Three v-SNAREs and Two t-SNAREs, Present in a Pentameric cis-SNARE Complex on Isolated Vacuoles, Are Essential for Homotypic Fusion (original) (raw)
Abstract
Vacuole SNAREs, including the t-SNAREs Vam3p and Vam7p and the v-SNARE Nyv1p, are found in a multisubunit “cis” complex on isolated organelles. We now identify the v-SNAREs Vti1p and Ykt6p by mass spectrometry as additional components of the immunoisolated vacuolar SNARE complex. Immunodepletion of detergent extracts with anti-Vti1p removes all the Ykt6p that is in a complex with Vam3p, immunodepletion with anti-Ykt6p removes all the Vti1p that is complexed with Vam3p, and immunodepletion with anti-Nyv1p removes all the Ykt6p in complex with other SNAREs, demonstrating that they are all together in the same cis multi-SNARE complex. After priming, which disassembles the cis-SNARE complex, antibodies to any of the five SNARE proteins still inhibit the fusion assay until the docking stage is completed, suggesting that each SNARE plays a role in docking. Furthermore, vti1 temperature-sensitive alleles cause a synthetic fusion-defective phenotype in our reaction. Our data show that vacuole-vacuole fusion requires a cis-SNARE complex of five SNAREs, the t-SNAREs Vam3p and Vam7p and the v-SNAREs Nyv1p, Vti1p, and Ykt6p.
Keywords: SNAREs, membrane fusion, yeast vacuoles, NSF, α-SNAP
The targeting of vesicles to their destination in the secretory pathway depends on several layers of specificity. The GTPases of the Rab/Ypt family are critical for virtually every vesicle trafficking step (Novick and Zerial, 1997). In addition, membrane proteins with cytosolic coiled-coil domains, termed SNAREs, are found on vesicles (v-SNAREs) and organelles (t-SNAREs). It has been proposed that SNAREs act in a lock-key mechanism to specify the docking of vesicles with their target membrane and even to catalyze their fusion (Söllner et al., 1993; Ferro-Novick and Jahn, 1994; Rothman, 1994; Hay and Scheller, 1997; Weber et al., 1998; Weis and Scheller, 1998).
SNAREs are found in multisubunit complexes together with two soluble proteins, the ATPase NSF and its cofactor α-SNAP, on organelles and on vesicle membranes (Walch-Solimena et al., 1995; Otto et al., 1997; Swanton et al., 1998; Ungermann et al., 1998a; Ungermann and Wickner, 1998). Studies of yeast vacuole fusion have shown that ATP hydrolysis by yeast NSF (Sec18p) causes release of yeast α-SNAP (Sec17p) from the membrane (Mayer et al., 1996) and disassembly of the SNARE complex (Söllner et al., 1993), enabling the individual SNAREs to participate in the downstream docking reaction (Nichols et al., 1997; Ungermann et al., 1998a). Docking of vesicles with their target membrane also involves tethering by velcro factors and Rab proteins (Pfeffer, 1996). In yeast, for example, Uso1p and the GTPase Ypt1p tether ER-derived vesicles to the Golgi apparatus before the action of SNAREs (Cao et al., 1998). Likewise, the mammalian Uso1p homologue p115 interacts with GM130 to promote the fusion of Golgi vesicles after mitosis (Löwe et al., 1998). Other possible tethering factors include rabaptin 5 (Stenmark et al., 1995), Vac1p (Burd et al., 1997), and EEA1 (Mills et al., 1998; Simonsen et al., 1998; Christoforidis et al., 1999). Recent studies on the homotypic fusion of yeast vacuoles suggest that the docking reaction can be subdivided into a reversible tethering reaction mediated by the GTPase Ypt7p and a subsequent pairing of the SNAREs in trans (Ungermann et al., 1998b). The mechanism of the final fusion step is still unclear. SNAREs have been implicated as fusion catalysts based on their ability to mediate lipid exchange in a reconstituted fusion assay (Weber et al., 1998). However, trans-SNARE pairs can be disassembled by Sec18p without influencing the fusion rate, suggesting that SNARE pairs may not be the proximal fusion catalysts (Ungermann et al., 1998b). Similarly, the fusion of cortical granules in sea urchin eggs can be preceded by a Ca2+-dependent disassembly of the SNARE complex without affecting the fusion rate (Coorssen et al., 1998; Tahara et al., 1998). Furthermore, yeast vacuoles require Ca2+ and calmodulin and a phosphatase for the fusion step per se (Conradt et al., 1992; Peters and Mayer, 1998), suggesting that these proteins act after the SNAREs.
We have identified a cis-SNARE complex on the vacuole membrane which contains the t-SNAREs Vam3p and Vam7p and the v-SNARE Nyv1p as well as Sec17p (α-SNAP), Sec18p (NSF), and LMA1 (Ungermann et al., 1998a; Ungermann and Wickner, 1998; Xu et al., 1998). We now show that two additional SNAREs, Vti1p and Ykt6p, are physically and functionally part of this complex. Vti1p has been previously characterized as an essential v-SNARE required for trafficking between the Golgi apparatus and the vacuole (Fischer von Mollard et al., 1997; Lupashin et al., 1997). Ykt6p was initially identified in a complex with the Golgi t-SNARE Sed5p (Søgaard et al., 1994). It does not have a transmembrane domain, but is prenylated and may partition between the cytosol and membranes (McNew et al., 1997). Both proteins are essential for viability, interact genetically, and were suggested to be involved in retrograde trafficking to the cis-Golgi membrane (Fischer von Mollard et al., 1997; Lupashin et al., 1997). Our data show that these proteins are components of a heteropentameric SNARE complex and that each of the subunits has a vital role in homotypic vacuole fusion.
Materials and Methods
Yeast Strains
Temperature-sensitive (ts)1 alleles in VTI1 were introduced into yeast strains BJ3505 and DKY6281 by transformation and loop in-loop out of plasmids containing the vti1 ts alleles and a URA3 marker at the VTI1 locus (Fischer von Mollard et al., 1997). Ura+ transformants were selected and Ura− clones which were generated in a second selection with 5-fluoroorotic acid were tested for loss of the wild-type VTI1 sequences by their ts growth and CPY-secretion phenotypes (Fischer von Mollard et al., 1997).
Biochemical Methods
Reagents were as described by Haas (1995), Mayer et al. (1996), and Haas and Wickner (1996). SDS-PAGE, immunoblotting using ECL (Haas et al., 1994), and purification of IgGs and his6-tagged Sec18p (Haas and Wickner, 1996) were as described. Rabbit antibodies were generated against Ni-NTA purified His6-Ykt6 protein and His6-Nyv1p that was overproduced in Escherichia coli. For coimmunoprecipitations, vacuoles were sedimented (10 min, 8,000 g, 4°C) after any priming reaction with ATP, washed with 500 μl PS buffer (10 mM Pipes/KOH, pH 6.8, 200 mM sorbitol), and detergent solubilized in 1 ml of buffer A (1% digitonin, 50 mM NaCl, 20 mM Hepes/KOH, pH 7.4, 2 mM EDTA, 1× PIC [Xu and Wickner, 1996], 1 mM PMSF, and 10 μg/ml α2-macroglobulin). The detergent extract was placed onto a nutator for 10 min at 4°C, the insoluble material was removed by centrifugation (10 min, 16,000 g), and the supernatant was applied to protein A–immobilized IgGs (Harlow and Lane, 1988; Ungermann et al., 1998a). Incubations, washes, and elution of bound proteins were as described (Ungermann et al., 1998a).
Purification of the Vam3p Complex
His6-Vam3p was immobilized on Aminolink resin (Pierce) and used as an affinity matrix to purify antibodies to Vam3p. Affinity-purified antibodies (200 ng) and an equal amount of nonimmune rabbit IgGs were covalently linked to 1 ml protein A–Sepharose (_Amersham_-Pharmacia; Harlow and Lane, 1988). Vacuoles were prepared by a batch purification. Cells from 6-liter overnight cultures were lysed with oxalyticase and DEAE dextran as described (Haas, 1995). After heat shock, cell lysates were chilled on ice, diluted with 15% Ficoll in PS buffer (200 mM sorbitol, 10 mM Pipes/ KOH, pH 6.8) to 4% Ficoll (final concentration), and transferred to 60Ti tubes (Beckman). Lysates were centrifuged (50,000 rpm, 4°C, 60 min, 60Ti rotor) and vacuoles harvested from the top, diluted 20-fold with cold PS buffer, and centrifuged (JA20, 10,000 rpm, 10 min, 4°C). The vacuole pellet was resuspended in PS buffer.
For purification of the SNARE complex, 26 mg of vacuoles was lysed in 10 ml of 1.5% Triton X-100, PBS (Harlow and Lane, 1988), pH 7.4, 2 mM EDTA, 1× PIC (Xu and Wickner, 1996), and 1 mM PMSF (lysis buffer). After 30 min at 4°C on a nutator, the detergent extract was centrifuged for 30 min in a 60Ti rotor at 4°C, 35,000 rpm. The supernatant was collected and incubated on a nutator for 1.5 h at 4°C with 3 ml of a protein A resin bearing nonimmune IgGs. The flow through was collected, reapplied to fresh resin, and incubated as before. Three such sequential preadsorption steps were performed. The sample was then halved. One half was applied to a control resin, the other to the immobilized affinity-purified antibodies to Vam3p. The detergent extracts were incubated with the resins for 18 h on a nutator at 4°C. The flow throughs were collected and the resins were washed with 50 ml of 150 mM, 350 mM, and 500 mM NaCl in lysis buffer. Bound proteins were eluted with 4 ml 0.1 M glycine/HCl, pH 2.6, 0.025% Triton X-100, precipitated by TCA, washed with 1 ml of ice-cold acetone, and dried at 56°C for 5 min. Aliquots were analyzed by SDS-PAGE and Coomassie blue–stained or transferred to nitrocellulose for immunoblotting.
Proteins were identified by comparing their tryptic peptide mass maps to the Saccharomyces cerevisiae sequence database (Jensen et al., 1998). Protein bands were excised from the gel, rinsed, and the protein samples were digested with trypsin in the gel matrix (Shevchenko et al., 1996). Extracted peptide mixtures were analyzed by matrix-assisted laser desorption/ionization (MALDI) time-of-flight mass spectrometry (REFLEX; Bruker Daltonics). The peptide mass maps were used to query a comprehensive sequence database for unambiguous protein identification (PeptideSearch software, provided by M. Mann and P. Mortensen, EMBL) (Jensen et al., 1996, 1997).
Vacuole Fusion
Vacuole fusion is measured by a biochemical complementation assay (Conradt et al., 1992; Haas et al., 1994). Vacuoles from DKY6821 have normal proteases but lack the membrane protein alkaline phosphatase. Vacuoles from BJ3505 accumulate alkaline phosphatase in the unprocessed and catalytically inactive “pro” form due to the deletion of the gene encoding the protease Pep4p. Incubation of a mixture of these vacuoles in reaction buffer at 27°C in the presence of cytosol and ATP leads to fusion, content mixing, and processing of pro-alkaline phosphatase by Pep4p. The active alkaline phosphatase is measured by a colorimetric assay at the end of the fusion reaction.
Vacuoles (Haas, 1995) were used immediately after isolation. The standard fusion reaction (30 μl) contained 3 μg of each vacuole type (BJ3505 and DKY6281) in reaction buffer (10 mM Pipes/KOH, pH 6.8, 200 mM sorbitol, 150 mM KCl, 0.5 mM MgCl2, 0.5 mM MnCl2), 0.5 mM ATP, 3 μg/ml cytosol, 3.5 U/ml creatine kinase, 20 mM creatine phosphate, and a protease inhibitor cocktail (PIC; Xu and Wickner, 1996) containing 7.5 μM pefabloc SC, 7.5 ng/ml leupeptin, 3.75 μM _o_-phenanthroline, and 37.5 ng/ml pepstatin. To reduce proteolysis in the coimmunoprecipitation experiments, only the protease A–deficient BJ3505 vacuoles were analyzed. One unit of fusion activity is defined as 1 μmol _p_-nitrophenol phosphate hydrolyzed per minute and milligram of BJ3505.
Results
Identification of Vti1p and Ykt6p as Part of the Vacuolar SNARE Complex
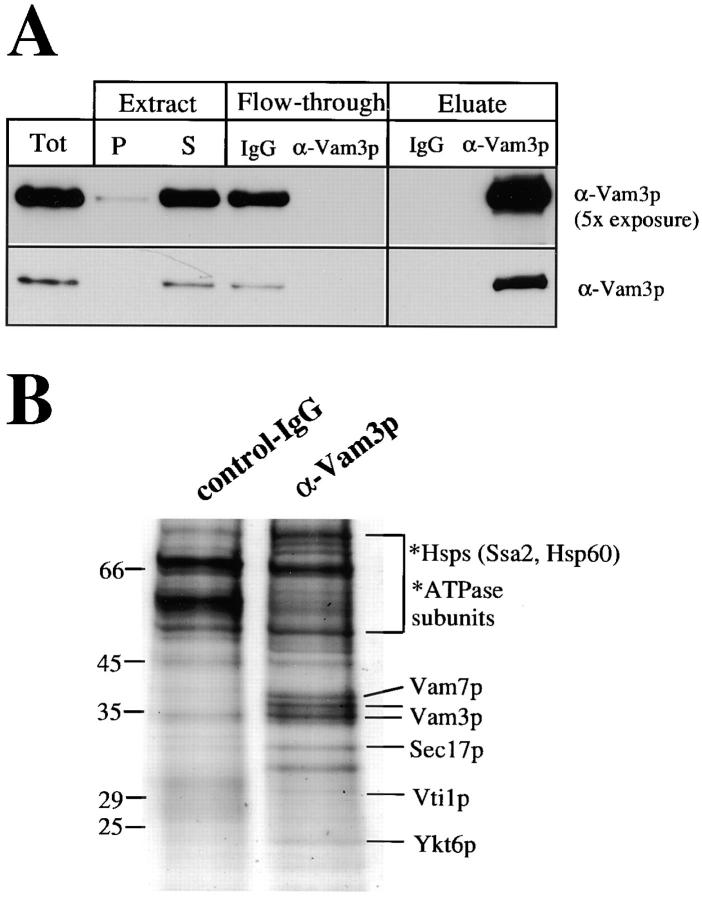
To identify proteins that interact with the vacuolar t-SNARE Vam3p, a detergent extract of vacuoles was incubated with immobilized affinity-purified antibodies to Vam3p or with a control IgG resin. Retained proteins were eluted from each column and analyzed by SDS-PAGE and immunoblotting (Fig. 1 A) or Coomassie staining (Fig. 1 B). Vam3p was solubilized completely under the experimental conditions and was retained specifically on the anti–Vam3p-affinity column (compare flow through and eluate in Fig. 1 A). The Coomassie-stained protein bands (Fig. 1 B) were identified by peptide mapping by MALDI mass spectrometry combined with sequence database searching (Jensen et al., 1998). In addition to Vam3p, Vam7p, and Sec17p, two additional SNARE proteins were specifically eluted from the anti-Vam3p column and identified by MALDI mass spectrometry: Vti1p (Fig. 1 C; Fischer von Mollard et al., 1997), an essential v-SNARE implicated in Golgi to vacuole trafficking, and Ykt6p (Fig. 1 D), a v-SNARE previously implicated in trafficking through the Golgi (Søgaard et al., 1994; McNew et al., 1997). The vacuole cis-SNARE complex is neither SDS resistant nor as stable as the exocytic complex in neurons (Hayashi et al., 1994, 1995; Otto et al., 1997). Thus, washing the column in high salt removed a substantial amount of Nyv1p, though its presence in the cis-SNARE complex was confirmed by immunoblotting (data not shown). Both Vti1p and Ykt6p were found in substoichiometric amounts, consistent with their association to Vam3p being salt-sensitive (data not shown). Because of the lability of this cis-SNARE complex during immunoisolation, we cannot be sure that we have identified all its constituents, and the isolation of functional cis-SNARE complex for analysis in a reconstituted fusion assay (Sato and Wickner, 1998) may reveal additional components.
Figure 1.
Identification of Vti1p and Ykt6p in a complex with Vam3p. Vacuoles (26 μg) were purified and detergent solubilized in 10 ml of buffer A as described in Materials and Methods. Aliquots (50 μl) were removed for analysis of the lysate before (Tot) and after centrifugation (S). The insoluble pellet was resuspended in 10 ml of lysate buffer and 50 μl was removed (P). Proteins were TCA-precipitated, washed with ice-cold acetone, and resuspended in SDS-sample buffer. The detergent extract was preadsorbed with nonimmune IgGs as described in Materials and Methods, then halved and incubated with a control IgG resin and with protein A–immobilized affinity-purified anti-Vam3p antibodies. An aliquot of the flow through (50 μl) from each column was saved and proteins were TCA-precipitated, acetone-washed, and resuspended in sample buffer as above. The columns were washed and specifically retained proteins were eluted as described in Materials and Methods, precipitated by TCA, washed with 1 ml ice-cold acetone, and dissolved in 50 μl of SDS-sample buffer. Aliquots of Tot, P, S, and the flow through together with 2 μl of the eluted samples were analyzed by SDS-PAGE, transferred to nitrocellulose, and immunoblotted for Vam3p (A). The remaining eluted proteins from both columns were also analyzed by SDS-PAGE and stained with Coomassie brilliant blue (B). Specific protein bands were excised and trypsin digested, and the resulting peptide mixtures were analyzed by MALDI mass spectrometry. Identified proteins are indicated. The peptide mass maps of Vti1p (C) (6 peptides, 30% amino acid sequence coverage) and Ykt6p (D) (8 tryptic peptides, 53% amino acid sequence coverage) are shown.
Though Vti1p was found previously in a complex with Vam3p (Holthuis et al., 1998), Ykt6p has so far only been described as a Golgi-specific SNARE (Søgaard et al., 1994; Lupashin et al., 1997; McNew et al., 1997). We therefore used coimmunoprecipitation to test whether antibodies to each protein would immunoprecipitate the vacuolar cis-SNARE complex in a manner similar to antibodies to Vam3p. This was indeed the case (Fig. 2 A, lanes 1, 3, and 5). Upon ATP/Sec18p/Sec17p-dependent priming, the cis-SNARE complex disassembled (lanes 2, 4, and 6) and cross-immunoprecipitation was lost. While each of the three antibodies immunoprecipitated a disproportionate amount of its cognate protein, reflecting partial SNARE complex disassembly or in vitro lability, each also precipitated the other SNARE complex components (Nyv1p, Vam7p, and Sec17p) in a similar proportion. The composition of the SNARE complex was also examined by an independent approach. All members of the SNARE complex cosediment in a glycerol velocity gradient, suggesting that they are largely in a complex with each other (Fig. 2 B). Vti1p and Ykt6p do not completely copurify with Vam3p, in agreement with their role in other trafficking reactions (Fig. 2 C; Fischer von Mollard et al., 1997; Lupashin et al., 1997). They are both present in a complex with Vam3p on the vacuole membrane and behave similarly to previously identified members of the vacuolar SNARE complex. The localization of Vti1p and Ykt6p to the vacuole does not depend on previously characterized members of the SNARE complex (Fig. 2 C).
Figure 2.
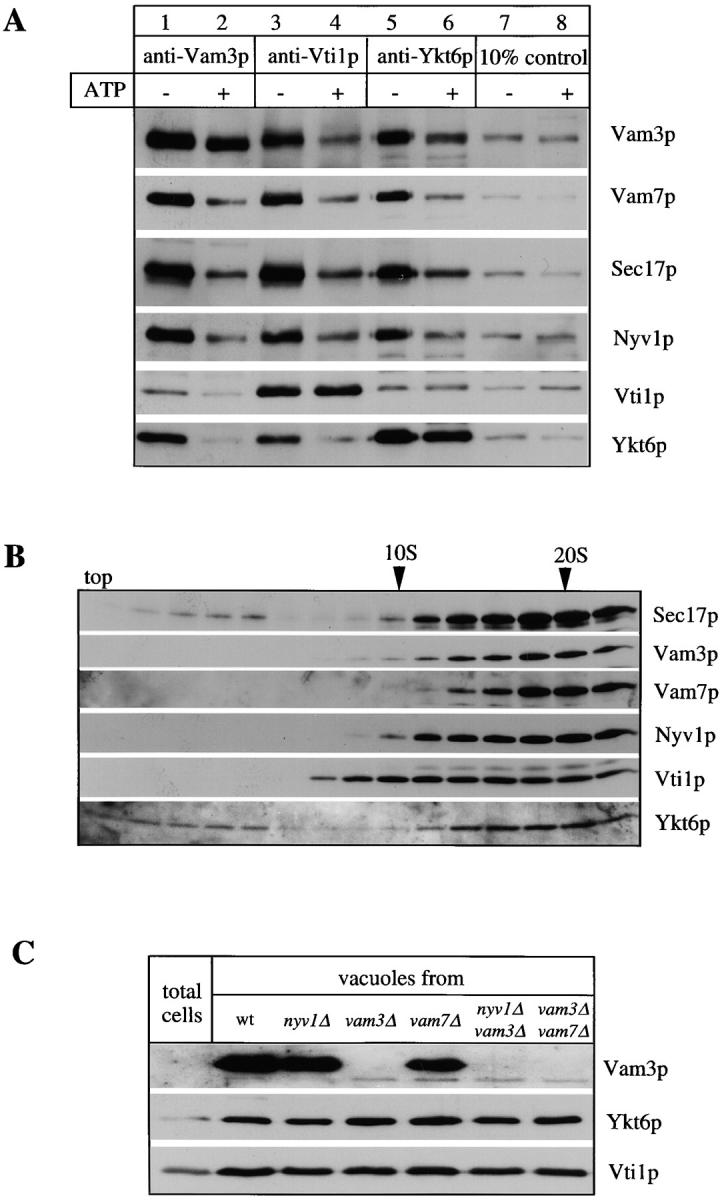
Vti1p and Ykt6p copurify with other members of the SNARE complex. (A) Coimmunoprecipitation analysis. Salt-washed vacuoles (100 μg) were resuspended in 750 μl of reaction buffer containing 1 mg/ml cytosol with or without ATP, 1.26 μg/ml his6-Sec18p, and an ATP-regenerating system (Haas, 1995). Reactions were incubated for 10 min at 27°C, vacuoles were reisolated (4 min, 8,000 g, 4°C), washed with 500 μl PS buffer, and detergent solubilized for 10 min at 0°C by adding 1 ml of lysis buffer (1% digitonin, 50 mM NaCl, 2 mM EDTA, 1 mM PMSF, 30 μM pefablock SC, 30 ng/ml leupeptin, 15 μM _o_-phenanthroline, 150 ng/ ml pepstatin). Insoluble material was removed by centrifugation (10 min, 20,000 g, 4°C) and protein complexes were analyzed by coimmunoprecipitation with protein A–immobilized antibodies to Vam3p, Vti1p (Fischer von Mollard et al., 1997), and Ykt6p as described (Ungermann et al., 1998). A portion (10%) of the total detergent extract was TCA precipitated. Proteins retained on the protein A–immunoglobulin beads were released by addition of 1 ml 0.1 M glycine/Cl, pH 2.6, precipitated by TCA, washed with ice-cold acetone and solubilized in SDS-sample buffer, analyzed by SDS-PAGE, transferred to nitrocellulose, and decorated with antibodies to Sec17p, Vam3p, Vam7p, Vti1p, Ykt6p, and Nyv1p. A greater percentage of Vti1p was typically dissociated from Ykt6p upon incubation with ATP than seen here (lanes 5 and 6; see, for example, lanes 3 and 4). (B) Vacuolar SNAREs comigrate on a glycerol gradient. Isolated wild-type vacuoles (300 μg) were solubilized for 10 min on ice as in A. The cleared detergent extract was loaded on a linear 10–34% glycerol gradient. The samples were centrifuged for 18 h at 4°C (40,000 rpm, Beckman SW41). Fractions (500 μl) were collected from top to bottom, proteins were precipitated with TCA, analyzed by SDS-PAGE, transferred to nitrocellulose, and decorated with antibodies to Vam3p, Vam7p, Sec17p, Ykt6p, Nyv1p, and Vti1p. The bottom of the gradient did not contain any detectable protein complexes and is not shown here. (C) Localization of Ykt6p and Vti1p to the vacuole. Proteins from total yeast extract and isolated wild-type and mutant vacuoles were resolved by SDS-PAGE and analyzed as in B. The Vam3p band in the total extract shows up only after long overexposure (data not shown), demonstrating the enrichment of Vam3p in the vacuolar fraction.
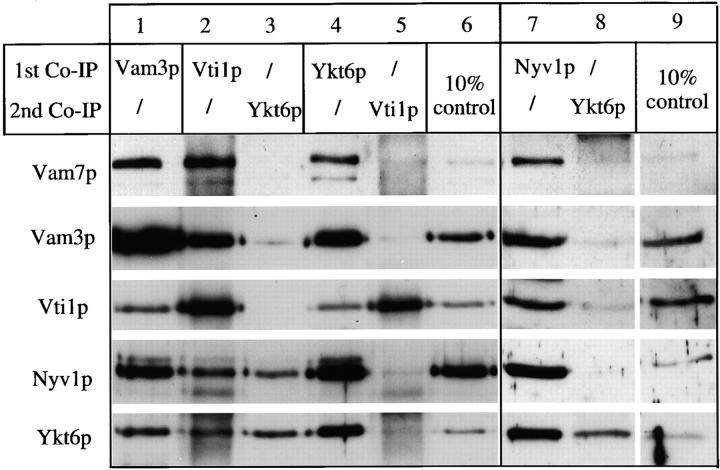
To determine directly whether Vti1p, Nyv1p, and Ykt6p are in the same SNARE complex with each other and with the other SNAREs, successive immunoprecipitations were performed (Fig. 3). The same SNAREs were recovered in immunoprecipitates with antibodies to Vam3p (Fig. 3, lane 1), Vti1p (lane 2), Ykt6p (lane 4), or Nyv1p (lane 7). Some Ykt6p remained in the supernatant after immunoprecipitation with antibody to Vti1p, but it was not in complex with Vam3p or Vam7p (lane 3). Similarly, some Vti1p remained in the supernatant after immunoprecipitation with antibody to Ykt6p, but it was not in complex with Vam3p or Vam7p (lane 5). Immunoprecipitation with antibody to Nyv1p (lane 6) removed almost all the Ykt6p, leaving only a small amount that is not in complex with other SNAREs (lane 7). We conclude that the cis-SNARE complex contains Vam3p, Vam7p, Vti1p, Nyv1p, and Ykt6p and is at least pentameric for SNAREs.
Figure 3.
The cis-SNARE complex is pentameric for SNAREs. Salt-washed vacuoles (100 μg) were detergent solubilized by incubation for 10 min at 0°C in 1 ml of lysis buffer. Insoluble material was removed by centrifugation (10 min, 20,000 g, 4°C) and protein complexes were analyzed by coimmunoprecipitation with protein A–immobilized antibodies to Vam3p, Vti1p, Ykt6p, and Nyv1p as described (Ungermann et al., 1998a). A portion (10%) of the total detergent extract was TCA precipitated (lanes 6 and 8). Supernatants of the first immunoprecipitation were incubated for another 2 h at 4°C with protein A–immobilized IgGs to Ykt6p (lanes 3 and 7) or Vti1p (lane 5) and washed as described (Ungermann et al., 1998a). The immunoprecipitations shown in lanes 1–6 and lanes 7–9 were done in independent experiments. Proteins retained on the protein A–immunoglobulin beads in the first or second immunoprecipitation were analyzed as described in Fig. 2.
Vti1p and Ykt6p Are Required for Homotypic Vacuole Fusion
The above physical data establish that Vtilp and Ykt6p are present in a cis-complex with Vam3p, Nyvlp, and Vam7p. However, we have noted (Ungermann et al., 1998b) that only a small percentage of the SNAREs enters the trans-complex, and thus functional criteria are needed to establish that each SNARE is part of a functional complex. Such experiments address the possibility that a small percentage of the SNAREs in a tetrameric complex might be the active complex for fusion, while the fifth SNARE was present but irrelevant for function. Three lines of evidence show that Vti1p is directly involved in the fusion reaction. First, antibodies to Vti1p inhibit fusion to a similar extent as Vam3p antibodies when added at different times to an ongoing reaction (Fig. 4 A). Antibodies to Vti1p, Sec17p, or Vam3p were added to aliquots from a fusion reaction at several times and fusion was continued for a total of 90 min at 27°C (Fig. 4 A). All antibodies inhibited the reaction thoroughly when added at the beginning. Whereas Sec17p completes its action during the early priming step (Mayer et al., 1996), Vam3p acts at a later docking stage and thus antibodies to Vam3p inhibit at later times (Nichols et al., 1997; Ungermann et al., 1998a). Antibodies to Vti1p inhibit the reaction in a kinetic fashion similar to Vam3p antibodies, suggesting that they may act at the same reaction. Second, Vti1p function depends on vacuole mixing. Vacuoles from the two tester strains were incubated in the presence of ATP in separate tubes. At the indicated times, aliquots from each tube were mixed in the absence or presence of antibodies to Vam3p, Vti1p, or Sec17p. Of these three proteins, only Sec17p function can be fulfilled early in the reaction (Fig. 4 B; Mayer et al., 1996). The reaction remains sensitive to antibodies to Vam3p and Vti1p, showing that the completion of the function of these proteins depends on vacuole contact. Third, previously characterized vti1 ts alleles (Fischer von Mollard et al., 1997) were introduced into the tester strains and analyzed in the fusion reaction. Vacuoles were purified from all six wild-type and vti1 ts mutant strains and tested in all combinations. To induce the phenotype of the ts allele, vacuoles were mixed and preincubated at the indicated temperatures without ATP for the times shown. The ts alleles are much more thermolabile in the protease-plus DKY background than in the protease-minus BJ vacuoles, perhaps because partially thermally altered mutant Vtilp is more susceptible to proteolysis. When combined with a wild-type partner, only the ts alleles in the DKY background show a ts phenotype which is strongly induced at elevated temperatures. Strikingly, combination of vacuoles with vti1 ts alleles leads to a synthetic fusion phenotype, as even vacuoles that were only preincubated on ice retained only 5–10% fusion activity (DKY vti1-1/BJ vti1-2 and DKY vti1-2/BJ vti1-2). However, both vacuole partners show up to 60% fusion with the wild-type partner. Though some of the effects observed may be due to enhanced protease sensitivity of the ts alleles of Vti1p, the synthetic fusion phenotype strongly implies that Vti1p is directly involved in the reaction, as priming (as judged by Sec17p release and SNARE complex disassembly) is not altered after induction of the ts phenotype (not shown). Finally, Ykt6p antibodies inhibit with similar kinetics as Vti1p antibodies when added to a fusion reaction (Fig. 4 D) and acquisition of resistance to antibodies to Ykt6p requires docking (Fig. 4 E).
Figure 4.

Vti1p and Ykt6p are part of the complex required for homotypic vacuole fusion. (A) Coincident acquisition of resistance to anti-Vam3p and anti-Vti1p during the fusion reaction. A 30-fold scale fusion reaction was started in the presence of ATP at 27°C. Aliquots (30 μl) were removed at indicated times and placed on ice or added to antibodies to Sec17p, Vam3p, or Vti1p and incubated at 27°C. Fusion reactions were incubated for a total of 90 min at 27°C before being assayed for alkaline phosphatase activity. (B) Vti1p action depends on vacuole docking. Two 10-fold reactions with vacuoles from either BJ3505 or from DKY6281 were incubated at 27°C in the presence of ATP. At indicated times, 15-μl portions were removed from each reaction and combined in the presence of buffer or antibodies to either Sec17p, Vam3p, or Vti1p and incubated for 90 min at 27°C. One aliquot was combined in the presence of buffer and placed on ice for the remaining reaction period. After 90 min, all reactions were analyzed for fusion. (C) Ts alleles in Vti1p cause a synthetic fusion phenotype. Cells were grown at 26°C overnight. Vacuoles were isolated from all strains and diluted in PS buffer to 0.3 mg/ ml. BJ and DKY vacuoles with wild-type, vti1-1, or vti1-2 alleles (10 μl) were combined and incubated at the indicated temperatures for 5 or 10 min and then chilled on ice. Cytosol, the ATP-regenerating system, and salts were added in a 10-μl volume and reactions were incubated for 90 min at 27°C and assayed for alkaline phosphatase. Wild-type fusion was set to 100%. (D) As in A, but antibodies to Ykt6p were added at the indicated times. (E) As in B, but antibodies to Yktp were added at the indicated times as noted.
Thus, three v-SNAREs, Nyv1p, Vti1p, and Ykt6p, are required for the docking stage of vacuole-vacuole fusion. Since they are dissociated from the cis-SNARE complex during priming, these data suggest that inhibition by antibody to each SNARE is not due to steric hindrance of access to another SNARE. Rather, these data indicate that each SNARE has a functional role in the reaction. The complete sensitivity of fusion to antibodies to Vti1p and Ykt6p, or to ts alleles in Vti1p, suggests that each of these proteins is fully involved in the reaction rather than being in redundant tetrameric complexes of Vam3p/Vam7p/ Nyv1p/Vti1p and Vam3p/Vam7p/Nyv1p/Ykt6p.
Discussion
Vti1p and Ykt6p are components of the vacuolar SNARE complex, as both proteins copurify with the SNARE complex and antibodies to both Vti1p and Ykt6p precipitate all the previously identified members of this complex. This does not simply reflect an exchange of SNAREs from other contaminating organelles into association with Vam3p in detergent extracts, as inhibition studies with antibodies to Vti1p and Ykt6p and the synthetic fusion phenotype of ts alleles in Vti1p in our tester vacuoles indicate that both proteins are part of a SNARE complex with a functional role in the fusion reaction. Kinetic inhibition curves with antibodies to Vti1p and Ykt6p are indistinguishable from those reported for antibodies to the previously identified vacuolar SNAREs Vam3p, Vam7p, and Nyv1p (Fig. 3; Nichols et al., 1997; Ungermann et al., 1998a; Ungermann and Wickner, 1998).
Our data establish that each of these SNAREs— Vam7p, Nyv1p, Vtilp, and Ykt6p—has a role in the reaction, though these roles need not be unique. Before priming, the effects of ts mutants (Fig. 4) or of deleting SNAREs (Nichols et al., 1997; Ungermann and Wickner, 1998) could be due to allosteric effects on neighboring SNARE complex subunits. Similarly, antibodies which bind to one SNARE could inactivate the function of a pentameric cis-SNARE complex by obstructing access of a crucial protein or ligand to another SNARE. However, these concerns are vitiated by the observation that all SNAREs are disassembled from the complex during ATP-dependent priming (Fig. 2 A) and thus are not associated during docking while the reaction remains sensitive to each anti-SNARE antibody during docking (Nichols et al., 1997; Ungermann et al., 1998a; Ungermann and Wickner, 1998; Fig. 4, A and D). The sensitivities to each of these antibodies is a strong argument that each subunit of the pentameric cis-SNARE complex has some role in the overall reaction.
Our data suggest that three v-SNAREs, Nyv1p, Vti1p, and Ykt6p, participate in the fusion reaction. This is not without precedent as Vti1p has been recovered in a complex with Vam3p (Holthuis et al., 1998) and Ykt6p has been shown to be a weak multicopy suppressor of Vti1p (Lupashin et al., 1997). What could be the role of three v-SNAREs in the vacuole fusion reaction? The resolution of the crystal structure of the neuronal SNARE complex (Sutton et al., 1998) and the analysis of the exocytic SNARE complex in yeast (Katz et al., 1998) and neurons (Poirier et al., 1998) have led to the proposal that the core of a SNARE complex consists of four parallel coiled-coil domains provided by three proteins: syntaxin, synaptobrevin, and SNAP-25 and their homologues. The alignment of all SNAREs at their coiled-coil domains identifies a conserved glutamine (Q) in one set of SNAREs (mainly t-SNAREs and some v-SNAREs like Bet1p and Vti1p) and a conserved arginine (R) in another set (most v-SNAREs including Nyv1p and Ykt6p; Fasshauer et al., 1998). Based on these findings, Fasshauer et al. (1998) propose that each SNARE complex consists of three Q-SNARE coiled-coils (e.g., one from syntaxin, and two from SNAP25) and one R-SNARE coiled-coil (e.g., one from synaptobrevin; Sutton et al., 1998). How does this compare to data for the vacuolar SNARE complex? We already know of five SNAREs in our complex, the t-SNARE Vam3p (or Q-SNARE), the SNAP-25/23 homologue Vam7p (Q), and the v-SNAREs Vti1p (Q), Nyv1p (R), and Ykt6p (R). Vam3p and Vam7p are found in a tight complex on the vacuole (Sato et al., 1998; Ungermann and Wickner, 1998). Whereas SNAP-25 provides two coiled-coil domains to the neuronal SNARE complex, Vam7p provides only one (Weimbs et al., 1997). The third Q-SNARE coiled-coil could therefore come from Vti1p, which has been previously considered a v-SNARE. Either Nyv1p or Ykt6p would then be the required R-SNARE. However, both proteins are part of the same cis-SNARE complex (Fig. 3) and antibodies to either protein inhibit the fusion reaction (Fig. 4, D and E; Ungermann et al., 1998a). Furthermore, vacuoles lacking Nyv1p fuse only poorly, if at all, with each other (Nichols et al., 1997), suggesting an essential role of Nyv1p in the fusion reaction. In fact, Nyv1p is not required for any of the trafficking reactions to the vacuole (Fischer von Mollard and Stevens, 1999), but appears to be exclusively reserved for vacuole fusion. Thus, at least portions of the intracellular pool of all five of these SNAREs are in a complex with each other, which may define a new, five coiled-coil core of a SNARE complex.
Not all of the vacuolar SNAREs are recovered in a cis complex. The proportion is highest with salt-washed vacuoles, possibly due to removal of Sec18p (not shown). This might reflect the lability of the complex or, alternatively, that only some of the SNAREs are complexed and a second population may exist in an uncomplexed form or in a complex with unidentified proteins. Vacuoles without Vam3p or Vam7p have no cis-SNARE complex and yet are still capable of fusion at a measurable rate (Ungermann et al., 1998a; Ungermann and Wickner, 1998). Furthermore, vacuoles without Vam3p do not need priming by Sec17p/Sec18p/ATP (Ungermann et al., 1998a), which suggests that SNAREs that are not in a cis complex can also participate in the homotypic fusion reaction. We have shown by deletion analysis, antibody inhibition, and the generation of ts alleles that each of the subunits has a critical role for the fusion reaction and that a complex of all SNAREs exists on the vacuole (Nichols et al., 1997; Ungermann et al., 1998a; Ungermann and Wickner, 1998; this study). However, we do not know whether the separate SNAREs or the cis-SNARE complex have distinct roles or specific activities. Previous work has shown that a detergent extract which was immunodepleted of SNAREs can be reactivated by addition of a 200-fold purified v-t-SNARE complex (Sato and Wickner, 1998). Future work will be necessary to establish the stoichiometry and functional roles of the five SNAREs during this reconstitution reaction.
Finding a role for Vti1p and Ykt6p in the vacuole-vacuole fusion reaction adds to a long list of trafficking reactions in which these proteins have been implicated (Fischer von Mollard et al., 1997; Lupashin et al., 1997; McNew et al., 1997; Holthuis et al., 1998). Ykt6p is unusual as a v-SNARE in that it is prenylated and appears to partition between cytosol and membranes (McNew et al., 1997). Subcellular localization of Ykt6p has therefore been difficult. Although Ykt6p was initially identified in a complex with the Golgi t-SNARE Sed5p (Søgaard et al., 1994), and may participate in trafficking between the ER and Golgi membranes (McNew et al., 1997), we find a significant portion of Ykt6p on the vacuole, suggesting a vital role for this protein in vacuole function. Vti1p has been recovered in complexes with organellar t-SNAREs along the secretory pathway: with Sed5p, the Golgi t-SNARE, with Pep12p, the endosomal t-SNARE, and with Vam3p (Fischer von Mollard et al., 1997; Holthuis et al., 1998; this study). Because of their interactions with multiple t-SNAREs, Vti1p, and Ykt6p cannot be the sole determinants of specificity in vesicular traffic. These proteins are likely to be involved in a retrieval and recycling of trafficking factors from late organelles to, for example, the Golgi apparatus (Fischer von Mollard et al., 1997; Lupashin et al., 1997; Bryant et al., 1998). Two other v-SNAREs have been implicated in retrograde trafficking reactions in yeast: Sft1p in retrograde transport within the Golgi stack (Banfield et al., 1995), and Sec22p for the trafficking of vesicles from the Golgi apparatus back to the ER (Spang and Scheckman, 1998).
The vacuolar t-SNARE Vam3p has a fundamental role in several trafficking reactions. It has been implicated in trafficking from the endosome to the vacuole (Darsow et al., 1997; Götte and Gallwitz, 1997), in the trafficking of AP3-dependent Golgi-derived vesicles to the vacuole (Cowles et al., 1997; Piper et al., 1997), in aminopeptidase I transport to the vacuole and autophagocytosis (Darsow et al., 1997), and in homotypic vacuole fusion as the final step of the inheritance of this organelle (Nichols et al., 1997). Deletion of Vam3p results in a clear delay of protein trafficking to the vacuole (Darsow et al., 1997; Nichols et al., 1997; Piper et al., 1997; Wada et al., 1997; Srivastava and Jones, 1998). However, vam3Δ vacuoles can be purified by the same floatation protocol as for wild-type vacuoles, albeit at somewhat lower yield. These vacuoles contain all vacuolar marker proteins at the same steady-state concentration (Nichols et al., 1997; Ungermann et al., 1998a, Ungermann and Wickner, 1998; Stefan and Blumer, 1999), they fuse with wild-type vacuoles with similar kinetics, and they show the same sensitivities to inhibitors of fusion as wild-type vacuoles (Nichols et al., 1997; Ungermann et al., 1998b). Though vam3Δ vacuoles are fragmented and of much smaller size (Darsow et al., 1997; Nichols et al., 1997; Wada et al., 1997), their normal protein content and behavior in the vacuole fusion reaction classifies them as vacuoles. This suggests that delivery of proteins to the vacuole, even if slow or of limited efficiency, can occur in a Vam3p-independent fashion and raises the question of how the t-SNARE requirement can be bypassed. The requirement for the vacuole SNARE complex in several reactions implies that other factors are required to add specificity to these trafficking reactions. Defining these factors and their functions may contribute to the understanding of how trafficking to and from this organelle is specified.
Acknowledgments
We thank Drs. James McNew and James Rothman for providing an antiserum to Ykt6p, used in initial studies, Søren Andersen for technical assistance with sample preparation for mass spectrometry; and G. Eitzen, K. Sato, A. Price, and Z. Xu for advice and critical comments on the manuscript.
This work was supported by grants from the National Institute of General Medical Sciences to the labs of W. Wickner (GM23377) and T. Stevens (GM32448), and from the Deutsche Forschungsgemeinschaft to C. Ungermann.
Abbreviations used in this paper
MALDI
matrix-assisted laser desorption/ionization
ts
temperature-sensitive
Footnotes
C. Ungermann's present address is Biochemie Zentrum Heidelberg (BZH), Universität Heidelberg, Im Neuenheimer Feld 326, 69120 Heidelberg, Germany.
References
- Banfield DK, Lewis MJ, Pelham HRB. A SNARE-like protein required for traffic through the Golgi complex. Nature. 1995;375:806–809. doi: 10.1038/375806a0. [DOI] [PubMed] [Google Scholar]
- Bryant N, Piper RC, Weissman LS, Stevens TH. Retrograde traffic out of the yeast vacuole to the TGN occurs via the prevacuolar/endosomal compartment. J Cell Biol. 1998;142:651–663. doi: 10.1083/jcb.142.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd CG, Peterson M, Cowles CR, Emr SD. A novel Sec18p/ NSF-dependent complex required for Golgi-to-endosome transport in yeast. Mol Biol Cell. 1997;8:1089–1104. doi: 10.1091/mbc.8.6.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Ballew N, Barlowe C. Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO (Eur Mol Biol Org) J. 1998;17:2156–2165. doi: 10.1093/emboj/17.8.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoforidis S, McBride HM, Burgoyne RD, Zerial M. The Rab5 effector EEA1 is a core component of endosomal docking. Nature. 1999;397:621–625. doi: 10.1038/17618. [DOI] [PubMed] [Google Scholar]
- Conradt B, Shaw J, Vida T, Emr SD, Wickner W. In vitro reactions of vacuole inheritance in Saccharomyces cerevisiae. . J Cell Biol. 1992;119:1469–1479. doi: 10.1083/jcb.119.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coorssen JR, Blank PS, Tahara M, Zimmerberg J. Biochemical and functional studies of cortical vesicle fusion: the SNARE complex and Ca2+ sensitivity. J Cell Biol. 1998;143:1845–1857. doi: 10.1083/jcb.143.7.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowles CR, Odorizzi G, Payne GS, Emr SD. The AP-3 adaptor complex is essential for cargo-selective transport to the yeast vacuole. Cell. 1997;91:109–118. doi: 10.1016/s0092-8674(01)80013-1. [DOI] [PubMed] [Google Scholar]
- Darsow T, Rieder SE, Emr SD. A multispecificity syntaxin homologue, Vam3p, essential for autophagic and biosynthetic protein transport to the vacuole. J Cell Biol. 1997;138:517–529. doi: 10.1083/jcb.138.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasshauer D, Sutton RB, Brunger AT, Jahn R. Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc Nat Acad Sci USA. 1998;95:15781–15786. doi: 10.1073/pnas.95.26.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro-Novick S, Jahn R. Vesicle fusion from yeast to man. Nature. 1994;370:191–193. doi: 10.1038/370191a0. [DOI] [PubMed] [Google Scholar]
- Fischer von Mollard G, Stevens TH. The Saccharomyces cerevisiaev-SNARE Vti1p is required for multiple membrane transport pathways to the vacuole. Mol Biol Cell. 1999;10:1719–1732. doi: 10.1091/mbc.10.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer von Mollard G, Notwehr S, Stevens TH. The yeast v-SNARE Vti1p mediates two vesicle transport pathways through interactions with the t-SNAREs Sed5p and Pep12p. J Cell Biol. 1997;137:1511–1524. doi: 10.1083/jcb.137.7.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götte M, Gallwitz D. High expression of the yeast syntaxin-related Vam3 protein suppresses the protein transport defects of a pep12null mutant. FEBS Lett. 1997;411:48–52. doi: 10.1016/s0014-5793(97)00575-9. [DOI] [PubMed] [Google Scholar]
- Haas A. A quantitative assay to measure homotypic vacuole fusion in vitro. Meth Cell Sci. 1995;17:283–294. [Google Scholar]
- Haas A, Wickner W. Homotypic vacuole fusion requires Sec17p (yeast α-SNAP) and Sec18p (yeast NSF) EMBO (Eur Mol Biol Org) J. 1996;15:3296–3305. [PMC free article] [PubMed] [Google Scholar]
- Haas A, Conradt B, Wickner W. G-protein ligands inhibit in vitro reactions of vacuole inheritance. J Cell Biol. 1994;126:87–97. doi: 10.1083/jcb.126.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow, E., and O. Lane. 1988. Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. 519–551.
- Hay JC, Scheller R. SNAREs and NSF in targeted membrane fusion. Curr Opin Cell Biol. 1997;9:505–512. doi: 10.1016/s0955-0674(97)80026-9. [DOI] [PubMed] [Google Scholar]
- Hayashi T, McMahon H, Yamasaki S, Binz T, Hata Y, Südhof TC, Niemann H. Synaptic vesicle membrane fusion complex: action of clostridial neurotoxins on assembly. EMBO (Eur Mol Biol Org) J. 1994;13:5051–5061. doi: 10.1002/j.1460-2075.1994.tb06834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Yamasaki S, Nauenburg S, Binz T, Niemann H. Disassembly of the reconstituted synaptic vesicle membrane fusion complex in vitro. EMBO (Eur Mol Biol Org) J. 1995;14:2317–2325. doi: 10.1002/j.1460-2075.1995.tb07226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthuis JCM, Nichols BJ, Dhruvakumar S, Pelham HRB. Two syntaxin homologues in the TGN/endosomal system in yeast. EMBO (Eur Mol Biol Org) J. 1998;17:113–126. doi: 10.1093/emboj/17.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen ON, Podtelejnikov AV, Mann M. Delayed extraction improves specificity in database searches by MALDI peptide maps. Rapid Commun Mass Spectrom. 1996;10:1371–1378. doi: 10.1002/(SICI)1097-0231(199608)10:11<1371::AID-RCM682>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Jensen ON, Podtelejnikov AV, Mann M. Identification of the components of simple protein mixtures by high-accuracy peptide mass mapping and database searching. Anal Chem. 1997;69:4741–4750. doi: 10.1021/ac970896z. [DOI] [PubMed] [Google Scholar]
- Jensen, O.N., M.R. Larsen, and P. Roepstorff. 1998. Mass spectrometric identification and microcharacterization of proteins from electrophoretic gels: strategies and applications. Proteins. Suppl. 2:74–89. [DOI] [PubMed]
- Katz L, Hanson PI, Heuser JE, Brennwald P. Genetic and morphological analyses reveal a critical interaction between the C-termini of two SNARE proteins and a parallel four helical arrangement for the exocytic SNARE complex. EMBO (Eur Mol Biol Org) J. 1998;17:6200–6209. doi: 10.1093/emboj/17.21.6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löwe M, Nakamura N, Warren G. Golgi division and membrane traffic. Trends Cell Biol. 1998;8:40–44. doi: 10.1016/s0962-8924(97)01189-6. [DOI] [PubMed] [Google Scholar]
- Lupashin VV, Pokrovskaya ID, McNew JA, Waters MG. Characterization of a novel yeast SNARE protein implicated in Golgi retrograde traffic. Mol Biol Cell. 1997;8:2659–2676. doi: 10.1091/mbc.8.12.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A, Wickner W, Haas A. Sec18p (NSF)-driven release of Sec17p (α-SNAP) precedes docking and fusion of yeast vacuoles. Cell. 1996;85:83–94. doi: 10.1016/s0092-8674(00)81084-3. [DOI] [PubMed] [Google Scholar]
- McNew JA, Søgaard M, Lampen N, Machida S, Ye RR, Lacomis L, Tempst P, Rothman JE, Söllner TH. Ykt6p, a prenylated SNARE essential for endoplasmic reticulum-Golgi transport. J Biol Chem. 1997;272:17776–17783. doi: 10.1074/jbc.272.28.17776. [DOI] [PubMed] [Google Scholar]
- Mills IG, Jones AT, Clague MJ. Involvement of the endosomal autoantigen EEA1 in homotypic fusion of early endosomes. Curr Biol. 1998;8:881–884. doi: 10.1016/s0960-9822(07)00351-x. [DOI] [PubMed] [Google Scholar]
- Nichols BJ, Ungermann C, Pelham HRB, Wickner W, Haas A. Homotypic vacuolar fusion mediated by v- and t-SNAREs. Nature. 1997;387:199–202. doi: 10.1038/387199a0. [DOI] [PubMed] [Google Scholar]
- Novick P, Zerial M. The diversity of rab proteins in vesicle transport. Curr Opin Cell Biol. 1997;9:496–504. doi: 10.1016/s0955-0674(97)80025-7. [DOI] [PubMed] [Google Scholar]
- Otto H, Hanson PI, Jahn R. Assembly and disassembly of a ternary complex of synaptobrevin, syntaxin and SNAP-25 in the membrane of synaptic vesicles. Proc Natl Acad Sci USA. 1997;94:6197–6201. doi: 10.1073/pnas.94.12.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters C, Mayer A. Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature. 1998;396:575–580. doi: 10.1038/25133. [DOI] [PubMed] [Google Scholar]
- Pfeffer SR. Transport vesicle docking: SNARE and associates. Annu Rev Cell Biol Dev Biol. 1996;12:441–461. doi: 10.1146/annurev.cellbio.12.1.441. [DOI] [PubMed] [Google Scholar]
- Piper RC, Bryant NJ, Stevens TH. The membrane protein alkaline phosphatase is delivered to the vacuole by a route that is distinct from the VPS-dependent pathway. J Cell Biol. 1997;138:531–545. doi: 10.1083/jcb.138.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier MA, Xiao W, Macosko JC, Chan C, Shin YK, Bennett MK. The synaptic SNARE complex is a parallel four-stranded helical bundle. Nat Struct Biol. 1998;5:765–769. doi: 10.1038/1799. [DOI] [PubMed] [Google Scholar]
- Rothman JE. Mechanisms of intracellular membrane fusion. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Sato K, Wickner W. Functional reconstitution of Ypt7p GTPase and a purified vacuole SNARE complex. Science. 1998;281:700–702. doi: 10.1126/science.281.5377.700. [DOI] [PubMed] [Google Scholar]
- Sato K, Darsow T, Emr SE. Vam7p, a SNAP-25–like molecule, and Vam3p, a syntaxin homolog, function together in yeast vacuolar protein trafficking. Mol Cell Biol. 1998;18:5308–5319. doi: 10.1128/mcb.18.9.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- Simonsen A, Lippe R, Christoforidis S, Gaullier JM, Brech A, Callaghan J, Toh BH, Murphy C, Zerial M, Stenmark H. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature. 1998;394:494–498. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- Søgaard M, Tani K, Ye RR, Geromanos S, Tempst P, Kirchhausen T, Rothman JE, Söllner T. A rab protein is required for the assembly of SNARE complexes in the docking of transport vesicles. Cell. 1994;78:937–948. doi: 10.1016/0092-8674(94)90270-4. [DOI] [PubMed] [Google Scholar]
- Söllner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Spang A, Scheckman R. Reconstitution of retrograde transport from the Golgi to the ER in vitro. J Cell Biol. 1998;143:589–599. doi: 10.1083/jcb.143.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A, Jones EW. Pth1/Vam3p is the syntaxin homolog at the vacuolar membrane of Saccharomyces cerevisiaerequired for the delivery of vacuolar hydrolases. Genetics. 1998;148:85–98. doi: 10.1093/genetics/148.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan CJ, Blumer KJ. A syntaxin homolog encoded by VAM3 mediates down-regulation of a yeast G protein-coupled receptor. J Biol Chem. 1999;274:1835–1841. doi: 10.1074/jbc.274.3.1835. [DOI] [PubMed] [Google Scholar]
- Stenmark H, Vitale G, Ullrich O, Zerial M. Rabaptin-5 is a direct effector of the small GTPase Rab5 in endocytic membrane fusion. Cell. 1995;83:423–432. doi: 10.1016/0092-8674(95)90120-5. [DOI] [PubMed] [Google Scholar]
- Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- Swanton E, Sheehan J, Bishop N, High S, Woodman P. Formation and turnover of NSF- and SNAP-containing “fusion” complexes occurs on undocked, clathrin-coated vesicle-derived membranes. Mol Biol Cell. 1998;9:1633–1637. doi: 10.1091/mbc.9.7.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara M, Coorssen J, Timmers K, Blank PS, Whalley T, Scheller R, Zimmerberg J. Calcium can disrupt the SNARE protein complex on sea urchin egg secretory vesicles without irreversibly blocking fusion. J Biol Chem. 1998;273:33667–33673. doi: 10.1074/jbc.273.50.33667. [DOI] [PubMed] [Google Scholar]
- Ungermann C, Wickner W. Vam7p, a vacuolar SNAP-25 homolog, is required for SNARE complex integrity and vacuole docking and fusion. EMBO (Eur Mol Biol Org) J. 1998;17:3269–3276. doi: 10.1093/emboj/17.12.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C, Nichols BJ, Pelham HRB, Wickner W. A vacuolar v-t-SNARE complex, the predominant form in vivo and on isolated organelles, is disassembled and activated for docking and fusion. J Cell Biol. 1998a;140:61–69. doi: 10.1083/jcb.140.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C, Sato K, Wickner W. Defining the function of trans SNARE pairs. Nature. 1998b;396:543–548. doi: 10.1038/25069. [DOI] [PubMed] [Google Scholar]
- Wada Y, Nakamura N, Ohsumi Y, Hirata A. Vam3p, a new member of syntaxin related protein, is required for vacuolar assembly in the yeast Saccharomyces cerevisiae. . J Cell Sci. 1997;110:1299–1306. doi: 10.1242/jcs.110.11.1299. [DOI] [PubMed] [Google Scholar]
- Walch-Solimena, C., J. Blasi, L. Edelmann, L., E.R. Chapman, G. Fischer von Mollard, and R. Jahn. 1995. The t-SNAREs syntaxin 1 and SNAP-25 are present on organelles that participate in synaptic vesicle recycling. J. Cell Biol. 128:637–645. [DOI] [PMC free article] [PubMed]
- Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Söllner TH, Rothman JE. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- Weimbs T, Low SH, Chapin SJ, Mostov KE, Bucher P, Hofmann K. A conserved domain is present in different families of vesicular fusion proteins: a new superfamily. Proc Natl Acad Sci USA. 1997;94:3046–3051. doi: 10.1073/pnas.94.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis WI, Scheller RH. Membrane fusion. SNARE the rod, coil the complex. Nature. 1998;395:328–329. doi: 10.1038/26354. [DOI] [PubMed] [Google Scholar]
- Xu Z, Wickner W. Thioredoxin is required for vacuole inheritance in Saccharomyces cerevisiae. . J Cell Biol. 1996;132:787–794. doi: 10.1083/jcb.132.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Sato K, Wickner W. LMA1 binds to vacuoles at Sec18p (NSF), transfers upon ATP hydrolysis to a t-SNARE (Vam3p) complex, and is released during fusion. Cell. 1998;93:1125–1134. doi: 10.1016/s0092-8674(00)81457-9. [DOI] [PubMed] [Google Scholar]