Epidemiology of liver cirrhosis and associated complications: Current knowledge and future directions (original) (raw)
Abstract
Cirrhosis causes a heavy global burden. In this review, we summarized up-to-date epidemiological features of cirrhosis and its complications. Recent epidemiological studies reported an increase in the prevalence of cirrhosis in 2017 compared to in 1990 in both men and women, with 5.2 million cases of cirrhosis and chronic liver disease occurring in 2017. Cirrhosis caused 1.48 million deaths in 2019, an increase of 8.1% compared to 2017. Disability-adjusted life-years due to cirrhosis ranked 16th among all diseases and 7th in people aged 50-74 years in 2019. The global burden of hepatitis B virus and hepatitis C virus-associated cirrhosis is decreasing, while the burden of cirrhosis due to alcohol and nonalcoholic fatty liver disease (NAFLD) is increasing rapidly. We described the current epidemiology of the major complications of cirrhosis, including ascites, variceal bleeding, hepatic encephalopathy, renal disorders, and infections. We also summarized the epidemiology of hepatocellular carcinoma in patients with cirrhosis. In the future, NAFLD-related cirrhosis will likely become more common due to the prevalence of metabolic diseases such as obesity and diabetes, and the prevalence of alcohol-induced cirrhosis is increasing. This altered epidemiology should be clinically noted, and relevant interventions should be undertaken.
Keywords: Causes, Cirrhosis, Complications, Cost, Epidemiology, Burden, Feature
Core Tip: The global burden of liver cirrhosis continues to rise. In 2017, there were 520000 new cases of cirrhosis and chronic liver disease. In 2019, cirrhosis caused 1.48 million deaths, an increase of 8.1% compared to 2017, and its disability-adjusted life-years ranked 16th among all diseases. The global burden of cirrhosis due to hepatitis B virus and hepatitis C virus infection is decreasing, while the burden of cirrhosis due to alcohol and nonalcoholic fatty liver disease is increasing rapidly. We also outlined the recent epidemiology of the major complications and hepatocellular carcinoma in cirrhosis.
INTRODUCTION
Cirrhosis is a consequence of chronic liver damage and inflammation, and it is characterized by diffuse hepatic fibrosis and normal liver structures being replaced by regenerative liver nodules[1,2]. As the end stage of chronic liver disease (CLD), it can be caused by a variety of conditions, such as alcohol consumption, nonalcoholic fatty liver disease (NAFLD), hepatitis viruses, and autoimmune diseases. The progressive course of cirrhosis can generally include asymptomatic stages, such as compensated cirrhosis, and decompensated stages, which are frequently associated with the development of a range of complications, such as ascites, gastro-esophageal variceal (GEV) bleeding, and hepatic encephalopathy (HE); furthermore, cirrhosis may advance to liver failure and lead to death[3]. These complications impose a heavy burden on global public health in terms of significant quality of life impairment and associated high mortality in patients[4].
Despite the global prevalence and disease burden of cirrhosis, there is less public awareness and concern regarding cirrhosis than for other common chronic diseases, such as congestive heart failure, chronic obstructive pulmonary disease, and chronic kidney disease[5]. Currently, there remains an insufficient understanding of the clinical relevance of cirrhosis, which can therefore lead to unnecessary disease progression and outcomes[5]. In this review, we comprehensively overview and synthesize the recent epidemiological features of cirrhosis and its complications and discuss the changing trends in epidemiology. This could provide definite value for the clinical diagnosis and management of cirrhosis.
METHODS
The PubMed electronic database was manually searched to obtain relevant literature. The reference lists of the primary included literature were also searched to identify potentially eligible articles. Only articles published in English were included. There was no restriction regarding the publication year. The index terms included "cirrhosis", "ascites", "spontaneous bacterial peritonitis", "variceal bleeding", "hepatic encephalopathy", "acute kidney injury", "hepatorenal syndrome", "infection", "hepatocellular carcinoma", "epidemiology", "prevalence", "incidence", "mortality", "disease burden", "hospitalization", and "cost". A critical evaluation was carried out for all studies included in this paper.
CURRENT EPIDEMIOLOGY OF LIVER CIRRHOSIS
Prevalence
The most recent data available on the global prevalence of cirrhosis are from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2017[6]. The GBD 2017 reported the burden of cirrhosis based on pooled epidemiological data from 195 countries and territories stratified by cause, age, and sex from 1990 to 2017. The results for prevalence are presented as numbers and age-standardized or age-specific rates per 100000 populations with 95% uncertainty intervals (UIs). In 2017, there were an estimated 112 (107-119) million cases of compensated cirrhosis and 10.6 (10.3-10.9) million cases of decompensated cirrhosis prevalent worldwide. This represented a huge increase compared to the prevalence figures for 1990, when 65.9 (63.4-68.7) million cases of compensated cirrhosis and 5.20 (5.08-5.32) million cases of decompensated cirrhosis were observed. The age-standardized prevalence of compensated cirrhosis increased from 1354.5 (1300.6-1411.7) per 100000 in 1990 to 1395.0 (1323.5-1470.5) in 2017, while decompensated cirrhosis increased from 110.6 (108.0-113.0) per 100000 in 1990 to 132.5 (128.6-136.2) in 2017. In 2017, 58.8% of compensated cirrhosis cases and 60.3% of decompensated cirrhosis cases were observed in males, suggesting that men suffer from cirrhosis at higher rates. In males, the age-standardized prevalence of compensated cirrhosis increased by 2.9% from 1990 to 2017; the prevalence of decompensated cirrhosis increased by 21.1%. In females, these figures were 3.5% and 18.1%, respectively. Overall, the prevalence of liver cirrhosis increased by 74.53% from 1990 to 2017[7].
At the regional level, the GBD 2017 also provided relevant epidemiological characteristics[6]. In 2017, the high-income Asia-Pacific region had the highest age-standardized prevalence of both compensated [2455.0 (2344.9-2575.8) per 100000] and decompensated [267.4 (259.8-275.1) per 100000] cirrhosis. Most cases in this region were from Japan and were largely due to hepatitis C. In contrast, Australia reported the lowest age-standardized prevalence of both compensated and decompensated cirrhosis, with hepatitis C also being the main etiology. High-income regions in North America showed the lowest age-standardized prevalence of compensated cirrhosis (mainly caused by hepatitis C), while the lowest prevalence of decompensated cirrhosis was found in South Asia. At the country level, Moldova, Taiwan (Province of China), and Slovakia had the highest prevalence of compensated cirrhosis, while for decompensated cirrhosis, the Philippines had the lowest prevalence, and Slovakia had the highest.
Etiology-specific statistics on the prevalence of cirrhosis are also currently available. In a recent systematic review (retrieved until August 1, 2021) that included 520 studies from 86 countries or territories (reporting a total of 1376503 patients with cirrhosis), 42% of patients with cirrhosis worldwide had hepatitis B virus infection (HBV), and 21% had hepatitis C virus infection (HCV)[8]. The prevalence of HBV infection in cirrhosis was higher in Africa and Asia (8%-61%) than in Europe, the Americas, and Oceania (3%-14%). In contrast, the prevalence of HCV infection in cirrhosis was considerably heterogeneous by country and region (12%-83%). However, in general, the overall prevalence of HBV and HCV exceeded 50% in most parts of Asia and Africa. In China, 68% [95% confidence interval (CI) 60%-74%] of patients with cirrhosis had HBV infection, while only 7% (95%CI 5%-9%) reported HCV infection. In 2017, the age-standardized prevalence of HBV-related compensated cirrhosis did not change significantly compared to 1990, but the prevalence of decompensated cirrhosis increased from 30.9 (95%UI 29.3-32.2) to 36.6 (95%UI 34.7-38.4) per 100000 population[6]. The age-standardized prevalence of HCV-associated compensated cirrhosis increased to 341.1 (314.1-368.7), and the prevalence of decompensated cirrhosis increased to 32.5 (30.6-34.5) per 100000 population in 2017[6]. Regarding cirrhosis due to alcohol consumption, the highest prevalence was recorded in Europe (16%-78%), the Americas (17%-52%), and Oceania (15%-37%), while the lowest was reported in Asia (0%-41%)[8]. In 2017, the global age-standardized prevalence for alcohol-related compensated cirrhosis remained stable compared to 1990 (288.1 in 2017 compared to 290 per 100000 in 1990). However, the global prevalence of alcohol-related decompensated cirrhosis increased from 1990 (30 in 2017 compared to 25.3 per 100000 in 1990)[6]. Another important cause that should not be overlooked is NAFLD-related cirrhosis. According to the aggregate data of the GBD 2017, there were 9.42 million (8.57-10.34) cases of compensated cirrhosis and 917000 (850000-986000) cases of decompensated cirrhosis attributed to non-alcoholic steatohepatitis (NASH) in 2017, showing an impressive increase compared to 1990[6]. The age-standardized prevalence of NASH-related compensated cirrhosis was 115.5 (105.0-126.5) per 100000 in 2017, indicating a 33.2% increase compared to 1990; the prevalence of decompensated cirrhosis showed a 54.8% increase to 11.3 (10.4-12.1) per 100000. However, there is a lack of recent global or regional reported epidemiological data on other causes of cirrhosis, such as drugs, autoimmune liver disease, and metabolic disorders.
Additional profiles were also provided by some newer regional or organizational epidemiological studies (Table 1). A recent study from the United States reported summary statistics on the prevalence and disease burden of digestive diseases in a commercially insured adult population for the period 2016 to 2018. Of the total population, 7297435 (24%) individuals had a diagnosis of digestive disease, and the prevalence of nonalcoholic cirrhosis in the digestive disease population was 0.389% compared to 0.090% in the overall population[9]. Gu _et al_[10] evaluated all hospital admissions within the diagnosis-related group (diagnosis based on ICD-10-GM codes) in Germany from 2005 to 2018. A total of 248085936 admissions were recorded during this period, of which 2302171 admissions were diagnosed with cirrhosis, reflecting a prevalence of 0.94%[10]. A cross-sectional study conducted in Japan in 2020 randomly selected 6000 general citizens from 2 cities, and 488 individuals underwent fatty liver and advanced fibrosis screening. The prevalence of cirrhosis based on liver stiffness measurement (LSM) was 1%, with a markedly higher prevalence in men than in women (1.6% compared to 0.4%)[11]. Finally, a study using a commercial medical claims database yielded an adult prevalence of 0.21% for cirrhosis in 2018 (estimated at 536856 cases)[12].
Table 1.
Recent local epidemiological data on liver cirrhosis in the general population
| Ref. | Country | Study population | Study period | Diagnostic methods | Presented data |
|---|---|---|---|---|---|
| [9] | US | 7297435 patients with a GI diagnosis in a commercial insurance database | 2016-2018 | ICD-10 code | Nonalcoholic cirrhosis prevalence: 0.389%; average inpatient cost: 43733 dollars; annualized total costs: 53214 dollars |
| [10] | Germany | All hospital admissions (248085936 patients) | 2005-2018 | ICD-10 code | Prevalence: 0.94% |
| [11] | Japan | 488 randomly selected individuals underwent fatty liver and advanced fibrosis screening | From October to November 2020 | Liver stiffness measurement | Prevalence: 1% |
| [12] | US | Adult patients in a commercial medical claims database | 2018 | ICD-9 or ICD-10 code | Prevalence: 0.21% |
| [28] | China | 503993 participants prospectively included in China Kadoorie Biobank | 2004-2008 (10 years of follow-up) | ICD-10 code | Incidence: 756.4 and 397.4 per 100000 among diabetic patients and nondiabetic patients, respectively |
| [30] | Korea | Adult patients in the HIRA-NPS database | 2012-2016 | ICD-9 code | Alcoholic cirrhosis incident cases: 7295 cases |
| [31] | Sweden | All patients at Halland Hospital | 2011-2018 | ICD-10 code and SNOMED code | Age-standardized incidence: 23.2 per 100000 person-years |
| [32] | Korea | Adult patients in the NHIS database | 2011-2015 | KCD-7 code | Primary biliary cirrhosis average annual cumulative incidence: 68.32 cases per 10000000 |
| [33] | Canada | Adult patients in the ICES databases | 1997-2016 | ICD-10 code | Age-standardized incidence: 70.6 in 1997 and 89.6 per 100000 person-years in 2016 |
| [34] | Canada | Children in the ICES databases | 1997-2017 | ICES-validated algorithm | Age- and sex-adjusted incidence: 2.7 in 1997 and 10.6 per 100000 person-years in 2017 |
| [44] | US | NIS | 2003-2017 | ICD-10 code | Alcoholic cirrhosis deaths in women: 14330 cases |
| [45] | Mexico | National Institute of Statistics and Geography | 2000-2017 | ICD-10 code | Alcoholic cirrhosis mortality rate: From 20.55 to 10.62 per 100000 |
| [54] | US | Adult patients in the NIS | 2008-2014 | ICD-9 code | Hospitalization costs: 7.37 billion dollars in 2014 |
In specific at-risk populations, there were significant increases in the prevalence of cirrhosis (Table 2). In a 2017-2018 cross-sectional study using data from the National Health and Nutrition Examination Survey (NHANES), which included 825 United States adults with type 2 diabetes who had reliable transient elastography results, the prevalence of cirrhosis was 7.7% (95%CI 4.8%-11.9%)[13]. Another study also analyzed NHANES data from 2017-2018 and found a 4.4% prevalence of suspected cirrhosis among patients with fatty liver disease[14]. A recent study utilizing NHANES data from 2001 to 2018 included 3115 HBsAg-negative/HBcAb-positive subjects in which the prevalence of cirrhosis/advanced liver fibrosis based on FIB-4 diagnosis was 3.76% (95%CI 2.80%-4.72%), notably higher than in the general US population[15]. A systematic review and meta-analysis summarized the prevalence of cirrhosis in HBV-infected populations in sub-Saharan Africa. A total of 17 studies were included, including 22 cohorts from 13 countries (13 with HBV infection alone and 9 with HIV/HBV coinfection). The prevalence was 4.1% (95%CI 2.6%-6.4%) in primary care or the general population and significantly higher at 12.7% (95%CI 8.6%-18.3%) in referral or tertiary care settings, with no effect of HIV coinfection on cirrhosis[16]. In a recent Spanish population survey on the prevalence of NASH-related liver fibrosis, the prevalence of cirrhosis in the current biopsy-proven NASH cohort (a total of 501 patients from 2015-2020) was 0.70% (95%CI 0.10%-4.95%)[17]. A survey analyzing the Korea National Health and Nutrition Examination Survey (KNHANES) between 2015 and 2019 reported prevalence rates of cirrhosis in metabolically healthy obesity and metabolically unhealthy obesity populations of 0.5% and 0.4%, respectively[18]. In the TARGET-NASH study conducted from August 2016 to March 2019, researchers revealed that lean participants had a lower prevalence of cirrhosis (22.6% vs 40.2% of nonlean participants), with almost half of the lean subjects being Asian. Lean Asians were half as likely to develop NAFLD-related cirrhosis as nonlean individuals [odds ratio (OR) 0.47; 95%CI 0.29-0.77][19]. Furthermore, different diagnostic tools can have varied diagnostic accuracy and cost-effectiveness for cirrhosis. A cost-effectiveness study found high diagnostic accuracy and cost-effectiveness of fibrosis-4 (FIB-4), followed by either vibration-controlled transient elastography (VCTE), magnetic resonance elastography (MRE), or liver biopsy[20]. In terms of the combination of diagnostic tools, FIB-4 + VCTE was the least costly combination, whereas the incremental cost-effectiveness ratios (ICERs) for the combination of FIB-4 and MRE were lower than those for FIB-4 and liver biopsy[20]. The prevalence of cirrhosis in older patients with multiple comorbidities has recently been addressed. In a study that included a cohort of 6,193 elderly patients admitted to acute medical wards between 2010 and 2018, 315 patients (5%) were diagnosed with cirrhosis[21]. Finally, a pooled meta-analysis including 15 studies with a total of 320777 patients suggested that among those receiving dialysis, the prevalence of cirrhosis was 5% and associated with higher mortality, with further analysis showing that hepatitis B and C, rather than diabetes, contributed to the increased risk of cirrhosis[22].
Table 2.
Epidemiology of cirrhosis in specific at-risk populations
| Ref. | Country | Study population | Study period | Diagnostic methods | Presented data |
|---|---|---|---|---|---|
| [13] | US | 825 adults with type 2 diabetes who had reliable TE results from the NHANES | 2017-2018 | TE | Prevalence: 7.7% |
| [14] | US | Patients with NAFLD from the NHANES | 2017-2018 | TE | Prevalence: 4.4% |
| [15] | US | 3115 HBsAg-negative/HBcAb-positive subjects from the NHANES | 2001-2018 | FIB-4 | Cirrhosis/advanced liver fibrosis prevalence: 3.76% |
| [16] | 13 countries in sub-Saharan Africa | HBV-infected population | 2012-2019 | TE, APRI and Fibrotest | Prevalence: 4.1% in primary care or the general population and 12.7% in referral or tertiary care settings |
| [17] | Spain | 501 biopsy-proven NASH patients with paired TE data from tertiary centers | 2015-2020 | TE | Prevalence: 0.70% |
| [18] | Korea | 27629 adults with MHO or MUHO from the KNHANES | 2015-2019 | Self-report survey or by an AST level ≥ 23.5 IU/L | Prevalence: 0.5% and 0.4% in MHO and MUHO, respectively |
| [19] | US | 3386 patients with NAFLD in the TARGET-NASH study | 2016-2019 | Pragmatic case definitions | Prevalence: 22.6% in lean patients |
| [21] | Italy | 6193 older subjects admitted to acute medical wards and included in the REPOSI registry | 2010-2018 | ICD-9 code | Prevalence: 5% |
| [22] | 10 countries in the world | 320777 dialysis patients | 1980-2019 | TE, histopathology, radiology, and ICD codes | Prevalence: 5% |
| [36] | NR | 902 patients with a Fontan circulation | NR | NR | Cumulative incidence: 27.5% |
| [39] | Japan | 1260 patients who underwent the Fontan procedure and survived to discharge from 9 institutions | From before 2011 to 2021 (median10.2 of years follow-up) | Biopsy or imaging or extrahepatic features | Cumulative incidence at 10, 20, and 30 years after the Fontan procedure: 0.9%, 11.6%, and 25.7%, respectively |
| [41] | Italy | All adults aged 30 years or older without cirrhosis in Rome | From 2001 follow up to 2015 | A validated algorithm | Crude incidence rate: 67 per 100000 person-years |
Incidence
The global number of incident cases of cirrhosis and CLD in 2017 was 5.2 million according to the GBD 2017, although the incidence was not available[23]. However, the latest global incidence of NASH-associated cirrhosis has been mentioned in recent publications using data from the GBD 2017[24,25]. In 2017, the global incidence of cirrhosis due to NASH was 367780 cases, an increase of approximately 105.56% compared to 1990 (178430 cases in 1990). The age-standardized incidence rate (ASR) was 4.81 (95%UI 4.38-5.28) per 100000 population in 2017 compared to 3.31 (95%UI 3.02-3.63) per 100000 population in 1990, with an estimated annual percentage change (EAPC) of 1.35% (95%CI 1.28-1.42%). Gender and regional differences in incidence were also observed. The incidence was higher in males than in females [5.54 (5.06-6.07) compared to 4.08 (3.69-4.49) per 100000 population], although the EAPC was higher in females. The middle-high sociodemographic index (SDI) region featured the highest incidence, while that of the low SDI was the lowest [6.14 (5.60-6.70) compared to 2.72 (2.43-3.05) per 100000 population]. A more pronounced increase in the ASR was recorded in Eastern Europe, Andean Latin America, and Central Asia, while the Asia Pacific region showed a decline. In terms of HBV and HCV-associated cirrhosis, the overall incidence has shown a relatively encouraging trend both in men and women. Veracruz _et al_[26] exploited GBD statistics from 2010-2019 to analyze the global incidence and mortality trends in HBV and HCV infection, cirrhosis, and hepatocellular carcinoma (HCC) over this period. The worldwide incidence of HBV-related cirrhosis decreased by 15% from 5.78 (95%CI 4.3-7.3) in 2010 to 4.91 (95%CI 3.5-6.5) in 2019 per 100000 individuals, with the greatest reduction occurring in Eastern Europe at 36%. This trend may be related to widespread HBC vaccination[27]. However, the 2019 incidence of HCV-associated cirrhosis amounted to 6.7 (95%CI 5.0-8.6) per 100000 population, an increase of 5.6%, with the greatest increase of 27.8% in central sub-Saharan Africa and the greatest decrease of 13.5% in the high-income Asia-Pacific region.
The China Kadoorie Biobank prospectively included 512891 adults (210222 men and 302669 women) aged 30-79 years in 10 geographically disaggregated sites. During the 10-year follow-up period, 2082 cases of cirrhosis occurred in 503993 participants with an excluded history of CLD. The incidence of cirrhosis among diabetic patients was 756.4 per 100000 compared to 397.4 per 100000 among nondiabetic patients, and the Cox regression yielded a hazard ratio (HR) of 1.81 (95%CI 1.57-2.09) for cirrhosis among diabetic patients[28]. Another modeling study employed a multicohort state-transition (Markov) model to predict the epidemiological trends in alcohol-related liver disease in the US from 2019 to 2040[29]. In this model, researchers modeled prevalence and mortality trends of decompensated cirrhosis in three projection scenarios, including a status quo scenario (current trends continued), a moderate intervention scenario (high-risk alcohol intake receded to 2001 levels), and a strong intervention scenario (high-risk alcohol intake trends decreased by 3.5% per year). In the status quo scenario, the age-standardized incidence of alcohol consumption-related decompensated cirrhosis was projected to increase from 9.9 (95%CI 9.3-10.9) cases per 100000 person-years in 2019 to 17.5 (15.8-18.4) cases per 100000 person-years in 2040, which would be a 77% increase. In the moderate intervention scenario, the age-standardized incidence of alcohol drinking-related decompensated cirrhosis would be expected to increase by 69% to 16.7 (95%CI 14.2-16.4) cases per 100000 person-years in 2040. Conversely, the incidence of alcohol-related decompensated cirrhosis associated under the strong intervention scenario would decrease by 11% compared to 2019, which is encouraging. From 2019 to 2040, the cumulative incidence was projected to reach 1118200 cases (95%CI 1005400-1123500), 1067000 (943400-1084600) and 786400 cases (711200-819300) for the status quo, moderate and strong intervention scenarios, respectively, with the strong intervention scenario achieving a 30% reduction compared to the status quo scenario. In a large national cohort study conducted in Korea, the incidence of alcoholic cirrhosis showed an overall increase between 2012 and 2016, from 1463 cases in 2012 to 1530 cases in 2016[30]. A Swedish population-based cohort study including 310000 inhabitants analyzed epidemiological trends in the incidence, causes, and complications of cirrhosis in the last decade (2011-2018). The incidence of cirrhosis was assessed at 23.2 per 100000 person-years, with a higher rate of 30.5 in men and 16.4 in women[31]. Stratifying by age showed the highest incidence in the 60-69 age group, and alcohol was the leading cause of all cases (50.5%). In a study conducted in South Korea using the National Health Insurance Service database, trends in the incidence of rare diseases were explored for the period 2011-2015. The average annual cumulative incidence of primary biliary cirrhosis was 68.32 cases per 10000000 and was increasing at an annual trend of 6.32[32]. A retrospective study conducted in Ontario, Canada, used health data from the period 1997-2016 to determine the incidence of cirrhosis in young birth cohorts. During this period, 165979 cases of cirrhosis were diagnosed, with an increasing trend in age-standardized incidence from 70.6 in 1997 to 89.6 per 100000 person-years in 2016. The incidence was higher in the younger birth year cohort than in the middle-aged birth cohort and was more evident in women[33].
In pediatric populations, the incidence and current trends of cirrhosis have also been reported in the recent literature. A population-based study from Ontario, Canada, analyzed changes in the incidence of cirrhosis in children from 1997-2017[34]. Over the past two decades, 2966 new cases of cirrhosis were diagnosed in children (median age 9 years), and the age- and sex-adjusted incidence of cirrhosis increased significantly by nearly fourfold (from 2.7. in 1997 to 10.6 per 100000 person-years in 2017). Notably, the most marked increases were identified in infants < 1 year and adolescents > 11 years old. After the age-period-cohort study, the authors found that children born in 2010 had twice the risk of developing cirrhosis than those born in 2001. Dong _et al_[35] prospectively included 139 children with biopsy-proven cirrhosis (median age at initial diagnosis 2 years) from January 2010 to January 2020, 93 of whom had a definite cause. HBV infection was the most common cause (33.3%), followed by glycogen storage disease (17.2%) and Wilson disease (15.1%).
The incidence of cirrhosis has also been investigated in specific populations in recent studies. A recent meta-analysis pooled 14 studies including 902 patients with Fontan circulation and estimated the incidence of cirrhosis in this population[36]. Fontan circulation is characterized by an increase in central venous pressure, which in turn affects back-stream veins and can lead to congestive hepatopathy known as Fontan-related liver disease[37,38]. There were 241 patients with reported cirrhosis following a mean follow-up period of 17.9 ± 4.5 years, with a cumulative incidence of 27.5% (95%CI 16.9%-34.4%). Another updated study included 1250 patients (median age 3.6 years, 47.5% female) who underwent their first Fontan procedure, with cirrhosis diagnosed in 5.8% of patients over a median follow-up period of 10.2 years. The cumulative incidence of cirrhosis at 10, 20, and 30 years after Fontan surgery was 0.9%, 11.6%, and 25.7%, respectively[39]. The high incidence of cirrhosis in this population can be a substantial disease burden and therefore should be taken seriously. A nested case control study conducted in Taiwan found that the presence of diabetes and associated extrahepatic complications, such as hypertensive cardiovascular disease and chronic kidney disease, were associated with an increased incidence of treatment-naïve HCV-related cirrhosis[40], suggesting an important role for metabolic risk factors in the increased risk of developing cirrhosis. A nationwide cohort study including approximately 1.2 million people aged 30 years or older in Rome analyzed the association between long-term exposure to air pollution and the incidence of cirrhosis, of which 10111 cases occurred during a 14-year follow-up period, yielding a crude incidence of 67 per 100000 person-years[41]. Long-term exposure to PM10, PM coarse, PM2.5, and NO2 was associated with the incidence of cirrhosis.
Mortality
The most recent GBD 2019 describing global mortality from cirrhosis is available. A recent systematic analysis of the GBD 2019 highlighted that the total number of deaths from cirrhosis worldwide was 1.43 million in 2019, an increase of 8.1% compared to the number of deaths in 2017 according to the GBD 2017 (1323000 cases)[42]. In a recent report, the GBD 2019 assessed the health progress of subnational regions in Ethiopia in 2019. In 2019, the all-cause age-standardized mortality rate was 993.52 (95%UI 914.97-1070.55), and for cirrhosis and other CLDs, it was 52.18 (95%UI 44.17-62.07) per 100000 population[43].
In 2017, 31.5% of cirrhosis deaths among men were caused by HBV, 25.5% by HCV, 27.3% by alcoholic liver disease (ALD), 7.7% by NASH, and 8.0% by other factors[6]. Among women, the proportion of deaths from cirrhosis due to HBV, HCV, ALD, NASH, and other causes was 24.0%, 26.7%, 20.6%, 11.3%, and 17.3%, respectively[6]. Deaths from hepatitis B-related cirrhosis were 321000 in 2019, representing 22% of all cirrhosis deaths. In 2017, the number of associated deaths was 384000 (29%), which indicated a 16.4% decrease in the mortality rate[42]. A previous study investigated trends in the incidence and mortality of acute infections, cirrhosis, and HCC by exploring the GBD for HBV and HCV from 2010-2019[26]. The 2019 global mortality rate for HBV-associated cirrhosis was 4.03 (95%CI 3.39-4.76) per 100000 population, showing a 23.2% reduction over this decade. The highest death rate for cirrhosis due to HBV infection was recorded in western sub-Saharan Africa at 16.49 (95%CI 12.69-21.35), while the lowest was seen in high-income North America at 0.35 (95%CI 0.29-0.42). The largest reduction in mortality compared to 2010 was in East Asia at 46.5%. The global mortality rate for HCV-related cirrhosis was 4.82 (95%CI 4.09-5.57) per 100000 in 2019, a 7.4% decrease compared to 2010. The highest mortality rate was 15.4 (95%CI 12.52-19.04) in Eastern sub-Saharan Africa, and the lowest was 1.79 (95%CI 1.41-2.25) per 100000 population in Western Europe. The greatest decrease of 23.9% was seen in the high-income Asia Pacific region, although several regions, such as the Caribbean and high-income North America, experienced an upward trend. In 2017, the global age-standardized mortality rate for ALD was 6.2 (5.7-6.9) and 2.1 (1.9-2.6) per 100000 for men and women, respectively[6]. The estimated number of deaths due to NASH cirrhosis worldwide in 2017 was 118,030, an increase of 90.7% compared to 1990, with an age-standardized death rate of 1.5 (1.3-1.6) per 100000 population, which was not significantly different compared to that of 1990[25].
The trend in the in-hospital burden of ALD among women was analyzed using data from the 2003 to 2017 National Inpatient Sample (NIS). In 2017, there were 14330 deaths from alcoholic cirrhosis (2.05% of all deaths), a relative decrease of 21.27% compared to 2003, although deaths from alcoholic hepatitis have increased rapidly[44]. Another study analyzed trends in mortality from alcohol-related cirrhosis in Mexico from 2000-2017 and found a decrease in mortality in all age groups, with the associated mortality rate falling from 20.55 to 10.62 per 100000 for all populations during this period[45]. However, there has been a rapid increase in alcohol consumption in the United States and other regions in recent years, and consequently, the mortality rate from ALD has shown a marked increase[46-49]. A study included underlying cause of death public-use data from January 1, 2017, to December 31, 2020, a dataset that contains death data for all United States citizens. Age-adjusted mortality from ALD increased from 13.1 (95%CI 12.9-13.3) to 16.9 (16.7-17.1) in men and 5.6 (5.4-5.7) to 7.7 (7.6-7.9) per 100000 in women[50].
Public health burden
In the latest analysis of the burden of 369 diseases and injuries in 204 countries and territories, the percentage of disability-adjusted life-years (DALYs) for cirrhosis and CLD at all ages in 2019 was 1.8 (95%UI 1.6-2.0), ranking 16th. The percentage increase in the number of DALYs compared to 1990 was 33.0 (22.4-48.2), while the age-standardized DALYs decreased by 26.8%[51]. In the age-stratified analysis, DALYs for cirrhosis in 2019 were ranked 12th at 2.8% of all diseases and injuries among individuals aged 25-49 years, 7th at 2.7% among individuals aged 50-74 years, and 19th at 1.1% among individuals aged 75 or older. Another recent study analyzed the impact of HBV and HCV infections on DALYs using data from the GBD 2010-2019. The 2019 DALYs for HBV cirrhosis decreased by 23% from 168.6 (95%CI 146.9-191.3) in 2010 to 129.8 (108.3-153.0), while the DALYs for HCV cirrhosis decreased by 8.2% to 146.2 (124.4-169.8) compared to that in 2010[52]. In 2019, HCV infection, alcohol, and HBV infection-related etiology were the most predominant sources of DALYs for cirrhosis, with prevalences of 26%, 24%, and 23%, respectively, and NAFLD contributed a relatively small proportion (8%) but showed a rapidly increasing trend[53]. In poorer countries, DALYs were higher, and cirrhosis due to HBV was the main source, whereas in wealthier countries, HCV and alcohol were the primary contributors. DALYs due to NAFLD cirrhosis are expected to become mainstream in the future in parallel with the epidemic of diabetes and obesity. Furthermore, the authors critically highlighted the current underestimation of the disease burden of cirrhosis (as compensated cirrhosis is currently considered no disability and decompensated cirrhosis only mild disability)[53].
The financial burden associated with hospitalized patients with cirrhosis was addressed in a recent study using data from the 2008-2014 NIS. Hospitalization costs for cirrhosis increased by 30.2% to $7.37 billion over the study period. Admissions for compensated and decompensated cirrhosis increased by 24% and 36%, respectively, while noncirrhotic populations dropped by 7.7%. The median length of stay (LOS) in the hospital was longer for cirrhosis than for other diseases. Implementing mechanical ventilation and complications associated with cirrhosis were the main drivers of the increased costs. More specifically, mechanical ventilation increased costs by 15%-152% in hospitalized patients with cirrhosis, and infection and nonhypertensive gastrointestinal bleeding led to increased costs in patients with compensated cirrhosis, while renal and infectious events were contributors to decompensated cirrhosis[54]. Jepsen _et al_[53] suggested that although the prevalence of cirrhosis was increasing, it would be simplistic to assume that costs for patients with cirrhosis were increasing, as the treatment currently provided for cirrhotic patients may be less or cheaper than before. However, the cost of NAFLD-related cirrhosis will likely continue to rise until an effective treatment becomes available[55].
EPIDEMIOLOGY OF MAJOR COMPLICATIONS
Ascites and ascites infection
Ascites is the most common complication in patients with cirrhosis and is defined as pathological fluid accumulation in the peritoneal cavity[56]. Ascites occurs only in the presence of portal hypertension, and approximately 75% of the occurrence of ascites is due to cirrhosis and portal hypertension[56,57]. The pathophysiological mechanisms may involve a complex interaction of the endogenous vasoactive system, portal hypertension, and renal dysfunction[58]. As a hallmark of decompensation, ascites has a prevalence of approximately 10% in patients with cirrhosis[58]. Approximately 60% of patients with compensated cirrhosis can develop this complication over a 10-year period, and it is associated with a high mortality rate of up to 50% within 3 years of onset[59,60]. A recent population-based study analyzed the epidemiology of ascites infection among patients with cirrhosis in Queensland, Australia, from 2008-2017. Of 103165 patients with cirrhosis, 16550 had ascites (16%)[61]. A further Korean study using a nationally representative database yielded a real-world burden of complications in patients with decompensated cirrhosis from 2016 to 2018, with ascites being the most common decompensated event (54.8%), followed by GEV bleeding, HE and hepatorenal syndrome (HRS)[62]. However, recent epidemiological information related to ascites in cirrhosis is relatively scarce.
Ascites infection is a frequent concurrent event in patients with cirrhosis and ascites, such as the prevalent spontaneous bacterial peritonitis (SBP) and, less commonly, fungal infections[63,64]. SBP is defined as spontaneous ascites infection in the absence of other causes of secondary peritoneal infection[65]. The diagnosis is based on the presence of > 250 polymorphonuclear cells/mm3 in the ascites fluid as the high negative culture rate (up to 60% has been reported)[66,67]. Admissions for ascites infections increased by 76% in Queensland, Australia, from 2008 to 2017[61]. Another recent retrospective study included 1035 patients with cirrhosis from a single center in Israel between 1996 and 2020. A total of 173 (16.7%) of the patients developed SBP, and positive ascites fluid cultures were demonstrated in 47.4% of the SBP cases[68]. A recent meta-analysis including 99 studies comprising 5861142 patients with cirrhosis summarized the prevalence, resistance, and outcomes of SBP in cirrhosis worldwide[69]. The pooled global prevalence of SBP was 17.12% (95%CI 13.63%-21.30%), with Africa having the highest prevalence (68.20%) and North America having the lowest (10.81%). The prevalence of community-acquired SBP was 6.05% (95%CI 4.32%-8.40%) compared with 11.11% (95%CI 5.84%-20.11%) for health care-related SBP. The prevalence of antibiotic-resistant microorganisms in SBP was 11.77% (95%CI 7.63%-17.73%), with methicillin-resistant Staphylococcus aureus (6.23%), broad-spectrum β-lactamase-producing microorganisms (6.19%) and vancomycin-resistant enterococci (1.91%) being predominant. The incidence of SBP in outpatient paracentesis among patients with asymptomatic cirrhosis was estimated at 2% (95%CI 1%-3%) in a recent meta-analysis that included 16 studies with 1532 patients[70]. The global pooled mortality rate for SBP was 30.61% (23.30%-39.06%), with in-hospital, 30-d and 90-d mortality rates of 23.38%, 25.64% and 37.64%, respectively[69].
Variceal bleeding
Varices can be observed in up to two-thirds of patients with cirrhosis, while the annual incidence rate is 8%-10% and the rate of progression to large varices is 10%-12% annually[71]. Variceal bleeding is a common complication in cirrhosis associated with high mortality, with portal hypertension being the major driver. The common forms of variceal bleeding are esophageal and gastric variceal bleeding and, less commonly, rectal variceal bleeding. GEV bleeding can be present in 25%-35% of patients, which can develop in 40% of compensated cirrhosis patients and 85% of decompensated cirrhosis patients[72]. The six-week mortality rate for acute variceal bleeding ranges from 15% to 25%[73]. In a study that included 1902 children younger than 18 years who suffered esophageal variceal bleeding in 50 hospitals in the US from 2004-2019, the mortality rate for variceal bleeding was 7.3% (increasing to 8.8% after 6 wk) and 20.1% for any cause[74]. A retrospective study enrolled all patients in the NIS from 2016-2019 who were discharged with a diagnosis of esophageal variceal bleeding (166760 cases, of which 32.7% were women), and found that males were associated with a higher mortality rate than females (9.91% compared to 8.31%, P value 0.008 after adjusting for confounders)[75]. However, there are relatively few relevant recent epidemiological reports.
HE
HE is a neuropsychiatric disorder in cirrhosis that is strongly associated with prognosis, and its clinical course can be divided into covert hepatic encephalopathy (CHE), which includes minimal hepatic encephalopathy (MHE) (cognitive deficits found on psychological tests) and Grade I HE, and overt hepatic encephalopathy (OHE), where clinically significant symptoms develop[76,77]. The median survival of patients with cirrhosis is significantly shorter at 0.95 years in those over 65 years after the diagnosis of HE was established[78].
The prevalence of CHE has been reported to be very high in patients with cirrhosis, but estimates vary considerably among studies depending on, for example, the diagnostic method and the severity of cirrhosis[79]. In a prospective multicenter study, the prevalence of MHE under the combined diagnostic criteria based on the critical flicker frequency (CFF) and Psychometric Hepatic Encephalopathy Score (PHES) was 18.2%, with 12.1% of patients having compensated cirrhosis and 22.5% of patients showing decompensated cirrhosis[80]. Another multicenter study validated the ability of the EncephalApp in diagnosing MHE. The prevalence of MHE was 51% for the norm-based EncephalApp, 37% for the PHES-based EncephalApp, and 54% for the inhibitory control test (ICT)-based EncephalApp[81]. In a recent study conducted in Turkey, the prevalence of MHE in compensated cirrhosis patients based on the PHES, CFF, and a combination of both was 29.8%, 27.4%, and 16.0%, respectively[82]. An attempt was made to examine the effect of single and combined diagnostic modalities in CHE. The prevalence of CHE varied among the different diagnostic sets, with rates of 18%, 25%, 29%, 35%, 37% and 54% for the PHES + ICT, ICT + Stroop EncephAlapp (StE), PHES + StE, ICT, PHES, and StE, respectively[83]. In addition, the underestimation of the burden of HE and other factors that may be regionally variable, such as smoking, diabetes, and alcohol intake, can impact the diagnosis of CHE[79], all contributing to the significant variability in the prevalence of CHE.
The incidence of OHE has also been described recently. A prospective study included 294 patients with Child A-B cirrhosis without previous HE from July 2016 to August 2018, with the incidence of OHE at one year being 14% in all patients, 10% in Child A patients, and increased to 25% in Child B patients[84]. A large population-based study included a randomized 20% of Medicare participants with cirrhosis and Part D prescription coverage from 2008-2014, with a total OHE incidence of 11.6 per 100 patient-years over a 5.25-year follow-up of 166,192 patients with cirrhosis (median age 65 years). Alcoholic cirrhosis and portal hypertension are key players in the development of OHE, and drug use, such as proton pump inhibitors (PPIs), benzodiazepines, gamma-aminobutyric acid and opioids, is also potentially relevant[85]. These findings indicate that other components may also be associated with the development of HE and influence the incidence. In fact, several factors, such as transjugular intrahepatic portosystemic shunts (TIPSs)[86], PPIs[87], albumin[88], sustained virological response (SVR) in HCV infection[89], and others, can contribute to the development of HE.
HE imposes a heavy burden on patients with cirrhosis, including increased hospitalization, costs, and readmissions, impairment of health-related quality of life (HRQOL), and decreased socioeconomic status[90]. During 2010-2014, data from the NIS show a 24.4% increase in the number of hospital admissions for HE and a 46.0% increase in the total cost of admissions (which reached $11.9 billion in the United States in 2014)[91]. HE-related 90-d readmissions comprised approximately 23.7% of patients with cirrhosis[92] and were significantly associated with readmission in patients with decompensated cirrhosis[93]. In a large multistate population-based study on the causes and rates of readmission in cirrhosis, HE was significantly correlated with both 30-d readmission and 90-d readmission, with adjusted ORs of 3.23 (95%CI 2.97-3.52) and 3.07 (2.86-3.30), respectively[94]. Moreover, HE is associated with an increased risk of falls and can cause serious outcomes leading to high comorbidity and mortality[95]. In socioeconomic terms, cognitive impairment due to HE has been shown to have a multilevel association with adverse outcomes of employment/income, driving ability, and HRQOL[79].
Acute kidney injury and HRS
Renal dysfunction is a common complication in patients with cirrhosis and ascites[96]. In 2015, the revised consensus of the International Club of Ascites defined acute kidney injury (AKI) in cirrhosis as an increase in serum creatinine (sCr) of 0.3 mg/dL in < 48 h or a 50% increase in sCr from baseline within the last 3 mo[97]. AKI comprises a variety of phenotypes, including functional AKI and structural AKI. Functional AKI includes volume-responsive prerenal azotemia (PRA) and HRS-AKI, while structural AKI presents with structural changes such as acute tubular necrosis (ATN). HRS-AKI (previously known as HRS-1) is defined as at least stage 2 or above AKI in patients with cirrhosis and ascites, while excluding other causes such as PRA and ATN[97]. HRS can thus be divided into HRS-AKI and HRS-non-AKI (previous HRS-2)[98].
In a prospective study of 405 patients with cirrhosis enrolled in 2016-2017, the prevalence of AKI was 19.3%, and survival was lower at 30 d and 90 d compared to that of non-AKI patients[99]. The prevalence of AKI ranges from 18.5% to 40.6% in some other regions[100-102]. A meta-analysis revealed that the prevalence of AKI in acute-on-chronic liver failure (ACLF) could be significantly increased to 41%[103]. The overall prevalence rates of PRA and ATN in patients with cirrhosis are 15%-45% and 15%-60%, respectively, which are higher than the 10-40% rate of HRS[104]. The prevalence of HRS in patients with decompensated cirrhosis was 3.6%, while the median LOS for HRS was 4 wk per year in a large representative Korean database from 2016-2018, significantly higher than that for patients with ascites (19 d) or GEV bleeding (13 d)[62]. A recent study that included patients with a primary diagnosis of HRS in the NIS from 2008-2018 found a notable increase in the number of HRS hospitalizations from 22864 in 2008 to 42985 in 2018; however, there was a decreasing trend in inpatient mortality (36.2% in 2008 to 25.7% in 2018)[105].
Infection
In addition to SBP, patients with cirrhosis are at substantially increased risk of developing infections, commonly urinary tract infections (UTIs), pneumonia, and soft tissue infections[106]. The most frequent types of infections in a study that included 877 hospitalized cirrhotic patients from 2011-2016 were UTI (33%), pneumonia (23%), SBP (14%), and bacteremia (11%)[107]. Using the Nationwide Readmissions Database from 2011-2014, the overall prevalence of infections was 29.2% in 1798830 admissions, including UTI (13.7%), pneumonia (8.9%), cellulitis (5.2%), Clostridioides difficile infection (CDI) (2.8%), and SBP (2.0%). Pneumonia, SBP, and CDI had notably higher mortality than cellulitis and UTI, and sepsis and organ failure were also more common. Pneumonia had the highest mortality in the multivariate regression analysis (OR 2.73, 95%CI 2.68-2.80) and caused multiple organ failure (OR 3.59, 95%CI 3.50-3.68)[108]. The prevalence of CDI in cirrhosis has shown an increasing trend at approximately 2.7% in 2014, while the mortality of CDI is on the decline, and in local hospitals, the incidence of CDI ranges from 4.9% to 18.8%[109]. In recent years, infections caused by multidrug-resistant organisms (MDRO) have posed a serious challenge in cirrhosis[110]. In a study conducted in Europe that prospectively included two series of cohorts of patients with decompensated cirrhosis in 2011 and 2017-2018, the prevalence of MDRO in culture-positive infections increased from 29.2% in 2011 to 38.0% in 2017-2018[111]. Another worldwide study enrolled 1302 patients with cirrhosis and infections at 46 centers (15 in Asia, 15 in Europe, 11 in South America, and 5 in North America) in 2015-2016 and found a 34% prevalence of MDROs with geographic variability (highest in Asia)[112].
EPIDEMIOLOGY OF HCC IN LIVER CIRRHOSIS
Primary liver cancer was the sixth most common and the third most deadly cancer in 2020, with HCC being the predominant phenotype[113]. According to the GBD 2019, the global age-standardized incidence rate, age-standardized mortality rate and age-standardized DALYs for liver cancer in 2019 were 6.51, 5.95, and 151.08 per 100000, respectively[114]. NASH is the fastest growing cause of liver cancer and is projected to continue to increase in the future[115]. In 2019, the most common contributing factor for liver cancer was hepatitis B (41%), followed by hepatitis C (28.5%), alcohol use (18.4%), NASH (6.8%) and other etiologies (5.3%)[115,116]. Cirrhosis is a precancerous lesion that predisposes patients to progressing to HCC. However, HCC can develop directly without the presence of cirrhosis in a proportion of individuals. In a large US multicenter study, 11.7% of 5,144 included HCC patients showed the absence of cirrhosis, with NAFLD (26.3%), HCV (12.1%) and HBV (10%) being the most common causes[117]. A recent meta-analysis concluded that 37% (95%CI 28%-46%) of patients with NAFLD-related HCC presented without cirrhosis[118]. The prevalence of NAFLD-related HCC was significantly higher in patients with cirrhosis than in those without (374.4/10000 vs 4.6/10000 persons)[119].
The epidemiology of HCC in patients with cirrhosis has recently been studied and is etiologically variable (Table 3). In a recent Swedish nationwide population-based cohort study, the incidence of HCC in the cirrhotic population was 23 per 1000 person-years (lowest in ALD at 15 per 1000 person-years and highest in viral hepatitis at 41 per 1000 person-years)[120]. The cumulative incidence of HCC in patients with cirrhosis at 5 and 10 years was 8.3% and 12.2%, respectively. At 10 years, the cumulative incidence was lowest in women with alcoholic cirrhosis (4.3%) and highest in men with viral hepatitis (26.6%)[120]. A study included two US prospective multiethnic contemporary cohorts of patients with cirrhosis, with a total enrolled population of 2733 patients with cirrhosis (19.0% had active HCV, 23.3% had cured HCV, 16.1% had ALD, and 30.1% had NAFLD). After 7,406 person-years of follow-up, the annual HCC incidence rate was 1.82%. The annual HCC incidence in patients with cured HCV, ALD and NAFLD was 1.71%, 1.32%, and 1.24%, respectively. The risk of developing HCC in patients with cured HCV cirrhosis was two-fold higher than that in patients with NAFLD (HR 2.04, 95%CI 1.24-3.35)[121]. Data on the mortality and public health burden of HCC in patients with cirrhosis are relatively scarce.
Table 3.
Epidemiology of hepatocellular carcinoma in patients with cirrhosis
| Ref. | Country | Study population | Study period | Study design | Epidemiology |
|---|---|---|---|---|---|
| [119] | US | 392800 NAFLD patients from 26 major integrated US healthcare systems | 2015-2020 | Retrospective cohort study | Prevalence: 374.4/10000 persons |
| [120] | Sweden | 15215 individuals with cirrhosis in the National Outpatient Register | 2001-2016 | Nationwide population-based cohort study | Incidence rate: 23 per 1000 person-years; cumulative incidence: 8.3% at 5 years and 12.2% (4.3% in women with alcoholic cirrhosis and 26.6% in men with viral hepatitis) at 10 years |
| [121] | US | 2733 patients with cirrhosis in two contemporary prospective multiethnic cohorts | 2016-2020 (with follow-up until June 30, 2021) | Prospective multiethnic cohort study | Annual incidence: 1.82% (1.71%, 1.32%, and 1.24% in cured HCV, ALD and NAFLD, respectively) |
| [122] | NA | 29444 patients with HCV cure | NA | Meta-analysis | Incidence: 2.1 per 100 person-years |
| [123] | China | 937 treatment-naïve adults with compensated HBV-induced cirrhosis | 2012-2015 (with follow-up until June 30, 2019) | Prospective cohort study | Cumulative incidence: 7.4% at 5 years |
| [124] | Korea | 359 patients with HBV-associated cirrhosis who were treated with ETV for at least 2 years | 2007-2012 (median follow-up of 82 mo) | Retrospective cohort study | Cumulative incidence: 4.7%, 15.9%, 21.8% and 32.9% at 3, 5, 7 and 9 years, respectively |
| [125] | US | 501 veterans with PBC and compensated cirrhosis | 2008-2016 (with follow-up until December 31, 2019) | Retrospective cohort study | Incidence: 0.6 and 0.7 person-years in UDCA responders and UDCA partial responders, respectively |
| [126] | US | 532 patients with PBC and compensated cirrhosis | 2008-2016 (with follow-up until June 30, 2020) | Retrospective cohort study | Incidence: 0.9 and 0.3 person-years in males and females, respectively |
| [129] | NA | 148333 patients with alcoholic cirrhosis | NA | Meta-analysis | Cumulative incidence: 1%, 2%, 3%, and 9% at 1, 3, 5, and 10 years, respectively |
| [131] | China | 1095 patients with decompensated cirrhosis | 2014-2019 | Retrospective cohort study | Incidence: 3.92% in alcoholic cirrhosis |
| [132] | China | 1515 patients with cirrhosis with alcoholism or/and HBV infection | 2005-2020 (with follow-up until June 30, 2021) | Retrospective cohort study | Annual incidence: 3.5% (5.9%, 3.6%, and 2.9% in HBV plus alcoholism, HBV only and alcoholism only patients, respectively) |
In a recent meta-analysis of patients with cured HCV, the incidence of HCC was 2.1 per 100 person-years and declined over time after the patients was cured[122]. A prospective study yielded a cumulative incidence of HCC of 7.4% at 5 years in patients with HBV cirrhosis receiving antiviral therapy[123], and partial virological response after two years of entecavir treatment was associated with an increased risk of HCC[124].
In a retrospective study that included 501 patients with primary biliary cholangitis and compensated cirrhosis, a total of 22 cases of HCC occurred during the study period (4.39%)[125], which is similar to the findings of another study (4.51%)[126]. In patients with primary sclerosing cholangitis and cirrhosis, the risk of HCC development is very low, although the risk of gallbladder cancer and cholangiocarcinoma is high[127].
The absolute risk of developing HCC in alcoholic cirrhosis seems to be lower than in viral hepatitis (annual incidence of approximately 2%-5%)[128]. A recent meta-analysis that included 18 studies outlined the incidence of HCC in alcoholic cirrhosis. After accounting for the competing risk of death without HCC, the cumulative incidence of HCC at 1, 3, 5, and 10 years was 1%, 2%, 3%, and 9%, respectively. The overall incidence of HCC in alcoholic cirrhosis was 8.29 (95%CI 4.77-14.39) per 1000 person-years[129]. However, the prognosis for HCC due to alcoholic cirrhosis appears to be worse[130]. Furthermore, alcohol consumption increases the incidence of HCC in HBV-related cirrhosis[131-133], while abstinence from alcohol significantly reduces the risk of developing HCC[133].
In a nationwide survey conducted in Japan, HCV-associated cirrhosis was the leading cause of HCC (60.3% of cases). The proportion of HCC from 2008 to 2016 due to hepatitis virus-related cirrhosis decreased, while HCC due to NASH and ALD-related cirrhosis increased from 1.5 to 7.2% and 8.5 to 18.6%, respectively[134].
The value of HCC surveillance in patients with cirrhosis remains to be addressed given the lack of sufficient randomized controlled trials to confirm the overall benefits and harms[135]. However, recent studies have provided robust evidence of the significance of HCC screening. A recent meta-analysis that included 59 cohort studies concluded that HCC surveillance was associated with improved early detection, curative treatment receipts and survival, although few studies weighed the benefits against the harms[136]. In another prospective cohort of patients with cirrhosis, HCC surveillance improved early detection, with physical damage observed in 8.8% of patients and mostly mild[137]. Furthermore, a survey performed in patients with cirrhosis found that patients were more concerned about early HCC detection than about potential surveillance harm[138]. A survey conducted in the United States showed that gastroenterology and hepatology providers also prefer HCC surveillance when the risk of HCC is below the threshold recommended for surveillance by professional societies[139].
Ultrasound with or without alpha-fetoprotein (AFP) is recommended for HCC surveillance, and the addition of AFP to ultrasound significantly increases the sensitivity of early HCC detection[140]. Clinical HCC surveillance is still underused in patients with cirrhosis. A meta-analysis noted that only 24% of patients were screened, and this underutilization occurred particularly in patients with alcohol- or NASH-related cirrhosis and those not followed in subspecialty gastroenterology clinics[141]. In a United States nationwide cohort of patients with cirrhosis, only 8.78% of patients were under surveillance for HCC[142]. A retrospective multicenter cohort study found that the main reason for barriers to surveillance was lack of surveillance orders or nonadherence[143]. Another United States survey identified patient-reported barriers to surveillance as knowledge deficits about HCC surveillance, cost, difficulty scheduling and transportation[144]. Individualized predictive modeling for risk stratification in patients with cirrhosis can facilitate and improve the cost-effectiveness of surveillance[145,146].
FUTURE DIRECTIONS
In the early 2020s, the outbreak and subsequent epidemic of coronavirus disease 2019 (COVID-19) imposed heavy and multifaceted consequences globally[147]. The effect of COVID-19 on cirrhosis has also been extensively researched. COVID-19 infection is associated with significantly higher morbidity and mortality in patients with liver cirrhosis[148-151]. The COVID-19 epidemic may also have implications for the etiology of cirrhosis. The prevalence of COVID-19 promotes alcohol consumption and is associated with liver disease[152-155] and metabolic disorders[156]. Therefore, the newer epidemiology of cirrhosis may change due to the COVID-19 epidemic.
Alcohol consumption and NAFLD-induced liver disease are growing rapidly. A nationwide study in the United States showed that the charges of alcoholic cirrhosis exceeded the cost of other causes of cirrhosis combined[157]. NAFLD and ALD-related cirrhosis will account for almost all newly diagnosed cases in Canada by 2040[158]. Alcohol intake can influence cirrhosis of any etiology[133,159-161]. Therefore, effective measures to prevent and reduce the associated contributing factors will likely help mitigate the epidemic. One study found that alcohol control policies can have a significant and immediate effect on mortality from cirrhosis[162]. Alcohol abstinence reduced HCC due to alcoholic cirrhosis, although only in patients without previous decompensated disease[163]. NAFLD is emerging as another major epidemic due to the prevalence of metabolic disorders such as obesity and diabetes, and there is currently no effective treatment for NAFLD. Cirrhosis due to NAFLD is expected to be a major component in the future, representing a shift in the associated epidemiology. Therefore, utilization of available interventions such as weight loss and available medications to minimize the progression of NAFLD and the detection of early liver fibrosis using effective and accurate tools will be instrumental in mitigating the risk of cirrhosis.
CONCLUSION
The latest epidemiological data revealed the heavy burden of cirrhosis globally (Table 4). In 2017, the age-standardized global prevalence of compensated cirrhosis was 1395.0 per 100000, compared to 132.5 per 100000 for decompensated cirrhosis. In 2019, cirrhosis caused 1.48 million deaths worldwide, an increase of 8.1% compared to 2017. In 2019, liver cirrhosis ranked 16th among all diseases for DALYs. The burden of cirrhosis due to HBV and HCV is declining, while the burden of NAFLD and alcohol consumption is mounting. Furthermore, there is currently a changing epidemiology of the major complications of cirrhosis (Figure 1). The burden of HCC in patients with cirrhosis is etiologically variable, and HCC due to NASH and alcohol intake is increasing.
Table 4.
Latest global epidemiological features of cirrhosis
| Ref. | Epidemiological figures | Latest research | Type or etiology of cirrhosis | Reported data |
|---|---|---|---|---|
| Prevalence | ||||
| [6] | Age-standardized prevalence | GBD 2017 | Cirrhosis | Compensated cirrhosis: 1395.0 (1323.5-1470.5); decompensated cirrhosis: 132.5 (128.6-136.2) per 100000 |
| [6] | Age-standardized prevalence | GBD 2017 | HBV-related cirrhosis | Compensated cirrhosis: 451.9 (420.0-485.9); decompensated cirrhosis: 36.6 (34.7-38.4) |
| [6] | Age-standardized prevalence | GBD 2017 | HCV-related cirrhosis | Compensated cirrhosis: 341.1 (314.1-368.7); decompensated cirrhosis: 32.5 (30.6-34.5) |
| [6] | Age-standardized prevalence | GBD 2017 | Alcohol-related cirrhosis | Compensated cirrhosis: 288.1 (267.5-311.3); decompensated cirrhosis: 30.0 (28.2-31.8) |
| [6] | Age-standardized prevalence | GBD 2017 | NASH-related cirrhosis | Compensated cirrhosis: 115.5 (105.0-126.5); decompensated cirrhosis: 11.3 (10.4-12.1) |
| Incidence | ||||
| [24] | Age-standardized incidence | GBD 2017 | NASH-related cirrhosis | 4.81 (4.38-5.28) |
| [26] | Age-standardized incidence | GBD 2019 | HBV-related cirrhosis | 4.91 (3.50-6.50) |
| [26] | Age-standardized incidence | GBD 2019 | HCV-related cirrhosis | 6.7 (5.0-8.6) |
| Mortality | ||||
| [26] | Age-standardized Mortality | GBD 2019 | HBV-related cirrhosis | 4.03 (3.39-4.76) |
| [26] | Age-standardized Mortality | GBD 2019 | HCV-related cirrhosis | 4.82 (4.09-5.57) |
| [25] | Age-standardized mortality | GBD 2017 | NASH-related cirrhosis | 1.5 (1.3-1.6) |
| Public health burden | ||||
| [52] | DALYs | GBD 2019 | HBV-related cirrhosis | 129.8 (95%CI 108.3-153.0) |
| [52] | DALYs | GBD 2019 | HCV-related cirrhosis | 146.2 (124.4-169.8) |
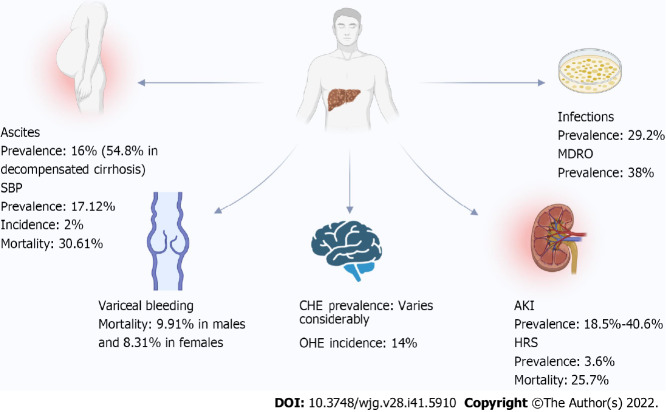
Figure 1.
The latest epidemiological data on the major complications of liver cirrhosis. The prevalence of covert hepatic encephalopathy depends on the means of diagnosis, the stage of cirrhosis, the underestimation of HE, and the presence of other factors affecting the prevalence. For the prevalence of infections, these data were obtained from the Nationwide Readmissions Database; therefore, the total population included readmissions. SBP: Spontaneous bacterial peritonitis; CHE: Covert hepatic encephalopathy; OHE: Overt hepatic encephalopathy; AKI: Acute kidney injury; HRS: Hepatorenal syndrome; MDRO: Multidrug-resistant organisms.
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interest in this study.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: August 27, 2022
First decision: September 25, 2022
Article in press: October 19, 2022
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kosmalski M, Poland; Metawea MI, Egypt; Sandoval C, Chile S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
Contributor Information
Yuan-Bin Liu, Department of Gastroenterology, Renmin Hospital of Wuhan University, Wuhan 430000, Hubei Province, China.
Ming-Kai Chen, Department of Gastroenterology, Renmin Hospital of Wuhan University, Wuhan 430000, Hubei Province, China. kaimingchen@163.com.
References
- 1.Kisseleva T, Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. 2021;18:151–166. doi: 10.1038/s41575-020-00372-7. [DOI] [PubMed] [Google Scholar]
- 2.Campana L, Esser H, Huch M, Forbes S. Liver regeneration and inflammation: from fundamental science to clinical applications. Nat Rev Mol Cell Biol. 2021;22:608–624. doi: 10.1038/s41580-021-00373-7. [DOI] [PubMed] [Google Scholar]
- 3.D'Amico G, Morabito A, D'Amico M, Pasta L, Malizia G, Rebora P, Valsecchi MG. Clinical states of cirrhosis and competing risks. J Hepatol. 2018;68:563–576. doi: 10.1016/j.jhep.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 4.Moon AM, Singal AG, Tapper EB. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clin Gastroenterol Hepatol. 2020;18:2650–2666. doi: 10.1016/j.cgh.2019.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398:1359–1376. doi: 10.1016/S0140-6736(21)01374-X. [DOI] [PubMed] [Google Scholar]
- 6.GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:245–266. doi: 10.1016/S2468-1253(19)30349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhai M, Long J, Liu S, Liu C, Li L, Yang L, Li Y, Shu B. The burden of liver cirrhosis and underlying etiologies: results from the global burden of disease study 2017. Aging (Albany NY) 2021;13:279–300. doi: 10.18632/aging.104127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alberts CJ, Clifford GM, Georges D, Negro F, Lesi OA, Hutin YJ, de Martel C. Worldwide prevalence of hepatitis B virus and hepatitis C virus among patients with cirrhosis at country, region, and global levels: a systematic review. Lancet Gastroenterol Hepatol. 2022;7:724–735. doi: 10.1016/S2468-1253(22)00050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathews SC, Izmailyan S, Brito FA, Yamal JM, Mikhail O, Revere FL. Prevalence and Financial Burden of Digestive Diseases in a Commercially Insured Population. Clin Gastroenterol Hepatol. 2022;20:1480–1487.e7. doi: 10.1016/j.cgh.2021.06.047. [DOI] [PubMed] [Google Scholar]
- 10.Gu W, Hortlik H, Erasmus HP, Schaaf L, Zeleke Y, Uschner FE, Ferstl P, Schulz M, Peiffer KH, Queck A, Sauerbruch T, Brol MJ, Rohde G, Sanchez C, Moreau R, Arroyo V, Zeuzem S, Welsch C, Trebicka J. Trends and the course of liver cirrhosis and its complications in Germany: Nationwide population-based study (2005 to 2018) Lancet Reg Health Eur. 2022;12:100240. doi: 10.1016/j.lanepe.2021.100240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagaoki Y, Sugiyama A, Mino M, Kodama H, Abe K, Imada H, Ouoba S, E B, Ko K, Akita T, Sako T, Kumada T, Chayama K, Tanaka J. Prevalence of fatty liver and advanced fibrosis by ultrasonography and FibroScan in a general population random sample. Hepatol Res. 2022 doi: 10.1111/hepr.13821. [DOI] [PubMed] [Google Scholar]
- 12.Potnis A, VanMeter S, Stange J. Prevalence of Hepatic Encephalopathy from a Commercial Medical Claims Database in the United States. Int J Hepatol. 2021;2021:8542179. doi: 10.1155/2021/8542179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciardullo S, Monti T, Perseghin G. High Prevalence of Advanced Liver Fibrosis Assessed by Transient Elastography Among U.S. Adults With Type 2 Diabetes. Diabetes Care. 2021;44:519–525. doi: 10.2337/dc20-1778. [DOI] [PubMed] [Google Scholar]
- 14.Kim D, Cholankeril G, Loomba R, Ahmed A. Prevalence of Fatty Liver Disease and Fibrosis Detected by Transient Elastography in Adults in the United States, 2017-2018. Clin Gastroenterol Hepatol. 2021;19:1499–1501.e2. doi: 10.1016/j.cgh.2020.08.017. [DOI] [PubMed] [Google Scholar]
- 15.Huang SW, Chen C, Kong HY, Huang JQ. Prevalence of Cirrhosis/Advanced Fibrosis Among HBsAg-Negative and HBcAb-Positive US Adults: A Nationwide Population-Based Study. Infect Dis Ther. 2022 doi: 10.1007/s40121-022-00680-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Surial B, Wyser D, Béguelin C, Ramírez-Mena A, Rauch A, Wandeler G. Prevalence of liver cirrhosis in individuals with hepatitis B virus infection in sub-Saharan Africa: Systematic review and meta-analysis. Liver Int. 2021;41:710–719. doi: 10.1111/liv.14744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calleja JL, Rivera-Esteban J, Aller R, Hernández-Conde M, Abad J, Pericàs JM, Benito HG, Serra MA, Escudero A, Ampuero J, Lucena A, Sánchez Y, Arias-Loste MT, Iruzubieta P, Romero-Gómez M, Augustin S, Crespo J. Prevalence estimation of significant fibrosis because of NASH in Spain combining transient elastography and histology. Liver Int. 2022;42:1783–1792. doi: 10.1111/liv.15323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mun H, So ES. Prevalence of liver cirrhosis based on the metabolic health and weight criteria: Report from the Korea National Health and Nutrition Examination Survey (KNHANES) data analysis. Ann Hepatol. 2022:100721. doi: 10.1016/j.aohep.2022.100721. [DOI] [PubMed] [Google Scholar]
- 19.Weinberg EM, Trinh HN, Firpi RJ, Bhamidimarri KR, Klein S, Durlam J, Watkins S, Reddy KR, Weiss M, Zink RC, Lok AS. Lean Americans With Nonalcoholic Fatty Liver Disease Have Lower Rates of Cirrhosis and Comorbid Diseases. Clin Gastroenterol Hepatol. 2021;19:996–1008.e6. doi: 10.1016/j.cgh.2020.06.066. [DOI] [PubMed] [Google Scholar]
- 20.Vilar-Gomez E, Lou Z, Kong N, Vuppalanchi R, Imperiale TF, Chalasani N. Cost Effectiveness of Different Strategies for Detecting Cirrhosis in Patients With Nonalcoholic Fatty Liver Disease Based on United States Health Care System. Clin Gastroenterol Hepatol. 2020;18:2305–2314.e12. doi: 10.1016/j.cgh.2020.04.017. [DOI] [PubMed] [Google Scholar]
- 21.De Vincentis A, Vespasiani-Gentilucci U, Costanzo L, Novella A, Cortesi L, Nobili A, Mannucci PM, Incalzi RA REPOSI Investigators. The multifaceted spectrum of liver cirrhosis in older hospitalised patients: analysis of the REPOSI registry. Age Ageing. 2021;50:498–504. doi: 10.1093/ageing/afaa150. [DOI] [PubMed] [Google Scholar]
- 22.Swift O, Sharma S, Ramanarayanan S, Umar H, Laws KR, Vilar E, Farrington K. Prevalence and outcomes of chronic liver disease in patients receiving dialysis: systematic review and meta-analysis. Clin Kidney J. 2022;15:747–757. doi: 10.1093/ckj/sfab230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhai M, Liu Z, Long J, Zhou Q, Yang L, Liu S, Dai Y. The incidence trends of liver cirrhosis caused by nonalcoholic steatohepatitis via the GBD study 2017. Sci Rep. 2021;11:5195. doi: 10.1038/s41598-021-84577-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang T, Xu J, Ye L, Lin X, Xu Y, Pan X, Weng X, Ye C, Fan L, Ren Y, Shan PF. Age, Gender and Geographic Differences in Global Health Burden of Cirrhosis and Liver Cancer due to Nonalcoholic Steatohepatitis. J Cancer. 2021;12:2855–2865. doi: 10.7150/jca.52282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veracruz N, Gish RG, Cheung R, Chitnis AS, Wong RJ. Global incidence and mortality of hepatitis B and hepatitis C acute infections, cirrhosis and hepatocellular carcinoma from 2010 to 2019. J Viral Hepat. 2022;29:352–365. doi: 10.1111/jvh.13663. [DOI] [PubMed] [Google Scholar]
- 27.Wong GL, Hui VW, Yip TC, Liang LY, Zhang X, Tse YK, Lai JC, Chan HL, Wong VW. Universal HBV vaccination dramatically reduces the prevalence of HBV infection and incidence of hepatocellular carcinoma. Aliment Pharmacol Ther. 2022;56:869–877. doi: 10.1111/apt.17120. [DOI] [PubMed] [Google Scholar]
- 28.Pang Y, Kartsonaki C, Turnbull I, Guo Y, Clarke R, Chen Y, Bragg F, Yang L, Bian Z, Millwood IY, Hao J, Han X, Zang Y, Chen J, Li L, Holmes MV, Chen Z. Diabetes, Plasma Glucose, and Incidence of Fatty Liver, Cirrhosis, and Liver Cancer: A Prospective Study of 0.5 Million People. Hepatology. 2018;68:1308–1318. doi: 10.1002/hep.30083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Julien J, Ayer T, Bethea ED, Tapper EB, Chhatwal J. Projected prevalence and mortality associated with alcohol-related liver disease in the USA, 2019-40: a modelling study. Lancet Public Health. 2020;5:e316–e323. doi: 10.1016/S2468-2667(20)30062-1. [DOI] [PubMed] [Google Scholar]
- 30.Kim HI, Park SY, Shin HP. Incidence and management patterns of alcohol-related liver disease in Korea: a nationwide standard cohort study. Sci Rep. 2021;11:6648. doi: 10.1038/s41598-021-86197-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaz J, Eriksson B, Strömberg U, Buchebner D, Midlöv P. Incidence, aetiology and related comorbidities of cirrhosis: a Swedish population-based cohort study. BMC Gastroenterol. 2020;20:84. doi: 10.1186/s12876-020-01239-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim SS, Lee W, Kim YK, Kim J, Park JH, Park BR, Yoon JH. The cumulative incidence and trends of rare diseases in South Korea: a nationwide study of the administrative data from the National Health Insurance Service database from 2011-2015. Orphanet J Rare Dis. 2019;14:49. doi: 10.1186/s13023-019-1032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flemming JA, Dewit Y, Mah JM, Saperia J, Groome PA, Booth CM. Incidence of cirrhosis in young birth cohorts in Canada from 1997 to 2016: a retrospective population-based study. Lancet Gastroenterol Hepatol. 2019;4:217–226. doi: 10.1016/S2468-1253(18)30339-X. [DOI] [PubMed] [Google Scholar]
- 34.Kehar M, Griffiths R, Flemming JA. Increasing Incidence of Cirrhosis Over the Past 2 Decades Among Children in Ontario, Canada. Am J Gastroenterol. 2022;117:189–192. doi: 10.14309/ajg.0000000000001564. [DOI] [PubMed] [Google Scholar]
- 35.Dong Y, Li A, Zhu S, Chen W, Li M, Zhao P. Biopsy-proven liver cirrhosis in young children: A 10-year cohort study. J Viral Hepat. 2021;28:959–963. doi: 10.1111/jvh.13501. [DOI] [PubMed] [Google Scholar]
- 36.Asbeutah AAA, Jefferies JL. Meta-Analysis of the Incidence of Liver Cirrhosis Among Patients With a Fontan Circulation. Am J Cardiol. 2022;177:166–167. doi: 10.1016/j.amjcard.2022.05.009. [DOI] [PubMed] [Google Scholar]
- 37.Hilscher MB, Wells ML, Venkatesh SK, Cetta F, Kamath PS. Fontan-associated liver disease. Hepatology. 2022;75:1300–1321. doi: 10.1002/hep.32406. [DOI] [PubMed] [Google Scholar]
- 38.Emamaullee J, Zaidi AN, Schiano T, Kahn J, Valentino PL, Hofer RE, Taner T, Wald JW, Olthoff KM, Bucuvalas J, Fischer R. Fontan-Associated Liver Disease: Screening, Management, and Transplant Considerations. Circulation. 2020;142:591–604. doi: 10.1161/CIRCULATIONAHA.120.045597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nii M, Inuzuka R, Inai K, Shimada E, Shinohara T, Kogiso T, Ono H, Ootsuki S, Kurita Y, Takeda A, Hirono K, Takei K, Yasukochi S, Yoshikawa T, Furutani Y, Shinozaki T, Matsuyama Y, Senzaki H, Tokushige K, Nakanishi T. Incidence and Expected Probability of Liver Cirrhosis and Hepatocellular Carcinoma After Fontan Operation. Circulation. 2021;144:2043–2045. doi: 10.1161/CIRCULATIONAHA.121.056870. [DOI] [PubMed] [Google Scholar]
- 40.Wang CH, Ou SF, Tseng YT. Long-term impact of certain coexisting extrahepatic unisystem and multisystem manifestations on trends in incidence of liver cirrhosis in treatment-naïve patients with chronic hepatitis C: A nested case-control study. Medicine (Baltimore) 2022;101:e29697. doi: 10.1097/MD.0000000000029697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orioli R, Solimini AG, Michelozzi P, Forastiere F, Davoli M, Cesaroni G. A cohort study on long-term exposure to air pollution and incidence of liver cirrhosis. Environ Epidemiol. 2020;4:e109. doi: 10.1097/EE9.0000000000000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.GBD 2019 Hepatitis B Collaborators. Global, regional, and national burden of hepatitis B, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol. 2022;7:796–829. doi: 10.1016/S2468-1253(22)00124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.GBD 2019 Ethiopia Subnational-Level Disease Burden Initiative Collaborators. Progress in health among regions of Ethiopia, 1990-2019: a subnational country analysis for the Global Burden of Disease Study 2019. Lancet. 2022;399:1322–1335. doi: 10.1016/S0140-6736(21)02868-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bertha M, Shedden K, Mellinger J. Trends in the inpatient burden of alcohol-related liver disease among women hospitalized in the United States. Liver Int. 2022;42:1557–1561. doi: 10.1111/liv.15277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yeverino-Gutiérrez ML, González-González MDR, González-Santiago O. Mortality From Alcohol-Related Liver Cirrhosis in Mexico (2000-2017) Front Public Health. 2020;8:524356. doi: 10.3389/fpubh.2020.524356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moon AM, Yang JY, Barritt AS 4th, Bataller R, Peery AF. Rising Mortality From Alcohol-Associated Liver Disease in the United States in the 21st Century. Am J Gastroenterol. 2020;115:79–87. doi: 10.14309/ajg.0000000000000442. [DOI] [PubMed] [Google Scholar]
- 47.Shirazi F, Singal AK, Wong RJ. Alcohol-associated Cirrhosis and Alcoholic Hepatitis Hospitalization Trends in the United States. J Clin Gastroenterol. 2021;55:174–179. doi: 10.1097/MCG.0000000000001378. [DOI] [PubMed] [Google Scholar]
- 48.Han S, Yang Z, Zhang T, Ma J, Chandler K, Liangpunsakul S. Epidemiology of Alcohol-Associated Liver Disease. Clin Liver Dis. 2021;25:483–492. doi: 10.1016/j.cld.2021.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoon JH, Jun CH, Kim JH, Yoon EL, Kim BS, Song JE, Suk KT, Kim MY, Kang SH. Changing Trends in Liver Cirrhosis Etiology and Severity in Korea: the Increasing Impact of Alcohol. J Korean Med Sci. 2021;36:e145. doi: 10.3346/jkms.2021.36.e145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deutsch-Link S, Jiang Y, Peery AF, Barritt AS, Bataller R, Moon AM. Alcohol-Associated Liver Disease Mortality Increased From 2017 to 2020 and Accelerated During the COVID-19 Pandemic. Clin Gastroenterol Hepatol. 2022;20:2142–2144.e2. doi: 10.1016/j.cgh.2022.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Veracruz N, Gish RG, Cheung R, Chitnis AS, Wong RJ. Global trends and the impact of chronic hepatitis B and C on disability-adjusted life years. Liver Int. 2022;42:2145–2153. doi: 10.1111/liv.15347. [DOI] [PubMed] [Google Scholar]
- 53.Jepsen P, Younossi ZM. The global burden of cirrhosis: A review of disability-adjusted life-years lost and unmet needs. J Hepatol. 2021;75 Suppl 1:S3–S13. doi: 10.1016/j.jhep.2020.11.042. [DOI] [PubMed] [Google Scholar]
- 54.Desai AP, Mohan P, Nokes B, Sheth D, Knapp S, Boustani M, Chalasani N, Fallon MB, Calhoun EA. Increasing Economic Burden in Hospitalized Patients With Cirrhosis: Analysis of a National Database. Clin Transl Gastroenterol. 2019;10:e00062. doi: 10.14309/ctg.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Allen AM, Van Houten HK, Sangaralingham LR, Talwalkar JA, McCoy RG. Healthcare Cost and Utilization in Nonalcoholic Fatty Liver Disease: Real-World Data From a Large U.S. Claims Database. Hepatology. 2018;68:2230–2238. doi: 10.1002/hep.30094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397–417. doi: 10.1016/j.jhep.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 57.Pericleous M, Sarnowski A, Moore A, Fijten R, Zaman M. The clinical management of abdominal ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: a review of current guidelines and recommendations. Eur J Gastroenterol Hepatol. 2016;28:e10–e18. doi: 10.1097/MEG.0000000000000548. [DOI] [PubMed] [Google Scholar]
- 58.Gordon FD. Ascites. Clin Liver Dis. 2012;16:285–299. doi: 10.1016/j.cld.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 59.Pedersen JS, Bendtsen F, Møller S. Management of cirrhotic ascites. Ther Adv Chronic Dis. 2015;6:124–137. doi: 10.1177/2040622315580069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garbuzenko DV, Arefyev NO. Current approaches to the management of patients with cirrhotic ascites. World J Gastroenterol. 2019;25:3738–3752. doi: 10.3748/wjg.v25.i28.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ratnasekera IU, Johnson A, Powell EE, Henderson A, Irvine KM, Valery PC. Epidemiology of ascites fluid infections in patients with cirrhosis in Queensland, Australia from 2008 to 2017: A population-based study. Medicine (Baltimore) 2022;101:e29217. doi: 10.1097/MD.0000000000029217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee H, Kim BK. Real-world clinical features, health-care utilization, and economic burden in decompensated cirrhosis patients: A national database. J Gastroenterol Hepatol. 2022 doi: 10.1111/jgh.15962. [DOI] [PubMed] [Google Scholar]
- 63.Gravito-Soares M, Gravito-Soares E, Lopes S, Ribeiro G, Figueiredo P. Spontaneous fungal peritonitis: a rare but severe complication of liver cirrhosis. Eur J Gastroenterol Hepatol. 2017;29:1010–1016. doi: 10.1097/MEG.0000000000000927. [DOI] [PubMed] [Google Scholar]
- 64.Fiore M, Chiodini P, Pota V, Sansone P, Passavanti MB, Leone S, Aurilio C, Pace MC. Risk of spontaneous fungal peritonitis in hospitalized cirrhotic patients with ascites: a systematic review of observational studies and meta-analysis. Minerva Anestesiol. 2017;83:1309–1316. doi: 10.23736/S0375-9393.17.12034-1. [DOI] [PubMed] [Google Scholar]
- 65.Mattos AA, Wiltgen D, Jotz RF, Dornelles CMR, Fernandes MV, Mattos ÂZ. Spontaneous bacterial peritonitis and extraperitoneal infections in patients with cirrhosis. Ann Hepatol. 2020;19:451–457. doi: 10.1016/j.aohep.2020.04.010. [DOI] [PubMed] [Google Scholar]
- 66.Rimola A, García-Tsao G, Navasa M, Piddock LJ, Planas R, Bernard B, Inadomi JM. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club. J Hepatol. 2000;32:142–153. doi: 10.1016/s0168-8278(00)80201-9. [DOI] [PubMed] [Google Scholar]
- 67.European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–460. doi: 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 68.Abu-Freha N, Michael T, Poupko L, Estis-Deaton A, Aasla M, Abu-Freha O, Etzion O, Nesher L. Spontaneous Bacterial Peritonitis among Cirrhotic Patients: Prevalence, Clinical Characteristics, and Outcomes. J Clin Med. 2021;11 doi: 10.3390/jcm11010227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tay PWL, Xiao J, Tan DJH, Ng C, Lye YN, Lim WH, Teo VXY, Heng RRY, Yeow MWX, Lum LHW, Tan EXX, Kew GS, Lee GH, Muthiah MD. An Epidemiological Meta-Analysis on the Worldwide Prevalence, Resistance, and Outcomes of Spontaneous Bacterial Peritonitis in Cirrhosis. Front Med (Lausanne) 2021;8:693652. doi: 10.3389/fmed.2021.693652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alotaibi A, Almaghrabi M, Ahmed O, Rodrigues D, Iansavichene A, Puka K, Gandhi R, Sey M, Patel K, Brahmania M. Incidence of spontaneous bacterial peritonitis among asymptomatic cirrhosis patients undergoing outpatient paracentesis: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2021;33:e851–e857. doi: 10.1097/MEG.0000000000002279. [DOI] [PubMed] [Google Scholar]
- 71.Alqahtani SA, Jang S. Pathophysiology and Management of Variceal Bleeding. Drugs. 2021;81:647–667. doi: 10.1007/s40265-021-01493-2. [DOI] [PubMed] [Google Scholar]
- 72.Baiges A, Hernández-Gea V. Management of Liver Decompensation in Advanced Chronic Liver Disease: Ascites, Hyponatremia, and Gastroesophageal Variceal Bleeding. Clin Drug Investig. 2022;42:25–31. doi: 10.1007/s40261-022-01147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khan F, Tripathi D. Role of early transjugular intrahepatic portosystemic stent-shunt in acute variceal bleeding: An update of the evidence and future directions. World J Gastroenterol. 2021;27:7612–7624. doi: 10.3748/wjg.v27.i44.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Molleston JP, Bennett WE Jr. Mortality, Risk Factors and Disparities Associated with Esophageal Variceal Bleeding in Children's Hospitals in the US. J Pediatr. 2021;232:176–182. doi: 10.1016/j.jpeds.2020.12.082. [DOI] [PubMed] [Google Scholar]
- 75.Sohal A, Chaudhry H, Dhaliwal A, Singla P, Gupta G, Sharma R, Dukovic D, Prajapati D. Gender differences in esophageal variceal bleeding in the United States. Ann Med. 2022;54:2115–2122. doi: 10.1080/07853890.2022.2104920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Häussinger D, Dhiman RK, Felipo V, Görg B, Jalan R, Kircheis G, Merli M, Montagnese S, Romero-Gomez M, Schnitzler A, Taylor-Robinson SD, Vilstrup H. Hepatic encephalopathy. Nat Rev Dis Primers. 2022;8:43. doi: 10.1038/s41572-022-00366-6. [DOI] [PubMed] [Google Scholar]
- 77.Ridola L, Faccioli J, Nardelli S, Gioia S, Riggio O. Hepatic Encephalopathy: Diagnosis and Management. J Transl Int Med. 2020;8:210–219. doi: 10.2478/jtim-2020-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tapper EB, Aberasturi D, Zhao Z, Hsu CY, Parikh ND. Outcomes after hepatic encephalopathy in population-based cohorts of patients with cirrhosis. Aliment Pharmacol Ther. 2020;51:1397–1405. doi: 10.1111/apt.15749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Louissaint J, Deutsch-Link S, Tapper EB. Changing Epidemiology of Cirrhosis and Hepatic Encephalopathy. Clin Gastroenterol Hepatol. 2022;20:S1–S8. doi: 10.1016/j.cgh.2022.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ampuero J, Montoliú C, Simón-Talero M, Aguilera V, Millán R, Márquez C, Jover R, Rico MC, Sendra C, Serra MÁ, Romero-Gómez M. Minimal hepatic encephalopathy identifies patients at risk of faster cirrhosis progression. J Gastroenterol Hepatol. 2018;33:718–725. doi: 10.1111/jgh.13917. [DOI] [PubMed] [Google Scholar]
- 81.Allampati S, Duarte-Rojo A, Thacker LR, Patidar KR, White MB, Klair JS, John B, Heuman DM, Wade JB, Flud C, O'Shea R, Gavis EA, Unser AB, Bajaj JS. Diagnosis of Minimal Hepatic Encephalopathy Using Stroop EncephalApp: A Multicenter US-Based, Norm-Based Study. Am J Gastroenterol. 2016;111:78–86. doi: 10.1038/ajg.2015.377. [DOI] [PubMed] [Google Scholar]
- 82.Özbaş B, Keskin O, Hecker H, Karahan I, Özbaş C, Kalkan Ç, Kartal A, Önder FO, Öncü BK, Gençdal G, Akyildiz M, Günşar F, Idilman R, Weissenborn K, Özütemiz Ö, Yurdaydin C. Determination of Turkish norms of psychometric tests for diagnosing minimal hepatic encephalopathy and proposal of a high sensitive screening test battery. Hepatol Int. 2021;15:1442–1455. doi: 10.1007/s12072-021-10207-5. [DOI] [PubMed] [Google Scholar]
- 83.Duarte-Rojo A, Allampati S, Thacker LR, Flud CR, Patidar KR, White MB, Klair JS, Heuman DM, Wade JB, Gavis EA, Bajaj JS. Diagnosis of covert hepatic encephalopathy: a multi-center study testing the utility of single versus combined testing. Metab Brain Dis. 2019;34:289–295. doi: 10.1007/s11011-018-0350-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tapper EB, Zhao L, Nikirk S, Baki J, Parikh ND, Lok AS, Waljee AK. Incidence and Bedside Predictors of the First Episode of Overt Hepatic Encephalopathy in Patients With Cirrhosis. Am J Gastroenterol. 2020;115:2017–2025. doi: 10.14309/ajg.0000000000000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tapper EB, Henderson JB, Parikh ND, Ioannou GN, Lok AS. Incidence of and Risk Factors for Hepatic Encephalopathy in a Population-Based Cohort of Americans With Cirrhosis. Hepatol Commun. 2019;3:1510–1519. doi: 10.1002/hep4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rudler M, Weiss N, Bouzbib C, Thabut D. Diagnosis and Management of Hepatic Encephalopathy. Clin Liver Dis. 2021;25:393–417. doi: 10.1016/j.cld.2021.01.008. [DOI] [PubMed] [Google Scholar]
- 87.Shi D, Zhou Z, Dai Y, Pan X, Cao Q. Proton Pump Inhibitor Therapy and Hepatic Encephalopathy Risk in Cirrhotic Patients: A Systematic Review with Meta-analysis. Clin Drug Investig. 2019;39:847–856. doi: 10.1007/s40261-019-00810-8. [DOI] [PubMed] [Google Scholar]
- 88.Bai Z, Guo X, Tacke F, Li Y, Li H, Qi X. Association of serum albumin level with incidence and mortality of overt hepatic encephalopathy in cirrhosis during hospitalization. Therap Adv Gastroenterol. 2019;12:1756284819881302. doi: 10.1177/1756284819881302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tapper EB, Parikh ND, Green PK, Berry K, Waljee AK, Moon AM, Ioannou GN. Reduced Incidence of Hepatic Encephalopathy and Higher Odds of Resolution Associated With Eradication of HCV Infection. Clin Gastroenterol Hepatol. 2020;18:1197–1206.e7. doi: 10.1016/j.cgh.2019.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Elsaid MI, John T, Li Y, Pentakota SR, Rustgi VK. The Health Care Burden of Hepatic Encephalopathy. Clin Liver Dis. 2020;24:263–275. doi: 10.1016/j.cld.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 91.Hirode G, Vittinghoff E, Wong RJ. Increasing Burden of Hepatic Encephalopathy Among Hospitalized Adults: An Analysis of the 2010-2014 National Inpatient Sample. Dig Dis Sci. 2019;64:1448–1457. doi: 10.1007/s10620-019-05576-9. [DOI] [PubMed] [Google Scholar]
- 92.Bajaj JS, Reddy KR, Tandon P, Wong F, Kamath PS, Garcia-Tsao G, Maliakkal B, Biggins SW, Thuluvath PJ, Fallon MB, Subramanian RM, Vargas H, Thacker LR, O'Leary JG North American Consortium for the Study of End-Stage Liver Disease. The 3-month readmission rate remains unacceptably high in a large North American cohort of patients with cirrhosis. Hepatology. 2016;64:200–208. doi: 10.1002/hep.28414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Seraj SM, Campbell EJ, Argyropoulos SK, Wegermann K, Chung RT, Richter JM. Hospital readmissions in decompensated cirrhotics: Factors pointing toward a prevention strategy. World J Gastroenterol. 2017;23:6868–6876. doi: 10.3748/wjg.v23.i37.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tapper EB, Halbert B, Mellinger J. Rates of and Reasons for Hospital Readmissions in Patients With Cirrhosis: A Multistate Population-based Cohort Study. Clin Gastroenterol Hepatol. 2016;14:1181–1188.e2. doi: 10.1016/j.cgh.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 95.Soriano G, Román E, Córdoba J, Torrens M, Poca M, Torras X, Villanueva C, Gich IJ, Vargas V, Guarner C. Cognitive dysfunction in cirrhosis is associated with falls: a prospective study. Hepatology. 2012;55:1922–1930. doi: 10.1002/hep.25554. [DOI] [PubMed] [Google Scholar]
- 96.Kumar R, Priyadarshi RN, Anand U. Chronic renal dysfunction in cirrhosis: A new frontier in hepatology. World J Gastroenterol. 2021;27:990–1005. doi: 10.3748/wjg.v27.i11.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Angeli P, Gines P, Wong F, Bernardi M, Boyer TD, Gerbes A, Moreau R, Jalan R, Sarin SK, Piano S, Moore K, Lee SS, Durand F, Salerno F, Caraceni P, Kim WR, Arroyo V, Garcia-Tsao G International Club of Ascites. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut. 2015;64:531–537. doi: 10.1136/gutjnl-2014-308874. [DOI] [PubMed] [Google Scholar]
- 98.Angeli P, Garcia-Tsao G, Nadim MK, Parikh CR. News in pathophysiology, definition and classification of hepatorenal syndrome: A step beyond the International Club of Ascites (ICA) consensus document. J Hepatol. 2019;71:811–822. doi: 10.1016/j.jhep.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 99.Moga L, Robic MA, Blasco-Perrin H, Cabarrou P, Mogno J, Guillaume M, Vinel JP, Péron JM, Bureau C. Acute kidney injury in patients with cirrhosis: Prospective longitudinal study in 405 patients. Clin Res Hepatol Gastroenterol. 2022;46:101822. doi: 10.1016/j.clinre.2021.101822. [DOI] [PubMed] [Google Scholar]
- 100.Thapa P, Kc S, Hamal AB, Sharma D, Khadka S, Karki N, Jaishi B, Tiwari PS, Vaidya A, Karki A. Prevalence of Acute Kidney Injury in Patients with Liver Cirrhosis. JNMA J Nepal Med Assoc. 2020;58:554–559. doi: 10.31729/jnma.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Arora MS, Kaushik R, Ahmad S, Kaushik RM. Profile of Acute Kidney Injury in Patients with Decompensated Cirrhosis at a Tertiary-Care Center in Uttarakhand, India. Dig Dis. 2020;38:335–343. doi: 10.1159/000504836. [DOI] [PubMed] [Google Scholar]
- 102.Duah A, Duah F, Ampofo-Boobi D, Addo BP, Osei-Poku F, Agyei-Nkansah A. Acute Kidney Injury in Patients with Liver Cirrhosis: Prevalence, Predictors, and In-Hospital Mortality at a District Hospital in Ghana. Biomed Res Int. 2022;2022:4589767. doi: 10.1155/2022/4589767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jiang W, Hu Y, Sun Y, Shen Y, Xun Y. Prevalence and short-term outcome of acute kidney injury in patients with acute-on-chronic liver failure: A meta-analysis. J Viral Hepat. 2020;27:810–817. doi: 10.1111/jvh.13287. [DOI] [PubMed] [Google Scholar]
- 104.Velez JCQ, Therapondos G, Juncos LA. Reappraising the spectrum of AKI and hepatorenal syndrome in patients with cirrhosis. Nat Rev Nephrol. 2020;16:137–155. doi: 10.1038/s41581-019-0218-4. [DOI] [PubMed] [Google Scholar]
- 105.Singh J, Dahiya DS, Kichloo A, Singh G, Khoshbin K, Shaka H. Hepatorenal syndrome: a Nationwide Trend Analysis from 2008 to 2018. Ann Med. 2021;53:2018–2024. doi: 10.1080/07853890.2021.1998595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Van der Merwe S, Chokshi S, Bernsmeier C, Albillos A. The multifactorial mechanisms of bacterial infection in decompensated cirrhosis. J Hepatol. 2021;75 Suppl 1:S82–S100. doi: 10.1016/j.jhep.2020.11.029. [DOI] [PubMed] [Google Scholar]
- 107.Choudry N, Sasso R, Rockey DC. Infection in Hospitalized Cirrhosis Patients: Changing Epidemiology and Clinical Features. Am J Med Sci. 2022;363:114–121. doi: 10.1016/j.amjms.2021.10.023. [DOI] [PubMed] [Google Scholar]
- 108.Atteberry P, Biederman B, Jesudian A, Lucero C, Brown RS Jr, Verna E, Sundaram V, Fortune B, Rosenblatt R. Mortality, sepsis, and organ failure in hospitalized patients with cirrhosis vary by type of infection. J Gastroenterol Hepatol. 2021;36:3363–3370. doi: 10.1111/jgh.15633. [DOI] [PubMed] [Google Scholar]
- 109.Liu Y, Chen M. Clostridioides difficile Infection in Liver Cirrhosis: A Concise Review. Can J Gastroenterol Hepatol. 2022;2022:4209442. doi: 10.1155/2022/4209442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fernández J, Piano S, Bartoletti M, Wey EQ. Management of bacterial and fungal infections in cirrhosis: The MDRO challenge. J Hepatol. 2021;75 Suppl 1:S101–S117. doi: 10.1016/j.jhep.2020.11.010. [DOI] [PubMed] [Google Scholar]
- 111.Fernández J, Prado V, Trebicka J, Amoros A, Gustot T, Wiest R, Deulofeu C, Garcia E, Acevedo J, Fuhrmann V, Durand F, Sánchez C, Papp M, Caraceni P, Vargas V, Bañares R, Piano S, Janicko M, Albillos A, Alessandria C, Soriano G, Welzel TM, Laleman W, Gerbes A, De Gottardi A, Merli M, Coenraad M, Saliba F, Pavesi M, Jalan R, Ginès P, Angeli P, Arroyo V European Foundation for the Study of Chronic Liver Failure (EF-Clif) Multidrug-resistant bacterial infections in patients with decompensated cirrhosis and with acute-on-chronic liver failure in Europe. J Hepatol. 2019;70:398–411. doi: 10.1016/j.jhep.2018.10.027. [DOI] [PubMed] [Google Scholar]
- 112.Piano S, Singh V, Caraceni P, Maiwall R, Alessandria C, Fernandez J, Soares EC, Kim DJ, Kim SE, Marino M, Vorobioff J, Barea RCR, Merli M, Elkrief L, Vargas V, Krag A, Singh SP, Lesmana LA, Toledo C, Marciano S, Verhelst X, Wong F, Intagliata N, Rabinowich L, Colombato L, Kim SG, Gerbes A, Durand F, Roblero JP, Bhamidimarri KR, Boyer TD, Maevskaya M, Fassio E, Kim HS, Hwang JS, Gines P, Gadano A, Sarin SK, Angeli P International Club of Ascites Global Study Group. Epidemiology and Effects of Bacterial Infections in Patients With Cirrhosis Worldwide. Gastroenterology. 2019;156:1368–1380.e10. doi: 10.1053/j.gastro.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 113.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 114.Huang DQ, Singal AG, Kono Y, Tan DJH, El-Serag HB, Loomba R. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell Metab. 2022;34:969–977.e2. doi: 10.1016/j.cmet.2022.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu Y, Zheng J, Hao J, Wang RR, Liu X, Gu P, Yu H, Yu Y, Wu C, Ou B, Peng Z. Global burden of primary liver cancer by five etiologies and global prediction by 2035 based on global burden of disease study 2019. Cancer Med. 2022;11:1310–1323. doi: 10.1002/cam4.4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xing QQ, Li JM, Dong X, Zeng DY, Chen ZJ, Lin XY, Pan JS. Socioeconomics and attributable etiology of primary liver cancer, 1990-2019. World J Gastroenterol. 2022;28:2361–2382. doi: 10.3748/wjg.v28.i21.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gawrieh S, Dakhoul L, Miller E, Scanga A, deLemos A, Kettler C, Burney H, Liu H, Abu-Sbeih H, Chalasani N, Wattacheril J. Characteristics, aetiologies and trends of hepatocellular carcinoma in patients without cirrhosis: a United States multicentre study. Aliment Pharmacol Ther. 2019;50:809–821. doi: 10.1111/apt.15464. [DOI] [PubMed] [Google Scholar]
- 118.Castellana M, Donghia R, Lampignano L, Castellana F, Zupo R, Sardone R, Pergola G, Giannelli G. Prevalence of the Absence of Cirrhosis in Subjects with NAFLD-Associated Hepatocellular Carcinoma. J Clin Med. 2021;10 doi: 10.3390/jcm10204638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pinyopornpanish K, Khoudari G, Saleh MA, Angkurawaranon C, Pinyopornpanish K, Mansoor E, Dasarathy S, McCullough A. Hepatocellular carcinoma in nonalcoholic fatty liver disease with or without cirrhosis: a population-based study. BMC Gastroenterol. 2021;21:394. doi: 10.1186/s12876-021-01978-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bengtsson B, Widman L, Wahlin S, Stål P, Björkström NK, Hagström H. The risk of hepatocellular carcinoma in cirrhosis differs by etiology, age and sex: A Swedish nationwide population-based cohort study. United European Gastroenterol J. 2022;10:465–476. doi: 10.1002/ueg2.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kanwal F, Khaderi S, Singal AG, Marrero JA, Loo N, Asrani SK, Amos CI, Thrift AP, Gu X, Luster M, Al-Sarraj A, Ning J, El-Serag HB. Risk factors for HCC in contemporary cohorts of patients with cirrhosis. Hepatology. 2022 doi: 10.1002/hep.32434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lockart I, Yeo MGH, Hajarizadeh B, Dore GJ, Danta M. HCC incidence after hepatitis C cure among patients with advanced fibrosis or cirrhosis: A meta-analysis. Hepatology. 2022;76:139–154. doi: 10.1002/hep.32341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wu X, Zhou J, Sun Y, Ding H, Chen G, Xie W, Piao H, Xu X, Jiang W, Ma H, Ma A, Chen Y, Xu M, Cheng J, Xu Y, Meng T, Wang B, Chen S, Shi Y, Kong Y, Ou X, You H, Jia J. Prediction of liver-related events in patients with compensated HBV-induced cirrhosis receiving antiviral therapy. Hepatol Int. 2021;15:82–92. doi: 10.1007/s12072-020-10114-1. [DOI] [PubMed] [Google Scholar]
- 124.Shin SK, Yim HJ, Kim JH, Lee CU, Yeon JE, Suh SJ, Jung YK, Kim YS, Kwon OS. Partial Virological Response after 2 Years of Entecavir Therapy Increases the Risk of Hepatocellular Carcinoma in Patients with Hepatitis B Virus-Associated Cirrhosis. Gut Liver. 2021;15:430–439. doi: 10.5009/gnl20074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.John BV, Khakoo NS, Schwartz KB, Aitchenson G, Levy C, Dahman B, Deng Y, Goldberg DS, Martin P, Kaplan DE, Taddei TH. Ursodeoxycholic Acid Response Is Associated With Reduced Mortality in Primary Biliary Cholangitis With Compensated Cirrhosis. Am J Gastroenterol. 2021;116:1913–1923. doi: 10.14309/ajg.0000000000001280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.John BV, Aitcheson G, Schwartz KB, Khakoo NS, Dahman B, Deng Y, Goldberg D, Martin P, Taddei TH, Levy C, Kaplan DE. Male Sex Is Associated With Higher Rates of Liver-Related Mortality in Primary Biliary Cholangitis and Cirrhosis. Hepatology. 2021;74:879–891. doi: 10.1002/hep.31776. [DOI] [PubMed] [Google Scholar]
- 127.Zenouzi R, Weismüller TJ, Hübener P, Schulze K, Bubenheim M, Pannicke N, Weiler-Normann C, Lenzen H, Manns MP, Lohse AW, Schramm C. Low risk of hepatocellular carcinoma in patients with primary sclerosing cholangitis with cirrhosis. Clin Gastroenterol Hepatol. 2014;12:1733–1738. doi: 10.1016/j.cgh.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 128.Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huang DQ, Tan DJH, Ng CH, Amangurbanova M, Sutter N, Lin Tay PW, Lim WH, Yong JN, Tang A, Syn N, Muthiah MD, Tan EXX, Dave S, Tay B, Majzoub AM, Gerberi D, Kim BK, Loomba R. Hepatocellular Carcinoma Incidence in Alcohol-Associated Cirrhosis: Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2022 doi: 10.1016/j.cgh.2022.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ganne-Carrié N, Nahon P, Chaffaut C, N'Kontchou G, Layese R, Audureau E, Chevret S CIRRAL group; ANRS CO12 CirVir group. Impact of cirrhosis aetiology on incidence and prognosis of hepatocellular carcinoma diagnosed during surveillance. JHEP Rep. 2021;3:100285. doi: 10.1016/j.jhepr.2021.100285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Guan X, Xing F, Li Y. Alcohol consumption increases the incidence of hepatocellular carcinoma in patients with hepatitis B cirrhosis but not in patients with hepatitis C cirrhosis. Eur J Gastroenterol Hepatol. 2021;33:1218–1221. doi: 10.1097/MEG.0000000000001837. [DOI] [PubMed] [Google Scholar]
- 132.Tsai MC, Yang SS, Lin CC, Wang WL, Hsu YC, Chen YS, Hu JT, Lin JY, Yu ML, Lin CW. Association of Heavy Alcohol Intake and ALDH2 rs671 Polymorphism With Hepatocellular Carcinoma and Mortality in Patients With Hepatitis B Virus-Related Cirrhosis. JAMA Netw Open. 2022;5:e2223511. doi: 10.1001/jamanetworkopen.2022.23511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Abassa KK, Wu XY, Xiao XP, Zhou HX, Guo YW, Wu B. Effect of alcohol on clinical complications of hepatitis virus-induced liver cirrhosis: a consecutive ten-year study. BMC Gastroenterol. 2022;22:130. doi: 10.1186/s12876-022-02198-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Enomoto H, Ueno Y, Hiasa Y, Nishikawa H, Hige S, Takikawa Y, Taniai M, Ishikawa T, Yasui K, Takaki A, Takaguchi K, Ido A, Kurosaki M, Kanto T, Nishiguchi S Japan Etiology of Liver Cirrhosis Study Group in the 54th Annual Meeting of JSH. The transition in the etiologies of hepatocellular carcinoma-complicated liver cirrhosis in a nationwide survey of Japan. J Gastroenterol. 2021;56:158–167. doi: 10.1007/s00535-020-01748-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jepsen P, West J. We need stronger evidence for (or against) hepatocellular carcinoma surveillance. J Hepatol. 2021;74:1234–1239. doi: 10.1016/j.jhep.2020.12.029. [DOI] [PubMed] [Google Scholar]
- 136.Singal AG, Zhang E, Narasimman M, Rich NE, Waljee AK, Hoshida Y, Yang JD, Reig M, Cabibbo G, Nahon P, Parikh ND, Marrero JA. HCC surveillance improves early detection, curative treatment receipt, and survival in patients with cirrhosis: A meta-analysis. J Hepatol. 2022;77:128–139. doi: 10.1016/j.jhep.2022.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Singal AG, Patibandla S, Obi J, Fullington H, Parikh ND, Yopp AC, Marrero JA. Benefits and Harms of Hepatocellular Carcinoma Surveillance in a Prospective Cohort of Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2021;19:1925–1932.e1. doi: 10.1016/j.cgh.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Woolen SA, Singal AG, Davenport MS, Troost JP, Khalatbari S, Mittal S, Siddiqui S, Fobar A, Morris J, Odewole M, Tapper EB, Pillai A, Parikh ND. Patient Preferences for Hepatocellular Carcinoma Surveillance Parameters. Clin Gastroenterol Hepatol. 2022;20:204–215.e6. doi: 10.1016/j.cgh.2021.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kim NJ, Rozenberg-Ben-Dror K, Jacob DA, Rich NE, Singal AG, Aby ES, Yang JD, Nguyen V, Pillai A, Fuchs M, Moon AM, Shroff H, Agarwal PD, Perumalswami P, Chandna S, Zhou K, Patel YA, Latt NL, Wong R, Duarte-Rojo A, Lindenmeyer CC, Frenette C, Ge J, Mehta N, Yao F, Benhammou JN, Bloom PP, Leise M, Kim HS, Levy C, Barnard A, Khalili M, Ioannou GN. Provider Attitudes Toward Risk-Based Hepatocellular Carcinoma Surveillance in Patients With Cirrhosis in the United States. Clin Gastroenterol Hepatol. 2022;20:183–193. doi: 10.1016/j.cgh.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tzartzeva K, Obi J, Rich NE, Parikh ND, Marrero JA, Yopp A, Waljee AK, Singal AG. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology. 2018;154:1706–1718.e1. doi: 10.1053/j.gastro.2018.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wolf E, Rich NE, Marrero JA, Parikh ND, Singal AG. Use of Hepatocellular Carcinoma Surveillance in Patients With Cirrhosis: A Systematic Review and Meta-Analysis. Hepatology. 2021;73:713–725. doi: 10.1002/hep.31309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yeo YH, Hwang J, Jeong D, Dang N, Kam LY, Henry L, Park H, Cheung R, Nguyen MH. Surveillance of patients with cirrhosis remains suboptimal in the United States. J Hepatol. 2021;75:856–864. doi: 10.1016/j.jhep.2021.04.042. [DOI] [PubMed] [Google Scholar]
- 143.Parikh ND, Tayob N, Al-Jarrah T, Kramer J, Melcher J, Smith D, Marquardt P, Liu PH, Tang R, Kanwal F, Singal AG. Barriers to Surveillance for Hepatocellular Carcinoma in a Multicenter Cohort. JAMA Netw Open. 2022;5:e2223504. doi: 10.1001/jamanetworkopen.2022.23504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Singal AG, Tiro JA, Murphy CC, Blackwell JM, Kramer JR, Khan A, Liu Y, Zhang S, Phillips JL, Hernaez R. Patient-Reported Barriers Are Associated With Receipt of Hepatocellular Carcinoma Surveillance in a Multicenter Cohort of Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2021;19:987–995.e1. doi: 10.1016/j.cgh.2020.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Audureau E, Carrat F, Layese R, Cagnot C, Asselah T, Guyader D, Larrey D, De Lédinghen V, Ouzan D, Zoulim F, Roulot D, Tran A, Bronowicki JP, Zarski JP, Riachi G, Calès P, Péron JM, Alric L, Bourlière M, Mathurin P, Blanc JF, Abergel A, Chazouillères O, Mallat A, Grangé JD, Attali P, d'Alteroche L, Wartelle C, Dao T, Thabut D, Pilette C, Silvain C, Christidis C, Nguyen-Khac E, Bernard-Chabert B, Zucman D, Di Martino V, Sutton A, Pol S, Nahon P ANRS CO12 CirVir group. Personalized surveillance for hepatocellular carcinoma in cirrhosis - using machine learning adapted to HCV status. J Hepatol. 2020;73:1434–1445. doi: 10.1016/j.jhep.2020.05.052. [DOI] [PubMed] [Google Scholar]
- 146.Singal AG, Chen Y, Sridhar S, Mittal V, Fullington H, Shaik M, Waljee AK, Tiro J. Novel Application of Predictive Modeling: A Tailored Approach to Promoting HCC Surveillance in Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2022;20:1795–1802.e2. doi: 10.1016/j.cgh.2021.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. 2020;383:2451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 148.Afify S, Eysa B, Hamid FA, Abo-Elazm OM, Edris MA, Maher R, Abdelhalim A, Abdel Ghaffar MM, Omran DA, Shousha HI. Survival and outcomes for co-infection of chronic hepatitis C with and without cirrhosis and COVID-19: A multicenter retrospective study. World J Gastroenterol. 2021;27:7362–7375. doi: 10.3748/wjg.v27.i42.7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mikolasevic I, Bozic D, Pavić T, Ruzic A, Hauser G, Radic M, Radic-Kristo D, Razov-Radas M, Puljiz Z, Milic S. Liver disease in the era of COVID-19: Is the worst yet to come? World J Gastroenterol. 2021;27:6039–6052. doi: 10.3748/wjg.v27.i36.6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kim D, Bonham CA, Konyn P, Cholankeril G, Ahmed A. Mortality Trends in Chronic Liver Disease and Cirrhosis in the United States, Before and During COVID-19 Pandemic. Clin Gastroenterol Hepatol. 2021;19:2664–2666.e2. doi: 10.1016/j.cgh.2021.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Marjot T, Webb GJ, Barritt AS 4th, Moon AM, Stamataki Z, Wong VW, Barnes E. COVID-19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol. 2021;18:348–364. doi: 10.1038/s41575-021-00426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gonzalez HC, Zhou Y, Nimri FM, Rupp LB, Trudeau S, Gordon SC. Alcohol-related hepatitis admissions increased 50% in the first months of the COVID-19 pandemic in the USA. Liver Int. 2022;42:762–764. doi: 10.1111/liv.15172. [DOI] [PubMed] [Google Scholar]
- 153.Huang W, Zhou H, Hodgkinson C, Montero A, Goldman D, Chang SL. Network Meta-Analysis on the Mechanisms Underlying Alcohol Augmentation of COVID-19 Pathologies. Alcohol Clin Exp Res. 2021;45:675–688. doi: 10.1111/acer.14573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Szajnoga D, Klimek-Tulwin M, Piekut A. COVID-19 lockdown leads to changes in alcohol consumption patterns. Results from the Polish national survey. J Addict Dis. 2021;39:215–225. doi: 10.1080/10550887.2020.1848247. [DOI] [PubMed] [Google Scholar]
- 155.Deutsch-Link S, Curtis B, Singal AK. Covid-19 and alcohol associated liver disease. Dig Liver Dis. 2022 doi: 10.1016/j.dld.2022.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Portincasa P, Krawczyk M, Smyk W, Lammert F, Di Ciaula A. COVID-19 and non-alcoholic fatty liver disease: Two intersecting pandemics. Eur J Clin Invest. 2020;50:e13338. doi: 10.1111/eci.13338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Barritt AS 4th, Jiang Y, Schmidt M, Hayashi PH, Bataller R. Charges for Alcoholic Cirrhosis Exceed All Other Etiologies of Cirrhosis Combined: A National and State Inpatient Survey Analysis. Dig Dis Sci. 2019;64:1460–1469. doi: 10.1007/s10620-019-5471-7. [DOI] [PubMed] [Google Scholar]
- 158.Flemming JA, Djerboua M, Groome PA, Booth CM, Terrault NA. NAFLD and Alcohol-Associated Liver Disease Will Be Responsible for Almost All New Diagnoses of Cirrhosis in Canada by 2040. Hepatology. 2021;74:3330–3344. doi: 10.1002/hep.32032. [DOI] [PubMed] [Google Scholar]
- 159.Llamosas-Falcón L, Shield KD, Gelovany M, Manthey J, Rehm J. Alcohol use disorders and the risk of progression of liver disease in people with hepatitis C virus infection - a systematic review. Subst Abuse Treat Prev Policy. 2020;15:45. doi: 10.1186/s13011-020-00287-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Peeraphatdit TB, Ahn JC, Choi DH, Allen AM, Simonetto DA, Kamath PS, Shah VH. A Cohort Study Examining the Interaction of Alcohol Consumption and Obesity in Hepatic Steatosis and Mortality. Mayo Clin Proc. 2020;95:2612–2620. doi: 10.1016/j.mayocp.2020.04.046. [DOI] [PubMed] [Google Scholar]
- 161.Rehm J, Patra J, Brennan A, Buckley C, Greenfield TK, Kerr WC, Manthey J, Purshouse RC, Rovira P, Shuper PA, Shield KD. The role of alcohol use in the aetiology and progression of liver disease: A narrative review and a quantification. Drug Alcohol Rev. 2021;40:1377–1386. doi: 10.1111/dar.13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tran A, Jiang H, Lange S, Manthey J, Štelemėkas M, Badaras R, Petkevičienė J, Radišauskas R, Room R, Rehm J. Can alcohol control policies reduce cirrhosis mortality? Liver Int. 2022;42:765–774. doi: 10.1111/liv.15151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rodríguez M, González-Diéguez ML, Varela M, Cadahía V, Andrés-Vizán SM, Mesa A, Castaño A, Alvarez-Navascués C. Impact of Alcohol Abstinence on the Risk of Hepatocellular Carcinoma in Patients With Alcohol-Related Liver Cirrhosis. Am J Gastroenterol. 2021;116:2390–2398. doi: 10.14309/ajg.0000000000001399. [DOI] [PubMed] [Google Scholar]