Vasculogenic Mimicry and Tumor Angiogenesis (original) (raw)
Abstract
Tumors require a blood supply for growth and hematogenous dissemination. Much attention has been focused on the role of angiogenesis—the recruitment of new vessels into a tumor from pre-existing vessels. However, angiogenesis may not be the only mechanism by which tumors acquire a microcirculation. Highly aggressive and metastatic melanoma cells are capable of forming highly patterned vascular channels in vitro that are composed of a basement membrane that stains positive with the periodic acid-Schiff (PAS) reagent in the absence of endothelial cells and fibroblasts. These channels formed in vitro are identical morphologically to PAS-positive channels in histological preparations from highly aggressive primary uveal melanomas, in the vertical growth phase of cutaneous melanomas, and in metastatic uveal and cutaneous melanoma. The generation of microvascular channels by genetically deregulated, aggressive tumor cells was termed “vasculogenic mimicry” to emphasize their de novo generation without participation by endothelial cells and independent of angiogenesis. Techniques designed to identify the tumor microcirculation by the staining of endothelial cells may not be applicable to tumors that express vasculogenic mimicry. Although it is not known if therapeutic strategies targeting endothelial cells will be effective in tumors whose blood supply is formed by tumor cells in the absence of angiogenesis, the biomechanical and molecular events that regulate vasculogenic mimicry provide opportunities for the development of novel forms of tumor-targeted treatments. The unique patterning characteristic of vasculogenic mimicry provides an opportunity to design noninvasive imaging techniques to detect highly aggressive neoplasms and their metastases.
Tumors require a blood supply to sustain growth. The tumor microcirculation plays a central role in the hematogenous dissemination of cancers. Considerable attention has been focused on the mechanisms by which tumors acquire their blood supply. It is a well-accepted paradigm that tumors recruit new blood vessels from the existing circulation 1 —angiogenesis—either from factors secreted by the tumor cells, as Folkman 2,3 has emphasized, or from surrounding stromal cells. 4 There are two variations on the theme of tumor angiogenesis: augmentation of the angiogenic response by progenitor endothelial cells, and vessel cooption. Asahara and associates 5 described the incorporation of endothelial cell progenitors (or angioblasts) from circulating peripheral blood into sites of ischemic-driven angiogenesis. Holash and associates 6 described a process of “vessel cooption” in which tumors coopt the existing vasculature, which regresses leading to massive necrosis, and the tumor is then vascularized at the periphery by tumor angiogenesis as described above.
We 7 recently described a novel process by which tumors develop a highly patterned microcirculation that is independent of angiogenesis: in aggressive primary and metastatic melanomas, the tumor cells generate acellular microcirculatory channels composed of extracellular matrix and lined externally by tumor cells. The de novo generation of vascular channels by aggressive and metastatic tumor cells is not strictly a vasculogenic event, because true vasculogenesis results in the de novo formation of endothelial cell-lined vessels. We therefore assigned the name “vasculogenic mimicry” to the process by which aggressive tumor cells generate non-endothelial cell-lined channels delimited by extracellular matrix.
The discovery of a mechanism by which an aggressive tumor generates its own network of vascular channels challenges the prevailing assumption that angiogenesis and related mechanisms are the only means by which a tumor acquires a blood supply. 7 Bissell 8 has further noted that vasculogenic mimicry poses challenges to the practice of surgical pathology and provides opportunities for the development of new imaging techniques and cancer treatment strategies.
Background
The patterned microcirculation characteristic of vasculogenic mimicry was first described in uveal (intraocular) melanoma. 9,10 Although cutaneous melanoma is more prevalent, one may exploit some unique biological properties of uveal melanoma to study critical issues in tumor progression and metastasis in a human model of cancer. 11
Cutaneous melanomas usually originate in the epidermal compartment and require a breach of the epidermal basement membrane for tumor cells to interact directly with the dermal mesenchyme. Although cutaneous melanoma may disseminate hematogenously, the first route of metastasis is usually to regional lymph nodes. Uveal melanomas, by contrast, develop within the mesenchyme of the choroid, ciliary body, or iris and do not have an intraepithelial growth phase. There are no lymphatics within the eye. Uveal melanoma, therefore, is an ideal human tumor system in which to study the biology of hematogenous dissemination of cancer. Moreover, uveal melanoma spreads first and preferentially to the liver, 12 making it an ideal human model to study organ targeted metastasis.
There are some important differences in the management of cutaneous and uveal melanoma. Cutaneous pigmented lesions are accessible to incision and excisional biopsy without significant morbidity. Patients have a general fear of losing vision that may be surpassed only by the threat to life posed by cancer, 11 and it is not possible to perform incisional biopsies of intraocular tumors without interfering with vision. Some ophthalmic oncologists perform fine-needle aspiration biopsies (FNAB) of intraocular tumors to distinguish between melanomas and lesions that simulate melanomas clinically such as metastases to the eye. 13-15 In so doing, they make only one pass into the neoplasm to avoid interfering with vision. Thus, the one-pass ophthalmic FNAB does not provide a broad sampling of the tumor (in other tissue sites, multiple passes into the lesion from different angles increases the likelihood of a representative sampling 16 ). The ophthalmic one-pass FNAB sampling of intraocular tumors does not yield material that is satisfactory for prognostication in cytologically heterogeneous neoplasms. 17-19
It is possible for a patient to harbor a significant quantity of metastatic disease to the liver and maintain normal hepatic enzymes. 20 Therefore, the application of any new therapy likely to be effective in treating metastatic melanoma would be most efficacious if applied before the metastatic tumor burden is great. The identification of a patient at high risk for metastasis at the time of diagnosis would then prompt the delivery of adjuvant therapy. 21 With the increasingly popular trend to avoid removal of an eye containing uveal melanoma by administering vision-sparing methods of primary tumor ablations such as radiation, hyperthermia, and laser treatments, it is likely that pathologists will not encounter any tissue from which to suggest a prognosis for the medical oncologist. It would therefore be helpful for those physicians who manage patients with uveal melanoma to be able to estimate the clinical course of a patient with a primary uveal melanoma by a noninvasive substitute for biopsy.
In 1984, we embarked on a series of studies to identify attributes of uveal melanoma that were both strong markers of tumor progression and that could be detected by a noninvasive imaging technique. Because the interior of the eye and its circulation can be visualized directly by angiography, our attention was directed first to the melanoma microcirculation.
The Microcirculation of Uveal Nevi
There are no animal models that accurately reflect the histology and behavior of primary human uveal melanoma. 22 Few animals develop these tumors spontaneously, and the transgenic models of pigmented intraocular tumors 23-26 are complicated by histological features that indicate retinal pigment epithelial differentiation (uveal melanomas develop from melanocytes of the iris, ciliary body or choroid and not from the retinal pigment epithelium; retinal pigment epithelial neoplasms are rarely encountered in humans 27 ).
Following the precedent of studying the histogenesis of primary cutaneous melanoma in animals following the application of 7,12-dimethylbenz[a]anthracene (DMBA), 28-30 Folberg et al 22,31 attempted to induce primary uveal melanocytic lesions by the repeated application of this carcinogen to the rabbit sclera. The rabbit, although not commonly used in carcinogenesis research, provides an eye whose interior is suitable for repeated photography, thereby affording the opportunity to visualize the clinical emergence of pigmented lesions from the normal tissues. It was possible to induce nevi in the choroid of pigmented rabbits but attempts to promote these lesions to melanomas were abandoned because the progressive corneal opacification precluded a clinical (funduscopic) view of emerging lesions.
The nevi induced in these pigmented rabbits provided an opportunity to study the histology of the earliest clinically detectable lesions. The choroid of these animals became thick with pigmented, cytologically bland melanocytes which appeared to accumulate around pre-existing choroidal vessels. These vessels are easily identified by histological examination because they appear to be evenly spaced throughout the choroidal tissues. Naumann et al 32 had earlier described the histological appearance of human uveal nevi in which he indicated that uveal nevi in humans also incorporate the pre-existing choroidal vessels. Thompson et al 33 had described the incorporation of pre-existing vessels by a neoplasm without destruction of the pre-existing vessels.
The Microcirculation of Uveal Melanomas
Most uveal melanomas (97%) contain pre-existing, endothelial cell-lined blood vessels of the type seen in experimentally induced choroidal nevi and in uveal nevi in humans (Figure 1) ▶ . 10 However, it is difficult to detect smaller microvessels in many uveal melanomas, especially in highly pigmented tumors in which the detection of chromogens from histochemical reactions is quite challenging.
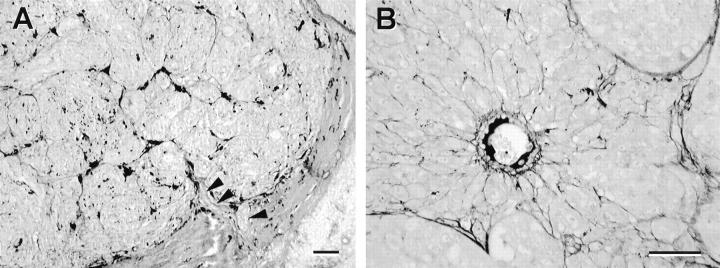
Figure 1.
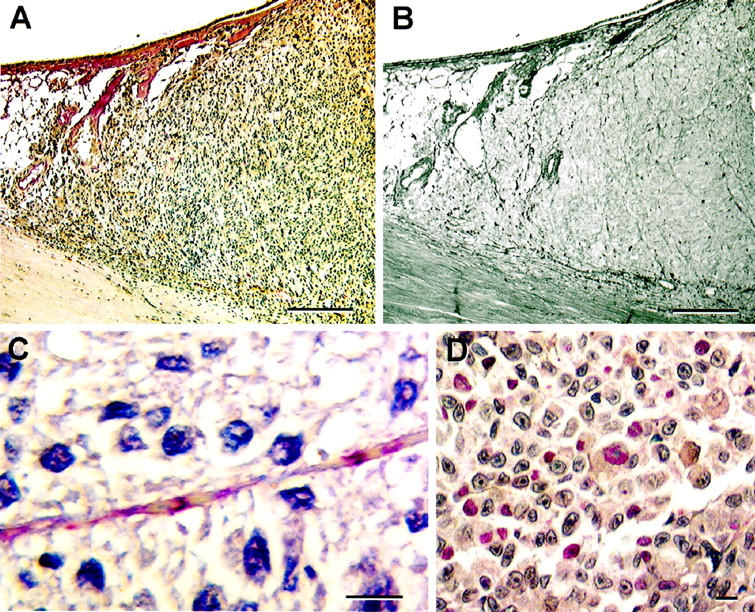
Normal choroidal vessels incorporated into uveal nevi and melanomas. A: Choroidal nevus. The nevus cells encircle four pre-existing choroidal vessels. B: Choroidal melanoma. At scanning magnification, few vessels are detected within the tumor, although some vessels are identified near the tumor’s edge (within the box). C: Choroidal melanoma. Higher magnification of boxed zone from B. The vessels at the tumor’s edge are lined completely by endothelial cells and these vessels have a prominent fibrous sheath, uncharacteristic of newly formed angiogenic vessels, but characteristic of normal choroidal vessels. Despite the size of this tumor, there is no evidence of necrosis. D: Normal choroidal vessel lined by endothelial cells (arrows) and invested with a distinctive fibrous connective tissue sheath is surrounded by epithelioid and spindle melanoma cells. The vessel is not damaged, and has been incorporated into the tumor. Original magnifications: A, scale bar, 250 μm; B, scale bar, 2 mm; C, scale bar, 100 μm; D, scale bar, 25 μm. A–D, hematoxylin-eosin.
Before the description of histochemical techniques to remove melanin pigmentation by peroxide bleaching after histochemical staining, 34 Folberg et al 9 explored the microcirculation of uveal melanomas with fluorescent-labeled Ulex europaeus agglutinin I (UEA-I) and laser scanning confocal microscopy to visualize the Ulex signal through melanin pigmentation. A variety of patterns of staining were identified including long, straight, vascular structures that were frequently arranged in parallel bundles and which occasionally cross-linked. _Ulex_-positive loops surrounding circular packets of tumor cells were also documented. These _Ulex_-positive channels were presumed to be endothelial cell-lined blood vessels. 9
A statistical analysis of the prognostic significance of these interconnected patterns of _Ulex_-positive structures required the study of a large series of tumors. It would have been impractical to use laser-scanning confocal microscopy to study a large series of tumors, and in the absence of continuous staining of these patterns by Ulex, Folberg et al 9 resorted to demonstrating these vascular channels by staining for the basal laminar matrix associated with these structures. Ophthalmic pathologists routinely employ the periodic acid-Schiff stain to highlight intraocular basement membranes of interest (such as Bruch’s membrane and Descemet’s membrane). The PAS stain highlighted the patterns demonstrated by Ulex in corresponding tissue sections. By omitting the hematoxylin counterstain, the visual confusion introduced by tumor cell nuclei was reduced and the PAS-positive patterns became more apparent. Further, by introducing a green filter into the light path of the microscope (or later, by selecting the green channel on digital images), the magenta color of the PAS-positive patterns were rendered vivid black color and easy to recognize.
In a pilot study, Folberg et al 9 examined 20 pairs of tumors matched for survival status (20 patients had died of metastatic melanoma and 20 had survived for 15 years or more disease-free). Each pair of tumors was also matched for size and location within the eye (confinement to the choroid or involvement of the ciliary body). The presence or absence of PAS-positive loops within the tumor was recorded for each tumor. The histological detection of closed PAS-positive loops was associated with the presence of other histological features predictive of metastasis: the presence of epithelioid melanoma cells by the modified Callender classification, 35 and mitotic figures.
With the histological identification of closed loops within uveal melanomas as a prognostically strong marker of tumor progression, attention was directed to the possibility of detecting other PAS-positive microcirculation-associated patterns in uveal melanoma that might be detectable by a noninvasive clinical test to serve as a surrogate for the invasive acquisition of tissue for examination by the pathologist. Folberg et al 10 later identified seven morphological patterns of PAS-positive channels in tissue sections of uveal melanomas (Figure 2) ▶ : straight channels, arrangements of parallel straight channels, straight channels that cross-link, arcs (incompletely closed loops), arcs with branching, closed loops, and networks (networks were defined arbitrarily as at least three back-to-back closed PAS-positive loops). These patterns were later found to be organized into two hierarchical groupings. 36 Tumors that contained parallel vessels with cross-linking also contained parallel channels and isolated straight channels, while tumors that contained networks also contained loops, arcs with branching and arcs without branching.
Figure 2.
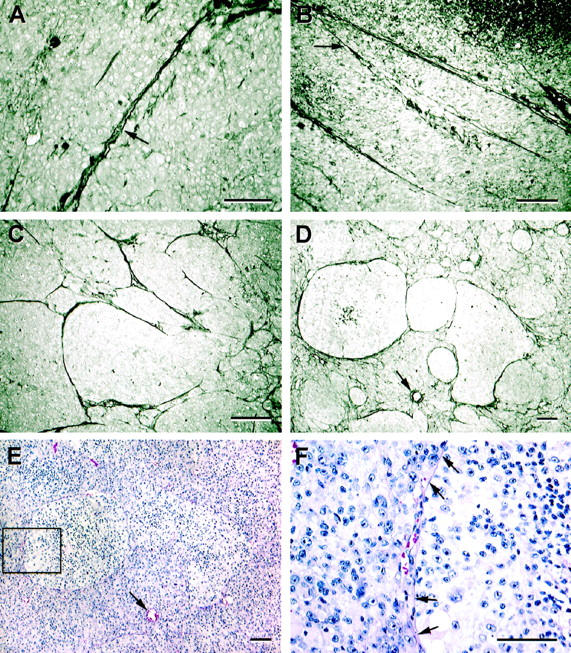
Patterns in primary uveal melanoma stained by the modified PAS stain (without hematoxylin counterstaining), compared with sections stained conventionally by hematoxylin-eosin. A: Straight channel. The channel splays open and contains circulating red blood cells (arrow). B: Parallel straight channels cross-link (arrow). C: Arcs (incomplete loops) are identified at the center of the micrograph, and a cluster of back-to-back complete loops is identified at the far right. D: Networks, defined as at least three back-to-back loops. Three large back-to-back loops are evident in the center of the micrograph above the normal choroidal vessel (arrow), but smaller complete loops are present throughout the upper half of the photomicrograph. E: Three pale-staining clusters of epithelioid melanoma cells correspond to the areas of tumor delimited by the large-diameter PAS-positive loops in the section adjacent to that shown in D (the arrow indicates the normal choroidal vessel for reference). The boxed area of the loop is illustrated at higher magnification in F. F: Red blood cells are identified within a space at the edge of the loop boxed in D. Arrows point to the contour of the loop highlighted here by hematoxylin-eosin. Original magnifications: A and F, scale bar, 50 μm.; B–E, scale bar, 100 μm. A–D, modified PAS without hematoxylin counterstain; 9 E and F, hematoxylin-eosin.
To obtain a more robust statistical analysis of the possible influence of microcirculation-associated PAS-positive histological patterns, Folberg et al 10 studied 234 eyes that had been removed for melanoma of the choroid or ciliary body. The prognostic significance of each of the PAS-positive patterns was tested. Kaplan-Meier survival curves generated from deaths secondary to metastatic melanoma indicated that at 10-year follow-up, the survival of patients whose tumors lacked cross-linked parallel vascular channels, loops, and networks was significantly better (91.7%, 91.1%, and 88.3%) than for patients whose tumor contained these patterns (56.9%, 55.4%, and 50.7%; P = 0.0001 for all comparisons; n = 234). Multivariate Cox proportional hazards models were generated that permitted the inclusion of conventional prognostic histological markers such as tumor size, location within the eye, the cell type according to the modified Callender classification, mitotic figures, and tumor infiltrating lymphocytes, and the presence or absence of PAS-positive vascular channel patterns, together with patient-related features such as age and gender. The presence of PAS-positive networks entered the model first (χ 2 = 40.84; P = 0.0001). Other significant variables included (in descending order of importance) tumor size, mitoses, cross-linking parallel vascular channels, the presence of tumor infiltrating lymphocytes, and male gender. Loops did not enter the model as an independent variable because networks, the most significant variable in the model, is composed of loops. In univariate models, the presence of arcs and arcs with branching was each associated with a significant mortality from metastatic melanoma.
The prognostic significance of these PAS-positive patterns, principally loops and networks, was confirmed subsequently by a number of independent laboratories. 37-41 There is a high degree of interobserver reliability in the histological detection of these patterns. 10,37,41
The prognostic association between any of the PAS-positive patterns depends on the mere detection of the pattern anywhere in the tissue section: the pattern is either present or absent. Because these patterns tend to be continuous (eg, arcs connect to loops which form networks), it is difficult to quantify patterns by counting discrete structures. However, one may measure the amount of tumor remodeling by patterns by calculating the percent of cross-sectional surface area in a histological preparation of tumor occupied by patterns of interest. Uveal melanoma lends itself to this technique because the entire cross-sectional area of the tumor can almost always be included in a standard glass microslide. Using this method, Mehaffey et al 42 associated death from metastatic melanoma with the presence of either networks or cross-linking parallel vessels that occupied 2% or more of cross-sectional area of tumor.
PAS-positive loops and networks were detected in hepatic metastases and in all secondary metastatic sites. 43 The ability for aggressive melanoma to form these patterns, therefore, did not appear to be dependent on the microenvironment of the eye, but rather represented an intrinsic property of this aggressive tumor cell phenotype.
The Vascular Nature of PAS-Positive Patterns in Uveal Melanoma
Foss et al 44,45 challenged the assertion that PAS-positive patterns in uveal melanomas were components of a microcirculation. Unable to demonstrate PAS-positive patterns by staining tissue sections for Factor-VIII related antigen, these investigators demonstrated an association between the number of points of tumor staining by Factor-VIII related antigen and survival. Following the protocol described by Weidner et al, 46 they assumed that every discrete point stained by this putative endothelial cell label represented a discrete blood vessel. Foss et al 44,45 discovered that PAS-positive patterns were associated with outcome in univariate models, but dropped out of multivariate models when “vessel counts” were allowed to enter the model.
In reviewing the work by Foss et al, 44,45 Folberg 47 and Rummelt et al 48 pointed out that by connecting discrete points labeled by Factor VIII-related antigen in the photomicrographs published by Foss et al, 45 one could demonstrate looping patterns in tissue sections of uveal melanoma. Parenthetically, even the initial studies from Folberg et al 9 using fluorescein-tagged Ulex and laser-scanning confocal microscopy suggested discontinuous labeling of loops, networks, and cross-linked parallel vascular channels.
Folberg et al 36 suggested that PAS-positive patterns in uveal melanoma were indeed a form of a tumor microcirculation for the following reasons. First, they 36 and others 41,44,49 labeled these PAS-positive patterns (albeit in a discontinuous fashion) with putative markers for vessels with Ulex, CD31, and CD34. Second, they traced these patterns directly to the vortex vein (Figure 3) ▶ 36 (the major venous drainage of the choroid) and to pre-existing vessels within the choroid (Figure 3) ▶ . 50 Third, they 36,51 performed three-dimensional reconstructions of _Ulex_-labeled PAS-positive patterns in uveal melanoma and demonstrated relatively flattened channels that branched and formed looping patterns.
Figure 3.
PAS-positive looping patterns connect to pre-existing normal vessels without intervening angiogenesis. A: Networks are traced to the vortex vein (arrowheads). There is no evidence of angiogenesis intervening between the networks and the vortex vein. B: Higher magnification of the normal vessel identified in Figure 2, D and E ▶ . The loops, which contain red blood cells (Figure 2F) ▶ connect directly to this pre-existing normal vessel without intervening angiogenesis. Original magnifications: A and B, scale bar, 50 μm.; A and B, modified PAS without hematoxylin counterstaining (Figure 3A ▶ modified from Folberg, et al 36 ).
At least three other observations argue for these patterns representing a functional microcirculation: 1) the absence of necrosis in uveal melanomas that measure 1 cm or more in diameter (Figure 1) ▶ suggests that these tumors are well-perfused: these tumors may lack histological evidence of internal angiogenesis but contain large areas of interconnected PAS-positive patterned channels 7 ; 2) red blood cells, often in a single-file (rouleaux) formation are frequently detected within these patterns (Figure 4) ▶ 7 ; and 3) ophthalmologists have detected looping patterns in uveal melanomas in patients using confocal imaging systems within seconds after injection of indocyanine green into the antecubital vein. 52-54 Additionally, the angiographic detection of looping patterns before removal of the eye has been correlated with the detection of PAS-positive looping patterns in histological sections of the corresponding tumors. 7,53
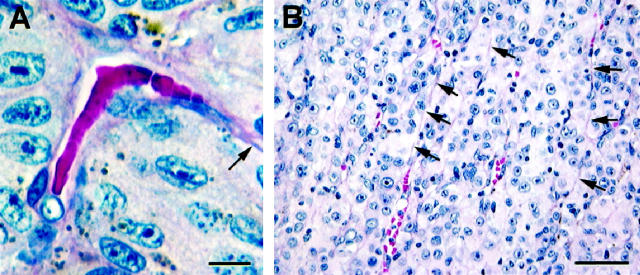
Figure 4.
Perfusion in vasculogenic mimicry patterns. A: Column of red blood cells in an arc without branching. Endothelial cell nuclei are not identified lining this channel. A thin layer of extracellular matrix (arrow) extends from this channel. B: Thin parallel channels that do not appear to be perfused with blood (arrows) splay open focally to reveal red blood cells in the lumen. None of these channels is lined by endothelium. Original magnifications: A, scale bar, 10 μm; B, scale bar, 50 μm; A and B, hematoxylin-eosin.
The Patterned Microcirculation of Uveal Melanoma: Is It Angiogenesis?
Foss et al 44,45,55 also argued that PAS-positive looping patterns identified by Folberg et al 10 could not have been vascular because of the topological arrangement of these patterns: vascular structures would not be expected to form looping patterns in two-dimensional histological sections.
What is the histological appearance of angiogenesis in intraocular tumors? Retinoblastoma, the most common intraocular tumor of children, is highly angiogenic and is characterized typically by a large number of vessels within tumors which are clearly lined by endothelium. Characteristically, zones of necrosis are present distal to the cuff of viable tumor cells surrounding the intratumoral blood vessels in retinoblastoma (Figure 5) ▶ . 56 Up-regulation of vascular endothelial growth factor has been demonstrated within these highly angiogenic tumors. 57,58 Curiously, despite evidence of florid intratumoral angiogenesis in retinoblastoma, deaths from metastatic retinoblastoma are vanishingly rare as long as the tumor is confined to the eye; the risk of mortality increases only when the tumor invades into the optic nerve, the uveal tract, or extends outside the eye. 59
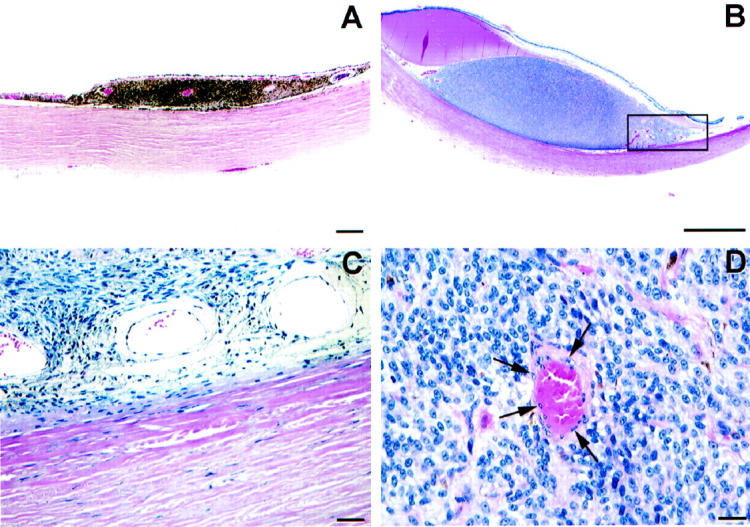
Figure 5.
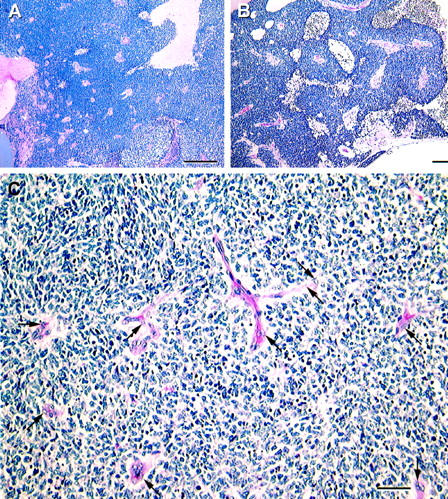
Angiogenesis in retinoblastoma. A: Numerous discrete vessels are identified. Note the zones of necrosis (left and upper right). B: Higher magnification. Viable tumor surrounds vessels in a cuff. Necrosis is identified farther away from the angiogenic vessel. C: With additional magnification, endothelial cell sprouting is identified histologically within the retinoblastoma tumor. Endothelial cells (arrows) are identified by light microscopy in every vessel. Original magnifications: A, scale bar, 500 μm; B, scale bar, 100 μm; C, scale bar, 50 μm. A–C, hematoxylin-eosin.
By contrast, one seldom sees the pattern of angiogenesis characteristic of retinoblastoma (with perivascular tumor cell cuffing around vessels that are clearly lined by endothelial cells interspersed with zones of necrosis, Figure 5 ▶ ) in tissue samples of primary human uveal melanoma. Significantly large zones of necrosis are seldom encountered within uveal melanomas. 41 Also, although Kvanta et al 57 detected VEGF mRNA by in situ hybridization within retinoblastoma, they were unable to do so in posterior uveal melanoma. Peer et al 60,61 have used uveal melanoma as negative controls for mRNA VEGF expression when studying classic examples of intraocular angiogenesis such as ischemic central retinal vein occlusion.
The interconnected channels characteristic of PAS-positive vascular channels are clearly different from the expected histological profile expected of tumor angiogenesis. Moreover, the incorporation of normal pre-existing choroidal vessels into uveal nevi and melanomas 33 (Figure 1) ▶ is clearly different from the mechanism of vascular cooption described by Holash et al 6 in which the inclusion of normal pre-existing vessels in the tumor results in destruction of the vessels, significant necrosis, and angiogenesis at the tumor periphery.
In light of these observations, the histology and ultrastructure of the PAS-positive channels in uveal melanoma was re-examined. The PAS-positive patterned channels were discovered to be lined externally by melanoma cells (Figure 4) ▶ but lacked an internal lining of endothelial cells by light and transmission electron microscopy. 7 Endothelial cells were detected lining the interior of pre-existing uveal vessel lumens incorporated into these tumors of the same type found in nevi 50 present within the same sections that contained PAS-positive patterned channels.
It is important to emphasize that a layer of extracellular matrix (corresponding to the PAS-positive channel lining) separated the blood column from the tumor cells. Thus, the red cells appeared to be contained within a tube of extracellular matrix. Tumor cells were apposed to the external surface of the tube. In this regard, the PAS-positive vascular channels in uveal melanoma are therefore different from the “angio-tumor complex” described by Lugassy et al 62 in which endothelial cells lining the interior of vessels are separated by laminin from melanoma cells (Lugassy et al 63 further proposed that melanoma cells migrate along the abluminal surface of endothelial cell-lined vessels, a process they term “extravascular migratory metastasis”).
The PAS-positive patterns of uveal melanoma were studied with conventional markers for endothelial cells including Factor VIII-related antigen, Ulex, CD31, CD34, and KDR (the flk receptor of vascular endothelial growth factor). 7 Although the endothelium in the vascular rich choroid adjacent to the tumor stained brilliantly with these markers, there was limited staining within tumors that contained the interconnected PAS-positive patterns such as loops, networks, and cross-linking parallel vascular channels (Figure 6) ▶ . At higher magnification, there was discrete labeling of these patterns with putative endothelial cell markers, but a careful examination of these labeling points indicated that the lumen contents (rather than the vessel walls) were stained, often discontinuously. 7 In some areas, the labeling was interrupted by the presence of red blood cells within the channel lumen (Figure 6) ▶ .
Figure 6.
Immunohistochemical staining of primary uveal melanoma with putative endothelial cell markers. A: Factor VIII-related antigen stains normal choroidal vessels (left of the tumor mass), but does not stain the interior of the tumor. B: Same tumor in A stained by the modified PAS stain without hematoxylin counterstain. Vasculogenic mimicry patterns are identified within the tumor. C: Primary uveal melanoma stained with Ulex europaeus agglutinin I. Note the intermittent staining of this vascular channel that contains red blood cells. The material between the red blood cells (plasma) stains with the Ulex lectin. The section is counterstained with hematoxylin, and despite the long segment of the channel illustrated, no endothelial cell nuclei are identified. There are no difficulties in identifying endothelial cell nuclei lining normal vessels incorporated into primary uveal melanomas (Figure 1) ▶ . D: CD31 staining tumor cells in the vicinity of vasculogenic mimicry patterns (not illustrated). Original magnifications: A and B, scale bar, 200 μm; C and D, scale bar, 10 μm. A: Factor VIII related antigen-hematoxylin; B: PAS without hematoxylin counterstain; C: Ulex europaeus agglutinin I with hematoxylin counterstain; D: CD31 counterstained with hematoxylin.
What explains the observation that endothelial cells are clearly present in normal vessels within the tumor but not within the anastomosing vascular channels outlined by PAS? One might argue that the interconnected PAS-positive patterns represent regressed angiogenesis: the endothelial cells are attacked, are destroyed, and leave behind their basal lamina. If this were correct, then the basal lamina of the PAS-positive patterns should resemble the basal laminar profiles of angiogenic blood vessels such as those demonstrated in retinoblastoma. However, the PAS-positive patterns do not appear to be vascular from the vantage point of topology. In fact, one might ask the following question: if highly invasive tumors are generally destructive of host tissues, what mechanisms permit a tropic growth of fragile endothelial cell sprouts to penetrate and survive within these tumors? 33
These issues prompted us to explore the hypothesis that the patterned PAS-positive vascular changes in uveal melanoma developed through mechanisms other than angiogenesis.
In Vitro Observations: Aggressive Uveal Melanoma Cells Are Capable of Generating Patterned Vascular Channels in the Absence of Endothelial Cells through Vasculogenic Mimicry
Daniels et al 64 and Hendrix et al 65,66 developed primary and metastatic uveal melanoma cell lines to explore the relationship between the aggressive tumor cell phenotype, and the generation of prognostically significant patterning in uveal melanomas. Aggressive uveal melanoma cells (but not non-aggressive cells) were found to produce type VI collagen which was thought to contribute to the histogenesis of these patterns. 64 Hendrix et al 65 described the relationship between the co-expression of mesenchymal (vimentin) and epithelial (keratin 8,18) intermediate filaments with respect to the invasive behavior of uveal melanoma cells, and found that this interconverted phenotype specifically expressed the c-met proto-oncogene which permitted the aggressive melanoma cells to respond to its ligand, hepatocyte growth factor/scatter factor (HGF/SF). 66 The relationship between aggressive melanoma cells that co-expressed vimentin and keratin 8,18 intermediate filaments was particularly interesting because these cells often aligned along the external walls of microvascular channels that conducted red blood cells, which did not appear to be lined by endothelial cells (Figure 7A) ▶ . This observation suggested further that the interconverted aggressive melanoma cell phenotype had a role in at least maintaining the patterned PAS-positive microcirculation of uveal melanomas.
Figure 7.
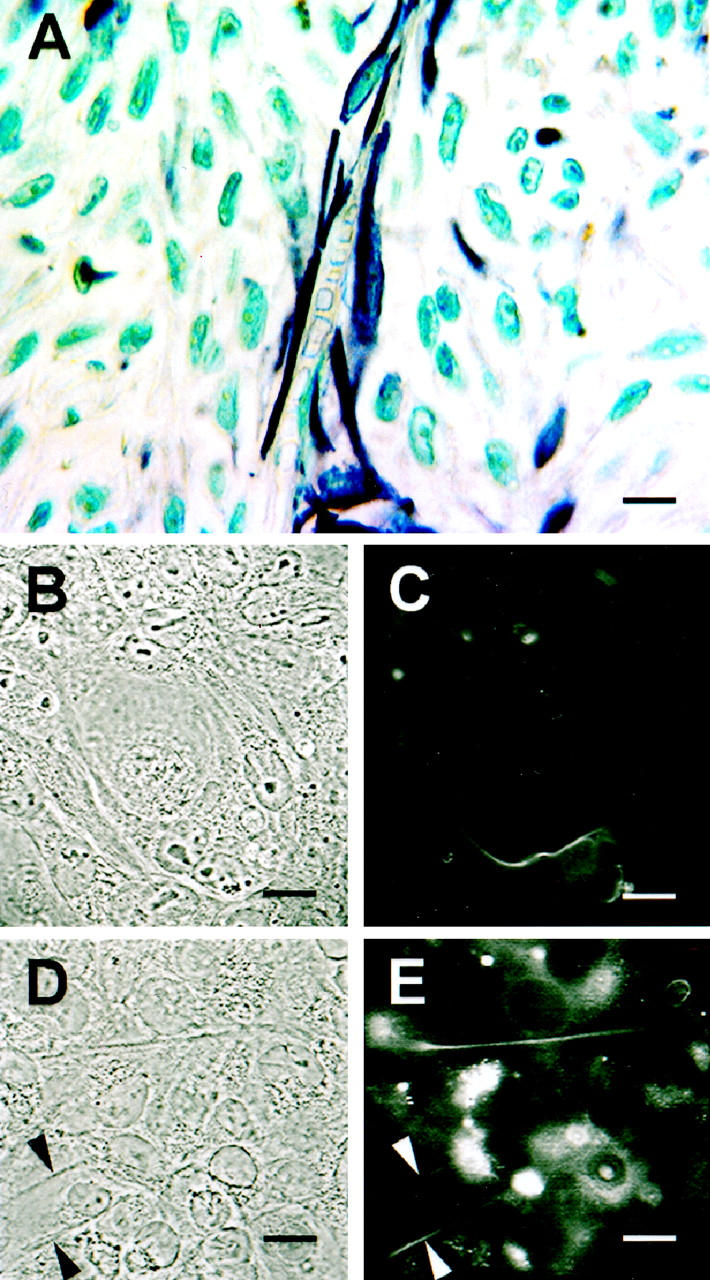
Cytokeratin expression in primary uveal melanoma and vasculogenic mimicry. A: Histological section of primary uveal melanoma. A channel containing red blood cells is lined externally by spindle melanoma cells that stain positive for pan-cytokeratin. Note the lack of endothelial cells along the inner channel wall. B–E:, Tissue cultures of metastatic uveal melanoma cell line MUM2B. B: Phase contrast showing loop encircling a small cluster of epithelioid melanoma cells. C: The same field illustrated in B photographed with fluorescence. The culture has been stained with antibody to keratins 8,18. Note the alignment of keratin-positive tumor cells alongside the looping pattern formed in vitro. D: Phase contrast of another aggressive melanoma culture showing two parallel straight channels. E: The same field illustrated in D photographed with fluorescence. The culture has been stained with antibody to keratins 8,18. Note the alignment of keratin-positive tumor cells alongside the straight channels, similar to that seen in tissue section (A). Original magnifications: A, scale bar, 10 μm; B–E, scale bar, 5 μm; A: pan-cytokeratin counterstained with hematoxylin; B and D: phase contrast; C and E: fluorescence (cultures labeled with antibody to keratins 8,18).
Maniotis et al 7 reported the unexpected finding that highly invasive and interconverted primary and metastatic uveal melanoma cell lines generated acellular channels in vitro (in three-dimensional cultures of Matrigel or Type I collagen) in the absence of endothelial cells or fibroblasts and without the addition of soluble growth factors such as bFGF, TGF-β, VEGF, PDGF. Thus, highly aggressive primary and metastatic tumor cells in vitro reconstituted channels that were interconnected into the patterns seen histologically in tissue samples of patients at high risk of dying from metastatic melanoma. By contrast, poorly aggressive uveal melanoma cells that were not interconverted (cells expressing vimentin but not co-expressing keratins 8,18) were incapable of generating channels under identical culture conditions as the aggressive cell lines, even after the induction of hypoxia, and the addition of conditioned media from the aggressive uveal melanoma cell lines and soluble growth factors. Furthermore, highly aggressive and metastatic melanoma cells expressing keratins 8,18 along with vimentin were frequently observed aligned outside the vascular channel wall (Figure 7, B–E) ▶ .
Maniotis et al 7 also demonstrated that the patterned channels generated by aggressive uveal melanoma cells in vitro were capable of conducting dye over short distances. They further demonstrated a striking comparison between the appearance of dye contained in the in vitro looping channels and those visualized angiographically in the tumors of patients after systemic injection of the dye, indocyanine green. 52,53
The differential ability of highly invasive and metastatic melanoma cell lines to generate patterned vascular channels (in comparison with poorly invasive melanoma cell lines) provided a biological basis for the use of these patterns in histological sections of human melanomas as a marker of tumor progression (Table 1) ▶ . Additionally, the generation of these patterned acellular channels by melanoma cells in the absence of endothelial cells provided an explanation for the histological appearance of patterned, matrix-lined vascular channels in melanomas that are not lined by endothelial cells.
Table 1.
Comparison between Patterned Circulatory Channels in Human Uveal Melanoma and in Vitro Melanoma-Generated Vascular Channels
| Channels in human uveal melanoma | In vitro melanoma cell-generated channels 7 |
|---|---|
| PAS-positive loops and networks | PAS-positive loops and networks |
| Association with death from metastatic melanoma | Formed only by aggressive uveal melanoma cells and not by poorly aggressive uveal melanoma cells |
| Absence of endothelial cell lining | Formed in the absence of endothelial cells, including fibroblasts |
| Loops can be visualized angiographically | Can transport dye in looping patterns |
Cutaneous Melanoma
Although uveal melanoma provides an interesting human model of cancer in which to study pure hematogenous dissemination of cancer, organ targeted metastasis, and the host response to a tumor that develops in an immunologically privileged site, uveal melanomas are considered rare tumors. The incidence of cutaneous melanoma is increasing and by contrast, cutaneous melanoma is a significant public health problem.
Busam et al 67 stained histological sections of primary cutaneous melanoma with UEA-1, CD34, and CD31 and failed to demonstrate any association between microvascular counts and outcome. In the course of this study, these investigators looked for the microcirculation patterns of uveal melanoma, 10 but did not identify them using these markers. In retrospect, Busam et al 67 stained for the presence of endothelial cells, whereas the patterned microcirculation of uveal melanoma was demonstrated in histological sections by staining for the matrix associated with the vascular channels using the PAS stain. If vasculogenic mimicry develops in cutaneous as well as uveal melanoma, then conventional markers for endothelial cells may not identify vasculogenic mimicry patterns.
Loops and networks can be demonstrated in the vertical growth phase of primary cutaneous melanoma (Figure 8) ▶ and in metastases from cutaneous melanoma 7,68 using the PAS stain without hematoxylin counterstaining. As in uveal melanoma, columns of red blood cells can be identified in these channels which are not lined by endothelial cells.
Figure 8.
Primary cutaneous melanoma, vertical growth phase. Networks are abundant. Original magnification: scale bar, 200 μm. PAS without hematoxylin counterstaining (tissue section courtesy of Prof. T. K. Das Gupta).
The identification of PAS patterns is not limited to histological observation. Maniotis et al 7 also demonstrated the generation of patterned vascular channels by a cell line of metastatic cutaneous melanoma under the same conditions for which the generation of patterned vascular channels was identified in cell lines derived from highly invasive primary and metastatic uveal melanoma. Therefore, the phenomenon of vasculogenic mimicry is not confined to the rare uveal melanoma, but appears both in vitro and in vivo in cutaneous melanoma. The prognostic significance of detecting looping vasculogenic mimicry patterns in histological sections of cutaneous melanoma is presently under investigation.
Counting Microvessels and Vasculogenic Mimicry
As mentioned above, some investigators 67,69-72 have not been able to establish a relationship between tumor vascularity as it is defined conventionally (the demonstration of microvessels by histochemical markers for endothelial cells) and outcome in cutaneous melanoma. On the other hand, some investigators 73,74 report an association between high “vascularity” and outcome in cutaneous melanoma while Ilmonen et al 75,76 recently reported that high vascularity was associated with a favorable outcome.
Likewise, there has been a significant difference of opinion in the value of counting microvessels in uveal melanoma. Lane et al 77 were unable to demonstrate a relationship between microvascular density and outcome from uveal melanoma, whereas Foss et al 44,55 and Makitie et al 78 demonstrated a relationship. The latter group cautioned that microvascular density may only be a “rough measure” of the relative vascularization rather than an exact number of vessels and cited several reasons for this insight: 1) some microvessels did not stain for endothelial cell markers (thus under-representing the number of vessels present); and 2) cell types other than endothelial cells might be labeled with these markers.
The discovery of vasculogenic mimicry in melanoma not only confirms the concerns of Makitie et al 78 but raises additional questions about the validity of counting structures that stain with putative endothelial cell markers as a measure of vascularization. When vasculogenic mimicry is present in a tumor (such as a melanoma), then pre-existing normal vessels 10,51 which contain endothelial cells will be labeled by the endothelial cell marker, making it difficult to equate “vascular density” with “angiogenesis” (the production of new vessels from pre-existing vessels). Additionally, the channels generated by tumor cells in vasculogenic mimicry may not stain with a variety of endothelial cell markers (because endothelial cells are not present in these channels) or the channels may stain in a discontinuous fashion because the contents of the lumen stain with these markers, assumed to be endothelial cell specific; 7 discontinuous staining of a vasculogenic mimicry channel may lead to the over-counting of one vascular structure (Figures 6 and 9) ▶ ▶ . Finally, tumor cells may themselves stain for endothelial cell markers (Figure 6) ▶ . 7 If the investigator uses slides that are stained to develop the chromagen for putative endothelial cell markers without counterstaining to identify the structure that is being labeled, then counting every labeling point may not accurately reflect the number of endothelial cell-lined vessels in the tumor.
Figure 9.
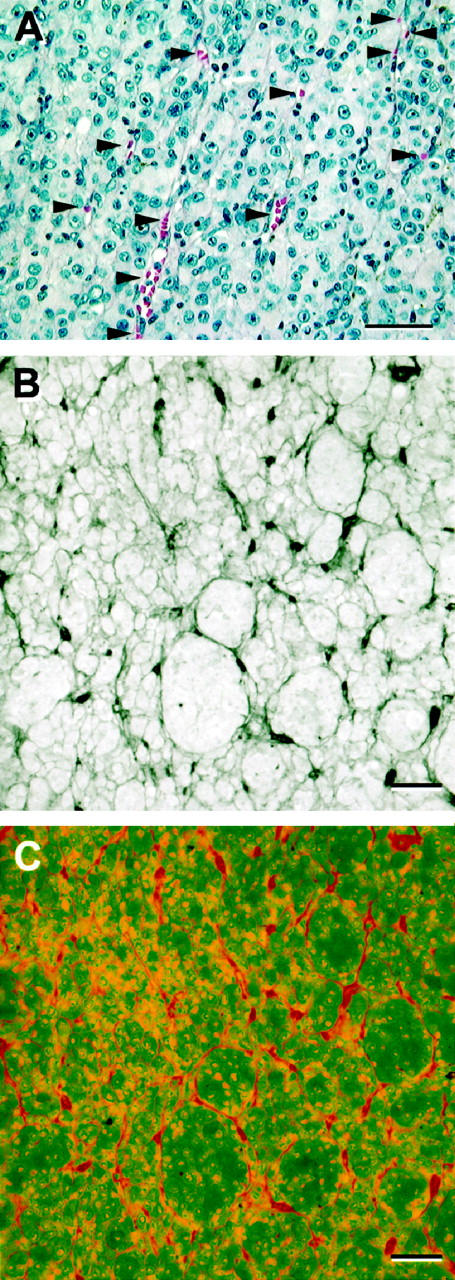
Perfusion of vasculogenic mimicry patterns may simulate the appearance of angiogenesis histologically. A: Primary uveal melanoma: perfusion in parallel vasculogenic mimicry channels. This is the same field illustrated in Figure 4B ▶ . Here, the areas in which the channels splay open and contain red blood cells are highlighted with arrowheads. Because the blood column itself stains with multiple putative endothelial cells markers such as Factor VIII-related antigen (Figure 6A) ▶ and Ulex (Figure 6) ▶ , as well as CD31, CD34, and KDR, 7 it is possible to count each focus of blood as a separate vessel if the tissue section is not counterstained with hematoxylin and one is not attuned to the existence of continuous vasculogenic mimicry patterns in the section. B and C: Co-localization of CD34 to PAS-positive loops and networks. A histological section of primary uveal melanoma containing multiple loops and networks was stained with CD34 (Texas Red chromagen) and counterstained subsequently by PAS without hematoxylin counterstaining. The tissue section was photographed Bio-Rad MRC-600 laser scanning confocal microscope (Bio-Rad, Cambridge, MA) by capturing both the direct illumination channel (for the PAS-positive patterns) and the rhodamine channel (for Texas Red). B: Back-to-back loops form networks. C: The same field illustrated in B, showing CD34 (in red) co-localizing to the loops by staining the lumen contents rather than endothelial cells. A pathologist looking only at the CD34 stain (C) might conclude erroneously that this is an angiogenic hot-spot. Original magnifications: A–C, scale bar, 50 μm; A, hematoxylin-eosin; B, CD34 and periodic acid-Schiff without hematoxylin counterstaining (direct illumination); C, CD34 and periodic acid-Schiff, rhodamine channel.
Many technical factors contribute to the accuracy of applying microvessel counts to prognosis by pathologists including the method of sampling and the selection of the marker for demonstrating microvessels. 79-81,81 Despite the popularity of using microvascular counts as a marker of tumor progression in many types of cancer, 81 there are a considerable number of reports that show no relationship between vascular counts and prognosis 67,69-72,77,82-107 and even a study that associates an increased vascular count with a longer rather than a shorter survival time. 96 It is possible that the presence of vasculogenic mimicry in tumor types other than melanoma may contribute to reports in which the association between vascular counting and outcome is not established.
Non-Endothelial Cell-Lined Vascular Channels in Animal and Human Tumors
Others 108-110 have hinted at the possibility of non-endothelial cell-lined channels in melanomas and other tumors. Jensen 108 described large sinusoids that were lined by tumor cells and not endothelial cells in the portion of a melanoma superficial to a break in Bruch’s membrane. Radnót and Antal 109 identified small vessels lined by endothelium in uveal melanoma (as did Folberg et al, 9 in an early study), but they also described vascular channels not lined by endothelium. Hammersen et al 111 identified cells lining the interior of vascular channels in animal models of melanoma and suggested that it would be difficult to identify these cells accurately by either transmission electron microscopy or with immunohistochemical stains. These authors suggested that mesenchymal cells and tumor cells may be incorporated into tumor blood vessels.
Konerding et al 110 described tumor-cell lined sinusoids in xenografts of melanomas and sarcomas that were clearly different from normal vessels. Konerding et al 110 also observed more of these abnormal tumor cell-lined vessels in the interior of tumors than normal endothelial cell-lined vessels. The scanning electron micrograph provided by Konerding et al 110 of flat, noodle-shaped vascular channels in a sarcoma that was not lined by endothelium is strikingly similar to three-dimensional reconstructions of the microcirculation of human uveal melanoma by Rummelt et al 51
Warren and Shubik 112 suggest from their in vivo observations of the vascularization of tumor implants in animals that an endothelial tropism develops adjacent to the tumor, and blood travels between loose cords of tumor cells as identified by transmission electron microscopy. The phenomenon described by Warren and Shubik 112 differs from vasculogenic mimicry in three key aspects: 1) capillary sprouts were observed to enter the tumor (angiogenesis) with leakage of red blood cells between tumor cells (one seldom sees leakage of red blood cells from the matrix-lined tubes characteristic of vasculogenic mimicry); 2) microthrombi were observed in the microcirculation (curiously, microthrombi are seldom observed in the patterned channels of vasculogenic mimicry, leading to speculation that either the tumor cell or the matrix outlining the vascular channel interferes with hemostasis); and 3) central necrosis was a feature of established animal tumors: central necrosis is not a feature typical of tumors containing vasculogenic mimicry patterns. 7
Nasu et al 113 implanted 0.1 mm 3 chunks of rat mammary carcinoma 13762 into transparent quartz chambers in female Fisher rats. They then recorded blood flow within the tumor 26 days after transplantation when the tumor measured 6 mm in mean diameter. By video microscopy, they observed back-to-back loops forming networks within these implants. Histological examination of these implants revealed little evidence of fibrous tissue adjacent to vascular structures within the tumor in contrast with fibrous connective tissue associated within the tissue surrounding the tumor. Moreover, these investigators demonstrated a uniform staining of Factor VIII related antigen in endothelial cells in the interstitium surrounding the tumor, but a non-uniform distribution within the tumor. They further observed that vessels within the tumor contained “extremely rare endothelial cells.” Of interest is the fact that they did not observe sprouting of new blood vessels and instead described the “primitive” flow of blood between tumor cells forming loops. This interesting observation differs from vasculogenic mimicry in two key aspects: 1) in vasculogenic mimicry, a layer of PAS-positive material of variable thickness separates the blood column from tumor cells (the red blood cells flow within channels formed by PAS-positive material as suggested by the in vitro reconstitution of these acellular channels by tumor cells), instead of blood coming directly in contact with tumor cells; and 2) vasculogenic mimicry requires the active participation of deregulated, highly aggressive tumor cells in the formation of a patterned non-endothelial cell-lined vascular channel, whereas Nasu et al 113 attribute the formation of non-endothelial cell-lined vascular channels to the passive sculpting of tumor by hemodynamic forces. Vasculogenic mimicry, therefore, is linked to the aggressive tumor cell phenotype; in the system described by Nasu et al, 113 it is not clear that the tumor plays any active role in establishing its circulation.
Vasculogenic Mimicry and Angiogenesis: The Issue of Compartmentalization
Before the discovery by Maniotis et al 7 that the PAS-positive acellular vascular channels were formed by tumor cells, some pathologists considered the patterns described by Folberg et al 10 to be a stromal response to the presence of the tumor as implied by terms used by some to describe these patterns: “fibrovascular loops.” 38,44,45 Foss et al 45 concluded that the PAS-positive patterns described by Folberg et al 10 were “mostly formed from connective tissue, including perivascular connective tissue.” The in vitro studies by Maniotis et al 7 do not support these conclusions: not only is the patterned tumor microcirculation of uveal melanoma generated by aggressive tumor cells themselves, these patterns form in the absence of fibroblasts, other stromal cells, and endothelial cells. 7
Uveal melanoma is distinctive because there is usually no induction of a stromal host response at the interface between the tumor and the surrounding host stroma (Figure 6) ▶ . Moreover, stromal ingrowth (a fibrovascular connective tissue stroma) is seldom seen within the expanding cellular compartment of most uveal melanomas unless the tumor has been treated previously by irradiation or necrosis is evident. However, uveal melanoma may not be unique: Birck et al 114 failed to demonstrate CD31-positive vessels within the expanding tumor mass in most cases of primary cutaneous melanoma.
In studying transplantable mouse mammary adenocarcinoma, Thompson et al 33 noted a greater number of vessels in the adjacent connective tissue than in the tumor itself. In studying human breast cancer, de Jong et al 115 noted a lower density of vessels within the tumor than in the stroma. These authors used this observation to caution pathologists who relate microvascular density to note whether their observations are taken from the cellular part of the tumor or the stroma. In prostatic carcinoma, Bigler et al 116 also demonstrated an increase in vascularity within the tumors, but the increased vascularity was confined to the stroma.
These observations suggest that the angiogenic response to tumors may be a component of the stromal compartment of tumors, rather than the tumor cell compartment. Indeed, in the initial description of tumor vessel counts as a marker of prognosis in breast cancer, Weidner et al 46 illustrated vascularity in the stromal compartment of the tumor adjacent to masses of tumor cells. Brown et al, 117 who likened the tumor stroma to a healing wound, recently observed 118 that the formation of a vascular rich stroma in breast cancer precedes invasion and suggested that breast cancer invades into a richly vascular stroma induced by the tumor. It is interesting to speculate that more aggressive tumors induce a more robust stromal response and that the intensity of the stromal response is the basis for relating counts of microvessels from histological sections to outcome. Along similar lines, one might argue that therapy targeted against angiogenesis would be expected to interfere with or reduce the stromal response to the cellular component of the tumor rather than affect the cellular compartment of the tumor directly.
The identification of vasculogenic mimicry in melanoma as an event separate from angiogenesis suggests that different tumor types may acquire their blood supply by different mechanisms. Uveal melanoma perhaps sits at one end of the spectrum. In the earliest phases of tumor growth, the uveal melanocytic neoplasm incorporates pre-existing vessels without destroying them, without provoking central necrosis, and without inducing angiogenesis at the tumor periphery (unlike cooption as described by Holash et al 6 ). Relatively indolent uveal melanomas do not show evidence of vasculogenic mimicry, but aggressive tumors develop a perfused microcirculation comprised of acellular vascular channels generated by the tumor cells themselves that generally precludes necrosis, even in relatively large tumors. The vascular channels generated by aggressive tumor cells hook up to either pre-existing vessels incorporated into the tumor 50 or to the venous drainage of the eye at the vortex vein (Figure 10) ▶ . 36 Angiogenesis may accompany focal zones of necrosis and may be seen after radiation treatment but does not play a significant role in supplying the tumor with a microcirculation.
Figure 10.
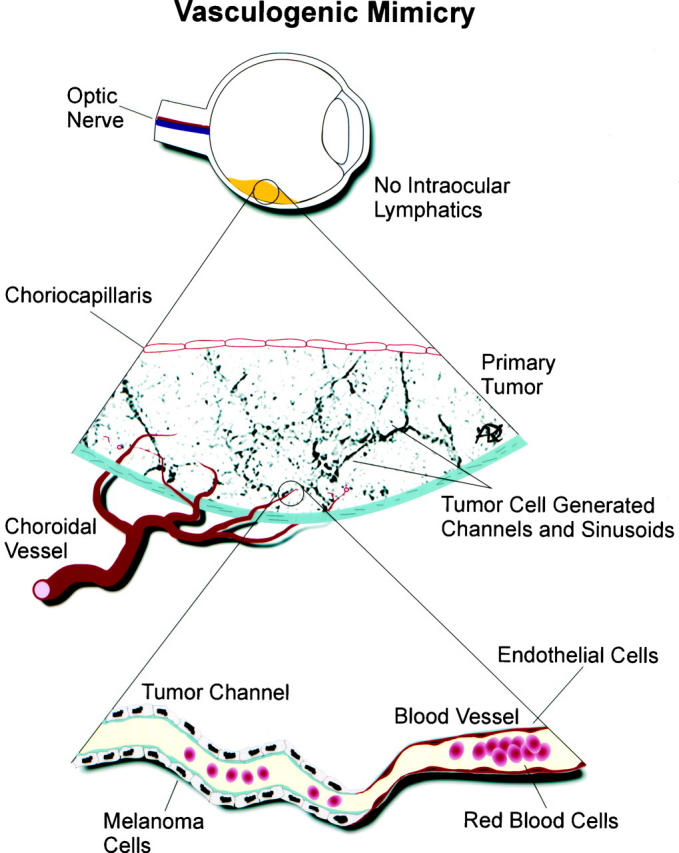
Diagrammatic scheme of vasculogenic mimicry. Uveal melanomas develop in an environment devoid of lymphatics. Aggressive tumors (but not non-aggressive tumors) form looping non-endothelial cell-lined channels that are delimited by PAS-positive material. These vasculogenic mimicry channels link directly to normal vessels in the choriocapillaris, the vortex vein, or normal vessels incorporated into the tumor without evidence of angiogenesis. Channels generated by tumor cells (vasculogenic mimicry) are lined externally by tumor cells, in contrast to blood vessels which are lined internally by endothelial cells. Diagram courtesy of Dr. Dawn Kirschmann.
Vasculogenic mimicry is also known to develop in cutaneous melanoma, and angiogenesis is seldom seen within the expanding cellular compartment of these tumors except adjacent to zones of ulceration or necrosis. However, vascularization in the dermis is a known component of the regression response to cutaneous melanoma. 119 The compartmentalization of vasculogenic mimicry to the cellular compartment and angiogenesis typically to the stromal response may account for the variability of associating vascular counts with prognosis in cutaneous melanoma.
Maniotis et al 7 showed that vasculogenic mimicry patterns form in vitro in the absence of hemodynamic forces, suggesting that the formation of a patterned non-endothelial cell-lined microcirculation may be an attribute of the tumor cell. The phenotypic properties of the tumor implants used in experiments by Warren and Shubik 112 and Nasu et al 113 were not described. If the animal tumor fragments used in these experiments contained vasculogenic mimicry patterns, then it is possible that the flow of blood from capillary sprouts that penetrated the tumor either dissected around tissue planes generated by tumor cell remodeling or that the flow of blood into the tumor hooked up with vasculogenic mimicry channels.
At the other end of the spectrum, there may be tumors that develop a prominent stromal vascular (angiogenic) response exclusive of vasculogenic mimicry. Until the various contributions of angiogenic stromal responses and non-angiogenic mechanisms are identified for different types of tumors, pathologists may wish to exercise caution in establishing and relying on conventional markers of tumor “vascularity” as prognostic markers, and those who are developing anti-cancer therapies by targeting angiogenesis may wish to exercise caution when interpreting their results. 111
Additional Observations from Animal Models
The microcirculation of xenografts may vary depending on the host and the tumor itself. Lauk et al 120 noted that in xenografts of human tumors, the cellular distribution and differentiation characteristics are retained, but the vascular density of the transplant is host specific. Wilson et al 121 cautioned that the organization of the microcirculation between different cell lines of prostate cancer varied, implying that each tumor may exert “unique influences” on the pattern of microvessel development.
The identification of vasculogenic mimicry as a mechanism for generating a blood supply to tumors independent of angiogenesis 7 also raises questions about the relevance of various animal models of cancer to their human counterparts. For example, it is possible that vasculogenic mimicry can be identified in animal models of cutaneous melanoma. Potgens et al 122 compared the formation of xenografts between human melanoma cell lines that had low and high levels of expression of vascular permeability factor (VPF or VEGF). Tumors that formed from cell lines with low VPF expression were characterized by vascularization by vessels of varying size with alternating zones of viability and necrosis. By comparison, the tumors that developed from a VPF-transfected melanoma cell line were characterized by vascularization around nodules of tumor cells. Curiously, both cell lines with high and low expression of VPF developed zones of tumor organized into lobules by anastomosing back-to-back loops of matrix that stained positive for laminin surrounding zones of viable tumor. These laminin-positive networks, identical in appearance to the PAS-positive patterns of uveal and cutaneous melanoma, were not identified with markers to mouse endothelial cells.
Potgens et al 123 studied this system further. They identified tracer material not only within the lumina of functional blood vessels, but also within the patterned (looping) “stromal septa” within the melanoma xenografts that “seemed to contain channels connecting the blood vessels.” They further observed that these looping channels that contained tracers only partly co-localized to putative markers for endothelial cells. It is not known from these studies if Potgens et al 122,123 assumed the presence of endothelial cells because of staining with endothelial cell markers, or if they confirmed the presence of endothelial cells by other means (as we demonstrated, many endothelial cell markers stain the luminal contents of the channels of vasculogenic mimicry_)._ Other suggestive evidence of vasculogenic mimicry is found in a report from Erhard et al 124 describing the lobular partitioning of cutaneous melanoma by septa that were positive for collagen Type IV and heparin sulfate proteoglycan: staining for an endothelial cell marker, PAL-E, showed marked staining of angiogenic vessels at the base of the lesion, but only sporadic staining within the septa.
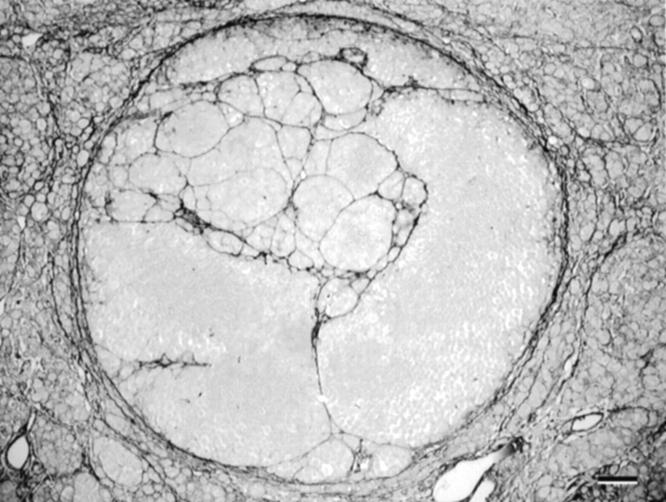
Most animal models of uveal melanoma are developed using xenografts of cell lines to immunosuppressed animals and yield inconsistent results. Grossniklaus 125 reported the development of looping PAS-positive patterns in an animal model of melanoma generated by implanting a mouse cutaneous melanoma cell line into mouse eyes. Recent evidence suggests that heterotopic injection of human uveal melanoma cells into the subcutaneous area of immunosuppressed mice resulted in a tumefaction with pronounced central necrosis, clear-cut evidence of angiogenesis (especially near the zones of necrosis and in the adjacent dermis), and an absence of looping patterns (Figure 11) ▶ associated with vasculogenic mimicry (M. J. C. Hendrix, A. J. Maniotis, and R. Folberg, unpublished data). As noted above, one seldom identifies large zones of necrosis in primary human uveal melanomas associated with angiogenesis (Figure 1) ▶ . However, these data must be interpreted cautiously because they are derived from an ectopic site in an animal model. Clearly, Killion et al 126 have emphasized the need to use orthotopic animal transplantation models in studying the effects of therapeutic agents.
Figure 11.
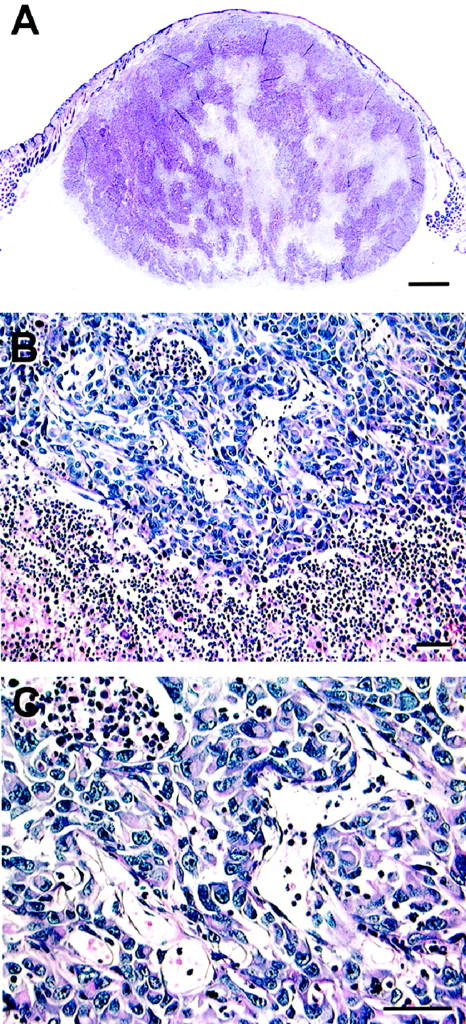
Animal model of uveal melanoma cell line implanted subcutaneously into immunosuppressed mouse. A: Scanning magnification. A tumor measuring 9 mm at its base developed from the subcutaneous injection of aggressive uveal melanoma cells. Note the presence of necrosis centrally which is not a feature typical of primary uveal melanoma (compare with the primary uveal melanoma shown in Figure 1B ▶ , which also measured 9 mm in diameter). B: Higher magnification of the interface between viable tumor and necrosis. Dilated endothelial cell-lined vessels are present adjacent to an area of necrosis (lower portion of the micrograph). C: Higher magnification of vessels shown in B. These vessels are lined by endothelial cells. Original magnifications: A, scale bar, 1 mm; B, scale bar, 50 μm; C, scale bar, 50 μm. A–C, hematoxylin-eosin.
Differences in the microcirculation of various animal models may help to explain the different responses of animal models to anti-angiogenic therapy. For example, anti-angiogenic compounds were capable of inducing long-term dormancy in a xenograft model of Lewis lung carcinoma. 127 By contrast, none of four anti-angiogenic compounds completely halted the growth of transgenically induced (primary) pancreatic cancers in a mouse model. 128 Paradoxically, in this transgenic model, it was not surprising that apoptosis was induced in endothelial cells treated with the cytostatic anti-angiogenic compounds, but it was intriguing that tumor cells in close apposition to capillaries were also frequently apoptotic. One might have expected the opposite: that oxygen and nutrient deprivation resulting from an impaired vasculature would preferentially affect hypoxic tumor cells most distal to capillaries. It is not known if anti-angiogenic compounds such as angiostatin 129 and endostatin 130 inhibit vasculogenic mimicry—the generation of acellular vascular channels by aggressive tumor cells independent of participation by endothelial cells.
There are, indeed, numerous variables that influence the characteristics of the microcirculation of tumors in animal models: orthotopic versus heterotopic injection, the nature of the animal host, the tumor type, and the presence or absence of vasculogenic mimicry versus angiogenesis. Therefore, it important to ensure that animal models represent accurately the microcirculation of the human counterpart before drawing conclusions about the efficacy or lack of efficacy of anti-angiogenic compounds.
Diagnostic Imaging
Vasculogenic mimicry generates a tumor microcirculation that is uniquely patterned and found in adults. Moreover, the putative biomechanical and proteolytic properties responsible for the generation of vasculogenic mimicry remodel the tumor cell compartment in a fashion different from that seen in normal tissues. Because vasculogenic mimicry is a hallmark of the aggressive cell phenotype in vitro and has been established as a strong marker of tumor progression from retrospective studies of human tumor tissue samples, attention has been focused on either imaging vasculogenic mimicry patterns directly by angiography, or imaging tumor remodeling by means of specialized ultrasonography to develop noninvasive substitutes for biopsy. Because vasculogenic mimicry is present in metastatic deposits from both uveal and cutaneous melanoma, the detection of this uniquely patterned microcirculation provides an opportunity to detect metastases.
Most of the research in clinical imaging of vasculogenic mimicry has focused on uveal melanoma. Within seconds after the injection of the dye, indocyanine green, it is possible to detect loops and networks within melanomas using a laser scanning confocal ophthalmoscopy device. 52,131
Mueller et al 53 presented preliminary data suggesting that the patterns imaged clinically with this technique reflect the histological vasculogenic mimicry patterns of prognostic significance. Before enucleation, two patients with melanomas of the choroid were studied by confocal laser scanning ophthalmoscopy after systemic injection of indocyanine green. The laser scanning confocal ophthalmoscope used in this study, the Heidelberg retinal angiogram (Heidelberg Engineering, Heidelberg, Germany), is capable of imaging retinal vessels as small as 10–15 μm (W. Freeman, personal communication). The angiogram of one patient revealed networks of back to back loops, and the angiogram of the other patient revealed only large, straight vessels typical of the normal choroidal circulation. Both eyes were opened by a special dissection technique (the anterior segments were removed by a coronal incision through the pars plana) to permit the pathologist a direct view of the tumor. 132 The vascular pattern of the retina seen on the angiogram was identified in each of these two tumors. The tumors were sectioned in a plane that corresponded exactly with the area on the angiogram that contained either the networks or the normal choroidal vessels by matching the bifurcation patterns of the overlying retinal vessels. Histological sections were stained both with hematoxylin-eosin and the periodic acid-Schiff stain without hematoxylin counterstaining. The eye that contained networks within the tumor angiographically also contained PAS-positive networks histologically without evidence of angiogenesis in the tumor section plane that corresponded to the angiogram; 7,53 the tumor that contained only normal choroidal vessels angiographically contained only large, choroidal vessels histologically without evidence of either PAS-positive networks or angiogenesis.
Following this initial description of correspondence between angiography and histology, Servetopoulou et al 133 studied 35 patients with choroidal melanoma using different angiographic techniques. They established an angiographic-histological correlation in 14 of 15 eyes studied by laser scanning confocal ophthalmoscopy and indocyanine green, and 7 of 8 eyes using digital fluorescein angiography. Conventional angiography provided a less accurate correlation: an angiographic-histological correlation was noted in only 11 of 26 cases. These findings suggest that the method of angiography used may be important in establishing angiographic-histological correlation. 54 The carefully conducted correlative studies by Mueller et al 53 and Servetopoulou et al 133 suggest that it is possible to image microcirculation patterns within choroidal melanomas that correspond precisely to histological patterns of prognostic significance.
It may also be possible to exploit the consequences of biomechanical remodeling within the tumor that generates the looping vasculogenic mimicry patterns by ultrasound parameter spectrum analysis. This specialized technique analyzes digitally recorded echoes returned from regions of tissue that provide statistical measures related to small spatial fluctuations in acoustic impedance. 134 In 1990, Coleman et al 135 originally attempted to use this technique to image the cell type within uveal melanomas (uveal melanomas containing epithelioid cells are associated with a worse prognosis than those tumors lacking these cells). They later acknowledged the fact that ultrasound parameter spectrum analysis could not image individual cells, and predicted that the patterns detected with this technique were “associated with tumor microregions such as intervascular nests of cells rather than directly with the individual size of cells.” 135 The following year, Coleman et al 136 showed that cell distribution patterns detected by parameter spectrum analysis were related to outcome of patients with uveal melanoma.
Correspondence between the acoustic scatterer sizes and the size of tumor microregions outlined by vasculogenic mimicry patterns suggested that ultrasound power spectrum analysis might be capable of identifying these patterns in tissue. Prospective studies in which ultrasonograms were obtained before removal of the eye have shown a very good correspondence between ultrasound power spectrum analysis and the histological presence of vasculogenic mimicry loops and networks. 137,138
Additional studies are underway to determine whether it is possible to image vasculogenic mimicry patterns in other primary tumors and their metastases for noninvasive prognostication.
Therapeutic Approaches Targeting Vasculogenic Mimicry
Maniotis et al 7 also reported an intriguing differential expression of multiple genes between poorly invasive and highly invasive uveal melanoma derived from the same tumor using hybridizations to cDNA microarrays. Approximately 210 known genes were expressed differentially. The overexpression of the TIE-1 gene in aggressive melanoma cells is intriguing because TIE-1 expression has heretofore been associated with the formation and maintenance of vessels by endothelial cells. 139 Easty et al 140 previously reported an association between TIE-1 expression and the metastatic phenotype in cutaneous melanoma. However, Maniotis et al 7 also discovered that genes for epithelial cell kinase and keratin 8 intermediate filament were overexpressed as well, suggesting a shared epithelial-like genotypic expression. Finally, these keratin and vimentin-positive melanoma cells also overexpressed the gene for Type VI collagen (confirming an earlier observation that aggressive uveal melanoma cells produce this component of the extracellular matrix 64 ), consistent with a mesenchymal phenotype. The cDNA microarray analysis therefore suggested a genetic reversion to a pluripotent embryonic-like genotype by highly aggressive melanoma cells.
It is intriguing to speculate that targeting one or more of these genes associated with the aggressive tumor cell phenotype might provide an avenue for therapeutic intervention. For example, Bissell 8 suggested that the overexpression of TIE-1 by aggressive melanoma cells might provide a common target for both angiogenesis and vasculogenic mimicry. It will be important to perform additional comparisons between cell lines to determine whether the profile of differential gene expression consistently discriminates between poorly and highly invasive cell lines, or if, as a result of generalized deregulation of gene expression, aggressive tumor cell lines show evidence of inconsistent gene differential gene expression. Moreover, it will be necessary to determine whether differential gene expression is reflected in corresponding overexpression of the gene product.
Contraction and remodeling of matrices has been linked to the ability of many types of cells to generate cords and tube-like structures in vitro. 141,142 Maniotis et al 7 demonstrated that highly invasive and metastatic uveal melanoma cells contracted floating collagen gels in vitro. By interfering with the ability of actin microfilaments to transduce forces throughout the tumor cell, it was possible to block the constriction of matrices reversibly with 1 μmol/L cytochalasin-D. Uveal melanoma cell lines have been shown to secrete gelatinolytic metalloproteinases, 143,144 and there is evidence associating expression of metalloproteinase-2 to metastases from uveal melanoma. 145
There are no known physiological analogs of vasculogenic mimicry. In fact, the generation of vascular channels by cytotrophoblasts in placental tissue 146-148 may represent the last normal vasculogenic event in the human. Because vasculogenesis and vasculogenic mimicry do not occur physiologically in the mature child or the adult, it may be possible to target the molecular mechanisms responsible for the generation and maintenance of vasculogenic mimicry and minimize the effects on normal physiological processes.
Note added in proof: It has been brought to our attention recently that Schneider et al identified networks angiographically using laser scanning confocal ophthalmoscopy and indocyanine green in 11 patients with uveal melanoma who subsequently had their eyes removed; PAS-positive networks were identified histologically in all 11 patients (Schneider U, Sobottka B, Inhoffen W, Kreissig I: Mikrovaskularisationsmuster maligner Melanome der Aderhaut: Vergleich von indozyaningrün-angiographischem und histopathologischem Befund. Ophthalmologe 1998, 95 (Suppl 1):53(abstract)). Additionally, Mueller et al studied 65 patients with choroidal melanocytic tumors by laser scanning confocal ophthalmoscopy and indocyanine green angiography. The tumors were then observed for evidence of growth. Angiographic detection of parallel vessels with cross-linking, arcs with branching, loops, and networks all showed a highly significant association with eventual tumor growth (Mueller AJ, Schaller UC, MUSIC Collaboration: Die Muenchen/San Diego/Iowa City Collaboration (MuSIC): Design, Charakterisierung des Kollektives und erste Ergebnisse. Ophthalmologe 1999, 96(Suppl 1):15 (abstract)). This is the first prospective study suggesting a prognostic role for the clinical imaging of vasculogenic mimicry patterns.
Footnotes
Address reprint requests to Dr. Robert Folberg, Department of Pathology, University of Illinois at Chicago, 446 CMW (M/C 847), 1819 W. Polk Street, Chicago, IL 60612. E-mail: rfolberg@uic.edu.
Supported by National Institutes of Health grants R01 EY10457 (to R.F.), R01 CA59702 (to M.J.C.H.) and R01 CA80318 (to M.J.C.H. and R.F.), by the University of Iowa Central Microscopy Research Facility, the Charles Hendrix Research Foundation and the University of Iowa Leading Woman Scientist Endowment (to M.J.C.H.), and in part by an unrestricted grant from Research to Prevent Blindness, Inc. Dr. Folberg is a Research to Prevent Blindness Senior Scientific Investigator.
References
- 1.Risau W: Mechanisms of angiogenesis. Nature 1997, 386:671-674 [DOI] [PubMed] [Google Scholar]
- 2.Folkman J: Tumor angiogenesis: therapeutic implications. N Engl J Med 1971, 285:1182-1186 [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Folkman J: Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996, 86:353-364 [DOI] [PubMed] [Google Scholar]
- 4.Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, Lu NF, Selig M, Nielsen G, Taksir T, Jain RK, Seed B: Tumor induction of VEGF promotor activity in stromal cells. Cell 1998, 94:715-725 [DOI] [PubMed] [Google Scholar]
- 5.Asahara T, Murohara T, Sullivan A, Silver M, van der ZR, Li T, Witzenbichler B, Schatteman G, Isner JM: Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997, 275:964–967 [DOI] [PubMed]
- 6.Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD, Wiegand SJ: Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science 1999, 284:1994-1998 [DOI] [PubMed] [Google Scholar]
- 7.Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LMG, Pe’er J, Trent JM, Meltzer PS, Hendrix MJC: Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol 1999, 155:739-752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bissell MJ: Tumor plasticity allows vasculogenic mimicry, a novel form of angiogenic switch—a rose by any other name? Am J Pathol 1999, 155:675-679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folberg R, Pe’er J, Gruman LM, Woolson RF, Jeng G, Montague PR, Moninger TO, Yi H, Moore KC: The morphologic characteristics of tumor blood vessels as a marker of tumor progression in primary human uveal melanoma: a matched case-control study. Hum Pathol 1992, 23:1298-1305 [DOI] [PubMed] [Google Scholar]
- 10.Folberg R, Rummelt V, Parys-Van Ginderdeuren R, Hwang T, Woolson RF, Pe’er J, Gruman LM: The prognostic value of tumor blood vessel morphology in primary uveal melanoma. Ophthalmology 1993, 100:1389–1398 [DOI] [PubMed]
- 11.Folberg R: Tumor progression in ocular melanomas. J Invest Dermatol 1993, 100:326S-331S [DOI] [PubMed] [Google Scholar]
- 12.McLean IW: The biology of haematogenous metastasis in human uveal malignant melanoma. Virchows Arch A Pathol Anat 1993, 422:433-437 [DOI] [PubMed] [Google Scholar]
- 13.Augsburger JJ, Shields JA, Folberg R, Lang WR, O’Hara BJ, Claricci JD: Fine needle aspiration biopsy in the diagnosis of intraocular cancer cytologic-histologic correlations. Ophthalmology 1985, 92:39-49 [DOI] [PubMed] [Google Scholar]
- 14.Shields JA, Shields CL, Ehya H, Eagle RC Jr, De Potter P: Fine-needle aspiration biopsy of suspected intraocular tumors. The 1992 Urwick Lecture. Ophthalmology 1993, 100:1677–1684 [DOI] [PubMed]
- 15.Eide N, Syrdalen P, Walaas L, Hagmar B: Fine needle aspiration biopsy in selecting treatment for inconclusive intraocular disease. Acta Ophthalmol Scand 1999, 77:448-452 [DOI] [PubMed] [Google Scholar]
- 16.DeMay RM: The art and science of cytopathology. 1996:pp 463-492 ASCP Press, Chicago
- 17.Folberg R, Augsburger JJ, Gamel JW, Shields JA, Lang WR: Fine-needle aspirates of uveal melanomas and prognosis. Am J Ophthalmol 1985, 100:654-657 [DOI] [PubMed] [Google Scholar]
- 18.Char DH, Kroll SM, Stoloff A, Kaleta-Michaels S, Crawford JB, Miller TR, Howes EL Jr, Ljung B-M: Cytomorphometry of uveal melanoma. Comparison of fine needle aspiration biopsy samples with histologic sections. Anal Quant Cytol Histol 1991, 13:293–299 [PubMed]
- 19.Shetlar DJ, Folberg R, Gass JD: Choroidal malignant melanoma associated with a melanocytoma. Retina 1999, 19:346-349 [DOI] [PubMed] [Google Scholar]
- 20.Donoso LA, Shields JA, Augsburger JA, Orth DH, Johnson P: Metastatic uveal melanoma: diffuse hepatic metastasis in a patient with concurrent normal serum enzyme levels and liver scan. Arch Ophthalmol 1985, 103:758. [DOI] [PubMed] [Google Scholar]
- 21.Mooy CM, De Jong PTVM: Prognostic parameters in uveal melanoma: a review. Surv Ophthalmol 1996, 41:215-228 [DOI] [PubMed] [Google Scholar]
- 22.Folberg R, Baron J, Reeves RD, Stevens RH, Tse DT: Primary melanocytic lesions of the rabbit choroid following topical application of 7,12-dimethylbenz[a]anthracene: preliminary observations. J Toxicol Cutan Ocul Toxicol 1990, 9:313-334 [Google Scholar]
- 23.Bradl M, Klein-Szanto A, Porter S, Mintz B: Malignant melanoma in transgenic mice. Proc Natl Acad Sci USA 1991, 88:164-168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anand R, Ma D, Alizadeh H, Comerford SA, Sambrook JF, Gething M-JH, McLean IW, Niederkorn JY: Characterization of intraocular tumors arising in transgenic mice. Invest Ophthalmol Vis Sci 1994, 35:3533-3539 [PubMed] [Google Scholar]
- 25.Syed NA, Windle JJ, Darjatmoko SR, Lokken JM, Steeves RA, Chappell R, Wallow IHL, Koop BA, Mangold G, Howes KA, Albert DM: Transgenic mice with pigmented intraocular tumors: tissue of origin and treatment. Invest Ophthalmol Vis Sci 1998, 39:2800-2805 [PubMed] [Google Scholar]
- 26.Kramer TR, Powell MB, Wilson MM, Salvatore J, Grossniklaus HE: Pigmented uveal tumours in a transgenic mouse model. Br J Ophthalmol 1998, 82:953-960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shields JA, Shields CL, Gunduz K, Eagle RC: Neoplasms of the retinal pigment epithelium: the 1998 Albert Ruedemann Sr, Memorial Lecture, part 2. Arch Ophthalmol 1999, 117:601-608 [DOI] [PubMed] [Google Scholar]
- 28.Pawlowski A, Haberman HF, Menon IA: Junctional and compound pigmented nevi induced by 9,10-dimethyl-1,2-benzanthracene in skin of albino guinea pigs. Cancer Res 1976, 36:2813-2831 [PubMed] [Google Scholar]
- 29.Pawlowski A, Lea PJ: Nevi and melanoma induced by chemical carcinogens in laboratory animals: similarities and differences with human lesions. J Cutan Pathol 1983, 10:81-110 [DOI] [PubMed] [Google Scholar]
- 30.Pawlowski A, Haberman HF, Menon IA: Skin melanoma induced by 7,12-dimethylbenzanthracene in albino guinea pigs and its similarities to skin melanomas of humans. Cancer Res 1980, 40:3652-3660 [PubMed] [Google Scholar]
- 31.Pe’er J, Folberg R, Massicotte SJ, Baron J, Parys-Van Ginderdeuren R, Zimmerman B, Meyer ML, Worsey H: Clinicopathologic spectrum of primary uveal melanocytic lesions in an animal model. Ophthalmology 1992, 99:977–986 [DOI] [PubMed]
- 32.Naumann G, Yanoff M, Zimmerman LE: Histogenesis of malignant melanomas of the uvea. I. Histopathologic characteristics of nevi of the choroid and ciliary body. Arch Ophthalmol 1966, 76:784-796 [DOI] [PubMed] [Google Scholar]
- 33.Thompson WD, Shiach KJ, Fraser RA, McIntosh LC, Simpson JG: Tumours acquire their vaculature by vessel incorporation, not vessel ingrowth. J Pathol 1987, 151:323-332 [DOI] [PubMed] [Google Scholar]
- 34.Kivela T: Immunohistochemical staining followed by bleaching of melanin: a practical method for ophthalmic pathology. Br J Biomed Sci 1995, 52:325-326 [PubMed] [Google Scholar]
- 35.McLean IW, Foster WD, Zimmerman LE, Gamel JW: Modifications of Callender’s classification of uveal melanoma at the Armed Forces Institute of Pathology. Am J Ophthalmol 1983, 96:502-509 [DOI] [PubMed] [Google Scholar]
- 36.Folberg R, Mehaffey M, Gardner LM, Meyer M, Rummelt V, Pe’er J: The microcirculation of choroidal and ciliary body melanomas. Eye 1997, 11:227-238 [DOI] [PubMed] [Google Scholar]
- 37.Sakamoto T, Sakamoto M, Yoshikawa H, Hata Y, Ishibashi T, Ohnishi Y, Inomata H: Histologic findings and prognosis of uveal malignant melanoma in Japanese patients. Am J Ophthalmol 1996, 121:276-283 [DOI] [PubMed] [Google Scholar]
- 38.McLean IW, Keefe KS, Burnier MN: Uveal melanoma: comparison of the prognostic value of fibrovascular loops, mean of the ten largest nucleoli, cell type and tumor size. Ophthalmology 1997, 104:777-780 [DOI] [PubMed] [Google Scholar]
- 39.Braun UC, Rummelt VC, Naumann GO: Diffuse malignant melanomas of the uvea. A clinico-pathologic study of 39 patients. Klin Monatsbl Augenheilkd 1998, 213:331-340 [DOI] [PubMed] [Google Scholar]
- 40.Seregard S, Spangberg B, Juul C, Oskarsson M: Prognostic accuracy of the mean of the largest nucleoli, vascular patterns, and PC-10 in posterior uveal melanoma. Ophthalmology 1998, 105:485-491 [DOI] [PubMed] [Google Scholar]
- 41.Makitie T, Summanen P, Tarkannen A, Kivela T: Microvascular loops and networks as prognostic indicators in choroidal and ciliary body melanomas. J Natl Cancer Inst 1999, 91:359-367 [DOI] [PubMed] [Google Scholar]
- 42.Mehaffey MG, Folberg R, Meyer M, Bentler SE, Hwang T, Woolson RF, Moore KC: Relative importance of quantifying area and vascular patterns in uveal melanoma. Am J Ophthalmol 1997, 123:798-809 [DOI] [PubMed] [Google Scholar]
- 43.Rummelt V, Mehaffey MG, Campbell RJ, Peer J, Bentler SE, Woolson RF, Naumann GOH, Folberg R: Microcirculation architecture of metastases from primary ciliary body and choroidal melanomas. Am J Ophthalmol 1998, 126:303-305 [DOI] [PubMed] [Google Scholar]
- 44.Foss AJE, Alexander RA, Jefferies LW, Hungerford JL, Harris AL, Lightman S: Microvessel count predicts survival in uveal melanoma. Cancer Res 1996, 56:2900-2903 [PubMed] [Google Scholar]
- 45.Foss AJE, Alexander RA, Hungerford JL, Harris AL, Cree IA, Lightman S: Re-assessment of the PAS patterns in uveal melanoma. Br J Ophthalmol 1997, 81:240-246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weidner N, Semple JP, Welch WR, Folkman J: Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med 1991, 324:1-8 [DOI] [PubMed] [Google Scholar]
- 47.Folberg R: Discussion of paper by Foss et al. Br J Ophthalmol 1997, 81:247-248 [Google Scholar]
- 48.Rummelt V, Naumann GH, Folberg R: Reassessment of the PAS patterns in uveal melanoma—reply. Br J Ophthalmol 1998, 82:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cicaloni B, Paterra N: The properties of intratumoral blood flow as a possible prognostic index in uveal melanoma—a study using color Doppler imaging. Ann Ophthalmol Glaucoma 1997, 29:225-230 [Google Scholar]
- 50.Rummelt V, Folberg R, Rummelt C, Gruman LM, Hwang T, Woolson RF, Yi H, Naumann GOH: Microcirculation architecture of melanocytic nevi and malignant melanomas of the ciliary body and choroid: a comparative histopathologic and ultrastructural study. Ophthalmology 1994, 101:718-727 [DOI] [PubMed] [Google Scholar]
- 51.Rummelt V, Gardner LM, Folberg R, Beck S, Knosp B, Moninger TO, Moore KC: Three-dimensional relationships between tumor cells and microcirculation using double cyanine-immunolabeling, laser scanning confocal microscopy and computer-assisted reconstruction: an alternative to cast corrosion preparations. J Histochem Cytochem 1994, 42:681-686 [DOI] [PubMed] [Google Scholar]
- 52.Schneider U, Gelisken F, Inhoffen W, Kreissig I: Indocyanine-green videoangiography of malignant melanomas of the choroid using the scanning laser ophthalmoscope. Ger J Ophthalmol 1996, 5:6-11 [PubMed] [Google Scholar]
- 53.Mueller AJ, Bartsch DU, Folberg R, Mehaffey MG, Boldt HC, Meyer M, Gardner LM, Goldbaum MH, Peer J, Freeman WR: Imaging the microvasculature of choroidal melanomas with confocal indocyanine green scanning laser ophthalmoscopy. Arch Ophthalmol 1998, 116:31-39 [DOI] [PubMed] [Google Scholar]
- 54.Mueller AJ, Freeman WR, Folberg R, Bartsch DU, Scheider A, Schaller U, Kampik A: Evaluation of microvascularization pattern visibility in human choroidal melanomas: comparison of confocal fluorescein with indocyanine green angiography. Graefes Arch Clin Exp Ophthalmol 1999, 237:448-456 [DOI] [PubMed] [Google Scholar]
- 55.Foss AE, Cree IA: Reassessment of the PAS patterns in uveal melanoma—reply. Br J Ophthalmol 1998, 82:101-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burnier MN, McLean IW, Zimmerman LE, Rosenberg SH: Retinoblastoma: the relationship of proliferating cells to blood vessels. Invest Ophthalmol Vis Sci 1990, 31:2037-2040 [PubMed] [Google Scholar]
- 57.Kvanta A, Steen B, Seregard S: Expression of vascular endothelial growth factor (VEGF) in retinoblastoma but not in posterior uveal melanoma. Exp Eye Res 1996, 63:511-518 [DOI] [PubMed] [Google Scholar]
- 58.Pe’er J, Neufeld M, Baras M, Gnessin H, Itin A, Keshet E: Rubeosis iridis in retinoblastoma: histologic findings and the possible role of vascular endothelial growth factor in its induction. Ophthalmology 1997, 104:1251-1258 [DOI] [PubMed] [Google Scholar]
- 59.Kopelman JE, McLean IW, Rosenberg SH: Multivariate analysis of risk factors for metastasis in retinoblastoma treated by enucleation. Ophthalmology 1987, 94:371-377 [DOI] [PubMed] [Google Scholar]
- 60.Peer J, Folberg R, Itin A, Gnessin H, Hemo I, Keshet E: Upregulated expression of vascular endothelial growth factor in proliferative diabetic retinopathy. Br J Ophthalmol 1996, 80:241-245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pe’er J, Folberg R, Itin A, Gnessin H, Hemo I, Keshet E: Vascular endothelial growth factor upregulation in human central retinal vein occlusion. Ophthalmology 1998, 105:412-416 [DOI] [PubMed] [Google Scholar]
- 62.Lugassy C, Eyden BP, Christensen L, Escande JP: Angio-tumoral complex in human malignant melanoma characterised by free laminin: ultrastructural and immunohistochemical observations. J Submicrosc Cytol Pathol 1997, 29:19-28 [PubMed] [Google Scholar]
- 63.Lugassy C, Shahsafaei A, Bonitz P, Busam KJ, Barnhill RL: Tumor microvessels in melanoma express the β-2 chain of laminin. Implications for melanoma metastasis. J Cutan Pathol 1999, 26:222-226 [DOI] [PubMed] [Google Scholar]
- 64.Daniels KJ, Boldt HC, Martin JA, Gardner LM, Meyer M, Folberg R: Expression of type VI collagen in uveal melanoma: role in pattern formation and tumor progression. Lab Invest 1996, 75:55-66 [PubMed] [Google Scholar]
- 65.Hendrix MJC, Seftor EA, Seftor RB, Gardner LM, Boldt HC, Meyer M, Peer J, Folberg R: Biologic determinants of uveal melanoma metastatic phenotype—role of intermediate filaments as predictive markers. Lab Invest 1998, 78:153-163 [PubMed] [Google Scholar]
- 66.Hendrix MJC, Seftor EA, Seftor REB, Gardner LM, Boldt HC, Meyer M, Pe’er J, Folberg R: Regulation of uveal melanoma interconverted phenotype by hepatocyte growth factor/scatter factor (HGF/SF). Am J Pathol 1998, 152:855-863 [PMC free article] [PubMed] [Google Scholar]
- 67.Barnhill RL, Busam KJ, Berwick M, Blessing K, Cochran AJ, Elder DE, Fandrey K, Karaoli T, White WL: Tumour vascularity is not a prognostic factor for cutaneous melanoma. Lancet 1994, 344:1237-1238 [DOI] [PubMed] [Google Scholar]
- 68.Nowak MA, Fatteh SM, Campbell TE: Glycogen-rich malignant melanomas and glycogen-rich balloon cell malignant melanomas—frequency and pattern of PAS positivity in primary and metastatic melanomas. Arch Pathol Lab Med 1998, 122:353-360 [PubMed] [Google Scholar]
- 69.Carnochan P, Briggs JC, Westbury G, Davies AJS: The vascularity of cutaneous melanoma: a quantitative histological study of lesions 0.85–1.25 mm in thickness. Br J Cancer 1991, 64:102-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Graham CH, Rivers J, Kerbel RS, Stankiewicz KS, White WL: Extent of vascularization as a prognostic indicator in thin (<0.76 mm) malignant melanomas. Am J Pathol 1994, 145:510-514 [PMC free article] [PubMed] [Google Scholar]
- 71.Binder M, Steiner A, Mossbacher U, Hunegnaw M, Wolff K, Pehamberger H: Quantification of vascularity in nodular melanoma and spitzs nevus. J Cutan Pathol 1997, 24:272-277 [DOI] [PubMed] [Google Scholar]
- 72.Barnhill RL, Piepkorn MW, Cochran AJ, Flynn E, Karaoli T, Folkman J: Tumor vascularity, proliferation, and apoptosis in human melanoma micrometastases and macrometastases. Arch Dermatol 1998, 134:991-994 [DOI] [PubMed] [Google Scholar]
- 73.Fallowfield ME, Cook MG: The vascularity of primary cutaneous melanoma. J Pathol 1991, 164:241-244 [DOI] [PubMed] [Google Scholar]
- 74.Srivastava A, Hughes LE, Woodcock JP, Laidler P: Vascularity in cutaneous melanoma detected by Doppler sonography and histology: correlation with tumour behaviour. Br J Cancer 1989, 59:89-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Murata J, Saiki I, Makabe T, Tsuta Y, Tokura S, Azuma I: Inhibition of tumor-induced angiogenesis by sulfated chitin derivatives. Cancer Res 1991, 51:22-26 [PubMed] [Google Scholar]
- 76.Ilmonen S, Kariniemi AL, Vlaykova T, Muhonen T, Pyrhonen S, Asko-Seljavaara S: Prognostic value of tumour vascularity in primary melanoma. Melanoma Res 1999, 9:273-278 [DOI] [PubMed] [Google Scholar]
- 77.Lane AM, Egan KM, Gragoudas ES, Yang J, Saornil MA, Alroy J, Albert D: An evaluation of tumour vascularity as a prognostic indicator in uveal melanoma. Melanoma Res 1997, 7:237-242 [DOI] [PubMed] [Google Scholar]
- 78.Makitie T, Summanen P, Tarkkanen A, Kivela T: Microvascular density in predicting survival of patients with choroidal and ciliary body melanoma. Invest Ophthalmol Vis Sci 1999, 40:2471-2480 [PubMed] [Google Scholar]
- 79.Hollingsworth HC, Kohn EC, Steinberg SM, Rothenberg ML, Merino MJ: Tumor angiogenesis in advanced stage ovarian carcinoma. Am J Pathol 1995, 147:33-41 [PMC free article] [PubMed] [Google Scholar]
- 80.Vermeulen PB, Gasparini G, Fox SB, Toi M, Martin L, McCulloch P, Pezzella F, Viale G, Weidner N, Harris AL, Dirix LY: Quantification of angiogenesis in solid human tumours—an international consensus on the methodology and criteria of evaluation. Eur J Cancer 1996, 32A:2474-2484 [DOI] [PubMed] [Google Scholar]
- 81.Weidner N: Tumoral vascularity as a prognostic factor in cancer patients—the evidence continues to grow. J Pathol 1998, 184:119-122 [DOI] [PubMed] [Google Scholar]
- 82.Van Hoef MEHM, Knox WF, Dhesi SS, Howell A, Schor AM: Assessment of tumour vascularity as a prognostic factor in lymph node negative invasive breast cancer. Eur J Cancer 1993, 29A:1141-1145 [DOI] [PubMed] [Google Scholar]
- 83.Vesalainen S, Lipponen P, Talia M, Alhava E, Syrjänen K: Tumor vascularity and basement membrane structure as prognostic factors in T1–2MO prostatic adenocarcinoma. Anticancer Res 1994, 17:709-714 [PubMed] [Google Scholar]
- 84.Bevilacqua P, Barbareschi M, Verderio P, Boracchi P, Caffo O, Dallapalma P, Meli S, Weidner N, Gasparini G: Prognostic value of intratumoral microvessel density, a measure of tumor angiogenesis, in node-negative breast carcinoma—results of a multiparametric study. Breast Cancer Res Treat 1995, 36:205-217 [DOI] [PubMed] [Google Scholar]
- 85.Bossi P, Viale G, Lee AKC, Alfano R, Coggi G, Bosari S: Angiogenesis in colorectal tumors: microvessel quantitation in adenomas and carcinomas with clinicopathological correlations. Cancer Res 1995, 55:5049-5053 [PubMed] [Google Scholar]
- 86.Busam KJ, Berwick M, Blessing K, Fandrey K, Kang S, Karaoli T, Fine J, Cochran AJ, White WL, Rivers J, Elder DE, Wen DRP, Heyman BH, Barnhill RL: Tumor vascularity is not a prognostic factor for malignant melanoma of the skin. Am J Pathol 1995, 147:1049-1056 [PMC free article] [PubMed] [Google Scholar]
- 87.Costello P, McCann A, Carney DN, Dervan PA: Prognostic significance of microvessel density in lymph node negative breast carcinoma. Hum Pathol 1995, 26:1181-1184 [DOI] [PubMed] [Google Scholar]
- 88.Dray TG, Hardin NJ, Sofferman RA: Angiogenesis as a prognostic marker in early head and neck cancer. Ann Otol Rhinol Laryngol 1995, 104:724-729 [DOI] [PubMed] [Google Scholar]
- 89.Goulding H, Rashid NFAR, Robertson JF, Bell JA, Elston CW, Blamey RW, Ellis IO: Assessment of angiogenesis in breast carcinoma: an important factor in prognosis? Hum Pathol 1995, 26:1196-1200 [DOI] [PubMed] [Google Scholar]
- 90.Kainz C, Speiser P, Wanner C, Obermair A, Tempfer C, Sliutz G, Reinthaller A, Breitenecker G: Prognostic value of tumour microvessel density in cancer of the uterine cervix stage IB to IIB. Anticancer Res 1995, 15:1549-1551 [PubMed] [Google Scholar]
- 91.Maclennan GT, Bostwick DG: Microvessel density in renal cell carcinoma: lack of prognostic significance. Urology 1995, 46:27-30 [DOI] [PubMed] [Google Scholar]
- 92.Miliaras D, Kamas A, Kalekou H: Angiogenesis in invasive breast carcinoma: is it associated with parameters of prognostic significance? Histopathology 1995, 26:165-169 [DOI] [PubMed] [Google Scholar]
- 93.Ohsawa M, Tomita Y, Kuratsu S, Kanno H, Aozasa K: Angiogenesis in malignant fibrous histiocytoma. Oncology 1995, 52:51-54 [DOI] [PubMed] [Google Scholar]
- 94.Tahan SR, Stein AL: Angiogenesis in invasive squamous cell carcinoma of the lip: tumor vascularity is not an indicator of metastatic risk. J Cutan Pathol 1995, 22:236-240 [DOI] [PubMed] [Google Scholar]
- 95.Fontanini G, Vignati S, Pacini F, Pollina L, Basolo F: Microvessel count: an indicator of poor outcome in medullary thyroid carcinoma but not in other types of thyroid carcinoma. Mod Pathol 1996, 9:636-641 [PubMed] [Google Scholar]
- 96.Lindmark G, Gerdin B, Sundberg C, Pahlman L, Bergstrom R, Glimelius B: Prognostic significance of the microvascular count in colorectal cancer. J Clin Oncol 1996, 14:461-466 [DOI] [PubMed] [Google Scholar]
- 97.Morphopoulos G, Pearson M, Ryder WD, Howell A, Harris M: Tumour angiogenesis as a prognostic marker in infiltrating lobular carcinoma of the breast. J Pathol 1996, 180:44-49 [DOI] [PubMed] [Google Scholar]
- 98.Sarbia M, Bittinger F, Porschen R, Dutkowski P, Willers R, Gabbert HE: Tumor vascularization and prognosis in squamous cell carcinomas of the esophagus. Anticancer Res 1996, 16:2117-2121 [PubMed] [Google Scholar]
- 99.Sulh MA, Greco MA, Jiang T, Goswami SB, Present D, Steiner G: Proliferation index and vascular density of giant cell tumors of bone: are they prognostic markers? Cancer 1996, 77:2044-2051 [DOI] [PubMed] [Google Scholar]
- 100.Chandrachud LM, Pendleton N, Chisholm DM, Horan MA, Schor AM: Relationship between vascularity, age and survival in non small-cell lung cancer. Br J Cancer 1997, 76:1367-1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gleich LL, Biddinger PW, Duperier FD, Gluckman JL, Angiogenesis: oral c, aquamous ml, metastases, outcomes: tumor angiogenesis as a prognostic indicator in T2–T4 oral cavity squamous cell carcinoma—a clinical-pathologic correlation. Head Neck Surg 1997, 19:276–280 [DOI] [PubMed]
- 102.Orre M, Lotfimiri M, Mamers P, Rogers PAW: Increased microvessel density in mucinous compared with malignant serous and benign tumours of the ovary. Br J Cancer 1998, 77:2204-2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pendleton N, Pazouki S, Heerkens E, Smither RL, Chisholm DM, Moore JV, Howell A, Horan MA, Schor AM: Relationships between different measurements of vascularity and clinico-pathological parameters in breast cancer. Anticancer Res 1998, 18:4565-4568 [PubMed] [Google Scholar]
- 104.Saenz NC, Heslin MJ, Adsay V, Lewis JJ, Leung DH, Laquaglia MP, Brennan MF: Neovascularity and clinical outcome in high-grade extremity soft tissue sarcomas. Ann Surg Oncol 1998, 5:48-53 [DOI] [PubMed] [Google Scholar]
- 105.Salvesen HB, Iversen OE, Akslen LA: Independent prognostic importance of microvessel density in endometrial carcinoma. Br J Cancer 1998, 77:1140-1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Takebayashi Y, Natugoe S, Baba M, Fukumoto T, Takao S, Akiba S, Akiyama SI, Aikou T: Angiogenesis in esophageal squamous cell carcinoma. Oncol Rep 1998, 5:401-404 [DOI] [PubMed] [Google Scholar]
- 107.Rubin MA, Buyyounouski M, Bagiella E, Sharir S, Neugut A, Benson M, de la Taille A, Katz AE, Olsson CA, Ennis RD: Microvessel density in prostate cancer: lack of correlation with tumor grade, pathologic stage, and clinical outcome. Urology 1999, 53:542-547 [DOI] [PubMed] [Google Scholar]
- 108.Jensen OA: Malignant melanoma of the choroid of a peculiar cavernous type: clinical manifestation by hemorrhage of the vitreous body. Arch Ophthalmol 1964, 72:337-340 [DOI] [PubMed] [Google Scholar]
- 109.Radnot M, Antal M: Vessels of intraocular malignant melanomas. Am J Ophthalmol 1979, 88:472-478 [DOI] [PubMed] [Google Scholar]
- 110.Konerding MA, Fait E, Dimitropoulou C, Malkusch W, Ferri C, Giavazzi R, Coltrini D, Presta M: Impact of fibroblast growth factor-2 on tumor microvascular architecture: a tridimensional morphometric study. Am J Pathol 1998, 152:1607-1616 [PMC free article] [PubMed] [Google Scholar]
- 111.Hammersen F, Endrich B, Messmer K: The fine structure of tumor blood vessels. I. Participation of non-endothelial cells in tumor angiogenesis. Int J Microcirc Clin Exp 1985, 4:31-43 [PubMed] [Google Scholar]
- 112.Warren BA, Shubik P: The growth of the blood supply to melanoma transplants in the hamster cheek pouch. Lab Invest 1966, 15:464-478 [PubMed] [Google Scholar]
- 113.Nasu R, Kimura H, Akagi K, Murata T, Tanaka Y: Blood flow influences vascular growth during tumour angiogenesis. Br J Cancer 1999, 79:780-786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Birck A, Kirkin AF, Zeuthen J, Hou-Jensen K: Expression of basic fibroblast growth factor and vascular endothelial growth factor in primary and metastatic melanoma from the same patients. Melanoma Res 1999, 9:375-381 [DOI] [PubMed] [Google Scholar]
- 115.de Jong JS, van Diest PJ, Baak JP: Heterogeneity and reproducibility of microvessel counts in breast cancer. Lab Invest 1995, 73:922-926 [PubMed] [Google Scholar]
- 116.Bigler SA, Deering RE, Brawer MK: Comparison of microscopic vascularity in benign and malignant prostate tissue. Hum Pathol 1993, 24:220-226 [DOI] [PubMed] [Google Scholar]
- 117.Brown LF, Van De Water L, Harvey VS, Dvorak HF: Fibrinogen influx and accumulation of cross-linked fibrin in healing wounds and in tumor stroma. Am J Pathol 1988, 130:455-465 [PMC free article] [PubMed] [Google Scholar]
- 118.Brown LF, Guidi AJ, Schnitt SJ, Van De WL, Iruela-Arispe ML, Yeo, Tk, Tognazzi K, Dvorak HF: Vascular stroma formation in carcinoma in situ, invasive carcinoma, and metastatic carcinoma of the breast. Clin Cancer Res 1999, 5:1041–1056 [PubMed]
- 119.Barnhill RL, Mihm MC, Jr, Ceballos PI: Angiogenesis and regressing cutaneous malignant melanoma. Lancet 1992, 339:991-992 [DOI] [PubMed] [Google Scholar]
- 120.Lauk S, Zeitman A, Skates S, Fabian R, Suit HD: Comparative morphometric study of tumor vasculature in human squamous cell carcinomas and their xenotransplants in athymic nude mice. Cancer Res 1989, 49:4557-4561 [PubMed] [Google Scholar]
- 121.Wilson MJ, Sinha AA: Human prostate tumor angiogenesis in nude mice—metalloprotease and plasminogen activator activities during tumor growth and neovascularization of subcutaneously injected matrigel impregnated with human prostate tumor cells. Anat Rec 1997, 249:63-73 [DOI] [PubMed] [Google Scholar]
- 122.Potgens AJG, Lubsen NH, Vanaltena MC, Schoenmakers JGG, Ruiter DJ, Dewaal RMW: Vascular permeability factor expression influences tumor angiogenesis in human melanoma lines xenografted to nude mice. Am J Pathol 1995, 146:197-209 [PMC free article] [PubMed] [Google Scholar]
- 123.Potgens AJG, van Altena MC, Lubsen NH, Ruiter DJ, de Waal RMW: Analysis of the tumor vasculature and metastatic behavior of xenografts of human melanoma cell lines transfected with vascular permeability factor. Am J Pathol 1996, 148:1203-1217 [PMC free article] [PubMed] [Google Scholar]
- 124.Erhard H, Dewaal RMW, Rietveld FJR, Broker EB, Ruiter DJ: Phenotype and morphology of tumor lymphatic vessels in horizontal and vertical growth phase melanoma: an immuno-electronmicroscopical study. Immunol Hum Melanoma 1996, 12:19-29 [Google Scholar]
- 125.Grossniklaus HE: Tumor vascularity and hematogenous metastasis in experimental murine intraocular melanoma. Trans Am Ophthalmol Soc 1998, 96:721-752 [PMC free article] [PubMed] [Google Scholar]
- 126.Killion JJ, Radinsky R, Fidler IJ: Orthotopic models are necessary to predict therapy of transplantable tumors in mice. Cancer Metastasis Rev 1998, 17:279-284 [DOI] [PubMed] [Google Scholar]
- 127.Boehm T, Folkman J, Browder T, Oreilly MS: Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature 1997, 390:404-407 [DOI] [PubMed] [Google Scholar]
- 128.Bergers G, Javaherian K, Lo KM, Folkman J, Hanahan D: Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Science 1999, 284:808-812 [DOI] [PubMed] [Google Scholar]
- 129.O’Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao YH, Sage EH, Folkman J: Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 1994, 79:315-328 [DOI] [PubMed] [Google Scholar]
- 130.O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J: Endostatin-an endogenous inhibitor of angiogenesis and tumor growth. Cell 1997, 88:277-285 [DOI] [PubMed] [Google Scholar]
- 131.Bartsch D-U, Weinreb RN, Zinser G, Freeman WR: Confocal scanning infrared laser ophthalmoscopy for indocyanine green angiography. Am J Ophthalmol 1995, 120:642-651 [DOI] [PubMed] [Google Scholar]
- 132.Folberg R, Verdick RE, Weingeist TA, Montague PR: Gross examination of eyes removed for ciliary body or choroidal melanoma. Ophthalmology 1986, 93:1643-1647 [DOI] [PubMed] [Google Scholar]
- 133.Servetopoulou F, Krause L, Angnastopoulos J, Bechrakis NE: Klinische Darstellung der Tumor-Mikrovaskularisation beim uvealen malignen Melanom. Ophthalmologe 1999, 96 (Suppl 1):152 (abstract)
- 134.Coleman DJ, Lizzi FL, Silverman RH, Rondeau MJ, Smith MD, Torpey JH: Acoustic biopsy as a means for characterization of intraocular tumors. Henkind P eds. Acta XXIV: International Congress of Ophthalmology. 1982, :pp 115-118 PA, Lippincott, Philadelphia [Google Scholar]
- 135.Coleman DJ, Silverman RH, Rondeau MJ, Lizzi FL, McLean IW, Jakobiec FA: Correlations of acoustic tissue typing of malignant melanoma and histopathologic features as a predictor of death. Am J Ophthalmol 1990, 110:380-388 [DOI] [PubMed] [Google Scholar]
- 136.Coleman DJ, Silverman RH, Rondeau MJ, Coleman JA, Rosberger D, Ellsworth RM, Lizzi FL: Ultrasonic tissue characterization of uveal melanoma and prediction of patient survival after enucleation and brachytherapy. Am J Ophthalmol 1991, 112:682-688 [DOI] [PubMed] [Google Scholar]
- 137.Coleman DJ, Rondeau MJ, Silverman RH, Folberg R, Rummelt V, Woods SM, Lizzi FL: Correlation of microcirculation architecture with ultrasound parameters of uveal melanoma. Eur J Ophthalmol 1995, 5:96-106 [DOI] [PubMed] [Google Scholar]
- 138.Silverman RH, Folberg R, Boldt HC, Rondeau MJ, Lloyd HO, Mehaffey MG, Lizzi FL, Coleman DJ: Correlation of ultrasound parameter imaging with microcirculatory patterns in uveal melanomas. Ultrasound Med Biol 1997, 23:573-581 [DOI] [PubMed] [Google Scholar]
- 139.McCarthy MJ, Crowther M, Bell PR, Brindle NP: The endothelial receptor tyrosine kinase tie-1 is upregulated by hypoxia and vascular endothelial growth factor. FEBS Lett 1998, 423:334-338 [DOI] [PubMed] [Google Scholar]
- 140.Easty DJ, Herlyn M, Bennett DC: Abnormal protein tyrosine kinase gene expression during melanoma progression and metastasis. Int J Cancer 1995, 60:129-136 [DOI] [PubMed] [Google Scholar]
- 141.Nicosia RF, Ottinetti A: Modulation of microvascular growth and morphogenesis by reconstituted basement membrane gel in three-dimensional cultures of rat aorta: a comparative study of angiogenesis in matrigel, collagen, fibrin, and plasma clot. In Vitro Cell Dev Biol 1990, 26:119-128 [DOI] [PubMed] [Google Scholar]
- 142.Vernon RB, Lara SL, Drake CJ, Iruelaarispe ML, Angello JC, Little CD, Wight TN, Sage EH: Organized type I collagen influences endothelial patterns during “spontaneous angiogenesis in vitro”: planar cultures as models of vascular development. In Vitro Cell Dev Biol Animal 1995, 31:120-131 [DOI] [PubMed] [Google Scholar]
- 143.Cottam DW, Rennie IG, Woods K, Parsons MA, Bunning RAD, Rees RC: Gelatinolytic metalloproteinase secretion patterns in ocular melanoma. Invest Ophthalmol Vis Sci 1992, 33:1923-1927 [PubMed] [Google Scholar]
- 144.Rennie IG: The Ashton Lecture: Uveal melanoma: the past, the present and the future. Eye 1997, 11:255-264 [DOI] [PubMed] [Google Scholar]
- 145.Vaisanen A, Kallioinen M, von Dickhoff K, Laatikainen L, Hoyhtya M, Turpeenniemi-Hujanen T: Matrix metalloproteinase-2 (MMP-2) immunoreactive protein—a new prognostic marker in uveal melanoma? J Pathol 1999, 188:56-62 [DOI] [PubMed] [Google Scholar]
- 146.Zhou Y, Damsky CH, Fisher SJ: Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? J Clin Invest 1997, 99:2152-2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, Damsky CH: Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J Clin Invest 1997, 99:2139-2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Damsky CH, Fisher SJ: Trophoblast pseudo-vasculogenesis: faking it with endothelial adhesion receptors. Curr Opin Cell Biol 1998, 10:660-666 [DOI] [PubMed] [Google Scholar]