Genetic Progression in the Pancreatic Ducts (original) (raw)
Precursors to Infiltrating Cancer
There are histologically well-defined precursors to invasive carcinoma of the uterine cervix, colon, breast, and other organs. This knowledge, coupled with effective screening tests for these preclinical neoplasms, has saved many lives that might otherwise have been lost to cancer. 1-4 For example, simple annual screening of asymptomatic individuals over the age of 50 for fecal occult blood can lead to the early detection of adenomas and cancers of the colorectum, and, remarkably, annual screening can reduce mortality from colorectal cancer by 33%. 1
By contrast, there is no effective screening test for early pancreatic cancer. Most patients with pancreatic cancer present late in the course of their disease after the cancer has spread beyond the gland. Therefore, pancreatic cancer is almost universally fatal. 5 This year it is estimated that ∼28,000 Americans will be diagnosed with and ∼28,000 will die from pancreatic cancer. 5 One cannot but believe that a sensitive and specific test for early pancreatic cancer would reduce this extraordinarily high mortality rate.
Early Pancreatic Cancer
The first step in developing a test for early pancreatic cancer is recognizing and defining the precursors to infiltrating pancreatic cancer. Although the recognition of an early form of cancer may be relatively easy for organs, such as the cervix and colon, which are accessible to non-invasive biopsy, the study of early pancreatic cancer is much more difficult because the organ can only be sampled using invasive procedures. Therefore, sampling generally does not occur until the patient is symptomatic and the disease is advanced. The recognition and general acceptance of early non-invasive precursor lesions in the pancreas has, therefore, lagged far behind the recognition of precursor lesions in other organs such as the uterine cervix, colon, breast, and prostate.
Early Clues
The first hint that there may be histologically distinct precursor lesions to infiltrating ductal adenocarcinoma of the pancreas came from Sheldon Sommers. 6 He noted pancreatic duct hyperplasias in 9% of people without cancer and similar lesions in over one-third of those with pancreatic cancer. The duct hyperplasias associated with cancer generally had a more advanced papillary architecture and cellular atypia. Cubilla and Fitzgerald extended those observations in a seminal study of over 300 pancreata and again found that papillary proliferative lesions in the ducts were more common in pancreata with cancer than they were in pancreata without cancer and that papillary proliferations with significant atypia were seen only in the pancreata with cancer. 7 These observations have been confirmed by other groups. 8-10 In addition, Furakawa et al showed, using three-dimensional mapping, that severely dysplastic areas often arise within zones of milder atypia, a spatial pattern supportive of a stepwise progression model. 8-10 Thus, morphological observations provided the first critical step in the recognition that intraductal proliferations in the pancreas are the precursors to invasive ductal cancer.
These morphological observations, however, had several significant limitations. First, although it was felt that these lesions in the pancreatic ducts were the precursors to infiltrating cancer, there was no direct evidence beyond morphological atypia that they actually progressed to infiltrating cancer. As noted earlier, the relative difficulty of biopsying the pancreas and the high operative mortality rate associated with pancreatic surgery make routine sampling the pancreas extremely treacherous. It was therefore difficult to determine the natural history of these duct lesions in the pancreas. Surgery on the pancreas has, however, become much safer, 11 and a growing number of patients who have had a portion of their pancreas removed are alive today. Some of these patients have had pancreatic duct lesions, providing a unique opportunity to study the natural history of these lesions. As predicted, some duct lesions do indeed progress to infiltrating carcinomas. 12,13 For example, Brat et al reported three patients who developed an infiltrating adenocarcinoma of the pancreas 17 months, 9 years, and 10 years after partial pancreatectomy revealed atypical papillary duct lesions in their pancreata. 12 Brockie et al have reported two similar cases. 14 Although such anecdotal cases do not provide information on the frequency and speed at which duct lesions progress, they do establish that some duct lesions in the pancreas can progress to infiltrating carcinoma.
Cancerization versus Invasive Neoplasia
The second limitation to the morphological observations described earlier, as Klöppel and colleagues have emphasized, is the fact that on careful morphological examination many of the atypical papillary lesions in pancreatic ducts can be shown to be intraductal growth of an invasive cancer. 15 This phenomenon, known as cancerization of the ducts, may account for a significant fraction of the markedly atypical papillary intraductal proliferations adjacent to infiltrating adenocarcinomas of the pancreas. Although cancerization of the ducts cannot account for the atypical papillary duct lesions seen in pancreata without cancer, 16 recognition of this phenomenon is essential for those studying the genetic alterations in these lesions.
Genetic Alterations in Duct Lesions
Though morphological observations and anecdotal case reports of progression established a foundation for the development of a progression model for pancreatic neoplasia, it was not until modern molecular genetic techniques were applied to the study of intraductal proliferations in the pancreas that such a model could be rigorously tested. The pathological term “hyperplasia” refers to an increase in cell numbers in the absence of neoplasia. If these lesions in the pancreatic ducts were simply hyperplastic, then they should not be clonal and they should not harbor functionally significant mutations in cancer-associated genes. If the lesions are merely intraductal growths of an invasive cancer, then they should be clonal and they should harbor the same mutations in cancer-associated genes as their associated infiltrating cancer. If, however, as the morphological observations would suggest, the duct lesions are indeed the precursors to invasive cancer, then the lesions should be clonal; they should harbor some, but not all, of the mutations found in their associated infiltrating cancer; and the prevalence of these genetic alterations should increase with increasing severity of dysplasia in the duct lesion. Pancreatic duct lesions have now been examined for loss of heterozygosity at a number of loci and for alterations in a number of genes and proteins including K-ras, HER-2/neu, p16, p53, DPC4, and BRCA2. In all instances the results of these analyses suggest that pancreatic duct lesions are the precursors to infiltrating ductal carcinoma. 16-23
K-ras
Activating point mutations in codon 12 of the K-ras oncogene are among the most common genetic alterations identified to date in infiltrating ductal carcinomas of the pancreas. 24 These mutations can be found in 80 to 95% of these cancers. Mutations of codon 13 and 61 occur only occasionally. 25 A number of investigators have microdissected the duct lesions of the pancreas and analyzed them for K-ras mutations. 20,26,27 The first genetic evidence of a progression model for these duct lesions was provided by Caldas et al. 26,27 They noted that activating point mutations in codon 12 of the K-ras gene occur in about half of the nonpapillary duct lesions, but were present in the vast majority of papillary, more advanced lesions. It is this progression in K-ras mutational frequency that suggests that K-ras is not necessarily the first change, or gatekeeper, required for pancreatic ductal neoplasia. 28
HER-2/neu
HER-2/neu is a member of the epidermal growth factor receptor family. Approximately 70% of infiltrating ductal carcinomas of the pancreas overexpress HER-2/neu. 18,29,30 Day et al examined the expression of HER-2/neu in a series of duct lesions using immunohistochemical labeling. 18 They found that although HER-2/neu was essentially not expressed in normal pancreatic duct epithelium, it was expressed in 82% of flat duct lesions, in 86% of papillary duct lesions without atypia, and in 92% of atypical papillary duct lesions. 18 The means of overexpression is unknown and does not seem to involve gene amplification.
p16
The p16 tumor-suppressor gene on chromosome 9p encodes for a regulator of the cell cycle. The p16 gene has been shown to be genetically mutated or its expression abrogated in duct lesions. 20,21 Although loss of the p16 gene appears to occur slightly later than K-ras activation, the prevalence of p16 gene loss also appears to increase with increasing degrees of atypia in duct lesions. For example, Wilentz et al, using immunohistochemical labeling for the p16 gene product, showed that 30% of flat duct lesions without significant atypia, 55% of papillary duct lesions without significant atypia, and 71% of papillary duct lesions with significant atypia had loss of expression of the p16 gene product. 20 In addition, the labeling pattern in the papillary duct lesions with atypia often differed from the patient’s infiltrating carcinoma, establishing that these lesions were not simply an artifact of cancerization of the ducts.
p53
The p53 tumor-suppressor gene on chromosome 17p is inactivated in 50 to 75% of infiltrating pancreatic carcinomas. 31-33 Although an imperfect marker of gene status, immunohistochemical labeling for the p53 gene product has been used to examine duct lesions for p53 inactivation. 22 For example, DiGiuseppe et al found abnormalities in p53 labeling in 2 of 17 (12%) of histologically high-grade lesions (carcinomas in situ). 22 By contrast, all histologically lower-grade duct lesions labeled normally, suggesting that p53 gene inactivation is a late event in genetic progression in pancreatic ducts.
DPC4
The DPC4 tumor-suppressor gene on chromosome 18q is inactivated in ∼55% of infiltrating pancreatic carcinomas, and immunohistochemical labeling for the DPC4 gene product has recently been shown to mirror DPC4 gene status. 34,35 Wilentz et al studied a large series of pancreata using the anti-Dpc4 antibody. Dpc4 expression was intact in all of the low-grade duct lesions they examined, whereas 30% of the histologically high-grade lesions had a complete loss of Dpc4 expression. 19 Thus, just as is true for the p53 gene, inactivation of the DPC4 gene appears to be a late event in the genetic progression of pancreatic cancer.
BRCA2
The BRCA2 tumor suppressor gene on chromosome 13q is inactivated in 7 to 10% of pancreatic carcinomas, and Goggins et al have recently demonstrated biallelic inactivation of BRCA2 in a histologically high-grade duct lesion. 23 In this study, of patients that carried a germline BRCA2 mutation, the remaining normal allele of BRCA2 was intact in all of the histologically lower-grade duct lesions, suggesting that BRCA2 inactivation, like DPC4 and p53 inactivation, occurs relatively late in neoplastic progression in the pancreas.
Loss of Heterozygosity
In this issue of The American Journal of Pathology, Yamano, Fujii, Takagati, Kadowaki, Watanabe, and Shirai report their studies of carefully microdissected duct lesions in which they detected loss of heterozygosity (LOH) using a panel of microsatellite markers. 17 The markers used included markers on chromosome arms 3p, 4q, 5q, 6q, 8p, 9p, 10q, 11q, 13q, 16q, 17p, and 18q. All histologically high-grade intraductal lesions exhibited LOH at more than one chromosomal locus, confirming the genetic complexity of this end of the progression model. The patterns of loss observed also confirm both the importance of the genetic targets and the sequence of gene inactivation in the genetic progression to pancreatic cancer. One allele of chromosome 9p (the location of the p16 gene) was lost in ∼13% of histologically low-grade duct lesions in dramatic contrast to the 90% of histologically high-grade duct lesions that had LOH of this chromosome arm. Chromosome arms 17p (the location of the p53 gene) and 18q (the location of the DPC4 gene) had LOH only in duct lesions with severe dysplasia. Their elegant technique also sets the stage for a cladistic understanding of neoplastic progression in the pancreas, genetically dissecting the heterogeneity that occurs after the initial invasive focus, as well as identifying the genetic combinations within the duct lesions that did not progress to invasive cancer.
Progression Model
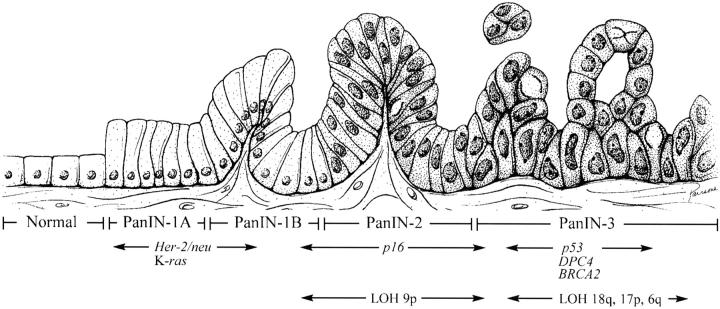
Taken together, these morphological, clinical, and genetic observations all suggest a progression model for pancreatic neoplasia (Figure 1) ▶ . 19,36 Just as there is a progression in the colorectum from adenoma to invasive cancer, so too is there a progression in the pancreas from intraductal proliferation to invasive ductal carcinoma. This progression is associated with increasing degrees of cytological and architectural atypia and with the accumulation of genetic alterations in cancer-associated genes. K-ras activation and HER-2/neu expression appear to occur relatively early, p16 inactivation is at an intermediate stage, and p53, DPC4, and BRCA2 inactivation are late.
Figure 1.
Progression model for pancreatic cancer. The progression from histologically normal epithelium to low-grade PanIN to high-grade PanIN (left to right) is associated with the accumulation of specific genetic alterations. (Artwork by Jennifer L. Parsons. Adapted from Reference 19 with permission.)
Implications of the Progression Model
This progression model for pancreatic carcinoma has a number of important implications. First, it suggests that the nomenclature used to designate proliferative duct lesions in the pancreas should reflect their neoplastic nature (most are clonal proliferations with genetic alterations in cancer-associated genes). The term “pancreatic intraepithelial neoplasia” (PanIN), as proposed by the National Cancer Institute-sponsored Pancreatic Think Tank held in Park City, Utah in September 1999, has been adopted. (For details on this nomenclature, see http://pathology.jhu.edu/pancreas/panin.) Second, this progression model suggests that, were the right tools to be developed, we might reasonably expect to be capable of detecting early, potentially curable pancreatic neoplasms. Third, this progression model suggests that, just as adenomas in the colon are a reasonable target for chemoprevention, so too are PanINs in the pancreas a potential target for chemoprevention trials. 37-39
Summary
The genetic progression described by Yamano et al in this issue of The American Journal of Pathology helps establish a progression model for pancreatic ductal carcinoma. This progression model has a number of important implications for the classification of lesions in the pancreas and for the future early detection and chemoprevention of pancreatic carcinoma.
Footnotes
Address reprint requests to Scott E. Kern, M.D., Johns Hopkins Medical Institutions, Cancer Research Building, 1650 Orleans Street, Room 451, Baltimore, MD 21231. E-mail: sk@jhmi.edu.
Supported by the National Institutes of Health Specialized Program of Research Excellence (SPORE) in Gastrointestinal Cancer (no. CA62924).
References
- 1.Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, Ederer F: Reducing mortality from colorectal cancer by screening for fecal occult blood: Minnesota Colon Cancer Control Study (published erratum appears in N Engl J Med 329:672; see comments). N Engl J Med 1993, 328:1365-1371 [DOI] [PubMed] [Google Scholar]
- 2.Boyes DA, Worth AJ, Fidler HK: The results of treatment of 4389 cases of preclinical cervical squamous carcinoma. J Obstet Gynaecol Br Commonw 1970, 77:769-780 [DOI] [PubMed] [Google Scholar]
- 3.Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O: Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet 1996, 348:1467-1471 [DOI] [PubMed] [Google Scholar]
- 4.Kronborg O, Fenger C, Olsen J, Bech K, Sondergaard O: Repeated screening for colorectal cancer with fecal occult blood test: a prospective randomized study at Funen, Denmark. Scand J Gastroenterol 1989, 24:599-606 [DOI] [PubMed] [Google Scholar]
- 5.Greenlee RT, Murray T, Bolden S, Wingo PA: Cancer statistics, 2000. CA Cancer J Clin 2000, 50:7-33 [DOI] [PubMed] [Google Scholar]
- 6.Sommers SC, Murphy SA, Warren S: Pancreatic duct hyperplasia and cancer. Gastroenterology 1954, 27:629-640 [PubMed] [Google Scholar]
- 7.Cubilla AL, Fitzgerald PJ: Morphological lesions associated with human primary invasive nonendocrine pancreas cancer. Cancer Res 1976, 36:2690-2698 [PubMed] [Google Scholar]
- 8.Furukawa T, Chiba R, Kobari M, Matsuno S, Nagura H, Takahashi T: Varying grades of epithelial atypia in the pancreatic ducts of humans: classification based on morphometry and multilvariate analysis and correlated with positive reactions of carcinoembryonic antigen. Arch Pathol Lab Med 1994, 118:227-234 [PubMed] [Google Scholar]
- 9.Kozuka S, Sassa R, Taki T, Masamoto K, Nagasawa S, Saga S, Hasegawa K, Takeuchi M: Relation of pancreatic duct hyperplasia to carcinoma. Cancer 1979, 43:1418-1428 [DOI] [PubMed] [Google Scholar]
- 10.Klöppel G, Bommer G, Rückert K, Seifert G: Intraductal proliferation in the pancreas and its relationship to human and experimental carcinogenesis. Virchows Arch A 1980, 387:221-233 [DOI] [PubMed] [Google Scholar]
- 11.Yeo CJ, Cameron JL, Lillemoe KD, Sitzmann JV, Hruban RH, Goodman SN, Dooley WC, Coleman J, Pitt HA: Pancreaticoduodenectomy for cancer of the head of the pancreas: 201 patients. Ann Surg 1995, 221:721-733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brat DJ, Lillemoe KD, Yeo CJ, Warfield PB, Hruban RH: Progression of pancreatic intraductal neoplasias (high-grade PanIN) to infiltrating adenocarcinoma of the pancreas. Am J Surg Pathol 1998, 22:163-169 [DOI] [PubMed] [Google Scholar]
- 13.Hruban RH, Wilentz RE, Goggins M, Offerhaus GJA, Yeo CJ, Kern SE: Pathology of incipient pancreatic cancer. Ann Oncol 1999, 10:S9-S11 [PubMed] [Google Scholar]
- 14.Brockie E, Anand A, Albores-Saavedra J: Progression of atypical ductal hyperplasia/carcinoma in situ of the pancreas to invasive adenocarcinoma. Ann Diag Pathol 1998, 2:286-292 [DOI] [PubMed] [Google Scholar]
- 15.Lüttges J, Schlehe B, Menke MA, Vogel I, Henne-Bruns D, Klöppel G: The K-ras mutation pattern in pancreatic ductal adenocarcinoma usually is identical to that in associated normal, hyperplastic, and metaplastic ductal epithelium. Cancer 1999, 85:1703-1710 [PubMed] [Google Scholar]
- 16.DiGiuseppe JA, Hruban RH, Offerhaus GJA, Clement MJ, van den Berg FM, Cameron JL, van Mansfeld ADM: Detection of K-ras mutations in mucinous pancreatic duct hyperplasia from a patient with a family history of pancreatic carcinoma. Am J Pathol 1994, 144:889-895 [PMC free article] [PubMed] [Google Scholar]
- 17.Yamano M, Fujii H, Takagaki T, Kadowaki N, Watanabe H, Shirai T: Genetic progression and divergence in pancreatic carcinoma. Am J Pathol 2000, 156:2123-2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Day JD, DiGiuseppe JA, Yeo CJ, Loi-Goldman M, Anderson S, Kern SE, Hruban RH: Immunohistochemical evaluation of HER-2/neu oncogene expression in pancreatic adenocarcinoma and pancreatic intraepithelial neoplasms. Hum Pathol 1996, 27:119-124 [DOI] [PubMed] [Google Scholar]
- 19.Wilentz RE, Iacobuzio-Donahue CA, Argani P, McCarthy DM, Parsons JL, Yeo CJ, Kern SE, Hruban RH: Loss of expression of Dpc4 in pancreatic intraepithelial neoplasia: evidence that DPC4 inactivation occurs late in neoplastic progression. Cancer Res 2000, 60:2002-2006 [PubMed] [Google Scholar]
- 20.Wilentz RE, Geradts J, Maynard R, Offerhaus GJA, Kang M, Goggins M, Yeo CJ, Kern SE, Hruban RH: Inactivation of the p16 (INK4A) tumor-suppressor gene in pancreatic duct lesions: loss of intranuclear expression. Cancer Res 1998, 58:4740-4744 [PubMed] [Google Scholar]
- 21.Moskaluk CA, Hruban RH, Kern SE: p16 and K-ras gene mutations in the intraductal precursors of human pancreatic adenocarcinoma. Cancer Res 1997, 57:2140-2143 [PubMed] [Google Scholar]
- 22.DiGiuseppe JA, Hruban RH, Goodman SN, Polak M, van den Berg FM, Allison DC, Cameron JL, Offerhaus GJA: Overexpression of p53 protein in adenocarcinoma of the pancreas. Am J Clin Pathol 1994, 101:684-688 [DOI] [PubMed] [Google Scholar]
- 23.Goggins M, Hruban RH, Kern SE: The late temporal pattern of BRCA2 inactivation in pancreatic intraductal neoplasia: evidence and implications. Am J Pathol 2000, 156:1767-1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hruban RH, van Mansfeld ADM, Offerhaus GJA, van Weering DHJ, Allison DC, Goodman SN, Kensler TW, Bose KK, Cameron JL, Bos JL: K-ras oncogene activation in adenocarcinoma of the human pancreas: a study of 82 carcinomas using a combination of mutant-enriched polymerase chain reaction analysis and allele-specific oligonucleotide hybridization. Am J Pathol 1993, 143:545-554 [PMC free article] [PubMed] [Google Scholar]
- 25.Wilentz RE, Goggins M, Redston M, Marcus VA, Adsay NV, Sohn TA, Kadkol SS, Yeo CJ, Choti M, Zahurak M, Johnson K, Tascilar M, Offerhaus GJA, Hruban RH, Kern SE: Genetic, immunohistochemical, and clinical features of medullary carcinomas of the pancreas: a newly described and characterized entity. Am J Pathol 2000, 156:1641-1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caldas C, Hahn SA, Hruban RH, Redston MS, Yeo CJ, Kern SE: Detection of K-ras mutations in the stool of patients with pancreatic adenocarcinoma and pancreatic ductal hyperplasia. Cancer Res 1994, 54:3568-3573 [PubMed] [Google Scholar]
- 27.Yanagisawa A, Ohtake K, Ohashi K, Hori M, Kitagawa T, Sugano H, Kato Y: Frequent c-Ki-ras oncogene activation in mucous cell hyperplasias of pancreas suffering from chronic inflammation. Cancer Res 1993, 53:953-956 [PubMed] [Google Scholar]
- 28.Kinzler KW, Vogelstein B: Gatekeepers and caretakers. Nature 1997, 386:761-763 [DOI] [PubMed] [Google Scholar]
- 29.Hall PA, Hughes CM, Staddon SL, Richman PI, Gullick WJ, Lemoine NR: The c-erbB-2 proto-oncogene in human pancreatic cancer. J Pathol 1990, 161:195-200 [DOI] [PubMed] [Google Scholar]
- 30.Satoh K, Sasano H, Shimosegawa T, Koizumi M, Yamazuki T, Mochizuki F, Kobayashi N, Okano T, Toyota T, Sawai T: An immunohistochemical study of the c-erbB-2 oncogene product in intraductal mucin-hypersecreting neoplasms and in ductal cell carcinomas of the pancreas. Cancer 1993, 72:51-56 [DOI] [PubMed] [Google Scholar]
- 31.Redston MS, Caldas C, Seymour AB, Hruban RH, da Costa L, Yeo CJ, Kern SE: p53 mutations in pancreatic carcinoma and evidence of common involvement of homocopolymer tracts in DNA microdeletions. Cancer Res 1994, 54:3025-3033 [PubMed] [Google Scholar]
- 32.Rozenblum E, Schutte M, Goggins M, Hahn SA, Panzer S, Zahurak M, Goodman SN, Sohn TA, Hruban RH, Yeo CJ, Kern SE: Tumor-suppressive pathways in pancreatic carcinoma. Cancer Res 1997, 57:1731-1734 [PubMed] [Google Scholar]
- 33.Pellegata NS, Sessa F, Renault B, Bonato MS, Leone BE, Solcia E, Ranzani GN: K-ras and p53 gene mutations in pancreatic cancer: ductal and nonductal tumors progress through different genetic lesions. Cancer Res 1994, 54:1556-1560 [PubMed] [Google Scholar]
- 34.Hahn SA, Schutte M, Hoque ATMS, Moskaluk CA, daCosta LT, Rozenblum E, Weinstein CL, Fischer A, Yeo CJ, Hruban RH, Kern SE: DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science 1996, 271:350–353 [DOI] [PubMed]
- 35.Wilentz RE, Su GH, Dai JL, Sparks AB, Argani P, Sohn TA, Yeo CJ, Kern SE, Hruban RH: Immunohistochemical labeling for Dpc4 mirrors genetic status in pancreatic: a new marker of DPC4 inactivation. Am J Pathol 2000, 156:37-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hruban RH, Goggins M, Parsons JL, Kern SE: Progression model for pancreatic cancer. Clin Cancer Res 2000 (in press) [PubMed]
- 37.Hong WK, Sporn MB: Recent advances in chemoprevention of cancer. Science 1997, 278:1073-1077 [DOI] [PubMed] [Google Scholar]
- 38.Tucker ON, Dannenberg AJ, Yang EK, Zhang F, Teng L, Daly JM, Soslow RA, Masferrer JL, Woerner BM, Koki AT, Fahey TJ: Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res 1999, 59:987-990 [PubMed] [Google Scholar]
- 39.Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, Booker SV, Robinson CR, Offerhaus GJ: Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med 1993, 328:1313-1316 [DOI] [PubMed] [Google Scholar]