Identification of Legionella pneumophila-Specific Genes by Genomic Subtractive Hybridization with Legionella micdadei and Identification of lpnE, a Gene Required for Efficient Host Cell Entry (original) (raw)
Abstract
Legionella pneumophila is a ubiquitous environmental organism and a facultative intracellular pathogen of humans. To identify genes that may contribute to the virulence of L. pneumophila, we performed genomic subtractive hybridization between L. pneumophila serogroup 1 strain 02/41 and L. micdadei strain 02/42. A total of 144 _L. pneumophila_-specific clones were sequenced, revealing 151 genes that were absent in L. micdadei strain 02/42. Low-stringency Southern hybridization was used to determine the distribution of 41 sequences, representing 40 open reading frames (ORFs) with a range of putative functions among L. pneumophila isolates of various serogroups as well as strains of Legionella longbeachae, L. micdadei, Legionella gormanii, and Legionella jordanis. Twelve predicted ORFs were L. pneumophila specific, including the gene encoding the dot/icm effector, lepB, as well as several genes predicted to play a role in lipopolysaccharide biosynthesis and cell wall synthesis and several sequences with similarity to virulence-associated determinants. A further nine predicted ORFs were in all L. pneumophila serotypes tested and an isolate of L. gormanii. These included icmD, the 5′ end of a pilMNOPQ locus, and two genes known to be upregulated during growth within macrophages, cadA2 and ceaA. Disruption of an L. pneumophila_-specific gene (lpg2222 locus tag) encoding a putative protein with eight tetratricopeptide repeats resulted in reduced entry into the macrophage-like cell line, THP-1, and the type II alveolar epithelial cell line, A549. The gene was subsequently renamed lpnE, for “_L. pneumophila entry.” In summary, this investigation has revealed important genetic differences between L. pneumophila and other Legionella species that may contribute to the phenotypic and clinical differences observed within this genus.
Legionella pneumophila is the major causative agent of Legionnaires' disease, a severe form of acute pneumonia that is responsible for 2 to 15% of cases of community-acquired pneumonia requiring hospitalization (57). L. pneumophila ubiquitously inhabits soil, biofilm, and aquatic environments, where it replicates within protozoa (25, 40, 49, 52, 55). Following the inhalation of aerosols containing L. pneumophila, the bacteria are internalized by alveolar macrophages and epithelial cells, where they replicate within an intracellular vacuole. The Legionella vacuole recruits the host GTPase, Rab1, and the v-SNARE, Sec22b, to establish a replicative vacuole surrounded by rough endoplasmic reticulum (RER) (30). The recruitment of RER correlates strongly with the virulence of L. pneumophila, and the manipulation of host cell trafficking pathways allows the bacteria to replicate to high numbers inside cells before eventual bacterial egress, host cell death, and the infection of new host cells (2, 39).
The establishment of the unique _Legionella_-containing vacuole (LCV) depends on the dot (“defective in organelle trafficking”)/icm (“intracellular multiplication”) type IV secretion system (8, 53), which secretes and translocates bacterial effector proteins to the LCV membrane and presumably to the cytosol of the host cell (44). Recently, several _dot/icm_-translocated effector proteins were identified, including a guanine nucleotide exchange factor, RalF, that recruits the host GTPase Arf1 to the LCV membrane; a large coiled-coiled protein of unknown function termed LidA; two effectors, LepA and LepB, with low homology to host SNARE proteins; and a group of DotF binding proteins, SidA to -H (14, 17, 34, 44). Interestingly, mutation of the genes encoding these proteins does not result in a significant reduction in intracellular replication within macrophages, suggesting there is much functional redundancy among these effectors (14, 17, 34, 44).
While there are more than 40 named species of Legionella, 80 to 90% of cases of Legionnaires' disease are caused by L. pneumophila serogroup 1 (7, 35, 59). Legionella micdadei and Legionella longbeachae are the next most common etiological agents of Legionnaires' disease and together account for approximately 2 to 5% of the disease worldwide (6, 43, 59). Interestingly, this trend does not hold true in Australia and New Zealand, where approximately 30% of Legionnaires' disease is attributed to L. longbeachae (59). Nevertheless, these epidemiological differences suggest that L. pneumophila, in particular serogroup 1, is more virulent than other Legionella species.
There have been several studies comparing the virulence traits of different Legionella spp., yet little is known about the genetic basis of these phenotypic differences. Unlike L. pneumophila, L. micdadei appears to replicate within a vacuole that does not recruit host RER and undergoes phagosome-lysosome fusion (26, 29, 48). Both L. longbeachae and L. micdadei replicate within human macrophage cell lines to a level comparable to that of L. pneumophila; however, they show varying abilities to replicate within protozoan hosts (26, 45). In addition, non-pneumophila species of Legionella are less cytotoxic to macrophages, alveolar epithelial cells, and protozoa (3, 26, 29). Although Southern hybridization analysis has revealed that strains of L. micdadei and L. longbeachae possess dot/icm homologues, the completeness, functionality, and expression of the type IV secretion system in either species have not been thoroughly examined (3, 24, 29).
In this study, we investigated genetic differences between L. pneumophila and L. micdadei. Low-stringency genomic subtractive hybridization between a serogroup 1 isolate of L. pneumophila and a clinical isolate of L. micdadei showed that many known virulence determinants of Legionella were L. pneumophila specific, including a novel gene involved in the uptake of L. pneumophila by host cells which is described in this study.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The bacterial strains used in this study are listed in Table 1. Legionella spp. were cultured on BCYE or inACES [_N_-(2-acetamido)-2-aminoethanesulfonic acid]-buffered yeast extract broth (AYE) (23). When necessary, ampicillin, kanamycin, and chloramphenicol were added to Legionella media at 100 μg/ml, 25 μg/ml, and 6 μg/ml, respectively. Escherichia coli DH5α was grown in Luria-Bertani (LB) broth or agar aerobically at 37°C. When required, ampicillin, kanamycin, and chloramphenicol were added to bacteriological media at 100 μg/ml, 100 μg/ml, and 12.5 μg/ml, respectively.
TABLE 1.
Bacterial strains and plasmids used in this study
| Bacterial strain or plasmid | Serogroup and characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| L. pneumophila | ||
| 130b (ATCC BAA-74) | O1; clinical isolate | 22 |
| 02/41 | O1; environmental isolate (Australia) | MDUa |
| 02/40 | O1; clinical isolate (Australia) | MDU |
| 03/41 | O1; environmental isolate (Australia) | MDU |
| 03/42 | O1; environmental isolate (Australia) | MDU |
| 03/43 | O1; environmental isolate (Australia) | MDU |
| 03/44 | O1; environmental isolate (Australia) | MDU |
| 03/45 | O1; environmental isolate (Australia) | MDU |
| 03/46 | O1; clinical isolate (Australia) | MDU |
| 03/47 | O1; clinical isolate (Australia) | MDU |
| 03/48 | O1; clinical isolate (Australia) | MDU |
| 03/49 | O1; clinical isolate (Australia) | MDU |
| 03/50 | O1; clinical isolate (Australia) | MDU |
| 03/54 | O3; environmental isolate (Australia) | MDU |
| 03/55 | O4; environmental isolate (Australia) | MDU |
| 03/56 | O4; environmental isolate (Australia) | MDU |
| 03/57 | O5; environmental isolate (Australia) | MDU |
| 03/59 | O6; environmental isolate (Australia) | MDU |
| 03/60 | O6; environmental isolate (Australia) | MDU |
| 03/61 | O7; environmental isolate (Australia) | MDU |
| 03/63 | O8; environmental isolate (Australia) | MDU |
| 03/64 | O8; environmental isolate (Australia) | MDU |
| lpnE::km | lpnE insertion mutant of 130b (Kmr) | This study |
| lpnE::km (pMIP:lpnE) | lpnE::km carrying pMIP:lpnE (Kmr Cmr) | This study |
| L. micdadei | ||
| 02/42 | Clinical isolate (Australia) | MDU |
| 02/43 | Quality assurance standard (Australia) | MDU |
| 03/67 | Environmental isolate (Australia) | MDU |
| L. longbeachae | ||
| ATCC 33462 | O1; type strain (U.S.) | 37 |
| A5H5 | Clinical isolate (Australia) | 19 |
| A4C5 | Clinical isolate (Australia) | 19 |
| D-1750 | Clinical isolate (U.S.) | 19 |
| D-493 | Clinical isolate (U.S.) | 19 |
| Atlanta-5 | Clinical isolate (U.S.) | 19 |
| D-880 | Clinical isolate (U.S.) | 19 |
| LA-24 | Clinical isolate (U.S.) | 19 |
| L6C9 | Environmental isolate (Australia) | 19 |
| K8B9 | Environmental isolate (Australia) | 19 |
| L. gormanii 03/69 | Environmental isolate (Australia) | MDU |
| L. jordanis 03/70 | Environmental isolate (Australia) | MDU |
| E. coli DH5α | F− Φ80d_lacZ_ΔM15 Δ(lacZYA-argF)U169 endA1 recA1 hsdR17 deoR thi-1 supE44 gyrA96 relA1 | Clontech |
| Plasmids | ||
| pCR-Script | High-copy cloning vector (Ampr) | Stratagene |
| pGEM-T Easy | 3,015-bp cloning vector | Promega |
| pMMB207 | RSF1010 derivative; IncQ oriT lacI_q P_tac Cmr | 41 |
| pMip | pMMB207 with the promoter region of mip cloned into _Sac_I/_Xba_I | This study |
| pMip:lpnE | pMip carrying lpnE and ribosome binding site, cloned into _Xba_I/_Pst_I | This study |
Tissue culture conditions and intracellular infection by Legionella spp.
The human monocytic cell line THP-1 and the human alveolar epithelial cell line A549 were maintained in RPMI 1640 medium, supplemented with 10% fetal bovine serum, in 5% CO2 at 37°C. For THP-1 infection, cells were seeded into 24-well tissue culture trays (Sarstedt, Leicestershire, United Kingdom) at a density of 5 × 105 cells/well and pretreated with 10−8 M phorbol 12-myristate 13-acetate for 36 to 48 h, in 5% CO2 at 37°C, to induce differentiation into macrophage-like adherent cells. Stationary-phase Legionella cells were added at a multiplicity of infection (MOI) of 5 and incubated for 2 h. Cells were treated with 100 μg/ml gentamicin for 1 h to kill extracellular bacteria and were subsequently incubated with tissue culture maintenance media. At specified time points, THP-1 cells were washed three times with PBS and treated with 0.1% (vol/vol) Triton X-100 or 0.01% digitonin for lysis and serial dilutions were plated on BCYE agar. For assessment of bacterial uptake, THP-1 lysis and bacterial recovery were performed immediately following gentamicin treatment and washing. Infection of A549 cells was performed as described for THP-1 cells, except that 2 × 105 cells/well were seeded approximately 20 h before infection with Legionella using an MOI of 100.
Electron microscopy.
THP-1 cells (2 × 106) were differentiated as described above in six-well tissue culture trays (Sarstedt) and infected at an MOI of 10. Infection proceeded for 5 h before cells were washed with phosphate-buffered saline (PBS) and fixed in 2.5% glutaraldehyde for 1 h. Cells were washed in PBS plus 5% sucrose and scraped from the tray surface before being pelleted and washed with PBS plus 5% sucrose followed by a postfixation step in 2.5% osmium tetroxide for 1 h. Following dehydration in a graded acetone series, the cell pellet was embedded in Epon-Araldite epoxy resin. Thin (0.5 μm) sections were stained with 10% uranyl acetate and 2.5% lead citrate before viewing under a Phillips CM12 electron microscope at 60 kV.
General DNA techniques.
Bacterial genomic DNA was extracted as described previously (4), and plasmid DNA was isolated using a QIAprep spin Miniprep kit (QIAGEN, Hilden, Germany). DNA-modifying enzymes including restriction endonucleases were used according to the manufacturer's instructions (Promega, Madison, WI). Dot blotting and Southern hybridization were carried out using standard protocols (4). For Southern hybridization, approximately 2 μg of HindIII- and BamHI-digested genomic DNA was separated by agarose gel electrophoresis and transferred to Biodyne PLUS membrane (Pall Corporation, Pensacola, Fla.). Hybridization with labeled probe was performed at 55°C, and membranes were washed under low-stringency conditions (0.5% sodium dodecyl sulfate at 55°C). L. pneumophila 02/41 DNA and L. micdadei 02/42 DNA were used as positive and negative controls, respectively. Probe DNA was labeled with digoxigen (DIG)-11-dUTP via incorporation during PCR or by random labeling, and DIG detection was performed according to the manufacturers' guidelines (Roche Diagnostics, Mannheim, Germany).
PCR techniques.
PCR amplification was performed on 200 ng of template DNA with approximately 0.25 μg of each primer per 25-μl reaction. The reaction conditions were standard with annealing temperatures adjusted for the oligonucleotide melting temperatures. Eleven pairs of oligonucleotides were designed from sequences of known L. pneumophila virulence genes for amplification from strains of interest. 5′-GCGATTATTTAAGCAGC and 5′-CTCTCAATCGTA ATGAG (annealing temperature, 44°C) yielded a 798-bp product within ralF, 5′-GGGTTATCGTGCAAGGC and 5′-GAAGCCAATGCCAAAGG (annealing temperature, 48°C) yielded a 991-bp fragment of dotA, 5′-GTATCGCCA AAGCAGCAC and 5′-GAGTTGGCTCATCGGGC (annealing temperature, 50°C) amplified 1,137 bp spanning dotD-B, 5′-CCGTCGAGCTTCACTTG and5′-CAAGACCAGCATCTCCC (annealing temperature, 50°C) yielded a 1,500-bp product within dotO, 5′-CACCTCGTGTTAAAGAG and 5′-CATGAA CAAAGCGGCTG (annealing temperature, 46°C) amplified 929 bp spanning icmR-Q, 5′-CTTCCTCAATCCCCCCC and 5′-CTACACCAGCCTCATCC (annealing temperature, 46°C) yielded a 783-bp product within plaA, 5′-CAGGAGTCAGTGTACTTG and 5′-CCAGGTTGTCCTGCTGC (annealing temperature, 50°C) amplified 845 bp spanning lspF-G, 5′-CCAATAACCCTTGCCTG and 5′-CGAGTGCTCGTAAAACG (annealing temperature, 48°C) amplified 996 bp spanning icmV-icmW, 5′-CGGGCTTTTCCAATGAG and 5′-GTTGGCAGAAAGTACCC (annealing temperature, 48°C) yielded a 1,029-bp fragment within lvhB4, 5′-GAAATTGGTGACTGCAGC and 5′-GGGCCATATGCA AGACC (annealing temperature, 48°C) yielded a 612-bp fragment within mip, and 5′-GGTGTGCCCGGTTACTC and 5′-GCTTGGCCTCCAGAGTG (annealing temperature, 48°C) amplified a 944-bp product within rtxA.
Genomic subtractive hybridization.
The PCR-Select bacterial genome subtraction kit (Clontech Laboratories, Palo Alto, CA) was used as described by the manufacturer. Due to the low GC content of Legionella spp. and the level of base pair mismatch between L. pneumophila and L. micdadei (0 to 30%) (42), the temperature of the subtractive hybridization was decreased to 35°C, minimizing the recovery of homologous sequences. Genomic DNA was digested with AluI to generate DNA fragments with a median size of 0.4 kb. DNA fragments were amplified following ligation with specific linkers, cloned into pGEM-T Easy (Promega), and transformed into E. coli DH5α competent cells.
Disruption of lpnE (lpg2222) in L. pneumophila 130b.
To generate an insertional mutation, a 1,149-bp fragment of lpnE was amplified by PCR with the primers 5′-CGGGATCCATGGACATGAAAAAATATATT and 5′-CCATCGATCTTTTGTCCATTGTCCG (annealing temperature, 42°C) and cloned into pCR-Script. A kanamycin resistance cassette (Km) was then ligated into the native EcoRI site of lpnE. This construct was introduced into L. pneumophila 130b by natural transformation for homologous recombination as described previously (56). Briefly, bacteria were incubated in AYE broth at 30°C with 10 μg/ml of pCR-Script:lpnE::Km until reaching an optical density greater than 1.5 at 660 nm before being spread onto the appropriate CYE plates. Kanamycin-resistant clones were assessed for replacement of lpnE with lpnE::Km and loss of pCR-Script by PCR and ampicillin sensitivity.
Construction of an lpnE transcomplementing vector.
To develop a transcomplementation vector with constitutive expression during host cell infection, the 448-bp promoter region of mip was cloned into the SacI/XbaI sites of pMMB207, producing pMIP (31). Full-length lpnE, including the predicted ribosome binding site, was amplified with 5′-GCTCTAGAGATAGCTCTTAAAAATAAGG and 5′-AAC TGCAGGAAAACAGGTAACAGGC (annealing temperature, 44°C) and cloned into the XbaI/PstI sites of pMIP. The resulting plasmid, pMip:lpnE, was introduced into L. pneumophila 130b lpnE::Km via electroporation as described previously (20).
Nucleotide sequencing and analysis.
Plasmid DNA was prepared for sequence analysis using a PRISM Ready reaction dye deoxy terminator cycle sequencing kit (Applied Biosystems, Foster City, CA). The nucleotide sequence of samples was determined by automated DNA sequencing using an Applied Biosystems 3730 DNA analyzer. DNA sequences were assembled using Sequencher 3.1.1 (Gene Codes Corp., MI). BLAST programs were used to determine nucleotide and amino acid homology with sequences in GenBank. The Columbia Genome Center Legionella Project (15) and http://genolist.pasteur.fr/LegioList/ (13) were utilized to obtain sequences of entire open reading frames (ORFs) and information on nucleotide sequence surrounding ORFs of interest.
Nucleotide sequence accession number.
All nucleotide sequences derived from L. pneumophila 02/41 have been submitted to GenBank under accession no. AY688214-29 and AY902807-909.
RESULTS
Characterization of L. pneumophila and L. micdadei strains for subtractive hybridization.
The intraspecies genetic heterogeneity among isolates of L. pneumophila is not well documented and even less well investigated for L. micdadei. Thus, to examine broadly the suitability of L. pneumophila and L. micdadei strains in our collection for subtractive hybridization, we tested two serogroup 1 L. pneumophila strains (02/40 and 02/41) and two strains of L. micdadei (02/42 and 02/43) for the presence of several known virulence-associated loci of L. pneumophila. These were ralF, dotA, dotD-B, dotO, icmR-Q, plaA, lspF-G, icmV-W, lvhB4, mip, and rtxA. L. pneumophila 02/41 was positive for all virulence loci by PCR, while L. pneumophila 02/40 was negative by PCR for ralF, dotA, and icmV-W (data not shown). In contrast, while both L. micdadei 02/42 and 02/43 were positive by PCR for lvhB4, these strains were negative by PCR for all other loci tested (data not shown). L. pneumophila 02/41 and L. micdadei 02/42 were then chosen for further comparison by genomic subtractive hybridization.
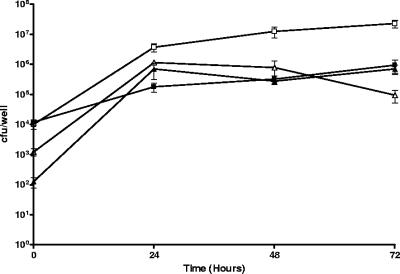
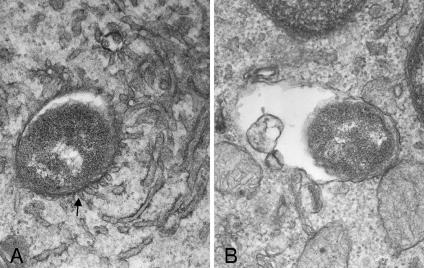
To examine any phenotypic differences between L. pneumophila 02/41 and L. micdadei 02/42 in their ability to survive within macrophages and epithelial cells, these strains were tested for their ability to replicate within the human macrophage-like cell line THP-1 and in A549 human alveolar epithelial cells, as well as for their ability to recruit RER to the replicative vacuole. As expected, both strains were able to replicate within macrophages and epithelial cells (Fig. 1); however, L. pneumophila 02/41 recruited RER to the vacuole 5 h after infection of THP-1 cells (Fig. 2A), whereas the L. micdadei 02/42 vacuole showed no association with RER at the same time point (Fig. 2B). Of the 10 individual THP-1 cells examined that were infected with L. pneumophila 02/41, all LCVs showed an association with RER. In contrast, none of the 10 individual THP-1 cells examined that were infected with L. micdadei 02/42 contained LCVs that were associated with RER.
FIG. 1.
Replication of L. pneumophila strain 02/41 (▴ and ▵) and L. micdadei strain 02/42 (▪ and □) in THP-1 macrophages (open) and A549 alveolar epithelial cells (solid). Results are expressed as the number of cell-associated bacteria and are the mean ± standard deviation of at least three independent experiments from duplicate wells.
FIG. 2.
Transmission electron microscopy of THP-1 cells infected with L. pneumophila strain 02/41 and L. micdadei strain 02/42. (A) 02/41. Magnification, ×45,000. An arrow indicates fusion of RER to the LCV. (B) 02/42. Magnification, ×45,000.
Genomic subtractive hybridization between L. pneumophila 02/41 and L. micdadei 02/42.
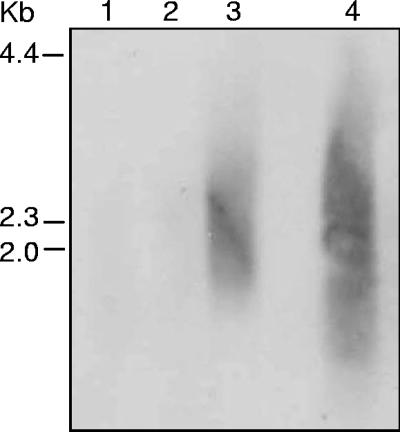
To determine the appropriate stringency for subtractive hybridization of L. pneumophila 02/41 with L. micdadei 02/42, we tested a range of hybridization temperatures, including 35°C, 45°C, and 55°C. Low-stringency conditions were used to ensure homologous sequences were subtracted, allowing for the average G+C content (38%) of L. pneumophila and up to 30% reported base pair mismatch between L. pneumophila and L. micdadei (42). The specificity of the PCR products obtained following subtractive hybridization was examined by low-stringency Southern hybridization of subtracted L. pneumophila 02/41 DNA probed with DIG-labeled L. micdadei genomic DNA. Subtracted DNA fragments amplified from the hybridization conducted at 35°C showed the least reactivity with L. micdadei DIG-labeled DNA (Fig. 3), and these products were subsequently cloned into pGEM-T Easy. A total of 152 clones were recovered, and these were tested by dot blot hybridization for reactivity with L. pneumophila 02/41 and L. micdadei 02/42 DIG-labeled genomic DNA. Eight clones (5.3%) hybridized with both L. pneumophila 02/41 and L. micdadei 02/42 genomic DNA and were classified as nonspecific. The remaining 144 clones only hybridized with L. pneumophila 02/41 DNA.
FIG. 3.
Southern hybridization of subtracted and PCR-amplified L. pneumophila 02/41 DNA fragments probed with randomly labeled AluI-digested L. micdadei 02/42 genomic DNA. Equal amounts of subtracted DNA were loaded in each lane. Lane 1, subtraction performed at 35°C; lane 2, subtraction performed at 45°C; lane 3, subtraction performed at 55°C; lane 4, AluI-digested L. micdadei 02/42 genomic DNA.
Identification of L. pneumophila 02/41-specific sequences.
Sequencing of the 144 _L. pneumophila_-specific clones showed an average clone size of 375 bp representing 151 putative and known L. pneumophila ORFs (see Table S1 in the supplemental material). Twenty-two clones were absent in at least one of the three sequenced L. pneumophila genomes, with only 1, clone 2B6, appearing to be strain specific, with no homologous nucleotide sequence in any of the sequenced L. pneumophila genomes (13, 15). This level of conservation between the three L. pneumophila genome sequences and L. pneumophila strain 02/41 supports the hypothesis that genes intrinsic to the function and virulence of L. pneumophila serogroup 1 may be identified by genomic comparisons with similar and less virulent Legionella spp. Further sequence analysis of the clones showed that 36% corresponded to hypothetical proteins and ORFs with no significant homologues and no known function. The remaining clones had a range of predicted functions and included 16 ORFs with known or putative roles in virulence, 10 putative regulators, and 13 genes putatively involved in cell wall and lipopolysaccharide (LPS) biosynthesis (see Table S1 in the supplemental material).
Distribution of selected sequences among L. pneumophila of different serogroups and other Legionella spp.
Of the 144 clones sequenced, 41 different sequences representing 40 ORFs were selected to cover a range of putative functions for testing by low-stringency Southern hybridization to examine their distribution among L. pneumophila strains of different serogroups as well as additional strains of L. micdadei, L. longbeachae, L. gormanii, and L. jordanis. Overall, the sequences fell into four broad groups. Twelve different ORFs (30%) were present only in L. pneumophila (group I, Table 2); a further 9 ORFs (22.5%) were present in all L. pneumophila strains tested and an isolate of L. gormanii (group II, Table 2); 15 ORFs (37.5%) were present in most or all strains of L. pneumophila, L. longbeachae, L. gormanii, and/or L. jordanis (group III, Table 2); and 4 ORFs (10%) were present in all Legionella spp., including L. jordanii and L. micdadei (group IV, Table 2).
TABLE 2.
Distribution of selected sequences among strains of L. pneumophila, L. gormanii, L. longbeachae, L. jordanis, and L. micdadei
| Clone namea | Corresponding L. pneumophila Philadelphia gene(s) or locus tag | Known or putative function |
|---|---|---|
| Group I | ||
| 2B7, B11, C12/F3, F8b | yvf, lpg0754, waaM, lpg0774 | LPS and lipidA biosynthesis |
| E10/2B5 | lpg2699 | Autolysin |
| 2E6 | lepB | Dot/Icm effector |
| 2C12 | lpg2222 | Hypothetical TPR repeat protein |
| G7 | ladC | Adenylate cyclase |
| C3, B5 | lpg2977, lpg1176 | Zn metalloproteases |
| D12, E12 | pleD, lpg1357 | Transcriptional regulators |
| Group II | ||
| D6 | icmD | Dot/Icm secretion system |
| C10 | pilM, pilN | Type IV pilus biosynthesis |
| 2C5 | clpB | Heat shock chaperone |
| C1/2C8 | lpg0919 | Zn metalloprotease |
| A8 | enhA | Unknown function |
| A3/B2 | cadA2 | Cadmium efflux ATPase |
| H1 | lpg2549 | Transcriptional regulator |
| Group III | ||
| B8 | rep | ATP-dependent DNA helicase |
| A6 | icmE | Dot/Icm secretion system |
| C2 | lspI, lspJ | Type II secretion system |
| H7/2C6, C6, F5/F6c | lpg0142, lpg1071, lpg2119 | Transposases |
| D1 | lpg1292, lpg1291 | Two-component response regulator |
| F11 | hisF, hisH | Amino acid metabolism |
| C9d | sidH | Dot/Icm effector |
| E6e | sidE | Dot/Icm effector |
| 2C11f | sidB | Dot/Icm effector |
| 2E3g | sidG | Dot/Icm effector |
| Group IV | ||
| A11,h E2,i G12j | lpg0991, lpg0986, lpg0987 | Hypothetical proteins |
| 2E10 | udk, fabI | Lipid metabolism |
The 12 different ORFs in group I included 2 genes identified previously, ladC and lepB (14, 47), 2 putative transcriptional regulators, 2 putative zinc-dependent protease genes, including one with homology to virulence-associated elastases (9); and 4 ORFs predicted to play a role in lipopolysaccharide and cell wall biogenesis. Two clones, E10 and 2B5, were found within a gene predicted to encode an N-acetylmuramoyl-l-alanine amidase. Autolysins are common among different bacterial species and are associated with various cellular processes such as cell growth, cell-wall turnover, motility, protein secretion, differentiation, biofilm formation, and pathogenicity (10, 11, 58). The eight ORFs present in group II comprised a putative transcriptional regulator and several genes previously identified in L. pneumophila, including cadA2, icmD, and ceaA, of which cadA2 and ceaA have been shown to be upregulated during intracellular growth (47). Other genes within this group included a homolog of enhA, the 5′ end of the pilMNOPQ locus, a putative Zn-dependent protease gene, and a homolog of clpB. Of the 15 ORFs in group III, clone D1 showed homology to a novel, intact two-component response regulator. The closest homologues of this response regulator comprise a group of DNA binding proteins highly homologous to CheY-like receiver domains involved in bacterial chemotaxis (21). Group III also included four ORFs, sidB, sidE, sidG, and sidH, which encode secreted substrates of dot/icm type IV secretion system which were identified using an interbacterial protein transfer technique (34). Group IV contained 4 ORFs, of which clones A11, G12, and E2 are clustered in a region of hypothetical and unknown ORFs on the basis of the L. pneumophila genome sequences. Clones A11, E2, G12, and 2E10 were the only sequences present in the two additional strains of L. micdadei tested, suggesting that their absence in the subtraction driver strain, L. micdadei 02/42, may be strain specific.
Characterization of an _L. pneumophila_-specific gene encoding a putative protein with eight tetratricopeptide repeats.
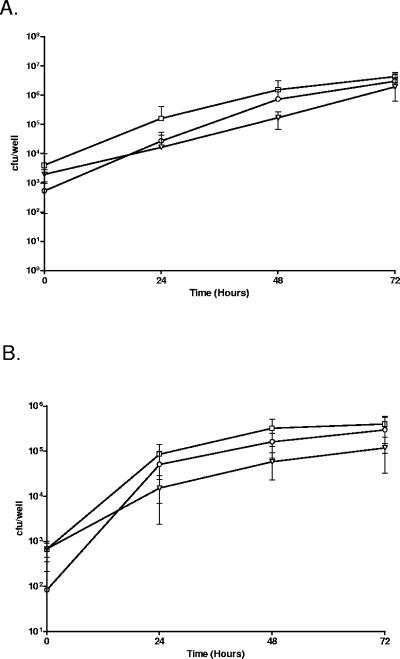
One of the putative virulence-associated genes present in group I included the locus tag lpg2222, which is predicted to encode a protein that shares similarity with EnhC. EnhC has been shown to play a role in enhancing the uptake of L. pneumophila into host cells (16). To determine if lpg2222 also contributes to the entry of L. pneumophila into host cells, we inactivated lpg2222 by insertion of a kanamycin resistance cassette. The resulting mutant showed no significant defect in intracellular replication during L. pneumophila infection of both the THP-1 and A549 cell lines (Fig. 4A and B). However, the lpg2222 mutant did exhibit reduced entry into THP-1 and A549 cells compared with wild-type L. pneumophila 130b, which was partially restored upon _trans_-complementation of the mutant with pMIP:lpnE (Table 3). Interestingly, although pMIP:lpnE was able to complement the entry defect, carriage of the plasmid appeared to interfere with intracellular replication of the _trans_-complemented lpg2222 mutant. pMIP:lpnE is derived from the IncQ plasmid, pMMB207, and previous studies have reported thatpMMB207 can interfere with intracellular replication of L.pneumophila (51, 54). This defect has been attributed to thepresence of mobilization factors on the plasmid that presumably interfere with Dot/Icm function (54), and this islikely to be the reason for the replication defect observed here.
FIG. 4.
Replication of derivatives of L. pneumophila strain 130b in THP-1 macrophages (A) and A549 alveolar epithelial cells (B). L. pneumophila 130b (□), lpnE::km (○) and lpnE::km (pMIP:lpnE) (▾). Results are expressed as the number of cell-associated bacteria and are the mean ± standard deviation of at least three independent experiments from duplicate wells.
TABLE 3.
L. pneumophila entry into THP-1 and A549 cell lines
| Strain | % of intracellular bacteria ina: | |
|---|---|---|
| THP-1 | A549 | |
| 130b | 0.40 ± 0.24 | 0.0063 ± 0.0036 |
| lpnE::km | 0.09 ± 0.07b | 0.0004 ± 0.0006b |
| lpnE::km (pMip:lpnE) | 0.21 ± 0.12c | 0.0046 ± 0.0022c |
The decrease in entry observed for the lpg2222 mutant was not the result of decreased attachment to host cells as the lpg2222 mutant demonstrated levels of attachment to both host cell lines similar to those of wild-type L. pneumophila 130b. Bacterial attachment was calculated as the percentage of the original inoculum that remained after infection for 2 h. In infected THP-1 cells, the percentages of cell-associated bacteria were 1.37% ± 0.70% for L. pneumophila 130b and 1.12% ± 0.71% for the lpg2222 mutant. Similarly the percentage of the inoculum associated with A549 cells for 130b was 0.50% ± 0.24%, and for the lpg2222 mutant it was 0.58% ± 0.29%. In light of these results, lpg2222 was renamed lpnE for “gene involved in L. pneumophila host cell entry.”
DISCUSSION
Outbreaks of Legionnaires' disease worldwide are almost exclusively due to infection with L. pneumophila, and this species is also responsible for the vast majority of sporadic cases of legionellosis (35). Although infection with non-pneumophila Legionella species can occur (43), overall, non-pneumophila species account for around only 10% of cases of legionellosis (59). While environmental factors and genetic regulation may contribute to this epidemiological difference, it is widely believed that L. pneumophila possesses species-specific virulence determinants that account for its greater prominence as a pathogen.
In this study, we identified specific genetic differences between a serogroup 1 strain of L. pneumophila and an isolate of L. micdadei. In the absence of a genome sequence for L. micdadei, the genetic comparison was done experimentally by genomic subtractive hybridization (1). Of the resulting 144 L. pneumophila 02/41-specific clones, many were genes with unknown function, but some known and putative virulence determinants were also identified as absent in L. micdadei.
Forty-one sequences, corresponding to 40 different predicted ORFs in the L. pneumophila Philadelphia, Paris, and Lens genome sequences (13, 15) covering a range of functions, were examined for their prevalence among various serogroups of L. pneumophila and other species of Legionella by low-stringency Southern hybridization. The results showed that 30% of these clones were L. pneumophila specific, and a further 22.5% were present in L. pneumophila and L. gormanii only.
Several genes involved in cell wall biosynthesis were identified in this study, including five genes within the L. pneumophila LPS biosynthesis gene locus. Clones 2B7 and B11, representing neighboring orf21 (lyrF) and orf22 (lpg0754), were L. pneumophila specific. This piece of data is consistent with a recent hybridization study showing that a probe encompassing orf21 to orf26 of the LPS biosynthesis locus hybridized to L. pneumophila of serogroups 2 to 14 but showed no hybridization to a range of non-pneumophila Legionella species (33). In this study we also showed that clone F11, spanning orf26 (hisF) and orf27 (hisH), hybridized to all strains of L. longbeachae and L. gormanii, suggesting that orf27 is shared among different species of Legionella. In addition, we found that clone F8, corresponding to orf3 (lpg0774) of the LPS biosynthesis locus, was present in all serogroup 1 strains of L. pneumophila as well as serogroups 4, 5, and 6. This distribution differs from that found by Luneberg et al., who reported that a probe spanning orf1 to orf4 hybridized only to serogroups 1 and 7 of L. pneumophila. This discrepancy suggests that the distribution of orf3 is highly variable among different Legionella species and strains.
A selection of known L. pneumophila virulence loci were also identified by this subtractive hybridization study. These included two members of the dot/icm type IV secretion system, icmD and icmE, and five genes that encode Dot/Icm effector proteins that are translocated into host cells, lepB, sidB, sidE, sidG, and sidH (14, 34). lepB encodes a putative SNARE homologue involved in nonlytic release of L. pneumophila from Dictyostelium discoideum that may contribute to the transmission of L. pneumophila by promoting the egress of bacteria from amoebae (14). Similar to ralF, which encodes another Dot/Icm effector, lepB was present only in strains of L. pneumophila (44). Interestingly, the same was not true for sidB, sidE, sidG, and sidH, which, as well as being present in all L. pneumophila serogroups tested, were found in most strains of L. longbeachae, L. jordanis, and L. gormanii used in this study. icmE (dotG) was present in L. gormanii and all L. longbeachae strains but absent in L. micdadei and L. jordanis. Conversely, icmD was found in L. pneumophila and L. gormanii but none of the other non-pneumophila species examined. A previous study investigating the distribution of icmD and icmE genes among different species of Legionella also showed that both loci were absent in L. micdadei and L. jordanis (42). Recently, species-specific heterogeneity was described for the icmR locus in L. pneumophila, L. longbeachae, and L. micdadei. The gene occupying this position in each species was unrelated by amino acid similarity but performed the same function as a chaperone for its cognate IcmQ protein (24). Overall, these data suggest that not only do the dot/icm loci vary in their organization and composition among different Legionella strains and species but that some Dot/Icm effector proteins, such as RalF and LepB, may be species specific (14, 44).
In this study we also identified three sequences corresponding to L. pneumophila genes expressed during replication within U937 macrophages (47). Of these, ladC encodes a putative adenylate cyclase which was L. pneumophila specific (group I), whereas cadA2 and ceaA were found in all L. pneumophila strains and in L. gormanii but were absent from all of the other species tested (group II). cadA2 and ceaA encode a putative cadmium efflux pump and a component of a chemiosmotic efflux system, respectively. Overall, 9.3% of ORFs identified in this study represent genes predicted to be involved in transport and efflux of various ions. The large number and apparent functional redundancy of dedicated transport systems in L. pneumophila have been described previously and may reflect the importance of detoxification and ion balance during intracellular replication of L. pneumophila (36, 47).
Among other group II sequences, we identified the 5′ region of the pilMNOPQ locus. The pil locus of L. pneumophila is essential for type IV pilus biogenesis, but only the prepilin peptidase encoded by pilD is also essential for type II protein secretion, intracellular infection, and virulence in A/J mice (32, 51). Interestingly we also identified lspJ, which is predicted to encode a pseudopilin of the Lsp type II secretion system. The Lsp type II protein secretion apparatus secretes a number of enzymes, including the zinc metalloprotease, ProA, and is required for growth of L. pneumophila in both Hartmannella vermiformis and Acanthamoeba castellanii (28, 50). A recent study has also shown that the Lsp system is essential for virulence in the A/J mouse model of infection (28, 46, 50, 51), implying an important role in virulence factor export. While this gene was absent in L. micdadei and L. jordanis, it was present in all strains of L. pneumophila, L. longbeachae, and L. gormanii.
Several unknown ORFs specific for L. pneumophila or L. pneumophila and L. gormanii were identified in this study that may represent novel virulence determinants. Three of these showed homology to proteases from other pathogens, including vibrolysin and elastases, which are known to contribute to the virulence of Vibro vulnificus, Pseudomonas aeruginosa, and Aeromonas hydrophila, respectively (12, 18, 38). In addition, we identified an _L. pneumophila_-specific ORF, locus tag lpg2222, with predicted similarity to the product of enhC. enhC is part of a locus involved in L. pneumophia entry into host epithelial cells and macrophages (16). Further characterization of lpg2222, termed lpnE, demonstrated that this gene was also required for full entry of L. pneumophila into THP-1 and A549 cells. This finding is consistent with the function of EnhC in enhancing Legionella entry presumably through the possession of multiple tetratricopeptide repeats (16). These repeat regions are associated with a range of functions in eukaryotic cells through their ability to mediate protein-protein interactions (27), and we predict that these regions may be important for LpnE-mediated interaction of L. pneumophila with host cells. Recently, the product of lpnE was identified by proteomic analysis as a protein upregulated during L. pneumophila growth in medium designed to induce resistance to stress, termed ERS (enhanced resistance to stress) treatment (5). The authors found that ERS treatment of L. pneumophila promoted Dot/Icm-independent entry into macrophages and amoebae, demonstrating that there was a correlation between lpnE expression and levels of bacterial uptake into host cells.
In summary, this study has identified distinct genetic differences between L. pneumophila and other species of Legionella. These differences encompassed many known and putative virulence determinants that may contribute to the increased virulence and unique intracellular lifestyle of L. pneumophila. Further work will help to define the importance of this genetic diversity to the pathogenesis of infections with both L. pneumophila and non-pneumophila species.
Supplementary Material
[Supplemental material]
Acknowledgments
We are indebted to Geoff Hogg and Agnes Tan from the Microbiological Diagnostic Unit, Public Health Laboratory, Department of Microbiology and Immunology, University of Melbourne, for the provision of L. pneumophila, L. micdadei, L. gormanii and L. jordanis strains; and Robyn Doyle and Michael Heuzenroeder, Institute of Medical and Veterinary Science, Adelaide, for L. longbeachae strains. We would also like to thank Alastair McEwan, University of Queensland, for sending us L. pneumophila 130b and Yih Teng Yap, University of Melbourne, for her technical assistance. The THP-1 and A549 cell lines were a kind gift from Ranjini Ganendren, Western Sydney Health Services.
H.J.N is the recipient of an Australian Post Graduate Award, and F.M.S. is the recipient of a Monash Graduate Scholarship. This work was supported by the Australian National Health and Medical Research Council.
Footnotes
REFERENCES
- 1.Akopyants, N. S., A. Fradkov, L. Diatchenko, J. E. Hill, P. D. Siebert, S. A. Lukyanov, E. D. Sverdlov, and D. E. Berg. 1998. PCR-based subtractive hybridization and differences in gene content among strains of Helicobacter pylori. Proc. Natl. Acad. Sci. USA 95**:**13108-13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alli, O. A. T., L.-Y. Gao, L. L. Pedersen, S. Zink, M. Radulic, M. Doric, and Y. Abu Kwaik. 2000. Temporal pore formation-mediated egress from macrophages and alveolar epithelial cells by Legionella pneumophila. Infect. Immun. 68**:**6431-6440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alli, O. A. T., S. Zink, K. N. von Lackum, and Y. Abu-Kwaik. 2003. Comparative assessment of virulence traits in Legionella spp. Microbiology 149**:**631-641. [DOI] [PubMed] [Google Scholar]
- 4.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1995. Current protocols in molecular biology. John Wiley and Sons, Inc., New York, N.Y.
- 5.Bandyopadhyay, P., H. Xiao, H. A. Coleman, A. Price-Whelan, and H. M. Steinman. 2004. Icm/Dot-independent entry of Legionella pneumophila into amoeba and macrophage hosts. Infect. Immun. 72**:**4541-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benin, A. L., R. F. Benson, and R. E. Besser. 2002. Trends in Legionnaires disease, 1980-1998: declining mortality and new patterns of diagnosis. Clin. Infect. Dis. 35**:**1039-1046. [DOI] [PubMed] [Google Scholar]
- 7.Benson, R. F., and B. S. Fields. 1998. Classification of the genus Legionella. Semin. Resp. Infect. 13**:**90-99. [PubMed] [Google Scholar]
- 8.Berger, K. H., and R. R. Isberg. 1993. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol. Microbiol. 7**:**7-19. [DOI] [PubMed] [Google Scholar]
- 9.Bever, R. A., and B. H. Iglewski. 1988. Molecular characterization and nucleotide sequence of the Pseudomonas aeruginosa elastase structural gene. J. Bacteriol. 170**:**4309-4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blackman, S. A., T. J. Smith, and S. J. Foster. 1998. The role of autolysins during vegetative growth of Bacillus subtilis. Microbiology 144**:**73-82. [DOI] [PubMed] [Google Scholar]
- 11.Cabanes, D., O. Dussurget, P. Dehoux, and P. Cossart. 2004. Auto, a surface associated autolysin of Listeria monocytogenes required for entry into eukaryotic cells and virulence. Mol. Microbiol. 51**:**1601-1614. [DOI] [PubMed] [Google Scholar]
- 12.Cascón, A., J. Yugueros, A. Temprano, M. Sánchez, C. Hernanz, J. M. Luengo, and G. Naharro. 2000. A major secreted elastase is essential for pathogenicity of Aeromonas hydrophila. Infect. Immun. 68**:**3233-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cazalet, C., C. Rusniok, H. Bruggemann, N. Zidane, A. Magnier, L. Ma, M. Tichit, S. Jarraud, C. Bouchier, F. Vandenesch, F. Kunst, J. Etienne, P. Glaser, and C. Buchrieser. 2004. Evidence in the Legionella pneumophila genome for exploitation of host cell functions and high genome plasticity. Nat. Genet. 36**:**1165-1173. [DOI] [PubMed] [Google Scholar]
- 14.Chen, J., K. S. de Felipe, M. Clarke, H. Lu, O. R. Anderson, G. Segal, and H. A. Shuman. 2004. Legionella effectors that promote non-lytic release from protozoa. Science 303**:**1358-1361. [DOI] [PubMed] [Google Scholar]
- 15.Chien, M., I. Morozova, S. Shi, H. Sheng, J. Chen, S. M. Gomez, G. Asamani, K. Hill, J. Nuara, M. Feder, J. Rineer, J. J. Greenberg, V. Steshenko, S. H. Park, B. Zhao, E. Teplitskaya, J. R. Edwards, S. Pampou, A. Georghiou, I. C. Chou, W. Iannuccilli, M. E. Ulz, D. H. Kim, A. Geringer-Sameth, C. Goldsberry, P. Morozov, S. G. Fisher, G. Segal, X. Qu, A. Rzhetsky, P. Zhang, E. Cayanis, P. J. De Jong, J. Ju, S. Kalachikov, H. A. Shuman, and J. J. Russo. 2004. The genomic sequence of the accidental pathogen Legionella pneumophila. Science 305**:**1966-1968. [DOI] [PubMed] [Google Scholar]
- 16.Cirillo, S. L., J. Lum, and J. D. Cirillo. 2000. Identification of novel loci involved in entry by Legionella pneumophila. Microbiology 146**:**1345-1359. [DOI] [PubMed] [Google Scholar]
- 17.Conover, G. M., I. Derre, J. P. Vogel, and R. R. Isberg. 2003. The Legionella pneumophila LidA protein: a translocated substrate of the Dot/Icm system associated with maintenance of bacterial integrity. Mol. Microbiol. 48**:**305-321. [DOI] [PubMed] [Google Scholar]
- 18.Cowell, B. A., S. S. Twining, J. A. Hobden, M. S. Kwong, and S. M. Fleiszig. 2003. Mutation of lasA and lasB reduces Pseudomonas aeruginosa invasion of epithelial cells. Microbiology 149**:**2291-2299. [DOI] [PubMed] [Google Scholar]
- 19.Doyle, R. M., N. P. Cianciotto, S. Banvi, P. A. Manning, and M. W. Heuzenroeder. 2001. Comparison of virulence of Legionella longbeachae strains in guinea pigs and U937 macrophage-like cells. Infect. Immun. 69**:**5335-5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doyle, R. M., T. W. Steele, A. M. McLennan, I. H. Parkinson, P. A. Manning, and M. W. Heuzenroeder. 1998. Sequence analysis of the mip gene of the soilborne pathogen Legionella longbeachae. Infect. Immun. 66**:**1492-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisenback, M. 1996. Control of bacterial chemtaxis. Mol. Microbiol. 20**:**903-910. [DOI] [PubMed] [Google Scholar]
- 22.Engleberg, N. C., D. J. Drutz, and B. I. Eisenstein. 1984. Cloning and expression of Legionella pneumophila antigens in Escherichia coli. Infect. Immun. 44**:**222-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feeley, J. C., R. J. Gibson, G. W. Gorman, N. C. Langford, J. K. Rasheed, D. C. Mackel, and W. B. Baine. 1979. Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila. J. Clin. Microbiol. 10**:**437-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldman, M., and G. Segal. 2004. A specific genomic location within the icm/dot pathogenesis region of different Legionella species encodes functionally similar but nonhomologous virulence proteins. Infect. Immun. 72**:**4503-4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fields, B. S. 1996. The molecular ecology of legionellae. Trends Microbiol. 4**:**286-290. [DOI] [PubMed] [Google Scholar]
- 26.Gao, L. Y., M. Susa, B. Ticac, and Y. Abu-Kwaik. 1999. Heterogeneity in intracellular replication and cytopathogenicity of Legionella pneumophila and Legionella micdadei in mammalian and protozoan cells. Microb. Pathog. 27**:**273-287. [DOI] [PubMed] [Google Scholar]
- 27.Goebl, M., and M. Yanagida. 1991. The TPR snap helix: a novel protein repeat motif from mitosis to transcription. Trends Biochem. Sci. 16**:**173-177. [DOI] [PubMed] [Google Scholar]
- 28.Hales, L. M., and H. A. Shuman. 1999. Legionella pneumophila contains a type II general secretion pathway required for growth in amoebae as well as for secretion of the Msp protease. Infect. Immun. 67**:**3662-3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joshi, A. D., and M. S. Swanson. 1999. Comparative analysis of Legionella pneumophila and Legionella micdadei virulence traits. Infect. Immun. 67**:**4134-4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kagan, J. C., M. P. Stein, M. Pypaert, and C. R. Roy. 2004. Legionella subvert the functions of Rab1 and Sec22b to create a replicative organelle. J. Exp. Med. 199**:**1201-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohler, R., A. Bubert, W. Goebel, M. Steinert, J. Hacker, and B. Bubert. 2000. Expression and use of the green fluorescent protein as a reporter system in Legionella pneumophila. Mol. Genet. Genom. 262**:**1060-1069. [DOI] [PubMed] [Google Scholar]
- 32.Liles, M. R., V. K. Viswanathan, and N. P. Cianciotto. 1998. Identification and temperature regulation of Legionella pneumophila genes involved in type IV pilus biogenesis and type II protein secretion. Infect. Immun. 66**:**1776-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luneberg, E., N. Zetzmann, D. Alber, Y. A. Knirel, O. Kooistra, U. Zahringer, and M. Frosch. 2000. Cloning and functional characterization of a 30 kb gene locus required for lipopolysaccharide biosynthesis in Legionella pneumophila. Int. J. Med. Microbiol. 290**:**37-49. [DOI] [PubMed] [Google Scholar]
- 34.Luo, Z. Q., and R. R. Isberg. 2004. Multiple substrates of the Legionella pneumophila Dot/Icm system identified by interbacterial protein transfer. Proc. Natl. Acad. Sci. USA 101**:**841-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marston, B. J., H. B. Lipman, and R. F. Breiman. 1994. Surveillance for Legionnaires' disease. Arch. Int. Med. 154**:**2417-2422. [PubMed] [Google Scholar]
- 36.McClain, M. S., M. C. Hurley, J. K. Brieland, and N. C. Engleberg. 1996. The Legionella pneumophila hel locus encodes intracellularly induced homologs of heavy-metal ion transporters of Alcaligenes spp. Infect. Immun. 64**:**1532-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKinney, R. M., R. K. Porschen, P. H. Edelstein, M. J. Bissett, P. P. Harris, S. P. Bondell, A. G. Steigerwalt, R. E. Weaver, M. E. Ein, D. S. Lindquist, R. S. Kops, and D. J. Brenner. 1981. Legionella longbeachae species nova, another etiological agent of human pneumonia. Ann. Intern. Med. 94**:**739-743. [DOI] [PubMed] [Google Scholar]
- 38.Miyoshi, S.-I., H. Nakazawa, K. Kawata, K.-I. Tomochika, K. Tobe, and S. Shinoda. 1998. Characterization of the hemorrhagic reaction caused by Vibrio vulnificus metalloprotease, a member of the thermolysin family. Infect. Immun. 66**:**4851-4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molmeret, M., D. M. Bitar, L. Han, and Y. A. Kwaik. 2004. Disruption of the phagosomal membrane and egress of Legionella pneumophila into the cytoplasm during the last stages of intracellular infection of macrophages and Acanthamoeba polyphaga. Infect. Immun. 72**:**4040-4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molmeret, M., M. Horn, M. Wagner, M. Santic, and Y. Abu Kwaik. 2005. Amoebae as training grounds for intracellular bacterial pathogens. Appl. Environ. Microbiol. 71**:**20-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morales, V. M., A. Backman, and M. Bagdasarian. 1991. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene 97**:**39-47. [DOI] [PubMed] [Google Scholar]
- 42.Morozova, I., X. Qu, S. Shi, G. Asamani, J. E. Greenberg, H. A. Shuman, and J. J. Russo. 2004. Comparative sequence analysis of the icm/dot genes in Legionella. Plasmid 51**:**127-147. [DOI] [PubMed] [Google Scholar]
- 43.Muder, R., and V. L. Yu. 2002. Infection due to Legionella species other than L. pneumophila. Clin. Infect. Dis. 35**:**990-998. [DOI] [PubMed] [Google Scholar]
- 44.Nagai, H., J. C. Kagan, X. Zhu, R. A. Kahn, and C. R. Roy. 2002. A bacterial guanine nucleotide exchange factor activates ARF on Legionella phagosomes. Science 295**:**679-682. [DOI] [PubMed] [Google Scholar]
- 45.Neumeister, B., M. Schöniger, M. Faigle, M. Eichner, and K. Dietz. 1997. Multiplication of different Legionella species in Mono Mac 6 cells and in Acanthamoebae castellanii. Appl. Environ. Microbiol. 63**:**1219-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Polesky, A. H., J. T. D. Ross, S. Falkow, and L. S. Tompkins. 2001. Identification of Legionella pneumophila genes important for infection of amoebas by signature-tagged mutagenesis. Infect. Immun. 69**:**977-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rankin, S., Z. Li, and R. R. Isberg. 2002. Macrophage-induced genes of Legionella pneumophila: protection from reactive intermediates and solute imbalance during intracellular growth. Infect. Immun. 70**:**3637-3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rechnitzer, C., and J. Blom. 1989. Engulfment of the Philadelphia strain of Legionella pneumophila within the pseudopod coils in human phagocytes. Comparison with other Legionella strains and species. APMIS 97**:**105-114. [PubMed] [Google Scholar]
- 49.Rogers, J., A. B. Dowsett, P. J. Dennis, J. V. Lee, and C. W. Keevil. 1994. Influence of temperature and plumbing material selection on biofilm formation and growth of Legionella pneumophila in a model potable water system containing complex microbial flora. Appl. Environ. Microbiol. 60**:**1585-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rossier, O., and N. P. Cianciotto. 2001. Type II protein secretion is a subset of the PilD-dependent processes that facilitate intracellular infection by Legionella pneumophila. Infect. Immun. 69**:**2092-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rossier, O., S. R. Starkenburg, and N. P. Cianciotto. 2004. Legionella pneumophila type II protein secretion promotes virulence in the A/J mouse model of Legionnaires' disease pneumonia. Infect. Immun. 72**:**310-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rowbotham, T. J. 1980. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J. Clin. Pathol. 33**:**1179-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Segal, G., M. Purcell, and H. A. Shuman. 1998. Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila genome. Proc. Natl. Acad. Sci. USA 95**:**1669-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Segal, G., and H. A. Shuman. 1998. Intracellular multiplication and human macrophage killing by Legionella pneumophila are inhibited by conjugal components of IncQ plasmid RSF1010. Mol. Microbiol. 30**:**197-208. [DOI] [PubMed] [Google Scholar]
- 55.Steele, T. W., C. V. Moore, and N. Sangster. 1990. Distribution of Legionella longbeachae serogroup 1 and other legionellae in potting soils in Australia. Appl. Environ. Microbiol. 56**:**2984-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stone, B. J., and Y. Abu Kwaik. 1999. Natural competence for DNA transformation by Legionella pneumophila and its association with expression of type IV pili. J. Bacteriol. 181**:**1395-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stout, J. E., and V. L. Yu. 1997. Legionellosis. N. Engl. J. Med. 337**:**682-687. [DOI] [PubMed] [Google Scholar]
- 58.Vuong, C., C. Gerke, G. A. Somerville, E. R. Fischer, and M. Otto. 2003. Quorum-sensing control of biofilm factors in Staphylococcus epidermidis. J. Infect. Dis. 188**:**706-718. [DOI] [PubMed] [Google Scholar]
- 59.Yu, V. L., J. F. Plouffe, M. C. Pastoris, J. E. Stout, M. Schousboe, A. Widmer, J. Summersgill, T. File, C. M. Heath, D. L. Paterson, and A. Chereshsky. 2002. Distribution of Legionella species and serogroups isolated by culture in patients with sporadic community-acquired pneumonia: an international collaborative study. J. Infect. Dis. 186**:**127-128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental material]