Blocking of Interleukin-17 during Reactivation of Experimental Arthritis Prevents Joint Inflammation and Bone Erosion by Decreasing RANKL and Interleukin-1 (original) (raw)
Abstract
Rheumatoid arthritis is characterized by an intermittent course of disease with alternate periods of remission and relapse. T cells, and in particular the T-cell cytokine interleukin-17 (IL-17), are expected to be involved in arthritic flares. Here, we report that neutralizing endogenous IL-17 during reactivation of antigen-induced arthritis prevents joint inflammation and bone erosion. Synovial IL-17 mRNA expression was clearly up-regulated during primary arthritis and was further enhanced after antigen rechallenge. Neutralization of IL-17 significantly prevented joint swelling at day 1 of flare and significantly suppressed joint inflammation and cartilage proteoglycan depletion at day 4, as assessed by histology. Blocking IL-17 also clearly reduced bone erosions. Cathepsin K, a marker of osteoclast-like activity, and synovial RANKL mRNA expression were both suppressed. The degree of bone erosions strongly correlated with the severity of joint inflammation, suggesting that anti-IL-17 treatment reduced bone erosion by suppressing joint inflammation. Interestingly, blocking IL-17 suppressed synovial expression of both IL-1β and tumor necrosis factor-α, whereas blocking IL-1 did not affect tumor necrosis factor-α levels. These data indicate that IL-17 is an important upstream mediator in joint pathology during flare-up of experimental arthritis.
Rheumatoid arthritis (RA) is a systemic joint disease characterized by progressive destructive joint inflammation. Although RA is considered an autoimmune disease, the autoantigen(s) is still not identified. Interestingly, CTLA4Ig treatment in patients with RA showed promising improvement of the ACR50 and ACR70 responses.1 This treatment blocks the interaction of CD80/86 on antigen-presenting cells with CD28 on naïve T cells. This interaction is required for optimal T-cell activation. This study supports the theory that (CD4+) T cells play a key role in the pathogenesis of RA.2–4 The contribution of T cells in the joints can be related to antigen-dependent and -independent mechanisms, such as direct cell contact, which can induce cytokine and protease production by macrophages and fibroblasts.5 Furthermore, RA is characterized by an intermittent course of the disease with alternate periods of remission and relapse. The cause of a flare-up reaction during RA is unclear, and both antigen-specific and antigen-nonspecific T cells may play a role.4
Studies in animal models for experimental arthritis have resulted in better understanding of the role of different cell types and various cytokines during arthritic flares. In antigen-induced arthritis, the capacity to develop arthritis can be transferred by lymphocytes,6 in particular by cell fractions enriched for T cells, and high numbers of T cells can be detected in the inflamed synovium after flare-up reaction.7 Reactivation of chronic streptococcal cell wall arthritis in Lewis rats could be prevented by removal of T lymphocytes,8 and successful suppression of antigen-induced arthritis (AIA)-flare in mice treated with anti-lymphocyte serum9 or anti-CD4 monoclonal antibodies10 also suggests an important role for T cells in this process. In addition, during reactivation of arthritis, tumor necrosis factor-α (TNF-α) is markedly produced in the joint and peaks at 6 hours after antigen rechallenge,11 suggesting a role for this cytokine at the onset of exacerbations. IL-1 is also clearly present in experimental flare reactions and is shown to be an important mediator in joint inflammation and cartilage pathology in exacerbations of murine arthritis.12
The discovery of the T-cell cytokine IL-17A in the synovium of RA patients raised the question of its role in (antigen-specific) flare-up reactions. IL-17A is a proinflammatory cytokine produced mainly by activated (memory) T cells. IL-17A, further referred to as IL-17, belongs to a growing family of related cytokines (IL-17A-F).13–16 Until now, only IL-17A has been shown to be involved in RA pathogenesis. Neutralizing IL-17 during collagen-induced arthritis (CIA) showed that IL-17 plays a role in the early and late stages of CIA.17,18 In addition, crossing IL-17-deficient mice with IL-1Ra-deficient mice prevented the spontaneous development of arthritis normally found in mice lacking IL-1-Receptor antagonist (IL-1Ra).19 These studies clearly show a role for IL-17 in the development of arthritis. However, the role of IL-17 in a flare-up reaction of arthritis remains to be elucidated.
In the present study, we used the AIA model because of its unique antigen-specific arthritic flare-reaction. After primary AIA, arthritis can be reactivated by a small amount of antigen, causing an antigen-specific flare-up reaction with an expected major role for memory T cells and their cytokines. AIA was provoked in C57Bl/6 mice, and flare-up was induced by a rechallenge with a small amount of antigen (methylated bovine serum albumin; mBSA) locally into the arthritic knee joint. The role of IL-17 was studied by neutralizing endogenous IL-17 with polyclonal anti-IL-17 antibodies. Here, we demonstrate the critical role of IL-17 in driving joint inflammation and cartilage depletion after antigen rechallenge. In addition, neutralizing IL-17 prevents bone erosion and results in suppressed synovial expression of proinflammatory cytokines (IL-1, TNF, and RANKL) and chemokines (KCs). These data reveal IL-17 as an important mediator in flare-up reaction, acting as an upstream mediator in destructive joint inflammation during an antigen-specific flare-up of experimental arthritis.
Materials and Methods
Animals
Male C57Bl/6 mice were obtained from Elevage-Janvier (Le Genest Saint Isle, France). Mice were housed in filter-top cages under specified pathogen-free conditions, and a standard diet and water were provided ad libitum. The mice were used between 10 and 12 weeks of age. All animal procedures were approved by the institutional ethics committee.
Materials
Methylated bovine serum albumin was purchased from Sigma-Aldrich (St. Louis, MO). TRIzol Reagent, oligo-dT primers, and Moloney murine leukemia virus reverse transcriptase were obtained from Life Technologies (Breda, The Netherlands). Primers were purchased from Biolegio (Malden, The Netherlands). SYBR Green Master Mix was purchased from Applied Biosystems (ABI/PE, Foster City, CA).
Induction of Antigen-Induced Arthritis
Mice were immunized with 100 μg of mBSA (Sigma), emulsified in 100 μl Freund’s complete adjuvant (Difco Laboratories, Detroit, MI). Injections were divided over both flanks and footpath of the forelegs. Heat-killed Bordetella pertussis (RIVM, Bilthoven, The Netherlands) was administered intraperitoneally as an additional adjuvant. Two subcutaneous booster injections with in total 50 μg mBSA/Freund’s complete adjuvant were given in the neck region 1 week after the initial immunization. Three weeks after these injections, primary AIA was induced by injecting 60 μg of mBSA in 6 μl of phosphate-buffered saline into the right knee joint, resulting in chronic arthritis. At week 3 of arthritis, 2 μg of mBSA was injected intraarticularly into the arthritic joint to induce a flare-up of the smoldering inflammation.
Neutralizing Antibodies for IL-17 and IL-1
Polyclonal rabbit antibodies were directed against murine recombinant IL-17 (mIL-17; R&D Systems, Minneapolis, MN) or against each type of murine recombinant IL-1, and were prepared and tested as previously described.18,20 Flare-up of antigen-induced arthritis was induced in C57Bl/6 mice as described above. Two hours before flare-up, a single injection with the polyclonal mIL-17 or mIL-1 antibodies (200 μl/mouse) was given intraperitoneally. As a control, the same amount of normal rabbit antibodies was injected.
RNA Isolation and RT Reaction
Mice were sacrificed by cervical dislocation, immediately followed by dissection of the patella with adjacent synovium. Two biopsies with a diameter of 3 mm were punched out using a biopsy punch (Stiefel, Wachtersbach, Germany), one from the lateral side and one from the medial side of the synovial tissue. Six patella specimens per experimental group were taken, and three lateral and three medial biopsies were pooled to yield two samples per group. The synovium samples were immediately frozen in liquid nitrogen. Synovium biopsy samples were ground to powder using a Microdismembrator II (Braun, Melsungen, Germany). Total RNA was extracted in 1 ml of TRIzol reagent, an improved single-step RNA isolation method based on the method described by Chomczynski and Sacchi.21 Thereafter, RNA was precipitated with isopropanol, washed with 70% ethanol, and re-dissolved in water. Isolated RNA was treated with DNase before being reverse transcribed into cDNA using oligo-dT primers and Moloney murine leukemia virus reverse transcriptase.
Quantitative Polymerase Chain Reaction (QPCR)
Quantitative real-time PCR was performed using the ABI/PRISM 7000 Sequence Detection System for quantification with Sybr Green and melting curve analysis (ABI/PE, Foster City, CA). Primer sequences for the reference gene GAPDH and other genes were as follows: 5′-GGC AAA TTC AAC GGC ACA-3′ (GAPDH forward), 5′-GTT AGT GGG GTC TCG CTC TG-3′ (GAPDH reverse), 5′-CAG GAC GCG CAA ACA TGA-3′ (IL-17 forward), 5′-GCA ACA GCA TCA GAG ACA CAG AT-3′ (IL-17 reverse), 5′-GGA CAG AAT ATC AAC CAA CAA GTG ATA-3′ (IL-1β forward), 5′-GTG TGC CGT CTT TCA TTA CAC AG-3′ (IL-1β reverse), 5′-CAG ACC CTC ACA CTC AGA TCA TCT-3′ (TNF-α forward), 5′-CCT CCA CTT GGT GGT TTG CTA-3′ (TNF-α forward), 5′-CTG AGG CCC AGC CAT TTG-3′ (RANKL forward), and 5′-GTT GCT TAA CGT CAT GTT AGA GAT CTT G-3′ (RANKL reverse). PCR conditions were as follows: 2 minutes at 50°C and 10 minutes at 95°C followed by 40 cycles of 15 seconds at 95°C and 1 minute at 60°C, with data collection in the last 30 seconds. For all PCRs, SYBR Green Master Mix was used in the reaction. Primer concentrations were 300 nmol/L. Efficiencies (E) for all PCR primers were determined from a serial dilution series of cDNA in water: GAPDH, E = 2.09; IL-17, E = 2.06; IL-1β, E = 2.08; TNF-α, E = 2.04; RANKL, E = 2.06; all expressed as the fold increase in fluorescence per PCR cycle. The correlation coefficients (_R_2) for the PCR dilution series were 0.997 for GAPDH, 0.974 for IL-17, 0.988 for IL-1β, 0.970 for TNF-α, and 0.990 for RANKL. PCRs for each sample were performed in duplicate. All PCRs were performed in a total volume of 25 μl with a maximum of 10 ng cDNA per reaction. In addition, we ran nontemplate controls in duplicate for each primer pair of our targeted genes. Relative quantification of the PCR signals was performed by comparing the cycle threshold value (CT), in duplicate, of the gene of interest of each sample with the CT values of the reference gene GAPDH. QPCR analysis for each sample was performed in duplicate.
99mTc Uptake Measurements
Joint swelling was measured by 99mTc pertechnetate uptake in the knee joint. This method was shown earlier to correlate well with histological findings. Briefly, mice were sedated with chloralhydrate and subsequently injected intraperitoneally with 20 μCi 99mTc. Thirty minutes thereafter, gamma radiation was assessed by use of a collimated Na-I-scintillation crystal with the knee in a fixed position. Arthritis was scored as the ratio of the 99mTc uptake in the right (R) and the left (L) knee joint. R:L ratios >1.1 were taken to indicate swelling of the right knee joint.
Histology
Total knee joints of mice were isolated at day 4 after flare induction. For standard histology, tissue was fixed for 4 days in 4% formaldehyde, decalcified in 5% formic acid, and subsequently dehydrated and embedded in paraffin. Standard frontal sections of 7 μm were mounted on SuperFrost slides (Menzel-Gläser, Braunschweig, Germany). Hematoxylin and eosin (H&E) staining was performed to study joint inflammation. The severity of inflammation in the knee joints was scored on a scale of 0–3 (0 = no cells, 1 = mild cellularity, 2 = moderate cellularity, and 3 = maximal cellularity). To study proteoglycan (PG) depletion from the cartilage matrix, sections were stained with safranin O followed by counterstaining with fast green. Depletion of PGs was determined using an arbitrary scale of 0 to 3, ranging from normal, fully stained cartilage to destained cartilage, fully depleted of PGs. Bone erosion was graded on a scale of 0 to 5, ranging from no damage to complete loss of the bone structure. Histopathological changes in the knee joint were scored in the patella and femur/tibia regions on five semiserial sections of the joint spaced 70 μm apart. Scoring was performed in a blindfolded manner by two independent observers.
Cathepsin K Staining
Osteoclast-activity was visualized by immunohistochemistry for cathepsin K. Joint sections (7 μm) were deparaffinized, rehydrated, and incubated with rabbit-anti-murine cathepsin K (a kind gift of Dr. E. Sakai, Department of Pharmacology, Nagasaki University School of Dentistry, Nagasaki, Japan) or with normal rabbit IgG (X0936; DAKO, Glostrup, Denmark) in phosphate-buffered saline containing 5% milk powder, 3% fetal calf serum, and 2% BSA. Subsequently, the sections were incubated with biotinylated swine-anti-rabbit IgG (E0431; DAKO) followed by labeling with streptavidin-horseradish-peroxidase (P0397; DAKO). Peroxidase was developed with diaminobenzidine as substrate. Sections were counterstained with hematoxylin for 1 minute.
Cytokine Measurements
To determine levels of several cytokines, including IL-1β, TNF-α, IL-17, and KC in patellae washouts, patellae with surrounding soft tissue consisting of the tendon and synovium were dissected in a standardized manner.17 Patellae were cultured in RPMI 1640 medium containing 0.1% BSA (200 μl/patella) for 1 hour at room temperature. Thereafter, supernatant was harvested and centrifuged for 5 minutes at 1000 × g. Cytokine levels were determined using the Luminex multianalyte technology. The BioPlex system in combination with multiplex cytokine kits (Bio-Rad Laboratories, Hercules, CA) was used. Cytokines were measured in 50 μl of patellae washout medium. The sensitivity of the multiplex kit was 5, <3, 12.5, and 5 pg/ml, respectively, for IL-1β, TNF-α, IL-17, and KC.
Statistical Analysis
Differences between experimental groups were tested using the Mann-Whitney _U_-test, unless stated otherwise. P values less than 0.05 were considered significant. Results are expressed as the mean ± SEM.
Results
Pronounced IL-17 Up-Regulation during Antigen-Induced Exacerbation of Experimental Arthritis
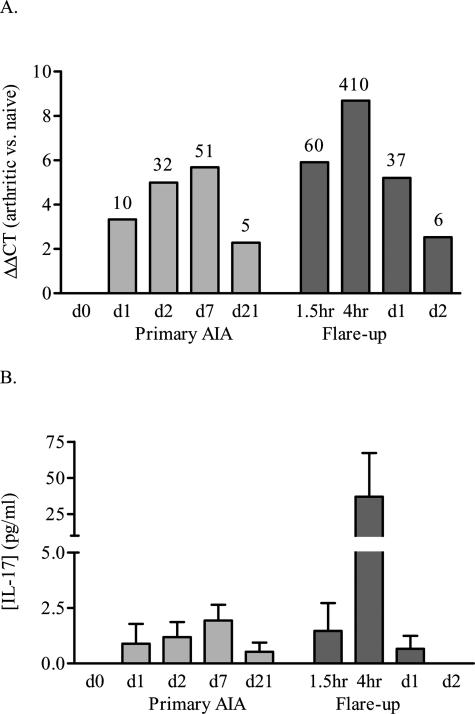
Shortly after intraarticular injection of 60 μg of mBSA in preimmunized mice, a primary chronic joint inflammation develops. During this primary AIA, IL-17 mRNA expression is up-regulated in the synovium at day 1 (Figure 1A) and even more enhanced at days 2 and 7 (Figure 1A). At day 21, IL-17 mRNA expression is low but still present in the synovium of arthritic mice (Figure 1A). Flare-up of arthritis is induced at day 21 by local injection of a small amount of antigen (2 μg mBSA) into the arthritic knee joint. Interestingly, after antigen rechallenge with only a minor amount of antigen, IL-17 mRNA expression rapidly increases and reaches much higher expression levels than during primary arthritis. A profound increase was already noted after 90 minutes, exceeding the expression levels during primary arthritis, and IL-17 expression peaked after 4 hours with a more than 400-fold increase compared with naive mice (Figure 1A). The expression of IL-17 protein correlated well with mRNA levels, with IL-17 being expressed in low amounts during primary AIA and clearly up-regulated 4 hours after antigen rechallenge (Figure 2B). This rapid and tremendous response during flare reaction suggests involvement of IL-17 produced by activated (memory) T cells located at the site of antigen exposure.
Figure 1.
Up-regulation of IL-17 mRNA (A) and protein (B) levels in synovium from arthritic C57Bl/6 mice during AIA. At various time points during primary AIA and flare-up, synovium biopsies were taken for QPCR analysis, and synovial washouts were collected for protein determination. ΔΔCT-value, the mRNA expression corrected for GAPDH and compared with naive mice (day 0). Labels on top of the bars mention the fold mRNA increase compared with naive mice. The QPCR results are the average mRNA expression of two groups, consisting of six pooled synovium biopsies, measured in duplicate. The protein data show the mean with its SEM of three to eight animals per group.
Figure 2.
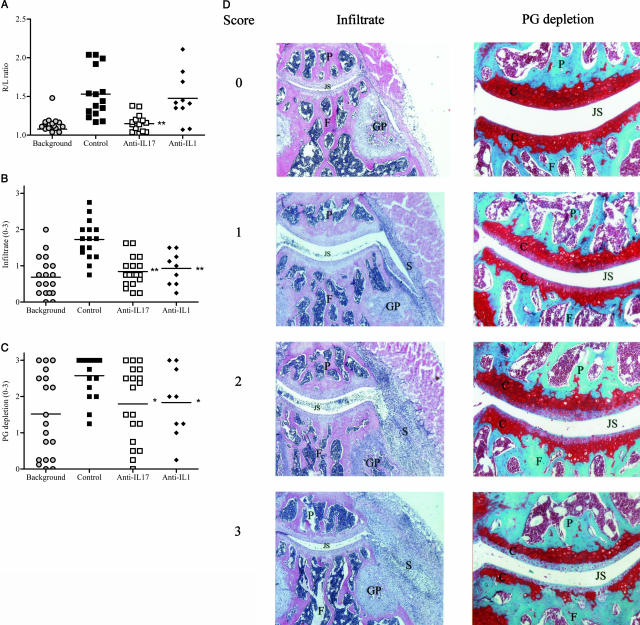
Histopathological effects of anti-IL-17 treatment during flare-up of antigen-induced arthritis. Blocking of IL-17 during flare-up of arthritis results in suppressed joint swelling (A), influx of proinflammatory cells (B), and cartilage PG depletion (C). D: Histological sections representative for our arbitrary scoring system for infiltrate and cartilage PG depletion (0–3). Joint swelling was measured by 99mTc uptake at day 1 of flare-up, and total knee joints were isolated at day 4 for histological analysis. Results are the mean of at least nine mice per group; *P < 0.05; **P < 0.005 versus control-treated group, by Mann Whitney _U_-test. Histological sections for infiltrate stained with H&E and for cartilage PG depletion stained with safranin O. P, patella; F, femur; S, synovitis; GP, growth plate; C, cartilage; JS, joint space (Original magnification, ×50 or ×100).
IL-17 Blocking in Exacerbation of Antigen-Induced Arthritis Suppresses Joint Swelling, Inflammation, and Cartilage Depletion
To investigate the importance of IL-17 produced by the synovial infiltrate during the flare-up reaction of arthritis, neutralizing rabbit-anti-mouse-IL-17 antibodies were injected intraperitoneally 2 hours before antigen rechallenge. As a control, normal rabbit antibodies or rabbit-anti-mouse-IL-1 antibodies were given.
Three weeks after induction of primary AIA and just before antigen rechallenge for the induction of a flare reaction, no significant joint swelling was found (99mTc measurement R/L ratio ≤1.1; Figure 2A, background). Despite the absence of joint swelling at day 21 of primary AIA, histological analysis showed some remaining smoldering joint inflammation and cartilage proteoglycan depletion in the background group (Figure 2, B and C). Figure 2D shows representative knee joint sections corresponding to the arbitrary scores for infiltrate and cartilage PG depletion of 0 to 3.
Local injection of 2 μg mBSA into the hypersensitive joint caused an acute joint swelling at day 1 (Figure 2A). Blocking IL-17 suppressed joint swelling significantly and was even more effective than blocking IL-1 (Figure 2A). Antigen rechallenge caused a marked aggravation of joint inflammation after antigen rechallenge in control-treated mice compared with unchallenged mice (background) (Figure 2B). Neutralizing IL-17 almost completely prevented this exacerbation of synovitis (Figure 2B) and showed comparable effectivity in suppressing joint inflammation as anti-IL-1 treatment. The flare-up reaction also resulted in a marked increase in cartilage PG depletion (Figure 2C). Arthritic mice treated with anti-IL-17 or anti-IL-1 antibodies showed clearly suppressed PG depletion of the cartilage (Figure 2C). These data indicate that blocking IL-17 is effective in preventing synovitis during an arthritic flare reaction and is as potent as blocking IL-1 to prevent cartilage PG depletion.
Neutralizing IL-17 Prevents Bone Erosion and Osteoclast-Like Activity
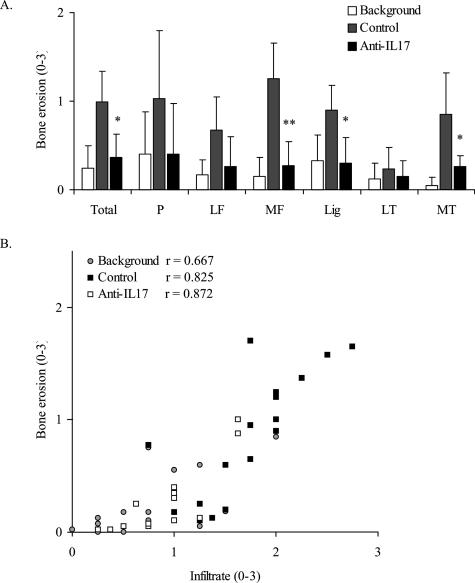
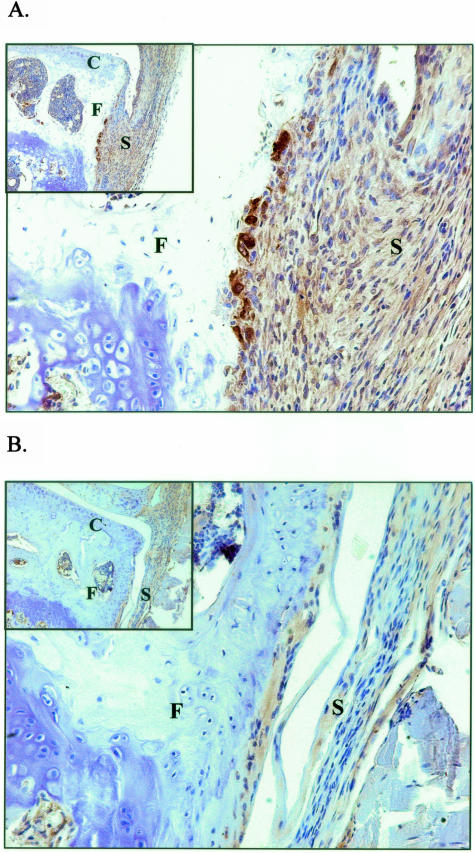
Histological analysis showed that the increased joint inflammation during flare-up of experimental arthritis is accompanied by marked bone erosions at day 4 (Figure 3A). In addition, immunostaining for cathepsin K, a marker for osteoclast-like activity, showed cathepsin K-positive multinucleate cells at sites of focal bone erosions in knee joints of rechallenged mice (Figure 4A). The degree of bone erosion was significantly suppressed by blocking IL-17 (Figure 3A), and also the osteoclast-like activity was clearly decreased during anti-IL-17 treatment (Figure 4B). Statistical analysis showed a strong correlation between the degree of bone erosions and the severity of joint inflammation during flare-up of AIA (Figure 3B). These findings suggest that neutralizing IL-17 prevents bone erosion by suppressing joint inflammation and osteoclastogenesis at the site of joint inflammation.
Figure 3.
Effect of anti-IL-17 treatment on bone erosion during flare-up of antigen-induced arthritis. Histological analysis at day 4 of flare-up shows that anti-IL-17 treatment reduces bone erosion in different parts of the murine knee joint (A) and that bone erosion is strongly correlated with the degree of joint inflammation (B). One group of mice was not flared to obtain background levels of joint pathology before flare-up of arthritis. P, patella; LF, lateral femur; MF, medial femur; Lig, ligament zone; LT, lateral tibia; MT, medial tibia. Results are the mean ± SEM of at least nine mice per group; *P < 0.01; **P < 0.005 versus control-treated group, by Mann Whitney _U_-test. Spearman R as calculated to determine the correlation between joint inflammation and bone erosion.
Figure 4.
Anti-IL-17 treatment during flare-up of experimental arthritis reduces cathepsin K staining as a marker for osteoclast-like activity. Arthritic mice were treated with control rabbit antibodies (A) or anti-IL-17 antibodies (B), and total knee joints were isolated at day 4 for histological analysis. F, femur; C, cartilage; S, synovium. Original magnification, ×100 and ×200. Pictures are representative of immunostainings on several joint sections of both groups.
Blocking IL-17 Results in Strong Down-Regulation of IL-1β, TNF-α, and RANKL
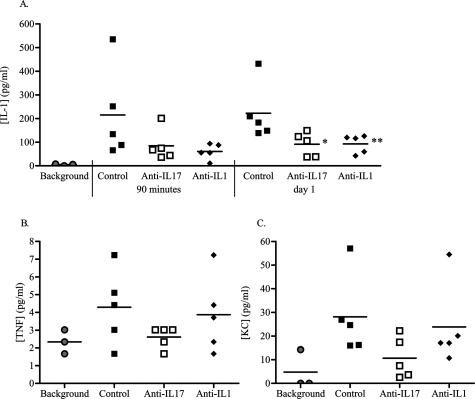
To examine the main processes regulated by IL-17 during the flare-up reaction, QPCR analysis on synovial biopsies from control and anti-IL-17-treated mice was performed. Arthritic control mice showed that mRNA expression for proinflammatory cytokines is clearly up-regulated at day 1 of flare-up (Table 1): IL-1β expression was increased more than 20-fold (4.4 PCR cycles), TNF-α circa fivefold (2.3 cycles), and IL-17 almost 40-fold (5.2 cycles). Antigen rechallenge also resulted in up-regulation of the osteoclast-differentiation factor RANKL for more than fivefold (2.4 PCR cycles) (Table 1). Anti-IL-17 treatment during flare-up almost completely prevented the up-regulation of IL-1 mRNA (Table 1), and cytokine analysis in synovial wash-outs of arthritic mice confirmed that IL-1 levels were significantly reduced by both anti-IL-17 and anti-IL-1 treatment (Figure 5A).
Table 1.
QPCR Results of Cytokine mRNA Levels in Murine Synovium at Day 1 of Flare of AIA with or without Anti-IL-17/IL-1 Treatment
| ΔΔCT (fold increase) | ||||
|---|---|---|---|---|
| IL-1β | TNFα | IL-17 | RANKL | |
| Control | 4.4 (21) | 2.3 (4.9) | 5.2 (37) | 2.4 (5.3) |
| Anti-IL-17 | 0.7 (1.6) | 1.0 (2.0) | 0 (0) | −1.2 (−2.3) |
| Anti-IL-1 | 3.6 (12) | 2.8 (7.0) | 3.4 (11) | 0.8 (1.7) |
Figure 5.
Neutralizing IL-17 or IL-1 during flare-up of arthritis reduces the levels of cytokines and chemokines in synovial washouts of arthritic mice. At various times after flare-induction, synovial washouts were collected and subsequently analyzed for cytokine and chemokine levels; IL-1β at 90 minutes and day 1 of flare-up (A); TNF-α at 90 minutes (B), and KCs at day 1 (C). Arthritic mice were treated with anti-IL-17, anti-IL-1, or control antibodies. One group of mice was not flared to obtain background expression levels before flare-up of arthritis. Results are the mean of five samples per group (n = 3 for background); *P < 0.05; **P < 0.01 versus control-treated group, by Mann Whitney _U_-test.
Interestingly, whereas IL-1 blocking hardly affected TNF expression, blocking IL-17 also reduced TNF-α mRNA and (although not significantly) TNF-α protein level (Table 1, Figure 5B), suggesting that TNF-α acts downstream of IL-17 during arthritic flares. Arthritic mice treated with anti-IL-17 antibodies also showed a trend for suppressed KC expression (Figure 5C), the murine equivalent of interleukin-8, and an important chemotactic factor for neutrophil recruitment. These data suggest that in addition to the proinflammatory capacity of IL-17 itself, this T-cell cytokine is an important upstream mediator driving the expression of TNF-α, IL-1β, RANKL, and KCs during reactivation of joint inflammation.
Discussion
In the present study, we clearly demonstrated the critical role of IL-17 in driving joint inflammation and cartilage depletion during flare-up of experimental arthritis. In addition, neutralizing IL-17 during antigen rechallenge prevents bone erosion and results in suppressed synovial expression of proinflammatory cytokines and chemokines.
The proinflammatory IL-17, mainly produced by activated (memory) T cells, is a likely contributor to the pathogenesis of rheumatoid arthritis. IL-17 is found in the synovium and synovial fluid of RA patients,22–24 and its proinflammatory and catabolic properties have been studied extensively both in vitro and in experimental arthritis. IL-17 stimulates the release of IL-1 and TNF by human macrophages25 and enhances the production of IL-6 and IL-8 by synovial fibroblasts.26 Both in vitro and in vivo experiments have shown cartilage breakdown by IL-17,27 effects that become more pronounced in combination with IL-1 or TNF.28 Blocking of endogenous IL-17 in the autoimmune CIA model showed that IL-17 plays a role in early stages of arthritis, but also later during disease progression.17,18 Furthermore, the spontaneous development of arthritis normally found in mice lacking IL-1Ra did not occur after crossing IL-17-deficient mice with IL-1Ra-deficient mice.19 Our recent study in mice deficient for IL-17R strongly suggested IL-17/IL-17R signaling to be a pivotal mechanism in turning a nondestructive macrophage-driven joint inflammation into a chronic T-cell-mediated destructive synovitis (unpublished data). These and other studies on IL-17 confirm the important role of this T-cell cytokine in onset and progression of arthritis. The novel findings in the present study show a critical role for IL-17 in the flare-up reaction of experimental arthritis, driving joint inflammation and bone erosion by regulating cytokine and chemokine expression.
In the model of AIA, flare-up can be induced in the arthritic knee joint by the local injection of a small amount of antigen (mBSA). This reactivation of a smoldering arthritis results in joint swelling, inflammation, and joint destruction. Previous studies in AIA-flare showed that T cells and the activation of T cells by antigen-presenting cells are required for flare-up of experimental arthritis.9,29 In our previous study with anti-IL-1 treatment in AIA-flare, IL-1 was found to be mainly involved in cartilage destruction. Joint swelling and early cell influx were for the greater part not IL-1-mediated,12 suggesting that other mediators are involved in these processes. In the present study, we selectively blocked the T-cell cytokine IL-17 with antibodies, and this markedly reduced joint swelling and inflammation during the antigen-induced flare-up. Furthermore, cartilage proteoglycan depletion was suppressed in the anti-IL-17-treated mice compared with the control group. Because IL-17 is highly up-regulated early after flare-up induction, neutralizing IL-17 protein prevents influx of inflammatory cells. This includes IL-17-producing activated T cells, leading to indirect down-regulation of mRNA expression of IL-17 in the synovium further on during the flare-up as was measured at day 1.
Besides the proinflammatory capacity of IL-17 itself, this T-cell cytokine plays an important role in driving synovial expression of other proinflammatory cytokines and chemokines. QPCR and cytokine analysis showed suppressed expression of IL-1, TNF-α, and KCs in mice treated with anti-IL-17 antibodies, suggesting that IL-17 acts as an upstream mediator driving joint inflammation. Anti-IL-17 treatment clearly reduced synovial TNF-α expression and completely prevented joint swelling, underlining the involvement of TNF-α in this process.30 In contrast, blocking IL-1 also resulted in reduced influx of inflammatory cells but failed to suppress joint swelling, possibly by lacking a reduction in TNF-α expression. Interestingly, our results also indicate that TNF-α acts downstream of IL-17 in the arthritis flare-up process and is not regulated by IL-1.
Down-regulation of IL-17 and its downstream mediators by anti-IL-17 treatment is probably not the only process by which joint inflammation is suppressed. Previous in vitro and ex vivo studies have shown the great capacity of IL-17 to synergize with TNF-α and IL-1 in the induction of other cytokines and chemokines31,32 and the degradation of cartilage33,34 and bone.35 The interaction of IL-17 with other cytokines during reactivation of experimental arthritis, in particular the additive and synergistic effects with TNF-α and IL-1, might play an important role in preventing joint pathology.
Synovial fluids from RA patients often contain clearly elevated levels of IL-17.23,24 This study demonstrated that antigen rechallenge in the arthritic joint induced marked bone erosion, and blocking of IL-17 was found to be highly effective in preventing this form of joint destruction. Previous anti-IL-17 treatment during rat adjuvant arthritis has also shown significant suppression of radiological scores.36 These findings suggest a possible beneficial effect of anti-IL-17 treatment in RA patients in the prevention of further bone damage during arthritic flares. The protective effect of neutralizing endogenous IL-17 reported here was supported by reduced synovial RANKL and cathepsin K expression. In this study, we found a strong correlation between the degree of bone erosion and the severity of joint inflammation. In the anti-IL-17-treated mice, the correlation between inflammation and bone erosion remained. Our findings suggest that the prevention of bone erosion by anti-IL-17 treatment can for a greater part be attributed to effective suppression of joint inflammation.
Data from the present study demonstrate the critical role of IL-17 in driving joint inflammation and cartilage depletion during flare-up of experimental arthritis. In addition, neutralizing IL-17 during antigen rechallenge prevents bone erosion and results in suppressed synovial expression of proinflammatory cytokines and chemokines. Because neutralizing IL-17 has been shown to be remarkably effective in this experimental model of arthritis exacerbation, blocking of IL-17 might be an interesting therapeutic target in the treatment of antigen-specific relapses during RA.
Footnotes
Address reprint requests to Marije Koenders, Radboud University Nijmegen Medical Center, Department of Rheumatology, Experimental Rheumatology and Advanced Therapeutics, 189, Geert Grooteplein Zuid 26-28, PO Box 9101, 6500 HB Nijmegen, The Netherlands. E-mail: m.koenders@reuma.umcn.nl.
Supported by the Dutch Arthritis Association (grant NR 00-1-302), by Veni Fellowship grant 906-02-038 (to E.L.) of the Netherlands Organization for Scientific Research, and by a grant from Novartis Pharma AG (Basel, Switzerland).
References
- Kremer JM, Westhovens R, Leon M, Di Giorgio E, Alten R, Steinfeld S, Russell A, Dougados M, Emery P, Nuamah IF, Williams GR, Becker JC, Hagerty DT, Moreland LW. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med. 2003;349:1907–1915. doi: 10.1056/NEJMoa035075. [DOI] [PubMed] [Google Scholar]
- Firestein GS. The T cell cometh: interplay between adaptive immunity and cytokine networks in rheumatoid arthritis. J Clin Invest. 2004;114:471–474. doi: 10.1172/JCI22651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goronzy JJ, Weyand CM. T-cell regulation in rheumatoid arthritis. Curr Opin Rheumatol. 2004;16:212–217. doi: 10.1097/00002281-200405000-00008. [DOI] [PubMed] [Google Scholar]
- Bennett SR, Falta MT, Bill J, Kotzin BL. Antigen-specific T cells in rheumatoid arthritis. Curr Rheumatol Rep. 2003;5:255–263. doi: 10.1007/s11926-003-0003-y. [DOI] [PubMed] [Google Scholar]
- Burger D, Dayer JM. The role of human T-lymphocyte-monocyte contact in inflammation and tissue destruction. Arthritis Res Ther. 2002;4:S169–S176. doi: 10.1186/ar558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idogawa H, Imamura A, Onda M, Umemura T, Ohashi M. Progression of articular destruction and the production of tumour necrosis factor-alpha in antigen-induced arthritis in rabbits. Scand J Immunol. 1997;46:572–580. doi: 10.1046/j.1365-3083.1997.d01-174.x. [DOI] [PubMed] [Google Scholar]
- Van de Loo FAJ, Arntz OJ, Bakker AC, Van Lent PLEM, Jacobs MJM, Van den Berg WB. Role of interleukin-1 in antigen-induced exacerbations of murine arthritis. Am J Pathol. 1995;146:239–249. [PMC free article] [PubMed] [Google Scholar]
- Brackertz D, Mitchell GF, Vadas MA, Mackay IR. Studies on antigen-induced arthritis in mice. III. Cell and serum transfer experiments. J Immunol. 1977;118:1645–1648. [PubMed] [Google Scholar]
- Buchner E, Brauer R, Schmidt C, Emmrich F, Kinne RW. Induction of flare-up reactions in rat antigen-induced arthritis. J Autoimmun. 1995;8:61–74. [PubMed] [Google Scholar]
- Van den Broek MF, Van Bruggen MCJ, Stimpson SA, Severijnen AJ, Van de Putte LBA, Van den Berg WB. Flare-up reaction of streptococcal cell-wall induced arthritis in Lewis and F344 rats: the role of lymphocytes-T. Clin Exp Immunol. 1990;79:297–306. doi: 10.1111/j.1365-2249.1990.tb05194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lens JW, Van den Berg WB, Van de Putte LBA, Berden JHM, Lems SPM. Flare-up of antigen-induced arthritis in mice after challenge with intravenous antigen: effects of pre-treatment with cobra venom factor and anti-lymphocyte serum. Clin Exp Immunol. 1984;57:520–528. [PMC free article] [PubMed] [Google Scholar]
- Jacobs MJM, Van den Hoek AEM, Van Lent PLEM, Van de Loo FAJ, Van de Putte LBA, Van den Berg WB. Role of IL-2 and IL-4 in exacerbations of murine antigen-induced arthritis. Immunology. 1994;83:390–396. [PMC free article] [PubMed] [Google Scholar]
- Li H, Chen J, Huang A, Stinson J, Heldens S, Foster J, Dowd P, Gurney AL, Wood WI. Cloning and characterization of IL-17B and IL-17C, two new members of the IL-17 cytokine family. Proc Natl Acad Sci USA. 2000;97:773–778. doi: 10.1073/pnas.97.2.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starnes T, Broxmeyer HE, Robertson MJ, Hromas R. Cutting edge: IL-17D, a novel member of the IL-17 family, stimulates cytokine production and inhibits hemopoiesis. J Immunol. 2002;169:642–646. doi: 10.4049/jimmunol.169.2.642. [DOI] [PubMed] [Google Scholar]
- Lee J, Ho WH, Maruoka M, Corpuz RT, Baldwin DT, Foster JS, Goddard AD, Yansura DG, Vandlen RL, Wood WI, Gurney AL. IL-17E, a novel proinflammatory ligand for the IL-17 receptor homolog IL-17Rh1. J Biol Chem. 2001;276:1660–1664. doi: 10.1074/jbc.M008289200. [DOI] [PubMed] [Google Scholar]
- Starnes T, Robertson MJ, Sledge G, Kelich S, Nakshatri H, Broxmeyer HE, Hromas R. Cutting edge: IL-17F, a novel cytokine selectively expressed in activated T cells and monocytes, regulates angiogenesis and endothelial cell cytokine production. J Immunol. 2001;167:4137–4140. doi: 10.4049/jimmunol.167.8.4137. [DOI] [PubMed] [Google Scholar]
- Lubberts E, Joosten LAB, Oppers B, Van den Bersselaar L, Coenen-de Roo CJ, Kolls JK, Schwarzenberger P, Van de Loo FA, Van den Berg WB. IL-1-independent role of IL-17 in synovial inflammation and joint destruction during collagen-induced arthritis. J Immunol. 2001;167:1004–1013. doi: 10.4049/jimmunol.167.2.1004. [DOI] [PubMed] [Google Scholar]
- Lubberts E, Koenders MI, Oppers-Walgreen B, Van den Bersselaar L, Coenen-de Roo CJJ, Joosten LAB, Van den Berg WB. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 2004;50:650–659. doi: 10.1002/art.20001. [DOI] [PubMed] [Google Scholar]
- Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci U S A. 2003;100:5986–5990. doi: 10.1073/pnas.1035999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Loo FAJ, Joosten LAB, Van Lent PLEM, Arntz OJ, Van den Berg WB. Role of interleukin-1, tumor-necrosis-factor-alpha, and interleukin-6 in cartilage proteoglycan metabolism and destruction: effect of in-situ blocking in murine antigen-induced and zymosan-induced arthritis. Arthritis Rheum. 1995;38:164–172. doi: 10.1002/art.1780380204. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate phenol chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chabaud M, Durand JM, Buchs N, Fossiez F, Page G, Frappart L, Miossec P. Human interleukin-17: a T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 1999;42:963–970. doi: 10.1002/1529-0131(199905)42:5<963::AID-ANR15>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N, Gillespie MT, Martin TJ, Suda T. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziolkowska M, Koc A, Luszczykiewicz G, Ksiezopolska-Pietrzak K, Klimczak E, Chwalinska-Sadowska H, Maslinski W. High levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanism. J Immunol. 2000;164:2832–2838. doi: 10.4049/jimmunol.164.5.2832. [DOI] [PubMed] [Google Scholar]
- Jovanovic DV, Di Battista JA, Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, Mineau F, Pelletier JP. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol. 1998;160:3513–3521. [PubMed] [Google Scholar]
- Hwang SY, Kim JY, Kim KW, Park MK, Moon Y, Kim WU, Kim HY. IL-17 induces production of IL-6 and IL-8 in rheumatoid arthritis synovial fibroblasts via NF-kappa B- and PI3-kinase/Akt-dependent pathways. Arthritis Res Ther. 2004;6:R120–R128. doi: 10.1186/ar1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L, Yin JP, Starovasnik MA, Hogue DA, Hillan KJ, Mort JS, Filvaroff EH. Pathways by which interleukin 17 induces articular cartilage breakdown in vitro and in vivo. Cytokine. 2001;16:10–21. doi: 10.1006/cyto.2001.0939. [DOI] [PubMed] [Google Scholar]
- Chabaud M, Lubberts E, Joosten LAB, Van den Berg WB, Miossec P. IL-17 derived from juxta-articular bone and synovium contributes to joint degradation in rheumatoid arthritis. Arthritis Res. 2001;3:168–177. doi: 10.1186/ar294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Broek MF, Van den Berg WB, Van de Putte LBA. Monoclonal anti-Ia antibodies suppress the flare up reaction of antigen-induced arthritis in mice. Clin Exp Immunol. 1986;66:320–330. [PMC free article] [PubMed] [Google Scholar]
- Van den Berg WB, Joosten LAB, Van de Loo FAJ. TNF alpha and IL-1 beta are separate targets in chronic arthritis. Clin Exp Rheumatol. 1999;17:S105–S114. [PubMed] [Google Scholar]
- Katz Y, Nadiv O, Beer Y. Interleukin-17 enhances tumor necrosis factor alpha-induced synthesis of interleukins 1,6, and 8 in skin and synovial fibroblasts: a possible role as a “fine-tuning cytokine” in inflammation processes. Arthritis Rheum. 2001;44:2176–2184. doi: 10.1002/1529-0131(200109)44:9<2176::aid-art371>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Chabaud M, Fossiez F, Taupin JL, Miossec P. Enhancing effect of IL-17 on IL-1-induced IL-6 and leukemia inhibitory factor production by rheumatoid arthritis synoviocytes and its regulation by Th2 cytokines. J Immunol. 1998;161:409–414. [PubMed] [Google Scholar]
- Koshy PJ, Henderson N, Logan C, Life PF, Cawston TE, Rowan AD. Interleukin 17 induces cartilage collagen breakdown: novel synergistic effects in combination with proinflammatory cytokines. Ann Rheum Dis. 2002;61:704–713. doi: 10.1136/ard.61.8.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bezooijen RL, Wee-Pals L, Papapoulos SE, Lowik CW. Interleukin 17 synergises with tumour necrosis factor alpha to induce cartilage destruction in vitro. Ann Rheum Dis. 2002;61:870–876. doi: 10.1136/ard.61.10.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabaud M, Miossec P. The combination of tumor necrosis factor alpha blockade with interleukin-1 and interleukin-17 blockade is more effective for controlling synovial inflammation and bone resorption in an ex vivo model. Arthritis Rheum. 2001;44:1293–1303. doi: 10.1002/1529-0131(200106)44:6<1293::AID-ART221>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Bush KA, Farmer KM, Walker JS, Kirkham BW. Reduction of joint inflammation and bone erosion in rat adjuvant arthritis by treatment with interleukin-17 receptor IgG1 Fc fusion protein. Arthritis Rheum. 2002;46:802–805. doi: 10.1002/art.10173. [DOI] [PubMed] [Google Scholar]