Flk2+ myeloid progenitors are the main source of Langerhans cells (original) (raw)
Abstract
Langerhans cells (LCs) are antigen-presenting cells (APCs) residing in the epidermis that play a major role in skin immunity. Our earlier studies showed that when skin is inflamed LCs are replaced by bone marrow-derived progenitor cells, while during steady-state conditions LCs are able to self-renew in the skin. Identification of the LC progenitors in bone marrow would represent a critical step toward identifying the factors that regulate LC generation as well as their trafficking to the skin. To determine LC lineage origin, we reconstituted lethally irradiated CD45.2 mice with rigorously purified lymphoid and myeloid progenitors from CD45.1 congenic mice. Twenty-four hours later, we exposed the mice to UV light to deplete resident LCs and induce their replacement by progenitors. Reconstitution with common myeloid progenitors (CMPs), common lymphoid progenitors (CLPs), granulocyte-macrophage progenitors (GMPs), or early thymic progenitors led to LC generation within 2 to 3 weeks. CMPs were at least 20 times more efficient at generating LCs than CLPs. LCs from both lineages were derived almost entirely from fetal liver kinase-2+ (Flk-2+) progenitors, displayed typical dendritic-cell (DC) morphology, and showed long-term persistence in the skin. These results indicate that LCs are derived mainly from myeloid progenitors and are dependent on Flt3-ligand for their development.
Introduction
Langerhans cells (LCs) belong to the family of dendritic cells (DCs) and are specifically localized in the epidermis and other squamous epithelia.1 As the primary antigen-presenting cells in the skin, both LCs and dermal dendritic cells play a major role in skin immunity.1,2 In addition, LCs are important for the onset of skin graft-versus-host disease3 and may also trigger the skin disease psoriasis.4 Like other DCs, LCs ingest foreign antigens and upon activation migrate to the T-cell areas of skin draining lymph nodes.1,5 Although LCs were identified in 1868 by Paul Langerhans,6 less is known about their developmental origin compared with other DC subsets. While the immune functions of LCs are well characterized, their origin remains controversial. LCs in mice express CD11b, which is otherwise found mainly on myeloid cells, but during activation these cells express CD8α, once considered as a marker of lymphoid cells.7 Moreover, murine LCs can be generated in vivo from thymic progenitors.8 Human LCs express the myeloid marker CD33 and can be generated in vitro from monocytes as well as from CD14+ cells present in the dermis,9 but they can also be generated from a lymphoid progenitor.10
In the mouse, a number of phenotypically defined bone marrow (BM) progenitor populations have been used to study the origin of DC subsets other than LCs. Common myeloid progenitors (CMPs) and common lymphoid progenitors (CLPs) have proved to be particularly useful in determining the lineage origin of DCs, as they give rise only to cells of myeloid and lymphoid lineage, respectively, with the exception of rare CMP-derived B cells.11,12 Other murine-cell populations that have been reported to give rise to DCs in adoptive transfer models are monocytes13 and a CD11c+/I-Ab- population present in blood14 and bone marrow.15 In addition to adoptive transfer models, several gene-deficient mouse models have been used to study the requirements for DC development.16-18 Of the factors identified in these models, only the lack of the growth factor TGF-β specifically impairs LC development.16
LCs originate from the BM,19 but unlike other DC subtypes, LCs are not replaced by BM-derived progenitor cells during steady-state conditions.20 Rather, under such conditions, LCs turn over slowly and their numbers are maintained by local precursors present in the skin. Recruitment of BM-derived LC progenitors to the skin requires an inflammatory signal that can be induced by UV light and depends on the local production of inflammatory cytokines such as CCL20.20,21 In the current study, we took advantage of this requirement to study the BM source of LCs. Our results show that, under the conditions studied, both lymphoid and myeloid progenitors can give rise to LCs. However, LC reconstitution by myeloid progenitors is at least 20 times more efficient, suggesting that LCs are mainly of myeloid origin.
Materials and methods
Animals
Four- to 8-week-old C57BL/6 mice (CD45.1) or congenic C57BL/6 mice (CD45.2) were obtained from Jackson Laboratories (Bar Harbor, ME) or the Stanford Animal Facility (Stanford, CA). All animals were maintained according to the PHS Policy for Humane Care and Use of Laboratory Animals.
Cytokines and media
Epidermal cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (Gibco/Invitrogen, Gaithersburg, MD), l-glutamine (5 mM; Gibco/Invitrogen), and penicillin G (100 U/mL) and streptomycin (100 μg/mL; Bio-Whittaker, Walkersville, MD). Recombinant murine GM-CSF and murine TNF-α were purchased from Peprotech (Rocky Hill, NJ) and reconstituted in sterile PBS. Cell staining and sorting were done in PBS with 2% FBS and 0.05% sodium azide.
Antibodies
The following fluorochrome-conjugated antibodies were produced in our laboratories: M1/70 (anti-Mac-1/CD11b), 8C5 (anti-Gr-1), 6B2 (anti-B220), KT-31 (anti-CD3), GK1.5 (anti-CD4), 53-6.7 (anti-CD8), A7R34 (anti-IL-7R/CD127), 2B8 (anti-c-Kit/CD117), 3C11 (anti-c-Kit/CD117), E13-161-7 (anti-Sca-1), 19XE5 (anti-Thy-1.1), A20.1.7 (anti-CD45.1), and AL1-4A2 (anti-CD45.2). Goat anti-rat IgG (PE or Cy5-PE conjugated) was purchased from Caltag (Burlingame, CA). Monoclonal antibodies to I-Ab, B220, CD3, CD4, CD8, CD11b, CD11c, CD16/CD32, CD19, CD34, CD44, CD45.1, CD45.2, Gr-1, and Flk2 and isotype controls were purchased from BDPharmingen (San Diego, CA). For visualization of biotinylated antibodies, streptavidin-conjugated PE, Cy5-PE, Cy7-PE, and Texas Red (Invitrogen, Carlsbad, CA) were used. CCR2 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and detected by a secondary anti-goat IgG-PE antibody (Caltag). CCR6-PE was purchased from R&D Systems (Minneapolis, MN).
Isolation of hematopoietic progenitor cells from BM
Progenitor populations were isolated from BM or thymus based on phenotype as previously described: hematopoietic stem cells (HSCs; Lin-/CD127-/c-Kithi/Scahi/Thy1.1lo)22; common myeloid progenitors (CMPs; Lin-/CD34+/CD16/32-/c-Kithi/Sca-); granulocyte/macrophage progenitors (GMPs; Lin-/CD34+/CD16/32+/c-Kithi/Sca-)23; common lymphoid progenitors (CLPs; Lin-/CD127+/c-Kitint/Scaint/Thy1.1-)24; thymic progenitors pro-T1 (Lin-/CD44hi/c-Kithi/Thy1.1+/CD25-) and pro-T2 (Lin-/CD44hi/c-Kithi/Thy1.1+/CD25+).25 All populations were double sorted on a FACSVantage SE (BD, San Jose, CA) to high purity (> 99%). Lineage specificity of progenitor populations was confirmed by flow cytometric analysis of spleen and thymus of mice that underwent transplantation.
Transplantation of congenic hematopoietic progenitor cells and recruitment to the skin
Mice received 9.5 Gy x-irradiation prior to intravenous injection of progenitors in 150 μL sterile PBS. In order to ensure survival, mice received 3 × 105 autologous BM cells together with the progenitors. Twenty-four hours after progenitor transfer, mice were exposed to UV light (wavelength: 254 nm; voltage: 8 W; source: 38 cm from target) for 20 minutes.
LC isolation
LCs were isolated from epidermal sheets of mouse ears as described previously20 to determine progenitor-derived LCs at 2 to 3 weeks after transplantation. Briefly, ears were incubated in 0.5% trypsin/5 mM EDTA, which allows separation of epidermal sheets from the dermis. The epidermal sheets were cultivated in RPMI/10% FBS supplemented with GM-CSF and TNF-α for 24 hours, and migratory cells were stained with I-Ab-FITC, CD45.1-PE and CD11c-APC, B220-TXR, and TCRγ/δ-biotin antibodies for flow cytometric analysis.
In situ immunofluorescence
Epidermal sheets for in situ immunofluorescence staining were obtained by shaving mouse ears and cultivating the dorsal and ventral halves of the ears in 0.5 M ammonium thiocyanate to separate the epidermis from the dermis. Epidermal sheets were then fixed with acetone and double-labeled with I-Ab-FITC/CD45.1-PE overnight followed by extensive washing and immersion in mounting media (Molecular Probes, Eugene, OR). Stainings were analyzed on a Leica microscope (Leica, Wetzlar, Germany) at a 10 × 40 magnification and pictures were processed using Openlab software (Improvision, Lexington, MA) and Adobe Photoshop software (Adobe Systems, San Jose, CA).
Cytospin preparations
Purified donor-derived (CD45.1+) epidermal Langerhans cells (CD11c+ I-Ab+ B220-TCRγδ-) were spun onto glass slides, dried overnight, stained for 1 minute in 0.3% Wright solution (Sigma, St Louis, MO), and rinsed in distilled water. Stainings were analyzed using a Leica microscope at a 1.6 × 10 × 40 magnification.
Reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was isolated using the TRIZOL Reagent (Invitrogen) according to the manufacturer's protocol. As cell numbers were below 1 × 104 cells, 10 μg/mL linear acrylamide (Ambion, Austin, TX) was used as a carrier. All RNA samples were treated with DnaseI (Invitrogen) to avoid genomic DNA contamination and reverse-transcribed into cDNA using the SuperScript First Strand Synthesis System (Invitrogen) according to the manufacturer's protocol. Oligonucleotide sequences and annealing temperatures used were as follows: Cd207 (Langerin) primer (forward) 5′-AAGAGTGATGCCCAGATGTTGAAA-3′ and (reverse) 5′-TTGGGGTGCGTGAAAAGTAATAGA-3′ (tm, 57°C), and the expected product length was 418 bp; Actb (β-actin) primer (forward) 5′ACGAGGCCCAGAGCAAGAGAGG-3′ and (reverse) 5′-AGCCACCGATCCACACAGAGT A-3′ (tm 68°C). CCR2 and CCR6 primers were used as described previously.26,27
Results
Myeloid progenitors give rise to epidermal LCs in vivo
To study the origin of LCs, lethally irradiated mice received transplants of defined BM progenitor populations obtained from congenic donors (Figure 1A). Twenty-four hours after transplantation, the mice were exposed to UV light to induce the recruitment of LC progenitors to the skin. As shown in Figure 1B, 2 weeks after CD45.2 recipients of 1 × 104 CD45.1+ CMPs or 4 × 104 CD45.1+ GMPs were exposed to UV light, donor-derived I-Ab/CD11c+ LCs were easily detected by flow cytometric analysis of epidermal-cell suspensions. On average, between 5% and 10% of the LCs were replaced by donor-derived cells following transplantation of 1 × 104 CMPs, with somewhat higher reconstitution if 3 × 104 CMPs were transplanted (Table 1). To ensure the purity of progenitor populations, all progenitors were double sorted. Flow cytometric analysis of spleen and thymus confirmed that each progenitor population gave rise to its expected lineages and not to other lineages. No lymphoid cells were ever obtained from GMPs, while rare B cells, but no T cells, could be detected after reconstitution with CMPs (Supplemental Figure S1, available at the Blood website; click on the Supplemental Figure link at the top of the online article).
Figure 1.

LCs develop from myeloid progenitors after UV light-induced skin inflammation. (A) Transplantation model. Lethally irradiated CD45.2 C57Bl/6 mice were injected intravenously with progenitor cells isolated from congenic CD45.1 mice plus 105 CD45.2 BM cells to ensure survival. Twenty-four hours after progenitor transplantation, mice received 20 minutes of UV light treatment to recruit progenitor cells to the skin. LC chimerism was analyzed by flow cytometry of epidermal cells. (B) Histogram plots show CD45.1 expression of epidermal cells isolated from mice 2 weeks after transplantation with 104 CMPs or 4 × 104 GMPs, or control mice. Control mice received transplants of autologous support BM cells only. Contour plots show CD11c/I-Ab expression profile of gated CD45.1+ and CD45.1- epidermal cells. Percentages of LCs derived from host- or donor-derived cells are indicated. Data are representative of at least 5 mice that underwent transplantation per progenitor population.
Table 1.
LC reconstitution by various progenitor populations at different time points after adoptive transfer
| Reconstitution of LCs and total epidermal cells by transplanted progenitors, % | |||||||
|---|---|---|---|---|---|---|---|
| 2 wk after transfer | 3 wk after transfer | 3 mo after transfer | |||||
| Progenitor population | Progenitor no, × 103 | dEC/tEC, % average (range) | dLC/tLC, % average (range) | dEC/tEC, % average (range) | dLC/tLC, % average (range) | dEC/tEC, % average (range) | dLC/tLC, % average (range) |
| HSCs | 3 | 7.14 (0.7-27.7) | 17.68 (7.1-35.2) | 2.58 (0.9-8.4) | 22.40 (11.5-40.2) | 2.55 (1.1-4.0) | 16.50 (3.5-23.1) |
| CMPs | 10 | 0.43 (0.07-1.1) | 8.72 (1.8-14.0) | ND | ND | 0.5 (0.24-1.0) | 5.15 (3.1-6.7) |
| CMPs | 30 | 0.58 (0.2-0.9) | 14.86 (4.3-29.2) | ND | ND | ND | ND |
| GMPs | 40 | 0.09 (0.01-0.17) | 1.60 (0.1-4.6) | ND | ND | 0.12 (0.04-0.29) | 1.59 (1.3-2.3) |
| CLPs | 10 | 0.04 (0-0.1) | 0.13 (0-0.6) | 0.07 (0.03-0.12) | 0.46 (0.1-1.2) | 0.48 (0.15-0.65) | 1.55 (0.6-2.4) |
| CLPs | 30 | 0.02 (0.01-0.04) | 0.39 (0.23-0.55) | 0.11 (0.09-0.13) | 0.45 (0.38-0.52) | ND | ND |
| Pro-T1 | 50 | 0.09 (0.03-0.2) | 0.55 (0-1.1) | 0.35 (0.06-0.81) | 3.44 (0.1-4.9) | ND | ND |
| Pro-T2 | 60 | 0.01 (0-0.02) | 0.09 (0-0.3) | 0.09 (0.03-0.25) | 0.28 (0-1.1) | ND | ND |
| Control | 0 | 0.02 (0-0.07) | 0 | 0.02 (0-0.06) | 0.04 (0-0.2) | 0.08 (0-0.18) | 0.12 (0-0.44) |
Epidermal LCs can be generated by lymphoid progenitors at low efficiency
To determine if lymphoid progenitor cells are also capable of giving rise to LCs after UV light-induced skin inflammation, mice received transplants of either CLPs or early thymic progenitors, such as pro-T1 or pro-T2 cells. At 2 weeks after transfer of 1 × 104 CLPs, few CLP-derived LCs could be detected (Figure 2A). As the maximum peak of thymic and splenic DCs generated by CLPs is reached at about 3 weeks after intravenous transplantation of 1 × 104 CLPs,12 we decided to evaluate the capacity of CLPs to give rise to LCs after 3 weeks. Indeed, a small but significant number of CLP-derived LCs were present in the epidermis at this time point, as determined by CD45.1 expression of CD11c+/I-Ab+ cells (Figure 2A). LC reconstitution efficiency by CLPs could not be increased by transplantation of higher CLP numbers (3 × 104 cells) (Table 1). On average, 0.46% of the LCs were CLP derived after 3 weeks compared with 8.7% CMP-derived LCs after 2 weeks (Table 1). As shown in Figure 2B, CLP-derived LCs did not express lymphoid markers, such as B220 or TCRγδ, suggesting that these cells were similar to conventional LCs. The lower LC reconstitution capacity of CLPs compared with CMPs was not due to a low repopulation capacity of CLPs, as significant numbers of CLP-derived lymphoid cells but no myeloid cells could be detected in spleen or thymus (Figure 2C and Supplemental Figure S1).
Figure 2.
LC reconstitution by lymphoid progenitor cells. (A) Histogram plots represent CD45.1 expression profile of epidermal-cell suspensions 2 and 3 weeks after adoptive transfer of either 104 CLPs or 5 × 104 pro-T1 or control cells (autologous BM only). Contour plots show corresponding I-Ab/CD11c expression profile of gated CD45.1+ and CD45.1- epidermal cells from mice that underwent transplantation. (B) Contour plot shows B220/TCRγδ profile of CLP-derived LCs (CD45.1+, I-Ab+, CD11c+ population) isolated from the epidermis of mice that received transplants of CLPs at 3 weeks after adoptive transfer. (C) Top contour plot shows CD45.1/CD45.2 expression profile of total spleen cells at 3 weeks after adoptive transfer of 104 CLPs. Bottom contour plot represents TCR/CD19 expression profile of gated CLP-derived (CD45.1+) spleen cells. (D) Chart shows average LC reconstitution (% of total LCs) obtained per 104 progenitor cells at 2 weeks (both myeloid and lymphoid progenitors) and 3 weeks (only CLPs, pro-T1, pro-T2) after adoptive transfer. ND indicates not determined. Data are representative of at least 5 transplantations per progenitor population with similar results.
Although CMPs and CLPs differ significantly in their LC generation capacity, nearly all donor-derived cells in the epidermis were LC independent of the progenitor population and the differences in total LC reconstitution efficiency are based mainly on variations of the overall presence of progenitor-derived cells in the skin (Figures 1, 2; Table 1). On a per-cell basis, CMPs are 67 times more efficient in generating LCs than CLPs after 2 weeks and still 19 times more efficient if compared with the optimum LC readout for CLPs at 3 weeks (Figure 2D; Table 1). The thymic progenitor populations, pro-T1 and pro-T2, were also able to generate LCs at low efficiency (Figure 2; Table 1). On a per-cell basis, LC reconstitution by pro-T1 3 weeks after transfer was comparable with CLPs, while reconstitution by pro-T2 was about 10 times less efficient than CLPs (Figure 2D).
Both myeloid- and lymphoid-derived LCs persist in the skin
In contrast to other DCs, in the absence of inflammation, LCs are capable of maintaining themselves in the skin for more than one year. To determine if the LCs derived from progenitor cells during inflammation can provide a durable source of LCs in the skin, we analyzed epidermal-cell suspensions for the presence of donor-derived LCs 3 months after transplantation and UV light treatment. At this time point, we still could detect both CMP- and CLP-derived LCs in the skin, and the ratio of CMP progenitor-derived LCs was in the same range as obtained after short-term analysis (Figure 3A; Table 1). In contrast, the LC reconstitution efficiency of CLPs increased over time, but remained substantially lower than that of CMPs (Figure 3B; Table 1). These results indicate that the optimum time point to detect LC reconstitution after CLP transfer is later than for other CLP-derived DC populations. Even after transplantation of the further differentiated GMP population, it was still possible to find GMP-derived LCs at 3 months after UV light-induced inflammation (Figure 3A). To exclude false-positive results due to contamination of transplanted progenitors with HSCs, spleen and thymus were analyzed for the presence of donor-derived CD45.1+ cells at the time of analysis and none was detected (data not shown).
Figure 3.
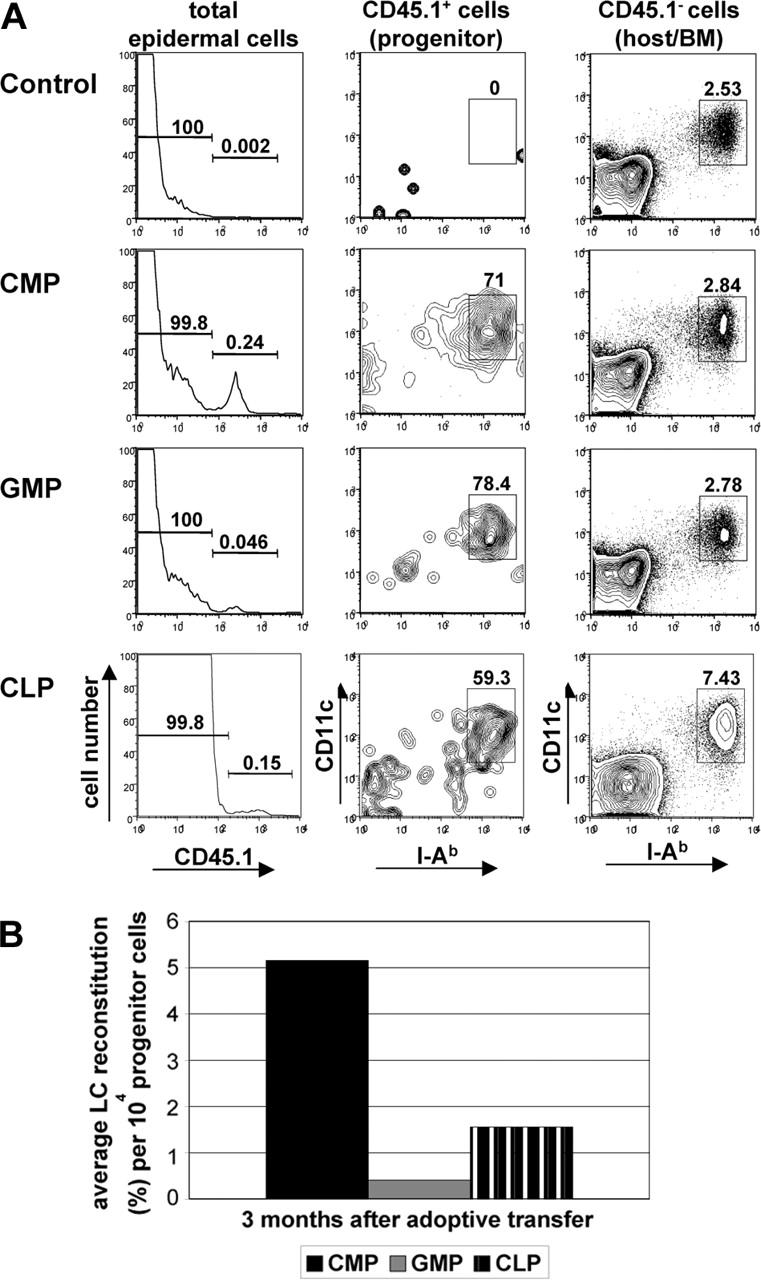
Progenitor-derived LCs persist in the skin. Epidermal cells were isolated from ears of chimeric mice 3 months after progenitor transfer. (A) Histograms in left panel show CD45.1 expression profile of total epidermal cells derived from CD45.2 mice reconstituted with either 104 CD45.1 CMPs, 4 × 104 GMPs, or 104 CLPs. Contour plots show CD11c/I-Ab expression profile of gated donor (CD45.1+) or host-derived (CD45.1-) epidermal cells. Percentages of LCs derived from transplanted cells (CD45.1+) or host cells (CD45.1-) are indicated. (B) Chart shows average LC reconstitution (% of total LCs) obtained per 104 progenitor cells at 3 months after adoptive transfer. Data are representative of at least 3 transplantations per progenitor population with similar results.
Progenitor-derived epidermal LCs display the same morphology as host-derived epidermal LCs
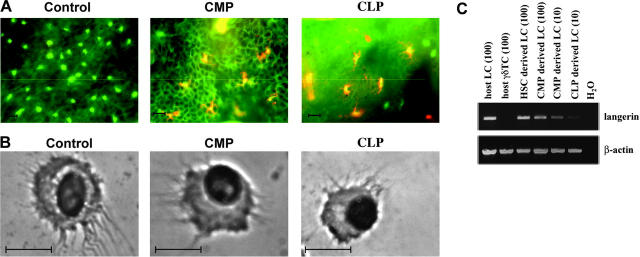
A distinguishing feature of LCs and other DCs is their characteristic morphology. To assess the morphology and distribution of donor-derived LCs, we double stained epidermal sheets obtained from mice that received transplants of progenitors with antibodies to I-Ab and CD45.1. The results confirmed the presence of CD45.1+/I-Ab+ DC-like cells located in the epidermis (Figure 4A). The cells always appeared in clusters, typically in areas devoid of host LCs. The morphology of progenitor-derived LCs was further assessed in cytospin preparations of sorted CD45.1+/CD11c+/I-Ab+/B220-/TCRγδ- cells. Both CMP- and CLP-derived LCs displayed typical DC morphology (Figure 4B). To further characterize myeloid- and lymphoid-derived LCs, we analyzed sorted CD45.1+/CD11c+/I-Ab+/B220-/TCRγδ- cells for expression of Cd207 mRNA, as Langerin is a reliable LC marker in both humans and mice. Langerin expression could be detected at similar levels in all sorted LC populations irrespective of their developmental origin (Figure 4C). Sorted epidermal γδT cells served as a negative control.
Figure 4.
Morphology of CLP- and CMP-derived LCs. (A) Immunofluorescence staining with anti-CD45.1-PE and anti-I-Ab-FITC of epidermal sheets prepared from mice 3 months after transplantation and UV light exposure with either 104 CD45.1 CMPs or 104 CD45.1 CLPs. Epidermal sheets from CD45.2 mice that received transplants of only autologous BM cells served as controls. Scale bar: 10 μm. (B) Cytospin preparations of sorted CMP- or CLP-derived LCs. Sorted CD45.1+/I-Ab+/CD11c+/B220-/TCRγδ- epidermal cells derived from mice that received transplants of either CMPs or CLPs at 3 months after transfer were spun onto glass slides and stained with Wright solution. Scale bar: 10 μm. (C) RT-PCR analysis of Cd207 expression in sorted CD45.1+/I-Ab+/CD11c+/B220-/TCRγδ- epidermal cells. c-DNA was prepared at 4 weeks after transplantation from mice reconstituted with either 104 CD45.1 CMPs or 104 CD45.1 CLPs. cDNAs from LC numbers used per PCR reaction are given in brackets. Actb primers acted as controls. Data are based on at least 2 experiments with similar results.
The capacity to generate LCs lies mainly in the Flk2+ fractions of CMPs and CLPs
Flt3-ligand (FL) represents an important growth factor for the generation of DC subsets in the spleen.28,29 To study the role of FL in LC development, we took advantage of the fact that both CMPs and CLPs are heterogeneous with respect to their expression of the FL receptor, fetal liver kinase-2 (Flk-2).28,29 When mice were reconstituted with Flk2+ and Flk2- fractions of CMPs and CLPs, only the Flk2+ progenitors from CLPs gave rise to LCs as determined by flow cytometry (Figure 5A) and immunofluorescence staining of epidermal sheets (Figure 5B). On average, 1.0% of total LCs was reconstituted with 1 × 104 Flk2+ CLPs at 3 weeks after adoptive transfer, which is about twice the number obtained after transfer of 1 × 104 unseparated CLPs (Figure 5C). This corresponds to the percentage of Flk2+ cells within the CLP population (50%-70%). For CMPs, the LC reconstitution by donor-derived cells averaged 11.4% 2 weeks after transfer of 1 × 104 Flk2+ CMPs, compared with 8.7% after transfer of 1 × 104 CMPs or 2.6% after transfer of 1 × 104 Flk2- CMPs (Figure 5C).
Figure 5.
The potential to reconstitute LCs resides preferentially in the fraction of CLPs and CMPs expressing the Flt3 receptor. (A) Histograms in upper panel show CD45.1 expression profile of total epidermal cells derived from CD45.2 mice reconstituted with 104 CD45.1 CMPs or CLPs either expressing or lacking Flk2. Contour plots show CD11c/I-Ab expression profile of gated donor (CD45.1+) epidermal cells derived from the respective progenitor populations. Epidermal cells were analyzed for the presence of progenitor-derived LCs at 2 weeks (CMPs) or 3 weeks (CLPs). Percentages of LCs derived from transplanted cells (CD45.1+) or host cells (CD45.1-) are indicated. (B) Epidermal sheets from mice were prepared from mice that received transplants of 104 CD45.1+ CMPs (Flk2+ or Flk2-) at 2 weeks after transplantation and from mice reconstituted with either 104 CD45.1 Flk2+ CLPs or CD45.1 Flk2- CLPs at 3 weeks after transplantation. Shown are the overlays of photomicrographs obtained after immunofluorescence staining of epidermal sheets with anti-CD45.1-PE (red) and anti-I-Ab-FITC (green). Scale bar: 100 μm. Data are representative of at least 3 transplantations for each progenitor population with similar results. (C) Chart shows average LC reconstitution (% of total LCs) obtained per 104 progenitor cells (Flk2+, Flk2-, or unseparated) at 2 weeks (CMP populations) and 3 weeks (CLP populations) after adoptive transfer. Data are representative of at least 3 transplantations per progenitor population with similar results.
Chemokine receptor expression on hematopoietic progenitors does not correlate with LC generation potential
As the recruitment of LC progenitors to the skin requires the presence of inflammatory chemokines, such as CCL20 and, specifically, the chemokine receptors CCR2 and CCR6 have been reported to be involved in this process,3,20 we determined the expression profile of those receptors in the progenitor populations. RT-PCR analysis for CCR2 and CCR6 demonstrates that only GMPs, and at lower levels CMPs, express CCR2, while none of the progenitors shows expression of CCR6 (Figure 6). Flow cytometric analysis of the progenitor populations after staining with either CCR2 or CCR6 antibodies confirms that CMPs and GMPs are the only progenitor populations that express CCR2. In contrast to the RT-PCR results, CMPs show slightly higher expression of CCR2 than GMPs (Figure 6B), pointing to posttranslational regulation of CCR2 surface expression. As expected, all progenitor populations were negative for CCR6 expression, confirming the RT-PCR results (Figure 6B). Therefore, the expression of these receptors (Figure 6) does not correlate completely with LC generation potential of the progenitors (Figures 1, 2, 3; Table 1).
Figure 6.
Chemokine receptor expression on progenitors does not correlate with LC generation potential. (A) RT-PCR analysis of CCR2 and CCR6 expression in purified progenitor populations and epidermal LCs. Actb primers acted as controls. Data are based on 2 experiments with similar results. (B) Histogram plots show CCR2 and CCR6 (bold lines) surface expression of progenitor populations. Dashed lines represent isotype control stainings. Data are representative of 2 experiments with similar results.
Discussion
Recently our laboratory has shown that LCs self-renew in the skin during steady-state conditions and are replaced by bone marrow-derived progenitors only during inflammatory conditions.20 Using an inflammatory model system, we could demonstrate for the first time that both CMPs and CLPs have the capacity to generate LCs in vivo. This has been shown both quantitatively by flow cytometry as well as qualitatively by microscopic analysis of epidermal sheets. Our data indicate that CMPs have a superior capacity to generate LCs, as we could detect a several-fold-higher reconstitution rate of LCs by CMPs in the skin than after transplantation of similar numbers of CLPs. In combination with our observation that LC reconstitution by myeloid progenitors is faster than by all tested lymphoid progenitors, we can conclude that mainly myeloid progenitors are recruited to the epidermis upon inflammation and replace the depleted resident LC population, especially as CMPs are about 10-fold more abundant in the BM than CLPs. Previously, the assumption that LCs are of myeloid origin was based exclusively on data from in vitro cultures, which showed that human blood monocytes and even a CD14+ dermal progenitor have the capacity to differentiate into LC-like cells that show the typical Birbeck granules and show expression of Langerin and CD1a.
To date, few studies have been performed to delineate the origin of LCs in vivo. One previously published report indicates a lymphoid origin of LCs.8 In this study, early thymic progenitors have been shown to give rise to LCs after 2 weeks. Although we confirmed that a small percentage of LCs can indeed be generated from early thymic progenitors, the percentages of pro-T1- or pro-T2-derived LCs were lower than those detected in the previous study and significantly lower than after transfer of CMPs. Unfortunately, the previous study did not evaluate the capacity of any myeloid progenitors or CLPs to give rise to LCs in comparison, so that the results are difficult to compare with our data. One reason for the higher LC generation capacity of thymic progenitors in the previous study might be due to the longer exposure to UV light, as LC depletion and inflammatory conditions are crucial to induce LC reconstitution by BM-derived progenitors. Furthermore, our study provides a detailed morphologic and phenotypic analysis of CLP- and CMP-derived LCs, as well as giving more insight into the mechanism of LC generation with regard to time course of LC generation and correlation to Flk2 and chemokine receptor expression on progenitor cells.
In a recent attempt to identify the LC precursors resident in the skin, dendritic epidermal leukocytes (DELs) isolated from fetal skin were shown to differentiate into LCs in vitro.30 Unfortunately, the capacity of DELs to generate LCs in vivo was not evaluated in this study.30 Whether such skin-resident LC precursors originate from CLPs or CMPs during inflammation, or represent an independent source of LCs, remains unanswered. Our observation that progenitor-derived LCs typically appeared in clusters suggests that CMP- and CLP-derived progenitors may continue to proliferate after they are recruited to the skin. Previously, CCR2 has been shown to play an important role in the recruitment of LC precursors to the skin,20 but as CCR2 expression on the progenitor populations does not correlate with the LC generation potential observed in our experiments, this further argues that mainly downstream progenitors, and not the transplanted BM progenitors, migrate to the skin. However, CCR2 expression might partially account for the superior LC potential of myeloid progenitors compared with lymphoid progenitors, as GMPs and CMPs but neither HSCs, CLPs, nor any of thymic progenitors show expression of CCR2. Both CMPs and GMPs can therefore potentially migrate directly to the skin, which might explain the faster LC reconstitution by myeloid progenitors.
The identification of murine hematopoietic progenitor populations has had a major impact on the ability to study the development of discrete leukocyte lineages, including DCs. Both “lymphoid” CD8α+ and “myeloid” CD11b+/CD8α- splenic DC subsets have been shown to be derived mainly from CMPs, while thymic DCs originate preferentially from lymphoid progenitors.11,12 Recently, even splenic CD11c+ CD45RA+ Ly6C+ CD11b- interferon-alpha-producing plasmacytoid dendritic cells (IPCs) have been identified as primarily myeloid derived, while the IPC population present in the thymus develops preferentially from CLPs.31,32 The different homing patterns of the various progenitor populations likely influence the developmental origins of DCs in particular tissues. Conversely, differences in tissue microenvironment might favor the development of DC precursors from either lymphoid or myeloid progenitors. Thus it is likely that the thymic environment may favor the development of lymphoid DCs, while splenic and peripheral tissues favor the development of myeloid-derived DCs and LCs, even though we cannot exclude that the inflammatory environment induced by UV light exposure might skew LC development toward the myeloid lineage.
Although UV light is the most effective inflammatory stimulus to induce LC chimerism, a variety of other inflammatory stimuli, such as the contact sensitizer DNFB, croton oil, or induction of skin irritation by tape stripping, are also capable of recruiting BM-derived LC progenitors to the skin (data not shown). Unfortunately, the levels of LC chimerism induced by these stimuli after reconstitution of C57Bl/6 mice with CD45.1 congenic BM were significantly lower than after UV light exposure. This did not allow us to analyze LC reconstitution after adoptive transfer of committed progenitors, which have a limited life span. Nevertheless, it is likely that the same progenitors that are the source of LCs in our model system (mainly Flk2+ CMPs) give rise to LCs during all inflammatory conditions that induce LC reconstitution by BM-derived progenitors. Replacement of LCs by BM-derived progenitors also occurs during the course of graft-versus-host disease (GVHD) after allogeneic BM transplantation. As induction of LC chimerism before allogeneic BM transplantation can also prevent GVHD,3 understanding the mechanisms responsible for LC development and recruitment during inflammatory conditions might prove useful in the development of new prophylactic therapeutic approaches to GVHD or other diseases involving skin inflammation.
While recent studies indicate that DC subsets in addition to LCs can be of both myeloid or lymphoid origin,11,12,31,32 it is still not clear if the developmental origins of these cells has an impact on their functional properties, although no major differences have been shown between CLP- or CMP-derived cells of the same DC subset. Unfortunately, the low numbers of progenitor-derived LCs prevented us from studying the capacity of these cells to ingest and present antigen or migrate to lymphoid organs. However, we could not detect any phenotypic or morphologic differences between LCs derived from CMPs or CLPs. Furthermore, all LC populations showed similar expression of Cd207 mRNA. Langerin, a typical LC marker, is a c-type lectin receptor that is involved in presentation of nonpeptide antigens to T cells33 and is also a potent inducer of Birbeck granules.34 Recent studies have confirmed that although other murine DC populations express Langerin, LCs are the only Langerin-expressing cells in the skin.35,36 Thus, despite their numeric differences, the LCs derived from myeloid and lymphoid progenitors appear to be indistinguishable from one another and from conventional LCs.
Flt3-ligand (FL) is an important growth factor that not only expands early hematopoietic progenitors but also promotes the development of both conventional DCs as well as plasmacytoid DCs (PDCs). Flk2 (fetal liver kinase), the cellular receptor for FL, is expressed on most early hematopoietic progenitors and the fact that both CMPs and CLPs contain Flk2- as well as Flk2+ populations allowed us to study the effect of Flk2 expression on LC generation.28 The much greater capacity of Flk2+ progenitors to generate LCs indicates that FL plays an important role not only in the development of conventional DCs and PDCs but also during LC development, confirming the promoting effect of FL on LC development observed in human in vitro cultures.37 Our finding that the Flk2+ fraction of hematopoietic progenitors is the main source of LCs is consistent with results observed previously for conventional DCs in the spleen28,29 and further supports the DC potential of Flk2+ progenitors.
In contrast to splenic and thymic DC populations, a direct effect of FL administration on LC development is difficult to determine in vivo, as FL injections do not increase total LC numbers in vivo,38 which is most likely due to limited numbers of available LC niches in the epidermis. In addition, FL expands but does not accelerate splenic DC generation from progenitors, therefore FL administration should have no direct effect on LC recovery after UV light-induced inflammation, which is also in accordance with previous data reporting no effect on LC recovery after LPS-induced depletion.38 We have shown before that splenic DC numbers derived from either CMPs or CLPs increase relative to the percentage of Flk2+ cells within the progenitor population, but independent of their lineage origin.28 It is therefore likely that in vivo FL administration has the same promoting effects on CMPs and CLPs during LC development in vivo as observed for conventional splenic DCs, with the exception that total LC numbers in the epidermis are not affected by FL.
Taken together, our results demonstrate for the first time that both CMPs and CLPs have the capacity to generate LCs in vivo. On a per-cell basis, CMP reconstituted LCs in the skin more efficiently and more rapidly than CLPs or early thymic progenitors. With the additional knowledge that CMPs are at least 10-fold more abundant than CLPs in the BM, we can conclude that mainly myeloid progenitors are recruited to the epidermis upon inflammation and replace the depleted resident LC population. Given the much greater capacity of Flk2+ progenitors to generate LCs, we also conclude that FL plays an important role in the development of LCs.
Supplementary Material
[Supplemental Figure]
Acknowledgments
We wish to thank Dr Pia Björck and Claudia Benike for critical reading of the paper.
Prepublished online as Blood First Edition Paper, November 1, 2005; DOI 10.1182/blood-2005-05-1878.
Supported by grants HL75462 and HL57443 (E.G.E.) and grant AI047458 (I.L.W) from the National Institutes of Health.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Stingl G, Tamaki K, Katz SI. Origin and function of epidermal Langerhans cells. Immunol Rev. 1980; 53: 149-174. [DOI] [PubMed] [Google Scholar]
- 2.Nestle FO, Nickoloff BJ. Dermal dendritic cells are important members of the skin immune system. Adv Exp Med Biol. 1995;378: 111-116. [DOI] [PubMed] [Google Scholar]
- 3.Merad M, Hoffmann P, Ranheim E, et al. Depletion of host Langerhans cells before transplantation of donor alloreactive T cells prevents skin graft-versus-host disease. Nat Med. 2004;10: 510-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morhenn VB. Langerhans cells may trigger the psoriatic disease process via production of nitric oxide. Immunol Today. 1997;18: 433-436. [DOI] [PubMed] [Google Scholar]
- 5.Schuler G, Steinman RM. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J Exp Med. 1985;161: 526-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langerhans P. Ueber die Nerven der Menschlichen Haut. Arch Path Anat Physiol. 1868;44: 325-337. [Google Scholar]
- 7.Romani N, Holzmann S, Tripp CH, Koch F, Stoitzner P. Langerhans cells: dendritic cells of the epidermis. Apmis. 2003;111: 725-740. [DOI] [PubMed] [Google Scholar]
- 8.Anjuere F, del Hoyo GM, Martin P, Ardavin C. Langerhans cells develop from a lymphoid-committed precursor. Blood. 2000;96: 1633-1637. [PubMed] [Google Scholar]
- 9.Larregina AT, Morelli AE, Spencer LA, et al. Dermal-resident CD14+ cells differentiate into Langerhans cells. Nat Immunol. 2001;2: 1151-1158. [DOI] [PubMed] [Google Scholar]
- 10.Canque B, Camus S, Dalloul A, et al. Characterization of dendritic cell differentiation pathways from cord blood CD34(+)CD7(+)CD45RA(+) hematopoietic progenitor cells. Blood. 2000;96: 3748-3756. [PubMed] [Google Scholar]
- 11.Traver D, Akashi K, Manz M, et al. Development of CD8alpha-positive dendritic cells from a common myeloid progenitor. Science. 2000;290: 2152-2154. [DOI] [PubMed] [Google Scholar]
- 12.Manz MG, Traver D, Miyamoto T, Weissman IL, Akashi K. Dendritic cell potentials of early lymphoid and myeloid progenitors. Blood. 2001;97: 3333-3341. [DOI] [PubMed] [Google Scholar]
- 13.Leon B, Martinez del Hoyo G, Parrillas V, et al. Dendritic cell differentiation potential of mouse monocytes: monocytes represent immediate precursors of CD8- and CD8+ splenic dendritic cells. Blood. 2004;103: 2668-2676. [DOI] [PubMed] [Google Scholar]
- 14.del Hoyo GM, Martin P, Vargas HH, Ruiz S, Arias CF, Ardavin C. Characterization of a common precursor population for dendritic cells. Nature. 2002;415: 1043-1047. [DOI] [PubMed] [Google Scholar]
- 15.Diao J, Winter E, Chen W, Cantin C, Cattral MS. Characterization of distinct conventional and plasmacytoid dendritic cell-committed precursors in murine bone marrow. J Immunol. 2004;173: 1826-1833. [DOI] [PubMed] [Google Scholar]
- 16.Borkowski TA, Letterio JJ, Farr AG, Udey MC. A role for endogenous transforming growth factor beta 1 in Langerhans cell biology: the skin of transforming growth factor beta 1 null mice is devoid of epidermal Langerhans cells. J Exp Med. 1996;184: 2417-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ichikawa E, Hida S, Omatsu Y, et al. Defective development of splenic and epidermal CD4+ dendritic cells in mice deficient for IFN regulatory factor-2. Proc Natl Acad Sci U S A. 2004;101: 3909-3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schiavoni G, Mattei F, Borghi P, et al. ICSBP is critically involved in the normal development and trafficking of Langerhans cells and dermal dendritic cells. Blood. 2004;103: 2221-2228. [DOI] [PubMed] [Google Scholar]
- 19.Katz SI, Tamaki K, Sachs DH. Epidermal Langerhans cells are derived from cells originating in bone marrow. Nature. 1979;282: 324-326. [DOI] [PubMed] [Google Scholar]
- 20.Merad M, Manz MG, Karsunky H, et al. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat Immunol. 2002;3: 1135-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dieu-Nosjean MC, Massacrier C, Homey B, et al. Macrophage inflammatory protein 3alpha is expressed at inflamed epithelial surfaces and is the most potent chemokine known in attracting Langerhans cell precursors. J Exp Med. 2000;192: 705-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikuta K, Kina T, MacNeil I, et al. A developmental switch in thymic lymphocyte maturation potential occurs at the level of hematopoietic stem cells. Cell. 1990;62: 863-874. [DOI] [PubMed] [Google Scholar]
- 23.Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404: 193-197. [DOI] [PubMed] [Google Scholar]
- 24.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91: 661-672. [DOI] [PubMed] [Google Scholar]
- 25.King AG, Kondo M, Scherer DC, Weissman IL. Lineage infidelity in myeloid cells with TCR gene rearrangement: a latent developmental potential of proT cells revealed by ectopic cytokine receptor signaling. Proc Natl Acad Sci U S A. 2002;99: 4508-4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boring L, Gosling J, Chensue SW, et al. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest. 1997;100: 2552-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merad M, Fong L, Bogenberger J, Engleman EG. Differentiation of myeloid dendritic cells into CD8alpha-positive dendritic cells in vivo. Blood. 2000;96: 1865-1872. [PubMed] [Google Scholar]
- 28.Karsunky H, Merad M, Cozzio A, Weissman IL, Manz MG. Flt3 ligand regulates dendritic cell development from Flt3+ lymphoid and myeloid-committed progenitors to Flt3+ dendritic cells in vivo. J Exp Med. 2003;198: 305-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D'Amico A, Wu L. The early progenitors of mouse dendritic cells and plasmacytoid predendritic cells are within the bone marrow hemopoietic precursors expressing Flt3. J Exp Med. 2003;198: 293-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang-Rodriguez S, Hoetzenecker W, Schwarzler C, Biedermann T, Saeland S, Elbe-Burger A. Fetal and neonatal murine skin harbors Langerhans cell precursors. J Leukoc Biol. 2005;77: 352-360. [DOI] [PubMed] [Google Scholar]
- 31.Shigematsu H, Reizis B, Iwasaki H, et al. Plasmacytoid dendritic cells activate lymphoid-specific genetic programs irrespective of their cellular origin. Immunity. 2004;21: 43-53. [DOI] [PubMed] [Google Scholar]
- 32.Karsunky H, Merad M, Mende I, Manz MG, Engleman EG, Weissman IL. Developmental origin of interferon-alpha-producing dendritic cells from hematopoietic precursors. Exp Hematol. 2005;33: 173-181. [DOI] [PubMed] [Google Scholar]
- 33.Hunger RE, Sieling PA, Ochoa MT, et al. Langerhans cells utilize CD1a and langerin to efficiently present nonpeptide antigens to T cells. J Clin Invest. 2004;113: 701-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valladeau J, Ravel O, Dezutter-Dambuyant C, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12: 71-81. [DOI] [PubMed] [Google Scholar]
- 35.Kissenpfennig A, Ait-Yahia S, Clair-Moninot V, et al. Disruption of the langerin/CD207 gene abolishes Birbeck granules without a marked loss of Langerhans cell function. Mol Cell Biol. 2005;25: 88-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bennett CL, van Rijn E, Jung S, et al. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol. 2005;169: 569-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strobl H, Bello-Fernandez C, Riedl E, et al. flt3 ligand in cooperation with transforming growth factor-beta1 potentiates in vitro development of Langerhans-type dendritic cells and allows single-cell dendritic cell cluster formation under serum-free conditions. Blood. 1997;90: 1425-1434. [PubMed] [Google Scholar]
- 38.Burnham K, Robb L, Scott CL, O'Keeffe M, Shortman K. Effect of granulocyte-macrophage colony-stimulating factor on the generation of epidermal Langerhans cells. J Interferon Cytokine Res. 2000;20: 1071-1076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental Figure]