Continual Low-Level Activation of the Classical Complement Pathway (original) (raw)
Abstract
There is evidence that the classical complement pathway may be activated via a “C1-tickover” mechanism, analogous to the C3-tickover of the alternative pathway. We have quantitated and characterized this pathway of complement activation. Analysis of freshly collected mouse and human plasma revealed that spontaneous C3 activation rapidly occurred with the generation of C3 fragments in the plasma. By the use of complement- and Ig-deficient mice it was found that C1q, C4, C2, and plasma Ig were all required for this spontaneous C3 activation, with the alternative complement pathway further amplifying C3 fragment generation. Study of plasma from a human with C1q deficiency before and after therapeutic C1q infusion confirmed the existence of a similar pathway for complement activation in humans. Elevated levels of plasma C3 were detected in mice deficient in complement components required for activation of either the classical or alternative complement pathways, supporting the hypothesis that there is continuous complement activation and C3 consumption through both these pathways in vivo. Blood stasis was found to stimulate C3 activation by classical pathway tick-over. This antigen-independent mechanism for classical pathway activation may augment activation of the complement system at sites of inflammation and infarction.
Keywords: innate immunity, alternative complement pathway, C3 tick-over, inflammation, deficiency
Introduction
The complement system consists of >30 proteins that contribute to many aspects of inflammation and host defense, including opsonization of foreign particles, cell lysis, and regulation of cellular immune responses.
There are three pathways by which effector functions of complement can be activated, namely the classical, mannose-binding lectin, and alternative pathways. The alternative pathway is distinct from the other two pathways in that specific pathogen recognition is not required for activation. Rather, it is in a state of continuous low-level activation as a result of spontaneous hydrolysis of the thioester group in native C3 1 2. This spontaneous “C3 tick-over” leads to C3b deposition on biological particles in plasma or on cell surfaces 1 3 4. Host cell surfaces are protected from subsequent complement activation mediated by the deposited C3b by the presence of a number of membrane-associated inhibitors 5. In contrast, foreign cells lack these inhibitory molecules resulting in complement activation and amplification via the alternative pathway.
The classical and mannose-binding lectin pathways share a number of proteins and employ homologous activating complexes that recognize specific pathogen molecules to induce complement activation. Ig bound to antigen activates the classical pathway through the binding of C1q which then activate C1r and C1s. Of the Ig classes antigen-complexed IgM and IgG efficiently bind complement and are the primary mediators of activation via the classical pathway. Among the IgG subclasses in humans, IgG3 and IgG1 bind and activate C1 readily, whereas IgG2 does so poorly, and IgG4 exhibits no activity 6 7 8. In the mouse, IgG2a, IgG2b, and IgG3 all efficiently activate C1, whereas IgG1, the murine homologue of human IgG4, is a very poor activator 7 9 10 11. On the other hand, induction of the mannose-binding lectin pathway is mediated by recognition of arrays of terminal mannose groups on pathogens by mannose-binding lectin (MBL) 12 13. MBL is a C-type lectin 14, structurally similar to C1q which, on binding its ligand, activates two serine proteases, MBL-associated serine protease-1 (MASP-1) 15 16 and MASP-2 17 18, which in turn activate the classical pathway complement proteins C4 and C2.
Consumption of complement with activation of C3 is known to occur ex vivo in blood from most species. For example, mouse complement is considered labile and weak as clotting mouse blood at room temperature leads to substantial losses in hemolytic complement activity 19, and cleavage of C3 10. However, despite the widespread knowledge that C3 activation occurs in blood ex vivo, the molecular basis of this spontaneous activation of complement has not been delineated.
In this paper we demonstrate that continuous activation of complement occurs via the classical pathway in the blood and plasma of mice and humans both in vitro and in vivo. Activation of complement appears to be mediated by soluble Ig, largely IgG, and results in the generation of C3 fragments in the plasma.
Materials and Methods
Mice.
C57BL/6 and recombinase-activating gene 2–deficient mice (RAG−/−) on a C57BL/6 background were used between 6–20 wk old and were housed under specific pathogen-free conditions at the John Curtin School of Medical Research (JCSMR). C1q-knockout (C1qa−/−) 20, C4−/− 21 22, factor B knockout (Bf−/−), and H2-Bf,C2−/− mice 23 were all generated as previously described. Mice were bred onto a C57BL/6 background and maintained under specific pathogen-free conditions at the Biological Services Unit, Hammersmith Hospital, London. At the time of their use the mice were 8–12 wk old. All animal experiments reported in this paper were approved by the Australian National University Animal Experimentation Ethics Committee, and experiments performed at Hammersmith Hospital were carried out according to institutional guidelines.
Blood and Plasma Isolation.
C57BL/6, RAG−/−, C4−/−, Bf−/−, H2-Bf,C2−/−, and C1qa−/− mice were exsanguinated from the chest cavity immediately after death of the mice by CO2 administration. The blood was collected directly into the anticoagulant heparin (5 U/ml) or hirudin (50 U/ml) and used in experiments ex vivo. Plasma was then isolated from the blood by centrifugation (800 g, 5 min, 4°C) to remove cells, and stored at 4°C until use. All mouse plasma was used within 2 h of collection.
Blood was collected from normal and C1q-deficient humans into heparin (5 U/ml), hirudin (50 U/ml), or heparin and 2 mM EDTA, pH 8.0. Plasma was then isolated from the blood by centrifugation (800 g, 5 min, 4°C) to remove cells, and either stored at 4°C or in frozen aliquots (−70°C) until use. Blood was also collected, as above, from a C1q-deficient patient immediately after the therapeutic intravenous infusion of 400 ml of Octaplas (solvent/detergent treated human plasma; Octapharma Ltd.), and the plasma stored at −70°C until use.
Cell Preparation.
Spleen cell suspensions were prepared from C57BL/6 mice by gentle dissociation of the spleen between two frosted slides into PBS/0.1% BSA, followed by uptake of the cell suspension once into a syringe through a 26 g needle. Cells were then pelleted (500 g, 5 min), before resuspension in Tris/NH4Cl (17 mM Tris, 140 mM NH4Cl, pH 7.2) to lyse erythrocytes. The cell suspension was then incubated at room temperature for 5 min before washing the cells twice in PBS/0.1% BSA (500 g, 5 min), and finally the cells were resuspended in PBS/0.1% BSA at 2.5 × 106 cells per ml and stored on ice.
Raji cells were provided by Hilary Warren (Canberra Hospital, Canberra, Australia) and cultured in RPMI 1640 supplemented with 10% FCS and antibiotics (120 mg/liter penicillin; 200 mg/liter streptomycin; and 200 mg/liter neomycin). For the in vitro complement assays the Raji cells were harvested and resuspended in PBS/0.1% BSA at 2.5 × 106 cells per ml and stored on ice until use.
Ex Vivo Assay of Complement Activation.
In some experiments, after collection into heparin (5 U/ml) or hirudin (50 U/ml) to prevent blood clotting, the blood was immediately aliquoted into 100-μl volumes and incubated at 37°C for up to 60 min. At different time points tubes were removed from the water bath, 2 ml of 500 mM EDTA, pH 8.0, added (10 mM final concentration), and the samples placed on ice. To induce immune complex (IC) formation 20 mg/ml rabbit anti–mouse Ig (Dako) was added to blood aliquots before incubation. Control blood was collected directly into EDTA (10 mM). Blood samples were then centrifuged (800 g, 5 min) to collect the plasma.
Complement activity was restored in frozen EDTA-containing human plasma samples (i.e., plasma containing 5 U/ml heparin, 2 mM EDTA) by the addition of exogenous Ca2+ (3 mM final concentration). Aliquots of the recalcified plasma were then incubated for 0, 5, 15, or 30 min at 37°C before the addition of 10 mM EDTA. Variations of this basic assay involved the addition of exogenous purified human C1q (20 μg/ml final concentration; Sigma-Aldrich) or Octaplas (final C1q concentration ∼20 μg/ml; Octapharma Ltd.) to recalcified plasma before incubation at 37°C.
Immunofluorescence Flow Cytometry for Plasma C3 Fragments.
Individual plasma samples (20 μl) were added to either 5 × 104 splenic lymphocytes (for mouse plasma) or 5 × 104 Raji cells (for human plasma) in 96-well v-bottomed plates (Costar) and incubated on ice for 30 min. Plates were then washed three times with PBS/0.1% BSA (300 g, 2 min, 4°C) before incubating the cells for 30 min on ice with C3-specific antibodies. In the case of mouse plasma samples, the splenic lymphocytes were incubated with a polyclonal goat anti–mouse C3-FITC antibody (Cappel; ICN Pharmaceuticals), with the splenic B cells being identified by a monoclonal anti–mouse B220-PE antibody (BD PharMingen). For human plasma samples, Raji cells were incubated with either a polyclonal goat anti–human C3-FITC antibody (Cappel; ICN Pharmaceuticals) or a polyclonal rabbit anti–human C3d-FITC antibody (Dako). Cells were finally washed three times with PBS/0.1% BSA (300 g, 2 min, 4°C) before performing two-color flow cytometry using a FACS® (Becton Dickinson). Data from 2 × 104 cells were analyzed using WinMDI software (Joseph Trotter, Scripps Research Institute, La Jolla, CA). In the case of mouse splenocytes, samples were analyzed by first gating on lymphocytes, based on forward and side scatter, followed by gating for B220+ B cells. The relative amount of C3 fragments on the membrane of mouse B220+ B cells or Raji cells was assessed on the basis of mean fluorescence intensity (MFI), in arbitrary units.
Immunoprecipitation and Western Blotting for Plasma C3.
Mouse plasma from ex vivo incubation experiments was diluted 1/250 in PBS (2 μl plasma per 500 μl PBS) in Eppendorf tubes and added to 10 ml of CNBr-activated Sepharose beads (Amersham Pharmacia Biotech) that had been conjugated with a polyclonal goat anti–mouse C3 antibody. The bead-plasma mixture was incubated at 4°C on a rotating wheel for 60 min, before washing of the beads three times with PBS (50 g, 20 s) to remove unbound plasma protein. The beads were then resuspended in 50 μl of reduced SDS-PAGE sample buffer, and boiled for 5 min to dislodge bound C3 fragments. C3 fragments were resolved on a 10% SDS-PAGE gel (Gradipore), transferred to a nitrocellulose filter (Bio-Rad Laboratories), and the filter was probed with a biotin-conjugated polyclonal goat anti–mouse C3 antibody followed by streptavidin-horseradish peroxidase (HRP; Amersham Pharmacia Biotech). Finally, bands were revealed by enhanced chemiluminescence (ECL; Amersham Pharmacia Biotech).
Complement Activation in RAG−/− Mouse Plasma Reconstituted with Ig.
The following antibodies were used for reconstitution experiments. Mouse myelomas of the following Ig classes and subclasses: IgG1 (MOPC-31c); IgG2aκ (UPC-10); IgG2aλ (HOPC-1); IgG2b (MOPC-141); IgG3 (FLOPC-21); IgM (TEPC 183); and IgA (TEPC 15), and mouse mAbs specific for human CD54 (IgG1, HA58), human CD158b (IgG2aκ, DX27), and rat α/β-TCR (IgG1, RT3). All these antibodies were purchased from Sigma-Aldrich. An IgG1 mAb specific for human CD32 (clone 8.7) 24, both as the whole IgG1 molecule and as a F(ab′)2 fragment was a kind donation from M. Hulett (John Curtin School of Medical Research). An IgG1 mouse mAb specific for human CD94 (WV2), and IgM mAbs specific for human CD94 (WV4), CD56 (WV5), and CD57 (WV7) were donated by H. Warren, Canberra Hospital, Canberra, Australia.
Blood was collected directly into the anticoagulant heparin (5 U/ml) and then 100-μl volumes were aliquoted into Eppendorf tubes containing the different purified Igs (50 μg/ml final concentration), as listed above. Control tubes contained PBS instead of the purified Igs, or EDTA to give a final concentration of 10 mM. All tubes were then incubated at 37°C for 10 min before addition of EDTA (10 mM final concentration). Tubes were finally centrifuged (800 g, 5 min) to separate the plasma, the plasma collected, and the amount of C3 fragments in the plasma determined by immunofluorescence flow cytometry (described previously).
Complement Activation in a Vascular Stasis Model.
Mice were injected intraperitoneally with 100 μl of heparin (20 U) in saline and 10 min later killed by CO2 administration. Immediately or at various time points after CO2 administration the mice were exsanguinated from the chest cavity and the blood collected directly into EDTA (20 mM final concentration). Plasma was then separated by centrifugation (800 g, 5 min) and the level of C3 fragments in the plasma determined by immunofluorescence flow cytometry (described previously).
Complement Levels in Mouse and Human Plasma.
C3 levels in mouse plasma were measured by rocket immuno-electrophoresis as described previously 25. Samples were electrophoresed for 20 h at 15°C in a 1% agarose L gel (Amersham Pharmacia Biotech) containing 3% polyclonal goat anti–mouse C3 antibody (Cappel; ICN Pharmaceuticals) in 0.07 M barbiturate buffer, pH 8.8. Acute phase mouse sera containing a known amount of C3 were used as a standard.
Human plasma C3 and C4 levels were measured by radial immunodiffusion using sheep anti–mouse C3 and sheep anti–mouse C4 antibodies (The Binding Site). Normal human sera containing known amounts of C3 and C4 were used as standards.
Statistical Analysis.
Data are shown as mean values ± standard deviation, unless otherwise stated. Student's t test was used for comparisons with differences considered significant when P < 0.05.
Results
C3 Fragments Accumulate to High Levels in Plasma Ex Vivo.
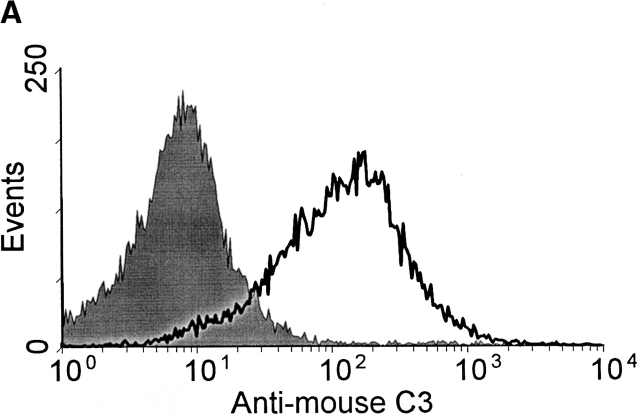
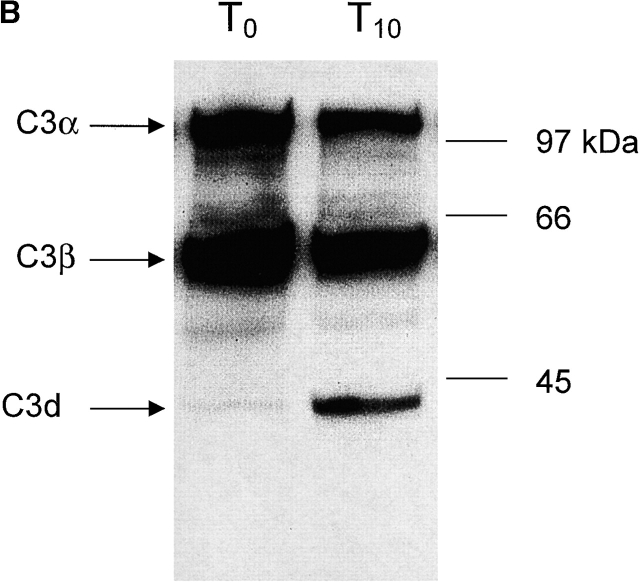
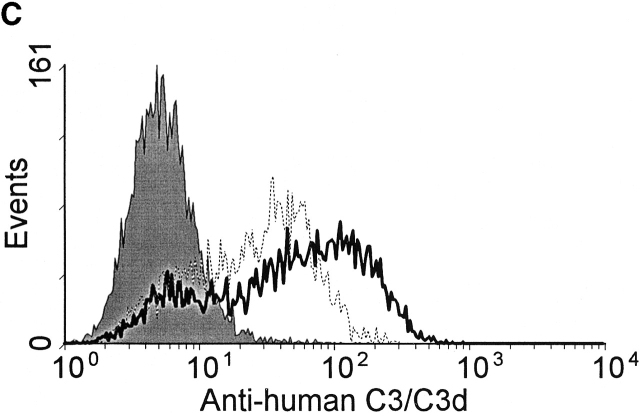
In initial experiments it was noted that complement activation, as measured by the generation of C3 fragments, rapidly occurred in heparinized mouse and human plasma incubated in vitro at 37°C. C3 fragments were detected by two methods; first by the specific binding of C3 fragments to complement receptor 1 and 2 (CR1/2) on mouse and human B cells in vitro, as detected by immunofluorescence flow cytometry (Fig. 1a and Fig. c), and second by Western blot detection of C3 fragments in mouse plasma (Fig. 1 B). Fig. 1 A shows a histogram of the binding of C3 fragments from mouse plasma incubated for 10 min ex vivo (open histogram) to B cells, with the B cell population being identified by B220 expression. Similarly, Fig. 1 C shows a histogram of the binding of C3 fragments from human plasma incubated for 30 min ex vivo (open histogram) to Raji cells, with bound C3 fragments being detected using antibodies specific for human C3 or the C3 fragment C3d. The binding of the mouse C3 fragments to B cells was dependent on complement receptors as blocking of CR1/2, with the antibody 7E9, prevented binding of the fragments (data not shown).
Figure 1.
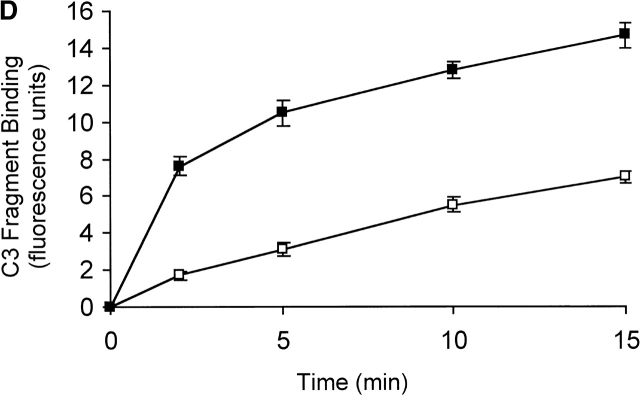
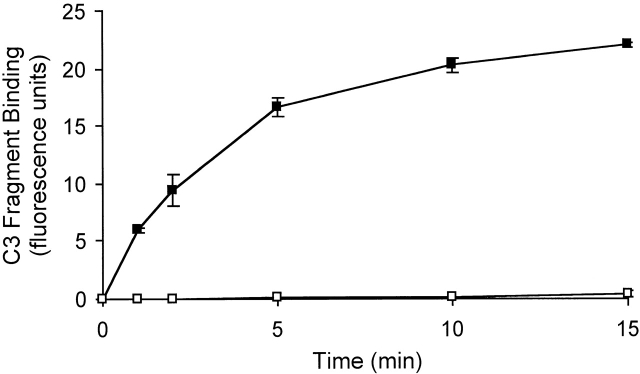
Detection of C3 fragments in mouse and human blood following incubation ex vivo at 37°C. (A) The level of C3 fragments in mouse blood collected directly into heparin and EDTA (T0) or collected into heparin and incubated at 37°C for 10 min before addition of EDTA (T10). The histograms represent the amount of C3 fragments detected on B220+ B cells following incubation of splenic lymphocytes with T0 plasma (filled histogram) or T10 plasma (open histogram), as measured by immunofluorescence flow cytometry. (B) Western blot analysis of C3 fragments in mouse plasma, as collected in A. C3 was immunoprecipitated from plasma samples with polyclonal anti–mouse C3 antibody conjugated Sepharose beads, and the Western blot was probed with biotin-labeled polyclonal anti–mouse C3 Ab and developed using streptavidin-HRP. Position of intact C3α and C3β chains and the C3dg fragments, that bind to CR1/2 on B cells (A), is indicated. (C) The level of C3 fragments in human blood collected directly into heparin and EDTA (T0) or into heparin and incubated at 37°C for 30 min before addition of EDTA (T30). The histograms represent the amount of C3 (solid line) or C3d (broken line) fragments detected on Raji cells after incubation with T0 plasma (filled histogram) or T30 plasma (open histograms), as measured by immunofluorescence flow cytometry. (D) The rate of formation of C3 fragments in blood from C57BL/6 mice collected into either heparin (□) or hirudin (▪) is depicted, as measured by the binding of C3 fragments to B220+ spleen cells using immunofluorescence flow cytometry, as shown in A. Error bars represent ± SD of means of triplicate assays.
The presence of C3 fragments in mouse plasma was confirmed by Western blot analysis of the C3 molecule (Fig. 1 B). In Fig. 1 B, lane T0, plasma was collected directly into 10 mM EDTA to prevent any complement activation ex vivo. Here, almost all of the C3 observed was in the native state with intact α and β chains accompanied by only a low level of the 40-kD C3 fragment, C3dg. In contrast, when blood was incubated for 10 min at 37°C ex vivo before addition of EDTA (Fig. 1 B, lane T10), then a large increase in the amount of C3dg fragments present was observed, a finding consistent with the flow cytometry data depicted in Fig. 1a and Fig. c.
As heparin was used in most assays, any potential involvement of heparin in spontaneous complement activation was investigated. Blood was collected from normal C57BL/6 mice or normal humans into either heparin, or an alternative anticoagulant, hirudin, which does not modulate complement activity 26. Comparable levels of C3 fragments were detected in human plasma collected in heparin or hirudin, after incubation at 37°C for up to 30 min in vitro (data not shown). In contrast, comparison of the mouse blood samples showed approximately a twofold greater level of spontaneous complement activation and C3 fragment generation in the presence of hirudin, compared with that observed in the presence of heparin (Fig. 1 D), showing that, if anything, heparin slightly inhibited the spontaneous complement activation. As a further control for any artefactual effects of the anticoagulant, blood was collected from C57BL/6 mice in the absence of heparin or any other anticoagulant, and after incubation of the blood in vitro, C3 fragments were shown to be present in the serum (data not shown). In addition, it is important to note that formation of the C3 fragments did not require the presence of RBCs or leukocytes as it occurred in plasma in the absence of any cells (data not shown).
We compared the amount of spontaneous C3 consumption that occurred in blood ex vivo with that induced by the presence of ICs. Blood was collected from C57BL/6 mice into heparin (5 U/ml) to prevent clotting in the absence or presence of 50 μg/ml of anti–mouse Ig to induce IC formation, and then incubated at 37°C for 5 min before the addition of 10 mM EDTA. In the presence of IC a very high level of C3 fragments was generated (MFI = 160) compared with those spontaneously generated (MFI = 6), demonstrating that spontaneous C3 consumption is slight compared with the level of activation induced by ICs. No C3 fragments were generated in blood from C1q-deficient mice even in the presence of ICs.
Spontaneous C3 Fragment Formation in Plasma Occurs via the Classical Complement Pathway.
To determine the complement activation pathway responsible for the spontaneous generation of C3 fragments ex vivo, the rate of formation of C3 fragments in blood from normal C57BL/6 mice was compared with that from C1qa−/−, C4−/−, Bf−/−, H2-Bf, C2−/−, and RAG−/− mice. Blood was collected from these mice into heparin (5 U/ml) to prevent clotting, and then incubated at 37°C for up to 15 min. At different time points EDTA (10 mM) was added to stop complement activity, and then the level of C3 fragments in the plasma of these samples was measured by immunofluorescence flow cytometry. By measuring the mean fluorescence of the B cells for bound C3 the relative level of C3dg fragments in the samples was quantified. Fig. 2 clearly shows that C3 fragments were rapidly generated in plasma from normal C57BL/6 mice. In contrast, there was a complete absence of C3 fragment generation in plasma from C1qa−/− mice. Table summarizes the rate of formation of C3 fragments in blood from normal, complement component–deficient, and Ig-deficient mice, and demonstrates a number of important points. First, no C3 fragment production was observed in plasma from C1qa−/−, C4−/−, and H2-Bf, C2−/− mice demonstrating that spontaneous C3 fragment generation involves activation through the classical complement pathway, and not the lectin or alternative pathways. However, in the absence of the alternative complement pathway (Bf−/− mice) a substantial reduction in the level of C3 fragments was observed (54 ± 26%), suggestive that this pathway is involved in amplification of C3 fragment generation initiated through the classical complement pathway. In addition, the major reduction of C3 fragment generation in the plasma of RAG−/− mice (16 ± 2%) suggests an important role for plasma Ig in complement activation.
Figure 2.
Spontaneous formation of C3 fragments in mouse plasma requires the classical complement pathway. The rate of formation of C3 fragments in heparin-containing plasma from C57BL/6 mice (▪), and C1qa−/− mice (□) is depicted, as measured by the binding of C3 fragments to B220+ spleen cells using immunofluorescence flow cytometry, as shown in Fig. 1 A. Error bars represent ± SD of means of triplicate assays.
Table 1.
Spontaneous Formation of C3 Fragments in Mouse Plasma Requires the Classical Complement Pathway and Plasma Ig
| In vitro incubation time | |||
|---|---|---|---|
| Mouse strain | 5 min | 10 min | 15 min |
| Normal | 100 ± 6 | 100 ± 5 | 100 ± 2 |
| Clqa−/− | 1 ± 1 | 1 ± 0 | 2 ± 1 |
| C4−/− | 0 ± 0 | 1 ± 1 | 1 ± 1 |
| Bf−/− | 30 ± 4 | 22 ± 14 | 54 ± 26 |
| H2-Bf,C2−/− | 4 ± 4 | 0 ± 0 | 0 ± 0 |
| RAG−/− | 11 ± 4 | 15 ± 2 | 16 ± 2 |
To determine if the classical complement pathway is also responsible for the spontaneous generation of C3 fragments in human blood ex vivo, the rate of formation of C3 fragments was compared in blood from normal and C1q-deficient patients. Blood was initially collected from normal and C1q-deficient humans directly into heparin (5 U/ml) and 2 mM EDTA and the plasma frozen at −70°C until use. The plasma samples were then recalcified by the addition of 3 mM Ca2+, and incubated for up to 30 min at 37°C. We have previously shown that this concentration of Ca2+ restored the spontaneous generation of C3 fragments (data not shown). As a control for these experiments spontaneous complement activation was measured in freshly collected heparinized blood from normal patients incubated for 30 min at 37°C directly ex vivo and compared with normal plasma collected in heparin and EDTA, frozen, and recalcified with 3 mM Ca2+ before incubation at 37°C. Similar levels of spontaneous complement activation were measured in both fresh and stored samples (data not shown).
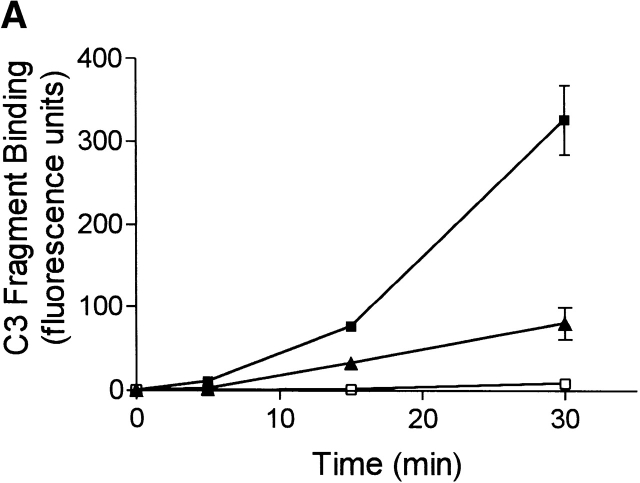
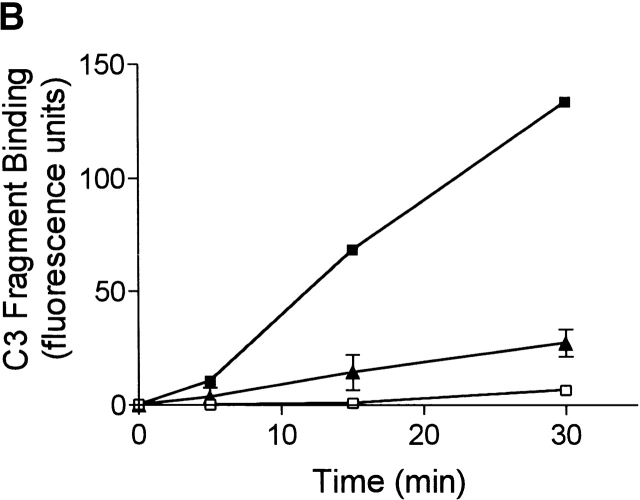
Spontaneous complement activation and generation of C3 fragments was absent in plasma from C1q-deficient patients (Fig. 3 A), as observed in C1qa−/− mice. Addition of 20 μg/ml of purified human C1q to the plasma from the C1q-deficient patients, previously shown to restore >80% classical complement pathway activity (data not shown), resulted in the restoration of the ability of the plasma to spontaneously generate C3 fragments to well above that of plasma from normal donors (Fig. 3 A). As controls, exogenous purified C1q was added to plasma from normal humans and did not enhance the level of C3 fragments generated. The plasma from C1q-deficient patients did not contain any detectable levels of anti-C1q antibodies (data not shown). In addition, blood was collected from a C1q-deficient patient who receives regular therapeutic infusions of Octaplas to temporarily replenish the plasma C1q levels (plasma C1q levels ∼20 μg/ml after infusion). In plasma collected from this C1q-deficient patient, immediately after the infusion of Octaplas, spontaneous generation of C3 fragments was completely restored and to a much higher level than that observed in normal humans (Fig. 3 B). The levels of C3 and C4 were not significantly increased in the patient's plasma after the Octaplas infusion, and the addition of Octaplas to plasma from normal humans in vitro did not enhance the level of spontaneous C3 fragment generation (data not shown).
Figure 3.
Spontaneous formation of C3 fragments in human plasma is C1q dependent. (A) Plasma from normal and C1q-deficient patients, containing heparin and EDTA, was recalcified and incubated at 37°C for up to 30 min. To some C1q-deficient plasma exogenous purified human C1q (20 μg/ml) was added before incubation. The rate of formation of C3 fragments in normal (▴, n = 8), C1q-deficient (□, n = 3), and reconstituted C1q-deficient (▪, n = 3) plasma is depicted, as measured by the binding of C3 fragments to Raji cells using immunofluorescence flow cytometry, as in Fig. 1 C. (B) The rate of formation of C3 fragments in plasma from normal individuals (▴, n = 8), and a C1q-deficient patient before (□) and immediately after (▪) infusion of Octaplas. All results are represented as means ± SD.
Exogenous Ig Activates Complement and Generates C3 Fragments in Plasma from RAG−/− Mice.
Plasma from RAG−/− mice was reconstituted with purified murine monoclonal Ig in vitro to examine more closely the ability of specific Ig isotypes to activate complement and generate C3 fragments. Table gives a complete list of the Igs tested and shows a number of important points. First, each monoclonal Ig tested exhibited a highly reproducible ability to activate C3 in RAG−/− plasma. Second, the Fc portion of IgG appeared to be required for C3 activation as, if this portion was removed from IgG1, it lost its ability to activate complement and generate C3 fragments in plasma. Third, all of the monoclonal IgGs tested were able to activate complement although there was considerable variation in activity both between isotypes and even within the isotypes tested. For example, the two IgG2aκ samples tested were both able to activate complement very efficiently compared with IgG2b. However, comparison between the IgG2aκ and IgG2aλ samples tested showed a 10–20-fold difference in the efficiency of complement activation and generation of C3 fragments. This result not only shows variation within the IgG2a isotype but also suggests that light-chain type may have an effect on the efficiency of C3 activation. Finally, all three IgM samples tested were only poor activators and one was unable to activate complement, suggesting that IgG probably plays a more important role in this phenomenon than IgM.
Table 2.
Ability of Different Mouse Igs to Induce C3 Fragment Generation in Plasma from RAG−/− Mice
| Mouse Ig isotype | Source | Clone | Specificity | Percent increase in C3 fragments generated |
|---|---|---|---|---|
| IgG1 | Hybridoma | 8.7 | Human CD32 | 69 ± 22 |
| F(ab)′2 IgG1 | Hybridoma | 8.7 | Human CD32 | 5 ± 5 |
| IgG1 | Myeloma | MPOC-31c | Unknown | 78 ± 13 |
| IgG1 | Hybridoma | WV2 | Human CD94 | 154 ± 8 |
| IgG1 | Hybridoma | R73 | Rat α/β TCR | 182 ± 14 |
| IgG1 | Hybridoma | HA58 | Human CD54 | 388 ± 32 |
| IgG2aκ | Myeloma | UPC-10 | β-2-6 fructosan | 734 ± 71 |
| IgG2aκ | Hybridoma | DX27 | Human CD158b | 315 ± 18 |
| IgG2aλ | Myeloma | HOPC-1 | Unknown | 34 ± 11 |
| IgG2b | Myeloma | MOPC-141 | Unknown | 36 ± 15 |
| IgG3 | Myeloma | FLOPC-21 | Unknown | 170 ± 8 |
| IgM | Myeloma | TEPC 183 | Unknown | 0 |
| IgM | Hybridoma | WV4 | Human CD94 | 67 ± 6 |
| IgM | Hybridoma | WV5 | Human CD56 | 82 ± 6 |
| IgM | Hybridoma | WV7 | Human CD57 | 61 ± 6 |
| IgA | Myeloma | TEPC 15 | Phosphoryl choline | 0 |
All myeloma preparations were analyzed by fast performance liquid chromatography (FPLC) to ensure that no Ig aggregates were present before use, and some preparations were also centrifuged (10 min, 20,000 g) to remove large aggregates, a procedure that was shown to have little or no effect on their ability to spontaneously activate complement (data not shown).
C3 Fragments Are Formed and Accumulate in Blood within the Circulation in a Vascular Stasis Model.
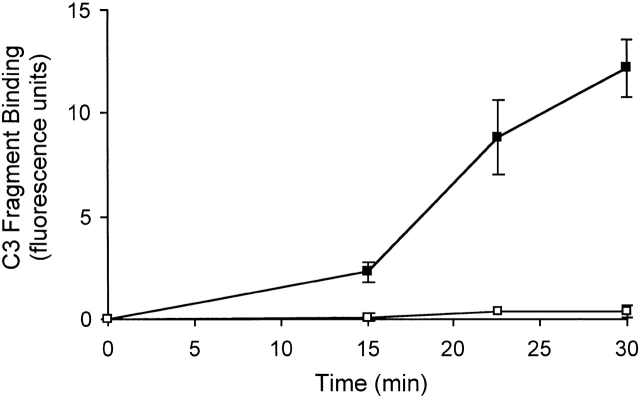
To test if C3 fragments are formed and accumulate in blood within the circulation as observed in vitro, a model of vascular stasis was established. Heparin was initially injected into mice to prevent clotting of blood in the vasculature, and then the mice were killed by CO2 administration. Such a procedure effectively creates stasis of the blood within the vasculature of the mice. At different time points after the death of the mice blood was collected directly into EDTA (10 mM) to prevent any further C3 fragments being generated ex vivo. As shown in Fig. 4, C3 fragments were generated in the blood of C57BL/6 mice within the circulation, beginning to appear 15 min after CO2 administration and then rapidly accumulating over the next 15 min, an effect that was not observed in the blood of C1qa−/− mice. Only low levels of C3 fragments were detected in the blood of mice during the first 15 min after CO2 administration (data not shown). The reason for the apparent lag time in vivo before the formation of the C3 fragments is unclear but may be due to a corresponding lag time in the induction of complete blood stasis and inactivation/saturation of clearance mechanisms. Table summarizes the rate of formation of C3 fragments generated within the circulation in normal, complement component–deficient, and Ig-deficient mice after induction of vascular stasis, and shows parallel findings to those observed in plasma from these mice using the in vitro model (Table ). Spontaneous complement activation within the circulation was totally dependent on the classical complement pathway components C1q, C4, and C2, highly dependent on Ig, and partially dependent on the alternative complement pathway.
Figure 4.
Formation of C3 fragments in mouse blood in vivo after vascular stasis requires the classical complement pathway. After injection of heparin (20 U) mice were killed by CO2 exposure, blood was collected from the mice at appropriate times after death of the mice, and the plasma analyzed for C3 fragments by immunofluorescence flow cytometry, as shown in Fig. 1 A. The rate of C3 fragment formation was examined in the plasma of C57BL/6 mice (▪) and C1qa−/− mice (□). Error bars represent ± SD of mean of triplicate assays. Data are representative of two separate experiments.
Table 3.
Formation of C3 Fragments in Blood after Vascular Stasis Requires the Classical Complement Pathway and Plasma Ig
| Vascular stasis time | ||
|---|---|---|
| Mouse strain | 15 min | 30 min |
| Normal | 100 ± 22 | 100 ± 11 |
| Clqa−/− | 5 ± 2 | 4 ± 2 |
| C4−/− | 0 ± 0 | 0 ± 0 |
| Bf−/− | 46 ± 33 | 19 ± 2 |
| H2-Bf,C2−/− | 0 ± 0 | 0 ± 0 |
| RAG−/− | 13 ± 2 | 4 ± 1 |
Elevated C3 and C4 Levels in Mice and Humans with Complement Deficiencies.
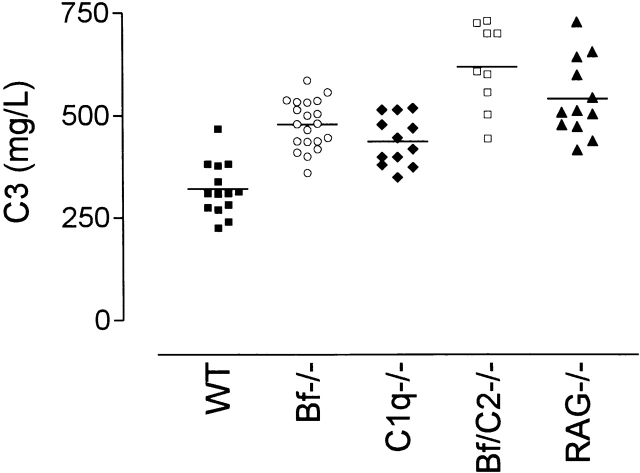
C3 has been shown to be continually consumed by the alternative complement pathway due to a low level of spontaneous hydrolysis of the native C3 molecule 1 2 that initiates formation of the alternative pathway C3 convertase. Consistent with this phenomenon, mice deficient in the alternative complement pathway (Bf−/−) had elevated levels of plasma C3 in comparison with wild-type C57BL/6 mice (Fig. 5 A, P = 0.0001). Surprisingly, elevated plasma C3 levels were also detected in mice deficient in C1q (C1qa−/−) and plasma Ig (RAG−/−) compared with normal C57BL/6 mice (Fig. 5 A, P = 0.001, P < 0.0001, respectively). The highest levels of plasma C3 were detected when mice were deficient in both the classical and alternative complement pathways (H2-Bf,C2−/−) in comparison with mice deficient in C1q (P = 0.0001) or factor B alone (P = 0.0001). Although the absence of Ig in the RAG−/− mice may explain the elevated C3 levels in these mice, it is also possible that other factors, including underlying infections, may also contribute to the raised C3 levels.
Figure 5.
Elevated plasma C3 levels in mice deficient in molecules essential for activation of both the classical and alternative complement pathways. Blood was collected from mice directly into EDTA and the level of plasma C3 determined by rocket immunoelectrophoresis. The plasma C3 levels from individual male C57BL/6 (▪, n = 14), Bf−/− (○, n = 20), C1qa−/− (♦, n = 12), H2-Bf,C2−/− (□, n = 9), and RAG−/− (▴, n = 12) mice is depicted, with horizontal bars denoting means. Similar trends in C3 levels were observed in female mice (data not shown).
In humans, plasma C3 levels were not elevated in C1q-deficient patients (median 0.91, range 0.84–1.34 g/liter) compared with normal individuals (median 0.97, range 0.84–1.27 g/liter), but plasma C4 levels were significantly elevated (P = 0.008) in the C1q-deficient patients (median 0.48, range 0.45–0.52 g/liter) compared with normal individuals (median 0.28, range 0.16–0.35 g/liter), consistent with previously published data 27 28.
Discussion
Activation of complement can occur via three pathways: the alternative, mannose-binding lectin, and classical. The alternative pathway has been shown to be continually undergoing a low level of spontaneous activation 1, whereas the classical and lectin pathways have been thought to require specific pathogen recognition for activation 29. Lachmann and colleagues first hypothesized that, analogous to the “C3-tickover” of the alternative pathway, the classical pathway may also be activated via a C1-tickover mechanism 30. When they added oxidized C2, a form that allows the stable formation of the classical pathway C3 convertase C4oxyC2, to normal human serum spontaneous cleavage of C3 was observed. Our findings confirm the existence of this pathway and show that C3 is continually activated through the classical complement pathway in the plasma of mice and humans.
Our initial observations demonstrated that, immediately after isolation of blood from mice and humans, C3d fragments accumulated to high levels (Fig. 1). Complement activation and formation of C3 fragments occurred via the classical pathway, as shown in Fig. 2 and Table , with no spontaneous C3 fragment generation in plasma deficient in C1q (C1qa−/− mice), C4 (C4−/− mice), or plasma Ig (RAG−/− mice). Furthermore, addition of exogenous Ig to the plasma from RAG−/− mice restored spontaneous complement activation and the generation of C3 fragments to similar levels to those found in the plasma of normal C57BL/6 mice (Table ), demonstrating the crucial role of Ig and the classical complement pathway in C3 fragment generation. Although the initial activation of complement occurred through the classical pathway, the alternative pathway was also required as isolated deficiency in factor B (Bf−/− mice) was associated with a decrease in the generation of C3 fragments (Table ). Presumably, C3 fragments generated through spontaneous activation of the classical pathway act to initiate alternative pathway C3 convertase formation, resulting in additional C3 consumption.
In humans, spontaneous complement activation and formation of C3 fragments also occurred via the classical pathway, as shown in Fig. 3 A by the absence of C3 fragment generation in plasma from C1q-deficient patients. The absence of spontaneous complement activation was solely due to a classical pathway deficiency as reconstitution of plasma from C1q-deficient patients, both in vitro by addition of purified C1q and in vivo by the infusion of Octaplas, was sufficient to restore the spontaneous generation of C3 fragments to levels above those observed in plasma from normal individuals (Fig. 3 B). The higher level of spontaneous complement activation observed, in comparison to normal donors, could have two explanations. The first is due to the increased levels of complement proteins in the pathway downstream to the deficient protein, allowing greater complement activation on restoration of the missing protein. The second explanation is that there may be elevated levels of circulating IC in the C1q-deficient individuals caused by abnormalities of IC processing known to occur in patients with hypocomplementaemia 31.
It should be noted that, due to the use of heparin as the anticoagulant in this study, the rate of spontaneous C3 fragment generation in mice, but not in humans, may have been underestimated, as demonstrated by the enhanced levels of C3 fragments generated in mouse plasma in the presence of hirudin. Such a result is not surprising as heparin is known to be anticomplementary 32 33 34 35.
In addition to observing the spontaneous formation of C3 fragments in plasma directly ex vivo, the fragments also formed within the circulation (Fig. 4, Table ). C3 fragments rapidly accumulated to high levels in the blood of C57BL/6 mice killed by CO2 administration (Fig. 4) after an initial 15-min lag phase, which presumably was required for establishment of complete blood stasis and inactivation/saturation of clearance mechanisms for C3 fragments. This activation of complement was also dependent on the classical pathway and the presence of Ig, with the alternative pathway being required for normal levels of spontaneous C3 fragment generation (Table ), as observed in the ex vivo situation.
C3 is continually consumed through the “tick-over” activation of the alternative complement pathway 1 2, therefore the absence of this pathway in factor B–deficient mice (Bf−/−) accounts for the elevated plasma C3 levels detected in these mice (Fig. 5). The observation that C1qa−/− and Ig-deficient (RAG−/−) mice also had elevated plasma C3 levels (Fig. 5) suggests that under normal conditions C3 is additionally being consumed through the classical pathway, a hypothesis consistent with the in vitro and in vivo data. In addition, even higher levels of plasma C3 were detected in mice deficient in both the classical and alternative pathways (H2-Bf,C2−/− mice) suggesting independent mechanisms of C3 consumption through the two activation pathways.
In humans, activation of complement in blood ex vivo is thought, at least in part, to be a consequence of complement activation by natural cold-reacting autoantibodies (agglutinins) 36 37. However, the presence of cold agglutinins in blood is unable to explain the complement activation observed in our system. In the ex vivo assay employed the human and mouse blood was incubated at 37°C immediately after collection and at no stage, before the addition of EDTA, was it cooled to allow the autoantibodies to bind to erythrocytes and activate complement. In addition, most cold agglutinins characterized are of the IgM isotype, but IgG rather than IgM appears to be the dominant Ig isotype that induced spontaneous complement activation in these experiments.
It has previously been reported that in mice the classical complement pathway is activated most effectively by IgM after binding of antigen, with IgG2a, IgG2b, and IgG3 also being good activators, whereas IgG1 is a very poor activator of complement 10 11. An intriguing aspect of this study is that there was a discrepancy between the ability of the different Ig isotypes to spontaneously activate C3 fragment generation, and their known ability to activate the classical complement pathway after antigen binding. For example, based on RAG−/− plasma reconstitution experiments (Table ), IgG appears to be a better spontaneous activator of complement than IgM. In addition, IgG1 antibodies induced spontaneous activation of complement, an isotype believed to be a poor activator of complement via the classical pathway. The explanations for these findings is not known. Possibly, secondary mediators may be involved that bind to and regulate the ability of antibodies to spontaneously activate complement. A potential candidate protein is histidine-rich glycoprotein (HRG) which has been shown to bind to the hinge region of some Igs, particularly IgGs, and also to bind C1q 38. Interestingly, the light-chain type (κ or λ) of an antibody also affected its ability to spontaneously activate complement (Table ), and the affinity of HRG for IgG is also dramatically affected by whether the IgG contains κ or λ light chains 39.
In addition to isotype variation, individual mAbs of each isotype varied considerably in their ability to spontaneously activate complement. This point was most evident in comparisons between different IgG1 and IgG2a mAbs (Table ) which ranged from the poorest through to the best spontaneous activators of C3 fragment formation. As antibodies of the same isotype only differ from each other in two aspects, namely the antigen-specific variable region and the level and type of glycosylation, these properties must either individually or in combination account for the observed variations. Neuberger and Rajewsky 11 also observed some differences between individual monoclonal IgG2 and IgM antibodies in their ability to fix complement and could partially explain this discrepancy as being due to the variable region and thus the affinity of the antibodies for specific antigen, in this case NP (4-hydroxy-3-nitrophenyl)acetyl. In our system it is possible that the antibodies tested were recognizing self-antigens in plasma, and that variations in the affinity of the different mAbs for specific plasma autoantigens may account for some of the variations in the observed ability of the antibodies to spontaneously activate complement.
Two possible mechanisms could account for the spontaneous complement activation induced by plasma Ig. First, a continual low level of circulating IC may be present in plasma. This is consistent with the observation that plasma from C1q-deficient patients, that is known to contain high levels of circulating IC, exhibited much higher levels of spontaneous complement activation than normal individuals when reconstituted with C1q. However, as discussed earlier, the ability of different Ig isotypes to spontaneously activate complement varies from their known ability to activate the classical complement pathway when bound within IC. Thus, an alternative explanation is that Ig-dependent complement activation may occur in plasma in the absence of specific antigen, via the continual spontaneous formation of unstable Ig aggregates, a process enhanced by linker proteins like HRG. Such a view is supported by the fact that Fc–Fc interactions have been shown to spontaneously occur between IgG molecules in IC 40 and the well known ability of the Fc portion of Ig to aggregate and form crystals 41. This aggregation may be encouraged or initiated by vascular stasis. Complement activation would therefore be augmented at sites of inflammation at which there may be thrombosis and vascular stasis, leading to Ig aggregate formation, providing a favorable environment for the activation of complement.
In conclusion, these findings indicate that C3 is consumed through the spontaneous activation of the classical complement pathway. This may represent an analogous mechanism to the continual tick-over of C3 via the alternative complement pathway. It may facilitate pathogen recognition by allowing the deposition of activated C3 fragments on foreign surfaces at sites of vascular stasis 1 2 3 4. An important corollary of this mechanism for spontaneous activation of the classical pathway, however, is that C3 fragments might accumulate after vascular stasis. This could contribute deleteriously to tissue injury in tissue infarction in which complement has been shown to play an important role 42 43.
Acknowledgments
We are grateful to Dr. Mark Hulett for the 8.7 mouse mAbs and its F(ab′)2 fragments, and to Dr. Hilary Warren for the WV2, WV4, WV5, and WV7 mouse mAbs. Thanks also to Sabine Gruninger and Geoff Osborne from the John Curtin School of Medical Research Flow Cytometry Unit for their technical assistance.
This work was supported by Wellcome Trust Biomedical Research Collaboration grant 063106 and Wellcome Trust Programme grant 054838.
Footnotes
Abbreviations used in this paper: HRG, histidine-rich glycoprotein; IC, immune complex; MBL, mannose-binding lectin; MFI, mean fluorescence intensity; RAG, recombinase-activating gene.
References
- Lachmann P.J., Hughes-Jones N.C. Initiation of complement activation. Springer Semin. Immunopathol. 1984;7:143–162. doi: 10.1007/BF01893018. [DOI] [PubMed] [Google Scholar]
- Pangburn M.K., Muller-Eberhard H.J. Relation of putative thioester bond in C3 to activation of the alternative pathway and the binding of C3b to biological targets of complement. J. Exp. Med. 1980;152:1102–1114. doi: 10.1084/jem.152.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M.K., Morrison D.C., Schreiber R.D., Muller-Eberhard H.J. Activation of the alternative complement pathwayrecognition of surface structures on activators by bound C3b. J. Immunol. 1980;124:977–982. [PubMed] [Google Scholar]
- Law S.K., Dodds A.W. C3, C4 and C5the thioester site. Biochem. Soc. Trans. 1990;18:1155–1159. doi: 10.1042/bst0181155. [DOI] [PubMed] [Google Scholar]
- Liszewski M.K., Farries T.C., Lublin D.M., Rooney I.A., Atkinson J.P. Control of the complement system. Adv. Immunol. 1996;61:201–283. doi: 10.1016/s0065-2776(08)60868-8. [DOI] [PubMed] [Google Scholar]
- Cooper N.R. The classical complement pathwayactivation and regulation of the first complement component. Adv. Immunol. 1985;37:151–216. doi: 10.1016/s0065-2776(08)60340-5. [DOI] [PubMed] [Google Scholar]
- Dangl J.L., Wensel T.G., Morrison S.L., Stryer L., Herzenberg L.A., Oi V.T. Segmental flexibility and complement fixation of genetically engineered chimeric human, rabbit and mouse antibodies. EMBO J. 1988;7:1989–1994. doi: 10.1002/j.1460-2075.1988.tb03037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenman D.E., Dorrington K.J., Painter R.H. The structure and function of immunoglobulin domains. II. The importance of interchain disulfide bonds and the possible role of molecular flexibility in the interaction between immunoglobulin G and complement. J. Immunol. 1975;114:1726–1729. [PubMed] [Google Scholar]
- Ey P.L., Russell-Jones G.J., Jenkin C.R. Isotypes of mouse IgG - I. Evidence for “non-complement-fixing” IgG1 antibodies and characterization of their capacity to interfere with IgG2 sensitization of target red blood cells for lysis by complement. Mol. Immunol. 1980;17:699–710. doi: 10.1016/0161-5890(80)90139-x. [DOI] [PubMed] [Google Scholar]
- Klaus G.G., Pepys M.B., Kitajima K., Askonas B.A. Activation of mouse complement by different classes of mouse antibody. Immunology. 1979;38:687–695. [PMC free article] [PubMed] [Google Scholar]
- Neuberger M.S., Rajewsky K. Activation of mouse complement by monoclonal mouse antibodies. Eur. J. Immunol. 1981;11:1012–1016. doi: 10.1002/eji.1830111212. [DOI] [PubMed] [Google Scholar]
- Weis W.I., Drickamer K., Hendrickson W.A. Structure of a C-type mannose-binding protein complexed with an oligosaccharide. Nature. 1992;360:127–134. doi: 10.1038/360127a0. [DOI] [PubMed] [Google Scholar]
- Turner M.W. Mannose-binding lectinthe pluripotent molecule of the innate immune system. Immunol. Today. 1996;17:532–540. doi: 10.1016/0167-5699(96)10062-1. [DOI] [PubMed] [Google Scholar]
- Holmskov U., Malhotra R., Sim R.B., Jensenius J.C. Collectinscollagenous C-type lectins of the innate immune defense system. Immunol. Today. 1994;15:67–74. doi: 10.1016/0167-5699(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Matsushita M., Fujita T. Activation of the classical complement pathway by mannose-binding protein in association with a novel C1s-like serine protease. J. Exp. Med. 1992;176:1497–1502. doi: 10.1084/jem.176.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita M., Endo Y., Fujita T. MASP1 (MBL-associated serine protease 1) Immunobiology. 1998;199:340–347. doi: 10.1016/S0171-2985(98)80038-7. [DOI] [PubMed] [Google Scholar]
- Thiel S., Vorup-Jensen T., Stover C.M., Schwaeble W., Laursen S.B., Poulsen K., Willis A.C., Eggleton P., Hansen S., Holmskov U. A second serine protease associated with mannan-binding lectin that activates complement. Nature. 1997;386:506–510. doi: 10.1038/386506a0. [DOI] [PubMed] [Google Scholar]
- Vorup-Jensen T., Jensenius J.C., Thiel S. MASP-2, the C3 convertase generating protease of the MBLectin complement activating pathway. Immunobiology. 1998;199:348–357. doi: 10.1016/S0171-2985(98)80039-9. [DOI] [PubMed] [Google Scholar]
- Borsos T., Cooper M. On the hemolytic activity of mouse complement. Proc. Soc. Exp. Biol. Med. 1961;107:227–237. [Google Scholar]
- Botto M., Dell'Agnola C., Bygrave A.E., Thompson E.M., Cook H.T., Petry F., Loos M., Pandolfi P.P., Walport M.J. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Genet. 1998;19:56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- Fischer M.B., Ma M., Goerg S., Zhou X., Xia J., Finco O., Han S., Kelsoe G., Howard R.G., Rothstein T.L., Kremmer E., Rosen F.S., Carroll M.C. Regulation of the B cell response to T-dependent antigens by classical pathway complement. J. Immunol. 1996;157:549–556. [PubMed] [Google Scholar]
- Wessels M.R., Butko P., Ma M., Warren H.B., Lage A.L., Carroll M.C. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc. Natl. Acad. Sci. USA. 1995;92:11490–11494. doi: 10.1073/pnas.92.25.11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P.R., Nash J.T., Theodoridis E., Bygrave A.E., Walport M.J., Botto M. A targeted disruption of the murine complement factor B gene resulting in loss of expression of three genes in close proximity, factor B, C2, and D17H6S45. J. Biol. Chem. 1998;273:1699–1704. doi: 10.1074/jbc.273.3.1699. [DOI] [PubMed] [Google Scholar]
- Ierino F.L., Hulett M.D., McKenzie I.F., Hogarth P.M. Mapping epitopes of human Fc gamma RII (CDw32) with monoclonal antibodies and recombinant receptors. J. Immunol. 1993;150:1794–1803. [PubMed] [Google Scholar]
- Pepys M.B., Dash A.C., Fielder A.H., Mirjah D.D. Isolation and study of murine C3. Immunology. 1977;33:491–499. [PMC free article] [PubMed] [Google Scholar]
- Fure E., Bergseth G., Brekke O.L., Mollnes T.E. Hirudin is an ideal anticoagulant for complement studies in a whole blood inflammatory model Immunopharmacology. 49 2000. 70(Abstr. 202) [Google Scholar]
- Pickering M.C., Botto M., Taylor P.R., Lachmann P.J., Walport M.J. Systemic lupus erythematosus, complement deficiency, and apoptosis. Adv. Immunol. 2000;76:227–324. doi: 10.1016/s0065-2776(01)76021-x. [DOI] [PubMed] [Google Scholar]
- Walport M.J., Davies K.A., Morley B.J., Botto M. Complement deficiency and autoimmunity. Ann. NY. Acad. Sci. 1997;815:267–281. doi: 10.1111/j.1749-6632.1997.tb52069.x. [DOI] [PubMed] [Google Scholar]
- Sim R.B., Reid K.B. C1molecular interactions with activating systems. Immunol. Today. 1991;12:307–311. doi: 10.1016/0167-5699(91)90004-D. [DOI] [PubMed] [Google Scholar]
- Mak L.W., Lachmann P.J., Majewski J. The activation of the C3b feedback cycle with human complement components. I. Through the classical pathway. Clin. Exp. Immunol. 1977;30:200–210. [PMC free article] [PubMed] [Google Scholar]
- Nash J.T., Taylor P.R., Botto M., Norsworthy P.J., Davies K.A., Walport M.J. Immune complex processing in C1q-deficient mice. Clin. Exp. Immunol. 2001;123:196–202. doi: 10.1046/j.1365-2249.2001.01459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeda S., Rosenberg R.D., Bing D.H. The binding properties of human complement component C1q. Interaction with mucopolysaccharides. J. Biol. Chem. 1983;258:785–791. [PubMed] [Google Scholar]
- Caughman G.B., Boackle R.J., Vesely J. A postulated mechanism for heparin's potentiation of C1 inhibitor function. Mol. Immunol. 1982;19:287–295. doi: 10.1016/0161-5890(82)90342-x. [DOI] [PubMed] [Google Scholar]
- Loos M., Volanakis J.E., Stroud R.M. Mode of interaction of different polyanions with the first (C1), the second (C2) and the fourth (C4) component of complement-II. Effect of polyanions on the binding of C2 to EAC4b. Immunochemistry. 1976;13:257–261. doi: 10.1016/0019-2791(76)90224-x. [DOI] [PubMed] [Google Scholar]
- Raepple E., Hill H.U., Loos M. Mode of interaction of different polyanions with the first (C1), the second (C2) and the fourth (C4) component of complement-I. Effect on fluid phase C1 and on C1 bound to EA or to EAC4. Immunochemistry. 1976;13:251–255. doi: 10.1016/0019-2791(76)90223-8. [DOI] [PubMed] [Google Scholar]
- Pruzanski W., Shumak K.H. Biologic activity of cold-reacting autoantibodies (second of two parts) New Engl. J. Med. 1977;297:583–589. doi: 10.1056/NEJM197709152971105. [DOI] [PubMed] [Google Scholar]
- Pruzanski W., Shumak K.H. Biologic activity of cold-reacting autoantibodies (first of two parts) New Engl. J. Med. 1977;297:538–542. doi: 10.1056/NEJM197709082971005. [DOI] [PubMed] [Google Scholar]
- Gorgani N.N., Parish C.R., Easterbrook Smith S.B., Altin J.G. Histidine-rich glycoprotein binds to human IgG and C1q and inhibits the formation of insoluble immune complexes. Biochemistry. 1997;36:6653–6662. doi: 10.1021/bi962573n. [DOI] [PubMed] [Google Scholar]
- Gorgani N.N., Parish C.R., Altin J.G. Differential binding of histidine-rich glycoprotein (HRG) to human IgG subclasses and IgG molecules containing κ and λ light chains. J. Biol. Chem. 1999;274:29633–29640. doi: 10.1074/jbc.274.42.29633. [DOI] [PubMed] [Google Scholar]
- Easterbrook-Smith S.B., Vandenberg R.J., Alden J.R. The role of Fc:Fc interactions in insoluble immune complex formation and complement activation. Mol. Immunol. 1988;25:1331–1337. doi: 10.1016/0161-5890(88)90048-x. [DOI] [PubMed] [Google Scholar]
- Porter R.R. Separation and isolation of fractions of rabbit γ-globulin containing the antibody and antigenic combining sites. Nature. 1958;182:670–671. doi: 10.1038/182670a0. [DOI] [PubMed] [Google Scholar]
- Griselli M., Herbert J., Hutchinson W.L., Taylor K.M., Sohail M., Krausz T., Pepys M.B. C-reactive protein and complement are important mediators of tissue damage in acute myocardial infarction. J. Exp. Med. 1999;190:1733–1740. doi: 10.1084/jem.190.12.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrand W.K., Niessen H.W., Wolbink G.J., Jaspars L.H., Visser C.A., Verheugt F.W., Meijer C.J., Hack C.E. C-reactive protein colocalizes with complement in human hearts during acute myocardial infarction. Circulation. 1997;95:97–103. doi: 10.1161/01.cir.95.1.97. [DOI] [PubMed] [Google Scholar]