DMP1 Depletion Decreases Bone Mineralization In Vivo: An FTIR Imaging Analysis (original) (raw)
. Author manuscript; available in PMC: 2006 May 2.
Published in final edited form as: J Bone Miner Res. 2005 Aug 22;20(12):2169–2177. doi: 10.1359/JBMR.050815
Abstract
The role of DMP1 in mineralization was analyzed by comparing bone mineral and matrix properties in _dmp1_-null female mice to heterozygous and wildtype controls by FTIR imaging spectroscopy. The observed decreased mineral content in dmp1 null mice indicates a key role for dmp1 in bone mineralization. Indirect effects of DMP1 on other systems also determine the KO phenotype.
Introduction
Dentin matrix protein 1 (DMP1), an acidic phosphorylated extracellular matrix protein, is highly expressed in mineralized tissues. In vitro, DMP1 peptides can promote or inhibit mineralization depending on the extent of phosphorylation, the peptide size, and concentration. To clarify the biological function of DMP1 protein on in vivo mineralization, this study analyzed bone properties of dmp1 knockout (KO) mice compared with heterozygous (HET) and wildtype (WT) controls.
Materials and Methods
Tibias from dmp1 KO and age-, sex-, and background-matched HET and WT mice at 4 and 16 weeks (_N_total = 60) were examined by Fourier transform infrared imaging (FTIRI), histology (n = 6 per genotype and age; N = 36), and geometry by μCT (n = 4 per genotype and age; N = 24). Serum ionic calcium and phosphate concentrations were also determined.
Results
The mineral-to-matrix ratios (spectroscopic parameter of relative mineral content) were significantly lower in dmp1 KO mice tibias compared with WT and HET at 4 and 16 weeks. The mineral crystallinity (crystal size/perfection) was significantly increased in dmp1 KO and HET mice relative to WT. Collagen cross-link ratios (a spectroscopic parameter related to the relative amounts of nonreducible/reducible collagen cross-links) in dmp1 KO were not significantly different from WT and HET. Based on μCT, cortical bone cross-sectional areas at 16 but not 4 weeks were significantly reduced in the KO compared with controls. Maximum, minimum, and polar cross-sectional moments of inertia were significantly lower in dmp1 KO than in HET at 16 weeks but not at 4 weeks. Histological analysis and μCT 3-D images suggested that dmp1 KO mice had osteomalacia. Dmp1 KO mice had significantly lower ionic calcium and phosphate concentrations relative to WT, whereas in the HET, values for phosphate were equivalent, and calcium values were decreased relative to WT values.
Conclusions
The findings of decreased mineral-to-matrix ratio and increased crystal size in bones of dmp1 KO mice suggest that DMP1 has multiple roles (both direct and indirect) in the regulation of postnatal mineralization. We suggest that direct effects on mineral formation, crystal growth, and indirect effects on regulation of Ca × P concentrations and matrix turnover all contribute to the dominant phenotype in the dmp1 KO mouse.
Keywords: dentin matrix protein-1, Fourier transform infrared imaging, mineralization, osteomalacia model, mouse, bone geometry
INTRODUCTION
Dentin matrix protein-1 (DMP1), initially named AG1(1), an acidic noncollagenous phosphoprotein, is a member of the SIBLING (small integrin binding ligand N-linked glycoprotein) family,(2) which includes osteopontin, bone sialoprotein, DMP1, dentin sialophosphoprotein (DSPP), enamelin, and matrix extracellular phosphoglycoprotein (MEPE). These proteins share some common features such as their location on human chromosome 4q21, their multiple phosphorylation sites, their highly acidic nature, and the presence of an arginine-glycine-aspartate (RGD) cell attachment domain. Each of these proteins is thought to play an important role in tissue mineralization.(1,3,4) Dmp1 was first cloned from dentin(1) and later found in bone,(5–7) cartilage,(8) and cementum.(9) Thus, it was originally thought to be specific to mineralized tissue. Recently, however, DMP1 was detected in a number of nonmineralized tissues including liver, muscle, pancreas, kidney, brain,(5,10) and salivary glands,(11) as well as in human lung adenocarcinoma,(12) phosphaturic mesenchymal tumors,(13) and cancers of the breast, uterus, and colon.(14) DMP1 is a multifunctional protein that has been found to regulate cell attachment(15) and cell differentiation,(16,17) to activate matrix metalloproteinase-9,(18) and has been postulated to play a significant role in biomineralization.(19)
In vitro, DMP1 acts as a hydroxyapatite (HA) crystal nucleator with very high calcium ion binding capability(20) and binds specifically to the N-telopeptide region of type I collagen in the gap region.(21) In addition, studies of fetal rat calvarial cell cultures showed that the expression of DMP1 is associated closely with “bone nodule” formation and mineralization.(8) These studies suggest that DMP1 is a promoter of mineralization. However, in a gelatin–gel diffusion system,(22) different forms of DMP1 act as nucleators or inhibitors. In the gelatin–gel system, the nonphosphorylated rDMP1 and the highly phosphorylated C-terminal 57K peptide of DMP1 act as apatite nucleators, the phosphorylated full-length bovine DMP1 expressed in marrow stromal cells is a mineralization inhibitor, and the rDMP1 phosphorylated in vitro had no effect on HA formation and growth.(23) Thus, further study is needed to understand the in situ effects of DMP1.
In vivo studies showed that embryo and newborn _dmp1_-null mice could not be distinguished visibly from wildtype littermates by whole skeleton radiography and Alizarin red/Alcian blue staining; femora of _dmp1_-null mice embryos did not show obvious abnormalities by H&E staining, although the _dmp1_-null mice had slightly expanded hypertrophic zones and modestly increased bone diameter compared with wildtypes.(24) Ye et al.(19) recently reported that dmp1 null mice postnatally displayed a profound tooth phenotype characterized by increased width of predentin with a reduced dentin wall thickness and dentin hypomineralization. In addition, vertebrae and long bones in postnatal _dmp1_-null mice were shorter and wider with delayed and malformed secondary ossification centers and an irregular and highly expanded growth plate, creating a phenotype resembling dwarfism with chondrodysplasia,(25) suggesting that DMP1 is critical for the postnatal development of mineralized tissues.
The purpose of this study was to clarify the role of DMP1 in bone mineralization in vivo. We used Fourier-transform infrared imaging (FTIRI) and μCT to characterize changes in both the mineral and matrix of the bones of dmp1 knockouts (KOs) and age-, sex-, and background-matched wild-types (WTs) and heterozygotes (HETs). FTIRI, in which an array detector is coupled to an infrared microscope allowing acquisition of multiple spectra simultaneously from selected areas of bone sections, provides unique spatially resolved information on the amount of mineral present, mineral crystallinity, and collagen properties. High-resolution μCT was used to provide 3-D information on bone geometry.
MATERIALS AND METHODS
Experimental animals
_Dmp1_-null mice targeted to replace the dmp1 exon 6 with a lacZ knock-in gene and a neo-cassette in embryonic stem cells were developed at University of Missouri-Kansas City as described elsewhere.(24) The heterozygous _dmp1_-null mice, verified by Southern blot analysis, were interbred to generate homozygotes in the C57BL/6 background. The Institutional Animal Care and Use Committees of University of Missouri-Kansas City and the National Institute of Environmental Health Sciences, NIH, approved all animal experimental protocols.
Ten tibias per genotype per age were obtained from WT, HET, and KO female mice at 4 and 16 weeks (_N_total = 60). Four tibias per group were cleaned of soft tissue, stored in 90% ethanol, and scanned by μCT as detailed below. The proximal ends of the other six tibias were cut off and embedded in Spur’s medium (Electron Microscopy Science, Hatfield, PA, USA). Longitudinal nondecalcified sections of the proximal ends of the tibia (four sections per bone) were cut by a HM360 microtome (Microm, Walldorf, Germany) at 2 μm thickness and mounted on barium fluoride (BaF2) infrared windows (Spectral Systems, Hopewell Junction, NY, USA) for FTIRI analyses. Another two sections per bone were cut at 4 μm thickness and stained by the von Kossa method and counterstained with neutral red(26) and Goldner’s trichrome.(27)
μCT
Tibial geometry was determined from μCT (Enhanced Vision Systems Model MS-8 In Vitro μCT scanner; GE Healthcare, London, Ontario, Canada). Tibias, cleaned of soft tissue, were scanned in saline (0.9% sodium chloride; Baxter Healthcare, Deerfield, IL, USA). 2-D projections of four tibias per scan were collected by Evolver software (GE Healthcare); isotropic voxel size was 12 × 12 × 12 μm. The hardware of the Enhanced Visions System μCT scanner is engineered to minimize beam hardening associated with polychromatic X-ray to ensure uniformity of the beam at the detector array. Filtering of the low-energy portion of the X-ray spectrum with aluminum, acrylic, and a saline bath results in a flat field where the attenuation within a uniform material is independent of spatial position. To minimize noise, a large number of views, increased frame averaging, and increased shutter exposure time were used, resulting in 4-h scans. Reconstruction of projections into CT volume data were accomplished by Beam software (GE Healthcare) with a modified Parker algorithm.(28,29) 2-D images, 3-D volume generation, and threshold analysis were calculated with Microview (GE Healthcare). Reconstructed grayscale volumes for each cortical subvolume at the middle of the tibias were extracted and segmented with the individual threshold defined by the auto-threshold function of Microview, namely generating the attenuation histogram of the volume of interest and fitting the data with the Otsu method.(30) We used the auto-threshold method because it segregates mineralized tissue based on the distribution for a given specimen of background and mineralized voxels and helps to reduce experimental error associated with temporal effects. A MATLAB (version 6.5; Mathworks, Natick, MA, USA) program based on an integrative method(31) was used to calculate the cross-sectional area, maximum cross-sectional moment of inertia (_I_max), minimum cross-sectional moment of inertia (_I_min), and polar cross-sectional moment of inertia (J). Moment of inertia is a geometric property of a beam that measures the distribution of mineral about a given axis, representing the ability to resist bending or torsion.
FTIRI
Tibia sections of 4- and 16-week-old mice were examined by FTIRI to acquire spectral images using either the Bio-Rad FTS-6000 Stingray system (BioRad, Cambridge, MA, USA) or the Perkin Elmer Spotlight Imaging system (Perkin Elmer Instruments, Shelton, CT, USA). The spectral resolution was 16 cm−1, and areas 400 × 400 μm2 were examined on both instruments. The spatial resolution was ~7 μm. Spectra were transferred to yield images corresponding to infrared band areas, peak height ratios, and integrated area ratios by a combination of instrument software and ISYS Chemical Imaging Software (v 2.1; Spectral Dimensions, Olney, MD, USA).(32,33) Background spectra were collected under identical conditions from the same BaF2 windows. IR data of three areas per anatomical bone site at primary spongiosa, secondary spongiosa, and cortex were collected (Fig. 1). After acquisition, spectra were truncated to allow analysis of the spectral data of interest and zero-corrected for the baseline, and the spectral contribution of Spur embedding media was subtracted using ISYS software.
FIG. 1.
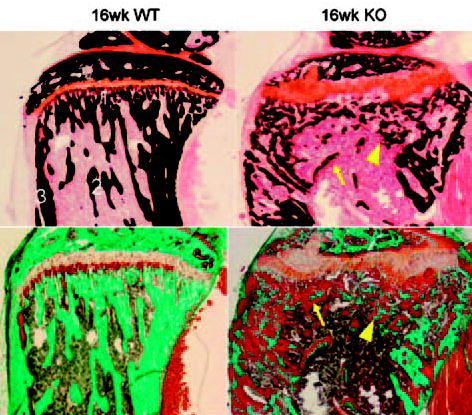
Photomicrographs of the proximal tibia of a 16-week-old _dmp1_-WT (left) and KO (right) mouse stained by the von Kossa (top) and Goldner’s trichrome staining (bottom) showing the morphologic changes resulted from DMP1 deficiency. Smaller and disorganized mineralized trabeculae (arrowhead) surrounded by widespread excessive osteoid, increased width of osteoid seams (arrow), irregular and expanded growth plate, enlarged metaphysis, smaller secondary ossification center, considerable cortex porosity in the dmp1 KO mice tibia compared with WT. Labels 1–3 in the first picture indicates anatomic sites examined by FTIRI: (1) primary spongiosa; (2) secondary spongiosa; (3) cortical bone.
Three spectroscopic parameters were calculated: mineral-to-matrix ratio, crystallinity, and collagen cross-link ratio (XLR). The mineral-to-matrix ratio (v1, v3 PO4 band [900–1200 cm−1]/amide I band [1590–1720 cm−1] integrated areas ratio) is a measure that corresponds to ash weight measurements.(34) Mineral crystallinity is a parameter that corresponds to the crystallite size and perfection as determined by X-ray diffraction, and was calculated from the intensity ratios of subbands at 1030 (stoichiometric apatite) and 1020 cm−1 (nonstoichiometric apatite).(33) XLR is a parameter reflecting the relative ratio of nonreducible and reducible collagen cross-links, expressed as the absorbance ratio at two specific wavenumbers (1660 and 1690 cm−1).(35) Details for the spectral processing methods and reproducibility of measurements are published elsewhere.(31,32,34–36) In the spectral images, pixels devoid of bone (no mineral and/or matrix spectral signature) were set equal to zero and masked to be excluded from calculations. The spectroscopic results were expressed as histograms describing the pixel distribution of the three parameters above, mean values, and SDs of the pixel distribution, and corresponding color-coded images were generated at the same time by ISYS. Means and SDs were averaged for multiple sites in each animal and among six different animals for each age and genotype using Microsoft EXCEL.
Biochemistry
Serum obtained by the cardiac puncture from WT, HET, and KO animals was analyzed for calcium and phosphate content by ion chromatography (DX-120 ion chromatograph; Dionex, Sunnyvale, CA, USA) as described elsewhere.(37)
Statistical analysis
Departures from normal distribution were assessed graphically and by the Kolmogorov-Smironov test. None of the outcome variables departed from normality. Data were analyzed by two-factor ANOVA with interaction. The first factor was genotype (dmp1 WT, HET, and KO) and the second factor was age (4 and 16 weeks). The ANOVA type I error rate was set at 0.05. If the interaction term between genotype and age was significant, the conclusion was drawn that the effect of genotype was dependent on age. This gave justification for examining effects of genotype separately for each age.(38) Posthoc comparisons were made using Tukey pairwise mean comparison tests. The Tukey experiment-wise error rate was set at 0.05. If the ANOVA interaction term was not significant, the main effects were examined. If the main effect of genotype was significant, contrasts among levels of genotype were tested. The contrast error rate was set at 0.05. Analyses were conducted using SYSTAT @ 9 Statistics I (SPSS, Chicago, IL, USA).
RESULTS
Dmp1 KO mice showed evidence of osteomalacia
In histological analysis, morphologic changes characteristic of osteomalacia were observed in the tibia sections of dmp1 KO mice: widespread excessive osteoid and increased width of osteoid seams at the surfaces of both cancellous and cortical bone, structure deformities, smaller and disorganized trabeculae, irregular and expanded growth plate, enlarged metaphysis, smaller secondary ossification center, and considerable cortical porosity (Fig. 1).
Geometric parameters were decreased in diaphysis of dmp1 KO mice tibias
Proximal tibias of dmp1 KO mice were shorter and wider than those of dmp1 WT and HET by μCT, with enlarged flaring metaphysis, expanded growth plates, smaller secondary ossification center, fewer trabeculae, thinner diaphyseal cortical walls, and larger marrow space (Fig. 2).
FIG. 2.
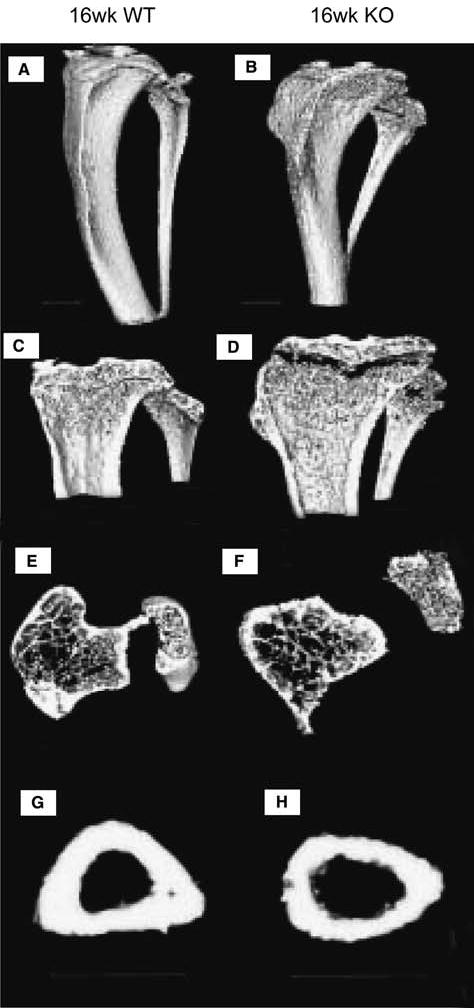
Representative 3-D rendered μCT images of 16-week dmp1 (A, C, E, and G) WT and (B, D, F, and H) KO mice tibias. Dmp1 (B) KO tibia showed enlarged metaphysis, abnormal shape and shorter length compared with (A) WT tibia. In longitudinal sections of tibias, significantly enlarged flared metaphysis, expanded growth plates, and smaller secondary ossification centers were seen in dmp1 (D) KO tibia relative to (C) WT. In 5-mm-thick cross-section under the growth plate, trabeculae were sparser in the dmp1 (F) KO than in the dmp1 (E) WT. In the 1-mm cross-section of tibia midshaft, thinner cortical wall and larger marrow space were observed in the dmp1 (H) KO compared with (G) WT. All scale bars are 1 mm.
Cross-sectional areas of the tibial midshaft were significantly smaller in dmp1 KO mice than in both dmp1 WT and HET mice at 16 weeks of age (Table 1). The cross-sectional moments of inertia including _I_max, _I_min, and J were significantly lower in dmp1 KO than in dmp1 HET at 16 weeks. No significant differences in these parameters were found comparing dmp1 WT and KO mice. In WT and HET mice at 16 weeks, cross-sectional areas and moments of inertia were larger than those of corresponding genotype mice at 4 weeks, indicating growth. In the dmp1 KO mice, only the cross-sectional area increased with age from 4 to 16 weeks; the moments of inertia did not get larger.
Table 1.
Microct Geometric Parameters of Midshaft Cortex of dmp1 WT, HET, and KO Tibias
| Cortex of tibia midshaft | |||
|---|---|---|---|
| WT | HET | KO | |
| 4-week-old mice | |||
| Cross-sectional area (mm2) | 0.37 ± 0.04 | 0.44 ± 0.03 | 0.36 ± 0.03 |
| _I_max (mm4) | 0.033 ± 0.003 | 0.044 ± 0.006 | 0.046 ± 0.014 |
| _I_min (mm4) | 0.020 ± 0.003 | 0.029 ± 0.002 | 0.032 ± 0.007 |
| J (mm4) | 0.053 ± 0.004 | 0.073 ± 0.007 | 0.077 ± 0.02 |
| Threshold (HU) | 1505 ± 86 | 1951 ± 130* | 1078 ± 148*† |
| 16-week-old mice | |||
| Cross-sectional area (mm2) | 0.63 ± 0.04* | 0.70 ± 0.05† | 0.47 ± 0.05‡§¶ |
| _I_max (mm4) | 0.074 ± 0.01* | 0.095 ± 0.015† | 0.053 ± 0.012¶ |
| _I_min (mm4) | 0.046 ± 0.005* | 0.057 ± 0.007† | 0.035 ± 0.006¶ |
| J (mm4) | 0.12 ± 0.01* | 0.15 ± 0.02† | 0.088 ± 0.017¶ |
| Threshold (HU) | 1677 ± 103 | 1760 ± 57 | 1635 ± 74 |
Mineral-to-matrix ratio decreased significantly in dmp1 KO mice
As shown in Fig. 3, at 4 and 16 weeks, mineral-to-matrix ratios of dmp1 KO mice in the cortex, primary spongiosa, and secondary spongiosa were significantly lower than those of WT and HET mice. Interestingly, the mineral-to-matrix ratio of 16-week dmp1 KO mice increased significantly from 4-week values, whereas WT and HET mice did not show significant increases in this parameter in primary and secondary spongiosa. These findings are shown by representative color-coded images of mineral-to-matrix ratio at cortex and secondary spongiosa of all genotypes at both ages (Figs. 4A and 4B).
FIG. 3.
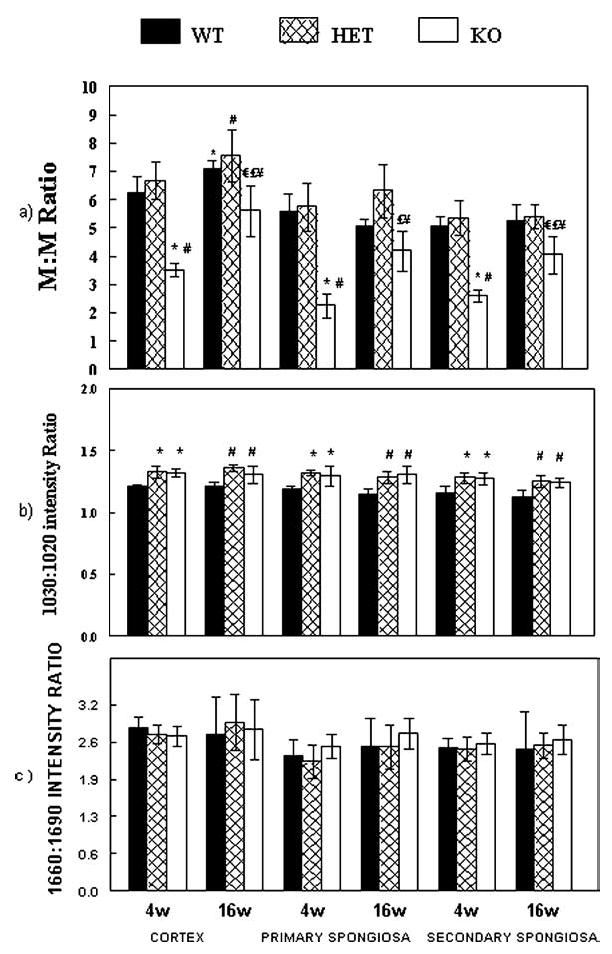
(A) Mineral-to-matrix ratio, spectroscopic mineral content, analyzed by FTIRI at cortex, primary spongiosa and secondary spongiosa of tibias of dmp1 WT, HET, and KO mice at 4 and 16 weeks. (B) Mineral crystal size and perfection (crystallinity; 1030/1020 intensity ratio) and (C) XLR (1660/1690 intensity ratio). *p < 0.05 vs. 4-week WT; #p < 0.05 vs. 4-week HET; € p < 0.05 vs. 16-week WT; £p < 0.05 vs. 16-week HET; ¥p < 0.05 vs. 4-week KO. All values are mean ± SD (n = 6).
FIG. 4.
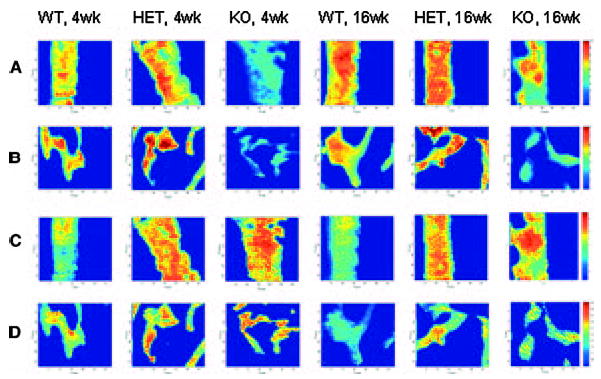
Typical FTIRIs of various spectral parameters in dmp1 WT, HET, and KO mice at 4 and 16 weeks. Representative images of mineral-to-matrix ratio at (A) the cortex and (B) secondary spongiosa and typical images of 1030/1020 ratio (crystallinity) at (C) the cortex and (D) secondary spongiosa. x and y axes represent pixel number. The numerical scales shown on the right side represent the intensity for each parameter in the same row and were kept consistent in WT, HET, and KO.
Crystallinity increased in dmp1 KO mice
Crystallinity, a measure of crystal maturity, a parameter that increases with bone age in normal animals, was significantly higher in the dmp1 KO mice with respect to WT mice at 4 and 16 weeks at all bone sites (Fig. 3B). However, there was not a significant age-dependent effect. Interestingly, this parameter was greater in the HET than in the KO mice at 4 and 16 weeks for cortical bone and was significantly greater than WT mice but not significantly different from KO mice for HET primary spongiosa and secondary spongiosa. Representative FTIRI images of 1030/1020 ratio at cortex and secondary spongiosa of all genotype mice at both ages show the intensity and spatial distribution of crystallinity in the same color scale (Figs. 4C and 4D).
Collagen cross-link ratio did not change in dmp1 KO mice
The collagen cross-link parameter, XLR, calculated from the intensity ratio 1660/1690 wavenumbers, were not significantly different in the dmp1 KO mice bones compared with values in similar bone sites of WT and HET mice at both ages (Fig. 3C).
Serum chemistry
Serum calcium and phosphate concentrations in mice of intermediate age (12 weeks) are summarized in Table 2. The KO mice had significantly lower ionic calcium and phosphate concentrations relative to WT mice, whereas in the HET mice, values for phosphate were equivalent and calcium values were decreased relative to WT values.
Table 2.
Serum Calcium and Phosphate Levels of dmp1 WT, HET, and KO Mice
| WT | HET | KO | |
|---|---|---|---|
| Serum Ca2+ (mM) | 2.95 ± 0.041 | 2.71 ± 0.063* | 2.48 ± 0.034† |
| Serum PO43−(mM) | 2.38 ± 0.085 | 2.41 ± 0.11 | 1.66 ± 0.11† |
| n | 10 | 9 | 14 |
DISCUSSION
Bone is a unique mineralized tissue composed of several cell types and a structured organic matrix impregnated with oriented hydroxyapatite crystals. Bone cells synthesize and secrete the bone matrix, consisting of type I collagen and noncollagenous proteins.(4) Although the detailed mineralization mechanism remains obscure, phosphorylated noncollagenous proteins including the SIBLING proteins have been shown both to nucleate formation of apatite crystals within collagen gap zones and to block the formation and growth of these crystals.(3,4,21,39–41) DMP1, a member of the SIBLING family, has very high calcium ion and apatite binding capabilities,(20) is secreted into the extracellular matrix during endochondral bone formation,(42) and may have a role in initial mineralization.
This study has shown that deletion of the dmp1 gene in mice results in bone changes reminiscent of those noted in osteomalacia,(43,44) decreased bone mineralization, and increased mineral crystallinity. _Dmp1_-null mice bones are less mineralized than their age-, background-, and sex-matched WT and HET counterparts, as reflected by a consistently decreased mineral-to-matrix ratio by FTIRI. Furthermore, the mineral crystallinity, as estimated from the 1030/1020 wavenumbers intensity ratio, was increased in the _dmp1_-KO mice bone relative to the corresponding WT mice. In addition, 16-week _dmp1_-KO mice had smaller cross-sectional area than dmp1 WT and HET mice and had lower cross-sectional moments of inertia compared with the HET but not with the WT mice.
The _dmp1_-KO mice had flared metaphyses, thinner cortices, and reduced diaphyseal moments of inertia that did not increase with age. This, as well as the decreased mineral content, would make the bones weaker. Whereas the widening of the metaphysic might be a response to the decreased mineralization, the failure of the bones to grow most likely is directly caused by the mineralization defect, although it might relate to the role of dmp1 in regulating bone cell differentiation.(45)
These findings support a role for DMP1 or DMP1 peptides in the mineralization process. Qin et al.(46,47) isolated two fragments of DMP1 protein from bone, namely the N-terminal 37-kDa fragment and C-terminal 57-kDa fragment, and indicated that DMP1 is proteolytically cleaved at four X-Asp bonds. Isolation of the complete autochthonous form of DMP1 protein has not been successful. The carboxyl-terminal 57-kDa fragment of DMP1 and the unphosphorylated recombinant protein acted as nucleators of hydroxyapatite.(20,21,23) In contrast, a phosphorylated full-length bovine DMP1 expressed in marrow stromal cells, and presumed to represent the actual intact DMP1 made in bone cells, was a mineralization inhibitor.(23) We thus speculate that, as the complete phosphorylated DMP1 is secreted into extracellular matrix, some proteinases cleave it into fragment(s) that initiate mineralization. Analogously, DSPP, another SIBLING member, considered to be an inactive precursor protein, is cleaved at X-Asp bonds to release a functional C-terminal fragment, dentin phosphoprotein (DPP), which is an extremely effective nucleator of hydroxyapatite.(3,39,46,48) It should be noted that the enzymes responsible for DMP1 proteolysis have not been conclusively identified, although BMP-1/Tolloid-like proteinases(49) and phosphate regulating gene with homologies to endopeptidases on the X chromosome (PHEX) enzyme(46) have been postulated to be involved. Steiglitz et al.(49) have successfully used BMP-1/Tolloid-like proteinases to cleave full-length DMP1-generating fragments similar in size to those previously isolated from bone and showed that fibroblasts from mouse embryos lacking this proteinase have deficient DMP1 processing.
In addition to its postulated function as a hydroxyapatite nucleator, DMP1 also is involved in calcium and phosphate metabolism through the kidney.(10) A kidney effect is suggested by the significantly reduced serum concentration of calcium and phosphate in the _dmp1_-KO as contrasted with the WT and HET mice. The kidney effect would have an indirect impact on mineralization but could account for much of the observed phenotype because lower Ca × P products, as seen in rickets and osteomalacia, would decrease the formation of new mineral and would tend to increase crystal size, because it is energetically easier for whatever crystals are present to grow in the presence of low Ca × P products than for new crystals to form.(50)
Similarly, because DMP1 can bind pro-MMP-9 to activate MMP-9,(18) an additional indirect effect that could account for the hypomineralization phenotype would be the need for DMP1 activation of MMP-9 for matrix modification before calcification. There are other activators of MMP-9, so this could be a minor effect. However, the MMP-9 KO also has impaired mineralization associated with blocked vascular invasion,(51) although frank osteomalacia has not been reported in MMP-9 KO mice, and there are other DMP1-independent pathways that can be used for MMP-9 activation.(18)
The increased crystallinity noted in the _dmp1_-KO mice in part can help distinguish between these mechanisms. The hydroxyapatite crystals formed in the presence of 57-kDa C-terminal DMP1 fragments were smaller than those formed in its absence,(23) indicating that DMP1 can regulate crystal growth. Thus, it is possible that the crystals are larger in the _dmp1_-KO mice because a regulator of growth is missing or because nucleation is impaired and thus fewer nuclei are available. Because there is a tendency for existing crystals to grow rather than for new crystals to form, especially in an environment in which Ca × P product is reduced, this could account for the increased crystallinity in the _dmp1_-KO mice. Crystals in animals with decreased or modified proteoglycans tend to be larger,(52,53) suggesting that the failure to activate MMP-9 probably is not a key factor in the mineralization defect in these animals, although it may account for the unusual shape of the growth plate, because vascular invasion would be impaired if MMP-9 were not activated. The lack of detectable difference in matrix age, reflected by XLR, implies that the matrix may not be persisting for a longer time, although the scatter in the data indicates that the matrix distribution in the WT mice tends to be much more heterogeneous than in the KO mice, implying that the KO matrix might be processed to different extents.
It is important to note that values for crystallinity in dmp1 HET mice were not intermediate between values for KO and WT mice, but were greater than KO values. This implies that this is a dominant trait and stresses the importance of dmp1 expression for normal development. Serum Ca and P levels in HET mice were intermediate between WT and KO mice; thus, the alterations in crystal size are probably not directly caused by a physicochemical effect, but are more likely dependent on direct actions of DMP1.
In conclusion, the findings of decreased mineral content and increased crystal size in _dmp1_-KO mice show depletion of DMP1 in vivo decreases bone mineralization and reveal DMP1 has multiple roles (both direct and indirect) in the regulation of postnatal mineralization. We suggest that direct effects on mineral formation and mineral crystal growth and indirect effects on regulation of Ca × P concentrations and matrix turnover all contribute to the dominant phenotype in the _dmp1_-KO mouse.
Acknowledgments
The authors thank Christopher Fritton for assistance with the μCT and MATLAB program and Dr Stephen B Doty and Orla O’Shea for assistance with the histology. This work was supported by National Institutes of Health Grants DE-04141 (ALB), AR0476121, and AR051587 (JQF). This study was conducted in a facility constructed with support from Research Facilities Improvement Program Grant C06-RR12538 from the National Center for Research Resources. The imaging data was obtained in the HSS Musculoskeletal Core Center (AR0476121). HFR is a recipient of a T32 fellowship (DE07294).
Footnotes
The authors have no conflict of interest.
References
- 1.George A, Sabsay B, Simonian PA, Veis A. Characterization of a novel dentin matrix acidic phosphoprotein: Implications for induction of biomineralization. J Biol Chem. 1993;268:12624–12630. [PubMed] [Google Scholar]
- 2.Fisher LW, Fedarko NS. Six genes expressed in bones and teeth encode the current members of the SIBLING family of proteins. Connect Tissue Res. 2003;44(Suppl 1):33–40. [PubMed] [Google Scholar]
- 3.Qin C, Baba O, Butler WT. Post-translational modifications of sibling proteins and their roles in osteogenesis and dentinogenesis. Crit Rev Oral Biol Med. 2004;15:126–136. doi: 10.1177/154411130401500302. [DOI] [PubMed] [Google Scholar]
- 4.Gokhale JA, Robey PG, Boskey AL 2001 The biochemistry of bone. In: Marcus R, Feldman D, Kelsey A (eds.) Osteoporosis, vol. 1. Academic Press, San Diego, CA, USA, pp. 107–188.
- 5.Hirst KL, Ibaraki-O’Connor K, Young MF, Dixon MJ. Cloning and expression analysis of the bovine dentin matrix acidic phosphoprotein gene. J Dent Res. 1997;76:754–760. doi: 10.1177/00220345970760030701. [DOI] [PubMed] [Google Scholar]
- 6.D’Souza RN, Cavender A, Sunavala G, Alvarez J, Ohshima T, Kulkarni AB, MacDougall M. Gene expression patterns of murine dentin matrix protein 1 (Dmp1) and dentin sialophosphoprotein (DSPP) suggest distinct developmental functions in vivo. J Bone Miner Res. 1997;12:2040–2049. doi: 10.1359/jbmr.1997.12.12.2040. [DOI] [PubMed] [Google Scholar]
- 7.Toyosawa S, Sato A, O’hUigin C, Tichy H, Klein J. Expression of the dentin matrix protein 1 gene in birds. J Mol Evol. 2000;50:31–38. doi: 10.1007/s002399910004. [DOI] [PubMed] [Google Scholar]
- 8.Feng JQ, Zhang J, Dallas SL, Lu Y, Chen S, Tan X, Owen M, Harris SE, MacDougall M. Dentin matrix protein 1, a target molecule for Cbfa1 in bone, is a unique bone marker gene. J Bone Miner Res. 2002;17:1822–1831. doi: 10.1359/jbmr.2002.17.10.1822. [DOI] [PubMed] [Google Scholar]
- 9.MacDougall M, Gu TT, Luan X, Simmons D, Chen J. Identification of a novel isoform of mouse dentin matrix protein 1: Spatial expression in mineralized tissues. J Bone Miner Res. 1998;13:422–431. doi: 10.1359/jbmr.1998.13.3.422. [DOI] [PubMed] [Google Scholar]
- 10.Terasawa M, Shimokawa R, Terashima T, Ohya K, Takagi Y, Shimokawa H. Expression of dentin matrix protein 1 (DMP1) in nonmineralized tissues. J Bone Miner Metab. 2004;22:430–438. doi: 10.1007/s00774-004-0504-4. [DOI] [PubMed] [Google Scholar]
- 11.Ogbureke KU, Fisher LW. Expression of SIBLINGs and their partner MMPs in salivary glands. J Dent Res. 2004;83:664–670. doi: 10.1177/154405910408300902. [DOI] [PubMed] [Google Scholar]
- 12.Chaplet M, de Leval L, Waltregny D, Detry C, Fornaciari G, Bevilacqua G, Fisher LW, Castronovo V, Bellahcene A. Dentin matrix protein 1 is expressed in human lung cancer. J Bone Miner Res. 2003;18:1506–1512. doi: 10.1359/jbmr.2003.18.8.1506. [DOI] [PubMed] [Google Scholar]
- 13.Toyosawa S, Tomita Y, Kishino M, Hashimoto J, Ueda T, Tsujimura T, Aozasa K, Ijuhin N, Komori T. Expression of dentin matrix protein 1 in tumors causing oncogenic osteomalacia. Mod Pathol. 2004;17:573–578. doi: 10.1038/modpathol.3800084. [DOI] [PubMed] [Google Scholar]
- 14.Fisher LW, Jain A, Tayback M, Fedarko NS. Small integrin binding ligand N-linked glycoprotein gene family expression in different cancers. Clin Cancer Res. 2004;10:8501–8511. doi: 10.1158/1078-0432.CCR-04-1072. [DOI] [PubMed] [Google Scholar]
- 15.Kulkarni GV, Chen B, Malone JP, Narayanan AS, George A. Promotion of selective cell attachment by the RGD sequence in dentine matrix protein 1. Arch Oral Biol. 2000;45:475–484. doi: 10.1016/s0003-9969(00)00010-8. [DOI] [PubMed] [Google Scholar]
- 16.Narayanan K, Srinivas R, Ramachandran A, Hao J, Quinn B, George A. Differentiation of embryonic mesenchymal cells to odontoblast-like cells by overexpression of dentin matrix protein 1. Proc Natl Acad Sci USA. 2001;98:4516–4521. doi: 10.1073/pnas.081075198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalajzic I, Braut A, Guo D, Jiang X, Kronenberg MS, Mina M, Harris MA, Harris SE, Rowe DW. Dentin matrix protein 1 expression during osteoblastic differentiation, generation of an osteocyte GFP-transgene. Bone. 2004;35:74–82. doi: 10.1016/j.bone.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Fedarko NS, Jain A, Karadag A, Fisher LW. Three small integrin binding ligand N-linked glycoproteins (SIBLINGs) bind and activate specific matrix metalloproteinases. FASEB J. 2004;18:734–736. doi: 10.1096/fj.03-0966fje. [DOI] [PubMed] [Google Scholar]
- 19.Ye L, MacDougall M, Zhang S, Xie Y, Zhang J, Li Z, Lu Y, Mishina Y, Feng JQ. Deletion of dentin matrix protein-1 leads to a partial failure of maturation of predentin into dentin, hypomineralization, and expanded cavities of pulp and root canal during postnatal tooth development. J Biol Chem. 2004;279:19141–19148. doi: 10.1074/jbc.M400490200. [DOI] [PubMed] [Google Scholar]
- 20.He G, Dahl T, Veis A, George A. Nucleation of apatite crystals in vitro by self-assembled dentin matrix protein 1. Nat Mater. 2003;2:552–558. doi: 10.1038/nmat945. [DOI] [PubMed] [Google Scholar]
- 21.He G, George A. Dentin matrix protein 1 immobilized on type I collagen fibrils facilitates apatite deposition in vitro. J Biol Chem. 2004;279:11649–11656. doi: 10.1074/jbc.M309296200. [DOI] [PubMed] [Google Scholar]
- 22.Silverman L, Boskey AL. Diffusion systems for evaluation of biomineralization. Calcif Tissue Int. 2004;75:494–501. doi: 10.1007/s00223-004-0019-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tartaix PH, Doulaverakis M, George A, Fisher LW, Butler WT, Qin C, Salih E, Tan M, Fujimoto Y, Spevak L, Boskey AL. Dentin matrix protein-1:In vitro effects on hydroxyapatite formation provide insights into in vivo functions. J Biol Chem. 2004;279:18115–18120. doi: 10.1074/jbc.M314114200. [DOI] [PubMed] [Google Scholar]
- 24.Feng JQ, Huang H, Lu Y, Ye L, Xie Y, Tsutsui TW, Kunieda T, Castranio T, Scott G, Bonewald LB, Mishina Y. The dentin matrix protein 1 (Dmp1) is specifically expressed in mineralized, but not soft, tissues during development. J Dent Res. 2003;82:776–780. doi: 10.1177/154405910308201003. [DOI] [PubMed] [Google Scholar]
- 25.Ye L, Mishina Y, Chen D, Huang H, Dallas SL, Dallas M, Kunieda TW, Tsutsui T, Boskey A, Bonewald LF, Feng JQ. Dmp1-deficient mice display severe defects in cartilage formation responsible for a chondrodysplasia-like phenotype. J Biol Chem. 2005;280:6197–6203. doi: 10.1074/jbc.M412911200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomori G 1952 Microscopic Histochemistry, 3rd ed. University of Chicago Press, Chicago, IL, USA.
- 27.Gruber HE. Adaptations of Goldner’s Masson trichrome stain for the study of undecalcified plastic embedded bone. Biotech Histochem. 1992;1:30–34. doi: 10.3109/10520299209110002. [DOI] [PubMed] [Google Scholar]
- 28.Feldkamp LA, Goldstein SA, Parfitt AM, Jesion G, Kleerekoper M. The direct examination of three-dimensional bone architecture in vitro by computed tomography. J Bone Miner Res. 1989;4:3–11. doi: 10.1002/jbmr.5650040103. [DOI] [PubMed] [Google Scholar]
- 29.Tommasini SM, Morgan TG, van der Meulen MCh, Jepsen KJ. Genetic variation in structure-function relationships for the inbred mouse lumbar vertebral body. J Bone Miner Res. 2005;20:817–827. doi: 10.1359/JBMR.041234. [DOI] [PubMed] [Google Scholar]
- 30.Otsu N. A threshold selection method from gray-level histograms. IEEE Trans Syst Man Cybern. 1979;9:62–66. [Google Scholar]
- 31.Nagurka ML, Hayes WC. An interactive graphics package for calculating cross-sectional properties of complex shapes. J Biomech. 1980;13:59–64. doi: 10.1016/0021-9290(80)90008-1. [DOI] [PubMed] [Google Scholar]
- 32.Marcott C, Reeder RC, Paschalis EP, Tatakis DN, Boskey AL, Mendelsohn R. Infrared microspectroscopic imaging of biomineralized tissues using a mercury-cadmium-telluride focal plane array detector. Cell Mol Biol. 1998;44:109–115. [PubMed] [Google Scholar]
- 33.Mendelsohn R, Paschalis EP, Boskey AL. Infrared spectroscopy, microscopy, and microscopic imaging of mineralizing tissues. Spectra-structure correlations from human iliac crest biopsies. J Biomed Opt. 1999;4:14–21. doi: 10.1117/1.429916. [DOI] [PubMed] [Google Scholar]
- 34.Boskey AL, Pleshko N, Doty SB, Mendelsohn R. Applications of Fourier transform infrared (FT-IR) microscopy to the study of mineralization in bone and cartilage. Cells Materials. 1992;2:209–220. [Google Scholar]
- 35.Paschalis EP, Verdelis K, Mendelsohn R, Boskey A, Yamauchi M. Spectroscopic determination of collagen cross-links in bone. J Bone Miner Res. 2001;16:1821–1828. doi: 10.1359/jbmr.2001.16.10.1821. [DOI] [PubMed] [Google Scholar]
- 36.Mendelsohn R, Paschalis EP, Sherman PJ, Boskey AL. IR microscopic imaging of pathological states and fracture healing of bone. Appl Spectrosc. 2000;54:1183–1191. doi: 10.1366/000370204322729405. [DOI] [PubMed] [Google Scholar]
- 37.Renfro JL, Maren TH, Zeien C, Swenson ER. Renal sulfate secretion is carbonic anhydrase dependent in a marine teleost, Pleuronectes americanus. Am J Physiol Renal Physiol. 1999;276:F288–F294. doi: 10.1152/ajprenal.1999.276.2.F288. [DOI] [PubMed] [Google Scholar]
- 38.Zar JH 1999 Biostatistical Analysis, 4th ed. Prentice Hall, Englewood Cliffs, NJ, USA.
- 39.Hunter GK, Hauschka PV, Poole AR, Rosenberg LC, Gold-berg HA. Nucleation and inhibition of hydroxyapatite formation by mineralized tissue proteins. Biochem J. 1996;317:59–64. doi: 10.1042/bj3170059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glimcher MJ. Mechanism of calcification: Role of collagen fibrils and collagen-phosphoprotein complexes in vitro and in vivo. Anat Rec. 1989;224:139–153. doi: 10.1002/ar.1092240205. [DOI] [PubMed] [Google Scholar]
- 41.Boskey AL. Osteopontin and related phosphorylated sialoproteins: Effects on mineralization. Ann NY Acad Sci. 1995;760:249–256. doi: 10.1111/j.1749-6632.1995.tb44635.x. [DOI] [PubMed] [Google Scholar]
- 42.Toyosawa S, Shintani S, Fujiwara T, Ooshima T, Sato A, Ijuhin N, Komori T. Dentin matrix protein 1 is predominantly expressed in chicken and rat osteocytes but not in osteoblasts. J Bone Miner Res. 2001;16:2017–2026. doi: 10.1359/jbmr.2001.16.11.2017. [DOI] [PubMed] [Google Scholar]
- 43.Vigorita VJ 1999 Orthopaedic Pathology. Lippincott Williams & Wilkins, Philadelphia, PA, USA.
- 44.Faibish D, Gomes A, Boivin G, Binderman I, Boskey A. Infrared imaging of calcified tissue in bone biopsies from adults with osteomalacia. Bone. 2005;36:6–12. doi: 10.1016/j.bone.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 45.Narayanan K, Srinivas R, Peterson MC, Ramachandran A, Hao J, Thimmapaya B, Scherer PE, George A. Transcriptional regulation of dentin matrix protein 1 by JunB and p300 during osteoblast differentiation. J Biol Chem. 2004;279:44294–44302. doi: 10.1074/jbc.M403511200. [DOI] [PubMed] [Google Scholar]
- 46.Qin C, Brunn JC, Cook RG, Orkiszewski RS, Malone JP, Veis A, Butler WT. Evidence for the proteolytic processing of dentin matrix protein 1. Identification and characterization of processed fragments and cleavage sites. J Biol Chem. 2003;278:34700–34708. doi: 10.1074/jbc.M305315200. [DOI] [PubMed] [Google Scholar]
- 47.Qin C, Brunn JC, Jones J, George A, Ramachandran A, Gorski JP, Butler WT. A comparative study of sialic acid-rich proteins in rat bone and dentin. Eur J Oral Sci. 2001;109:133–141. doi: 10.1034/j.1600-0722.2001.00001.x. [DOI] [PubMed] [Google Scholar]
- 48.Boskey AL, Maresca M, Doty S, Sabsay B, Veis A. Concentration-dependent effects of dentin phosphophorin in the regulation of in vitro hydroxyapatite formation and growth. Bone Miner. 1990;11:55–65. doi: 10.1016/0169-6009(90)90015-8. [DOI] [PubMed] [Google Scholar]
- 49.Steiglitz BM, Ayala M, Narayanan K, George A, Greenspan DS. Bone morphogenetic protein-1/Tolloid-like proteinases process dentin matrix protein-1. J Biol Chem. 2004;279:980–986. doi: 10.1074/jbc.M310179200. [DOI] [PubMed] [Google Scholar]
- 50.Boskey AL 2001 Bone mineralization, In: Cowin SC (ed.) Bone Biomechanics, 3rd ed. CRC Press, Boca Raton, FL, USA, pp. 5.1–5.34.
- 51.Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boskey AL, Maresca M, Armstrong AL, Ehrlich MG. Treatment of proteoglycan aggregates with physeal enzymes reduces their ability to inhibit hydroxyapatite proliferation in a gelatin gel. J Orthop Res. 1992;10:313–319. doi: 10.1002/jor.1100100302. [DOI] [PubMed] [Google Scholar]
- 53.Boskey AL, Maresca M, Wikstrom B, Hjerpe A. Hydroxyapatite formation in the presence of proteoglycans of reduced sulfate content: Studies in the brachymorphic mouse. Calcif Tissue Int. 1991;49:389–393. doi: 10.1007/BF02555848. [DOI] [PubMed] [Google Scholar]