Regulated Secretion from Hemopoietic Cells (original) (raw)
The process of regulated secretion (reviewed in Bugress and Kelly, 1987) is critical for the correct biological functioning of many different cells of the immune system, most of which are derived from the hemopoietic lineage (Table ). For example, T lymphocytes use regulated secretion to selectively destroy appropriate targets recognized by the T cell receptor, while mast cells degranulate in response to IgE cross-linking to counter parasitic infection. Unlike conventional secretory cells (e.g., exocrine and endocrine cells) which use a separate organelle for the storage and release of their secretory products (Fig. 1 a), cells of the hemopoietic lineage use lysosomes to store and release their secretory products (Fig. 1 b; Griffiths 1996). These organelles have been termed secretory lysosomes. Although the lysosomal nature of the secretory granules found in several hemopoietic cells has been known for many years, recent evidence supports the idea that secretory lysosomes may use specialized mechanisms for sorting and secretion, which differ from those found in conventional secretory cells. Interestingly, a small number of nonhemopoietic cells also use these mechanisms, suggesting that secretory lysosomes may represent an early form of regulated secretion.
Table 1.
Cell Types Using Secretory Lysosomes
| Cell type | Receptors triggering exocytosis | Function | Lysosomal soluble secreted products | Lysosomal membrane proteins relocalized by secretion | Secretory proteins identified |
|---|---|---|---|---|---|
| T cells | T cell receptor | Target cell killing | Perforin | Fas ligand | |
| Granzymes | CTLA-4 | ||||
| CD63 | |||||
| Mast cells | Fc receptor | Parasite defense | Histamine | MHC class II | SNAP 23 (Guo et al. 1998) |
| Serotonin | CD63 | Rab 3D (Tuvim et al.) | |||
| Synaptotagmins II, III, | |||||
| and V (Baram et al. 1999) | |||||
| Eosinophils | Fc receptor | Parasite defense | Major basic protein | ||
| Basophils | Fc receptor | Inflammatory | Histamine | ||
| Neutrophils | Fc receptor | Inflammatory | Chemo-attractants | CD63 | Syntaxin 4, VAMP 2, SCAMP |
| Phagocytosis | CD66 | (Brumell et al. 1995) | |||
| Platelets | Collagen and Fc | Clotting | Clotting factors | CD63 | |
| receptors | CD40 ligand | ||||
| Macrophages | Phagocytosis | Lysosome “secretes” | MHC class II | ||
| Antigen presentation | into phagosome | ||||
| Dendritic cells | Antigen presentation | MHC class II | |||
| B cells | Antigen presentation | MHC class II | |||
| Ig secretion | |||||
| Osteoclast | Bone resorption | Lysosomal hydrolases | |||
| Renal tubular | Kidney function | ||||
| Melanocytes | Secrete melanin for | ||||
| pigmentation | Melanin |
Figure 1.
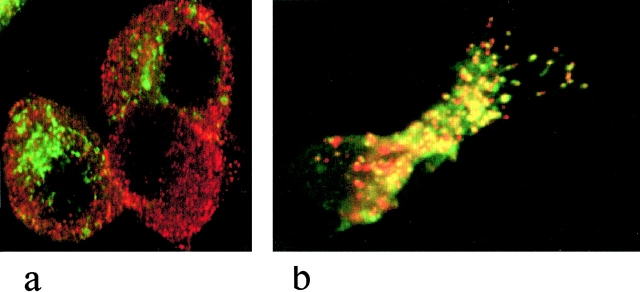
(a) Conventional secretory cells contain separate lysosomes and secretory granules. PC12 cells were transfected with the lysosomal protein CD63, tagged with GFP and costained with an antibody to secretogranin II, a secretory granule protein, in red. No overlap of the two compartments is observed. (b) Cells with secretory lysosomes package both lysosomal proteins and secretory proteins in the same compartment. A cytotoxic T lymphocyte is costained with the lysosomal membrane protein LAMP-2 (red) and the soluble secreted protein granzyme A (green). Extensive overlap of the two markers demonstrates colocalization in the same organelle (yellow).
Secretory Lysosomes: A Mixture of Two Organelles
Secretory lysosomes are a mixture of lysosomes and secretory granules on many different levels. Secretory lysosomes contain both the hydrolases and membrane proteins characteristic of lysosomes as well as the specialized secretory products of different cell types. Functionally, they serve both as the lysosome of the cell and as the secretory granule. The acidic pH is optimal for the action of lysosomal hydrolases and contributes to keeping secretory products inactive before release. As is true for lysosomes, proteins can reach secretory lysosomes by both endocytic and biosynthetic routes as demonstrated by HRP uptake and the delivery of newly synthesized proteins. Morphologically, secretory lysosomes are a mixture of the multilamellar structures characteristic of lysosomes and the dense cores characteristic of secretory granules. Studies on secretory lysosome biogenesis in T lymphocytes indicate that the dense core forms and enlarges within a multivesicular structure (Griffiths and Argon 1995). In mature T lymphocytes, there is a high degree of overlap between secretory and lysosomal markers, suggesting that the majority of lysosomes are secretory lysosomes (Stinchcombe, J.C., and G.M. Griffiths, unpublished observation). However, in neutrophils, lysosomal structures lacking VAMP-2 can be identified alongside other VAMP-2–labeled granules (Brumell et al. 1995), suggesting that these cells may possess both true lysosomes and secretory lysosomes (Table ).
The mechanisms that regulate the sorting of proteins to the secretory lysosomes are also a mixture of those used to target lysosomal proteins and secretory granule proteins in conventional cells, although in hemopoietic cells the proteins are targeted to the same organelle. For example, the soluble secretory granzymes of T lymphocytes follow the mannose-6-phosphate pathway used by lysosomal hydrolases to reach the granules (Griffiths and Isaaz 1993). Other soluble proteins of secretory lysosomes, such as perforin in T lymphocytes, are able to complex with proteoglycans (Masson et al. 1990) and may be sorted by selective condensation into the dense core, as has been suggested for chromogranins in endocrine secretory cells (Chanat and Huttner 1991). Membrane-bound proteins that are expressed only in cells with secretory lysosomes, such as GMP-17 (Medley et al. 1996) or CTLA-4 (McNeil and Steinhardt 1997), possess the tyrosine-based sorting motifs found in lysosomal membrane proteins that enable selective sorting to the secretory lysosome. Recent data suggest that specialized mechanisms for sorting to secretory lysosomes may also exist. This arises from the observation that the membrane-bound protein of T lymphocytes, Fas ligand, is differentially sorted in hemopoietic and nonhemopoietic cells. The cytosolic tail of this protein preferentially sorts Fas ligand to secretory lysosomes in hemopoietic cells, but is unable to do so in nonhemopoietic cells in which it is expressed directly on the cell surface (Dossi and Griffiths, 1999). Mutagenesis of the tail demonstrates that a proline-rich domain is required for sorting to secretory lysosomes (Bossi, G., and G.M. Griffiths, unpublished observation). One possible mechanism for the differential sorting might, therefore, involve the interaction of this proline-rich domain with an SH3-domain containing protein that is preferentially expressed in cells with secretory lysosomes.
The Advantages of Secretory Lysosomes for Hemopoietic Cells
The regulated secretion of the lysosomal compartment by hemopoietic cells provides an important pathway for controlling the release of both soluble and membrane proteins in many cells from this lineage. Cells of the immune system have both effector and regulator functions, and often delivery of both soluble and membrane proteins needs to be tightly controlled. For example, the secretory lysosomes of T lymphocytes can contain not only soluble proteins required for destruction of virally infected and tumorigenic targets (such as perforin and granzymes), but also membrane-bound proteins that are essential for controlling the immune response, e.g., Fas ligand (Dossi and Griffiths, 1999) and CTLA-4 (Thompson and Allison 1997). Recognition of the target cells via the T cell receptor triggers kinesin-driven movement of the secretory lysosomes along microtubules to the point of membrane contact between the T lymphocyte and target (reviewed in Griffiths and Argon 1995). The secretory lysosome membranes then fuse with the plasma membrane, acting not only to release the soluble proteins (e.g., perforin), but also to deliver the membrane proteins (e.g., Fas ligand) to the site of interaction. Delivery of these proteins via the secretory lysosome allows the T lymphocyte to first store these proteins within the cell and then control the precise location and timing of their release so that they are perfectly focused on the target cell. This is important in ensuring the specificity of the T lymphocyte response and in preventing damage to bystander cells which are not recognized by the T cell receptor.
In some cells it appears that not only the lysosome can be released but other prelysosomal compartments can also fuse with and deliver proteins to the plasma membrane. This mechanism is particularly important in hemopoietic cells expressing MHC class II. MHC class II is present within the cell in a prelysosomal compartment, termed the MIIC, and can be relocalized to the cell surface (Rodriguez et al. 1995; Wubbolts et al. 1996; Raposo et al. 1997). Since MHC class II presents peptides from extracellular pathogens that need to be taken up and degraded by the cell, the exocytosis of a compartment from the degradative pathway on the way to the lysosome is therefore ideal for efficient antigen presentation. Curiously, secretion of this multivesicular compartment not only translocates proteins of the outer membrane to the cell surface but also results in the release of small internal vesicles, termed exosomes, which may themselves have important biological effects (Zitvogel et al. 1998).
The Secretory Machinery Required for Lysosome Release
Several of the key proteins involved in secretion of conventional secretory granules are found in cells with secretory lysosomes. For example, both v- and t-SNARES (Brumell et al. 1995; Guo et al. 1998), as well as Rab proteins (Tardieux et al. 1992) and synaptotagmins (Baram et al. 1999), have been found to be associated with secretory lysosomes in hemopoietic cells (Table ). This indicates that some of the machinery involved in regulating the release of secretory lysosomes is common to that used by conventional secretory cells. However, some of the critical components of the secretory machinery are specific to cells with secretory lysosomes. The most compelling evidence comes from the human autosomal recessive disease, Chediak-Higashi syndrome (CHS). In this disease, all lysosomes are abnormally enlarged but with no obvious effect on the endocytic and degradative roles of the mutant lysosomes (Burkhardt et al. 1993). Similarly, the secretory function of conventional secretory cells is normal in CHS. What is particularly interesting about CHS is that the cell types that are functionally impaired all seem to be those with secretory lysosomes. In the case of T lymphocytes it has been demonstrated that the secretory lysosomes are unable to be secreted (Baetz et al. 1995). These observations implicate the defective protein in a unique aspect of secretory lysosome release which is not required for exocytosis of secretory granules in conventional secretory cells. The fact that replacement of cells of the hemopoietic lineage by bone marrow transplantation can be successfully used to treat patients with CHS supports this hypothesis (Fischer et al. 1986). The gene that is defective in CHS has now been cloned from both humans and mice (Barbosa et al. 1996; Nagle et al. 1996; Perou et al. 1996). The sequences predict homologous cytosolic proteins of ∼400 kD. The gene is expressed in the majority of tissues examined, consistent with the abnormally sized lysosomes found in all CHS cell types. The most direct clue as to the function of the protein comes from experiments in which the wild-type protein is overexpressed in mutant fibroblasts. This results in the production of abnormally small lysosomes, suggesting that the protein is involved in lysosomal fission (Perou et al. 1997).
Albinism and Lysosome Secretion: What's the Link?
One of the most intriguing clues from CHS regarding secretory lysosome biogenesis and secretion is the observation that the defect results in partial albinism. This is most apparent in the mouse model of the disease, the beige mouse, due to its partial albino coat color compared with the wild-type strain from which it arose. The critical link is the melanocyte, which, although not arising from the hemopoietic lineage, also possesses secretory lysosomes. These organelles, known as melanosomes, secrete the pigment melanin which then enters keratinocytes and gives rise to coat color (reviewed in Orlow 1995). Defective pigmentation in CHS has two important implications. First, secretory lysosomes are not entirely restricted to cells derived from the hemopoietic lineage. And second, other forms of albinism may reflect defects in secretory lysosome biogenesis and release. Since albinism requires sorting of proteins required in melanin synthesis, as well as polarization and secretory steps, then mutants may reflect different stages of this process. The examples that have already emerged show that this is the case.
Recent studies on two human autosomal recessive diseases that give rise to partial albinism have produced some intriguing findings concerning the link between albinism and secretory lysosome biogenesis. The first is Griscelli's syndrome that has a mouse homologue known as dilute. Griscelli's syndrome is clinically related to CHS in that the patients show selective immunodeficiency and partial albinism (Klein et al. 1994). The defective gene in the dilute mouse was shown to be the myosin Va heavy chain (Mercer et al. 1991) and the human lesion has been shown to encode the same protein (Pastural et al. 1997). In wild-type melanocytes, melanosomes are concentrated in the peripheral dendrites from which they are released. However, in dilute mice the melanosomes are more concentrated in the center of the cell. In wild-type cells, myosin Va colocalizes with melanosomes in the dendrites (Wu et al. 1997). Recent studies suggest that myosin Va is important in capturing melanosomes that reach the periphery, since overexpression of a dominant negative myosin Va in wild-type cells dramatically depletes melanosome accumulation at the periphery (Wu et al. 1998). The selective nature of these diseases, which affect both hemopoietic cells and melanocytes, raises the intriguing possibility that myosin Va may also play a critical role in the secretory lysosome polarization which is required for secretion in many hemopoietic cell types.
The second disease to shed light on the link between albinism and cells with secretory lysosomes is Hermansky-Pudlak syndrome (HPS). HPS is an autosomal recessive disease of humans resulting in partial albinism and defects in lysosomal secretion which has several mouse models (reviewed in Swank et al. 1998). Since the defective genes in the different mouse models map to at least 10 different loci, it seems likely that defects in multiple genes result in a similar phenotype (Swank et al. 1998). Recent studies demonstrate that HPS and its mouse models reflect defects in a lysosomal sorting pathway. Two different proteins have been identified so far. One has been termed HPS1 and encodes a 79-kD novel transmembrane protein of unknown function (Kantheti et al. 1998; Feng et al. 1999). The HPS1 sequence provides few clues as to the function of this protein and there is no significant homology to other known proteins. The other protein that has been found to be defective is the adaptor protein AP3. The mocha mouse is defective in the Δ subunit of AP3, while pearl mice are defective in the β3A subunit (Barbosa et al. 1996; Gardner et al. 1997). Some HPS patients which show normal expression of HPS1 have also been shown to be defective in the β3A subunit of the AP3 adaptor protein (DellAngelica et al. 1999). Several lysosomal membrane proteins are mis-sorted in fibroblasts derived from these patients. Findings from earlier studies of HPS and its related mouse models which indicate that both melanocyte function and lysosome secretion are affected by these mutations, suggest that AP3 may be especially important in transporting proteins to both melanosomes and secretory lysosomes. Intriguingly, all of these mutants have also been reported to be defective in the secretion lysosomal hydrolases from kidneys, another cell type that uses secretory lysosomes (Swank et al. 1998). Together, these studies demonstrate that many of these albinism mutants may provide important clues in understanding the different steps of the secretory mechanisms used by cells with secretory lysosomes.
Is Secretion a Property of All Lysosomes?
Although secretory lysosomes are predominantly used by cells of the hemopoietic lineage, there are clearly some nonhemopoietic cells which use a lysosomal compartment for regulated secretion, such as melanocytes (Orlow 1995) and renal tubular cells (Swank et al. 1998). This suggests that the secretion of lysosomes might be a more widespread phenomenon which has simply been enhanced in cells of the hemopoietic lineage. Several observations support this idea.
First, lysosomes from nonsecretory cells can be secreted. It has been shown that both Chinese hamster ovary and normal rat kidney cells can be induced to secrete their lysosomes in response to influx of high levels of calcium (Coorssen et al. 1996; Rodriguez et al. 1997). Although the percentage of the total lysosomal population responding to the signal is generally low (10%, compared with 60% in cells with secretory lysosomes) and high levels of calcium are required, these observations suggest that there may be secretion-competent lysosomes in these cells. Similarly, trypanosomes seem to be able to trigger calcium-mediated fusion of host cell lysosomes at the cell surface during their invasion into a variety of nonhemopoietic mammalian cells (Tardieux et al. 1992; Rodriguez et al. 1995, Rodriguez et al. 1996). Again only a sub-population of lysosomes respond to the trypanosomal signal in fibroblasts. These differences in the level of lysosomal secretion may simply reflect variations in the number of secretion-competent lysosomes in different cell types.
Second, there is evidence that repair of the plasma membrane may involve a process of lysosomal secretion. Wounding of the plasma membrane studied in many nonhemopoietic cell types results in a calcium flux which causes membranes of endocytic origin to fuse with the plasma membrane (Miyake and McNeil 1995). In this way, lysosomal exocytosis could be an important mechanism in wound healing required by all cell types (McNeil and Steinhardt 1997; Caler et al. 1998).
Studies from Dictyostelium suggest that secretion of lysosomes may represent a primitive secretory system. Dictyostelium are also able to secrete their lysosomal contents and a number of mutants in this process have been isolated, demonstrating that several groups of genes are required for secretion (Ebert et al. 1990). Many of these genes also appear to be important in the development of the multicellular stage of the Dictyostelium life cycle. Of particular interest is the observation that one of the genes required for cytokinesis bears strong homology to the gene that is defective in CHS (De Lozanne, A., personal communication). Many of the secretory mutants which block polarized membrane delivery in Saccharomyces are also required for cytokinesis (reviewed in Finger and Novick 1998) and may also be involved in polarized delivery of secretory lysosomes. The existence of genes required for lysosomal secretion in Dictyostelium related to those required for lysosomal secretion in mammals suggests a strong evolutionary conservation of these mechanisms.
Taken together, these findings suggest that the mechanism of regulated secretion of lysosomes used by hemopoietic cells is present in many cell types and may represent a primitive secretory system which has simply been enhanced in cells of the hemopoietic system. If this is the case, then it is likely that the specialized secretory granules used for regulated secretion from exocrine and endocrine cells are a later evolutionary development. Intriguingly, although conventional endocrine and exocrine secretory cells contain distinct lysosomes and secretory storage compartments they contain a post-Golgi intermediate, the immature granule, on the pathway to these distinct organelles in which the newly synthesized secretory and lysosomal proteins coexist (Klumperman et al. 1998; Kuliawat et al. 1997). This compartment has several features in common with secretory lysosomes: it is acidic, performs some proteolytic functions, and can be simulated to secrete in a calcium-dependent manner (reviewed in Arvan and Castle 1998). It may be that the immature granule is an evolutionary vestige of a secretory lysosome but that in these cells the secretory and lysosomal functions have subsequently become separated. In contrast, regulated secretory cells of the hemopoietic lineage have optimized a more primitive secretory mechanism and use it to regulate the release of soluble and membrane proteins.
Acknowledgments
The authors wish to acknowledge Lesley Page for 1 a and Jim Kaufman for critical reading of the manuscript.
References
- Arvan P., Castle D. Sorting and storage during secretory granule biogenesislooking backward and looking forward. Biochem. J. 1998;332:593–610. doi: 10.1042/bj3320593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baetz K., Isaaz S., Griffiths G.M. Loss of cytotoxic T lymphocyte function in Chediak-Higashi syndrome arises from a secretory defect that prevents lytic granule exocytosis. J. Immunol. 1995;154:6122–6131. [PubMed] [Google Scholar]
- Baram D., Adachi R., Medalia O., Tuvim M., Dickey B.F., Mekori Y.A., Sagi-Eisenberg R. Synaptotagmin II negatively regulates Ca2+-triggered exocytosis of lysosomes in mast cells. J. Exp. Med. 1999;189:1649–1658. doi: 10.1084/jem.189.10.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa M.D., Nguyen Q.A., Tchernev V.T., Ashley J.A., Detter J.C., Blaydes S.M., Brandt S.J., Chotai D., Hodgman C., Solari R.C., Lovett M., Kingsmore S.F. Identification of the homologous beige and Chediak-Higashi syndrome genes [published erratum appears in Nature 385:97] Nature. 1996;382:262–265. doi: 10.1038/382262a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossi G., Griffiths G.M. Degranulation plays an essential part in regulating cell surface expression of Fas ligand in T cells and natural killer cells. Nat. Med. 1999;5:90–96. doi: 10.1038/4779. [DOI] [PubMed] [Google Scholar]
- Brumell J.H., Volchuk A., Sengelov H., Borregaard N., Cieutat A.M., Bainton D.F., Grinstein S., Klip A. Subcellular distribution of docking/fusion proteins in neutrophils, secretory cells with multiple exocytic compartments. J. Immunol. 1995;155:5750–5759. [PubMed] [Google Scholar]
- Burgess T.L., Kelly R.B. Constitutive and regulated secretion of proteins. Annu. Rev. Cell Biol. 1987;3:243–293. doi: 10.1146/annurev.cb.03.110187.001331. [DOI] [PubMed] [Google Scholar]
- Burkhardt J.K., Wiebel F.A., Hester S., Argon Y. The giant organelles in beige and Chediak-Higashi fibroblasts are derived from late endosomes and mature lysosomes. J. Exp. Med. 1993;178:1845–1856. doi: 10.1084/jem.178.6.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caler E.V., Vaena de Avalos S., Haynes P.A., Andrews N.W., Burleigh B.A. Oligopeptidase B-dependent signaling mediates host cell invasion by Trypanosoma cruzi. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:4975–4986. doi: 10.1093/emboj/17.17.4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanat E., Huttner W.B. Milieu-induced, selective aggregation of regulated secretory proteins in the trans-Golgi network. J. Cell Biol. 1991;115:1505–1519. doi: 10.1083/jcb.115.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Angelica E.C., Shotelersuk V., Aguilar R.C., Gahl W.A., Bonifacino J.S. Altered trafficking of lysosomal proteins in Hermansky-Pudlak syndrome due to mutations in the beta 3A subunit of the AP-3 adaptor. Mol. Cell. 1999;3:11–21. doi: 10.1016/s1097-2765(00)80170-7. [DOI] [PubMed] [Google Scholar]
- Ebert D.L., Jordan K.B., Dimond R.L. Lysosomal enzyme secretory mutants of Dictyostelium discoideum . J. Cell Sci. 1990;96:491–500. doi: 10.1242/jcs.96.3.491. [DOI] [PubMed] [Google Scholar]
- Feng L., Seymour A.B., Jiang S., To A., Peden A.A., Novak E.K., Zhen L., Rusiniak M.E., Eicher E.M., Robinson M.S., Gorin M.B., Swank R.T. The beta3A subunit gene (Ap3b1) of the AP-3 adaptor complex is altered in the mouse hypopigmentation mutant pearl, a model for Hermansky-Pudlak syndrome and night blindness. Hum. Mol. Genet. 1999;8:323–330. doi: 10.1093/hmg/8.2.323. [DOI] [PubMed] [Google Scholar]
- Finger F.P., Novick P. Spatial regulation of exocytosislessons from yeast. J. Cell Biol. 1998;142:609–612. doi: 10.1083/jcb.142.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A., Griscelli C., Friedrich W., Kubanek B., Levinsky R., Morgan G., Vossen J., Wagemaker G., Landais P. Bone-marrow transplantation for immunodeficiencies and osteopetrosiseuropean survey, 1968–1985. Lancet. 1986;2:1080–1084. doi: 10.1016/s0140-6736(86)90477-0. [DOI] [PubMed] [Google Scholar]
- Gardner J.M., Wildenberg S.C., Keiper N.M., Novak E.K., Rusiniak M.E., Swank R.T., Puri N., Finger J.N., Hagiwara N., Lehman A.L. The mouse pale ear (ep) mutation is the homologue of human Hermansky- Pudlak syndrome. Proc. Natl. Acad. Sci. USA. 1997;94:9238–9243. doi: 10.1073/pnas.94.17.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G.M. Secretory lysosomes-a special mechanism of regulated secretion in haemopoieitic cells. Trends Cell Biol. 1996;6:329–332. doi: 10.1016/0962-8924(96)20031-5. [DOI] [PubMed] [Google Scholar]
- Griffiths G.M., Isaaz S. Granzymes A and B are targeted to the lytic granules of lymphocytes by the mannose-6-phosphate receptor. J. Cell Biol. 1993;120:885–896. doi: 10.1083/jcb.120.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G.M., Argon Y. Structure and biogenesis of lytic granules. Curr. Top. Microbiol. Immunol. 1995;198:39–58. doi: 10.1007/978-3-642-79414-8_3. [DOI] [PubMed] [Google Scholar]
- Guo Z., Turner C., Castle D. Relocation of the t-SNARE SNAP-23 from lamellipodia-like cell surface projections regulates compound exocytosis in mast cells. Cell. 1998;94:537–548. doi: 10.1016/s0092-8674(00)81594-9. [DOI] [PubMed] [Google Scholar]
- Kantheti P., Qiao X., Diaz M.E., Peden A.A., Meyer G.E., Carskadon S.L., Kapfhamer D., Sufalko D., Robinson M.S., Noebels J.L., Burmeister M. Mutation in AP-3 delta in the mocha mouse links endosomal transport to storage deficiency in platelets, melanosomes, and synaptic vesicles. Neuron. 1998;21:111–122. doi: 10.1016/s0896-6273(00)80519-x. [DOI] [PubMed] [Google Scholar]
- Klein C., Philippe N., Le Deist F., Fraitag S., Prost C., Durandy A., Fischer A., Griscelli C. Partial albinism with immunodeficiency (Griscelli syndrome) J. Pediatr. 1994;125:886–895. doi: 10.1016/s0022-3476(05)82003-7. [DOI] [PubMed] [Google Scholar]
- Klumperman J., Kuliawat R., Griffith J.M., Geuze H.J., Arvan P. Mannose 6-phosphate receptors are sorted from immature secretory granules via adaptor protein AP-1, clathrin, and syntaxin 6-positive vesicles. J. Cell Biol. 1998;141:359–371. doi: 10.1083/jcb.141.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuliawat R., Klumperman J., Ludwig T., Arvan P. Differential sorting of lysosomal enzymes out of the regulated secretory pathway in pancreatic beta-cells. J. Cell Biol. 1997;137:595–608. doi: 10.1083/jcb.137.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P.S., Bradshaw J., Greene J., Peach R., Bennett K.L., Mittler R.S. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity. 1996;4:535–543. doi: 10.1016/s1074-7613(00)80480-x. [DOI] [PubMed] [Google Scholar]
- Masson D., Peters P.J., Geuze H.J., Borst J., Tschopp J. Interaction of chondroitin sulfate with perforin and granzymes of cytolytic T-cells is dependent on pH. Biochemistry. 1990;29:11229–11235. doi: 10.1021/bi00503a011. [DOI] [PubMed] [Google Scholar]
- McNeil P.L., Steinhardt R.A. Loss, restoration, and maintenance of plasma membrane integrity. J. Cell Biol. 1997;137:1–4. doi: 10.1083/jcb.137.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medley Q.G., Kedersha N., O'Brien S., Tian Q., Schlossman S.F., Streuli M., Anderson P. Characterization of GMP-17, a granule membrane protein that moves to the plasma membrane of natural killer cells following target cell recognition. Proc. Natl. Acad. Sci. USA. 1996;93:685–689. doi: 10.1073/pnas.93.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J.A., Seperack P.K., Strobel M.C., Copeland N.G., Jenkins N.A. Novel myosin heavy chain encoded by murine dilute coat colour locus [published erratum appears in Nature 352:547] Nature. 1991;349:709–713. doi: 10.1038/349709a0. [DOI] [PubMed] [Google Scholar]
- Miyake K., McNeil P.L. Vesicle accumulation and exocytosis at sites of plasma membrane disruption. J. Cell Biol. 1995;131:1737–1745. doi: 10.1083/jcb.131.6.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle D.L., Karim M.A., Woolf E.A., Holmgren L., Bork P., Misumi D.J., McGrail S.H., Dussault B.J., Jr., Perou C.M., Boissy R.E. Identification and mutation analysis of the complete gene for Chediak-Higashi syndrome. Nat. Genet. 1996;14:307–311. doi: 10.1038/ng1196-307. [DOI] [PubMed] [Google Scholar]
- Orlow S.J. Melanosomes are specialized members of the lysosomal lineage of organelles. J. Invest. Dermatol. 1995;105:3–7. doi: 10.1111/1523-1747.ep12312291. [DOI] [PubMed] [Google Scholar]
- Pastural E., Barrat F.J., Dufourcq-Lagelouse R., Certain S., Sanal O., Jabado N., Seger R., Griscelli C., Fischer A., de Saint Basile G. Griscelli disease maps to chromosome 15q21 and is associated with mutations in the myosin-Va gene. Nat. Genet. 1997;16:289–292. doi: 10.1038/ng0797-289. [DOI] [PubMed] [Google Scholar]
- Perou C.M., Moore K.J., Nagle D.L., Misumi D.J., Woolf E.A., McGrail S.H., Holmgren L., Brody T.H., Dussault B.J., Jr., Monroe C.A. Identification of the murine beige gene by YAC complementation and positional cloning. Nat. Genet. 1996;13:303–308. doi: 10.1038/ng0796-303. [DOI] [PubMed] [Google Scholar]
- Perou C.M., Leslie J.D., Green W., Li L., Ward D.M., Kaplan J. The Beige/Chediak-Higashi syndrome gene encodes a widely expressed cytosolic protein. J. Biol. Chem. 1997;272:29790–29794. doi: 10.1074/jbc.272.47.29790. [DOI] [PubMed] [Google Scholar]
- Raposo G., Nijman H.W., Stoorvogel W., Liejendekker R., Harding C.V., Melief C.J., Geuze H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G., Tenza D., Mecheri S., Peronet R., Bonnerot C., Desaymard C. Accumulation of major histocompatibility complex class II molecules in mast cell secretory granules and their release upon degranulation. Mol. Biol. Cell. 1997;8:2631–2645. doi: 10.1091/mbc.8.12.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A., Rioult M.G., Ora A., Andrews N.W. A trypanosome-soluble factor induces IP3 formation, intracellular Ca2+ mobilization and microfilament rearrangement in host cells. J. Cell Biol. 1995;129:1263–1273. doi: 10.1083/jcb.129.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A., Samoff E., Rioult M.G., Chung A., Andrews N.W. Host cell invasion by trypanosomes requires lysosomes and microtubule/kinesin-mediated transport. J. Cell Biol. 1996;134:349–362. doi: 10.1083/jcb.134.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A., Webster P., Ortego J., Andrews N.W. Lysosomes behave as Ca2+-regulated exocytic vesicles in fibroblasts and epithelial cells. J. Cell Biol. 1997;137:93–104. doi: 10.1083/jcb.137.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank R.T., Novak E.K., McGarry M.P., Rusiniak M.E., Feng L. Mouse models of Hermansky Pudlak syndromea review. Pigment Cell Res. 1998;11:60–80. doi: 10.1111/j.1600-0749.1998.tb00713.x. [DOI] [PubMed] [Google Scholar]
- Tardieux I., Webster P., Ravesloot J., Boron W., Lunn J.A., Heuser J.E., Andrews N.W. Lysosome recruitment and fusion are early events required for trypanosome invasion of mammalian cells. Cell. 1992;71:1117–1130. doi: 10.1016/s0092-8674(05)80061-3. [DOI] [PubMed] [Google Scholar]
- Thompson C.B., Allison J.P. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7:445–450. doi: 10.1016/s1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]
- Tuvim M.J., Adachi R., Chocano J.F., Moore R.H., Lampert R.M., Zera E., Romero E., Knoll B.J., Dickey B.F. Rab3D, a small GTPase, is localized on mast cell secretory granules and translocates to the plasma membrane upon exocytosis. Am. J. Respir. Cell Mol. Biol. 1999;20:79–89. doi: 10.1165/ajrcmb.20.1.3279. [DOI] [PubMed] [Google Scholar]
- Wu X., Bowers B., Wei Q., Kocher B., Hammer J.A., III. Myosin V associates with melanosomes in mouse melanocytesevidence that myosin V is an organelle motor. J. Cell Sci. 1997;110:847–859. doi: 10.1242/jcs.110.7.847. [DOI] [PubMed] [Google Scholar]
- Wu X., Bowers B., Rao K., Wei Q., Hammer J.A., III. Visualization of melanosome dynamics within wild-type and dilute melanocytes suggests a paradigm for myosin V function in vivo. J. Cell Biol. 1998;143:1899–1918. doi: 10.1083/jcb.143.7.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wubbolts R., Fernandez-Borja M., Oomen L., Verwoerd D., Janssen H., Calafat J., Tulp A., Dusseljee S., Neefjes J. Direct vesicular transport of MHC class II molecules from lysosomal structures to the cell surface. J. Cell Biol. 1996;135:611–622. doi: 10.1083/jcb.135.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L., Regnault A., Lozier A., Wolfers J., Flament C., Tenza D., Ricciardi-Castagnoli P., Raposo G., Amigorena S. Eradication of established murine tumors using a novel cell-free vaccinedendritic cell-derived exosomes. Nat. Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]