Dissection of a functional interaction between the DNA translocase, FtsK, and the XerD recombinase (original) (raw)
Summary
Successful bacterial circular chromosome segregation requires that any dimeric chromosomes, which arise by crossing over during homologous recombination, are converted to monomers. Resolution of dimers to monomers requires the action of the XerCD site-specific recombinase at dif in the chromosome replication terminus region. This reaction requires the DNA translocase, FtsKC, which activates dimer resolution by catalysing an ATP hydrolysis-dependent switch in the catalytic state of the nucleoprotein recombination complex. We show that a 62-amino-acid fragment of FtsKC interacts directly with the XerD C-terminus in order to stimulate the cleavage by XerD of BSN, a dif-DNA suicide substrate containing a nick in the ‘bottom’ strand. The resulting recombinase–DNA covalent complex can undergo strand exchange with intact duplex dif in the absence of ATP. FtsKC-mediated stimulation of BSN cleavage by XerD requires synaptic complex formation. Mutational impairment of the XerD–FtsKC interaction leads to reduction in the in vitro stimulation of BSN cleavage by XerD and a concomitant deficiency in the resolution of chromosomal dimers at dif in vivo, although other XerD functions are not affected.
Introduction
After termination of bacterial chromosome replication, the two sister chromosomes need to be physically separated before segregation can be completed. Two potential impediments to such separation are catenation of the two monomeric sister chromosomes and circular chromosome dimer formation by crossing over during homologous recombination (reviewed in Espeli and Marians, 2004; Barre and Sherratt, 2005). Topoisomerase action is required for decatenation, while XerCD site-specific recombination at the recombination site dif, located in the ter region converts bacterial chromosome dimers to monomers. FtsK, a 1329-amino-acid integral membrane protein that localizes to the FtsZ ring, functions directly in dimer resolution and facilitates decatenation by topoisomerase IV (Espeli et al., 2003). In addition, FtsK may contribute directly to decatenation during XerCD recombination at dif (Ip et al., 2003). The 179-amino-acid N-terminal membrane domain of FtsK is required for cytokinesis, while the ∼500-amino-acid C-terminal domain (FtsKC) is a DNA translocase that functions in chromosome segregation and dimer resolution (Draper et al., 1998; Yu et al., 1998; Aussel et al., 2002). Therefore, FtsK links chromosome segregation with cell division via its C- and N-terminal domains. The DNA sequence-directed translocation activity of FtsKC promotes synapsis of dif sites in the vicinity of the septum before recombination activation (Pérals et al., 2000; Bigot et al., 2004; Barre and Sherratt, 2005; Pease et al., 2005). FtsKC action may also facilitate chromosome segregation by organizing newly replicated ter regions at midcell (Lesterlin et al., 2004).
It has been proposed previously that FtsKC activates XerCD recombination at dif by catalysing an ATP-dependent switch in the conformation of XerCD–dif nucleoprotein complex, thereby allowing XerD to initiate recombination (Aussel et al., 2002). Here we dissect the activation process by showing that a biochemically active form of FtsKC, FtsK50C (Aussel et al., 2002), can stimulate cleavage by XerD of a _dif_-DNA suicide substrate, BSN, containing a nick in the ‘bottom’ strand. This occurs only in synaptic complexes containing two BSN DNA fragments. Furthermore, we show that interaction between a 62-amino-acid non-motor subdomain of FtsKC and the C-terminus of XerD is required for stimulation of BSN cleavage. This stimulation can also lead to intermolecular recombination between BSN and intact linear dif duplex in the absence of ATP.
Results and discussion
FtsK50C stimulates XerD-mediated cleavage of BSN DNA
Previous work has demonstrated that FtsK50C-dependent activation of a complete Xer recombination reaction at dif requires ATP hydrolysis by FtsK50C and a DNA extension adjacent to the XerD binding site on at least one of the two participating dif sites (Aussel et al., 2002; Massey et al., 2004). This reaction requires a specific interaction between FtsK50C and the XerCD recombinase that leads to an ATP hydrolysis-dependent switch in the catalytic state of the recombination complex, so that recombination is initiated by XerD to give a Holliday junction (HJ) intermediate that is resolved to complete recombinant product by XerC (Aussel et al., 2002; Yates et al., 2003).
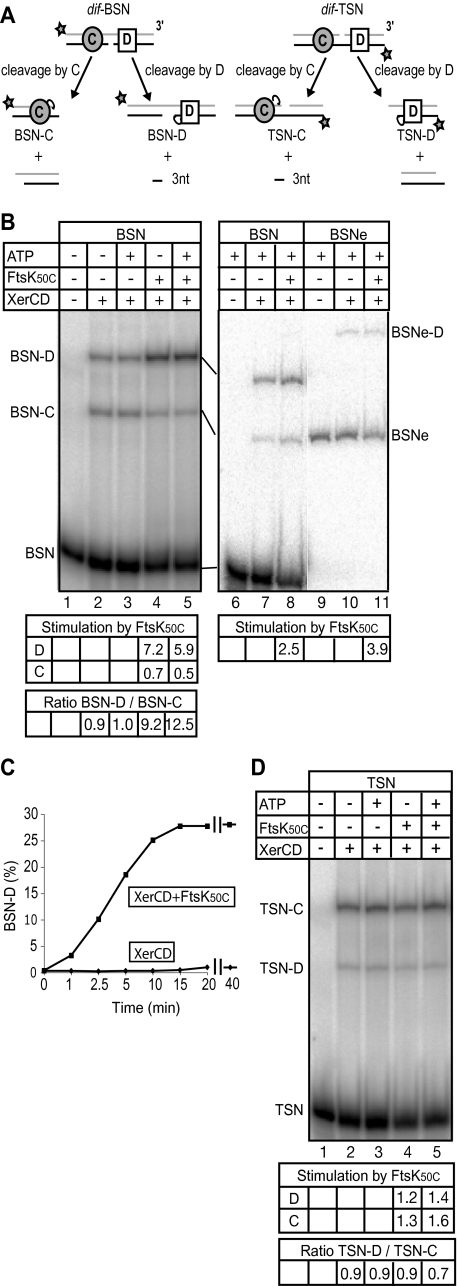
In order to dissect the molecular basis of this activation mechanism, we designed experiments to test whether FtsK50C, or parts of it, could activate the initial chemical step of the recombination reaction, cleavage of DNA by XerCD to form a recombinase–DNA covalent complex. Short DNA substrates containing the 28 bp dif site with a nick in either of the two strands at the centre of the dif site can be used to monitor recombinase-mediated DNA cleavage, as after cleavage a trinucleotide can diffuse away, or the DNA can dissociate into two fragments, thereby capturing the covalent complex between the recombinase and dif(Fig. 1A; Nunes-Düby et al., 1987; Blakely et al., 2000).
Fig. 1.
FtsK50C stimulates XerD-mediated cleavage of BSN.
- Schematic of XerCD-mediated cleavages of nicked suicide substrates, BSN and TSN. XerC (circle) binds to the left half-site of dif while XerD (square) binds to the right half-site. A 6 bp central region separates the two binding sites. The 28 bp dif site is flanked by 17 bp or 13 bp DNA segments. XerC and XerD cleave the top (grey) and bottom (black) strand of dif respectively. The position of the 5′ radiolabel is indicated by a star. The BSNe substrate carries a 205 bp extension adjacent to XerD binding site (not shown). Cleavage by either recombinase gives a diagnostic labelled DNA fragment with covalently attached recombinase.
- The 60 min 37°C reactions of the indicated reagents were analysed on a 0.1% SDS-6% PAGE. The levels of FtsK50C-mediated stimulation of DNA cleavage by XerD and XerC are shown, as are the ratios of cleavage by XerD as compared with XerC. The levels of FtsK50C-mediated stimulation of DNA cleavage by XerD varied between 2.5- and 32-fold after 60 min reactions in different experiments. In part, this results from day-to-day variations in FtsK50C-specific activity. Within experiments using a given set of protein dilutions, cleavage activities can be reliably compared.
- Time-course of XerD-mediated cleavage of BSN substrate in the presence and absence of FtsK50C. The percentage of substrate DNA converted to BSN-XerD with respect to time is plotted. The level of stimulation by FtsK50C as judged by initial rates is > 10-fold in this experiment.
- XerCD cleavage reactions with TSN substrate.
Whereas in vitro recombination between intact dif sites requires FtsK50C, ATP hydrolysis and a DNA extension on the XerD binding site of dif (Aussel et al., 2002; Massey et al., 2004), XerD-mediated cleavage of BSN, a 54 bp dif substrate with a nick in the bottom strand, was stimulated by FtsK50C, in a reaction that was independent of ATP (Fig. 1B and C). Consistent with this, Walker A and Walker B mutants of FtsK50C, which should be unable to bind ATP, or hydrolyse ATP, respectively, were also able to stimulate cleavage by XerD (data not shown). Addition of a 200 bp extension to the XerD binding side of BSN generated BSNe, whose cleavage by XerD was stimulated by FtsK50C to a comparable extent as BSN. We conclude that activation of BSN cleavage by XerD does not require the loading of FtsK50C onto duplex DNA, as the 13 bp flank adjacent to the XerD binding site of BSN is insufficient to facilitate FtsK50C loading onto DNA (Massey et al., 2004).
There was no stimulation of XerC-mediated cleavage by FtsK50C on these substrates (Fig. 1B), consistent with our demonstration that FtsK50C activates XerD in a complete XerCD dif intermolecular recombination reaction (Aussel et al., 2002). Furthermore, FtsK50C did not stimulate XerD-mediated cleavage of TSN, a substrate with the nick in the top stand of the dif central region that undergoes cleavage by XerC preferentially (Fig. 1D).
These results suggest that a configuration in which XerD is potentially active within synaptic complexes can more readily be adopted with BSN than with duplex dif and that the formation of such synaptic complexes with BSN is stimulated by FtsK50C without the need for ATP hydrolysis.
A 62-amino-acid fragment of FtsKC is sufficient to stimulate cleavage of BSN by XerD
In vivo experiments designed to map which region of FtsKC is responsible for a productive interaction with XerCD–dif identified a 142-amino-acid region of FtsKC that contains sequences responsible for species specificity in this interaction. This region of Escherichia coli (Ec) FtsKC is responsible for interacting specifically with the Ec Xer recombination machinery while the equivalent domain derived from Haemophilus influenzae (Hi) interacts specifically with the Hi Xer recombination machinery (Yates et al., 2003). The 142-amino-acid region contains an 81-amino-acid C-terminal segment that is relatively non-conserved in sequence between E. coli and H. influenzae (residues 1249–1329). We define this as the γ subdomain of FtsKC(Fig. 2A). The C-terminal 126 residues of Ec FtsKC, containing γ, were fused to maltose-binding protein (MBPγ; FtsK residues 1203–1329), as were truncated N- and C-terminal γ derivatives (MBPγ1, residues 1203–1267; and MBPγ2, residues 1268–1329).
Fig. 2.
A 62-amino-acid region of the FtsKCγ subdomain can stimulate DNA cleavage by XerD.
- A schematic of FtsK, FtsK50C and the maltose-binding protein (MBP) fusion derivatives.
- The 60 min 37°C reactions of BSN with the indicated reagents were analysed by SDS-PAGE as in Fig. 1.
Purified MBPγ and MBPγ2 each stimulated DNA cleavage of BSN by XerD, whereas MBPγ1 showed no activity (Fig. 2B). Activation was independent of ATP hydrolysis, as expected for proteins lacking the ATP-dependent motor β domain of FtsKC. We conclude that determinants for interaction with XerCD reside in the C-terminal 62 amino acids of Ec FtsKC, and that these are sufficient to stimulate BSN cleavage by XerD.
The FtsKCγ subdomain promotes intermolecular XerCD-dependent strand exchange between BSN and intact duplex dif
As a consequence of the ability of γ to stimulate cleavage of BSN by XerD, we reasoned that FtsK50C and γ might be able to stimulate strand exchange between BSN and an intact dif duplex, without the need for ATP. In order to test this, recombinase-mediated cleavage and strand exchange in reactions containing radiolabelled BSN, and an unlabelled intact DNA fragment containing dif flanked by 197 bp and 200 bp DNA segments (197_dif_200) were assayed. Incubation of the two DNA substrates with XerCD and FtsK50C in the absence of ATP generated, in addition to the expected recombinase–BSN covalent complexes, a spectrum of novel radiolabelled DNA species, most of which were dependent on FtsK50C(Fig. 3A and B). The same recombinant profile was obtained with FtsK50C and MBPγ (Fig. 3A), confirming that this intermolecular recombination is independent of the translocation activity of FtsK50C. A comparison of product profiles before and after proteinase K treatment showed which products had recombinase covalently bound, while experiments utilizing maltose-binding fusion derivatives of the recombinases determined which covalent complexes with DNA had XerC or XerD bound (data not shown).
Fig. 3.
The FtsKCγ subdomain can stimulate intermolecular recombination between BSN and intact _dif_-DNA substrates.
- The 60 min 37°C reactions containing radiolabelled BSN and 197_dif_200, an unlabelled intact linear DNA duplex, and the indicated reagents were analysed by 0.1% SDS-4% PAGE. BSN-C, covalent complex of BSN fragment and XerC; BSN-D, covalent product of BSN and XerD; LP, nicked or gapped recombinant product of BSN and 197_dif_200; LP-D, XerD covalent complex of gapped LP; HJ, nicked or gapped HJ; HJ-C, covalent complex of XerC with a HJ that has lost a DNA arm; HJ-D, covalent complex of XerD and a gapped HJ (see C).
- Time-course of reaction of radiolabelled BSN, 197_dif_200 DNA, and XerCD in the absence (left) and presence of FtsK50C (right). DNA species as labelled in (A).
- The proposed sequence of steps leading to generation of the observed radiolabelled products initiated by XerD. Completion of a pair of strand exchanges by XerD requires that the 3 nt fragment (represented by three dots) released by BSN cleavage by XerD does not diffuse out of the synaptic complex so that its 3′ end can be used to attack the phosphotyrosyl bond on 197_dif_200. A gapped HJ can arise by hydrolysis of the phosphotyrosine in HJ-D. The nick in BSN and subsequent products is shown by a small arrow.
A Holliday junction with XerD covalently bound (HJ-D) formed as quickly as BSN-D when FtsK50C was present (Fig. 3B, right). Therefore, intermolecular complexes can form and react efficiently under the reaction conditions used. Assuming that the FtsK50C-dependent products arise from reactions initiated by XerD, HJ-D, which has a 3 nt gap, must have arisen from two XerD-mediated cleavages, one of which is accompanied by strand exchange (Fig. 3C). Nicked HJs, which are present in low amount only at later times, could result from two completed strand exchanges by XerD; this is expected to occur inefficiently as it would require that the 3 nt oligonucleotide be retained in the recombining complex. Both HJ-D and HJ can be processed by a pair of XerC-mediated strand exchanges, generating linear recombinant products, LP-D and LP respectively. We imagine that it is easier for XerC strand exchanges to occur on HJ than on HJ-D. Formally, LP-D could also arise from XerD-mediated cleavage of linear nicked recombinant product (LP), although this is unlikely because LP-D accumulates before LP. Gapped LP or HJ could also arise from LP-D or HJ-D by phosphotyrosine hydrolysis.
Although the data on the relative amounts of reactions products and the kinetics of their production and turnover support the hypothesis that all of the observed FtsKC-dependent products arise from reactions that were initiated by XerD, we cannot yet formally prove that this is the case. Nevertheless, we note that if strand exchange by XerC precedes XerD in reactions with synapsed duplexes, it would generate labelled intermediates [nicked HJ (HJ) and XerC covalently linked to a three-armed junction (HJ-C)]. Consistent with this, HJ and HJ-C are the two FtsKC-independent products resulting from strand exchange between BSN and 197_dif_200 (Fig. 3B, left). We expect these reactions to be initiated by XerC. Although these initial products might be acted on by XerD to produce BSN-D, HJ-D, LP-D and LP in the presence of FtsK50C, we believe this unlikely because the reaction kinetics of intermolecular product formation show that the products of XerD-mediated cleavage and strand exchange appear before any that could be produced by XerC (Fig. 3B, right). Furthermore, the kinetics of HJ-C and HJ appearance and turnover are not readily compatible with them being processed by XerD. Indeed, dif HJ intermediates made by XerC do not show significant resolution by XerD under any conditions tested and we have never been able to show stimulation of XerD activity by FtsKC on HJ intermediates (Arciszewska et al., 2000; Massey et al., 2004). In contrast, intact or nicked dif HJs are excellent substrates for resolution by XerC (not shown).
These results on FtsK50C-dependent intermolecular recombination support the view that the stimulation of XerD-mediated cleavage of BSN by FtsK50C represents a reaction that is functionally relevant. Also consistent with this conclusion is the observation that FtsK50C-dependent stimulation of BSN cleavage requires the presence of both XerC and XerD (data not shown).
FtsK50C-mediated stimulation of BSN cleavage by XerD occurs in synaptic complexes
The results above, which show that intermolecular complexes containing two duplexes form and react quickly, are consistent with the idea that the FtsK50C-dependent stimulation of BSN cleavage by XerD requires synaptic complex formation. In order to test this hypothesis, we first determined whether FtsK50C-independent cleavage of BSN by XerC and XerD is synapsis-dependent, by assaying cleavage reaction efficiency on TSN and BSN as a function of DNA concentration. In all of the experiments, a constant level of radiolabelled BSN or TSN was mixed with unlabelled BSN or TSN in varying amounts before reaction with the indicated proteins. The 3 min, 15 min and 60 min reactions were analysed (Fig. 4 and not shown). If cleavage of labelled BSN or TSN is synapsis-independent, its efficiency (measured by initial rate) will not vary as a function of concentration of unlabelled dif substrate (at saturating protein concentrations). In contrast, if stimulation is synapsis-dependent, the efficiency will increase as a function of DNA concentration. The results were unambiguous with substrate TSN; cleavage by XerC is synapsis-dependent, the level of cleavage increasing as DNA concentration increases from 5 to 45 nM. In contrast, cleavage by XerD on this substrate does not increase as intermolecular reaction between two duplexes increases and therefore is synapsis-independent. The complementary result was obtained with BSN; cleavage by XerD is synapsis-dependent, while cleavage by XerC is synapsis-independent (not shown). Consistent with the synapsis-independent cleavage of BSN by XerC, we observed that high concentrations of XerC alone can cleave BSN; we would not expect that XerC alone can mediate synaptic complex formation. We note that in Cre–loxP recombination, the _K_D for synapsis is ∼10 nM; our results are consistent with the K_D for XerCD–_dif synapsis being of a similar order. If this is the case, at the high DNA and protein concentrations we use, much of the radiolabelled DNA will be in synaptic complexes (Ghosh et al., 2005).
Fig. 4.
Synapsis-dependent and -independent reactions. Varying concentration of TSN (top) or BSN (bottom), radiolabelled with an invariant concentration of radiolabelled DNA. Reactions were analysed by SDS-PAGE after 3 min, 15 min and 60 min reactions, with essentially the same overall results, although some of the 3 min reactions had very low levels of cleavage for one or the other recombinases. The 15 min (TSN) and 60 min (BSN) reactions are shown. The concentration dependence of the FtsKC-stimulated cleavage of BSN by XerD was essentially identical to BSN-D cleavage alone (the difference between the two BSN-D curves). FtsK50C had no effect on the TSN reactions or on cleavage of BSN by XerC (not shown).
Next, we tested whether the FtsK50C-stimulated cleavage of BSN by XerD is synapsis-dependent. Again the result was unequivocal; the stimulated cleavage like all catalysis by XerD on BSN is synapsis-dependent, with the concentration dependence being identical to that of cleavage of BSN by XerD in the absence of FtsK50C. This result provides a possible explanation of why FtsK50C fails to stimulate cleavage by XerD on TSN. The stimulation by FtsK50C requires formation of synaptic complexes that are in the XerD-active configuration; TSN cleavage by XerD is synapsis-independent. Also consistent with the synapsis-dependent cleavage of BSN by XerD is the observation that XerD alone can cleave BSN (not shown). This cleavage is not stimulated by FtsK50C, thereby reinforcing the view that FtsK50C-stimulated cleavage by XerD requires synaptic complex formation, as XerD alone is unlikely to mediate synapsis between two BSN fragments. Finally, FtsK50C failed to stimulate cleavage of BSN by XerD in the presence of XerC[Y275F], which is mutated for the catalytic tyrosine (not shown); the equivalent mutants of Cre are synapsis-defective (G.D. Van Duyne, pers. comm.).
The demonstration that cleavage of BSN by XerD, in the presence of XerC, is synapsis-dependent reinforces the view that all FtsKC-mediated effects on XerCD recombination are mediated by promoting the formation of heterotetrameric synaptic complexes that have a conformation appropriate for catalysis by XerD (later). Furthermore, our observation with TSN and BSN that catalysis by one recombinase is synapsis-dependent and the other independent (with a switch in which recombinase requires synapsis as one goes from TSN to BSN) is reminiscent of the demonstration that in Cre–loxP recombination, ‘bottom strand’ cleavage in vitro requires synapsis, while ‘top strand’ cleavage is synapsis-independent, although in these latter experiments duplexes with a bridging phosphorothioate were used rather than nicked duplexes (Ghosh et al., 2005). Finally, our results show the impact that a strand-specific nick has on which productive synaptic complex can form and react. Similarly, the _loxP_–Cre experiments demonstrate how specific synaptic complex formation directs a preferred order of strand exchange.
The γ subdomain of FtsKC interacts with XerD
In order to determine which components of the XerCD–dif recombination machinery are interacting with FtsK50C, His-tagged derivatives of each of the recombinases were immobilized on cobalt-agarose affinity resin and tested for their ability to interact with FtsK50C and its derivatives. Initial experiments showed that His-tagged variants of either XerC or XerD retained their non-His-tagged recombinase partner on the affinity resin, with both recombinases being co-eluted by 300 mM imidazole, thereby demonstrating that a XerC–XerD interaction can be assayed in the absence of DNA (Fig. 5A).
Fig. 5.
The FtsKCγ subdomain interacts directly with XerD.
- XerCD interact in the absence of _dif_-DNA. Analysis by SDS-PAGE of recombinase interactions. 1. The indicated His-tagged recombinase is applied to cobalt agarose resin. L, protein loaded; F, flow through fraction; W, wash fraction. 2. Then the second indicated recombinase is applied to the washed column indicated in 1. E, proteins eluted by 300 mM imidazole; other abbreviations as in 1. Untagged XerCD did not bind to the columns under these binding conditions (controls).
- FtsK50C interacts with XerD. The sequential loading of His-tagged recombinase or FtsK50C to cobalt agarose resin, column washing, and then application of a second non-His-tagged protein, followed by 300 mM imidazole elution was as in (A). I–IV show the indicated protein combinations.
- MBPγ2 interacts with XerD. Sequential loading, washing and elution of the indicated proteins were as in (A). The elutions only are shown.
- MBPγ specifically competes with FtsK50C. Sequential loadings and elutions as indicated.
Immobilized HisXerD also specifically retained FtsK50C, with both proteins co-eluting with imidazole (Fig. 5B, II). In contrast, pre-bound HisXerC did not retain FtsK50C (Fig. 5B, I), although pre-bound HisXerC retained FtsK50C in the presence of XerD. In this case, all three proteins co-eluted with imidazole (not shown). Additionally, XerD, but not XerC, was retained efficiently on the cobalt agarose resin pre-loaded by HisFtsK50C (Fig. 5B, III, IV). The small amount of XerC that co-elutes with FtsK50C could reflect a weak interaction between FtsK50C and XerC. We conclude that FtsK50C interacts strongly with XerD, irrespective of the presence of XerC. These interactions are ATP-independent.
MBPγ and MBPγ2 behaved like FtsK50C. They were retained by immobilized HisXerC-XerD, and co-eluted with XerCD in the presence of imidazole (Fig. 5C). This retention was not dependent on XerC when HisXerD was immobilized (not shown). MBPγ1 was not retained by immobilized HisXerD, thereby confirming that the γ interaction with XerD is mediated by γ2. FtsK50C bound to immobilized HisXerC-XerD could also be eluted by MBPγ, confirming the specificity of the interaction (Fig. 5D). Confirmatory experiments using FLAG- or intein-tagged derivative of FtsK50C also showed specific binding of XerD, but not XerC (data not shown). We conclude that the 62-amino-acid γ2 segment of FtsKC contains the determinants for the ATP-independent interaction with XerD.
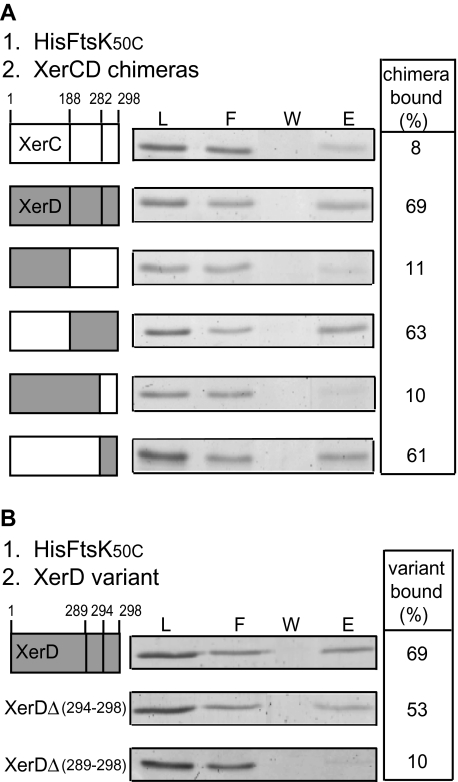
The extreme C-terminus of XerD interacts with the γ subdomain of FtsK_C_
XerC and XerD belong to the tyrosine recombinase family, which have relatively little amino acid sequence homology, despite being structurally highly related and sharing a common catalytic mechanism (Esposito and Scocca, 1997; Sherratt and Wigley, 1998). We have taken advantage of the 67% sequence divergence between XerC and XerD to construct chimeric recombinases that are part XerC and part XerD (Ferreira et al., 2003). These chimeras were exploited in order to identify which part of XerD interacts with FtsK50C by testing their ability to interact with immobilized HisFtsK50C (Fig. 6A). The results were unambiguous; the specificity of interaction with FtsK50C resided in the extreme C-terminal region of XerD (residues 282–298). Further analysis using XerD variants carrying deletions in the C-terminal region revealed that XerD deleted for its C-terminal five amino acids was proficient in interaction with FtsK50C (Fig. 6B). In contrast, a deletion of the 10 or 15 C-terminal residues gave a protein that no longer showed an interaction with FtsK50C, despite being able to bind to _dif_-DNA (Fig. 6B; data not shown; Spiers and Sherratt, 1997). We therefore conclude that the region of XerD carrying determinants necessary for interaction with FtsKC is located between amino acids 282 and 292. Whether these residues are sufficient for the interaction is not known.
Fig. 6.
The C-terminal region of XerD interacts with FtsK50C.
- The indicated XerCD chimeras were tested for their ability to interact with immobilized HisFtsK50C by using the sequential loading and elution protocol described in Fig. 5. Abbreviations as in Fig. 5A. Per cent protein bound was calculated from percentage E/L.
- The indicated XerD variants carrying deletions at the C-terminus were tested for their ability to bind to immobilized HisFtsK50C. Abbreviations as in Fig. 5A.
As Ec XerCD interacts in vivo with Ec FtsKC but not with Hi FtsKC (Yates et al., 2003), we expected to find differences between Ec and Hi XerD in this C-terminal region. Five such differences identify amino acids that are unique to XerD, yet are absent in XerC (Fig. 7A). Intriguingly, this region of γ-proteobacterial XerD is relatively highly diverged as compared with the comparable region in XerC. We constructed Ec XerD variants carrying amino acids present in Hi XerD at four of the five positions (Fig. 7A). We assessed double and quadruple substitution XerD variants for their ability to interact with immobilized HisFtsK50C and for their ability to be stimulated by FtsK50C in a _dif_-BSN cleavage assay. The results of both assays show that determinants for XerD interaction with FtsKC are located within these four residues (Fig. 7B). Variants XerD[KR] and XerD[ER], each carrying two substitutions, showed only slightly reduced activity, while the variant having the quadruple substitution, XerD[KR..ER], had an activity similar to Hi XerD, consistent with the species specificity residing in this region.
Fig. 7.
XerD variants carrying substitutions at R288, Q289, Q292 and Q293 lack the ability to interact with FtsK50C.
- Amino acid sequence alignment of C-terminal regions of Ec XerD, Hi XerD and Ec XerC. The amino acid residue 282–293 region of Ec XerD (horizontal line) has been shown by deletion analysis (Fig. 6) to be necessary for the interaction with FtsK50C. Differences in amino acid sequence between Ec XerD and Hi XerD within this region are indicated (stars), as are the four residues targeted for mutagenesis (bold). The active site tyrosine (residue 279) is also indicated (ovoid).
- Interactions between the XerD variants and FtsK50C. The indicated XerD variants were assayed for their ability to bind to immobilized HisFtsK50C (open bars) and to stimulate cleavage of BSN in the presence of FtsK50C (grey bars). The activity of the variants is expressed as a percentage of the activity of wild-type Ec XerD.
- In vivo analysis of XerCD recombination at plasmid-borne dif in the presence of XerD or its variants. The SpR reporter plasmids carried either _dif_-KmR-dif (d) or _psi_-KmR-psi (p) cassettes in which two dif or two psi sites were directly oriented. Plasmid DNA preparations obtained from stationary-phase cultures of strains carrying reporter plasmids and vectors expressing XerD or XerD variants were analysed by agarose electrophoresis. The efficiencies of dif and psi plasmid resolution in the presence of XerD and XerD[KR..ER] were quantified by scoring for the loss of KmR marker upon transformation of the DNA samples shown into a Xer– strain. Five hundred transformants from each DNA sample were analysed.
- Efficiency of chromosome dimer resolution by FtsK-dependent recombination at dif, as judged by growth competition (Bigot et al., 2004). Competition was assessed at 10 generation intervals between the strains (KmR or TpR) expressing the indicated XerD variant and wild-type XerD from appropriate plasmids in a chromosomal XerD– background. A complementary competition experiment in which the chromosomal antibiotic resistance markers were exchanged led to the same results (not shown).
We reasoned that if these four amino acids are specifically involved in the interactions with FtsKC, their substitution should not affect the ability of Ec XerD to mediate recombination of DNA substrates that do not require activation by Ec FtsKC, for example, the plasmid recombination site, psi (Colloms et al., 1996). We therefore compared the proficiency of XerD and the XerD variants in supporting in vivo recombination between directly repeated plasmid-borne dif or psi recombination sites flanking a KmR cassette (Fig. 7C). All of the XerD variants completely resolved the psi reporter plasmid. In contrast, XerD[KR..ER], containing the quadruple substitution, showed no detectable dif resolution (note that resolution at dif is generally less efficient than at psi with wild-type XerCD). Transformation of the plasmid DNA into an Xer– strain allowed a quantitative measure of resolution at dif by scoring the ratio of KmS SpR/KmR SpR transformants. Whereas 14% of dif reporter plasmids had resolved in a wild-type XerD+ strain, 2% had resolved with the strain carrying the XerD quadruple substitution (Fig. 7C).
Escherichia coli strains lacking chromosomal dif, functional xerCD or ftsK C genes, are defective in chromosome segregation, largely as a consequence of failing to resolve chromosome dimers (Recchia et al., 1999; Barre and Sherratt, 2005). We therefore reasoned that the presence of the Ec XerD[KR..ER] variant, which is unable to interact with Ec FtsKC, should lead to a defect in dimer resolution and consequent chromosome segregation, despite its ability to recombine plasmid psi sites. To test this we used a co-culture competition assay, which provides a sensitive measure of the efficiency of chromosome dimer resolution (Fig. 7D; Pérals et al., 2000; Bigot et al., 2004). Two differentially marked strains (TpR or KmR), lacking a functional copy of their chromosomal xerD gene, carried related plasmids that expressed either wild-type XerD or an XerD variant. After mixing the cultures in a 1:1 ratio they were grown to stationary phase, diluted 1000-fold, grown back to stationary phase and so on until 40 generations had been reached in medium that selects for maintenance of the plasmid. The relative proportions of the two strains were assessed every 10 generations by comparing viable counts on Ap agar with those on Km Ap. The XerD+ strain out-competed the XerD– strain as expected, while the XerD quadruple substitution was almost as poor in competition as the XerD– strain. These results correlate well with the plasmid resolution data and reinforce our conclusion that the XerD[RQ.QQ] region carries major determinants for interaction with FtsKC, rather than for core recombinase activity.
Mapping regions of γ subdomain that interact with XerD
Species specificity in FtsKC–XerCD interactions in vivo (Yates et al., 2003) was also demonstrated in vitro in BSN cleavage reactions (Fig. 8A). E. coli XerD-mediated cleavage of BSN is efficiently stimulated in reactions containing Ec XerCD and Ec MBPγ, but not in reactions containing Hi MBPγ. Similarly, Hi MBPγ, but not Ec MBPγ, could stimulate cleavage of BSN by Hi XerCD.
Fig. 8.
Mapping the FtsK γ region that interacts with XerD.
- Species specificity of the FtsKC–XerCD interaction between E. coli and H. influenzae in vitro. The indicated 60 min reactions were analysed by 0.1% SDS-6% PAGE.
- Amino acid sequence comparison between the γ2 regions of E. coli and H. influenzae. The elements of the Ec FtsKγ secondary structure derived from structure predictions are shown above the sequence; α-helices as horizontal grey lines, β-strand as a black line. The region of most notable sequence divergence is shown in bold font.
- FtsKγ variants were tested for binding to immobilized Ec HisXerD (open bars) and for their ability to stimulate _dif_-BSN DNA cleavage mediated by XerD (grey bars). The results of both assays are expressed as a percentage of the Ec MBPγ activity (100%).
- Paradigm of Cre–loxP recombination as applied to the activation of XerD by FtsKC. In a tetrameric recombination complex, only two of the four recombinase molecules are in an active state at any given time (either XerC or XerD in the case of XerCD). In the absence of FtsKC, XerCD–dif forms an ‘XerC-active’ synaptic complex preferentially (Hallet et al., 1999). In this conformation, XerC is active as a consequence of its C-terminal donor region interacting within the acceptor region of an XerD molecule bound to the same duplex; this positions the catalytic tyrosine of the active XerC molecule adjacent to the scissile phosphate (view from the C-terminal side of the complex). The switch from the ‘XerC-active’ to the ‘XerD-active’ configuration in a heterotetramer requires either dissolution of all recombinase–recombinase interactions and reformation of new interactions that lead to an altered DNA conformation, or dissociation of each DNA duplex from one of its recombinase binding sites and reformation of a complex with altered DNA paths. The action of FtsKC could be through pathway a, in which it directs the formation of the XerD-active state on duplex, and/or through pathway b, where the heterotetramer is the substrate for FtsKC action.
In order to gain some insight into where the possible determinants of interaction with XerD are located in γ2, we made comparison of the γ2 regions from Ec and Hi FtsKC (Fig. 8B). This comparison revealed a short region of amino acid sequence divergence, TEKRKA, in Ec FtsK, predicted to lie mainly in a loop between two sequence-conserved α-helices. A more detailed comparison of this region among γ-proteobacteria supported the view that this region might interact with XerD (not shown).
Therefore, Ec MBPγ variants in which whole or parts of the TEKRKA sequence were substituted by amino acids from the Hi region were constructed. These variants were assayed for their ability to bind immobilized Ec HisXerD and for their ability to stimulate cleavage of BSN by Ec XerD (Fig. 8C). The results show that replacement of TEKRKA of Ec MBPγ by the Hi sequence, INTGTT, resulted in almost complete loss of interaction with Ec XerD, with the activity of this variant being similar to that of Hi MBPγ. Variants MBPγ[T1277I] and MBPγ[E1278N] had similar activities as Ec MBPγ (not shown), while variants MBPγ[K1279T] and MBPγ[R1280G] showed slightly reduced activity. Ec MBPγ[K1281T] had moderately reduced activity, while Ec MBPγ[A1282T] activity was dramatically reduced, being similar to Hi MBPγ. We conclude that the ‘KRKA’ C-terminal region of XerD is involved in the FtsKC–XerD interaction and that the strength of this interaction correlates well with the ability to stimulate cleavage of BSN by XerD.
New insight into FtsKC-dependent chromosome dimer resolution
In the work reported here, we have initiated dissection of a highly conserved interaction between a recombination machine and a DNA translocase, a reaction that plays a central role in the late stages of bacterial chromosome segregation. In particular, we have characterized the specific interaction that leads to activation of the XerD recombinase. The recognition between a small region of the FtsKCγ subdomain and a region close to the C-terminus of XerD is necessary for the stimulation of BSN cleavage by XerD, and for the reaction of such a complex with intact linear dif duplex in the absence of ATP binding or hydrolysis. This interaction is functionally relevant because its impairment leads to the in vivo loss of XerCD recombination at dif and the concomitant loss of chromosomal dimer resolution. As yet we do not know whether this interaction alone is sufficient for FtsKC to activate XerD, although all of the determinants for the interaction reside in the 62-amino-acid γ subdomain.
The structures of a whole range of intermediates in Cre–loxP recombination (Gopaul and Van Duyne, 1999) provide a paradigm that can be applied to FtsKC-dependent XerCD recombination at dif (Fig. 8D). In this paradigm, the heterotetrameric synaptic complex can adopt one of two configurations, ‘XerC-active’ (bottom), or ‘XerD-active’ (right). The active recombinase has its C-terminal ‘donor’ region engaged into a specific ‘acceptor’ region of its partner recombinase bound to the same duplex. This positions the catalytic tyrosine of the active recombinase close to the scissile DNA phosphodiester. Donor–acceptor interactions between recombinases bound to the different duplexes participate in synapsis. In the absence of FtsKC, the XerCD–dif heterotetrameric complex preferentially adopts the ‘XerC-active’ conformation when the duplex DNA is intact. In order for the ‘XerD-active’ configuration to be adopted, FtsKC must convert a ‘XerC-active’ heterotetramer to the ‘XerD-active’ heterotetramer through a remodelling reaction (pathway b), and/or stimulate the formation of the ‘XerD-active’ state on duplex (pathway a), two such duplexes forming a ‘XerD-active’ heterotetramer. The latter pathway could occur before synapsis or after a ‘XerC-active’ synaptic complex has been dissociated by FtsKC action. In either scenario, we propose that the specific interaction of FtsKC with XerD identified here promotes formation of the ‘XerD-active’ state. Formally, this could be achieved by compromising the interaction that leads to XerC being active in the absence of FtsKC and/or facilitating the interaction that makes XerD active. Our data are most easily accommodated in a model in which the interaction of the C-terminus of XerD with an acceptor region in XerC is facilitated, as the C-terminal region of XerD, identified as being necessary for the interaction with FtsKC, is more likely to comprise part of the putative XerD donor region than the XerD acceptor region (Hallet et al., 1999). Because of the expected intimate association of XerD with XerC in a synaptic complex, it is not impossible that FtsKC might additionally interact, although weakly, with XerC during the formation of the ‘XerD-active’ state. Examination of the Cre–loxP structures suggests that a recombinase C-terminal donor region docked with the acceptor region of a partner recombinase would have the potential to interact with a surface-located peptide ligand present in the γ subdomain.
The ability of FtsKC derivatives to stimulate the formation of an ‘XerD-active’ synaptic complex on BSN is facilitated by the bottom strand nick present in BSN, which allows formation of some ‘XerD-active’ heterotetrameric state in the absence of FtsKC. Presumably DNA flexibility arising from the nick allows the ‘XerD-active’ conformation to form, thereby relieving the requirement for ATP-dependent remodelling by FtsKC. We do not know whether FtsKC, in addition, stimulates catalysis by XerD once the ‘XerD-active’ synaptic complex has formed; we have no evidence to support the existence of such a second step.
The conversion of an ‘XerC-active’ heterotetramic complex, which forms readily in the absence of FtsKC, to an ‘XerD-active’ complex requires either the dissolution of all initial recombinase–recombinase interactions and the creation of new ones, or a switch in the path of DNA in the synaptic complex that would require recombinase–DNA interactions be broken and re-made (Fig. 8D). Whether this would require a complete dissociation of the heterotetrameric complex into two separated duplexes is unclear. We imagine that when the FtsKCγ subdomain fragment interacts with XerD bound to BSN, a single γ determinant interacts with a single XerD molecule, although we do not yet know whether both XerD molecules in a heterotetrameric BSN complex need to undergo such interactions for stimulation of cleavage by XerD. With wild-type multimeric FtsK, multiple γ subdomains are potentially available for binding to the recombinases.
Future experiments need to address precisely how interaction of FtsKC with XerCD leads to recombination activation in a two-duplex heterotetrameric complex, how DNA sequence-directed FtsKC translocation facilitates the simple synapsis of distant dif sites, and how FtsKC switches from being a DNA translocase to a nucleoprotein remodelling machine once it encounters XerCD bound to _dif_-DNA.
Experimental procedures
Bacterial strains
In vivo assays of XerCD and FtsKC function were carried out in Ec AB1157 derivatives (Bachmann, 1972). DS9008 and DS9028 are _xerD_– derivatives marked by KmR and TpR respectively (Blakely et al., 1993; D.J. Sherratt, unpublished).
Recombinant proteins
Recombinant proteins were produced using standard methods. MBP fusions to FtsK50Cγ fragments were constructed by PCR amplifying the desired region of ftsK and cloning into the pMAL-c2x vector (NEB) using EcoRI and HindIII restriction sites. Amino acid substitutions in Ec MBPγ and Ec XerD were obtained by site-directed mutagenesis using mutagenic primers. Ec XerCD, XerCD chimeras, FlFtsK50C and Hi XerCD were purified as described previously (Subramanya et al., 1997; Ferreira et al., 2003; Aussel et al., 2002; Yates et al., 2003). His-tagged Ec XerC and XerD, XerD deletions and XerD point mutants were affinity purified on nickel resin followed by chromatography on a heparin column. His-tagged FtsK50C was purified on nickel resin followed by heparin and DEAE columns. MBP-FtsKγ variants were purified on amylose resin and concentrated in spin concentrators.
Intermolecular recombination and suicide substrate assays
A 425 bp, unlabelled, _dif_-containing substrate was produced by PCR on pMIN33 (Blakely et al., 1993). Short linear or nicked, _dif_-containing, DNA fragments were produced by annealing appropriate oligonucleotides, followed by purification by polyacrylamide gel electrophoresis (PAGE). The longest strand was 32P-labelled at the 5′ end before annealing. BSNe was constructed by ligating a 200 bp fragment to BSN; its radiolabel was at the same position as that in BSN.
Recombination reactions were carried out in 10 µl of reaction buffer (10 mM Tris-HCl pH 7.5, 1 mM DTT, 15 mM MgCl2) and contained 0.5 mg ml−1 BSA, 60 mM NaCl and 8% glycerol, 2.5 mM ATP, 2 nM labelled DNA, 2 nM unlabelled DNA, 500 nM XerC, 250 nM XerD, 500 nM FtsK50C or 1–5 µM MBP-FtsKγ derivatives. Cleavage of suicide substrates used the same conditions other than that unlabelled DNA was absent, except in the experiment shown in Fig. 4. FtsK50C or MBP-FtsKγ derivatives were added last to start the reactions. Reactions were incubated at 37°C for up to 60 min. Products were analysed by 0.1% SDS, 4% or 6% PAGE in Tris-borate buffer (TBE). Gels were scanned and quantified using a Fuji FLA 3000 fluorimager and ImageQuant software.
In experiments designed to test whether synapsis of two duplexes is required for the action of FtsKC a constant amount of radiolabelled TSN or BSN DNA (5 nM) was mixed with increasing concentrations of the same unlabelled DNA fragment (overall concentration range 5–45 nM). Poly(dI-dC)-poly(dI-dC) was added to a final concentration of 125 µg ml−1 and sufficient XerCD and FtsK50C were added to ensure saturation at the highest DNA concentration.
Physical interaction assay
His-tagged proteins were loaded and immobilized on cobalt chelated affinity agarose (Pierce) in 25 mM Tris-HCl pH 7.2, 150 mM NaCl and 40 mM imidazole. After series of washes to remove any unbound His-tagged protein, the untagged protein of interest was loaded. Proteins that were not retained due to physical interaction with His-tagged protein were removed in the subsequent washing steps. Interaction was assessed on 0.1% SDS-10% PAGE gels following a selective co-elution of the interacting proteins by 300 mM or 400 mM imidazole. The amounts of proteins in samples were determined by staining the gel with SYPR Orange, followed by scanning in a Fuji FLA 3000 fluorimager and subsequent quantification.
In vivo plasmid resolution assay
An E. coli strain deleted for xerD (DS9028) was co-transformed with plasmid carrying wild-type xerD gene or its variant, expressed from the p_lac_ promoter (pRM132, ApR; Blakely et al., 1993), and with a low copy number reporter plasmid, containing either a _dif_-KmR-dif cassette (pFX142, SpR; Aussel et al., 2002) or a _psi_-KmR-psi cassette (pIZ18, SpR). Transformants were plated on LB agar supplemented with Km, Ap and glucose (1%). A population of approximately 20 colonies was inoculated into LB Sp, Ap liquid culture and grown to A600 of 0.4, induced with IPTG (100 µM) and grown overnight. Plasmid DNA was extracted and separated on 1% agarose-TAE gels, stained with SybrGreen and scanned on a Fuji FLA3000 fluorimager. Resolution was quantified by transformation of DNA samples into a Xer– strain and determination of the fraction of SpR transformants that are KmR.
Co-culture competition assay
This assay is used to assess the efficiency of FtsKC-dependent chromosome dimer resolution (Pérals et al., 2000; Bigot et al., 2004). A pair of strains, each lacking a functional xerD gene (DS9008, KmR and DS9028 TpR) were transformed with a pUC-derived plasmid carrying either a wild-type xerD gene or xerD variant under p_lac_ promoter control. Cells were grown in LB Ap until A600 = 0.4, induced with IPTG (100 µM), mixed 1:1, diluted and grown in serial cultures for 40 generations. The relative ratios of colony-forming units of both strains in cultures were assessed at 10 generations time points by plating on LB Ap Km agar and LB Ap agar.
Acknowledgments
We thank Greg Van Duyne for stimulating discussions and Katie Christoffers for technical assistance. This work was supported by the Wellcome Trust. J.Y. was supported by a MRC studentship for postgraduate training and I.Z. by a Helmore postgraduate award of Oxford University.
References
- Arciszewska LK, Baker RA, Hallet B, Sherratt DJ. Coordinated control of XerC and XerD catalytic activities during Holliday junction resolution. J Mol Biol. 2000;299:391–403. doi: 10.1006/jmbi.2000.3762. [DOI] [PubMed] [Google Scholar]
- Aussel L, Barre FX, Aroyo M, Stasiak A, Stasiak AZ, Sherratt DJ. FtsK is a DNA motor protein that activates chromosome dimer resolution by switching the catalytic state of the XerC and XerD recombinases. Cell. 2002;108:195–205. doi: 10.1016/s0092-8674(02)00624-4. [DOI] [PubMed] [Google Scholar]
- Bachmann BJ. Pedigrees of some mutant strains of Escherichia coli K12. Bacteriol Rev. 1972;36:525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barre FX, Sherratt DJ. Chromosome dimer resolution. In: Higgins NP, editor. The Bacterial Chromosome. Washington DC: American Society for Microbiology Press; 2005. pp. 513–524. [Google Scholar]
- Bigot S, Corre J, Louarn JM, Cornet F, Barre FX. FtsK activities in Xer recombination, DNA mobilization and cell division involve overlapping and separate domains of the protein. Mol Microbiol. 2004;54:876–886. doi: 10.1111/j.1365-2958.2004.04335.x. [DOI] [PubMed] [Google Scholar]
- Blakely G, May G, McCulloch R, Arciszewska LK, Burke M, Lovett ST, Sherratt DJ. Two related recombinases are required for site-specific recombination at dif and cer in E. coli K12. Cell. 1993;75:351–361. doi: 10.1016/0092-8674(93)80076-q. [DOI] [PubMed] [Google Scholar]
- Blakely GW, Davidson AO, Sherratt DJ. Sequential strand exchange by XerC and XerD during site-specific recombination at dif. J Biol Chem. 2000;275:9930–9936. doi: 10.1074/jbc.275.14.9930. [DOI] [PubMed] [Google Scholar]
- Colloms SD, McCulloch R, Grant K, Neilson L, Sherratt DJ. Xer-mediated site-specific recombination in vitro. EMBO J. 1996;15:1172–1181. [PMC free article] [PubMed] [Google Scholar]
- Draper GC, McLennan N, Begg K, Masters M, Donachie WD. Only the N-terminal domain of FtsK functions in cell division. J Bacteriol. 1998;180:4621–4627. doi: 10.1128/jb.180.17.4621-4627.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeli O, Marians KJ. Untangling intracellular DNA topology. Mol Microbiol. 2004;52:925–931. doi: 10.1111/j.1365-2958.2004.04047.x. [DOI] [PubMed] [Google Scholar]
- Espeli O, Lee C, Marians KJ. A physical and functional interaction between Escherichia coli FtsK and topoisomerase IV. J Biol Chem. 2003;278:44639–44644. doi: 10.1074/jbc.M308926200. [DOI] [PubMed] [Google Scholar]
- Esposito D, Scocca JJ. The integrase family of tyrosine recombinases: evolution of a conserved active site domain. Nucleic Acids Res. 1997;25:3605–3614. doi: 10.1093/nar/25.18.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira H, Butler-Cole B, Burgin A, Baker R, Sherratt DJ, Arciszewska LK. Functional analysis of the C-terminal domains of the site-specific recombinases XerC and XerD. J Mol Biol. 2003;330:15–27. doi: 10.1016/s0022-2836(03)00558-8. [DOI] [PubMed] [Google Scholar]
- Ghosh K, Lau CK, Gupta K, Van Duyne GD. Preferential synapsis of loxP sites drives ordered strand exchange in Cre–loxP site-specific recombination. Nature Chem Biol. 2005;1:275–282. doi: 10.1038/nchembio733. [DOI] [PubMed] [Google Scholar]
- Gopaul DN, Van Duyne GD. Structure and mechanism in site-specific recombination. Curr Opin Struct Biol. 1999;9:14–20. doi: 10.1016/s0959-440x(99)80003-7. [DOI] [PubMed] [Google Scholar]
- Hallet B, Arciszewska LK, Sherratt DJ. Reciprocal control of catalysis by the tyrosine recombinases XerC and XerD: an enzymatic switch in site-specific recombination. Mol Cell. 1999;4:949–959. doi: 10.1016/s1097-2765(00)80224-5. [DOI] [PubMed] [Google Scholar]
- Ip SCY, Bregu M, Barre FX, Sherratt DJ. Decatenation of DNA circles by FtsK-dependent Xer site-specific recombination. EMBO J. 2003;22:6399–6407. doi: 10.1093/emboj/cdg589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesterlin C, Barre FX, Cornet F. Genetic recombination and the cell cycle: what we have learned from chromosome dimers. Mol Microbiol. 2004;54:1151–1160. doi: 10.1111/j.1365-2958.2004.04356.x. [DOI] [PubMed] [Google Scholar]
- Massey TH, Aussel L, Barre FX, Sherratt DJ. Asymmetric activation of Xer-site-specific recombination by FtsK. EMBO Rep. 2004;5:399–404. doi: 10.1038/sj.embor.7400116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes-Düby SE, Matsumoto L, Landy A. Site-specific recombination intermediates trapped with suicide substrates. Cell. 1987;50:779–788. doi: 10.1016/0092-8674(87)90336-9. [DOI] [PubMed] [Google Scholar]
- Pease JP, Levy O, Cost GJ, Gore J, Ptacin JL, Sherratt DJ, et al. Sequence-directed DNA translocation by purified FtsK. Science. 2005;307:586–590. doi: 10.1126/science.1104885. [DOI] [PubMed] [Google Scholar]
- Pérals K, Cornet F, Merlet Y, Delon I, Louarn JM. Functional polarization of the Escherichia coli chromosome terminus: the dif site acts in chromosome dimer resolution only when located between long stretches of opposite polarity. Mol Microbiol. 2000;36:33–43. doi: 10.1046/j.1365-2958.2000.01847.x. [DOI] [PubMed] [Google Scholar]
- Recchia GD, Aroyo M, Wolf D, Blakely G, Sherratt DJ. FtsK-dependent and -independent pathways of Xer site-specific recombination. EMBO J. 1999;18:5724–5734. doi: 10.1093/emboj/18.20.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherratt DJ, Wigley DB. Conserved themes but novel activities in recombinases and topoisomerases. Cell. 1998;93:149–152. doi: 10.1016/s0092-8674(00)81566-4. [DOI] [PubMed] [Google Scholar]
- Spiers AJ, Sherratt DJ. Relating primary structure to function in the Escherichia coli XerD site-specific recombinase. Mol Microbiol. 1997;24:1071–1082. doi: 10.1046/j.1365-2958.1997.4171784.x. [DOI] [PubMed] [Google Scholar]
- Subramanya HS, Arciszewska LK, Baker RA, Bird LE, Sherratt DJ, Wigley DB. Crystal structure of the site-specific recombinase, XerD. EMBO J. 1997;16:5178–5187. doi: 10.1093/emboj/16.17.5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J, Aroyo M, Sherratt DJ, Barre FX. Species specificity in the activation of Xer recombination at dif by FtsK. Mol Microbiol. 2003;49:241–249. doi: 10.1046/j.1365-2958.2003.03574.x. [DOI] [PubMed] [Google Scholar]
- Yu XC, Weihe EK, Margolin W. Role of the C-terminus of FtsK in Escherichia coli chromosome segregation. J Bacteriol. 1998;180:6424–6428. doi: 10.1128/jb.180.23.6424-6428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]