Leishmania major Reaches Distant Cutaneous Sites Where It Persists Transiently while Persisting Durably in the Primary Dermal Site and Its Draining Lymph Node: a Study with Laboratory Mice (original) (raw)
Abstract
So far, studies of Leishmania persistence in mice have used injections of parasites administered either intravenously in the tail vein or subcutaneously in the footpad. These routes poorly reflect the natural conditions when the sandfly delivers metacyclic promastigotes intradermally. In this study B10D2 and BALB/c mice were inoculated within the ear dermis with 104 Leishmania major metacyclic promastigotes. The parasite load was monitored by quantitative PCR in different tissues from the dermal inoculation site to distant tissues. The two sites of multiplication and persistence of parasites were the site of L. major inoculation and the draining lymph node (DLN), with a different pattern in the two mouse inbred lines. These two organs were the only sites harboring parasites 12 months postinoculation, with the DLN of BALB/c mice harboring around 107 parasites, a stable load from months 3 to 12. In these two sites, 8 and 12 months after inoculation, interleukin 4 (IL-4), gamma interferon, and inducible nitric oxide synthase transcripts parallel the parasite load while IL-10 transcript levels remain high. In addition, at early time points until month 3, parasite DNA was also detected in distant tissues such as the contralateral noninoculated ear or the tail skin, indicating that blood was at least transiently disseminating the parasites. In contrast, L. major DNA in liver, spleen, and femoral bone marrow remained sporadic in mice of both lines. This study is discussed within the framework of Leishmania transmission from the vertebrate host to the sandfly vector, a complex process still poorly understood.
Leishmaniasis currently affects some 12 million individuals in 88 countries, and at least 350 million people are exposed to the risk of the Leishmania parasite inoculation (see the World Health Organization information at http://www.who.int/emc/diseases/leish/leis.html). It is well established that in Leishmania transmission areas, individuals may harbor the parasites at very low levels, without developing symptoms. These individuals, considered asymptomatic carriers, are likely to transmit the parasite to the hematophagous sandfly vector or, as shown recently, via blood transfusion (16). Moreover, Leishmania spp. may persist in cured hosts after drug therapy (for a review, see reference 1) and be reactivated after immunosuppression, for instance, in human immunodeficiency virus-infected people (3). The capacity of the parasite to establish persistent infection as a means to achieve its transmission and hence maintenance of its life cycle is a process common to many parasites (9, 10). Little is known on the mechanisms underlying persistence of Leishmania parasites in their vertebrate host and transmission to the sandflies, pool-feeder hematophagous insects expected to recover transmissible parasites from the dermis. In a recent study using C57BL/6 mice, described as mice resistant to L. major, Stenger et al. (21) showed that a small number of parasites may persist in the regional lymph node, the spleen, and in some cases also at the site of the former lesion after resolution of this primary lesion. Persistence of parasites was paralleled with a sustained expression of inducible nitric oxide synthase (iNOS), and the treatment of mice with a selective inhibitor of iNOS switches on a massive replication of the parasites in the tissue and caused recrudescence of cutaneous leishmaniasis (21). However, the previous studies on persistence of Leishmania in the mouse model used either intravenous injection of the parasite in the tail vein (15) or subcutaneous injection in the footpad (21). These routes of injection poorly reflect what happens under natural conditions. Indeed, when the _Leishmania_-carrying sandflies take their blood meal on vertebrate hosts, they deliver metacyclic promastigotes within the dermis. Not only the local processes driven by the parasites but also the dissemination of the parasites within the distant mouse tissues may depend on the route of inoculation. This was shown with L. donovani by Melby et al. (18). These authors observed that the spleens of mice inoculated intravenously in the tail vein were highly parasitized, as expected, while the spleens of mice infected subcutaneously in the footpad were not. Therefore, in our laboratory, a model of parasite inoculation within the dermis of the mouse ear has been established (5, 6) and is closer to the natural way of infection than the above-mentioned methods. In the present study, we have set up a model and readout assays for tracing the dissemination to and the duration of the persistence of L. major in different tissues of BALB/c and B10D2 mice, after inoculation of metacyclic promastigotes within the dermis of one ear. We have developed a quantitative PCR method for monitoring the parasite burden and load. In long-term inoculated mice, the Leishmania organisms do persist mainly at the site of inoculation (the ear) and its draining lymph node. At 8 and 12 months post inoculation, we have observed that the levels of interleukin 4 (IL-4), gamma interferon (IFN-γ), and iNOS but not IL-10 transcripts in these inoculated ears and draining lymph nodes of long-term-infected mice, either resistant (B10D2) or susceptible (BALB/c), paralleled the parasite burden. As far as the parasite transmission from the vertebrate host to the vector is concerned, the most notable finding from these studies is that early transient dissemination via the blood does allow parasites to reach other distant dermal sites such as the contralateral ear and tail skin, two sites where they persist at a low level for at least 3 months. The next step will be to determine whether these Leishmania parasites residing in these clinically silent tissues are transmissible and will be indeed taken up by the sandflies from these otherwise accessible dermal sites.
MATERIALS AND METHODS
Animals, parasite inoculum, and parasite intradermal delivery.
Female BALB/c and B10D2 mice were purchased from Harlan (Gannat, France) and used for infection at 6 to 8 weeks of age. L. major strain NIH 173 (MHOM/IR/-/173) was cultured at 26°C in Hosmem-II medium (7) supplemented with 10% heat-inactivated fetal calf serum (Dutscher, Brumath, France), 100 U of penicillin per ml, and 100 μg of streptomycin (Seromed, Berlin, Germany) per ml.
Infective-stage metacyclic promastigotes were kindly provided by N. Courret in our Unit. Briefly, they were isolated from stationary culture (5 days old) by negative selection using peanut agglutinin (Vector Laboratories Inc., Burlingame, Calif.) (13, 19). Mice received injections in the dermis of the right ear with 104 metacyclic parasites in a volume of 10 μl of phosphate-buffered saline (PBS) using a 27.5-G needle as described in reference 5. The basis of the ear was always avoided as a site of inoculation. The evolution of the lesion was monitored by measuring, on 10 mice at each time point, the thickness of the ears with a direct-reading vernier caliper and was expressed as (thickness of inoculated ear − thickness of uninoculated ear).
Tissue sampling for monitoring parasite burden by PCR.
At week 2 and months 1, 3, 6, 8, and 12 postinfection, 3 to 10 mice were killed at each time point for monitoring the distribution of the parasites in the different tissues, using a quantitative PCR assay. Blood was sampled at the retroorbital sinus, prior to the killing of the animals, and was kept in EDTA (0.1 M). The following tissues were sampled: retromaxillar draining lymph nodes, spleen, liver, both ears separately, bone marrow from the two femurs, and a piece of tail skin. Tissues were removed by using different scissors or scalpels to avoid contamination and were minced with Potter grinders and then carefully homogenized in 1.5-ml microtubes with single-use blue pellet pestles (Polylabo, Paris, France) in PBS. Aliquots of the homogenates were stored at −20°C until DNA extraction.
DNA extraction.
DNA was isolated from the tissue homogenates using the InstaGene DNA purification matrix (Bio-Rad, Ivry s/Seine, France). Homogenates (50 μl) were mixed with 200 μl of DNA purification matrix, incubated for 45 min at 56°C and 8 min at 98°C, and then centrifuged at 10,000 × g for 5 min. DNA from blood samples was purified from 50 μl aliquots using the InstaGene Whole Blood purification kit (Bio-Rad). Samples of the supernatants (2 μl) were used for PCR analysis.
PCR amplification.
Specific detection of Leishmania DNA was carried out using the primers forward, 5′-CCTATTTTACACCAACCCCCAGT-3′(JW11), and reverse, 5′-GGGTAGGGGCGTTCTGCGAAA-3′ (JW12), which amplify a 116-bp fragment of the minicircle kinetoplast DNA (kDNA) of L. major, present at ca. 10,000 copies in each parasite (4, 11). Extracted DNA (2 μl) was mixed with a solution containing 10 mM Tris-HCl (pH 9.0), 50 mM KCl, 0.1% Triton X-100, 2 mM MgCl2, a 250 μM concentration of each deoxynucleotide triphosphate, 10 pmol of each primer, and 0.5 U of Taq polymerase (Promega, Charbonnières, France), in a 40-μl final volume. PCR was performed with an automated thermocycler (PCR-Express; Hybaid, Ashford, United Kingdom), with which the annealing temperature was optimized from 48 to 60°C. A hot-start procedure was used to increase specificity. After an initial denaturation (4 min at 94°C), 30 cycles (denaturation for 1 min at 64°C, annealing for 30 s at 58°C, elongation for 30 s at 72°C) were carried out and PCR was terminated by a final extension at 72°C for 10 min. Each sample was tested in duplicate. Negative control tubes which received 2 μl of water instead of DNA extract were included in each run of PCR to detect any amplicon contamination.
Sensitivity and reproducibility of the PCR based assay of kDNA of L. major.
The reproducibility of extraction of Leishmania DNA from the different tissues and the impact of putative PCR inhibitory processes were evaluated with mimics of infected tissues. Homogenates from different tissues, or PBS as a control, were spiked with 10-fold increasing numbers of L. major promastigotes, extracted in duplicate and subjected to PCR with Leishmania specific primers. PCR products were quantified from ethidium bromide-stained agarose gels and scanned with ImageQuant for Windows. An internal standard was loaded on each gel.
Quantitative PCR by dilution limits.
For tissues highly infected, DNA was extracted from serial dilutions of homogenates, and six replicates of each dilution were subjected to PCR. The number of parasites in each tissue was determined from the highest dilution at which Leishmania DNA could be detected by PCR.
Quantification of iNOS, IL-4, IL-10, and IFN-γ transcripts.
At months 8 and 12 postinfection, whole ears, retromaxillar lymph nodes, and a piece of tail skin were collected, and the transcripts of iNOS, IL-4, IL-10, and IFN-γ were quantified by competitive reverse transcription-PCR as described in reference 12. Briefly, total RNA was isolated using the RNeasy kit (Qiagen, Hilden, Germany) and reverse transcribed. Reverse transcripts (cDNA) were quantified using a PCR method involving coamplification of cDNA with an internal standard. The standards were generated by addition of 2 to 4 bp to the sequence of the wild-type DNA molecules, which generated restriction sites specific for either wild-type or standard DNAs. After restriction endonuclease digestion, the equivalence between coamplified cDNA and standard DNA was monitored on agarose gel. To eliminate variations due to RNA extraction and cDNA synthesis steps, quantification of each transcript in a given sample was expressed with respect to a constant number (106) of β-actin copies.
RESULTS
Monitoring of the symptoms at the inoculation site, the right ear.
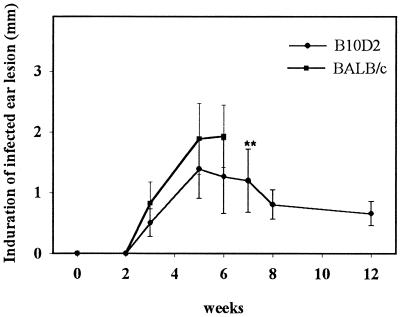
When 104 L. major NIH 173 metacyclic promastigotes were inoculated into the ear dermis, the dermal lesions were first detectable around week 3 in mice of both inbred lines (Fig. 1). In BALB/c mice, the increase of thickness was higher than that in B10D2 mice, peaked around week 5, and was followed by tissue necrosis and loss of tissue around week 6 and onwards, leaving clean scars. Of note, in a few BALB/c mice, necrosis was never observed up to 12 months. In B10D2 mice, the thickness of the inoculated ear peaked around week 5 as well and then declined slowly. The thickness of contralateral noninoculated ears remained constant throughout the experiment, and no external clinical symptoms were observed.
FIG. 1.
Evolution of induration of the inoculated right ear for B10D2 or BALB/c mice after intradermal inoculation of 104 L. major (NIH 173) metacyclic promastigotes into the ear dermis. Measurements are expressed as the difference of thickness between inoculated ear and noninoculated ear. The data represent mean values ± standard deviations (error bars) (n = 10). ∗∗, after week 6, the inoculated ear of most BALB/c mice showed necrosis and loss of tissue and therefore the thickness of induration could not be measured further.
Detection and quantitation of Leishmania DNA: reproducibility and sensitivity of PCR.
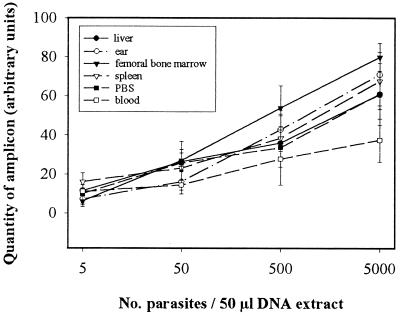
The putative impact of tissue origin on PCR yield when amplifying Leishmania target kDNA was checked with tissue homogenates from naïve BALB/c or B10D2 mice spiked with known numbers of in vitro-grown L. major promastigotes. After 30 cycles of amplification, parasite concentrations ranging from 102 to 105 per ml of tissue homogenate were detectable in a linear way, i.e., 5 to 5 × 103 parasites per DNA extract in a 50-μl sample and the origin of tissue did not significantly influence the yield of PCR, except when parasites were recovered from blood, which led to a lower number of amplicon molecules (Fig. 2). All the tissue homogenates from naïve mice were PCR negative with the Leishmania specific primers used, even after 40 cycles of PCR. For a given tissue, the reproducibility of Leishmania DNA extraction was checked (not shown). Under the experimental conditions used, the thresholds of detection levels, after 30 cycles of amplification, were approximately 20 parasites/ml of blood, 10 parasites/femoral bone marrow or lymph node, 20 parasites/ear, 40 parasites/piece of tail, and 100 parasites/spleen or liver.
FIG. 2.
Standard curves of detection of kDNA of L. major in different tissue homogenates. Tenfold numbers of in vitro-grown L. major NIH 173 were spiked to tissue homogenates prior to DNA extraction and PCR. Data represent the means ± standard deviations (error bars) of at least two DNA extractions. kDNA amplicons were quantified by scanning agarose gels with Ampli Quant Windows software.
At different time points after intradermal inoculation, the tissues were assessed by PCR for the presence of Leishmania DNA. In a first PCR survey of samples, a qualitative detection of DNA was performed using undiluted homogenates (Table 1). The quantity of PCR amplicons was estimated by comparison to a standard curve using determined concentrations of L. major promastigotes. Then, the tissues harboring a parasite load of >102 per organ, i.e., the inoculated ears and their lymph nodes and the contralateral noninoculated ears, were reanalyzed after serial dilutions of DNA extracts (Fig. 3).
TABLE 1.
Detection of L. major kDNA by PCR in different distant tissues of BALB/c or B10D2 mice inoculated intradermally in the right ear with 104 metacyclic promastigotesa
| Mouse | Tissue or organ | No. of PCR-positive mice/no. of mice analyzed at time postinoculation | |||||
|---|---|---|---|---|---|---|---|
| 2 wk | 1 mo | 3 mo | 6 mo | 8 mo | 12 mo | ||
| BALB/c | Right ear (inoculation site) | 4/4 | 4/4 | 4/4 | 3/3 | 5/9 | 8/10 |
| Left ear | 3/4 | 3/4 | 3/4 | 0/3 | 0/9 | 0/10 | |
| Draining lymph nodeb | 4/4 | 4/4 | 4/4 | 3/3 | 8/9 | 7/10 | |
| Tail skin | 2/4 | 1/4 | 1/4 | 2/3 | 0/9 | NDc | |
| Blood | 4/4 | 4/4 | 4/4 | 0/3 | ND | ND | |
| Femoral bone marrow | 1/4 | 0/4 | 1/4 | 0/3 | ND | ND | |
| Spleen | 4/4 | 4/4 | 3/4 | 0/3 | ND | ND | |
| Liver | 0/4 | 0/4 | 2/4 | 0/3 | ND | ND | |
| B10D2 | Right ear (inoculation site) | 4/4 | 4/4 | 4/4 | 3/3 | 2/5 | 5/10 |
| Left ear | 3/4 | 3/4 | 4/4 | 0/3 | 0/5 | 0/10 | |
| Draining lymph nodeb | 4/4 | 4/4 | 4/4 | 1/3 | 3/5 | 3/10 | |
| Tail skin | 4/4 | 4/4 | 2/4 | 2/3 | 0/5 | ND | |
| Blood | 1/4 | 2/4 | 2/4 | 0/3 | ND | ND | |
| Femoral bone marrow | 0/4 | 0/4 | 2/4 | 0/3 | ND | ND | |
| Spleen | 0/4 | 0/4 | 1/4 | 0/3 | ND | ND | |
| Liver | 1/4 | 1/4 | 1/4 | 0/3 | ND | ND |
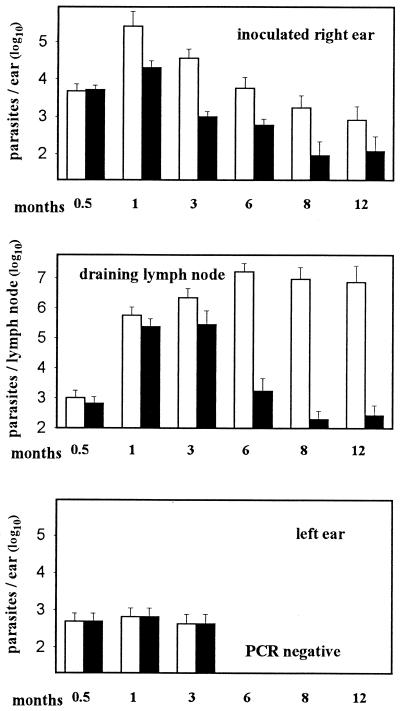
FIG. 3.
Monitoring of the parasitic load in B10D2 (black histograms) or BALB/c (white histograms) mice after intradermal inoculation of 104 L. major metacyclic promastigotes into the right ear dermis. Parasitic load is estimated by L. major kDNA PCR (see Fig. 2). Histograms represent mean values + standard deviations (error bars).
L. major does persist durably in the inoculation site and its draining lymph node, but is there a unique profile of IL-4, IFN-γ, iNOS, and IL-10 transcripts in both sites?
The parasite burden in the site of inoculation (right ear), followed a similar pattern in BALB/c and B10D2 mice, with a peak at month 1 followed by a decrease until month 12 (Fig. 3). However, in susceptible mice (BALB/c), the estimated numbers of parasites remained approximately 10 times higher than that in resistant mice, despite the fact that in the inoculated ear of BALB/c mice there was a loss of tissue from month 3 onwards. In resistant mice, the mean numbers of parasites remaining at the site of inoculation were nevertheless estimated at ca. 103 parasites at month 6 and were still 102 at month 12. The parasite numbers in noninoculated left ears were similar in both mouse inbred lines and never exceeded ca. 103 parasites per ear until month 3. In contrast to what happened in the ears, the estimated parasite numbers in the retromaxillar lymph node draining the inoculated right ear, rapidly increased in BALB/c and B10D2 mice until month 3. However, in susceptible BALB/c mice, the numbers of parasites reached a plateau of ca. 107 parasites per node, while a sharp decrease was observed in resistant mice.
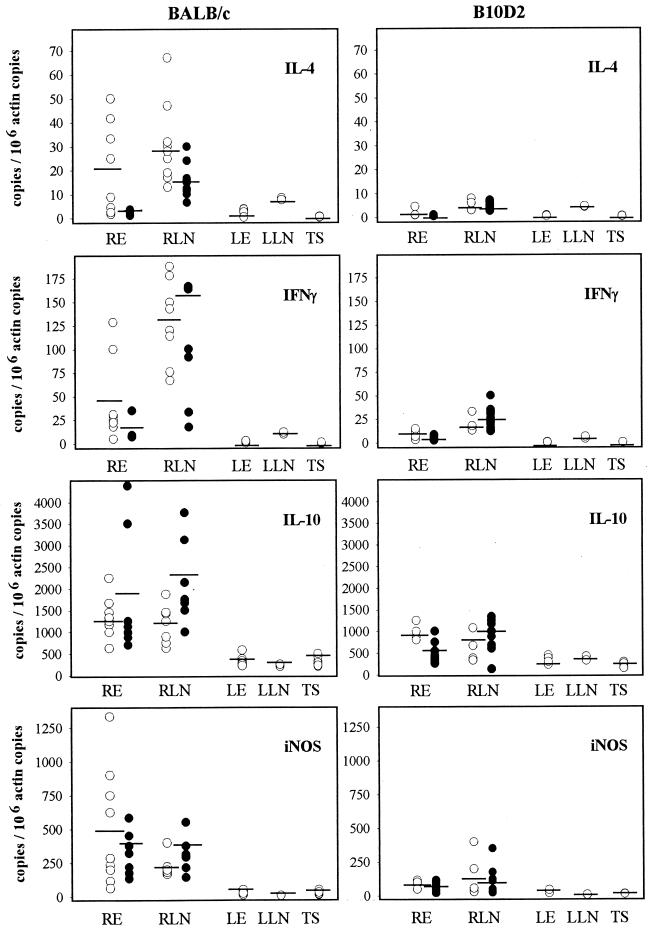
Transcripts of IL-4, IL-10, IFN-γ, and iNOS were quantified in long-term-infected mice at months 8 and 12 in the ears and in the retromaxillar lymph nodes and at month 8 only in a piece of the tail skin (Fig. 4). In BALB/c mice, the levels of the transcripts of IL-4, IFN-γ, IL-10, and iNOS at month 8 were significantly higher in the inoculated site (right ear) (P < 0.004) and its draining lymph node (P < 0.01) than in respective contralateral tissues. The levels of transcripts in left ears and their draining lymph nodes were similar to those observed in tail skins, which are close to the levels measured in the skin of naïve BALB/c mice (J. H. Colle, unpublished data). At month 12, the levels of transcripts in the different tissues were not significantly different than at month 8, except for IL-4 in the inoculation site (right ear) and its draining lymph node (right lymph node), where the levels of transcripts significantly decreased between months 8 and 12 (P < 0.01).
FIG. 4.
Quantitation by RT-PCR of mRNA transcribed from IL-4, IFN-γ, IL-10, and iNOS genes in ears, draining lymph nodes and distant skin (tail) of BALB/c or B10D2 mice. Tissues analyzed are right ear (RE), right retromaxillary lymph node (RLN), left ear (LE), left retromaxillary lymph node (LLN), and tail skin (TS) at month 8 (open circles) and RE and RLN at month 12 (black circles). Data of LE and LLN at month 12 were very similar to those of month 8 and are not shown on this figure. Circles represent individual mice, and bars indicate the means. The number of each transcript is normalized to 106 β-actin copies.
In mice which controlled the lesions and the parasite burden (B10D2), the level of the IL-4 transcripts was very low in both ears and both lymph nodes and not significantly different from the basic level observed in tail skin. The levels of IFN-γ and iNOS transcripts were slightly higher in the right ears and draining lymph node than in the respective contralateral tissue. However, this slight difference was only statistically significant for ears. Globally, when BALB/c and B10D2 mice are compared, the levels of IL-4, IFN-γ, and iNOS transcripts were higher in infected ears and their draining lymph nodes of BALB/c than in those of B10D2 mice (P < 0.004, for IL-4 and IFN-γ in right lymph node). Special attention must be drawn to IL-10: the levels of this transcript remained higher in infected ears and their draining lymph nodes than in contralateral tissues, in mice of both lines. In tail skin, the levels of all transcripts were close to that observed at homeostasis, whatever the mouse lines. In B10D2 mice, the levels of all transcripts did not significantly vary between months 8 and 12, in the different tissues.
Presence of a stable number of parasites over 3 to 6 months in distant cutaneous sites: a clinically silent transient process.
Two weeks after inoculation, the first time point we selected in the present study, Leishmania DNA was detected in the site of inoculation and its draining lymph node in all mice analyzed but also was detected in distant tissues such as the noninoculated ear (left ear), tail skin, and blood in most of mice, which indicated at least the transient presence of parasites in the blood (Table 1). Parasite load in those organs remained stable and low (∼103/contralateral ear), and no parasite DNA was recovered after month 3 in blood and contralateral ears and after month 6 in tail skins, whatever the mouse line (Table 1). Detection of DNA in liver and bone marrow was sporadic in both mouse lines and never exceeded 5,000 parasites per liver and 50 parasites per femur bone marrow. All the spleens of BALB/c mice were PCR positive until month 3, and five out of nine were PCR positive at month 6; however, the parasite burden in this organ remained low (<500 parasites per spleen). In contrast, the spleens of B10D2 mice were parasite DNA negative.
It is important to note that no clinical symptoms were detected over the period of observation (12 months).
DISCUSSION
In this work is compared, for the first time, the extent and duration of the multifocal distribution of L. major disseminating from the dermal site where they were initially delivered. Following intradermal delivery of 104 L. major metacyclic promastigotes in one ear, parasite presence and load were estimated in the draining lymph nodes, the blood, the blood-filtering tissues, and distant cutaneous sites, such as the contralateral ear and the tail skin.
In terms of clinical symptoms in the different cutaneous sites loaded with or reached by the parasites, the only sites where symptoms develop until tissue destruction, with clean scars, are the inoculated ears of BALB/c mice. In the inoculated ears of B10D2 mice, a transient lesion was observed which healed without loss of tissue and without scar or with a minimal fibrotic scar. In all other cutaneous tissues which were reached by parasites, i.e., the contralateral ears and tail skin, no clinical symptom was observed.
If data on the mouse immunological determinants of the early steps of infection—following subcutaneous inoculation of large number of stationary phase promastigotes—are now well established (14, 20), data on the late steps of this long term process are still lacking, especially when parasites have been delivered intradermally. In this study we have analyzed the amounts of transcripts of IL-4, IL-10, IFN-γ, and iNOS in 8- and 12-month-infected mice. Globally at month 8, except for IL-10, we have observed that the levels of those transcripts are related to the parasite load and long-term persistence, especially in the lymph node of BALB/c mice where ∼107 parasites persist. Similar features have been noticed when biopsy specimens of human patients infected by L. major in Tunisia have been monitored for the same transcripts plus IL-12 transcripts: a positive association between IL-12 and IFN-γ transcripts and the presence of parasites in the lesion was pinpointed (17). These authors have also observed that the biopsy specimens of the patients exhibiting a higher level of IL-10, IL-12, and IFN-γ transcripts had an unfavorable evolution of the lesion. In the present study, at month 12, the overall transcript profile did not dramatically change even if the parallel fluctuations of IFN-γ, IL-10, and iNOS transcripts are notable in the lymph node of BALB/c mice, where the parasites persist at a very high level.
The most notable finding from this study is the rapid and multifocal distribution of the parasites in the mouse body after intradermal inoculation of 104 NIH 173 metacyclic promastigotes, which followed a similar pattern in mice of both inbred lines. Rapid and at least transient distribution of the parasites was observed in the blood compartment and in hairless cutaneous sites distant from the inoculation site, such as the tail skin or the contralateral ear. However, in contrast to study using different routes of inoculation with large doses of parasites, the liver, spleen, and femoral bone marrow of either BALB/c or B10D2 mice remained sporadically parasitized in this study. Therefore, particular attention should be paid to the route of parasite delivery in model studies, to mimic as much as possible the natural situation (6).
It remains now to be determined (i) whether the parasites which circulate and reach cutaneous distant tissue are free or intracellular and if so, which leukocyte subsets act as shuttles (phagocytic mononuclear cells or dendritic leukocytes are relevant candidates); (ii) whether the parasites are transported directly via the venous or indirectly via the lymphatic network; and (iii) in these distant cutaneous tissues, what local immunological and nonimmunological features allow the transient persistence (0.5 to 3 months) of a stable number of parasites while their number increases in the inoculated sites and DLN during the same period at least in BALB/c mice.
The cutaneous tissues distant from the inoculation site, e.g., contralateral ears or tail skin, remained parasitized for 3 to 5 months after initial infection not only in mice with destructive lesions but also in mice which were able to control the lesions. This indicates that most of these mice probably remain, during this period, efficient reservoirs for transmission to sandflies, even in absence of reinfection, which should be checked by feeding sandflies on long-term-infected mice. This also emphasizes the interest of further studies in field situations as well to determine the presence of Leishmania in the lesion-face skin of putative reservoir hosts, at least rodents. The large range of PCR primers now available to accurately detect the presence of Leishmania DNA should facilitate such epidemiological studies (2, 8, 22).
ACKNOWLEDGMENTS
This work was supported by grants from the Délégation Générale pour l'Armement (contract DGA 95/150) and from the Institut Pasteur. S. Sidjanski received a fellowship from Hoffmann-Laroche Foundation and Novartis Foundation.
We thank Nathalie Courret for providing Leishmania metacyclic promastigotes and Karim Sebastien for taking care of the mice used in this study.
REFERENCES
- 1.Aebischer T. Recurrent cutaneous leishmaniasis: a role for persistent parasites. Parasitol Today. 1994;10:25–28. doi: 10.1016/0169-4758(94)90353-0. [DOI] [PubMed] [Google Scholar]
- 2.Alexander B, Lozano C, Barker D C, McCann S H E, Adler G H. Detection of Leishmania (Viannia) braziliensis complex in wild mammals from colombian coffee plantations by PCR and DNA hybridization. Acta Trop. 1998;69:41–50. doi: 10.1016/s0001-706x(97)00114-9. [DOI] [PubMed] [Google Scholar]
- 3.Alvar J, Canavate C, Gutierrez-Solar B, Jimenez M, Laguna F, Lopez-Velez R, Molina R, Moreno J. Leishmania and human immunodeficiency virus coinfection: the first 10 years. Clin Microbiol Rev. 1997;10:298–319. doi: 10.1128/cmr.10.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker D C, Gibson L J, Kennedy W P K, Nasser A A A A, Williams R H. The potential of using recombinant DNA species specific probes for the identification of tropical Leishmania. Parasitology. 1986;91(Suppl.):S139–S174. doi: 10.1017/s0031182000085747. [DOI] [PubMed] [Google Scholar]
- 5.Belkaid Y, Jouin H, Milon G. A method to recover, enumerate and identify lymphomyeloid cells present in an inflammatory dermal site: a study in laboratory mice. J Immunol Methods. 1996;199:5–25. doi: 10.1016/s0022-1759(96)00117-2. [DOI] [PubMed] [Google Scholar]
- 6.Belkaid Y, Kamhawi S, Modi G, Valenzuela J, Noben-Trauth N, Rowton E, Riberio J, Sacks D L. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J Exp Med. 1998;188:1941–1953. doi: 10.1084/jem.188.10.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berens R L, Marr J J. An easily prepared defined medium for cultivation of Leishmania donovani promastigotes. J Parasitol. 1978;64:160. [PubMed] [Google Scholar]
- 8.Berrahal F, Mary C, Roze M, Berenger A, Escoffier K, Lamouroux D, Dunan S. Canine leishmaniasis: identification of asymptomatic carriers by polymerase chain reaction and immunoblotting. Am J Trop Med Hyg. 1996;55:273–277. doi: 10.4269/ajtmh.1996.55.273. [DOI] [PubMed] [Google Scholar]
- 9.Bogdan C, Gessner A, Solbach W, Röllinghoff M. Invasion, control and persistence of Leishmania parasites. Curr Opin Immunol. 1996;8:517–525. doi: 10.1016/s0952-7915(96)80040-9. [DOI] [PubMed] [Google Scholar]
- 10.Bogdan C, Röllinghoff M. How do protozoan parasites survive inside macrophages. Parasitol Today. 1999;15:22–28. doi: 10.1016/s0169-4758(98)01362-3. [DOI] [PubMed] [Google Scholar]
- 11.Brewster S, Aslett M, Barker D C. Kinetoplast DNA minicircle database. Parasitol Today. 1998;14:437–438. doi: 10.1016/s0169-4758(98)01330-1. [DOI] [PubMed] [Google Scholar]
- 12.Colle J H, Falanga P B, Singer M, Hevin B, Milon G. Quantitation of messenger RNA by competitive RT-PCR: a simplified read out assay. J Immunol Methods. 1997;210:175–184. doi: 10.1016/s0022-1759(97)00186-5. [DOI] [PubMed] [Google Scholar]
- 13.Courret N, Prina E, Mougneau E, Saraiva E M, Sacks D L, Glaichenhaus N, Antoine J C. Presentation of the Leishmania antigen LACK by infected macrophages is dependent upon the virulence of the phagocytosed parasites. Eur J Immunol. 1999;29:762–773. doi: 10.1002/(SICI)1521-4141(199903)29:03<762::AID-IMMU762>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 14.Launois P, Louis J A, Milon G. The fate and persistence of Leishmania major in mice of different genetic backgrounds: an example of exploitation of the immune system by intracellular parasites. Parasitology. 1997;115:S25–S32. doi: 10.1017/s0031182097001777. [DOI] [PubMed] [Google Scholar]
- 15.Leclercq V, Lebastard M, Belkaid Y, Louis J, Milon G. The outcome of the parasitic process initiated by Leishmania infantum in laboratory mice: a tissue-dependent pattern controlled by the Lsh and MHC loci. J Immunol. 1996;157:4537–4545. [PubMed] [Google Scholar]
- 16.Le Fichoux Y, Quaranta J F, Aufeuvre J P, Lelièvre A, Marty P, Suffia I, Rousseau D, Kubar J. Occurrence of Leishmania infantum in asymptomatic blood donors living in an area of endemicity in Southern France. J Clin Microbiol. 1999;37:1953–1957. doi: 10.1128/jcm.37.6.1953-1957.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louzir H, Melby P C, Ben Salah A, Marrakchi H, Aoun K, Ben Ismail R, Dellagi K. Immunological determinants of disease evolution in localized cutaneous leishmaniasis due to Leishmania major. J Infect Dis. 1998;177:1687–1695. doi: 10.1086/515297. [DOI] [PubMed] [Google Scholar]
- 18.Melby P C, Yang Y Z, Cheng J, Zhao W. Regional differences in the cellular immune response to experimental cutaneous or visceral infection with Leishmania donovani. Infect Immun. 1998;66:18–27. doi: 10.1128/iai.66.1.18-27.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sacks D L, Hieny S, Sher A. Identification of cell surface carbohydrate and antigenic changes between noninfective and infective developmental stages of Leishmania major promastigotes. J Immunol. 1985;135:564–569. [PubMed] [Google Scholar]
- 20.Solbach W, Laskay T. The host response to Leishmania infection. Adv Immunol. 2000;74:275–317. doi: 10.1016/s0065-2776(08)60912-8. [DOI] [PubMed] [Google Scholar]
- 21.Stenger S, Donhauser N, Thüring H, Röllinghof M, Bogdan C. Reactivation of latent leishmaniasis by inhibition of inducible nitric oxide synthase. J Exp Med. 1996;183:1501–1514. doi: 10.1084/jem.183.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Telleria J, Bosseno M F, Tarifa T, Buitrago R, Martinez E, Torrez M, Le Pont F, Brenière S F. Putative reservoirs of Leishmania amazonensis in a sub-andean focus of Bolivia identified by kDNA polymerase chain reaction. Mem Inst Oswaldo Cruz. 1999;94:5–6. doi: 10.1590/s0074-02761999000100002. [DOI] [PubMed] [Google Scholar]