Immunization of Macaques with Formalin-Inactivated Respiratory Syncytial Virus (RSV) Induces Interleukin-13-Associated Hypersensitivity to Subsequent RSV Infection (original) (raw)
Abstract
Respiratory syncytial virus (RSV) is a major cause of severe respiratory disease in infants and the elderly. RSV vaccine development has been hampered by results of clinical trials in the 1960s, when formalin-inactivated whole-RSV preparations adjuvated with alum (FI-RSV) were found to predispose infants for enhanced disease following subsequent natural RSV infection. We have reproduced this apparently immunopathological phenomenon in infant cynomolgus macaques and identified immunological and pathological correlates. Vaccination with FI-RSV induced specific virus-neutralizing antibody responses accompanied by strong lymphoproliferative responses. The vaccine-induced RSV-specific T cells predominantly produced the Th2 cytokines interleukin-13 (IL-13) and IL-5. Intratracheal challenge with a macaque-adapted wild-type RSV 3 months after the third vaccination elicited a hypersensitivity response associated with lung eosinophilia. The challenge resulted in a rapid boosting of IL-13-producing T cells in the FI-RSV-vaccinated animals but not in the FI-measles virus-vaccinated control animals. Two out of seven FI-RSV-vaccinated animals died 12 days after RSV challenge with pulmonary hyperinflation. Surprisingly, the lungs of these two animals did not show overt inflammatory lesions. However, upon vaccination the animals had shown the strongest lymphoproliferative responses associated with the most pronounced Th2 phenotype within their group. We hypothesize that an IL-13-associated asthma-like mechanism resulted in airway hyperreactivity in these animals. This nonhuman primate model will be an important tool to assess the safety of nonreplicating candidate RSV vaccines.
Respiratory syncytial virus (RSV) is a member of the family Paramyxoviridae, genus Pneumovirus (3). While associated with relatively mild upper respiratory tract disease in immunocompetent adults, RSV infection is a major cause of severe lower respiratory tract disease in infants, immunocompromised individuals, and the elderly (7). No vaccine against this important pathogen is currently licensed (5). Three major obstacles hamper the development of a safe and effective RSV vaccine. First, the highest RSV-related morbidity and mortality are seen in infants below 6 months of age. A vaccine would therefore have to be effective in the presence of maternally derived RSV-specific virus-neutralizing (VN) antibodies and in children with a relatively immature immune system. Second, natural RSV infection does not induce sustained protective immunity. Therefore, a vaccine should ideally induce a better immune response than natural infection. Both points argue against a classical live attenuated vaccine and favor a subunit vaccine approach. However, development of nonreplicating RSV vaccines has been seriously hampered by a third complication. During vaccine trials in the 1960s, experimental formalin-inactivated whole-RSV (FI-RSV) vaccines were found to predispose infants to enhanced pulmonary disease upon subsequent RSV infection (17). Studies using murine models of RSV infection have demonstrated that alum-adjuvated FI-RSV is a strong inducer of Th2 cells, which proved to be the most important mediators of immunological hypersensitivity (23). However, histopathological characterization of FI-RSV-mediated enhanced disease by using other animal models has shown that patterns of inflammation may vary substantially (10, 33), and the relevance of the different animal models continues to be subject of debate (32).
Hypersensitivity related to vaccination with formalin-inactivated vaccines is not a unique phenomenon for RSV; it has also been reported for measles virus (MV), another member of the Paramyxoviridae family, which is not antigenically related to RSV. Vaccination with FI-MV predisposed infants for atypical measles upon natural MV infection. This syndrome was characterized by high fever, pneumonia, abnormal rash, and lymphadenopathy (9). It is interesting that FI-RSV-induced hypersensitivity was observed in a majority of vaccinees during the first RSV season after vaccination, whereas atypical measles was observed in a minority of the vaccinees and only years after FI-MV vaccination. In contrast to what was found for RSV infection, low levels of MV-specific VN antibodies confer complete protection against measles. Therefore, susceptibility to atypical measles in an FI-MV-vaccinated subject will most probably require the waning of humoral immunity in the presence of retained MV-specific cellular immunity (8). Due to the lack of a suitable MV model for small laboratory animals, few studies to clarify the underlying mechanism of atypical measles have been performed. However, the recent reproduction of atypical measles in a rhesus macaque model pointed to a mechanism of immune complex formation and Th2-mediated eosinophilic hypersensitivity responses (28, 29). It may be speculated that similar aberrant immune responses, elicited by either FI-RSV or FI-MV, result in distinct clinical syndromes, determined by the distribution of viral antigens in tissue upon natural infection with these viruses (11).
Studies of FI-RSV-related immunopathogenesis in rodent models have contributed to our understanding of the underlying mechanisms. However, there are important differences between rodent and primate immune systems. Therefore, vaccination-and-challenge models for nonhuman primates are important for additional mechanistic studies, as well as for the evaluation of the efficacy and safety of candidate RSV vaccines. Several monkey species have been shown to be susceptible to RSV infection (1, 35, 41). Studies focusing on the histopathology of FI-RSV-related enhanced pulmonary disease in African green monkeys (Cercopithecus aethiops) (16) and in bonnet monkeys (Macaca radiata) (30) have yielded largely conflicting data.
We have previously developed a measles vaccination-and-challenge model for cynomolgus macaques (Macaca fascicularis), which was used for preclinical testing of the safety and efficacy of candidate measles vaccines (36, 38, 39). The availability of macaque-specific immunological reagents allows detailed studies of the virus-specific immune response in these animals. Here, we have used the same species to reproduce FI-RSV-mediated enhanced disease, with FI-MV-immunized animals as a control group. We characterized the RSV-specific humoral and cellular immune responses upon vaccination and challenge to identify correlates of immunopathology.
MATERIALS AND METHODS
Vaccine preparations.
RSV (Long strain) was grown on HEp-2 cells and harvested at an early stage of the cytopathic effect. Supernatant was centrifuged (15 min, 1,500 × g) to remove cellular debris, and subsequently the virus was pelleted by ultracentrifugation (Beckman; SW28; 4 h at 25,000 rpm). The pellet was resuspended in TEN buffer (20 mM Tris-HCl, 1 mM EDTA, 0.15 M NaCl [pH 7.8]). MV antigen (Edmonston strain; produced on Vero cells) was kindly provided by R. S. van Binnendijk (Bilthoven, The Netherlands). Both virus preparations were dialyzed against TEN buffer, adjusted to a protein concentration of 1 mg/ml, and inactivated by addition of 37% formalin (1:4,000; 3 days at 37°C). The final preparations, which did not contain infectious virus as shown by cell culture, were adjusted to a protein concentration of 0.2 mg/ml and frozen in aliquots at −70°C.
A vaccine dose was prepared by mixing FI-RSV or FI-MV (10 μg of total protein) with 1 ml of Adju-Phos (kindly provided by Superfos Biosector, Frederikssund, Denmark) and incubating the mixture for 30 min at room temperature. The vaccine was administered intramuscularly in the gluteal muscle in two 0.5-ml doses divided over the two legs.
Study design.
In a first experiment, eight infant cynomolgus macaques were vaccinated with either FI-RSV (animals RS1 to -4) or FI-MV (animals MV1 to -4). All animals received three vaccinations with 4-week intervals. EDTA-blood samples were collected every 2 weeks. Approximately 3 months after the third vaccination, all animals were challenged with RSV. Subsequently, five more infant cynomolgus macaques were vaccinated by using the same materials and experimental design; three received FI-RSV (animals RS5 to -7) and two received FI-MV (animals MV5 and -6). During this last experiment, one animal of each group was sacrificed for histopathological analysis at days 7 (animals RS5 and MV5) and 13 (animals RS7 and MV6) after challenge.
All animals were between 5 and 12 months of age at the moment of first vaccination, with the exception of MV1, which was 19 months old. During the vaccination periods the animals were housed in groups with their mothers. During the challenge period the animals were housed in smaller standard cages (three or four animals per cage). The animal study was approved by the Local Animal Ethics Committee of the Erasmus MC, Rotterdam, The Netherlands, and was carried out in accordance with animal experimentation guidelines.
Virus neutralization.
Virus neutralization assays were carried out as previously described (31, 36). Briefly, twofold serial dilutions of heat-inactivated (30 min, 56°C) macaque EDTA-plasma samples were tested for their ability to neutralize 100 50% tissue culture infectious doses (TCID50) of RSV (Long strain) or MV (Edmonston strain). The VN titer was defined as the reciprocal of the plasma dilution at which 50% of the wells showed cytopathic effect, as calculated from triplicate measurements by the method of Reed and Muench (34).
Lymphoproliferation.
For production of antigens for in vitro stimulations, RSV (Long strain) and MV (Edmonston strain) were grown in Vero cells. After clarification the virus was pelleted by ultracentrifugation and resuspended in RPMI 1640 medium and inactivated with β-propiolactone (BPL; Sigma-Aldrich, St. Louis, Mo.). A cell control antigen was produced by processing freeze-thawed Vero cells in an identical manner.
Macaque peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation and resuspended in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum and 1% heat-inactivated pooled macaque serum. PBMC were cultured in 96-well round-bottom plates (2 × 105 cells in 200 μl per well) in the presence of BPL-RSV, BPL-MV, or BPL-Vero antigen at previously determined optimal concentrations. Each lymphoproliferation assay was carried out in duplicate, and the resulting culture supernatants (125 μl per well) were harvested after 3 and 5 days, respectively, and frozen. The supernatant was replaced with 75 μl of culture medium, 3H-labeled thymidine (0.5 μCi per well) was added, and cell-associated radioactivity was measured the following day.
Cytokine ELISAs.
Macaque cytokines (interleukin-2 [IL-2], IL-4, IL-5, IL-10, IL-13, and gamma interferon [IFN-γ]) were measured in culture supernatants by macaque-specific enzyme-linked immunosorbent assay (ELISA) systems (U-Cytech, Utrecht, The Netherlands) in accordance with the manufacturer's instructions. IL-2 and IFN-γ were measured in supernatants harvested after 3 days of culture, while IL-4, IL-5, IL-10, and IL-13 were measured in supernatants harvested after 5 days of culture.
Challenge virus.
A non-tissue-culture-adapted wild-type RSV A strain (nasal lavage of an infant hospitalized with RSV in 1996) was passaged in an RSV-seronegative cynomolgus macaque. Virus could be reisolated from throat swab samples between days 4 and 9 after infection and from a bronchoalveolar lavage (BAL) sample collected 7 days after challenge, but viral titers remained low. In an attempt to achieve higher titers, a second macaque was first immunocompromised by intramuscular administration of rabbit anti-human thymocyte globulin (ATG; National Institute of Public Health and the Environment, Bilthoven, The Netherlands; 80 mg/kg of body weight). Two days after ATG administration, almost all lymphocytes had been depleted from peripheral blood, as measured by a routine hematology analyzer (Sysmex; Myco Instrumentation Source, Renton, Wash.). At this point the animal was inoculated with a positive throat swab sample and a BAL sample from the first animal (intranasally and intratracheally, respectively). The total challenge dose as estimated from isolations on HEp-2 cells was 2 × 103 TCID50. ATG administration was continued at days 0, 2, and 6 after infection. RSV could be reisolated over a prolonged period of time (until day 21 after infection), but virus titers still remained relatively low. Pooled throat swab samples of this animal collected 6, 9, and 12 days after infection were used to inoculate another ATG-treated cynomolgus macaque with virtually identical results. Virus could be reisolated until day 26 after infection, but titers remained below 104 TCID50 per ml. An isolate from BAL cells of the third monkey was passaged three times in vitro in tertiary cynomolgus macaque kidney cells and subsequently aliquotted and stored at −135°C. The titer of this challenge stock as measured on HEp-2 cells was 2.4 × 106 TCID50 per ml. The challenge virus stock (as well as the FI-RSV and FI-MV preparations) was checked by reverse transcription PCR (RT-PCR) for the presence of coronavirus, enterovirus, human metapneumovirus, influenza A virus, influenza B virus, and rhinovirus genomic sequences, which could not be demonstrated.
Challenge.
The RSV challenge virus was thawed, sonicated in a cup sonicator for 3 min, and diluted in phosphate-buffered saline (PBS; 1 in 7.5). Subsequently, 3 ml (approximately 106 TCID50) was inoculated intratracheally, just below the larynx. EDTA-blood samples were collected at days 0, 6, 13, 17, and 22 after challenge for collection of plasma and PBMC. BAL samples were collected 6 days before challenge and at days 3, 6, 9, and 13 after challenge. Chest X-ray photographs were made at days 0, 3, 6, and 9 after challenge, and animals RS1 and RS2 were given chest X rays at day 12 after challenge.
BALs.
BAL samples were collected by intratracheal infusion and subsequent recovery of 10 ml of PBS with a flexible catheter (Cordis). BAL samples were pelleted, resuspended in PBS, and counted. For RT-PCR, 5 × 105 BAL cells were frozen as a dry pellet at −70°C. Cytospin slides were later stained (May-Grünwald-Giemsa), and differential cell counts were obtained by light microscopy, with 1,000 cells per slide counted.
Semiquantitative RT-PCR.
RNA from 5 × 105 BAL cells was isolated with the High Pure viral RNA kit (Roche Diagnostics, Almere, The Netherlands). Tenfold dilutions (100 to 10−4) were prepared in RNase-free water, and RT-PCR was performed using a single-tube reaction. The forward primer was 5′-TTAACCAGCAAAGTGTTA-3′ (RSV fusion protein gene positions 567 to 584), and the reverse primer was 5′-TTTGTTATAGGCATATCATTG-3′ (complementary to positions 808 to 788). PCR products were hybridized with a 32P-labeled oligonucleotide probe (5′-GACTACTAGAGATTACCAGGG-3′; F gene positions 697 to 711). A positive RT-PCR was defined as an amplified fragment of the correct size (243 bp) which hybridized with the specific probe. Results are shown as the highest RNA dilution at which the RT-PCR was still found positive.
Pathology.
Necropsy samples were stored in 10% neutral-buffered formalin (lungs after inflation with formalin), embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin. For quantification of eosinophils in different parts of the respiratory tract, cells in 10 arbitrarily chosen high power fields (40× objective) were counted.
Statistical analysis.
Data were analyzed by using a mixed-model analysis of variance after log transformation of the dependent variable (see Fig. 4) or by using the Wilcoxon matched-pair signed-rank test (see Fig. 2 and 5).
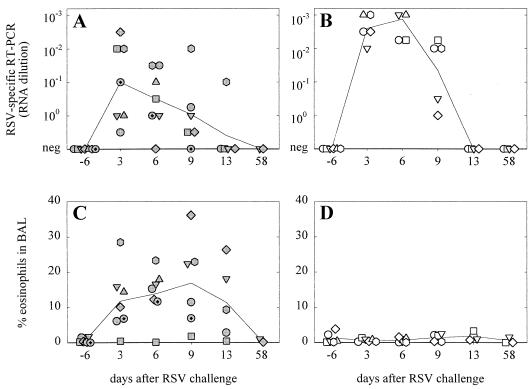
FIG. 4.
Semiquantitative RSV-specific RT-PCR signals (A and B) and percentages of eosinophils (C and D) in BAL samples collected at different time points after RSV challenge. Symbols represent the measurements of the individual animals (the correlation between symbols and animals is the same as that for Fig. 1), while lines connect the geometric means of the groups over time. Both parameters were significantly different between the two groups (mixed analysis of variance; P < 0.01).
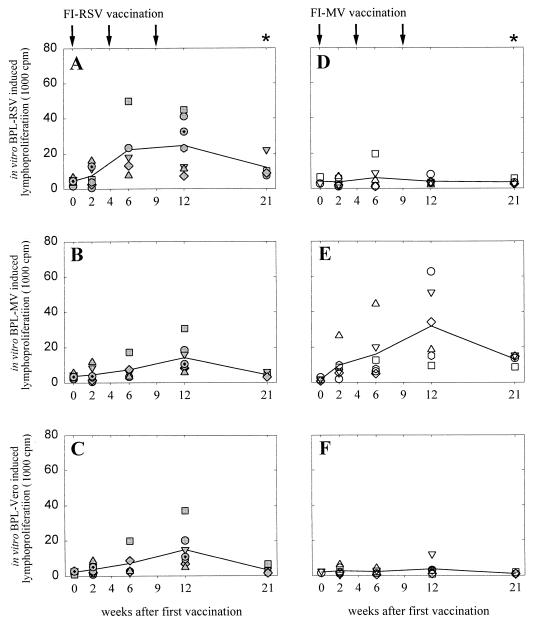
FIG. 2.
Proliferative PBMC responses of macaques vaccinated with FI-RSV (A to C) or FI-MV (D to F) after in vitro stimulation with BPL-RSV (A and D), BPL-MV (B and E), or BPL-Vero antigen (C and F). Arrows, times of vaccination; asterisks, times of RSV challenge. Symbols represent the means of the individual animals (the correlation between symbols and animals is the same as that for Fig. 1), while lines connect the geometric means of the groups over time.
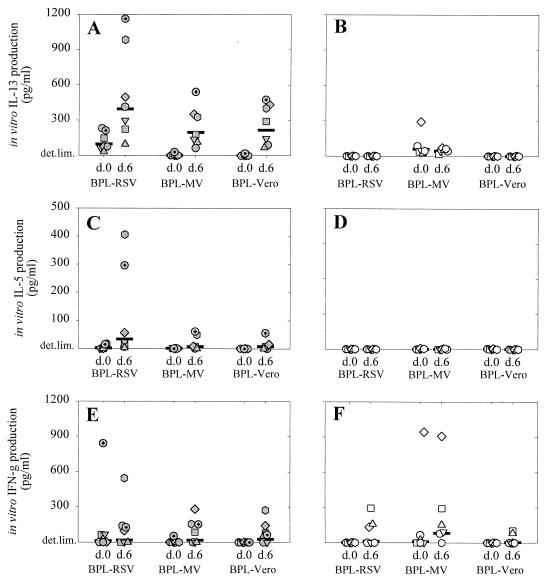
FIG. 5.
IL-13 (A and B), IL-5 (C and D), and IFN-γ (E and F) levels in culture supernatants of PBMC collected from macaques vaccinated with FI-RSV (A, C, and E) or FI-MV (B, D, and F) at days 0 and 6 after RSV challenge (indicated on the x axes). PBMC were stimulated in vitro with BPL-RSV, BPL-MV, or BPL-Vero antigen as indicated. Symbols represent the measurements of the individual animals (the correlation between symbols and animals is the same as that for Fig. 1), while horizontal lines represent the geometric mean values of the groups.
RESULTS
Virus-specific immune responses after vaccination.
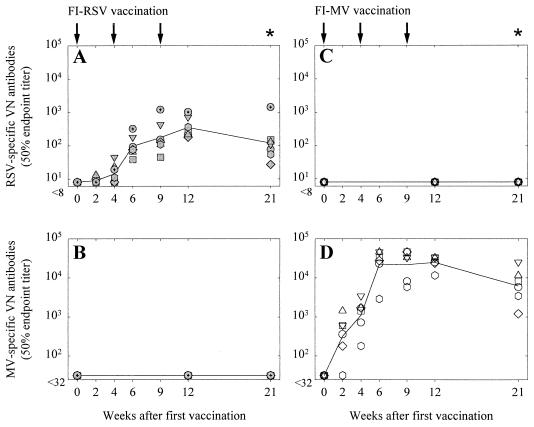
Infant macaques were immunized three times with FI-RSV (n = 7) or as a control with FI-MV (n = 6) formulated in alum. Virus-specific VN antibody responses in both groups were measured, and peak titers in FI-RSV-vaccinated animals (range, 102 to 103) were lower than those in FI-MV-vaccinated animals (range, 104 to 105; Fig. 1A and D, respectively). In most FI-RSV-vaccinated animals peak titers were reached after the third vaccination, while the highest titers induced by FI-MV were reached after the second vaccination. No cross-reactive VN antibody responses in the FI-RSV- or FI-MV-vaccinated animals were observed (Fig. 1B and C, respectively). In the FI-RSV-vaccinated animals more than in the FI-MV-vaccinated animals, HEp-2 cell- or Vero antigen-specific plasma antibodies were detected by ELISA (results not shown).
FIG. 1.
RSV-specific (A and C) and MV-specific (B and D) VN antibody responses measured in plasma samples of macaques vaccinated with FI-RSV (A and B) or FI-MV (C and D). Arrows, times of vaccination; asterisks, times of RSV challenge. Symbols represent the titers in the individual animals, while lines connect the geometric means of the groups over time. Animals RS1 to RS6 and MV1 to MV6 are represented shaded and open circles, squares, triangles, diamonds, and hexagons, respectively. Animal RS7 is represented by a shaded circle with a plus.
PBMC of macaques vaccinated with FI-RSV demonstrated strong proliferative responses after in vitro stimulation with BPL-inactivated RSV antigen (Fig. 2A). However, these responses were not significantly higher than those observed after stimulation with BPL-MV (Fig. 2B), BPL-Vero antigen (Fig. 2C), or medium alone (not shown). In most FI-RSV-vaccinated animals slightly higher proliferative PBMC responses were observed upon stimulation with BPL-RSV, suggesting that both RSV-specific and nonspecific responses were present. In contrast, PBMC of the FI-MV-vaccinated animals showed strong proliferative responses to stimulation with BPL-MV (Fig. 2E), which were significantly higher than those observed after stimulation with BPL-RSV (Fig. 2D) or BPL-Vero antigen (Fig. 2F; P < 0.05). In both groups the highest lymphoproliferative responses were observed after the third vaccination.
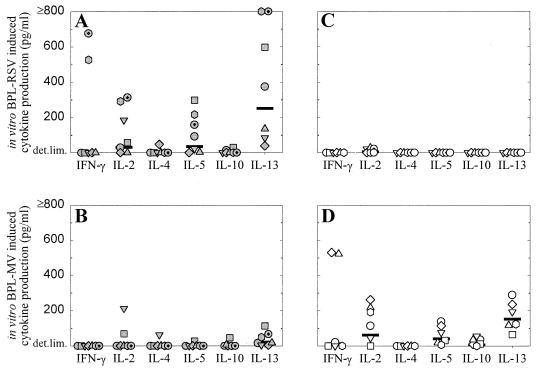
Supernatants of PBMC collected 3 weeks after the third vaccination and stimulated in vitro with BPL-RSV or BPL-MV were used to measure cytokine levels. In the FI-RSV-vaccinated animals the predominant cytokines measured upon in vitro stimulation with BPL-RSV were IL-13, IL-5, and IL-2, although PBMC of two animals also produced IFN-γ (Fig. 3A). The specificity of these responses was illustrated by the observation that cytokine levels in supernatants of the same PBMC stimulated in vitro with BPL-MV were low or undetectable (Fig. 3B). The inverse pattern was observed for the PBMC cultures of FI-MV-vaccinated animals, which showed significant IL-13, IL-5, and IL-2 responses (and IFN-γ responses in two animals) upon in vitro BPL-MV stimulation (Fig. 3D) but no detectable cytokine production upon in vitro BPL-RSV stimulation (Fig. 3C).
FIG. 3.
Cytokine levels in supernatants of PBMC collected from macaques 3 weeks after the third vaccination with FI-RSV (A and B) or FI-MV (C and D) and stimulated in vitro with BPL-RSV (A and C) or BPL-MV (B and D). The cytokines measured are indicated on the x axis, and levels are presented above the detection limits. Symbols represent the measurements of the individual animals (the correlation between symbols and animals is the same as that for Fig. 1), while horizontal lines represent the geometric mean values for the groups.
RSV challenge.
Three months after the last vaccination all animals were challenged intratracheally with 106 TCID50 of a macaque-adapted wild-type RSV strain. At that point, all FI-RSV-vaccinated animals still had RSV-specific VN antibody titers ranging from 30 to 1,500 (Fig. 1A). With our experimental setup, it proved difficult to detect clinical symptoms. Respiration frequencies were measured at each sampling point after catching, but these proved to be strongly influenced by capture-induced stress and showed no differences between the groups. Cyanosis was never observed, but abnormal breathing in some animals was found by auscultation. Oxygen saturation was measured for five animals and was found to be reduced on days 3, 6, and 9 after challenge (data not shown). Although virus isolations from throat swabs and BAL samples on HEp-2 cells were attempted, little or no RSV was reisolated (data not shown). Semiquantitative RT-PCR on BAL cells indicated that pulmonary viral loads of the FI-RSV-vaccinated animals were between 1 and 2 log units lower than those in the FI-MV-vaccinated animals (P < 0.01; Fig. 4A and B, respectively).
Hypersensitivity in FI-RSV-vaccinated macaques induced by RSV challenge.
Phenotypic analysis of BAL cells collected at different time points after RSV challenge demonstrated infiltration of eosinophils in six out of seven FI-RSV-vaccinated monkeys, compared to none of the six FI-MV-vaccinated control animals (P < 0.01; Fig. 4C and D, respectively).
This apparent hypersensitivity response was associated with a rapid boosting of the primed Th2 responses, as illustrated by the significantly increased levels of IL-13 in supernatants of in vitro-BPL-RSV-stimulated PBMC of the FI-RSV-vaccinated macaques collected on day 6 after challenge compared to those in supernatants collected on day 0 (Fig. 5A; P < 0.05). These cytokines were not detected in supernatants of similarly stimulated PBMC from the FI-MV-vaccinated macaques (Fig. 5B and D). IL-13 and to a lesser extent IL-5 were also detected in PBMC from the FI-RSV-vaccinated animals stimulated in vitro with BPL-MV and BPL-Vero antigen and collected on day 6 after challenge (Fig. 5A and C) but not in PBMC from the FI-MV-vaccinated animals (Fig. 5B and D), indicating an in vivo priming of the cells producing these Th2 cytokines in the first group of animals. No significant differences between the two groups in IFN-γ levels in the supernatants of PBMC cultures collected 6 days after challenge were found (Fig. 5E and F).
Histopathological characterization of hypersensitivity responses.
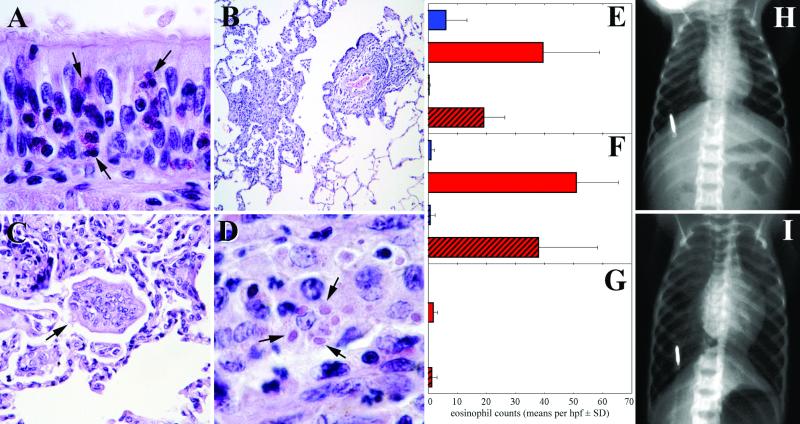
To characterize the pulmonary histopathology associated with the eosinophilia observed in BAL samples of the FI-RSV-vaccinated animals, animals of each group were sacrificed on day 7 (animals MV5 and RS5) and day 13 (animals MV6 and RS7) after challenge. In the FI-RSV-primed animals (RS5 and RS7), but not in the FI-MV-primed animals, marked eosinophilic tracheobronchitis and bronchiolitis, characterized by diffuse infiltration of mainly eosinophils and some neutrophils in the epithelium and submucosa, were found (Fig. 6A), with intercellular edema and disturbance of the histological architecture. In addition, a focal-to-diffuse eosinophilic periarteritis of the pulmonary arteries was observed. Quantification of eosinophil densities in different parts of the respiratory tract showed significantly higher numbers of eosinophils in the FI-RSV-primed animals than in the FI-MV-vaccinated animals, with the highest numbers found in the walls of bronchi and bronchioles and fewer found in the alveolar lumina (Fig. 6E to G). Irrespective of their vaccination histories, all four euthanized animals had multifocal-to-locally extensive bronchointerstitial pneumonia characterized by multifocal epithelial necrosis and the presence of macrophages and neutrophils in the alveolar lumina (Fig. 6B). In addition, multinucleated syncytial cells and intracytoplasmic inclusion bodies characteristic of RSV infection were present in all four animals (Fig. 6C and D, respectively). These lesions were less extensive and more chronic in the animals euthanized 13 days after challenge, indicative of a resolving infection.
FIG. 6.
(A) Diffuse infiltration of eosinophils (arrows) in the bronchial wall, as seen in the FI-RSV-primed monkeys euthanized at 7 or 13 days after challenge; (B to D) multifocal infiltration of inflammatory cells in the pulmonary parenchyma (B) with multinucleated syncytial cells (arrow; C) and eosinophilic intracytoplasmic inclusion bodies (arrows; D) characteristic of RSV infection, as seen in all four animals euthanized at 7 or 13 days after challenge irrespective of their vaccination history; (E to G) quantification of eosinophil numbers in bronchial (E) and bronchiolar (F) walls and in alveolar lumina (G) of FI-MV-primed (blue bars) and FI-RSV-primed (red bars) animals euthanized at 7 (solid bars) or 13 (hatched bars) days after challenge; (H and I) chest X rays of one of the FI-RSV-primed animals that died at day 12 after challenge, showing the normal image at day 9 (H) and pulmonary hyperinflation at day 12 (I). Bright vertical marks, identification chip; hpf, high-power field; SD, standard deviation.
Two FI-RSV-immunized animals (RS1 and RS2) were found in a moribund state 12 days after challenge. No infiltrates were visible by chest X-ray photography, but both animals showed a clear pulmonary hyperinflation (as shown for animal RS2 in Fig. 6H and I). One of the animals died, while the other had to be euthanized. No abnormalities were found in the lungs of these animals by necropsy and histopathological examination. However, minor lesions may have been masked due to suboptimal fixation of the tissue specimens. It is noteworthy that after vaccination these two animals had the highest vaccine-induced lymphoproliferative responses (Fig. 2A to 2C), which were of the most pronounced Th2 phenotype in this group (high IL-13 and IL-5 production in the absence of detectable IFN-γ; Fig. 3A). Average pulmonary viral loads measured by RT-PCR analysis in these two animals were not divergent (Fig. 4A), but the percentages of eosinophils in their BAL cells were lower than average (RS1) or virtually undetectable (RS2; Fig. 4C).
DISCUSSION
Infant cynomolgus macaques were immunized with FI-RSV formulated in alum and subsequently challenged intratracheally with RSV. Infant macaques immunized with FI-MV were used as controls. Animals of both groups developed a multifocal bronchointerstitial pneumonia with characteristic multinucleated syncytial cells, but only the FI-RSV-immunized animals developed a superimposed diffuse eosinophilic tracheobronchitis and bronchiolitis. Two of the seven FI-RSV-immunized animals, which had the most prominent Th2 responses upon vaccination, developed pulmonary hyperinflation and died 12 days after challenge. Surprisingly, limited and incomplete study of the lungs of these two animals did not show obvious inflammatory lesions.
Characterization of the vaccine-induced T-cell immune responses indicated that vaccination with FI-RSV induced both virus-specific and nonspecific immune responses, while vaccination with FI-MV induced largely virus-specific responses. This suggests that our FI-RSV preparation, more than our FI-MV preparation, contained immunogenic cellular or serum proteins. It has been found that formalin-inactivated mock antigen can predispose rodents to hypersensitivity upon RSV challenge, supposedly as a result of nonviral proteins present in the challenge stock (2, 27). We therefore cannot exclude the possibility that the immunopathology observed in FI-RSV-primed and RSV-challenged animals was at least in part due to recall of these responses by nonviral proteins present in the challenge stock.
Both FI-RSV and FI-MV vaccination induced strong lymphoproliferative responses, which is in accordance with observations in infants in vaccine trials in the 1960s (18, 19). Measurement of cytokine levels in culture supernatants showed that both vaccine preparations induced virus-specific T cells, which predominantly produced IL-13, IL-5, and IL-2 upon stimulation with BPL-inactivated virus antigens. Although IL-13 was detected in supernatants of PBMC stimulated with the homologous antigen in both groups of animals after vaccination, RSV challenge boosted these responses only in the FI-RSV group.
IL-13 is thought to play a key role in the development of asthma and airway hyperreactivity (AHR) (4, 13, 42), while IL-5 is a hematopoietic growth factor responsible for growth and differentiation of eosinophils (12). Both IL-13 and IL-5 have also been implicated in FI-RSV-mediated hypersensitivity in BALB/c mice (15, 40). In addition, FI-RSV priming and subsequent RSV challenge have been found to exacerbate AHR in mice (26), which was probably mediated by IL-13 (20, 25, 37). IL-13 is also a down-regulating factor of macrophage function, in particular production of IL-12 (6), which could explain the previously observed altered IL-12 synthesis in macaques primed with FI-MV and challenged with wild-type MV (29).
Why did two of the FI-RSV-vaccinated animals develop pulmonary hyperinflation 12 days after challenge in the absence of cellular infiltrates in their lungs? The timing is in agreement with a T-cell- and cytokine-mediated mechanism. We hypothesize that the underlying mechanism was not reminiscent of a normal RSV bronchiolitis but rather resulted from an asthma-like pathogenesis associated with a smooth muscle-mediated airway obstruction (14, 21). Remarkably, the two animals that died in our study had almost undetectable (RS2) or relatively low (RS1) eosinophil counts in their BAL cells. The highly skewed Th2 responses in these two animals may have resulted in a distinct type of antiviral immune response compared to those of the other five FI-RSV-immunized monkeys. This biological variation can probably be attributed to the use of outbred non-specific-pathogen-free animals and could also be expected in humans.
We observed significant eosinophilia in the BAL samples obtained from six out of seven FI-RSV-primed macaques upon RSV challenge. Eosinophils have also been described as an important marker of FI-RSV-mediated enhanced disease in BALB/c mice (23), but in cotton rats and cattle the major lesions observed were rather characterized by the presence of neutrophils (10, 33). In the two infants who died in 1967 the major inflammatory cells were also neutrophils (32), which favors the use of the cotton rat and bovine models. However, in our study the two macaques that died may not have been representative of the whole group in their histopathological presentation and did not show substantial inflammation at the moment of death. The availability of immunological reagents to study the RSV-specific immune response both in cattle and in cotton rats will, it is hoped, shed more light on the pathogenesis of the enhanced disease in these species, allowing comparisons with the mouse model and the present study.
Semiquantitative RSV-specific RT-PCR on BAL samples suggested that the viral loads in the lungs of the FI-RSV-vaccinated animals were 1 to 2 log units lower than those in the FI-MV-vaccinated animals. This is in accordance with virus shedding data for FI-RSV-vaccinated African green monkeys (16) but is in contrast to the results of a recent study of FI-RSV immunization in bonnet monkeys, which showed increased replication of RSV following challenge (30). However, in the latter study FI-RSV vaccination resulted in low lymphoproliferative and VN antibody responses, while in our study these responses were more pronounced. VN antibodies have been shown to confer protection against RSV lower respiratory tract infection (7), so in our study the FI-RSV-induced immune responses may well have resulted in a combination of partial protection and predisposition for immunopathology. Whether virus replication is reduced or increased may be determined by the balance between the antiviral effects of the RSV-specific immune response on the one hand and the numbers and phenotype of infiltrating cells promoting virus replication on the other.
In conclusion, we developed an FI-RSV immunopathology model for infant cynomolgus macaques and identified virus-specific IL-13 and IL-5 production upon vaccination as the immunological correlate of hypersensitivity responses upon intratracheal RSV challenge. The observation of hypersensitivity responses in humans and in animal models upon priming with FI-RSV (23), FI-MV (28), FI-parainfluenza virus 3 (24), a formalin-inactivated mock antigen (2), RSV-G protein (22), or dendritic cells cultured in fetal bovine serum (23a) and subsequent intratracheal challenge with the homologous antigens suggests a common mechanism for these events. We hypothesize that this underlying mechanism is a systemic priming for strong Th2 responses followed by an intrapulmonary recall, largely irrespective of the nature of the antigen. The macaque RSV model will allow the assessment of both the efficacy and safety of candidate nonreplicating RSV vaccines before embarking on clinical trials in humans.
Acknowledgments
This work was supported by financial contributions from the Sophia Foundation for Medical Research (grant no. 243) and from the EC 5th FWP (grant no. QLK2-1999-01044).
We thank Rob van Binnendijk, Bea van′t Land, Paul Mulder, Guido van der Net, Frank van der Panne, Suzanne Pas, Wim Roest, Koert Stittelaar, Jolanda Voermans, and members of the Departments of Hematology and Clinical Chemistry, Lung Disease, and Throat, Nose, and Ear Disease for their contributions.
REFERENCES
- 1.Belshe, R. B., L. S. Richardson, W. T. London, D. L. Sly, J. H. Lorfeld, E. Camargo, D. A. Prevar, and R. M. Chanock. 1977. Experimental respiratory syncytial infection of four species of primates. J. Med. Virol. 1**:**157-162. [DOI] [PubMed] [Google Scholar]
- 2.Boelen, A., A. C. Andeweg, J. Kwakkel, W. Lokhorst, T. M. Bestebroer, J. Dormans, and T. G. Kimman. 2000. Both immunisation with a formalin-inactivated respiratory syncytial virus (RSV) vaccine and a mock antigen vaccine induce severe lung pathology and a Th2 cytokine profile in RSV-challenged mice. Vaccine 19**:**982-991. [DOI] [PubMed] [Google Scholar]
- 3.Collins, P. L., K. McIntosh, and R. M. Chanock. 1996. Respiratory syncytial virus, p. 1313-1351. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 4.Corry, D. B. 1999. IL-13 in allergy: home at last. Curr. Opin. Immunol. 11**:**610-614. [DOI] [PubMed] [Google Scholar]
- 5.Crowe, J. E., Jr. 2002. Respiratory syncytial virus vaccine development. Vaccine 20**:**S32-S37. [DOI] [PubMed] [Google Scholar]
- 6.De Waal Malefyt, R., C. G. Figdor, R. Huijbens, S. Mohan-Peterson, B. Bennett, J. Culpepper, W. Dang, G. Zurawski, and J. E. De Vries. 1993. Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes. Comparison with IL-4 and modulation by IFN-gamma or IL-10. J. Immunol. 151**:**6370-6381. [PubMed] [Google Scholar]
- 7.Domachowske, J. B., and H. F. Rosenberg. 1999. Respiratory syncytial virus infection: immune response, immunopathogenesis, and treatment. Clin. Microbiol. Rev. 12**:**298-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fulginiti, V. A., and J. H. Arthur. 1969. Altered reactivity to measles virus. Skin test reactivity and antibody response to measles virus antigens in recipients of killed measles virus vaccine. J. Pediatr. 75**:**609-616. [DOI] [PubMed] [Google Scholar]
- 9.Fulginiti, V. A., J. J. Eller, A. W. Downte, and C. H. Kempe. 1967. Altered reactivity to measles virus. Atypical measles in children previously immunized with inactivated virus vaccines. JAMA 202**:**101-106. [DOI] [PubMed] [Google Scholar]
- 10.Gershwin, L. J., E. S. Schelegle, R. A. Gunther, M. L. Anderson, A. R. Woolums, D. R. Larochelle, G. A. Boyle, K. E. Friebertshauser, and R. S. Singer. 1998. A bovine model of vaccine enhanced respiratory syncytial virus pathophysiology. Vaccine 16**:**1225-1236. [DOI] [PubMed] [Google Scholar]
- 11.Graham, B. S. 1995. Pathogenesis of respiratory syncytial virus vaccine-augmented pathology. Am. J. Respir. Crit. Care Med. 152**:**S63-S66. [DOI] [PubMed] [Google Scholar]
- 12.Greenfeder, S., S. P. Umland, F. M. Cuss, R. W. Chapman, and R. W. Egan. 2001. Th2 cytokines and asthma. The role of interleukin-5 in allergic eosinophilic disease. Respir. Res. 2**:**71-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grunig, G., M. Warnock, A. E. Wakil, R. Venkayya, F. Brombacher, D. M. Rennick, D. Sheppard, M. Mohrs, D. D. Donaldson, R. M. Locksley, and D. B. Corry. 1998. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 282**:**2261-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirst, S. J. 2000. Airway smooth muscle as a target in asthma. Clin. Exp. Allergy 30(Suppl. 1)**:**54-59. [PubMed] [Google Scholar]
- 15.Johnson, T. R., and B. S. Graham. 1999. Secreted respiratory syncytial virus G glycoprotein induces interleukin-5 (IL-5), IL-13, and eosinophilia by an IL-4-independent mechanism. J. Virol. 73**:**8485-8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kakuk, T. J., K. Soike, R. J. Brideau, R. M. Zaya, S. L. Cole, J.-Y. Zhang, E. D. Roberts, P. A. Wells, and M. W. Wathen. 1993. A human respiratory syncytial virus (RSV) primate model of enhanced pulmonary pathology induced with a formalin-inactivated RSV vaccine but not a recombinant FG subunit vacine. J. Infect. Dis. 167**:**553-561. [DOI] [PubMed] [Google Scholar]
- 17.Kim, H. W., J. G. Canchola, C. D. Brandt, G. Pyles, R. M. Chanock, K. E. Jensen, and R. H. Parrott. 1969. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 89**:**422-434. [DOI] [PubMed] [Google Scholar]
- 18.Kim, H. W., S. L. Leikin, J. Arrobio, C. D. Brandt, R. M. Chanock, and R. H. Parrott. 1976. Cell-mediated immunity to respiratory syncytial virus induced by inactivated vaccine or by infection. Pediatr. Res. 10**:**75-78. [DOI] [PubMed] [Google Scholar]
- 19.Krause, P. J., J. D. Cherry, M. J. Naiditch, J. Deseda-Tous, and E. J. Walbergh. 1978. Revaccination of previous recipients of killed measles vaccine: clinical and immunologic studies. J. Pediatr. 93**:**565-571. [DOI] [PubMed] [Google Scholar]
- 20.Lukacs, N. W., K. K. Tekkanat, A. Berlin, C. M. Hogaboam, A. Miller, H. Evanoff, P. Lincoln, and H. Maassab. 2001. Respiratory syncytial virus predisposes mice to augmented allergic airway responses via IL-13-mediated mechanisms. J. Immunol. 167**:**1060-1065. [DOI] [PubMed] [Google Scholar]
- 21.Mazzarella, G., L. Stendardi, M. Grazzini, and G. Scano. 2000. Mechanisms involved in airway obstruction: the role of smooth muscle. Allergy 55(Suppl. 61)**:**46-48. [DOI] [PubMed] [Google Scholar]
- 22.Openshaw, P. J., S. L. Clarke, and F. M. Record. 1992. Pulmonary eosinophilic response to respiratory syncytial virus in mice sensitized to the major surface glycoprotein G. Int. Immunol. 4**:**493-500. [DOI] [PubMed] [Google Scholar]
- 23.Openshaw, P. J. M., F. J. Culley, and W. Olszewska. 2002. Immunopathogenesis of vaccine-enhanced RSV disease. Vaccine 20**:**S27-S31. [DOI] [PubMed] [Google Scholar]
- 23a.Ostler, T., and S. Ehl. 2002. A cautionary note on experimental artifacts induced by fetal calf serum in a viral model of pulmonary eosinophilia. J. Immunol. Methods 268**:**211-218. [DOI] [PubMed] [Google Scholar]
- 24.Ottolini, M. G., D. D. Porter, V. G. Hemming, and G. A. Prince. 2000. Enhanced pulmonary pathology in cotton rats upon challenge after immunization with inactivated parainfluenza virus 3 vaccines. Viral Immunol. 13**:**231-236. [DOI] [PubMed] [Google Scholar]
- 25.Peebles,R. S., Jr., K. Hashimoto, R. D. Collins, K. Jarzecka, J. Furlong, D. B. Mitchell, J. R. Sheller, and B. S. Graham. 2001. Immune interaction between respiratory syncytial virus infection and allergen sensitization critically depends on timing of challenges. J. Infect. Dis. 184**:**1374-1379. [DOI] [PubMed] [Google Scholar]
- 26.Peebles, R. S., Jr., J. R. Sheller, R. D. Collins, K. Jarzecka, D. B. Mitchell, and B. S. Graham. 2000. Respiratory syncytial virus (RSV)-induced airway hyperresponsiveness in allergically sensitized mice is inhibited by live RSV and exacerbated by formalin-inactivated RSV. J. Infect. Dis. 182**:**671-677. [DOI] [PubMed] [Google Scholar]
- 27.Piedra, P. A., P. R. Wyde, W. L. Castleman, M. W. Ambrose, A. M. Jewell, D. J. Speelman, and S. W. Hildreth. 1993. Enhanced pulmonary pathology associated with the use of formalin-inactivated respiratory syncytial virus vaccine in cotton rats is not a unique viral phenomenon. Vaccine 11**:**1415-1423. [DOI] [PubMed] [Google Scholar]
- 28.Polack, F. P., P. G. Auwaerter, S. H. Lee, H. C. Nousari, A. Valsamakis, K. M. Leiferman, A. Diwan, R. J. Adams, and D. E. Griffin. 1999. Production of atypical measles in rhesus macaques: evidence for disease mediated by immune complex formation and eosinophils in the presence of fusion-inhibiting antibody. Nat. Med. 5**:**629-634. [DOI] [PubMed] [Google Scholar]
- 29.Polack, F. P., S. J. Hoffman, W. J. Moss, and D. E. Griffin. 2002. Altered synthesis of interleukin-12 and type 1 and type 2 cytokines in rhesus macaques during measles and atypical measles. J. Infect. Dis. 185**:**13-19. [DOI] [PubMed] [Google Scholar]
- 30.Ponnuraj, E. M., A. R. Hayward, A. Raj, H. Wilson, and E. A. F. Simoes. 2001. Increased replication of respiratory syncytial virus (RSV) in pulmonary infiltrates is associated with enhanced histopathological disease in bonnet monkeys (Macaca radiata) pre-immunized with a formalin-inactivated RSV vaccine. J. Gen. Virol. 82**:**2663-2674. [DOI] [PubMed] [Google Scholar]
- 31.Power, U. F., T. N. Nguyen, E. Rietveld, R. L. de Swart, J. Groen, A. D. M. E. Osterhaus, R. de Groot, N. Corvaia, A. Beck, N. Bouveret-le-Cam, and J.-Y. Bonnefoy. 2001. Safety and immunogenicity of a novel recombinant subunit respiratory syncytial virus vaccine (BBG2Na) in healthy young adults. J. Infect. Dis. 184**:**1456-1460. [DOI] [PubMed] [Google Scholar]
- 32.Prince, G. A., S. J. Curtis, K. C. Yim, and D. D. Porter. 2001. Vaccine-enhanced respiratory syncytial virus disease in cotton rats following immunization with Lot 100 or a newly prepared reference vaccine. J. Gen. Virol. 82**:**2881-2888. [DOI] [PubMed] [Google Scholar]
- 33.Prince, G. A., A. B. Jenson, V. G. Hemming, B. R. Murphy, E. E. Walsh, R. L. Horswood, and R. M. Chanock. 1986. Enhancement of respiratory syncytial virus pulmonary pathology in cotton rats by prior intramuscular inoculation of formalin-inactivated virus. J. Virol. 57**:**721-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27**:**493-497. [Google Scholar]
- 35.Simoes, E. A. F., A. R. Hayward, E. M. Ponnuraj, J. P. Straumanis, K. R. Stenmark, H. L. Wilson, and P. G. Babu. 1999. Respiratory syncytial virus infects the Bonnet monkey, Macaca radiata. Pediatr. Dev. Pathol. 2**:**316-326. [DOI] [PubMed] [Google Scholar]
- 36.Stittelaar, K. J., L. S. Wyatt, R. L. de Swart, H. W. Vos, J. Groen, G. van Amerongen, R. S. van Binnendijk, S. Rozenblatt, B. Moss, and A. D. M. E. Osterhaus. 2000. Protective immunity in macaques vaccinated with a modified vaccinia virus Ankara-based measles vaccine in the presence of passively acquired antibodies. J. Virol. 74**:**4236-4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tekkanat, K. K., H. F. Maassab, D. S. Cho, J. J. Lai, A. John, A. Berlin, M. H. Kaplan, and N. W. Lukacs. 2001. IL-13-induced airway hyperreactivity during respiratory syncytial virus infection is STAT6 dependent. J. Immunol. 166**:**3542-3548. [DOI] [PubMed] [Google Scholar]
- 38.van Binnendijk, R. S., M. C. M. Poelen, G. van Amerongen, P. De Vries, and A. D. M. E. Osterhaus. 1997. Protective immunity in macaques vaccinated with live attenuated, recombinant and subunit measles vaccines in the presence of passively acquired antibodies. J. Infect. Dis. 175**:**524-534. [DOI] [PubMed] [Google Scholar]
- 39.van Binnendijk, R. S., R. W. J. van der Heijden, G. van Amerongen, F. G. C. M. UytdeHaag, and A. D. M. E. Osterhaus. 1994. Viral replication and development of specific immunity in macaques after infection with different measles virus strains. J. Infect. Dis. 170**:**443-448. [DOI] [PubMed] [Google Scholar]
- 40.Waris, M. E., C. Tsou, D. D. Erdman, S. R. Zaki, and L. J. Anderson. 1996. Respiratory syncytial virus infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like cytokine pattern. J. Virol. 70**:**2852-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weltzin, R., V. Traina-Dorge, K. Soike, J.-Y. Zhang, P. Mack, G. Soman, G. Drabik, and T. P. Monath. 1996. Intranasal monoclonal IgA antibody to respiratory syncytial virus protects rhesus monkeys against upper and lower respiratory tract infection. J. Infect. Dis. 174**:**256-261. [DOI] [PubMed] [Google Scholar]
- 42.Wills-Karp, M., J. Luyimbazi, X. Xu, B. Schofield, T. Y. Neben, C. L. Karp, and D. D. Donaldson. 1998. Interleukin-13: central mediator of allergic asthma. Science 282**:**2258-2261. [DOI] [PubMed] [Google Scholar]