Neural Correlates of Processing Valence and Arousal in Affective Words (original) (raw)
. Author manuscript; available in PMC: 2008 Mar 17.
Published in final edited form as: Cereb Cortex. 2006 May 12;17(3):742–748. doi: 10.1093/cercor/bhk024
Abstract
Psychological frameworks conceptualize emotion along 2 dimensions, “valence” and “arousal.” Arousal invokes a single axis of intensity increasing from neutral to maximally arousing. Valence can be described variously as a bipolar continuum, as independent positive and negative dimensions, or as hedonic value (distance from neutral). In this study, we used functional magnetic resonance imaging to characterize neural activity correlating with arousal and with distinct models of valence during presentation of affective word stimuli. Our results extend observations in the chemosensory domain suggesting a double dissociation in which subregions of orbitofrontal cortex process valence, whereas amygdala preferentially processes arousal. In addition, our data support the physiological validity of descriptions of valence along independent axes or as absolute distance from neutral but fail to support the validity of descriptions of valence along a bipolar continuum.
Keywords: amygdala, arousal, emotion, fMRI, orbitofrontal cortex, valence
Introduction
Emotional affect has been conceptualized along 2 dimensions: valence, which describes the extent of pleasure or sadness, and arousal, which describes the extent of calmness or excitation (Russell 1980; Lang and others 1993). These dimensions are commonly treated as independent factors, but real-world experience suggests that they are often correlated. More negative stimuli tend to have a higher intensity (few pleasant things are felt as intensely as truly unpleasant things), and higher intensity tends to amplify valence (strong blackcurrant juice is much more pleasant than weak blackcurrant juice). Interactions of this nature often make it difficult to dissociate these 2 dimensions.
One way to determine the extent of interdependence between intensity and arousal is to examine the neural responses associated with processing affective stimuli. Two recent neuroimaging studies (Anderson and others 2003; Small and others 2003) have attempted to do this by teasing apart the neural coding of chemosensory valence and intensity in the human brain. The first (Anderson and others 2003) showed that amygdala activation varies with the intensity but not the valence of presented odors, whereas activity in orbitofrontal cortex varies with the valence but not the intensity. The second (Small and others 2003) showed a similar dissociation between amygdala and orbitofrontal cortex during the processing of gustatory stimuli. These data suggest that distinct brain regions mediate the analysis of “quantitative” and “qualitative” aspects of chemosensory perception and also that the affective representations of intensity and valence may draw upon dissociable neural substrates. A strong correlation between the intensity ratings of words and the arousal dimension as proposed by Lang and others (1993) has been established (Bensafi and others 2002) using both subjective reports and measurement of autonomic responses, but the manner in which amygdala and orbitofrontal cortex respond to the valence and arousal dimensions of affective words remains unexplored. An aim of the current study was to determine whether the dissociation between valence and arousal that has been observed in the chemosensory domain also holds for these abstract stimuli (for prior efforts in this area, see Dolcos and others 2004; Kensinger and Corkin 2004).
Psychological arousal is generally envisaged as increasing along a single axis from most calm to most exciting. It is more difficult to establish this type of description for valence because it encapsulates both positive and negative affect. Traditional formulations (Wundt 1924; Lang and others 1993; Barrett and Russell 1998) have proposed an intuitively appealing bipolar continuum of valence that varies from most happy to most sad (Fig. 1A) (Russell 1989, 1991); however, psychological studies showing that self-reported degrees of happiness and sadness do not correlate (Nowlis 1965) suggest that this description may be oversimplified. This work has given rise to other models that propose positive (i.e., appetitive) and negative (i.e., aversive) valence as independent dimensions that do not share a common axis (Watson and Tellegen 1988; Cacioppo and Berntson 1994) (Fig. 1B). As with dissociating valence and arousal, one way to measure the validity of the various models for valence is by examining their neural substrate. To date, the neuroimaging literature is contradictory with respect to this issue: it suggests the involvement of distinct neural processes for positive and negative emotions while simultaneously demonstrating that they recruit shared neural circuits (for relevant reviews, see Baxter and Murray 2002; Phan and others 2004, 2005). Furthermore, recent reports (Winston and others 2003, 2005; Cunningham and others 2004) have proposed a novel framework in which aspects of emotion are coded on a single axis but in a hedonic manner (e.g., as difference from neutral), regardless of whether they are positive or negative, generating a U-shaped curve (Fig. 1C). This U-shaped model of valence should not be confused with the arousal dimension: the former depicts the extent of pleasantness/unpleasantness, whereas the later describes intensity of a stimulus. Observations that the amygdala responds to both happy and sad facial expressions (Winston and others 2003), as well as to both positive and negative odors (Winston and others 2005), are in keeping with the U-shaped model for valence. In the current study, we aimed to explicitly examine the brain activity correlating with each of the 3 models of valence.
Figure 1.
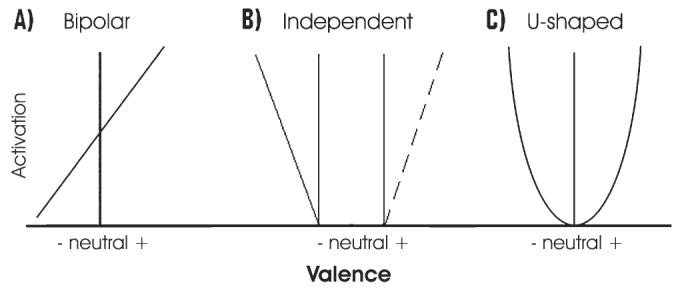
Three unidimensional models of emotional valence. In the bipolar distribution (A), activation increases from most negative to most positive. In the independent model (Wundt 1924; Lang and others 1993; Barrett and Russell 1998) (B), activation increases in an independent way for positive and negative valences (Watson and Tellegen 1988; Cacioppo and Berntson 1994). In the U-shaped distribution (C), valence increases from most neutral to most intense regardless of whether positive or negative (Winston and others 2003, 2005; Cunningham and others 2004). In the psychological models upon which these constructs are based (e.g., Wundt 1924; Watson and Tellegen 1988; Lang and others 1993; Cacioppo and Berntson 1994; Barrett and Russell 1998), “activation” was intended as an unspecified psychological response. In the context of brain imaging, however, this can easily be translated as neural activity or even blood oxygen level–dependent response.
The current study had 2 objectives. First, we aimed to tease apart activity associated with processing valence and arousal and to compare our findings in symbolic word stimuli with those already described in the chemosensory domain (Anderson and others 2003; Small and others 2003; Winston and others 2005), with the hypothesis of a double dissociation between amygdala responses to arousal and orbitofrontal responses to valence. Second, we aimed to determine whether 1 of the 3 models for valence, namely, bipolar, independent, and U-shaped models (Fig. 1), better describes neural activity underlying emotional processing, with the hypothesis that one model would correlate significantly with activity in the emotional system, whereas the others would not. To achieve these objectives, we used functional magnetic resonance imaging to quantify regional brain activity while subjects processed a series of emotional words. We then calculated the extent to which the brain response correlated with alterations in valence as defined by each model.
Materials and Methods
Subjects
Nineteen right-handed English first language subjects (9 females, mean age 30) with no history of neurological or psychiatric disease participated in this experiment. One subject was omitted due to his failure to respond to more than 50% of stimuli. Subjects were compensated for their time and travel expenses. The project was approved by the Joint Ethics Committee of the National Hospital and Institute of Neurology.
Task
We used words from the affective norms for English words (ANEW) standardized list (Bradley and Lang 1999); valence and arousal attributes are provided for these words: for valence on a scale of 1, very unpleasant, to 9, very pleasant, and for arousal on a scale of 0, not arousing, to 9, very arousing. The experimental stimuli consisted of 124 words with a valence rating >7 (positive) and 124 words with a valence rating <3 (negative), the words being chosen from all levels of arousal. Importantly, there were no significant differences in arousal attributes between the positive and negative words (see Fig. 2 for the distribution of valence/arousal scores), and valence and arousal were correlated (r = 0.42 and −0.19 in positive and negative words, respectively, with a significance of P < 0.01 in both cases). The words were presented for 1 s, with a 2-s inter stimulus interval, in a fully randomized order. Subjects performed a self-referential task, indicating (by button presses using the right hand) whether each word could be used to describe themselves (i.e., yes/no).
Figure 2.
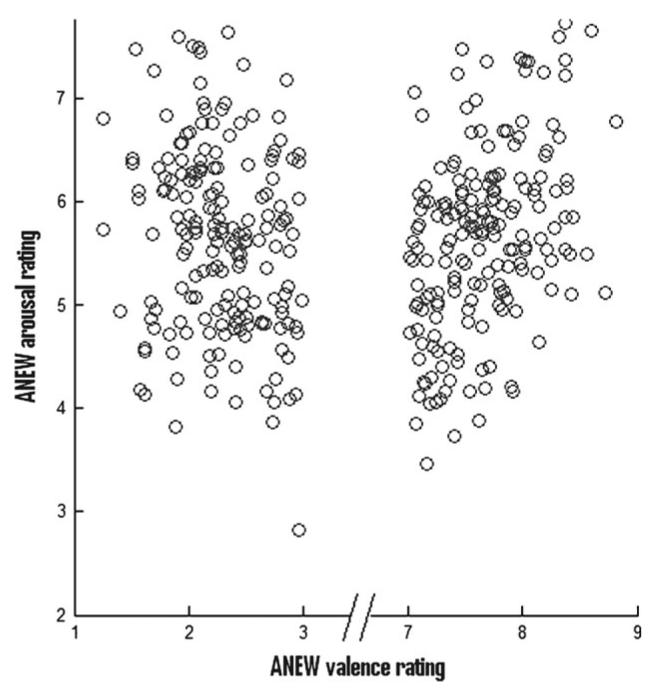
Distribution of our word stimuli in terms of valence and arousal. Normative valence, as taken from the ANEW (Bradley and Lang 1999) database, is shown on the x axis, and normative arousal is shown on the y axis.
Functional Imaging
Functional data were acquired using a Siemens Allegra 3T scanner. Gradient-echo echo-planar _T_2*-weighted image (EPI) volumes were composed of 44- × 2-mm-thick slices (repetition time of 2.86 s), covering the whole brain. We used a sequence that was optimized for sensitivity to responses in the orbitofrontal cortex and medial temporal lobe structures (Deichmann and others 2003). Acquisition was tilted at an oblique angle of 30 degrees to the anterior–posterior commissure line, and a preparation pulse (duration of 1 ms, amplitude of −2 mT/m) was used in the slice selection direction. Data were acquired in one continuous session consisting of 300 volumes.
Analysis of Functional Imaging Data
Functional data were analyzed using SPM2 (http://www.fil.ion.ucl.ac.uk/spm/). The first 6 volumes of the session were discarded, and the remaining volumes were realigned and corrected for slice timing differences. A mean image was produced and volumetrically normalized to the SPM2 EPI template (in Montreal Neurological Institute space). The images were then transformed to the standard anatomical volume using these parameters and smoothed with an 8-mm kernel.
Modeling and Contrasts
To characterize functional responses, the data were examined using a 2-level random-effects analysis. At the first level, we examined the responses of individual subjects by modeling the presentation times of positive and negative words (separately) along with 4 parametric regressors for each word: the valence rating, the arousal rating, the interaction between valence and arousal, and the subjects' response. Valence and arousal ratings were taken from the standardized ANEW database (Bradley and Lang 1999). The interaction term was created by multiplying the mean corrected ratings (valence × arousal = interaction). Parameter estimates reflecting the height of the hemodynamic response function for each of these regressors were calculated at each voxel. Contrast images were then calculated separately for each parametric regressor. The resulting images were used in the second-level random-effects analyses that combined data across subjects.
A series of contrasts were performed in order to examine the neural correlates of the 3 models of valence (independent, linear, and U-shaped). First, to ensure that parametric regressors explained the majority of valence related activity, we compared all positive words with all negative words. Second, we checked for activities correlating with self-referential responses by comparing words designated as self-descriptive with those designated as nonself-descriptive. Next, we created a second-level analysis of variance (ANOVA) with 6 factors (valence, arousal, and their interaction, separately for positive and negative words) in order to examine the neural correlates of valence and arousal. This design ensured that variance could only be explained by one factor, thus allowing a complete separation between responses associated with valence and arousal, despite the fact that these dimensions were correlated in our stimuli. Using this ANOVA, we examined parametric activity associated with the independent model by calculating activities associated with parametrically increasing valence (from most neutral to most strongly valenced) separately in positive and negative words. To ensure that results for the bipolar and U-shaped models were not driven by positive or negative words alone, we used global conjunctions (Friston and others 2005) across both valences to examine these models. Thus, activity associated with the U-shaped model was calculated using a conjunction across increasing valence in both positive and negative words. Activity associated with the linear model was calculated using a conjunction across increasing valence in positive words and decreasing valence (from most negative to most neutral) in negative words. Our analysis of activities relating to arousal and to the interaction term (valence × arousal) was performed in the same way: parametric increases were first examined separately in positive and negative words and then a functional conjunction was performed across the 2 valences in order to calculate activities shared across increases.
All second-level analyses relied upon 1-way _t_-tests using the relevant contrast images or a global conjunction across 2 contrasts. The statistical images resulting from these analyses were inclusively masked using a priori regions of interest defined by the Wake Forest University Pickatlas (http://www.fmri.wfubmc.edu/download.htm). These regions included an emotion matrix (amygdala, orbitofrontal cortex, subgenual cingulate, genual cingulate, striatum, brain stem/midbrain/pons, and insula). Data were thresholded at P = 0.05 using small volume correction for these regions and in lateral orbitofrontal cortex for a 15-mm-radius sphere centered on coordinates reported in Small and others 2003. An auxiliary (uncorrected) threshold of P = 0.001 was used to define the spatial extent of clusters forming the basis of the corrected inference.
Results
Behavioral Data
Subjects designated an average of 50% of the presented words as self-descriptive and 50% as nonself-descriptive. In order to check for correlations between the yes/no responses and the valence or arousal of words, we calculated the correlation coefficients between valence and yes/no responses and between arousal and yes/no responses for each individual. We then used a simple _t_-test to compare the correlation coefficients with zero. This revealed a weak but significant correlation (P = 0.02, mean correlation coefficient = −0.15) between valence and self-description, indicating that subjects tended to respond “yes” for more positive information. There was no significant correlation between arousal and yes/no responses (P = 0.22).
Functional Imaging Data
A discrete set of regions are implicated in processing valence and arousal in response to emotional stimuli: the amygdala (Baxter and Murray 2002; LeDoux 2003; Phan and others 2004), orbitofrontal cortex (Rolls 2000; O'Doherty, Kringelbach, and others 2001; O'Doherty, Rolls, and others 2001; O'Doherty and others 2003; Rolls and others 2003, corresponding to Brodmann areas 10, 11, 47), subgenual cingulate (corresponding to Brodmann area 25), genual cingulate (corresponding to Brodmann area 32), striatum (Phillips and others 2003; Phan and others 2004, 2005), brain stem/midbrain/pons (O'Doherty and others 2002), and insula (Critchley, Corfield, and others 2000; Critchley, Elliott, and others 2000; Critchley and others 2002, 2004; corresponding to Brodmann area 13). We limited our analysis to these areas using region of interest defined using the Wake Forest University Pickatlas (http://www.fmri.wfubmc.edu/download.htm). Our findings are summarized in Tables 1 and 2.
Table 1.
Peaks of activation significantly modulated by valence within our regions of interest (orbitofrontal cortex, subgenual and anterior cingulate, insula, amygdala, brain stem, pons, and striatum as defined by the Wake Forest University Pickatlas), reported in standardized Montreal Neurological Institute coordinates
| Cluster size | Peak T | SVC P | Peak Z | x, y, z (mm) | Anatomical region |
|---|---|---|---|---|---|
| Valence (increasing)—positive words | |||||
| 21 | 3.9 | 0.03 | 3.7 | 42, 42, −10 | Lateral orbitofrontal cortex** |
| 13 | 3.8 | 0.04 | 3.6 | 30, 20, −8 | Anterior insula** |
| 9 | 3.4 | 0.05 | 3.3 | 32, 46, −14 | Lateral orbitofrontal cortex** |
| Valence (increasing)—negative words | |||||
| 24 | 3.7 | 0.04 | 3.6 | 34, 22, −24 | Posterior insula** |
| 20 | 3.6 | 0.01 | 3.5 | −2, 46, 10 | Anterior cingulate** |
| 7 | 3.5 | 0.06 | 3.4 | −2, 26, −10 | Subgenual cingulate |
| 3 | 3.3 | 0.05 | 3.2 | 50, 28, −12 | Lateral orbitofrontal cortex** |
| 13 | 3.6 | 0.07 | 3.5 | −14, −16, −22 | Brain stem |
| 18 | 3.6 | 0.03 | 3.5 | 0, 26, −10 | Subgenual cingulate** |
| 5 | 3.3 | 0.06 | 3.2 | 12, 38, −12 | Subgenual cingulate |
| Valence (increasing U-shaped model) | |||||
| 67 | 2.6 | 0.01 | 4 | 36, 20, −18 | Lateral orbitofrontal cortex** |
| 33 | 2.4 | 0.03 | 3.7 | −2, 42, 12 | Anterior cingulate** |
| 32 | 2.3 | 0.05 | 3.6 | 0, 26, −4 | Subgenual cingulate** |
| 18 | 2.3 | 0.09 | 3.6 | −54, 26, −4 | Operculum |
| 2 | 2.1 | 0.01 | 3.3 | 50, 42, −14 | Lateral orbitofrontal cortex** |
Table 2.
Peaks of activation significantly modulated by arousal and by the interaction of valence and arousal within our regions of interest (orbitofrontal cortex, subgenual and anterior cingulate, insula, amygdala, brain stem, pons, and striatum as defined by the Wake Forest University Pickatlas), reported in standardized Montreal Neurological Institute coordinates
| Cluster size | Peak T | SVC P | Peak Z | x, y, z (mm) | Anatomical region |
|---|---|---|---|---|---|
| Arousal—negative words | |||||
| 37 | 3.9 | 0.03 | 3.7 | 12, −14, −8 | Midbrain** |
| 11 | 3.7 | 0.05 | 3.6 | −32, 6, 10 | Middle insula** |
| 4 | 3.5 | 0.07 | 3.4 | −32, −14, 14 | Posterior insula |
| 7 | 3.5 | 0.07 | 3.4 | 40, 38, −12 | Lateral orbitofrontal cortex |
| 3 | 3.4 | 0.02 | 3.3 | −24, −2, −12 | Amygdala** |
| 1 | 3.2 | 0.03 | 3.1 | 16, 10, −2 | Putamen** |
| Arousal—positive words | |||||
| 37 | 3.9 | 0.01 | 3.7 | 8, 18, −6 | Head of caudate** |
| 11 | 3.7 | 0.05 | 3.6 | 4, 22, −8 | Subgenual cingulate** |
| Arousal—both positive and negative words | |||||
| 106 | 2.7 | 0.01 | 4.2 | 12, 12, −6 | Ventral striatum** |
| 21 | 2.4 | 0.05 | 3.7 | −28, 16, −8 | Anterior insula** |
| 2 | 2.1 | 0.07 | 3.3 | 14, −10, −2 | Pallidum |
| 4 | 1.6 | 0.10 | 2.7 | −20, −8, −14 | Amygdala |
| Interaction—negative words | |||||
| 14 | 4.7 | 0.00 | 4.4 | 18, −20, 22 | Tail caudate** |
| 32 | 4 | 0.01 | 3.8 | 0, 50, −14 | Medial orbitofrontal cortex** |
| 2 | 3.6 | 0.06 | 3.5 | −38, −20, 2 | Posterior insula |
| 11 | 3.6 | 0.03 | 3.5 | −8, 34, −14 | Medial orbitofrontal cortex** |
| 2 | 3.4 | 0.09 | 3.3 | −32, −8, 18 | Middle insula |
| 2 | 3.3 | 0.07 | 3.2 | 18, 22, 10 | Head of caudate |
| 1 | 3.3 | 0.03 | 3.2 | −18, 0, 4 | Putamen** |
| 3 | 3.2 | 0.07 | 3.1 | −4, 58, −6 | Medial orbitofrontal cortex |
Valence
To detect activity associated with differences between positive and any negative words not modeled by the parametric regression, we compared the mean activities associated with these categories (e.g., positive vs. negative). To detect activity associated with self-referential responding, we compared self-descriptive with nonself-descriptive words. There were no significant responses to either of these contrasts within our regions of interest.
To characterize responses associated with each of the 3 models of valence, we examined activity correlating with normative valence ratings of word stimuli using different combinations of parametric regressors. The independent model was tested by examining modulation by valence for positive and negative words separately, the U-shaped model was tested using a conjunction across activities correlating with the increasing intensity of valence in positive and negative words, and the bipolar model was tested using a conjunction across increasing valence in positive words and decreasing valence in negative words. This analysis revealed robust activity in the first 2 cases but no significant response in the case of the bipolar model.
Regions significantly modulated by increasing valence in positive words (dark red in Fig. 3A,B) comprised foci in right lateral orbitofrontal cortex and in anterior insula. Regions significantly modulated by increasing valence in negative words (blue in Fig. 3A,C) included right posterolateral orbitofrontal cortex, right medial orbitofrontal cortex, and medial subgenual cingulate (see Table 1 for a complete list of activities).
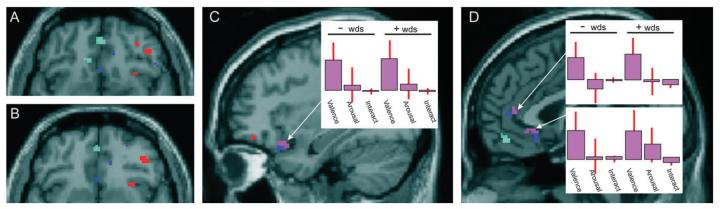
Figure 3.
Valence: Areas of activity significantly modulated by changing valence are rendered onto the SPM canonical brain at P < 0.001. Activity associated with increasingly positive valence is shown in red, activity associated with increasingly negative valence in blue, activity associated with the conjunction of these 2 (U-shaped model) in purple, and activity associated with the interaction of increasingly negative valence and arousal in cyan. Parameter estimates are shown for lateral orbitofrontal cortex (C), anterior cingulate (D top), and subgenual cingulate (D bottom). Note that responses in these regions followed the U-shaped model, that is, activity was modulated by increasingly intense valence in both positive and negative words. Parameter estimates represent (from left to right) valence, arousal, and the interaction of valence and arousal in negative (left) and positive (right) words.
The U-shaped model (purple in Fig. 3) revealed large regions of activity in posterior–lateral orbitofrontal cortex (Fig. 3C) and subgenual cingulate (Fig. 3D). Another region showing the U-shaped pattern was the anterior cingulate (Fig. 3D) (see Table 1 for a complete list of activities).
Arousal
To characterize brain regions involved in processing arousal, we examined activity correlating with the normative arousal ratings of word stimuli. This was implemented separately in positive and negative words and also in a conjunction across both valences.
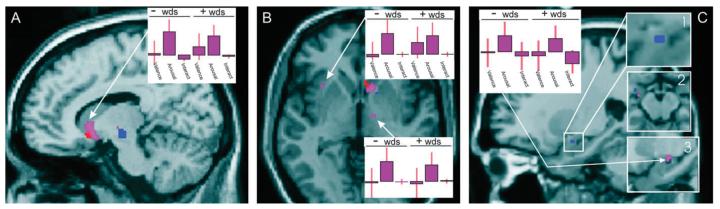
Increasing arousal in positive words modulated activity in the ventral striatum (red in Fig. 4A) and subgenual cingulate. Increasing arousal in negative words modulated activity (blue in Fig. 4) in the midbrain (Fig. 4A,B), left insula (Fig. 4B), left dorsal amygdala (Fig. 4C), and putamen (Fig. 4B). The conjunction of increasing arousal in both positive and negative words revealed shared activity in the ventral striatum (purple in Fig. 4A,B), pallidum (Fig. 4B), left lateral anterior insula (Fig. 4B), and, at a more lenient threshold of P < 0.005, left dorsal amygdala (see Table 2 for a complete list of activities). No significant modulation was found in the conjunction of correlations with decreasing arousal.
Figure 4.
Arousal: Areas of activity significantly modulated by arousal are rendered onto the SPM canonical brain at P < 0.001. The head of putamen, which is significantly modulated by increasing arousal in positive words, is shown in red (A), regions modulated by increasing arousal in negative words, including brain stem (A), pallidum (B), and amygdala (C), are shown in blue. Regions of activity shared between increasing arousal in positive and negative words (independent of valence), including ventral striatum (A, B), anterior insula (B), pallidum (B), and amygdala (C), are shown in purple. Insets in (C) show amygdala responses to both negative words (C1, 2, blue) and U-shaped model (C2,3, purple). Regions modulated by the interaction of increasingly negative valence and arousal are shown in cyan (C). Parameter estimates are shown for the activities shown in purple (correlating with increasing arousal in both positive and negative words) in ventral striatum (A), anterior insula (upper B), and pallidum (lower B), and amygdala (C). Parameter estimates represent (from left to right) valence, arousal, and the interaction of valence and arousal in negative (left) and positive (right) words. Activity in the amygdala is shown in (C), with insets showing the same at a visualization threshold of P < 0.005, sagittal view (C1,3) and coronal view (C2).
Interaction
To detect brain regions where activity was modulated by an interaction between arousal and valence, we searched for an evoked response that varied as a function of the interaction of normative ratings (valence × arousal). This was implemented for positive and negative words and for their conjunction. Significant interactions were observed in negative words alone and involved several foci within the left medial orbitofrontal cortex and striatum (right head of caudate and left putamen) (Fig. 3A,B,D). Other significant responses are listed in Table 2.
Discussion
A primary goal of this study was to characterize the functional neuroanatomy supporting the processing of valence and arousal in abstract word stimuli. Our findings extend work in the chemosensory domain showing a double dissociation between these dimensions by demonstrating that subregions of orbitofrontal and subgenual cortex respond to normative word valence, whereas responses in insula, basal ganglia, and amygdala vary with normative word arousal. The second aim of this study was to dissociate activity underpinning the 3 models of valence: bipolar model, independence of positive and negative affect, and U-shaped model (Fig. 1). Our results show distinct orbitofrontal responses in association with positive and negative items (independent models, Fig. 1B), as well as regions of shared activity (U-shaped model, Fig. 1C). By contrast, because no activity was uniquely attributable to the bipolar model (Fig. 1A), our findings do not support this construct as a descriptor of neural responses underlying the processing of word valence.
On the Comparison of Abstract Words and Chemosensory Stimuli
In chemosensory stimuli like taste and smell, the distinction between valence and intensity is indisputable. In abstract representational stimuli like words, however, the situation is less clear. Although subjects can produce distinct ratings for the valence and arousal of words, the behavioral differentiation of these attributes is often subtle. Our data demonstrate a functional dissociation between those brain regions modulated by the valence and those modulated by the arousal of abstract words, indicating that these 2 dimensions are processed in a distinct manner. In line with the chemosensory literature (Anderson and others 2003; Small and others 2003) and a recent study of affective pictures (Anders and others 2004), our findings show a double dissociation between orbitofrontal cortex and amygdala in coding for valence and arousal, respectively. This suggests that the valenced or arousing properties of abstract words or pictures draw upon neural processes similar to those evoked by valence and intensity in taste and smell (but see also Cunningham and others 2004; Dolcos and others 2004; Kensinger and Corkin 2004).
It is important to note that our data are not entirely in keeping with the dissociation observed in other modalities. We found significant modulation of the amygdala in response to increasing arousal in both positive and negative words, but with a preferential response in negative words. Furthermore, the dorsal location of the amygdala activations we observed differs notably from the more medial amygdala activity evoked by intensity in the chemosensory domain (Anderson and others 2003; Small and others 2003) and in the processing of attitudes (Cunningham and others 2004). Additionally, although orbitofrontal modulation correlated with increases in valence, a small region also correlated with changes in arousal. These discrepancies may reflect subtle differences in the processing of chemosensory stimuli and abstract words.
On the 3 Models of Valence
The suggestion of functional subdivision in the orbitofrontal cortex, with different regions specialized to process different components of positive or negative valence, is not new. The chemosensory literature (e.g., Zald and Pardo 2000; Anderson and others 2003; Small and others 2003) has repeatedly demonstrated left lateralization of orbitofrontal responses to negative stimuli and right lateralization of orbitofrontal responses to positive stimuli. Other work (O'Doherty, Kringelbach, and others 2001; Gottfried and others 2002; O'Doherty and others 2003; Small and others 2003) has shown differential responses in medial and lateral orbitofrontal cortex for positive versus negative or rewarding versus punishing stimuli. Our data show neither right/left nor medial/lateral patterns, a discrepancy that may be due to either the limited scope of these proposed frameworks or the abstract representative nature of our word stimuli.
Our demonstration that different regions of orbitofrontal cortex are modulated by increasing positivity and increasing negativity suggests that these 2 dimensions are best captured when examined separately, thus arguing for the independence of positive and negative valence. The concomitant observation of activity modulated by increasing the value of both valences shows that parts of the orbitofrontal cortex also code for the absolute value of valence, thus demonstrating a physiological basis for the U-shaped scheme. This latter result is in line with the observation of activity in left posterior–lateral orbitofrontal cortex in association with both positively and negatively valenced taste (Small and others 2003) and, to a lesser extent, with a study showing modulation of ventromedial prefrontal cortex and amygdala by the amount of expression in a morphed face (Winston and others 2003). We found no significant response in association with the bipolar model and were therefore unable to support the validity of this formulation as a descriptor of physiological responses to valence.
Several authors (Winston and others 2003; Cunningham and others 2004) have recently argued that a U-shaped model for valence would allow coding for a generalized form of “salience,” thus aiding in the direction of attention toward behaviorally important goals. In addition to this type of coding, it is clearly important to be able to distinguish between positive and negative stimuli and also to gauge degrees of valence in each category relative to the other (think of a situation in which minor negative stimuli must be overcome in order to achieve a very rewarding goal). Our observation of orbitofrontal activities in association with independent and U-shaped, but not bipolar, models suggests that, at least for symbolic word stimuli, orbitofrontal cortex may not provide this type of relative representation. It is likely that other regions (potentially higher cognitive areas) are recruited for judgment of emotional relativity, although this may only be true for abstract stimuli such as valenced words.
On the Interaction of Valence and Arousal
We found that large regions of activity were modulated by the interaction of normative valence and arousal in negative words. This indicates a parametrically varying valence-specific interdependence of these 2 dimensions in the observed regions (tail of caudate, medial orbitofrontal cortex, and other areas). We did not observe a parametrically modulated interaction for positive words but did find that subgenual cingulate and head of caudate responded to increasing arousal in positive but not negative words, whereas other regions (including insula and amygdala) responded to increasing arousal in negative but not positive words. These emotion-specific responses to arousal are reminiscent of a recent study (Winston and others 2005) demonstrating that the amygdala only responds to the intensity of odors when the odor stimuli are also strongly valenced. This type of positive/negative -specific interaction shows that emotional context can influence the way in which we respond to arousing information, suggesting a top down modulation of some aspects of arousal that is akin to the 2-factor model of emotional feeling (Schachter and Singer 1962) in which cognitive appraisal of context informs an interpretation of bodily arousal. The observation that amygdala responses to arousing stimuli occur only in an emotional context also calls into question the proposal that amygdala responds to arousal but not valence, suggesting instead that this structure may be coding for more general value and the interaction between valence and arousal (for a more complete argument, see Winston and others 2005). Because we did not examine neutral stimuli in the current study, our data cannot inform this debate.
Our data demonstrate that some regions respond independently to both valence and arousal. The subgenual cingulate, for instance, responds both to valence (negative words and U-shaped model) and to arousal in negative words. This overlap joins the interactions above in suggesting that valence and arousal are not fully dissociated in these structures.
On Self-Referential Responding
Self-referential processing has been associated with activity in the medial prefrontal cortex (Gusnard and others 2001; Fossati and others 2003; Northoff and Bermpohl 2004). Because our subjects indicated whether each word could or could not be used as a self-descriptor, we checked our data for potentially confounding activity related to this type of introspection. We found no significant difference between activities associated with words designated as self-descriptive and those designated as nonself-descriptive. The absence of such differences may be due to the similarity of evaluative processing for both stimulus types because introspective processing of a similar type has been shown to activate medial prefrontal cortex regardless of its outcome (Fossati and others 2003). It could also be due to the abstract nature of our word stimuli. We did find a self-referential effect in our behavioral data, with the probability that words were classified as self-descriptive correlating significantly with their positiveness. This result is in line with prior work showing that nondepressed subjects exhibit a positive bias on self-referential tasks (Tagami 2002), as well as studies noting that depressed subjects tend to exhibit a negative bias (e.g., Tagami 2002; Dalgleish and others 2004).
In conclusion, we sought to determine whether motivational attributes of symbolic stimuli (words) are represented within the neural systems that have evolved for processing more ecological stimuli such as taste and smell. Our results show that normative arousal ratings of affective words correlate with activity in the ventral striatum, anterior insula, and amygdala, regions commonly modulated by increasing intensity in primary chemosensory stimuli. This result demonstrates that abstract stimuli can recruit the same structures as primary sensory stimuli and thus implies that top down cognitive processing can draw upon primitive systems that commonly respond to more direct stimulation. As a secondary goal of this study, we aimed to examine 3 different ways of modeling valence as descriptors of physiological responses. Surprisingly, our results support the independent model in which positive and negative information are treated separately and the newly proposed U-shaped model in which valence is calculated as distance from neutral irrespective of whether it is positive or negative but not the traditional bipolar model in which valence increases from most negative to most positive.
Acknowledgments
PAL was supported by the European Union FET programme PRESENCIA IST-2001-37927 and HDC by the Wellcome Trust. This work was carried out under a programme grant for the Wellcome Trust to RJD. We are grateful to the technical staff and analysis group at the Functional Imaging Laboratory for invaluable assistance and to 3 anonymous reviewers for helpful suggestions and comments.
Footnotes
Conflict of Interest: None declared.
References
- Anders S, Lotze M, Erb M, Grodd W, Birbaumer N. Brain activity underlying emotional valence and arousal: a response-related fMRI study. Hum Brain Mapp. 2004;23(4):200–209. doi: 10.1002/hbm.20048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, Gabrieli JD, Sobel N. Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci. 2003;6(2):196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Barrett L, Russell J. Independence and bipolarity in the structure of current affect. J Pers Soc Psychol. 1998;74:967–984. [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3(7):563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Bensafi M, Rouby C, Farget V, Bertrand B, Vigouroux M, Holley A. Autonomic nervous system responses to odours: the role of pleasantness and arousal. Chem Senses. 2002;27(8):703–709. doi: 10.1093/chemse/27.8.703. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Affective norms for English words (ANEW): stimuli, instruction manual, and affective ratings. Gainesville, FL: The center for research in psychophysiology, University of Florida; 1999. [Google Scholar]
- Cacioppo J, Berntson G. Relationship between attitudes and evaluative space: a critical review, with emphasis on the separability of positive and negative substrates. Psychol Bull. 1994;115:401–423. [Google Scholar]
- Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ. Cerebral correlates of autonomic cardiovascular arousal: a functional neuroimaging investigation in humans. J Physiol. 2000;523(Pt 1):259–270. doi: 10.1111/j.1469-7793.2000.t01-1-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Elliott R, Mathias CJ, Dolan RJ. Neural activity relating to generation and representation of galvanic skin conductance responses: a functional magnetic resonance imaging study. J Neurosci. 2000;20(8):3033–3040. doi: 10.1523/JNEUROSCI.20-08-03033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ. Fear conditioning in humans: the influence of awareness and autonomic arousal on functional neuroanatomy. Neuron. 2002;33(4):653–663. doi: 10.1016/s0896-6273(02)00588-3. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7(2):189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Raye CL, Johnson MK. Implicit and explicit evaluation: fMRI correlates of valence, emotional intensity, and control in the processing of attitudes. J Cogn Neurosci. 2004;16(10):1717–1729. doi: 10.1162/0898929042947919. [DOI] [PubMed] [Google Scholar]
- Dalgleish T, Spinks H, Golden AM, du Toit P. Processing of emotional information in seasonal depression across different cognitive measures. J Abnorm Psychol. 2004;113(1):116–126. doi: 10.1037/0021-843X.113.1.116. [DOI] [PubMed] [Google Scholar]
- Deichmann R, Gottfried JA, Hutton C, Turner R. Optimized EPI for fMRI studies of the orbitofrontal cortex. Neuroimage. 2003;19(2 Pt 1):430–441. doi: 10.1016/s1053-8119(03)00073-9. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: an event-related fMRI study. Neuroimage. 2004;23(1):64–74. doi: 10.1016/j.neuroimage.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Graham SJ, Grady C, Keightley ML, Craik F, Mayberg H. In search of the emotional self: an fMRI study using positive and negative emotional words. Am J Psychiatry. 2003;160(11):1938–1945. doi: 10.1176/appi.ajp.160.11.1938. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Penny WD, Glaser DE. Conjunction revisited. Neuroimage. 2005;25(3):661–667. doi: 10.1016/j.neuroimage.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, Deichmann R, Winston JS, Dolan RJ. Functional heterogeneity in human olfactory cortex: an event-related functional magnetic resonance imaging study. J Neurosci. 2002;22(24):10819–10828. doi: 10.1523/JNEUROSCI.22-24-10819.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Corkin S. Two routes to emotional memory: distinct neural processes for valence and arousal. Proc Natl Acad Sci USA. 2004;101(9):3310–3315. doi: 10.1073/pnas.0306408101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30(3):261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23(4–5):727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends Cogn Sci. 2004;8(3):102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Nowlis V. Research with the mood adjective check list. In: Tomkins SS, Izard CE, editors. Affect, cognition, and personality. Oxford, UK: Springer; 1965. pp. 352–389. [Google Scholar]
- O'Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4(1):95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F. Representation of pleasant and aversive taste in the human brain. J Neurophysiol. 2001;85(3):1315–1321. doi: 10.1152/jn.2001.85.3.1315. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Winston J, Critchley H, Perrett D, Burt DM, Dolan RJ. Beauty in a smile: the role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia. 2003;41(2):147–155. doi: 10.1016/s0028-3932(02)00145-8. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;(5):815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57(3):210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager TD, Taylor SF, Liberzon I. Functional neuroimaging studies of human emotions. CNS Spectr. 2004;9(4):258–266. doi: 10.1017/s1092852900009196. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry. 2003;54(5):515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex and reward. Cereb Cortex. 2000;10(3):284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Rolls ET, O'Doherty J, Kringelbach ML, Francis S, Bowtell R, McGlone F. Representations of pleasant and painful touch in the human orbitofrontal and cingulate cortices. Cereb Cortex. 2003;13(3):308–317. doi: 10.1093/cercor/13.3.308. [DOI] [PubMed] [Google Scholar]
- Russell JA. A circumplex model of affect. J Pers Soc Psychol. 1980;39:1161–1178. [Google Scholar]
- Russell JA. Measures of emotion. In: Plutchik R, Kellerman H, editors. Emotion: theory, research, and experience. San Diego, CA: Academic Press; 1989. p. 83. [Google Scholar]
- Russell JA. Culture and the categorization of emotions. Psychol Bull. 1991;110:426–450. doi: 10.1037/0033-2909.110.3.426. [DOI] [PubMed] [Google Scholar]
- Schachter S, Singer JE. Cognitive, social, and physiological determinants of emotional states. Psychol Rev. 1962;69:379–399. doi: 10.1037/h0046234. [DOI] [PubMed] [Google Scholar]
- Small DM, Gregory MD, Mak YE, Gitelman D, Mesulam MM, Parrish T. Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron. 2003;39(4):701–711. doi: 10.1016/s0896-6273(03)00467-7. [DOI] [PubMed] [Google Scholar]
- Tagami K. Negative bias on self-referent processing in depression: focused on mood congruent effects. Shinrigaku Kenkyu. 2002;73(5):412–418. doi: 10.4992/jjpsy.73.412. [DOI] [PubMed] [Google Scholar]
- Watson D, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Winston JS, Gottfried JA, Kilner JM, Dolan RJ. Integrated neural representations of odor intensity and affective valence in human amygdala. J Neurosci. 2005;25(39):8903–8907. doi: 10.1523/JNEUROSCI.1569-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston JS, O'Doherty J, Dolan RJ. Common and distinct neural responses during direct and incidental processing of multiple facial emotions. Neuroimage. 2003;20(1):84–97. doi: 10.1016/s1053-8119(03)00303-3. [DOI] [PubMed] [Google Scholar]
- Wundt W. An introduction to psychology. London: Allen & Unwin; 1924. [Google Scholar]
- Zald DH, Pardo JV. Functional neuroimaging of the olfactory system in humans. Int J Psychophysiol. 2000;36(2):165–181. doi: 10.1016/s0167-8760(99)00110-5. [DOI] [PubMed] [Google Scholar]