Molecular and Conventional Epidemiology of Mycobacterium tuberculosis in Botswana: a Population-Based Prospective Study of 301 Pulmonary Tuberculosis Patients (original) (raw)
Abstract
Little is known about patterns of tuberculosis (TB) transmission among populations in developing countries with high rates of TB and human immunodeficiency virus (HIV) infection. To examine patterns of TB transmission in such a setting, we performed a population-based DNA fingerprinting study among TB patients in Botswana. Between January 1997 and July 1998, TB patients from four communities in Botswana were interviewed and offered HIV testing. Their Mycobacterium tuberculosis isolates underwent DNA fingerprinting using IS_6110_ restriction fragment length polymorphism, and those with matching fingerprints were reinterviewed. DNA fingerprints with >5 bands were considered clustered if they were either identical or differed by at most one band, while DNA fingerprints with ≤5 bands were considered clustered only if they were identical. TB isolates of 125 (42%) of the 301 patients with completed interviews and DNA fingerprints fell into 20 different clusters of 2 to 16 patients. HIV status was not associated with clustering. Prior imprisonment was the only statistically significant risk factor for clustering (risk ratio, 1.5; 95% confidence interval, 1.1 to 2.0). In three communities where the majority of eligible patients were enrolled, 26 (11%) of 243 patients overall and 26 (25%) of 104 clustered patients shared both a DNA fingerprint and strong antecedent epidemiologic link. Most of the increasing TB burden in Botswana may be attributable to reactivation of latent infection, but steps should be taken to control ongoing transmission in congregate settings. DNA fingerprinting helps determine loci of TB transmission in the community.
DNA fingerprinting of Mycobacterium tuberculosis has complemented conventional epidemiologic methods in the investigation of outbreaks and nosocomial transmission of both drug-sensitive (10, 17, 23, 27) and drug-resistant (4, 12, 19) tuberculosis (TB) and of laboratory cross-contamination (22). In addition, many investigators have used M. tuberculosis DNA fingerprinting in population-based cohorts of TB patients to determine the proportions of TB disease attributable to recent transmission versus reactivation, to delineate risk factors for recent acquisition of M. tuberculosis infection and TB disease, and to describe the sites of the greatest transmission of infection. Large urban studies in the United States and Europe (3, 5, 13, 20, 21, 24) demonstrated that between 32% (Baltimore, Md. [5]) and 59% (Los Angeles, Calif. [3]) of TB patients had isolates that fell into clusters of DNA fingerprints and thus may have had recently acquired infection and disease rather than reactivation of latent infection. In a rural state in the United States, 33% of TB patients had clustered isolates, and epidemiologic links could be identified for only 42% of these persons despite extensive interviews (8). Risk factors for clustering have been identified in many of these studies. Human immunodeficiency virus (HIV) (2, 21) has been found to be independently associated with clustering in some studies, but not in others (5, 13). In the United States, exposure to TB patients in congregate settings such as homeless shelters (3, 13), substance abuse centers (8), and prisons (8) has also been associated with probable recent M. tuberculosis transmission.
The overwhelming burden of TB is in nonindustrialized countries, where very few population-based studies of the molecular epidemiology of M. tuberculosis have been conducted, where HIV prevalence is often high, and where the epidemiology of TB may be quite different from that in industrialized nations. As Cohn and O'Brien (9) have highlighted, restriction fragment length polymorphism analysis in developing countries may help answer questions regarding community and nosocomial M. tuberculosis transmission and HIV-associated TB and thereby provide guidance for the investment of resources for TB control. Most published studies from Africa have been limited to the examination of DNA fingerprints of isolates from small samples of TB patients (14, 16, 29), including a study conducted in Botswana during 1995 and 1996 that provided data in preparation for the present study (18). Wilkinson et al. (28) reported results of a study of DNA fingerprinting and conventional contact tracing on 246 TB patients in rural South Africa, showing that approximately 40% of isolates were in clusters and that epidemiologic links could be established for 27% of clustered patients. A second South African study demonstrated that recent M. tuberculosis transmission rather than relapse was responsible for most cases of recurrent TB (26).
To examine the frequency and patterns of transmission in an African setting with both high HIV and high TB rates, we performed a population-based study in Botswana. We hypothesized that a significant proportion of disease would be due to recently transmitted TB rather than reactivation of TB and that HIV might be a risk factor for recent transmission. We also hoped to identify sites of TB transmission. Botswana is in southern Africa and had a population of approximately 1.7 million in 1997. TB rates in the country increased from 202 per 100,000 in 1989 (6) to 537 per 100,000 in 1999 (7), and 1999 HIV sentinel surveillance revealed HIV seroprevalence rates of 36% among women presenting for routine antenatal care (1). Botswana does not have a high rate of immigration, but the population is highly mobile, with many individuals traveling between home village, place of work, farm, and cattle post. This study, which was conducted in four communities over an 18-month period, was designed to (i) examine the utility of M. tuberculosis molecular epidemiology in communities with high TB and HIV prevalence, (ii) identify persons and settings associated with transmission of M. tuberculosis in these communities, and (iii) evaluate the effect of HIV infection on the transmission of M. tuberculosis.
MATERIALS AND METHODS
Patient enrollment.
Eligible patients were persons ≥18 years of age diagnosed with TB between January 1997 and March 1998 with at least one acid-fast bacillus (AFB)-positive sputum smear and who resided (at the time of TB diagnosis) within a 30-min driving radius of one of the four study sites: Kanye (a village of 26,000), Lobatse (a town of 29,000), Francistown (a city of 92,000), and Gaborone (the capital city, with a population of 192,000). All sites had full-time study nurses who, at least twice per week, visited their respective laboratories and identified patients with AFB-positive sputum smears. Smear-positive samples were sent to the National TB Reference Laboratory in Gaborone for culture on Lowenstein-Jensen medium; only patients with culture-positive TB were ultimately included in the study, as M. tuberculosis DNA fingerprints were needed for all patients in order to answer the study questions. Patients with AFB-positive sputum smears were sought by the study nurse, who obtained informed consent, conducted an interview, and offered optional HIV testing and tuberculin skin tests (TSTs). The TSTs were performed using 2 tuberculin units of R23 tuberculin by the Mantoux technique and were read at 48 to 72 h after placement. HIV testing was performed in Botswana for patients giving informed consent, using two enzyme-linked immunosorbent assays (ELISAs) for HIV types 1 and 2 run in parallel (Welcozyme [Burroughs Wellcome, London, Great Britain] and Detect [Biochem Immunosystems, Montreal, Canada]). Two positive ELISAs were considered positive for HIV infection, and two negative ELISAs were considered negative for HIV infection. No discordant ELISA results for any given patient were found. Confirmatory Western blotting was not performed. This study was approved by the human-subjects review boards of the Ministry of Health, Botswana, and the Centers for Disease Control and Prevention.
DNA fingerprinting and analysis.
Cultures positive for M. tuberculosis were shipped to the Centers for Disease Control and Prevention mycobacteriology laboratory, where standardized DNA fingerprinting was performed using IS_6110_-based restriction fragment length polymorphism (25). DNA fingerprints were analyzed and compared using the Bio Image Whole Band Analyzer version 3.3 software (Genomic Solutions, Ann Arbor, Mich.). Autoradiographs of DNA fingerprints were also compared visually. Using published criteria (8), DNA fingerprints with >5 bands were considered matched if they were identical or if they differed by at most one band, while DNA fingerprints with ≤5 bands were considered matched only if they were identical. An isolate that matched at least one other isolate, and the patients from whom these isolates were obtained, were considered to form a cluster.
Epidemiologic investigation.
Structured questionnaires with options for open-ended answers were administered to all participants by trained study nurses in a setting that ensured privacy. Surrogate interviews were not conducted in the case of patient death or absence. Information collected included demographics, history of current and previous TB, known or suspected TB contacts, all places and dates of residence, schooling, hospitalization, incarceration, employment, recreational activities, and travel. Clustered patients were sought for a second, open-ended interview to further establish or strengthen potential epidemiologic connections in place, time, and person among cluster members. Participants were considered to share a strong epidemiologic link if they had been in the same workplace, hospital, prison, household, cattle post, or farm at overlapping times.
Analysis.
Differences between the populations of the study sites were examined using chi-square and Wilcoxon rank sum tests. To examine the association between demographic, medical, and social factors and clustering, we calculated univariate risk ratios and 95% confidence intervals (CI) using Epi Info version 6.02 (11); statistical significance (P ≤ 0.05) was assessed using the Mantel-Haenszel chi-square or Fisher exact test.
RESULTS
A total of 439 (64%) of 685 eligible AFB-smear positive patients had sputum specimens submitted for culture. DNA fingerprints were obtained from 373 (85%) of these patients, 301 (81%) of whom had interviews completed. Of the 66 patients without DNA fingerprints, 59 had repeatedly contaminated cultures and 7 had an isolate from which no fingerprint could be obtained despite three or more attempts. The proportions of eligible patients in the study sites who were both interviewed and had a DNA fingerprint were 65 of 79 (84%) in Kanye, 84 of 163 (52%) in Lobatse, 94 of 145 (65%) in Francistown, and 58 of 298 (20%) in Gaborone (Table 1). Because only a small proportion of eligible patients in Gaborone had DNA fingerprints and were interviewed, our ability to find links between these patients may have been compromised, so these patients underwent a separate analysis of risk factors for clustering but have been included in comparisons of demographic data.
TABLE 1.
DNA fingerprint clusters, demographics, and HIV data by study site
| Study site(s) | Median age (yr)a | No. (%) | No. HIV positive/no. tested (% positive) | ||
|---|---|---|---|---|---|
| Eligibleb | In clusters | Male | |||
| All four (n = 301) | 34 | 685 (44) | 125 (42) | 196 (65) | 109/157 (69) |
| Three used in primary analysisc(n = 243) | 35 | 387 (63) | 104 (43) | 159 (65) | 77/118 (65) |
| Kanye (n = 65) | 41 | 79 (82) | 31 (48) | 53 (82) | 11/26 (42) |
| Lobatse (n = 84) | 35 | 163 (52) | 34 (41) | 56 (67) | 16/27 (59) |
| Francistown (n = 94) | 32 | 145 (65) | 39 (41) | 50 (53) | 50/65 (77) |
| Gaborone (n = 58) | 34 | 298 (20) | 21 (36) | 36 (62) | 32/39 (82) |
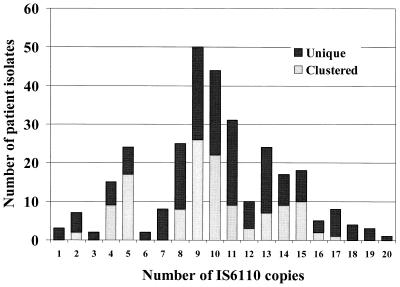
A total of 125 (42%) of the 301 participants belonged to one of 20 different clusters; each cluster was comprised of 2 to 16 patients (Fig. 1). When only the 250 fingerprints with >5 bands were included, 96 (38%) fell into a cluster. Band numbers ranged from 1 to 20; isolates had a median number of nine bands, and 51 isolates (17%) for which DNA fingerprints were obtained had ≤5 bands. Of the 125 patients in clusters, second interviews were conducted with 87 (70%); the remaining 38 patients either had died or could not be located for a second interview.
FIG. 1.
Distribution of IS_6110_ copies in M. tuberculosis DNA fingerprints among 301 interviewed patients with unique and clustered isolates in Botswana.
The proportion of patients whose isolates clustered did not differ significantly between the different sites, although the rate of clustering was somewhat lower in Gaborone than in the other areas (Table 1). Among the 243 patients with DNA fingerprints at the other three sites, 104 (43%) had clustered isolates. The median patient age was 34 years (range, 17 to 90 years; two patients age 17 years were erroneously enrolled despite study criteria and were included in the analysis). Patients from the two smaller towns, Kanye and Lobatse, were more likely to be older, male, and HIV negative than patients from Gaborone and Francistown. One hundred fifty-seven patients (52%) agreed to HIV testing, of whom 109 (69%) were HIV positive. Eighty-one patients (27%) had TSTs placed and read, of whom 29 (36%) had a TST result of ≥10 mm and 2 (3%) had a TST result of between 5 and 9 mm; the remaining 50 patients (62%) had a TST result of less than 5 mm. The mean TST indurations among 58 HIV-positive and 11 HIV-negative patients who had TSTs read were 4 and 11 mm, respectively (P < 0.001). The proportions of these patients overall and at each site with these factors did not change when only those patients whose DNA fingerprints contained >5 bands were evaluated.
Table 2 lists potential risk factors for clustering among patients in Kanye, Lobatse, and Francistown with both clustered and nonclustered isolates. One hundred four (43%) of 243 patients enrolled at these three sites had isolates that fell into one of 20 clusters. Neither gender nor age was identified as a risk factor for clustering. Of the 118 patients from the three sites who underwent HIV testing, 35 (63%) of 56 patients with clustered isolates and 42 (68%) of 62 patients with unique isolates were HIV positive (P = 0.6); HIV positivity was not associated with clustering even after stratifying by study site. Patients with a previous history of imprisonment were more likely to be in a cluster (relative risk [RR], 1.5; 95% CI, 1.1 to 2.0; P = 0.04). However, no other factors reached statistical significance in their association with clustering in these three study sites, including prior hospital work, hospitalization, mine work, history of TB, or potential exposure to TB patients in other congregate settings. The same risk factors were evaluated for all 301 patients with DNA fingerprints (including those from Gaborone); the only risk factor that approached statistical significance was previous imprisonment (RR, 1.3; 95% CI, 1.0 to 1.9; P = 0.06). Finally, the same set of risk factors for clustering was evaluated for each site individually. Among patients from Gaborone, regular church attendance was associated with clustering (RR, 3.7; 95% CI, 1.6 to 8.7; P = 0.001), as were female sex (RR, 2.0; 95% CI, 1.1 to 5.0; P = 0.02) and reported history of prior TB contact (RR, 1.9; 95% CI, 1.0 to 3.8; P = 0.06). Among patients from Kanye, regular church attendance was associated with a lower risk of clustering (RR, 0.6; 95% CI, 0.4 to 1.0; P = 0.05). In Lobatse, prior mine work was associated with clustering (RR, 2.0; 95% CI, 1.2 to 3.4; P = 0.02), and prior imprisonment (RR, 1.7; 95% CI, 1.0 to 2.9; P = 0.09) approached statistical significance. No risk factors were associated with clustering in Francistown. When only the 250 patients with DNA fingerprints yielding >5 bands were included in a separate analysis of these same risk factors, none of the results listed in Table 2 changed significantly except of the association between prior imprisonment and clustering, which was no longer significant (RR, 1.3; 95% CI, 0.9 to 2.0).
TABLE 2.
Potential risk factors for clustering among patients with clustered and unique M. tuberculosis DNA fingerprints at three study sites in Botswanaa
| Potential risk factor for clustering | No. (%) with risk factor | RR (95% CI) | ||
|---|---|---|---|---|
| Total (n = 243) | Among patients with clustered isolates (n = 104) | Among patients with unique isolates (n = 139) | ||
| Age of <35 yr | 121 (50) | 53 (51) | 68 (49) | 1.1 (0.8–1.4) |
| Male | 160 (66) | 71 (68) | 89 (64) | 1.2 (0.8–1.5) |
| HIV positiveb | 77 (65) | 35 (63) | 42 (68) | 0.9 (0.6–1.3) |
| Previous imprisonmentc | 38 (16) | 22 (21) | 16 (12) | 1.5 (1.1–2.0) |
| History of contact with TB patient | 122 (50) | 51 (49) | 71 (51) | 1.0 (0.7–1.3) |
| Previous employment as miner | 61 (25) | 28 (27) | 33 (24) | 1.1 (0.8–1.5) |
| Previous work in a hospital | 7 (3) | 3 (3) | 4 (3) | 1.0 (0.4–2.4) |
| Previous hospitalization | 153 (63) | 65 (63) | 88 (63) | 1.0 (0.7–1.3) |
| Previous Botswana Defense Force work | 4 (2) | 2 (2) | 2 (1) | 1.2 (0.4–3.2) |
| Previous dormitory stay (any) | 29 (12) | 11 (11) | 18 (13) | 0.9 (0.5–1.4) |
| Regular bar attendance | 149 (61) | 59 (57) | 90 (65) | 0.8 (0.6–1.1) |
| Regular church attendance | 121 (50) | 49 (47) | 72 (52) | 0.9 (0.7–1.2) |
| Previous travel outside Botswana of >1 mo | 29 (12) | 11 (11) | 18 (13) | 0.9 (0.5–1.4) |
| History of previous TB | 32 (13) | 16 (15) | 16 (12) | 1.2 (0.8–1.8) |
| Presence of Mycobacterium bovis BCG scar | 123 (53) | 52 (53) | 71 (53) | 1.0 (0.7–1.3) |
Primary and secondary interviews were carefully reviewed for all members within each cluster to determine whether epidemiologic links could be established among the 104 patients in Kanye, Lobatse, and Francistown whose isolates fell into the 20 identified clusters. Patients from Gaborone were once again omitted from this analysis since only a small fraction of eligible TB patients were enrolled at this site. A strong epidemiologic connection could be found for 26 (25%) of the 104 clustered patients in 9 of the 20 clusters. A strong epidemiologic connection was found in 19 (24%) of the 80 clustered patients in these three sites whose DNA fingerprints had >5 bands, a proportion similar to that for patients with any band number. The median number of members in clusters that contained patients with strong epidemiologic links did not differ significantly from the median number in clusters that did not contain patients with strong links (9 and 10 members, respectively). Eighteen pairs were identified among the 26 patients. In four of the nine clusters, epidemiologic links were identified between two patients (total of four pairs), an additional two clusters included three patients in whom epidemiologic links could be established among all three (total of six pairs), and the remaining four clusters contained three patients each, but in these instances two of the patients could be linked with the third patient but not with each other (total of eight pairs). Five pairs spent time on farms close to one another at overlapping time periods, all between 1995 and 1997 (three of these five pairs arose from a group of three patients who all shared the same DNA fingerprint and were all on nearby farms at the same time). Three pairs with the same DNA fingerprint shared the same workplace at the same time (from 1993 to 1997, 1992 to 1997, and 1993 to 1997); all three of these patients were at the same workplace. One pair frequented the same bar during the same period of time (most recently in 1997). Three pairs had all been imprisoned at the same prison: one pair in 1994, one pair from 1995 through 1998, and one pair in 1997. Members of three other pairs worked at the same South African mines at the same time as the other member of the pair with whom they shared the same M. tuberculosis DNA fingerprint (although each of the three pairs worked at a different mine). One of these pairs worked at the same mine in 1976; one pair did so from 1977 through 1987; and one pair did so from 1979 through 1980. Members of two pairs of patients had been previously hospitalized in the same hospital at the same time as the other member of the pair, one pair in 1994 and one pair in 1997 (with one pair hospitalized on the same ward). One mother-daughter pair who lived together between 1945 and 1997 shared the same DNA fingerprint, and they volunteered one another's names when asked about known previous or current TB contacts.
When only clustered patients with DNA fingerprints containing >5 bands were evaluated, 13 pairs among the 19 patients with a strong epidemiologic link were identified (compared with 18 pairs among the 26 patients, as described above). All five members of the three pairs mentioned above who spent overlapping time in the same jail had isolates with identical DNA identical fingerprints that had five bands. An additional two patients who had been hospitalized at the same time and place had identical DNA fingerprints with four bands.
DISCUSSION
Few studies performed in developing countries have combined conventional and molecular epidemiology. Our study, conducted in a country with very high TB and HIV prevalences, found that 42% of adult patients with AFB smear-positive pulmonary TB had isolates that clustered. This is similar to the results of Wilkinson et al. (28) in South Africa, who found that 45% of isolates from smear-positive pulmonary TB patients in one health district were in clusters. Most studies from developed countries suggest that clustered isolates represent recent transmission and have found that 30 to 60% of isolates from city-based studies fall into clusters. The rate of clustering detected in this study is somewhat higher than the 33% rate found in a primarily rural U.S. state, Arkansas (8). It is, however, somewhat surprising that we did not find a higher rate of clustering in Botswana, a country with high TB incidence rates. This may in part be due to the fact that the population in Botswana is highly mobile, moving frequently between villages, towns, and farms; patients whose isolates may have fallen into clusters and who may have been identified as epidemiologic links could thus have developed active TB outside of the study sites. It may also be because 37% of the eligible patients in the three study areas on which the analysis is focused either were not interviewed or did not have a DNA fingerprint; this could potentially lead to underestimation of recent transmission if epidemiologic links were missed due to exclusion of these patients. It is also possible that reactivation TB indeed accounts for approximately half of TB disease in Botswana, a country in which the majority of persons are likely to have been infected with TB by the time they reach adulthood. Our study also provides evidence, however, that ongoing transmission is occurring in congregate settings. As Glynn et al. (15) have highlighted, it is important to consider DNA fingerprinting studies in the context of factors such as the proportion of TB patients in a given population whose isolates are included in the study, case and cluster definitions, geographical area, time window, patient age, TB incidence, HIV infection, and frequency of immigration, etc., and to exercise caution in describing TB patients with isolates in clusters as having been recently infected with TB.
With regard to risk factors for clustering, we found that prior imprisonment was a risk factor when all sites were analyzed together, as has been previously observed in the United States (8), although this association disappeared when only patients whose DNA fingerprints contained >5 bands were included in the analysis. HIV infection was not associated with clustering in this study. This could be because the number of HIV-negative patients was too small to detect a difference. Regular attendance at church, previous mine work, reported prior TB contact, and female sex were associated with clustering at some of the individual sites.
We found that 25% of clustered patients overall and 24% of clustered patients with DNA fingerprints containing >5 bands had strong evidence of prior connection with at least one other cluster member; overall, this group represented only 11% of patients at the three sites included in the analysis, and a recent connection was evident for only 7% of patients. Our results for clustered patients are similar to those of Wilkinson et al. (28), who found an epidemiologic link between 27% of clustered patients; to those of Braden et al. (8), who detected evidence of an epidemiologic connection between 42% of patients in clusters; and to those of Frieden et al. (13), who found that 32% of clustered patients had epidemiologic links. (However, caution is required in making such comparisons, because definitions of epidemiologic links varied across these studies.) Although it is possible that clustering of isolates is due to the random presence of endemic M. tuberculosis strains, and some of the epidemiologic connections in our study could be due to chance, it is unlikely that 25% would have such strong connections due to chance alone. The lack of identification of strong epidemiologic links for 75% of clustered patients may be due to reasons already mentioned for the low rate of clustering overall, namely, a highly mobile population in Botswana and the fact that 37% of eligible patients at the three primary study sites could not be included in the study. Furthermore, identification of epidemiologic links relied upon detailed recall of prior life events by study participants. Finally, the presence of clustering does not by necessity mean recent transmission (15).
The results from interviews suggest that jails, mines, hospitals, workplaces, bars, and farms may be sites of casual TB transmission in Botswana. Only one pair of family members was found among those whose isolates clustered, suggesting that much of the M. tuberculosis transmission between adults in Botswana is occurring in jails, hospitals, and community social settings.
Our study is limited by the fact that 37% of eligible patients either were not interviewed or did not have a DNA fingerprint at the three sites for which detailed epidemiologic results are presented; we are thus likely to have underestimated the degree of recent M. tuberculosis transmission and to have missed avenues of transmission in the community. Indeed, the proportion of patients with clustered isolates was highest (48%) in Kanye, the site where the largest proportion of eligible patients was enrolled, and it was the lowest (36%) in Gaborone, the site where the fewest eligible patients were enrolled. This study was also limited in that secondary typing on DNA fingerprints with up to five bands was not performed; however, isolates from only 17% of patients had fewer than five IS_6110_ copies, and of the 250 patients whose DNA fingerprints had more than five bands, 97 (38%) clustered, a proportion that does not differ from the 43% clustering found when DNA fingerprints with ≤5 bands are included (P = 0.7). Furthermore, analyses among all patients as well as among only those with DNA fingerprints containing >5 copies have been presented here.
This study confirms that DNA fingerprinting combined with patient interview, while labor-intensive and not routinely practicable for TB control programs, describes novel sites of M. tuberculosis transmission that may be difficult to ascertain by regular contact tracing, and it supports the hypothesis that DNA fingerprint clustering in communities with high TB and HIV incidence is often indicative of person-to-person spread of M. tuberculosis in the community. Increased attention to case finding among prison inmates and mine workers for identification and treatment of their TB is warranted, as is prevention of M. tuberculosis transmission in hospital settings, wherever possible. A focus on early identification and treatment of smear-positive TB patients remains of great importance. Our data also suggest that, even in countries with high rates of TB and HIV, the majority of TB disease may be due to the reactivation of latent infection, as the majority of patients in this study did not have clustered isolates or identifiable epidemiologic links. Preventive therapy of latent M. tuberculosis infection remains an important potential individual and population TB control strategy that is not routinely used in developing countries, and it should be seriously considered for implementation, particularly among persons living with HIV.
ACKNOWLEDGMENTS
This work was supported by the Division of Tuberculosis Elimination, Centers for Disease Control and Prevention, Atlanta, Ga.
We thank Thandiwe Mavunga and Mothusi Bareetseng for their assistance with data management (The BOTUSA Project, Gaborone, Botswana); Patricia Chilesi and Ally Kombe for their assistance with mycobacteriology in Francistown (Microbiology Laboratory, Nyangabgwe Hospital, Francistown, Botswana); Kelebetse Thakadu, Collinah Jikijela, and Felix Matseka for mycobacteriology in Gaborone (National Tuberculosis Reference Laboratory, Gaborone, Botswana); and Beverly Metchock and Jack Crawford for their assistance with obtaining the M. tuberculosis DNA fingerprints (Division of AIDS, STD and Tuberculosis Laboratory Research, National Center for Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, Ga.).
REFERENCES
- 1.AIDS/STD Unit, Botswana Ministry of Health. Sentinel surveillance report 1999. Gaborone, Botswana: AIDS/STD Unit, Ministry of Health; 1999. [Google Scholar]
- 2.Alland D, Kalkut G E, Moss A R, McAdam R A, Hahn J A, Bosworth W, Drucker E, Bloom B R. Transmission of tuberculosis in New York City. N Engl J Med. 1994;330:1710–1716. doi: 10.1056/NEJM199406163302403. [DOI] [PubMed] [Google Scholar]
- 3.Barnes P F, Yang Z, Preston-Martin S, Pogoda J M, Jones B E, Otaya M, Eisenbach K D, Knowles L, Harvey S, Cave M D. Patterns of tuberculosis transmission in central Los Angeles. JAMA. 1997;278:1159–1163. [PubMed] [Google Scholar]
- 4.Beck-Sague C, Dooley S W, Hutton M D, Otten J, Breeden A, Crawford J T, Pitchenik A E, Woodley C, Cauthen G, Jarvis W R. Hospital outbreak of multidrug-resistant Mycobacterium tuberculosis infections: factors in transmission to staff and HIV-infected patients. JAMA. 1992;268:1280–1286. doi: 10.1001/jama.1992.03490100078031. [DOI] [PubMed] [Google Scholar]
- 5.Bishai W R, Graham N M H, Harrington S, Pope D S, Hooper N, Astemborski J, Sheely L, Vlahov D, Glass G E, Chaisson R E. Molecular and geographic patterns of tuberculosis transmission after 15 years of directly observed therapy. JAMA. 1998;280:1679–1684. doi: 10.1001/jama.280.19.1679. [DOI] [PubMed] [Google Scholar]
- 6.Botswana National Tuberculosis Program. Annual report. Gaborone, Botswana: Epidemiology Section, Ministry of Health; 1989. [Google Scholar]
- 7.Botswana National Tuberculosis Program. Annual report. Gaborone, Botswana: Epidemiology Section, Ministry of Health; 1999. [Google Scholar]
- 8.Braden C R, Templeton G L, Cave M D, Valway S, Onorato I M, Castro K G, Moers D, Yang Z, Stead W W, Bates J H. Interpretation of restriction fragment length polymorphism analysis of Mycobacterium tuberculosis isolates from a state with a large rural population. J Infect Dis. 1997;175:1446–1452. doi: 10.1086/516478. [DOI] [PubMed] [Google Scholar]
- 9.Cohn D L, O'Brien R F. The use of restriction fragment length polymorphism analysis for epidemiological studies of tuberculosis in developing countries. Int J Tuberc Lung Dis. 1998;2:16–26. [PubMed] [Google Scholar]
- 10.Daley C L, Small P M, Schecter G F, Schoolnik G K, McAdam R A, Jacobs W R, Hopewell P C. An outbreak of tuberculosis with accelerated progression among persons infected with the human immunodeficiency virus. N Engl J Med. 1992;326:231–235. doi: 10.1056/NEJM199201233260404. [DOI] [PubMed] [Google Scholar]
- 11.Dean A G, Dean J A, Coulombier D, Burton A H, Brendel K A, Smith D C, Dicker R C, Sullivan K M, Fagan R F. Epi Info, version 6.02: a word processing, database, and statistics program for public health on IBM-compatible microcomputers. Altanta, Ga: Centers for Disease Control and Prevention; 1995. [Google Scholar]
- 12.Edlin B R, Tokars J I, Grieco M H, Crawford J T, Williams J, Sordillo E M, Ong K R, Kilburn J O, Dudley S W, Castro K G. An outbreak of multidrug-resistant tuberculosis among hospitalized patients with the acquired immunodeficiency syndrome. N Engl J Med. 1992;326:1514–1521. doi: 10.1056/NEJM199206043262302. [DOI] [PubMed] [Google Scholar]
- 13.Frieden T R, Woodley C L, Crawford J T, Lew D, Dooley S M. The molecular epidemiology of tuberculosis in New York City: the importance of nosocomial transmission and laboratory error. Tuberc Lung Dis. 1996;77:407–413. doi: 10.1016/s0962-8479(96)90112-4. [DOI] [PubMed] [Google Scholar]
- 14.Gillespie S H, Kennedy N, Ngowi F I, Fomukong N G, al-Maamary S, Dale J W. Restriction fragment length polymorphism analysis of Mycobacterium tuberculosis isolated from patients with pulmonary tuberculosis in northern Tanzania. Trans R Soc Trop Med Hyg. 1995;89:335–338. doi: 10.1016/0035-9203(95)90571-5. [DOI] [PubMed] [Google Scholar]
- 15.Glynn J R, Bauer J, de Boer A S, Borgdorff M W, Fine P E, Godfrey-Faussett P, Vynnycky E. Interpreting DNA fingerprint clusters of Mycobacterium tuberculosis. European Concerted Action on Molecular Epidemiology and Control of Tuberculosis. Int J Tuberc Lung Dis. 1999;3:1055–1060. [PubMed] [Google Scholar]
- 16.Hermans P W, Messadi F, Guebrexabher H, Van Soolingen D, de Haas P E W, Heersma H, de Neeling H, Ayoub A, Portaels F, Frommel D, Zribi M, van Embden J D A. Analysis of the population structure of Mycobacterium tuberculosis in Ethiopia, Tunisia, and the Netherlands: usefulness of DNA typing for global tuberculosis epidemiology. J Infect Dis. 1995;171:1504–1513. doi: 10.1093/infdis/171.6.1504. [DOI] [PubMed] [Google Scholar]
- 17.Kiers A, Drost A P, van Soolingen D, Veen J. Use of DNA fingerprinting in international source case finding during a large outbreak of tuberculosis in the Netherlands. Int J Tuberc Lung Dis. 1997;1:239–245. [PubMed] [Google Scholar]
- 18.Lockman S, Sheppard J D, Mwasekaga M, Kenyon T A, Binkin N J, Braden C R, Woodley C L, Rumisha D L, Tappero J W. DNA fingerprinting of a national sample of Mycobacterium tuberculosis isolates, Botswana, 1995–1996. Int J Tuberc Lung Dis. 2000;4:1–4. [PubMed] [Google Scholar]
- 19.Ritacco V, Di Lonardo M, Reniero A, Ambroggi M, Barrera L, Dambrosi A, Lopez B, Isola N, de Kantor I N. Nosocomial spread of human immunodeficiency virus-related multidrug-resistant tuberculosis in Buenos Aires. J Infect Dis. 1997;176:637–642. doi: 10.1086/514084. [DOI] [PubMed] [Google Scholar]
- 20.Safi H, Aznar J, Palomares J C. Molecular epidemiology of Mycobacterium tuberculosis strains isolated during a 3-year period (1993 to 1995) in Seville, Spain. J Clin Microbiol. 1997;35:2472–2476. doi: 10.1128/jcm.35.10.2472-2476.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Small P M, Hopewell P C, Singh S P, Paz A, Parsonnet J, Ruston D C, Schecter G F, Daley C L, Schoolnik G K. The epidemiology of tuberculosis in San Francisco: a population-based study using conventional and molecular methods. N Engl J Med. 1994;330:1703–1709. doi: 10.1056/NEJM199406163302402. [DOI] [PubMed] [Google Scholar]
- 22.Small P M, McClenny N B, Singh S P, Schoolnik G K, Tompkins L S, Mickelsen P A. Molecular strain typing of Mycobacterium tuberculosis to confirm cross-contamination in the mycobacteriology laboratory and modification of procedures to minimize occurrence of false-positive cultures. J Clin Microbiol. 1993;31:1677–1682. doi: 10.1128/jcm.31.7.1677-1682.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabet S R, Goldbaum G M, Hooton T M, Eisenach K D, Cave M D, Nolan C M. Restriction fragment length polymorphism analysis detecting a community-based tuberculosis outbreak among persons infected with human immunodeficiency virus. J Infect Dis. 1994;169:189–192. doi: 10.1093/infdis/169.1.189. [DOI] [PubMed] [Google Scholar]
- 24.Van Deutekom H, Gerritsen J J J, van Soolingen D, van Ameijden E J C, van Embden J D A, Coutinho R A. A molecular epidemiological approach to studying the transmission of tuberculosis in Amsterdam. Clin Infect Dis. 1997;25:1071–1077. doi: 10.1086/516072. [DOI] [PubMed] [Google Scholar]
- 25.van Embden J D A, Cave M D, Crawford J T, Dale J W, Eisenach K D, Gicquel B, Hermans P, Martin C, McAdam R, Shinnick T M. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993;31:406–409. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Rie A, Warren R, Richardson M, Victor T C, Gie R P, Enarson D A, Beyers N, van Helden P D. Exogenous reinfection as a cause of recurrent tuberculosis after curative treatment. N Engl J Med. 1999;341:1174–1179. doi: 10.1056/NEJM199910143411602. [DOI] [PubMed] [Google Scholar]
- 27.Wilkinson D, Crump J, Pillay M, Sturm A W. Nosocomial transmission of tuberculosis in Africa documented by restriction fragment length polymorphism. Trans R Soc Trop Med Hyg. 1997;91:318. doi: 10.1016/s0035-9203(97)90090-0. [DOI] [PubMed] [Google Scholar]
- 28.Wilkinson D, Pillay M, Crump J, Lombard C, Davies G R, Sturm A W. Molecular epidemiology and transmission of Mycobacterium tuberculosis in rural Africa. Trop Med Int Health. 1997;2:747–753. doi: 10.1046/j.1365-3156.1997.d01-386.x. [DOI] [PubMed] [Google Scholar]
- 29.Yang Z H, Mtoni I, Chonde M, Mwasekaga M, Fuursted K, Askgard D S, Bennedsen J, de Haas P E W, van Soolingen D, van Embden J D A, Andersen A B. DNA fingperprinting and phenotyping of Mycobacterium tuberculosis isolates from human immunodeficiency virus (HIV)-seropositive and HIV-seronegative patients. J Clin Microbiol. 1995;33:1064–1069. doi: 10.1128/jcm.33.5.1064-1069.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]