CD8+ T cell-mediated CXC chemokine receptor 4-simian/human immunodeficiency virus suppression in dually infected rhesus macaques (original) (raw)
Abstract
We coinfected rhesus macaques with CXC chemokine receptor 4- and CC chemokine receptor 5-specific simian/human immunodeficiency viruses (SHIVs) to elucidate the basis for the early dominance of R5-tropic strains seen in HIV-infected humans. We found no intrinsic barrier to the transmission and dissemination of high-dose X4-SHIV in the dually infected macaques. In animals that maintained a viral set point, the R5 virus predominated. The time of appearance of R5 dominance coincided with the development of virus-specific immunity (3–6 weeks postinfection), suggestive of differential immune control of the two viruses. Indeed, after depletion of CD8+ T cells in the coinfected animals, X4 virus emerged, supporting the concept that differential CD8+ T cell-mediated immune control of X4- and R5-SHIV replication is responsible for the selective outgrowth of R5 viruses. These findings provide critical insights into a key question related to HIV pathogenesis and have important implications for the development and testing of antiviral vaccines and therapeutics.
It has been well documented that viruses that use the CC chemokine receptor 5 (CCR5) coreceptor for entry (R5 strains) dominate in the early stages of infection in HIV-1-infected individuals (1). With progression to AIDS, variants that use the CXC chemokine receptor 4 (CXCR4) (X4 strains) or both (R5X4 viruses) coreceptors emerge in about half of infected individuals and are associated with a more rapid rate of CD4+ T cell loss (2–5). Various mechanisms including a selective barrier against X4 variant transmission at the port of entry and/or selective amplification of R5 strains have been proposed to explain the observation of early R5 dominance (6, 7). Indeed, high levels of the CXCR4-blocking ligand, SDF-1, is constitutively expressed by genital epithelial cells at sites of HIV transmission and propagation (8), and intestinal epithelial cells express CCR5 and not CXCR4, selectively transferring R5 but not X4 viruses to CD4+ T cells in the lamina propria (9). Furthermore, immature dendritic cells present at mucosal tissues favor the replication of R5 viruses, facilitating their transport to regional lymph nodes for dissemination (10, 11). However, X4 viruses are responsible for the rare infection of individuals lacking the CCR5 coreceptor (12), and anecdotal transmission cases have been reported in humans where both X4 and R5 viruses could be detected early in infection, with R5 detected exclusively shortly thereafter (3, 13–17). These latter findings suggest that there are no intrinsic barriers to the transmission, amplification, and dissemination of X4 viruses in humans. Thus, precisely where and how selection for R5 virus occurs early in infection remained poorly understood.
Previously we described the generation of X4-SHIVSF33A and R5-SHIVSF162P3 pathogenic simian/human immunodeficiency viruses (SHIVs) through adaptation or rapid serial passage in rhesus macaques, respectively (18–20). In vitro, both viruses are neutralization-resistant (21, 22), but X4-SHIVSF33A replicates with faster kinetics in CD4+ T lymphocytes and is more cytopathic (unpublished observations). In vivo, single application of a cell-free pathogenic X4- or R5-SHIV onto the intact genital mucosa of rhesus macaque results in virus transmission and disease (19, 23). Thus, similar to findings in humans, X4 viruses can also transmit and disseminate vaginally in macaques under high-dose challenge settings. Despite comparable viral load, infection of macaques with X4-SHIVSF33A is accompanied by a rapid and severe depletion of peripheral blood and lymph node CD4+ T lymphocytes, whereas infection with R5 SHIVSF162P3 causes a more protracted loss (18). Infections with pathogenic SHIVs of different coreceptor usage, therefore, recapitulate the different stages of HIV infection in humans: R5 SHIV infection provides a model of early HIV infection with transient CD4+ T cell decline, whereas X4-SHIV infection reproduces the precipitous CD4+ T cell decline observed in patients infected with X4 isolates, which frequently emerge in late-stage disease. X4-SHIVSF33A and R5-SHIVSF162P3, therefore, provide the tools to address the underlying mechanistic basis of R5 dominance in cochallenged animals.
Materials and Methods
Virus, Animal Inoculations, and Plasma Viral RNA Measurements. Derivation of the pathogenic isolates SHIVSF162P3 and SHIVSF33A has been described (18–20). Viral stocks were propagated and titered in rhesus peripheral mononuclear cells. Tissue culture 50% infectious dose per ml was calculated to be 3.7 × 103 for SHIVSF162P3 and 6.3 × 103 for SHIVSF33A. For in vivo infection, Macaca mulatta (rhesus macaques) were exposed once to 1 ml of each virus individually or to a mixture of cell-free SHIVSF162P3 and SHIVSF33A by either the i.v. or intravaginal (IVAG) route. Macaques were not hormonally treated or experimentally cycled for IVAG inoculations, and high doses of infectious virus were used to achieve high infection rates. For data shown in Figs. 1, 2, 3, an equal volume of the two viruses equivalent to a 1:2 infectious dose of SHIVSF162P3/SHIVSF33A was used. For data in Figs. 4 and 5, animals were challenged with a 3:1 infectious dose of SHIVSF162P3/SHIVSF33A. Animals were housed individually at the Tulane Regional Primate Research Center in accordance with the Guide for the Care and Use of Laboratory Animals, and all procedures were approved by the Institutional Animal Care and Use Committee. Before inoculation and sample collection, animals were anesthetized with ketamine HCl. Animals undergo monthly physical examination that includes hematology and blood chemistry measurements, and those displaying clinical symptoms of simian AIDS (SAIDS) are killed. Plasma viral RNA levels were determined by using a branched-DNA signal amplification assay (Bayer Diagnostics, Emeryville, CA).
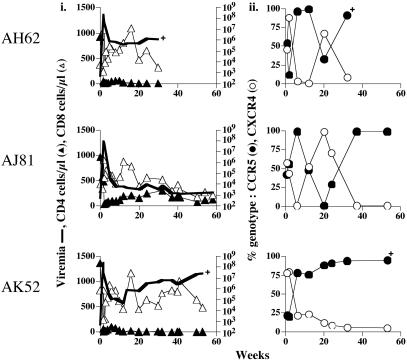
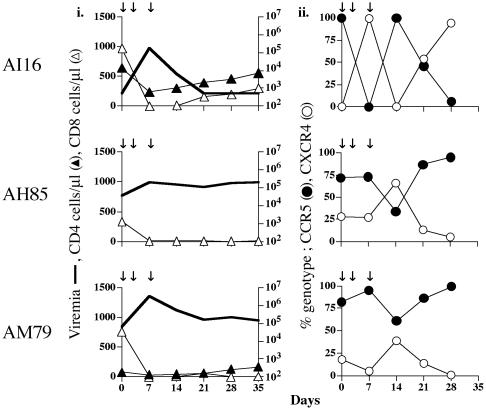
Fig. 1.
Viral replication, T cell subsets, and viral genotype in i.v. dually infected macaques. (i) Peripheral blood was collected in EDTA at designated time intervals and separated into plasma and cellular components. The absolute copies of viral RNA per ml of plasma (viremia, thick line) were determined by branched-DNA analysis (Bayer Diagnostics). The absolute number of CD3+CD4+ (▴) or CD3+CD8+(▵) cells per μl of blood as determined by Tru-Count FACS analysis. +, time that macaques were killed due to development of clinical symptoms of SAIDS. (ii) Percentage of CCR5- (•) or CXCR4- (○) specific env clones amplified by RT-PCR from plasma.
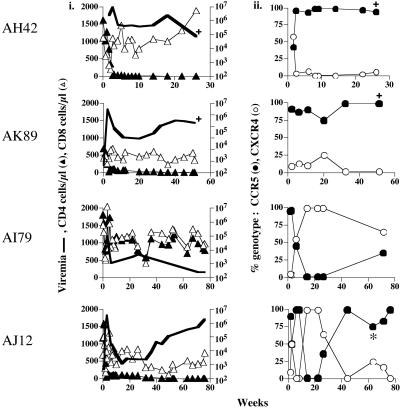
Fig. 2.
Virologic, immunologic, and genotypic measurements in IVAG dually infected macaques. The symbols are as described for Fig. 1. *, Presence of recombinant virus.
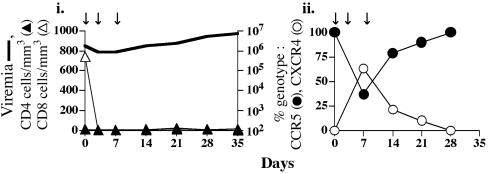
Fig. 3.
Changes in genotype of replicating virus after CD8+ T cell depletion in dually infected macaques. AJ12 was administered the mAb cM-T807 on days 0, 3, and 7 (arrows). Viral load and T cell subsets (i) and viral genotype (ii) were analyzed as described for Fig. 1.
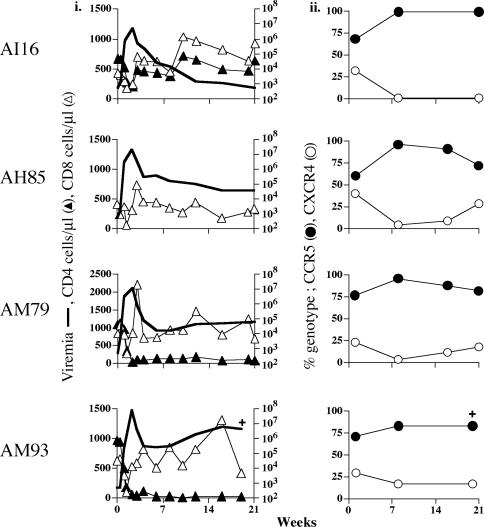
Fig. 4.
Dominance of R5-SHIVSF162P3 after i.v. inoculation of macaques. The symbols are as described for Fig. 1.
Fig. 5.
X4-SHIVSF33A emerged in i.v. dual-infected macaques after CD8+ T cell depletion. Viral load and T cell subsets (i) and viral genotypes (ii) were determined as described for Fig. 1. The arrows indicate time of administration of the anti-CD8 mAb cM-T807.
Viral Genotyping. The genotype of replicating virus in coinfected macaques was determined as follows. Viral RNA from clarified plasma was isolated by using QIAamp viral RNA mini kit (Qiagen, Valencia, CA) and reverse-transcribed by using random hexamers and Superscript reverse transcriptase (GIBCO/BRL). Transcripts were amplified in a thermocycler by using the HIV-1 _env_-specific primers ED7 and ED8 that were designed to amplify viral sequences from diverse clades (24). The sequence of the major viral variant of SHIVSF33A and SHIVSF162P3 are identical to the env primers in 40/41 and 41/41 nucleotides, respectively (Fig. 6, which is published as supporting information on the PNAS web site, www.pnas.org). Amplified products were gel-purified, ligated into TOPO TA cloning vector (Invitrogen), and used to transform electrocompetent Escherichia coli provided by the supplier (Invitrogen). Recombinant plasmid DNAs were prepared, and sequencing was performed with M13F universal primer by using the ABI BigDye terminator (v3) cycle sequencing kit (Applied Biosystems) and analyzed by using sequencher 4.0 software (Gene Codes, Ann Arbor, MI). Appropriate mixing and titration experiments were performed with either plasmids (pSF33A and pSF162P3) or viral RNA purified from macaques individually infected with SHIVSF33A or SHIVSF162P3 as targets to verify that the env primers used do not preferentially amplify either viral species. Additionally, independent viral RNA extractions, RT-PCR, and subcloning for multiple plasma samples were conducted to minimize the possibility of sampling bias. The relative proportional representation of X4- and R5-SHIVs in the product amplified by RT-PCR from plasma samples was estimated by scoring at least 22 clones (22–60 clones per sample) as being either SHIVSF33A or SHIVSF162P3.
Lymphocyte Immunophenotyping and mAb Treatment. EDTA anti-coagulated whole blood was stained with fluorescence-labeled control antibodies (IgG1) or with Leu-3a-phycoerythrin for CD4, Leu-2a-peridinin chlorophyll protein for CD8 (from Becton Dickinson), and FN-18-FITC for CD3 (BioSource International, Camarillo, CA). T cell subsets were enumerated by using TruCount absolute count tubes (Becton Dickinson) according to manufacturer instructions. Flow cytometry was performed by using the FACSCaliber and analyzed by using flojo software. For in vivo depletion of CD8+ T cells, animals were injected s.c. with a 10-mg/kg dose of the mouse/human chimeric anti-human CD8 mAb cM-T807 followed by further i.v. injections of 5-mg/kg doses 3 and 7 days later (25, 26). Animals were monitored for T cell subsets by using the TruCount method. The anti-CD8 mAb DK25 (DAKO), which reproducibly resolves CD8+ cells in the presence of cM-T807, was used in these analyses.
Results
X4-SHIVSF33A and R5-SHIVSF162P3 Transmitted and Replicated with Comparable Efficiencies When Inoculated Singly into Macaques. The ability of X4-SHIVSF33A and R5-SHIVSF162P3 to establish infection when inoculated singly by the i.v. and IVAG routes was determined and compared (Table 1). We found that SHIVSF162P3 infected 2 of 2 (100% transmission efficiency) and 7 of 9 (78% transmission efficiency) macaques by the i.v. and IVAG routes, respectively, with peak plasma viremia of 105 to 108 RNA copies per ml. X4-SHIVSF33A also infected 2 of 2 animals (100% transmission efficiency) by the i.v. route and 6 of 12 animals by the IVAG route (50% transmission efficiency), with acute plasma viremia comparable to that seen in R5-SHIVSF162P3-infected animals. Thus, in the macaque model, there is no physical barrier against high-dose X4 virus transmission at the port of entry. Furthermore, there is no significant difference in the degree of amplification/dissemination of the two viruses, as indicated by the comparable levels of acute viremia. Coinfection of macaques with the two viruses, therefore, was performed to determine whether both viruses could be transmitted under this experimental setting and whether the R5 virus establishes dominance thereafter.
Table 1. Comparison of transmission, replication, and pathogenesis of X4-SHIVSF33A and R5-SHIVSF162P3.
| Route | Virus | Infected/exposed | Peak viremia | Viral set point | SAIDS/infected | Time to SAIDS | Pathology |
|---|---|---|---|---|---|---|---|
| i.v. | X4-SHIVSF33A | 2/2 | 107 | 105 to 107 | 2/2 | 26 and 40 weeks | Severe loss of peripheral and lymphoid CD4+ T cells, MAI, PCP |
| R5-SHIVSF162 P3 | 2/2 | 107 | 102 to 106 | 1/2 | 26 weeks | Gradual loss of CD4+ PBMC severe loss of GALT CD4+ T cells, MAI, PCP | |
| IVAG | X4-SHIVSF33A | 6/12 | 105 to 108 | 103 to 106 | 6/6 | 30 weeks to 3 years | Severe loss of peripheral and lymphoid CD4+ T cells, MAI, PCP |
| R5-SHIVSF162 P3 | 7/9 | 105 to 108 | 102 to 106 | 4/7 | 24-104 weeks | Gradual loss of CD4+ PBMC, severe loss of GALT CD4+ T cells, MAI, PCP |
R5 Dominance in Macaques Coinoculated i.v. with R5- and X4-Specific SHIVs. Three rhesus macaques were inoculated i.v. with a 1:2 R5/X4 infectious dose of the identical viral stocks that were used for individual infections (Table 1). Longitudinal blood samples were obtained and analyzed for T cell subsets and peripheral viral replication. In addition to viral load, the percentage of R5 and X4 viruses present in plasma during acute and chronic infections was determined by sequence analysis. We found that after challenge, all three inoculated animals showed a dramatic and sustained drop in peripheral CD4+ T cells, indicative of the presence of the X4 variant (Fig. 1_i_) (20, 23). Within the first 1–2 weeks of infection, both R5 and X4 viruses were readily amplified from plasma of infected macaques, with X4 viruses accounting for 44–88% of the total circulating viral population (Fig. 1_ii_). This is consistent with reports of early X4 virus detection in humans (3, 13, 15, 16, 27) and indicates that there is no barrier to the i.v. transmission and dissemination of X4-SHIVSF33A in cochallenged animals. All three infected macaques seroconverted by 3–4 weeks postinfection (wpi) (data not shown). Coincidental with this onset of acquired antiviral immunity, R5 virus became dominant. In the two macaques with sustained high viral loads (AH62 and AK52; Fig. 1_i_), R5 virus persisted as the majority species at later time points as well (Fig. 1_ii_). In the animal (AJ81) with a low viral set point (<1,500 copies per ml of plasma), thought to be indicative of effective antiviral immune control, neither virus replicated preferentially over time. When expressed in terms of RNA copies per ml, it is apparent that X4 virus replication was not silent even in the animals where R5 virus was dominant (Fig. 7, which is published as supporting information on the PNAS web site). Macaques AH62 and AK52 were killed at 32 and 53 wpi, respectively, with clinical symptoms of SAIDS. Notably, the X4 variant was detected in animal AH62 at late-stage disease. These observations of early R5 outgrowth and emergence of X4 strains late in disease are reminiscent of the findings in HIV-infected humans (2–5, 28).
Similar Patterns of R5 Dominance in IVAG Coinfected Macaques. To address the impact of transmission route in R5 dominance, seven rhesus macaques were inoculated IVAG with the identical SHIVSF162P3/SHIVSF33A mixture as the i.v. exposed macaques, and longitudinal samples were obtained during acute and chronic infection. Four of the seven macaques exposed IVAG were infected, an efficiency comparable to that seen for IVAG transmission with either virus alone (Table 1). Similar to the findings for the i.v. inoculated macaques, IVAG infection was accompanied by a precipitous and sustained drop in peripheral CD4+ T cells. The only exception was macaque AI79, which had substantially lower peak viremia and viral set point (Fig. 2_i_). Genotypic analysis showed that both viruses were transmitted, indicating that there is no intrinsic barrier to X4 virus transmission at the mucosal surface in cochallenged macaques (Fig. 2_ii_). In macaques with sustained viral replication (AH42 and AK89), indicative of ineffective antiviral immunity, CCR5 dominance was established early and persisted. AH42 and AK89 were killed 26 and 51 wpi, respectively, with clinical symptoms of SAIDS. In the animal with a low peak viremia and viral set point (AI79), indicative of strong immune control, X4-SHIVSF33A constituted the majority of circulation virus. The fourth macaque (AJ12) showed a more complex pattern. Both R5 and X4 virus were present in plasma immediately after infection, but R5 dominated within 3–6 weeks, a time coincidental with seroconversion. Infection in AJ12 was then transiently controlled (<5 × 103 RNA copies per ml of plasma) between 10 and 20 weeks with virus consisting mainly of X4 variants. From 20 to 40 weeks, however, a marked increase in plasma viremia was noted (Fig. 2_i_). Concurrently, R5 virus increased in frequency and became the dominant genotype. At a later time point (as denoted by the asterisk in Fig. 2_ii_), a recombinant form was detected with sequence analysis identifying a crossover between SHIVSF162P3 and SHIVSF33A env. The envelope of the recombinant clone maintained CCR5 specificity with no expanded coreceptor utilization (data not shown). The R5 dominance associated with increased plasma viremia in AJ12 is consistent with the R5 dominance in macaques with high viral set point in both i.v. (AH62 and AK52; Fig. 1 i and ii) and IVAG (AH42 and AK89; Fig. 2 i and ii) challenged animals. Collectively, these findings suggest that R5-SHIVSF162P3 is more resistant to antiviral immune control in these dually infected macaques.
Emergence of X4 Virus After CD8+ T Cell Depletion in Chronic IVAG Coinfection. Acquired immune responses have been demonstrated to control both HIV infection of humans and simian immunodeficiency virus (SIV) infection of macaques, although the requirements for protective immune responses are not clearly understood. Neutralization-resistant variants emerge rapidly in vivo (29–31), in support of a role of antibodies in virus containment. Indeed, all infected macaques seroconverted by 3–4 wpi (data not shown). However, both X4-SHIVSF33A and R5-SHIVSF162P3 are resistant to neutralization with sera collected from the coinfected animals at the time of R5 dominance (data not shown), arguing against a role of antibodies in the selective outgrowth of the R5 virus in dually infected macaques. Cellular immune responses, particularly antiviral cytotoxic T lymphocytes (CTLs), are also important in controlling HIV and SIV infections. Anti-HIV CTL responses develop within weeks of infection, coincident with a rapid decline in plasma viremia, and in chronic infection these levels are inversely correlated with viral set points (32, 33). Macaques experimentally depleted of CD8+ CTLs during either acute or chronic SIV infection generally have a rapid increase in plasma viremia (25, 34), and CTL-escape variants are detected early (35). To assess whether CTLs differentially control X4 and R5 virus replication in dually infected macaques, we treated macaque AJ12 at the time of R5 dominance (76 wpi) with a chimeric mouse-human antibody that recognizes the CD8 molecule. This antibody has been shown to deplete CD8+ T cells in both blood and lymph node compartments of macaques (25, 26). On the day of CD8+ T cell depletion, AJ12 had considerable levels of plasma viremia (106 to 107 RNA copies per ml of plasma) that consisted primarily of R5 virus (99%) (Fig. 3).
Within 3 days after administration of the first dose of anti-CD8 antibody, CD8+ T cells were undetectable in the peripheral blood (Fig. 3_i_). Seven days after the initiation of CD8+ T cell depletion, the genotype of the replicating virus reversed from predominantly R5 (99%) to only 37% of env clones being of the R5 genotype (Fig. 3_ii_). The R5 genotype gradually regained dominance, increasing to pre-CD8+ T cell-depletion values by 28 days after depletion. CD8+ T cells did not regenerate in the periphery (Fig. 3_i_), and AJ12 was killed due to SAIDS at 35 days post antibody treatment (81 weeks postinoculation). The burst of X4-SHIVSF33A virus replication seen after CD8+ T cell depletion in AJ12 suggests selective suppression of SHIVSF33A virus replication by cellular immune responses.
Common Theme of X4 Virus Emergence After CD8+ T Cell Depletion in i.v. Coinfected Macaques. To extend our observations in AJ12, we infected four rhesus macaques i.v. with a 3:1 R5-SHIVSF162P3/X4-SHIVSF33A infectious-dose mixture (Fig. 4). This modification was made in an attempt to mimic the predominance of R5 viruses in the inocula of most exposed individuals (3, 6, 15, 17). We found that the extent of acute peripheral CD4+ T cell loss varied between macaques, consistent with the lower inoculum size of the X4 virus and depending on the level of virus replication. Of note, the CD4+ T cells of macaque AH85 did not react with the anti-CD4 mAb used (Fig. 4_i_). Viral load was sustained in three of the four infected animals (AH85, AM79, and AM93; 105 to 107 copies of RNA per ml of plasma) but was contained efficiently in macaque AI16 (<103 copies of RNA per ml of plasma) at 21 wpi (Fig. 4_i_). Accordingly, peripheral CD4+ T cell loss was dramatic and sustained in animals AM79 and AM93 but relatively modest in AI16, with absolute CD4+ T cell numbers in this animal returning to preinfection baseline values after the acute phase of infection. One week postinoculation, both R5 and X4 viruses could be detected in all the inoculated animals, with the proportion of X4 viruses varying from 23% to 32% among the macaques (Fig. 4_ii_). The percentage of R5 virus increased in the chronic phase (7 wpi), representing 72–99% of total plasma viremia. Macaque AM93, with the highest viral load, failed to develop anti-SHIV antibodies and was killed at 20 wpi with signs of SAIDS. The remaining three macaques underwent in vivo CD8+ T cell depletion at 21 wpi (Fig. 5).
A 1- to 2-log rise in plasma viremia was associated with experimentally induced CD8+ T cell depletion in all three macaques (Fig. 5_i_). CD8+ T cell repopulation was not observed in macaque AM79, and this animal was killed with SAIDS 6 weeks after depletion (week 27 postinoculation). A gradual increase in the absolute number of CD8+ T cells was seen in macaque AI16 beginning 14 days after depletion, but repopulation in the other macaque (AH85) seemed to be slower. After CD8+ T cell depletion, both X4 and R5 viruses expanded (Fig. 8, which is published as supporting information on the PNAS web site). However, the relative proportion of the two viruses changed, with an increase in the percentage of replicating virus of the X4 genotype in all three animals (Fig. 5_ii_). This emergence of the X4 virus was transient in the animals with high viral load and/or severe peripheral CD4+ T cell loss (AM79 and AH85) but was more sustained in macaque AI16. AI16 had low viral load and had regained preinfection CD4+ T cell numbers at the time of CD8+ T cell depletion. The 2-log rise in viremia 7 days after the administration of anti-CD8 mAbs in AI16 was accompanied by total replacement of R5-SHIVSF162P3 with X4-SHIVSF33A. But, infection was rapidly controlled again at 14–21 days post CD8+ T cell depletion (Fig. 5_i_), with neither virus predominating at these time points (Fig. 5_ii_). This fluctuation in viral genotypes seen in AI16 is consistent with patterns observed in other coinfected macaques that had suffered less CD4+ T cell loss and controlled viremia (AJ81, Fig. 1_ii_; AI79, Fig. 2_ii_). Overall, the emergence of X4 viruses after CD8+ T cell depletion in these i.v. inoculated animals is in agreement with the data obtained from IVAG challenged macaque AJ12 (Fig. 3), supporting the notion of an immunological basis for the selective outgrowth of the SHIVSF162P3 virus.
Discussion
The dual infection of macaques with X4-SHIVSF33A and R5-SHIVSF162P3 presented here consistently reproduces a key feature of HIV infection in humans, that is, early R5 dominance. Importantly, we show that both viruses can be detected at the first samplings (1–2 wpi) of plasma regardless of the transmission route, but R5-SHIVSF162P3 dominates after the development of antiviral immune response (3–6 wpi). This R5 dominance is seen primarily in those animals that did not control the infection efficiently (9 of 11 macaques), as evidenced by a substantial viral load (Figs. 1, 2, and 4). Our findings suggest that both viruses are transmitted, but R5 virus replication is more resilient in the presence of host antiviral immune responses. Indeed, the emergence of the X4-SHIVSF33A in four of four animals treated with anti-CD8 mAb at the time of R5-SHIVSF162P3 dominance (Figs. 3 and 5) demonstrates a critical role of CD8+ lymphocytes in mediating suppression of X4 virus replication.
The observations made after CD8+ T cell depletion suggest that CD8+ T cell-mediated control of infection is more effective in controlling X4-SHIVSF33A virus replication, providing an immunological basis for R5 dominance. Alternatively, the disruption of immune cell homeostasis by CD8 immunodepletion might be creating conditions (e.g., up-regulation of CXCR4 on CD4+ T cell populations and/or down-regulation of the SDF-1 chemokine) that favor X4 virus replication. Further studies are required to investigate these possibilities. It should be noted that the R5-SHIVSF162P3 isolate used here is not intrinsically resistant to CD8+ CTL immune responses. Administration of the anti-CD8 antibody to two SHIVSF162P3 singly infected macaques during chronic infection (157 wpi) resulted in a 1- to 3-log rise in plasma viremia (data not shown). Furthermore, R5-SHIVSF162P3 as well as X4-SHIVSF33A virus replication increased after CD8+ T cell depletion in macaques AI16, AH85, and AM79 (Fig. 8); it is only the relative proportion of the two viruses that changed (Fig. 5). The two viruses therefore show differential rather than all-or-none susceptibility to CTL suppression in the dually infected macaques.
Notably, suppression of X4-SHIVSF33A replication is not observed in singly infected macaques (Table 1) but only when R5-SHIVSF162P3 is also present. We suggest that target cell competition between the two viruses in vivo can explain this observation. We hypothesize that R5-SHIVSF162P3 has a higher level of tropism for tissue macrophages than X4-SHIVSF33A, and that infected macrophages are less susceptible to CD8+ T cell-mediated immune control. Together, these virologic and immunologic properties define the selective early growth advantage of R5 virus as follows. In the presence of R5 viruses, X4 virus replication is relatively restricted to the CD4+ T cell compartment that can be controlled more effectively by CD8+ T cells. In support of our hypothesis is the marked preference for X4 strains to infect CD4+ T cells and for R5 viruses to infect macrophages (36). Infected macrophages are long-lived (37, 38), important reservoirs of virus in vivo (39, 40), and relatively resistant to CTL-mediated lysis when compared with infected CD4+ T lymphocytes in vitro (41). Further investigations with additional X4- and R5-specific SHIVs that differ in macrophage tropism will be required to test this hypothesis as well as to establish the generality of our observations and their relevance to HIV infection.
In summary, dual infection of macaques with high doses of the X4-SHIVSF33A and R5-SHIVSF162P3 viruses provides insights into the underlying basis for R5 dominance in human-exposure cases where both viruses are successfully transmitted (3, 13–17). Our findings do not exclude the possibility that, under the low-dose exposure scenarios that are seen for most human HIV-1 infections, barriers exist at the port of entry that are more effective against X4 variant transmission (6–11). Additionally, other viral properties such as replication capacity (42) and target cell tropism (3, 27) are also likely to play a role in determining selective X4 or R5 virus transmission. Nonetheless, our findings showing differential control of X4- and R5-virus replication by the host immune response have important implications not only for viral pathogenesis but also for the design and development of antiviral therapeutics and vaccines as well. Foremost is the cause of the “phenotypic or coreceptor switch” observed in 50% of HIV-infected individuals (2–5, 28). This may not be due to R5 virus adaptation/evolution, a primary concern in the use of CCR5 inhibitors (43), but rather to the emergence of archived X4 variants in the terminal stages of infection when the immune system collapses. In other words, the emergence of X4 virus may be the result and not the cause of immune failure. Additionally, if indeed both R5 and X4 variants are being transmitted to newly infected individuals, then the use of entry antagonists as anti-HIV microbicides will need to be effective against both X4 and R5 viruses. Finally, for testing the efficacy of candidate vaccines in the nonhuman primate models, particularly those that primarily induce CTL responses, the inclusion of CCR5-tropic viruses as challenge strains will be crucial.
Supplementary Material
Supporting Figures
Acknowledgments
We thank Drs. Keith Reimann and Joern Schmitz (Beth Israel Deaconess Medical Center, Boston) for providing anti-CD8 mAb [the production of which is supported by a Public Health Service Resource Award R24RR16001 (to Keith Reimann)]; Allen Mayer, Peter Balfe, Peter Joglam, and Wendy Chen (Aaron Diamond AIDS Research Center) for discussion and technical assistance; and the animal husbandry staff at Tulane National Primate Research Center. This work was supported in part by National Institutes of Health Grants AI 46980 and RR00164 and the Division of AIDS.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CCR, CC chemokine receptor; CXCR, CXC chemokine receptor; SHIV, simian/human immunodeficiency virus; IVAG, intravaginal(ly); SAIDS, simian AIDS; wpi, weeks postinfection; CTL, cytotoxic T lymphocyte.
References
- 1.Fauci, A. S. (1996) Nature 384**,** 529–534. [DOI] [PubMed] [Google Scholar]
- 2.Koot, M., Keet, I. P., Vos, A. H., de Goede, R. E., Roos, M. T., Coutinho, R. A., Miedema, F., Schellekens, P. T. & Tersmette, M. (1993) Ann. Intern. Med. 118**,** 681–688. [DOI] [PubMed] [Google Scholar]
- 3.Schuitemaker, H., Koot, M., Kootstra, N. A., Dercksen, M. W., de Goede, R. E., van Steenwijk, R. P., Lange, J. M., Schattenkerk, J. K., Miedema, F. & Tersmette, M. (1992) J. Virol. 66**,** 1354–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng-Mayer, C., Seto, D., Tateno, M. & Levy, J. A. (1988) Science 240**,** 80–82. [DOI] [PubMed] [Google Scholar]
- 5.Tersmette, M., Gruters, R. A., de Wolf, F., de Goede, R. E., Lange, J. M., Schellekens, P. T., Goudsmit, J., Huisman, H. G. & Miedema, F. (1989) J. Virol. 63**,** 2118–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu, T., Mo, H., Wang, N., Nam, D. S., Cao, Y., Koup, R. A. & Ho, D. D. (1993) Science 261**,** 1179–1181. [DOI] [PubMed] [Google Scholar]
- 7.Bomsel, M. & David, V. (2002) Nat. Med. 8**,** 114–116. [DOI] [PubMed] [Google Scholar]
- 8.Agace, W. W., Amara, A., Roberts, A. I., Pablos, J. L., Thelen, S., Uguccioni, M., Li, X. Y., Marsal, J., Arenzana-Seisdedos, F., Delaunay, T., et al. (2000) Curr. Biol. 10**,** 325–328. [DOI] [PubMed] [Google Scholar]
- 9.Meng, G., Wei, X., Wu, X., Sellers, M. T., Decker, J. M., Moldoveanu, Z., Orenstein, J. M., Graham, M. F., Kappes, J. C., Mestecky, J., et al. (2002) Nat. Med. 8**,** 150–156. [DOI] [PubMed] [Google Scholar]
- 10.Granelli-Piperno, A., Delgado, E., Finkel, V., Paxton, W. & Steinman, R. M. (1998) J. Virol. 72**,** 2733–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reece, J. C., Handley, A. J., Anstee, E. J., Morrison, W. A., Crowe, S. M. & Cameron, P. U. (1998) J. Exp. Med. 187**,** 1623–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheppard, H. W., Celum, C., Michael, N. L., O'Brien, S., Dean, M., Carrington, M., Dondero, D. & Buchbinder, S. P. (2002) J. Acquired Immune Defic. Syndr. 29**,** 307–313. [DOI] [PubMed] [Google Scholar]
- 13.Groenink, M., Fouchier, R. A., de Goede, R. E., de Wolf, F., Gruters, R. A., Cuypers, H. T., Huisman, H. G. & Tersmette, M. (1991) J. Virol. 65**,** 1968–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baur, A., Schwarz, N., Ellinger, S., Korn, K., Harrer, T., Mang, K. & Jahn, G. (1989) Lancet 2**,** 1045. [DOI] [PubMed] [Google Scholar]
- 15.Cornelissen, M., Mulder-Kampinga, G., Veenstra, J., Zorgdrager, F., Kuiken, C., Hartman, S., Dekker, J., van der Hoek, L., Sol, C., Coutinho, R., et al. (1995) J. Virol. 69**,** 1810–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roos, M. T., Lange, J. M., de Goede, R. E., Coutinho, R. A., Schellekens, P. T., Miedema, F. & Tersmette, M. (1992) J. Infect. Dis. 165**,** 427–432. [DOI] [PubMed] [Google Scholar]
- 17.Pratt, R. D., Shapiro, J. F., McKinney, N., Kwok, S. & Spector, S. A. (1995) J. Infect. Dis. 172**,** 851–854. [DOI] [PubMed] [Google Scholar]
- 18.Harouse, J. M., Gettie, A., Tan, R. C., Blanchard, J. & Cheng-Mayer, C. (1999) Science 284**,** 816–819. [DOI] [PubMed] [Google Scholar]
- 19.Harouse, J. M., Gettie, A., Eshetu, T., Tan, R. C., Bohm, R., Blanchard, J., Baskin, G. & Cheng-Mayer, C. (2001) J. Virol. 75**,** 1990–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luciw, P. A., Mandell, C. P., Himathongkham, S., Li, J., Low, T. A., Schmidt, K. A., Shaw, K. E. & Cheng-Mayer, C. (1999) Virology 263**,** 112–127. [DOI] [PubMed] [Google Scholar]
- 21.Cheng-Mayer, C., Brown, A., Harouse, J., Luciw, P. A. & Mayer, A. J. (1999) J. Virol. 73**,** 5294–5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu, M., Harouse, J. M., Gettie, A., Buckner, C., Blanchard, J. & Cheng-Mayer, C. (2003) J. Virol. 77**,** 989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harouse, J. M., Tan, R. C., Gettie, A., Dailey, P., Marx, P. A., Luciw, P. A. & Cheng-Mayer, C. (1998) Virology 248**,** 95–107. [DOI] [PubMed] [Google Scholar]
- 24.Delwart, E. L., Shpaer, E. G., Louwagie, J., McCutchan, F. E., Grez, M., Rubsamen-Waigmann, H. & Mullins, J. I. (1993) Science 262**,** 1257–1261. [DOI] [PubMed] [Google Scholar]
- 25.Schmitz, J. E., Kuroda, M. J., Santra, S., Sasseville, V. G., Simon, M. A., Lifton, M. A., Racz, P., Tenner-Racz, K., Dalesandro, M., Scallon, B. J., et al. (1999) Science 283**,** 857–860. [DOI] [PubMed] [Google Scholar]
- 26.Schmitz, J. E., Simon, M. A., Kuroda, M. J., Lifton, M. A., Ollert, M. W., Vogel, C. W., Racz, P., Tenner-Racz, K., Scallon, B. J., Dalesandro, M., et al. (1999) Am. J. Pathol. 154**,** 1923–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van't Wout, A. B., Kootstra, N. A., Mulder-Kampinga, G. A., Albrecht-van Lent, N., Scherpbier, H. J., Veenstra, J., Boer, K., Coutinho, R. A., Miedema, F. & Schuitemaker, H. (1994) J. Clin. Invest. 94**,** 2060–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Connor, R. I., Sheridan, K. E., Ceradini, D., Choe, S. & Landau, N. R. (1997) J. Exp. Med. 185**,** 621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albert, J., Abrahamsson, B., Nagy, K., Aurelius, E., Gaines, H., Nystrom, G. & Fenyo, E. M. (1990) AIDS 4**,** 107–112. [DOI] [PubMed] [Google Scholar]
- 30.Richman, D. D., Wrin, T., Little, S. J. & Petropoulos, C. J. (2003) Proc. Natl. Acad. Sci. USA 100**,** 4144–4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei, X., Decker, J. M., Wang, S., Hui, H., Kappes, J. C., Wu, X., Salazar-Gonzalez, J. F., Salazar, M. G., Kilby, J. M., Saag, M. S., et al. (2003) Nature 422**,** 307–312. [DOI] [PubMed] [Google Scholar]
- 32.Koup, R. A., Safrit, J. T., Cao, Y., Andrews, C. A., McLeod, G., Borkowsky, W., Farthing, C. & Ho, D. D. (1994) J. Virol. 68**,** 4650–4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McMichael, A. J. & Rowland-Jones, S. L. (2001) Nature 410**,** 980–987. [DOI] [PubMed] [Google Scholar]
- 34.Jin, X., Bauer, D. E., Tuttleton, S. E., Lewin, S., Gettie, A., Blanchard, J., Irwin, C. E., Safrit, J. T., Mittler, J., Weinberger, L., et al. (1999) J. Exp. Med. 189**,** 991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allen, T. M., O'Connor, D. H., Jing, P., Dzuris, J. L., Mothe, B. R., Vogel, T. U., Dunphy, E., Liebl, M. E., Emerson, C., Wilson, N., et al. (2000) Nature 407**,** 386–390. [DOI] [PubMed] [Google Scholar]
- 36.Simmons, G., Wilkinson, D., Reeves, J. D., Dittmar, M. T., Beddows, S., Weber, J., Carnegie, G., Desselberger, U., Gray, P. W., Weiss, R. A., et al. (1996) J. Virol. 70**,** 8355–8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gartner, S., Markovits, P., Markovitz, D. M., Kaplan, M. H., Gallo, R. C. & Popovic, M. (1986) Science 233**,** 215–219. [DOI] [PubMed] [Google Scholar]
- 38.Orenstein, J. M., Fox, C. & Wahl, S. M. (1997) Science 276**,** 1857–1861. [DOI] [PubMed] [Google Scholar]
- 39.Igarashi, T., Brown, C. R., Endo, Y., Buckler-White, A., Plishka, R., Bischofberger, N., Hirsch, V. & Martin, M. A. (2001) Proc. Natl. Acad. Sci. USA 98**,** 658–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perelson, A. S., Essunger, P., Cao, Y., Vesanen, M., Hurley, A., Saksela, K., Markowitz, M. & Ho, D. D. (1997) Nature 387**,** 188–191. [DOI] [PubMed] [Google Scholar]
- 41.Schutten, M., van Baalen, C. A., Guillon, C., Huisman, R. C., Boers, P. H., Sintnicolaas, K., Gruters, R. A. & Osterhaus, A. D. (2001) J. Virol. 75**,** 2706–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller, C. J., Alexander, N. J., Sutjipto, S., Lackner, A. A., Gettie, A., Hendrickx, A. G., Lowenstine, L. J., Jennings, M. & Marx, P. A. (1989) J. Virol. 63**,** 4277–4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farber, J. M. & Berger, E. A. (2002) Proc. Natl. Acad. Sci. USA 99**,** 1749–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Figures