinel. However, further studies comparing the effects of AGN-241751 and rapastinel directly are needed to test any differences in therapeutic efficacy and to determine whether preclinical findings translate to clinical efficacy of AGN-241751.
NMDAR enhancers like rapastinel and sarcosine can reverse the structural and functional alterations of PFC after chronic stress by activating mTOR-dependent protein synthesis and increasing expression levels of synaptic proteins, implicated in neuronal plasticity, in rodents [16, 34, 49]. Consistent with these findings, AGN-241751 treatment acutely activated Akt/mTOR signaling as revealed by increased phosphorylation of Akt and p70s6k (downstream mTOR substrate for activating protein translation) and increased the levels of GluA1 and PSD95 in the PFC, but not hippocampus, suggesting that this NMDAR PAM can enhance excitatory neuronal plasticity in the PFC of mice. Furthermore, a single dose of AGN-241751 exerts rapid antidepressant-like effect and reverses CUS-induced behavioral deficits, including anhedonia and depressive-like behaviors in mice for at least 4 days after administration, suggesting that antidepressant-like behavioral effects of AGN-241751 lasts well beyond its bioavailability (systemic or brain exposure). We postulate that these sustained effects are mediated by initial activation of NMDARs and Akt/mTOR signaling that subsequently trigger structural and functional synaptic plasticity. In support of this hypothesis, AGN-241751 was reported to enhance long-term plasticity in the mPFC [18]. PET-ligand-based brain imaging studies of synaptic density markers could be used to test the neuronal plasticity hypothesis for AGN-241751 in depressed patients.
The observation that both NMDAR antagonist (ketamine) and NMDAR enhancers produce similar antidepressant-like effects raises the question of how drugs that have opposing actions on NMDAR function exert similar behavioral actions. Since NMDARs are expressed on both excitatory and inhibitory neurons, we propose that opposing effects of these drugs on NMDARs of each neuronal population in mPFC may underlie the similar behavioral actions of an NMDAR antagonist and NMDAR enhancers. In support of this hypothesis, we have recently shown that GluN2B on inhibitory neurons of mPFC is the initial cellular target for ketamine’s antidepressant-like effects [22]. In contrast to ketamine’s action, we demonstrate here that GluN2B on excitatory neurons of mPFC is essential for the antidepressant-like behavioral effects of the NMDAR PAM AGN-241751. Although, AGN-241751 could enhance NMDAR-mediated currents in both excitatory and PV-inhibitory neurons, there was no change in its behavioral effects when GluN2B was knocked down from inhibitory neurons, suggesting that enhancing NMDAR-mediated currents in GABAergic neurons of the mPFC is not sufficient to alter depression-like behaviors. These results are in line with our recent study demonstrating that GluN2B in excitatory neurons mediate the actions of the NMDAR PAM rapastinel [36], suggesting that positive modulation of NMDARs on glutamatergic neurons of mPFC produces antidepressant-like effects. Consistent with the current study, optogenetic activation of mPFC glutamatergic neurons is sufficient and necessary to produce rapid and sustained antidepressant-like effects [50]. One limitation of the current study is that these behavioral studies following conditional GluN2B KD animals were not performed following exposure to CUS, however, we note that these experiments were performed in socially isolated mice, which induces depressive-like molecular and behavioral deficits in rodents [51–53]. Future studies in well-established stress models would be helpful to substantiate these findings.
In conclusion, AGN-241751 produces rapid antidepressant-like effects and alleviates CUS-induced behavioral deficits, which may involve an increase in levels of synaptic proteins implicated in synaptic plasticity. Antidepressant-like effects of AGN-241751 are mediated by GluN2B-containing NMDARs on excitatory neurons. Thus, our findings provide new mechanistic insights into the cell-type-specific role of GluN2B in the actions of NMDAR enhancers which can further be explored to develop next-generation antidepressants.
Funding and disclosure
This research was supported by National Institute of Mental Health grants MH093897 and MH105910 (RSD) and a research grant from Allergan Inc., New Jersey. RSD has received consulting fees from Taisho, Johnson & Johnson, and Naurex, and grant support from Taisho, Johnson & Johnson, Naurex, Allergan, Navitor, Lundbeck, Relmada, and Lilly. PB is an employee of Allergan Inc., New Jersey. MRP was supported by MH077681 from the National Institutes of Health. All other authors declare no competing interests.
Supplementary information
Acknowledgements
We thank Xiao Yuan Li for her help with genotyping of mouse lines.
Author contributions
SP, PB, and RSD designed the study. SP wrote the paper. MRP edited the paper. SP, R-JL, MW, and A-NS conducted experiments, analyzed data, and interpreted the results. All authors reviewed and approved the final paper.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at (10.1038/s41386-020-00882-7).
References
- 1.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–858. [DOI] [PMC free article] [PubMed]
- 2.Lépine JP, Briley M. The increasing burden of depression. Neuropsychiatr Dis Treat. 2011;7(Suppl 1):3–7. doi: 10.2147/NDT.S19617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53:649–59. doi: 10.1016/s0006-3223(03)00231-2. [DOI] [PubMed] [Google Scholar]
- 4.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 5.Curtin SC, Warner M, Hedegaard H. Increase in suicide in the United States, 1999–2014. NCHS Data Brief. 2016;241:1–8. [PubMed] [Google Scholar]
- 6.Krystal JH, Sanacora G, Blumberg H, Anand A, Charney DS, Marek G, et al. Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Mol Psychiatry. 2002;7(Suppl 1):S71–80. doi: 10.1038/sj.mp.4001021.. [DOI] [PubMed] [Google Scholar]
- 7.Krystal JH, Sanacora G, Duman RS. Rapid-acting glutamatergic antidepressants: the path to ketamine and beyond. Biol Psychiatry. 2013;73:1133–41. doi: 10.1016/j.biopsych.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duman RS, Shinohara R, Fogaça MV, Hare B. Neurobiology of rapid-acting antidepressants: convergent effects on GluA1-synaptic function. Mol Psychiatry. 2019;24:1816–32. doi: 10.1038/s41380-019-0400-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–4. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 10.Moghaddam B, Krystal JH. Capturing the angel in “angel dust”: twenty years of translational neuroscience studies of NMDA receptor antagonists in animals and humans. Schizophr Bull. 2012;38:942–9. doi: 10.1093/schbul/sbs075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan CJA, Curran HV. Ketamine use: a review. Addiction. 2012;107:27–38. doi: 10.1111/j.1360-0443.2011.03576.x. [DOI] [PubMed] [Google Scholar]
- 12.Burgdorf J, Zhang XL, Nicholson KL, Balster RL, Leander JD, Stanton PK, et al. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology. 2013;38:729–42. doi: 10.1038/npp.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burch R, Preskorn S, Bastin L, Yu W, Burgdorf J, Moskal J. Adjunctive GLYX-13 induces prolonged efficacy in subjects with major depressive disorder (MDD) Neuropsychopharmacology. 2014;39:S335. [Google Scholar]
- 14.Donello JE, Banerjee P, Li YX, Guo YX, Yoshitake T, Zhang XL, et al. Positive N-Methyl-D-Aspartate Receptor Modulation by Rapastinel Promotes Rapid and Sustained Antidepressant-Like Effects. Int J Neuropsychopharmacol. 2019;22:247–59. doi: 10.1093/ijnp/pyy101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang CC, Wei IH, Huang CL, Chen KT, Tsai MH, Tsai P, et al. Inhibition of glycine transporter-I as a novel mechanism for the treatment of depression. Biol Psychiatry. 2013;74:734–41. doi: 10.1016/j.biopsych.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 16.Chen KT, Tsai MH, Wu CH, Jou MJ, Wei IH, Huang CC. AMPA receptor-mTOR activation is required for the antidepressant-like effects of sarcosine during the forced swim test in rats: insertion of AMPA receptor may play a role. Front Behav Neurosci. 2015;9:162. doi: 10.3389/fnbeh.2015.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malkesman O, Austin DR, Tragon T, Wang G, Rompala G, Hamidi AB, et al. Acute d-serine treatment produces antidepressant-like effects in rodents. Int J Neuropsychopharmacol. 2012;15:1135–48. doi: 10.1017/S1461145711001386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banerjee P, Donello J, Li YX, Bertelsen K, Gu Y-X, Zhang X-L, et al. AGN-241751, an orally bioavailable positive NMDA receptor modulator, exhibits rapid and sustained antidepressant-like effects in rodents. Biol Psychiatry. 2019;85:S348. [Google Scholar]
- 19.Chan SY, Matthews E, Burnet PW. On or off?: modulating the N-methyl-D-aspartate receptor in major depression. Front Mol Neurosci. 2017;9:169. doi: 10.3389/fnmol.2016.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papadia S, Hardingham GE. The dichotomy of NMDA receptor signaling. Neuroscientist. 2007;13:572–9. doi: 10.1177/10738584070130060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tasic B, Menon V, Nguyen TN, Kim TK, Jarsky T, Yao Z, et al. Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat Neurosci. 2016;19:335–46. doi: 10.1038/nn.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerhard DM, Pothula S, Liu RJ, Wu M, Li XY, Girgenti MJ, et al. GABA interneurons are the cellular trigger for ketamine’s rapid antidepressant actions. J Clin Invest. 2020;130:1336–49. doi: 10.1172/JCI130808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuchikami M, Thomas A, Liu R, Wohleb ES, Land BB, DiLeone RJ, et al. Optogenetic stimulation of infralimbic PFC reproduces ketamine’s rapid and sustained antidepressant actions. Proc Natl Acad Sci. 2015;112:8106–11.. doi: 10.1073/pnas.1414728112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–64.. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt HD, Duman RS. Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology. 2010;35:2378–91. doi: 10.1038/npp.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwata M, Ota KT, Li XY, Sakaue F, Li N, Dutheil S, et al. Psychological stress activates the inflammasome via release of adenosine triphosphate and stimulation of the purinergic type 2X7 receptor. Biol Psychiatry. 2015;80:12–22. doi: 10.1016/j.biopsych.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 27.Wohleb ES, Wu M, Gerhard DM, Taylor SR, Picciotto MR, Alreja M, et al. GABA interneurons mediate the rapid antidepressant-like effects of scopolamine. J Clin Invest. 2016;126:2482–94. doi: 10.1172/JCI85033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Isingrini E, Camus V, Le Guisquet AM, Pingaud M, Devers S, Belzung C. Association between repeated unpredictable chronic mild stress (UCMS) procedures with a high fat diet: a model of fluoxetine resistance in mice. PLoS ONE. 2010;5:e10404. doi: 10.1371/journal.pone.0010404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–67. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 30.Kato T, Duman RS. Rapastinel, a novel glutamatergic agent with ketamine-like antidepressant actions: convergent mechanisms. Pharm Biochem Behav. 2020;188:172827. doi: 10.1016/j.pbb.2019.172827. [DOI] [PubMed] [Google Scholar]
- 31.Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- 32.Miller OH, Yang L, Wang CC, Hargroder EA, Zhang Y, Delpire E, et al. GluN2B-containing NMDA receptors regulate depression-like behavior and are critical for the rapid antidepressant actions of ketamine. Elife. 2014;3:e03581. doi: 10.7554/eLife.03581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller OH, Bruns A, Ben Ammar I, Mueggler T, Hall BJ. Synaptic regulation of a thalamocortical circuit controls depression-related behavior. Cell Rep. 2017;20:1867–80. doi: 10.1016/j.celrep.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–61. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008;28:631–7. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- 36.Pothula S, Kato T, Liu RJ, Wu M, Gerhard D, Shinohara R, et al. Cell-type specific modulation of NMDA receptors triggers antidepressant actions. Mol Psychiatry. 2020. 10.1038/s41380-020-0796-3. [DOI] [PubMed]
- 37.Wilkinson ST, Sanacora G. A new generation of antidepressants: an update on the pharmaceutical pipeline for novel and rapid-acting therapeutics in mood disorders based on glutamate/GABA neurotransmitter systems. Drug Discov Today. 2019;24:606–15. doi: 10.1016/j.drudis.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hunt DL, Castillo PE. Synaptic plasticity of NMDA receptors: mechanisms and functional implications. Curr Opin Neurobiol. 2012;22:496–508. doi: 10.1016/j.conb.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skolnick P, Layer RT, Popik P, Nowak G, Paul IA, Trullas R. Adaptation of N-methyl-D-aspartate (NMDA) receptors following antidepressant treatment: implications for the pharmacotherapy of depression. Pharmacopsychiatry. 1996;29:23–6. doi: 10.1055/s-2007-979537. [DOI] [PubMed] [Google Scholar]
- 40.Radley JJ, Morrison JH. Repeated stress and structural plasticity in the brain. Ageing Res Rev. 2005;4:271–87. doi: 10.1016/j.arr.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 41.Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–20. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- 42.Goldwater DS, Pavlides C, Hunter RG, Bloss EB, Hof PR, McEwen BS, et al. Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience. 2009;164:798–808. doi: 10.1016/j.neuroscience.2009.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gourley SL, Kedves AT, Olausson P, Taylor JR. A history of corticosterone exposure regulates fear extinction and cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology. 2009;34:707–16. doi: 10.1038/npp.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. 2012;73:962–77. doi: 10.1016/j.neuron.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:70–5. doi: 10.1016/j.pnpbp.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nowak G, Ordway GA, Paul IA. Alterations in the N-methyl-d-asparatate (NMDA) receptor complex in the frontal cortex of suicide victims. Brain Res. 1995;675:157–64. doi: 10.1016/0006-8993(95)00057-w. [DOI] [PubMed] [Google Scholar]
- 47.Khan MA, Houck DR, Gross AL, Zhang XL, Cearley C, Madsen TM, et al. NYX-2925 Is a Novel NMDA Receptor-Specific Spirocyclic-β-Lactam That Modulates Synaptic Plasticity Processes Associated with Learning and Memory. Int J Neuropsychopharmacol. 2018;21:242–54. doi: 10.1093/ijnp/pyx096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Widman AJ, McMahon LL. Disinhibition of CA1 pyramidal cells by low-dose ketamine and other antagonists with rapid antidepressant efficacy. Proc Natl Acad Sci USA. 2018;115:E3007–16.. doi: 10.1073/pnas.1718883115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu RJ, Duman C, Kato T, Hare B, Lopresto D, Bang E, et al. GLYX-13 produces rapid antidepressant responses with key synaptic and behavioral effects distinct from ketamine. Neuropsychopharmacology. 2017;42:1231–42.. doi: 10.1038/npp.2016.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hare BD, Shinohara R, Liu RJ, Pothula S, DiLeone RJ, Duman RS. Optogenetic stimulation of medial prefrontal cortex Drd1 neurons produces rapid and long-lasting antidepressant effects. Nat Commun. 2019;10:223. doi: 10.1038/s41467-018-08168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berry A, Bellisario V, Capoccia S, Tirassa P, Calza A, Alleva E, et al. Social deprivation stress is a triggering factor for the emergence of anxiety- and depression-like behaviors and leads to reduced brain BDNF levels in C57BL/6J mice. Psychoneuroendocrinology. 2012;37:762–72. doi: 10.1016/j.psyneuen.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 52.Ieraci A, Mallei A, Popoli M. Social isolation stress induces anxious-depressive-like behavior and alterations of neuroplasticity-related genes in adult male mice. Neural Plast. 2016;2016:6212983. doi: 10.1155/2016/6212983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mumtaz F, Khan MI, Zubair M, Dehpour AR. Neurobiology and consequences of social isolation stress in animal model-A comprehensive review. Biomed Pharmacother. 2018;105:1205–22.. doi: 10.1016/j.biopha.2018.05.086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.