Poly(I:C) and Lipopolysaccharide Innate Sensing Functions of Circulating Human Myeloid Dendritic Cells Are Affected In Vivo in Hepatitis C Virus-Infected Patients (original) (raw)
Abstract
The role of peripheral dendritic cells (DCs) in hepatitis C virus (HCV) infection is unclear. To determine if persistent infection exerts an inhibitory pressure on HCV-specific innate responses, we analyzed DC function in blood through quantification of cell-associated HCV RNA levels in conjunction with multiparametric flow cytometry analysis of pathogen recognition receptor-induced cytokine expression. Independently of the serum viral load, fluorescence-activated cell sorter-purified total DCs had a wide range of cell-associated HCV genomic RNA copy numbers (mean log10, 5.0 per 106 cells; range, 4.3 to 5.8). Here we report that for viremic patients with high viral loads in their total DCs, the myeloid DC (MDC) subset displayed impaired expression of interleukin-12 (IL-12) and tumor necrosis factor alpha (TNF-α) but normal IL-6 or chemokine CCL3 expression in response to poly(I:C) and lipopolysaccharide (LPS). IL-6-expressing cells from this subgroup of viremic patients demonstrated a significant increase (sixfold more) in TNF-α− IL-12− cell frequency compared to healthy donors (mean, 38.8% versus 6.5%; P < 0.0001), indicating a functional defect in a subpopulation of cytokine-producing MDCs (∼6% of MDCs). Attenuation of poly(I:C) and LPS innate sensing was HCV RNA density dependent and did not correlate with viremia or deficits in circulating MDC frequencies in HCV-infected patients. Monocytes from these patients were functionally intact, responding normally on a per-cell basis following stimulation, independent of cell-associated HCV RNA levels. Taken together, these data indicate that detection of HCV genomic RNA in DCs and loss of function in the danger signal responsiveness of a small proportion of DCs in vivo are interrelated rather than independent phenomena.
Hepatitis C virus (HCV) chronic infectious disease is proving to be a public health problem of growing importance. The inadequacy and loss of HCV-specific CD4+ T-cell help are widely perceived as the principal factors responsible for the failure of CD8+ T cells to afford protection against the highly mutable HCV genome (13, 41). Nonetheless, the precise mechanisms operating within the host which result in the failure of CD4+ T-cell immunity and allow HCV to establish persistent infection have remained enigmatic (38). In this regard, the role of CD11chi myeloid dendritic cells (MDCs) is of interest because these peripheral antigen-presenting cells display extraordinary capacities to link innate and adaptive immunity (18). Indeed, diphtheria toxin-based in vivo depletion of CD11c+ DCs abrogates priming of T cells in response to infectious pathogens in otherwise immunocompetent mice (19). Despite careful execution of experiments, the exact contribution of MDCs to any individual aspect of HCV immunity remains controversial.
It has been reported that CD4+ T-cell activation (allogeneic responses) is decreased in HCV-viremic patients (3, 21, 42). This is corrected upon clearance of cell-associated viral RNA from MDCs after 4 weeks of ribavirin/alpha interferon (IFN-α) antiviral therapy (43), indicating that weak HCV-specific CD4+ T-cell responses are perhaps due to defects in DC-mediated innate immune functions. In contrast, other observations (26) have been taken to indicate that the deficiency in HCV-specific CD4+ T-cell activity is not attributable to virus-induced dysfunctions in these cells or their precursors (DC independent), even though DC-derived signals may be essential for the T-cell activation function itself. Accordingly, the use of in vitro cytokine-driven culture systems for chronic infection is reported to result in functionally intact CD14+ monocyte-derived DCs (albeit to various degrees in different studies) (24, 26, 32, 35). However, it has remained equally likely that the DC population in question was generated from nondefective precursors in the in vitro system, as these studies demonstrated the potential for functional DC development from monocytes rather than the existence of functional DC subsets in the periphery. The seemingly paradoxical conclusions drawn from those observations highlight the importance of carrying out functional measurements on cells with minimal manipulation. Most of the relevant studies presumed an equivalent function of the peripheral counterparts, a hypothesis supported by data showing that tumor necrosis factor alpha (TNF-α)-activated MDCs from viremic patients are functionally competent, with a CD4+ T-cell clonal expansion capacity equal to that of healthy donors (27). While that finding indicates that TNF receptor signaling is not affected in peripheral MDCs, the extent of activation of these cells in response to multiple innate inputs known to relay distinct danger signals (e.g., Toll-like receptors [TLRs]) remained unexplored.
In vivo, the requirement that DCs directly recognize a pathogen-specific molecule to effectively induce the efferent functions of helper CD4+ T cells after activation cannot be replaced by inflammatory cytokines (e.g., TNF-α) (39). TLR3, -7, and -8 expressed on MDCs act principally as viral sensors, recognizing, in a replication-independent way, conserved molecular motifs in virus genomic RNA (1, 14, 23). The signals that originate from these TLR sensors are those required to drive antigen-specific CD4+ T-helper-cell differentiation in vivo (31, 36, 37, 45). Recently, one study reported that reduced TLR3-induced interleukin-12 (IL-12) production correlated with lower frequencies of HCV-specific CD4+ T-cell help in viremic patients (2). Notably, HCV NS3/4A serine protease can function as an antagonist of TLR3-dependent innate sensing through its ability to specifically target its signaling adaptor, TRIF, for proteolytic cleavage (25). Inhibition of this protease activity enables host recognition of HCV RNA (9, 40). Loss of TLR3 sensing, as reported by Anthony et al. (2), may therefore relate to the ability of HCV to physically interact with MDCs and cause disruptions of TLR-mediated innate recognition of HCV components in these cells. However, in vivo data substantiating this view are scarce, as the function of TLRs in innate immunity with respect to HCV has not been studied extensively.
For this study, we took advantage of a sensitive and precise multiparametric flow cytometry assay based on the induction of intracellular cytokines after very short-term (6-hour) stimulation of Golgi transport-inhibited DCs with TLR agonists and readdressed the question of whether innate functions of MDCs are intact in HCV-positive subjects. We report the attenuation of poly(I:C) (TLR3) and lipopolysaccharide (LPS) (TLR4) innate sensing potential in a small proportion of CD14− CD33+ major histocompatibility complex class II-bright (MHC-IIbr) cytokine-producing MDCs from viremic patients, as long as the total DCs in those patients contained high levels of cell-associated HCV genomic RNA (≥5.0 log10 copies per 106 cells). These results provide the first clear evidence of an association between HCV and a loss of function of the danger signal responsiveness of MDCs in the presence of ongoing viremia.
(This work was carried out as partial fulfillment of I. G. Rodrigue-Gervais' Ph.D. thesis at the Université de Montréal, Montréal, Québec, Canada.)
MATERIALS AND METHODS
Patients and control individuals.
Blood samples were obtained from 18 HCV-infected patients enrolled in the drug addiction cohort and the hepatology experimental unit of the Centre Hospitalier de l'Université de Montréal (CHUM), Hôpital Saint-Luc. The clinical and demographic characteristics of the patients studied are shown in Table 1. Antiviral therapy had been discontinued at least 1 year prior to sample collection. Clinical protocols conformed to the ethical guidelines of our institutions. Samples were obtained with the informed consent of the subjects.
TABLE 1.
Clinical and demographic characteristics of HCV-infected patientse
| Patient (n = 18) | Age (yr)/sex | HCV genotype | Viremiaa (IU ml−1) | Therapy | Outcome | Presence of HCV RNAb | % MDCsc | Groupd | |
|---|---|---|---|---|---|---|---|---|---|
| PBMCs | DCsf | ||||||||
| P06 | 42/M | 1a | 216,000 | None | CH | + | 5.02 | 0.33 | CP-D |
| P18 | 24/M | 1a | 284,000 | None | CH | + | 4.95 | 0.11 | NA |
| P19 | 39/M | 1a | 90,300 | None | CH | + | 4.97 | 0.10 | NA |
| P20 | 30/M | 1a | 1,400,000 | None | CH | + | 5.09 | 0.14 | CP-D |
| P23 | 52/F | 1 | 348,000 | Riba+pIFN-α | NR | + | 5.15 | 0.25 | CP-D |
| P24 | 50/M | 1 | 5,495,409 | Riba+pIFN-α | NR | − | <4.35* | NA | CP-N |
| P25 | 20/M | 1 | 1,148,154 | Riba+pIFN-α | NR | − | <4.35* | NA | CP-N |
| P26 | 47/M | 1 | 2,884,032 | Riba+pIFN-α | NR | + | NA | NA | CP-D |
| P27 | 25/M | 1 | 2,344,228 | Riba+pIFN-α | NR | NA | NA | NA | CP-N |
| P28 | 46/M | 1b | 4,940,000 | Riba+pIFN-α | NR | + | 4.42 | 0.56 | CP-N |
| P29 | 59/F | 1 | 2,880,000 | Riba+pIFN-α | NR | + | 5.30 | 0.23 | CP-D |
| P30 | 49/M | 1 | 809,000 | IFN-α | NR | + | 5.80 | 0.16 | CP-D |
| P31 | 49/M | 1 | ≤50 | None | SR | + | 4.26 | 0.19 | CP-N |
| P32 | 43/F | 1 | 3,690,000 | None | CH | + | 5.80 | 0.21 | CP-D |
| P33 | 33/F | 1 | 3,410,000 | None | CH | + | 4.53 | 0.17 | CP-N |
| P34 | 74/M | 1 | 282,000 | None | CH | + | NA | 0.32 | CP-N |
| P35 | 49/M | 1 | ≤50 | Riba+pIFN-α | SVR | − | <4.47* | 0.28 | CP-N |
| P37 | 48/M | 1 | NA | Riba+pIFN-α | NR | + (14/17 [82%]) | 4.91 | NA | CP-D |
Primary cell isolation, cell culture, and cell stimulation.
Peripheral blood was collected in heparin preparation tubes (Vacutainer; Becton Dickinson, Franklin, NJ). Fresh peripheral blood mononuclear cells (PBMCs) were separated by Ficoll-Paque density gradient centrifugation, and cultures were performed as described previously (15). In brief, PBMCs were seeded in 75-cm2 culture flasks at a concentration of 10 × 106 cells per ml, cultured at 37°C with 5% CO2, and used within 12 to 16 h of isolation for all stimulations and sorting experiments. PBMCs were stained with directly conjugated anti-CD19-fluorescein isothiocyanate (anti-CD19-FITC) (HIB19), anti-HLA-DR-peridinin chlorophyll protein (PerCP) (L243; MHC-II), anti-CD3-allophycocyanin (anti-CD3-APC) (UCHT1), and anti-CD14-APC-Cy7 (MφP9) cell surface antibodies from BD Pharmingen and then sorted. In cell sorting experiments, about 1 × 105 blood CD3− CD14− CD19− MHC-II+ DCs were sorted from 1 × 108 cells on a FACSAria flow cytometer, using DiVa v4.0 software (Beckton Dickinson, La Jolla, CA). Subpopulation purity was always above 99%. For cytokine production experiments, cells were stimulated for 6 h at 37°C in 96-well U-bottomed deep-well plates (1.25 × 106 cells per well) with either 0.1 μg/ml LPS (Escherichia coli O55:B5; Sigma-Aldrich) or 25 μg/ml poly(I:C) (Amerhsam Biosciences) (final concentrations), and the secretion inhibitor brefeldin A (10 μg/ml; Sigma-Aldrich) was added for intracellular cytokine detection in the last 5 h of stimulation. Control conditions included the absence of agonists (medium alone) to control for spontaneous, non-agonist-specific cytokine synthesis. In some experiments, healthy donor PBMCs were stimulated in the presence of SB202190 (10 μM) to impose a blockade of p38 mitogen-activated protein kinase activity (see Fig. 2).
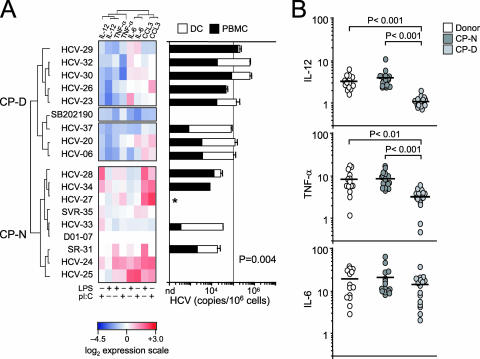
FIG. 2.
MDCs display impaired response to poly(I:C) and LPS in viremic patients. PBMCs were cultured for 1 h in the absence or presence of poly(I:C) or LPS before the addition of brefeldin A (10 μg/ml) for a further 5 h. Cells were recovered and stained for the indicated intracellular proteins. (A) (Left) Heat map indicating log2 changes in IL-6, IL-12, TNF-α, and CCL3 protein expression profiles for CD14− CD33+ MHC-IIbr MDCs from patients, treated with poly(I:C) or LPS for 6 h, versus protein expression in the same population from healthy donors. Expression signatures for 7 healthy blood donors (D01 to D07), 2 resolvers (SR-P31 and SVR-P35), and 14 viremic (HCV) patients were grouped according to similarity, using the complete linkage hierarchical clustering algorithm in MeV software (left margin). Ratios of the different fluorescent intracellular antibodies (columns) were determined by calculating log2(MFIstimulated/MFIunstimulated) and subtracting healthy donor sample means. Each square in the grid corresponds to a flow cytometry file containing approximately 5,000 CD14− CD33+ MHC-IIbr cell events. Protein expression changes relative to healthy donors are indicated by the color intensity scale (red, more expression than that of healthy donors; blue, less expression than that of healthy donors; and white, no change relative to healthy donors). SB202190 (10 μM for 30 min prior to stimulation) served as a positive control for down-regulation of the analyzed inflammatory mediators. (Right) Association between HCV RNA abundance in PBMCs (black bars), FACS-purified total DCs (CD3− CD19− CD14− MHC-II+; white bars), and cytokine expression profiles (CP). The asterisk indicates that HCV quantification was not done due to an unavailability of cell samples. The solid vertical line at 105 on the x axis represents a reference point. Statistical analysis was done by Fisher's two-tailed exact probability test on DCs (P = 0.004). (B) Groupwise comparisons between healthy donors and CP-N and CP-D subjects. IL-12, TNF-α, and IL-6 increases above baseline (_x_-fold) (MFIstimulated/MFIunstimulated) are shown for each cluster. Combined data for poly(I:C) and LPS responses and averages (solid horizontal lines) are shown. Statistical comparisons between groups were calculated by one-way ANOVA with Tukey's test.
Flow cytometry.
Surface and intracellular staining was done using directly conjugated antibodies and Cascade Yellow-streptavidin (Molecular Probes) according to standard techniques. The following antibodies were used: anti-CD3-phycoerythrin-Cy7 (anti-CD3-PE-Cy7) (SK7), anti-CD4-APC-Cy7 (RPAT4), anti-CD11c-APC (B-ly6), anti-CD14-FITC (M5E2), anti-CD14-PE (M5E2), anti-CD16-FITC (3G8), anti-CD19-PE-Cy7 (SJ25C1), anti-CD33-PerCP-Cy5.5 (P67.6), anti-CD123-PE-Cy5.5 (9F5), anti-HLA-DR-biotin (L243), anti-HLA-DR-APC-Cy7 (L243), anti-IL-6-PE (MQ2-6A3), anti-CCL3-PE (11A3), anti-IL-12 p40/70-APC (C11.5), anti-TNF-α-PE-Cy7 (MAb11) (all from BD Pharmingen), anti-CD62L-phycoerythrin Texas Red (ECD) (DREG56) (Immunotech), and anti-CD303-FITC (AC144) (Miltenyi Biotec). Fluorescence-activated cell sorting (FACS) data were acquired on an LSRII flow cytometer, using FACS DiVa v4.0 software. A minimum of 1 × 106 events in the live-cell gate, as defined by forward and side scatter, were accumulated for each sample, in duplicate. MDCs appear as a uniform cluster of CD33+ CD14− HLA-DR (MHC-II)br cells, allowing them to be distinguished from monocytes and lymphocytes. Responses to stimuli were considered positive if the frequency of IL-6 cytokine-producing MDCs was three or more times the frequency in medium alone. Cytokine clustering analysis was done as described previously (17) by inputting the log2 ratio of mean fluorescence intensity (MFI) values for intracellular FACS staining positive events (MFIstimulated/MFIunstimulated) into MeV array software (http://www.tigr.org/software/tm4/mev.html). For these clustering analyses, protein expression in the CD14− CD33+ MHC-IIbr MDC population from HCV patients treated with stimuli was presented as the log2 change versus protein expression in the MDC population from healthy donors.
HCV RNA quantification.
Primers specific for the 5′-untranslated region of HCV and the human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene (Table 2) were defined for the strain H77 and human sequences (GenBank accession numbers AF011751 and BC020308, respectively). The amplicon for HCV, defined by the HCV 5′/3′-out primers and amplified from the p90/HCV FL-long plasmid, was cloned into the pCR2.1 Topo vector (Invitrogen, Carlsbad, CA) together with the GAPDH amplification product (amplified from human lymphocyte RNA by using the GAPDH 3′/5′-out primer pair). This plasmid was used to generate standard curves for a real-time quantitative PCR-based assay. Quantification was performed with LightCycler hybridization probe technology (Roche Diagnostics Systems, Somerville, NJ). Cellular RNAs were extracted using RNeasy Mini columns (QIAGEN, Valencia, CA) per the manufacturer's instructions and were reverse transcribed for 30 min using Omniscript reverse transcriptase (QIAGEN). After reverse transcription (RT), PCR amplification was performed (5 min of initial denaturation at 95°C, followed by 45 s at 94°C, 60 s at 60°C, and 2 min at 72°C for 20 cycles) using the outer 3′/5′ primer pairs. The linearity of this first-round assay was demonstrated in duplicate experiments of up to 20 cycles, using a maximum of 1 μg of Huh7 clone 9-13 replicon RNA (unpublished data). These PCR conditions were used for all subsequent experiments. PCR conditions in the LightCycler experiments, performed with 1/10 of the initial PCR product, were as follows: 60 s of initial denaturation at 95°C, followed by 1 s at 95°C, 10 s at 60°C, and 15 s at 72°C for 40 cycles. Fluorescence measurements were performed at the end of the annealing steps. An HCV RNA replicon calibrator synthesized from plasmid pHCVrepAB12 with a T7-Megascript in vitro transcription kit (Ambion, Austin, TX) was used in all experimental runs. This highly sensitive nested quantitative RT-PCR (qRT-PCR) assay allows the detection of ≥100 HCV sense-strand copies per 106 cells. HCV was quantified in duplicate at the RT level for all studied samples. Healthy blood donors routinely tested in all experimental runs failed to give detectable signals for HCV RNA.
TABLE 2.
Oligonucleotides used for real-time RT-PCR quantification
| Primer or probe | Sequence (5′-3′)a |
|---|---|
| PCR primers | |
| HCV-out5 | CGTCTAGCCATGGCGTTAGTA |
| HCV-out3 | CGGTTGGTGTTTCTTTTGGTT |
| HCV-in5 | TCTGCGGAACCGGTGAGTA |
| HCV-in3 | CAAGCACCCTATCAGGCAGTA |
| GAPDH-out5 | GGTCGGAGTCAACGGATT |
| GAPDH-out3 | GCCATCACGCCACAGTT |
| GAPDH-in5 | TTCCATGGCACCGTCAA |
| GAPDH-in3 | GTCCTTCCACGATACCAAA |
| Strand-tag | GCTCATGGTGGCGAATAA |
| LightCycler hybridization probes | |
| HCV-P1 | ATTTGGGCGTGCCCCCGCAAGAf |
| HCV-P2 | XCTAGCCGAGTAGTGTTGGGTCGCGAAAGGCp |
| GAPDH-P1 | GCTCCTGGAAGATGGTGATGGGATTf |
| GAPDH-P2 | XCCATTGATGACAAGCTTCCCGTTCTCp |
Statistics.
Statistical analysis was performed using the Vassar website (http://faculty.vassar.edu/lowry/VassarStats.html) and the GraphPad Prism v3.0 statistical software package. All tests were two-tailed, and P values of <0.05 were considered significant.
RESULTS
Circulating total DCs from viremic patients contain genomic viral RNAs.
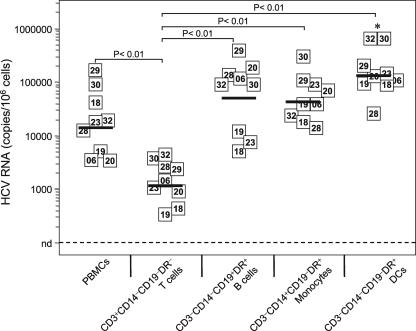
In trying to relate virus-induced loss of function with anomalies in innate sensing pathways of peripheral DCs, we first explored the nature of the immune subsets that interact with HCV and act as potential targets for this virus in vivo. PBMCs with measurable HCV RNA from viremic patients were sorted into T cells, B cells, monocytes, and total DCs by four-color flow cytometry (n = 9) and subjected to a real-time qRT-PCR assay (≥100 copies/106 cells) that allowed for accurate quantification of sense-strand HCV RNA molecules in peripheral leukocytes from infected patients (Fig. 1). Quantification of cell-associated viral RNA loads in sorted leukocytes confirmed that HCV genomic RNA is detectable within CD19+ B lymphocytes (mean log10 value, 4.7 per 106 cells; range, 3.6 to 5.5). We found a significantly lower HCV RNA level in peripheral CD3+ T cells than in all other peripheral subpopulations analyzed (one-way analysis of variance [ANOVA]; P < 0.0001), supporting the notion that T cells do not constitute a preferential viral interaction partner in vivo. Detection of viral RNA in peripheral DCs was consistent with other findings showing that this cellular compartment unambiguously interacts with HCV (12, 42, 43). Surprisingly, however, CD3− CD14− CD19− MHC-II+ total DCs had high cell-associated viral loads (mean log10 value, 5.2 per 106 cells; range, 4.4 to 5.8). In contrast to the case in a previous study (5), CD14+ monocytes were unusually positive for HCV RNA (mean log10 value, 4.6 per 106 cells; range, 4.1 to 5.4), with levels very similar to those of B cells but significantly lower than those of total DCs in a pairwise comparison (Student's correlated t test; P = 0.005). No correlation existed between the serum viral load (ranging from 4.9 to 6.7 log10 IU ml−1) (Table 1) and HCV genomic RNA level per cell in FACS-examined total DCs or monocytes (data not shown), arguing against passive plasma viral particle adsorption into mononuclear blood phagocytes as the source of the detected viral RNA. Consequently, we have used the quantitative detection of genomic HCV RNA as a reliable surrogate marker of a specific interaction between the virus and DCs, not as one of actual infection, in the following experiments. The consistent presence of genomic HCV RNA and its high content within total DCs indicate that these cells are targets of HCV in the periphery, with the potential to constitute an immunologically relevant host if this interaction was associated with dampened activation of the innate immune response in viremic patients.
FIG. 1.
HCV RNA contents in circulating immune blood cell subpopulations from viremic patients. HCV sense-strand RNA copy numbers in FACS-purified blood T cells (CD3+), B cells (CD19+), monocytes (CD14+), and total DCs (CD3− CD14− CD19− HLA-DR+) from nine viremic patients were quantified by LightCycler hybridization probe technology. The data are the averaged values for replicates from two independent measurements expressed as absolute numbers of HCV RNA molecules per 106 cells. Each square indicates the detection of viral RNA from a patient, with the corresponding identification number inside the square. The solid horizontal lines represent the average for each group. Differences between T cells and other subsets, as calculated by one-way ANOVA with Tukey's test, are shown above the graph. *, P < 0.01 for DCs versus PBMCs.
MDCs display impaired expression of IL-12 and TNF-α, but not IL-6 or CCL3, in response to poly(I:C) and LPS in viremic patients.
Next, we tested the hypothesis that the association of HCV with DCs in vivo would lead these cells to react to poly(I:C) and LPS in an inappropriate or sensitized manner in HCV-viremic patients and would be related to disease progression. PBMC samples from 14 HCV-viremic and 2 aviremic clinical resolvers (n = 16) were used to survey, by intracellular flow cytometry, the innate sensing potentials of MDCs and monocytes by exposing cells to stimuli and measuring downstream cytokine signatures at the single-cell level. We computed FACS cytokine expression levels [log2(MFIstimulated/MFIunstimulated)] and built a heat map indicating the log2 changes in IL-6, IL-12, TNF-α, and CCL3 protein expression profiles for CD14− CD33+ MHC-IIbr patient MDCs treated with poly(I:C) or LPS versus protein expression in the MDC population from age-matched healthy donors (n = 7) (Fig. 2A). Each square in the grid represents, in a color gradient, the expression of one intracellular marker (as measured by the MFI) in response to one stimulus for each patient. Surprisingly, we observed differences in the strengths of the MDC responses between viremic patients after stimulation. As an example, although patients HCV-P28 and HCV-P29 are both viremic nonresponders (Table 1), their MDCs exhibited very different cytokine induction patterns following poly(I:C) and LPS stimulation (Fig. 2A). To understand this dichotomy in responses between viremic patients, we grouped them with unsupervised hierarchical clustering algorithms used in microarray gene expression analysis (as described in Materials and Methods). This approach enabled the investigation of whether the patients with response anomalies showed functional similarities to each other and differed from those with normal responses. By using a cluster analysis, we identified two main groups of viremic patients with key differences in their poly(I:C)- and LPS-induced cytokine expression profiles (Fig. 2A). Each was termed a distinct cytokine profile (CP) cluster based on the primary nature of the response potential of the cluster. CP-defective (CP-D) patients (8 of 16 patients) displayed less cytokine (IL-12 and TNF-α) expression in response to agonist stimulation than did healthy donors. CP-normal (CP-N) patients (8 of 16 patients) displayed a response potential similar to that of healthy donors. Both IL-12 and TNF-α expression was significantly decreased in CP-D patients compared to that in age-matched healthy donors and CP-N patients (Fig. 2B), indicating that the expression of these two cytokines is jointly affected in viremic patients. Interestingly, IL-6 and chemokine CCL3 expression levels were not observed to differ in patient subgroups relative to healthy donors and each other (Fig. 2A and B). Notably, aviremic subjects SR-P31 and SVR-P35 exhibited normal cytokine expression and clustered within the CP-N group.
Attenuation of innate sensing in MDCs is associated with high HCV RNA abundance in total DCs.
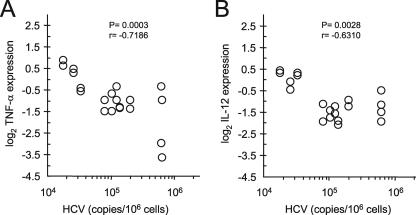
The picture that emerges from the unsupervised cluster analysis reveals a role for a virus-dependent loss of function as the cause of the innate sensing dysfunction in MDCs. This is apparent in that 6/9 patients (HCV-P24, -P25, -P33, and -P34, SR-P31, and SVR-P35) with low levels of HCV RNA in PBMCs (<104 copies) showed normal poly(I:C)- and LPS-potentiated expression profiles, in contrast to 5/6 patients (HCV-P23, -P26, -P29, -P30, and -P32) for whom poor responses were associated with high cell-associated HCV RNA levels in PBMCs (≥104 copies) and a CP-D clustering (Fig. 2A, black bars). Similarly, CP-D patients had high levels of HCV RNA in total DCs (≥105 copies; 6/7 patients) compared to CP-N patients (<105 copies; 6/6 patients) (Fig. 2A, white bars, and Table 1). These results demonstrate that in most cases, inhibition of cytokine expression is linked to the level of viral RNA in carrier cells. In fact, Fisher's exact probability test indicated that attenuation of poly(I:C)- and LPS-induced cytokine up-regulation was not independent of the level of HCV RNA copy abundance in total DCs (P = 0.004) (Fig. 2A, right panel). Thus, for every CP-D subject, whether naive or a nonresponder, DCs had a 3 to 23 times (mean, 10-fold) higher cell-associated virus content than that in CP-N patients. Furthermore, the abundance of viral RNA quantified in total FACS-examined DCs negatively correlated with poly(I:C)- and LPS-mediated induction of IL-12 and TNF-α in MDCs (P = 0.0028 and P = 0.0003, respectively) (Fig. 3). Finally, since CP-D patients did not have higher levels of viremia than CP-N patients (mean log10 value, 6.1 ± 0.5 and 6.3 ± 0.5, respectively; P = 0.37 by Student's t test) (Table 1), it is unlikely that this defect was due to nonspecific plasma viral particle adsorption. Thus, our findings support the idea that emergence of defective MDCs in the periphery is a result of virus-induced dysfunctions rather than the sole consequence of exposure of MDCs to chronic viral disease, as proposed previously (35). They also demonstrate that the cell-associated HCV RNA load in DCs is predictive of TLR responsiveness independent of viremia: DCs containing increasing amounts of HCV RNA are more functionally exhausted in that they are less able to respond to danger stimuli ex vivo. Altogether, these data provide evidence that HCV can specifically attenuate danger signal activation of innate immune functions of blood MDCs in viremic patients.
FIG. 3.
Poly(I:C) and LPS responsiveness in MDCs is associated with HCV RNA levels in total DCs. Dot plots show TNF-α (A) and IL-12 (B) responses (log2) as a function of HCV RNA copy numbers in FACS-purified DCs. Data points (two per patient) represent the mean levels of cytokine expression as log2 changes versus those of the donors for two independent measurements. A negative correlation was observed for HCV-infected patients between HCV RNA and IL-12 or TNF-α expression (n = 10). Correlation statistics were analyzed using the Spearman rank correlation test.
Selective attenuation of poly(I:C) and LPS innate sensing in a subpopulation of MDCs in viremic patients.
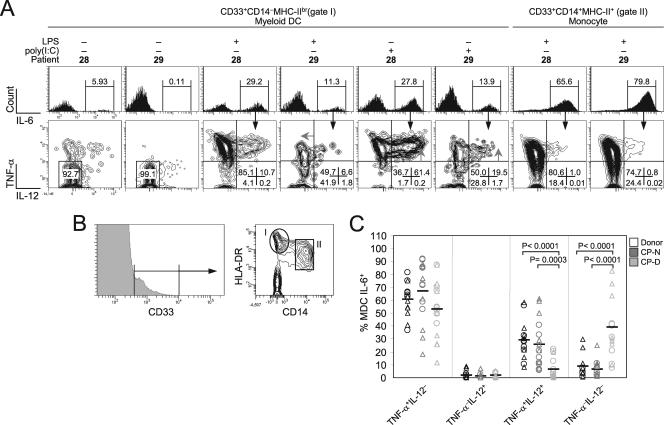
The higher levels of HCV RNA in CP-D patients could be due to a large number of copies in a small number of cells or to a small number of copies in nearly all DCs (of which ∼50% are MDCs). To reconcile the apparent low HCV RNA copy number per DC with the functional defects observed in MDCs (which until now were shown as an absolute level of cytokine expression for the whole MDC population), we analyzed the patterns of IL-12 and TNF-α production at the single-cell level to demonstrate that only a small proportion of the MDC population from these patients is nonfunctional for cytokine production. All patients analyzed consistently displayed approximately equivalent levels of IL-6 expression per cell (Fig. 2B, bottom panel), demonstrating that induction of IL-6 was not inhibited by HCV. In conjunction with the finding that essentially all TNF-α+ IL-12−, TNF-α− IL-12+, and TNF-α+ IL-12+ MDCs expressed IL-6 in healthy donors (data not shown), these observations indicated that IL-6 acts as a key molecular marker of cells activated to produce cytokines (TNF-α and IL-12), making this a traceable subset in defective patients, and that such an analysis should not lead to missed HCV-caused MDC dysfunctions.
Direct ex vivo analysis of CD14− CD33+ MHC-IIbr IL-6+ MDC responsiveness to poly(I:C) and LPS revealed striking differences at the single-cell level for the patients tested that confirmed the MFI results shown in Fig. 2. Figure 4 illustrates these different patterns in two-dimensional contour plots of the expression of IL-12 and TNF-α for two representative patients after gating on IL-6+ MDCs (∼30% of MDCs). Similar to the case for healthy donors, poly(I:C) and LPS stimulated both high IL-12 and TNF-α expression in MDCs from patient HCV-P28 (CP-N), and high dual production took place simultaneously in individual cells (Fig. 4A, bottom row). Conversely, patient HCV-P29 (CP-D) demonstrated a loss of cytokine-secreting effectors with both agonists (13.5-fold more TNF-α− IL-12− cells than those of patient HCV-P28). This resulted in a lower expression output at the single-cell level, as indicated by the diminished fluorescence intensity shift in both intracellular cytokines (Fig. 4A, gray arrows). This observation applied to the majority of the patients: CP-D patients exhibited sixfold higher frequencies of TNF-α− IL-12− cells than did CP-N patients (38.8% versus 6.5%; P < 0.0001 by Mann-Whitney U test) (Fig. 4C). This increase in doubly negative effectors paralleled a significant decrease in TNF-α+ IL-12+ cell frequency (fourfold less) in the CP-D subgroup compared to that for CP-N patients (mean, 6.3% versus 25.7%; P = 0.0003) (Fig. 4C), indicating that a subpopulation of cytokine-producing MDCs (∼6% of MDCs) in the CP-D subgroup of patients expressed abnormal levels of TNF-α and IL-12 following poly(I:C) or LPS stimulation. In contrast, LPS-activated CD14+ CD33+ MHC-II+ IL-6+ monocytes had essentially identical expression of TNF-α and IL-12 on a per-cell basis for all patients analyzed (Fig. 4A, rightmost panels, and data not shown), challenging the idea that nonspecific interactions with HCV or TLR-mediated chronic stimulation in vivo (7) is the underlying cause for the loss of function in the responsiveness of the MDCs. Thus, defective production of cytokines at the single-cell level is confined to a small population of responding MDCs (∼3% of total DCs, defined as IL-6+), appears to be cell type specific, and is a selective process restricted among viremic patients (Fig. 2). Furthermore, this analysis highlights that limited functionality is observed in only 6% of MDCs and is not a generalized characteristic of all MDCs in affected viremic patients.
FIG. 4.
Selective attenuation of poly(I:C) and LPS innate sensing in IL-6-expressing MDCs of CP-D viremic patients. (A) Flow cytometry plots of IL-6 (x axis [top row]), TNF-α, and IL-12 (y and x axes, respectively [bottom row]) expression following stimulation in two representative patient samples (of 16), namely, HCV-P28 and HCV-P29. Histograms are gated on total MDCs (gate I) or monocytes (gate II), as shown in panel B. Arrowheads in dot plots indicate a small population of bright cells that was absent from P29. Numbers in bottom right corners and above bracketed lines indicate the percentages of cells in the designated areas. (B) CD14− CD33+ MHC-IIbr (gate I) and CD14+ CD33+ MHC-II+ (gate II) populations used for FACS analysis in the experiments shown in panel A. (C) Frequencies of IL-6+ MDCs positive for TNF-α and IL-12 for all patients analyzed by FACS after poly(I:C) (triangles) and LPS (circles) activation. Groupwise comparisons between healthy donors (n = 7) and CP-N (n = 8) and CP-D (n = 8) subjects were determined with the Mann-Whitney rank sum test and are shown at the top of the graph.
Variability in MDC frequency does not account for loss of function of poly(I:C) and LPS innate sensing in the IL-6+ subset.
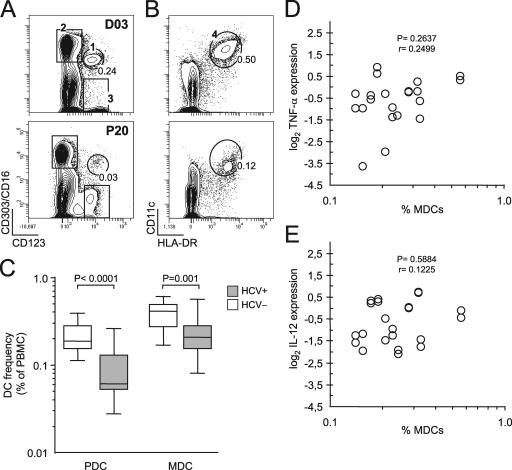
As mentioned above, a subpopulation of activated MDCs (IL-6+) from viremic patients responded less strongly to the agonists tested, expressing smaller amounts of TNF-α and IL-12 on a per-cell basis. A close look at Fig. 4A (top row) shows that patient HCV-P29 had a reduced frequency of IL-6-positive cells (half that of patient P28), hinting at the involvement of population heterogeneity (i.e., disappearance of circulating MDCs) (Table 1) as a further cause of the differential response outcome. The absence of a small population of CD14− CD33+ MHC-IIbr IL-6+ cells with the brightest intensities of TNF-α and IL-12 staining might be accounted for by the numerical disappearance of the cytokine-producing cells from the periphery rather than their inability to synthesize normal levels at the single-cell level (intrinsic defect). To address this issue, we enumerated DC subsets in blood, using 10 immunophenotypic surface markers simultaneously with eight-color flow cytometry (Fig. 5). Consistent with other reports (19, 26), we found that HCV-infected patients had 1.7-fold lower frequencies of circulating MDCs than did healthy donors (mean, 0.24% ± 0.03% and 0.40% ± 0.03%, respectively; P = 0.001 by Mann-Whitney U test) (Fig. 5C). Lower frequencies (2.4-fold) of plasmacytoid DCs (PDCs) also characterized the same patients (mean, 0.09% ± 0.01% and 0.22% ± 0.02% for patients and healthy controls, respectively; P < 0.0001) (Fig. 5C). These results demonstrate that frequencies of circulating DC subsets are decreased during HCV infection. However, an absence of relationship was observed between MDC frequencies and cytokine production at the single-cell level in response to either agonist (Fig. 5D and E). The absence of correlation reflects the fact that some patients with relatively high MDC counts in blood had weak poly(I:C) and LPS responses, as in the case of HCV-P06 (0.33%), whereas some patients with normal functions had some of the lowest MDC frequencies (e.g., patient P33, with a frequency of 0.17%) (see Table 1 and Fig. 2 for functional profiles). Thus, the patients with the lowest frequencies were not necessarily the patients with defects in cytokine expression, and consequently, we can eliminate the variability arising from intersubject differences in MDC subset frequencies as an alternative explanation, or even a contributing factor, to virus-dependent single-cell intrinsic defects. This finding further supports the concept that virus-induced interference with innate functions in vivo affects the capacity of a small population of blood MDCs of viremic patients to adequately respond to activating danger signals.
FIG. 5.
Influence of HCV on peripheral blood DC subset frequencies. Freshly isolated uncultured PBMCs (day 0) were surface stained for eight-color multiparametric flow cytometry. (A and B) Flow cytometry dot plots representative of circulating frequencies of CD3− CD19− gated PDCs (A) and MDCs (B) are shown for healthy volunteers (D03 [top]) and HCV-infected patients (P20 [bottom]). Gate 1 corresponds to CD303+ CD123br CD4hi CD11c− CD14− CD62L+ HLA-DR+ PDCs, and gate 4 corresponds to CD11c+ CD14− CD16− CD4+ CD123dim CD62L+ HLA-DRbr MDCs. In gate 3, CD123+ CD303− cells, which correspond to basophilic granulocytes, did not stain for HLA-DR, and gate 2 shows CD303− CD16+ NK lymphocytes and monocytes. For each DC gate, the fraction of cells relative to the total number of PBMCs is indicated as a percentage. (C) Box-and-whisker-plot representation of circulating frequencies of CD303+ PDCs and CD11c+ MDCs in HCV-infected individuals (gray boxes; n = 13) and healthy age-matched controls (white boxes; n = 7), measured as percentages of total PBMCs collected after Ficoll separation. The ends of the boxes define the 25th and 75th percentiles, a horizontal line indicates the median, and bars define the 5th and 95th percentiles. P values were determined with the Mann-Whitney rank sum test and are shown at the top of the graph (HCV+ versus HCV−). (D and E) There is no correlation between TNF-α and IL-12 cytokine production potentials and MDC frequencies in HCV-infected patients. Correlation statistics were analyzed using the Spearman rank correlation test.
DISCUSSION
There is a lack of consensus behind the concept of virus-induced impairment of DC function during chronic HCV infection. In previous work, monocytes were isolated from HCV-infected individuals and differentiated into DCs during a 1-week culture period with high doses of granulocyte-macrophage colony-stimulating factor and IL-4, which likely enhanced antigen-presenting cell capacity and overcame any immunologic defect in DCs present in vivo. In contrast, we have mapped danger signal inhibition in the CD14− CD33+ MHC-IIbr MDC blood subset within individual patient samples by clustering patients based on poly(I:C)- and LPS-mediated cytokine up-regulation outcomes. By comparing two similarly viremic groups with distinct functional outcomes, we discovered that responses to stimuli were significantly linked to the abundance of HCV genomic RNA in FACS-purified total DCs for a subgroup of patients (cluster CP-D) and did not correlate with either viremia or deficits in circulating MDC frequencies for HCV-infected patients. The results presented herein confirm the presence of impaired MDCs in some viremic subjects (2, 4, 29) and demonstrate that overall frequencies of nonfunctional cytokine-producing MDCs are considerably small (<3% of overall DCs in blood), indicating the absence of global defects in innate recognition of danger signals by DCs. The activities measured here are more likely to reflect the complex interactions that occur in vivo. For example, most studies have shown that it is possible to engineer phenotypically and functionally intact monocyte precursor-derived DCs from chronically infected individuals (24, 26, 32), and in keeping with this, we found that monocytes from these patients were functionally intact.
Collectively, these data indicate that detection of HCV genomic RNA in DCs and loss of function in the danger signal responsiveness of a small population of MDCs in vivo are interrelated rather than independent phenomena. Clearance of cell-associated viral RNA from MDCs of HCV-infected patients after 4 weeks of ribavirin/IFN-α antiviral therapy was reported to restore their CD4+ T-cell activation potential (43). Due to practical limitations, HCV qRT-PCR analysis could be performed exclusively with purified bulk DCs, not MDCs, in our study. As a consequence, the dynamics of HCV copy numbers in blood DCs and the interplay with functional impairment of pathogen recognition receptor innate sensing responses are highly tempered because of the presence of a large (and variable) fraction of cells not expected to be defective (27) or harboring HCV RNA (20). Furthermore, the fraction of impaired MDCs (defined as IL-6+ TNF-α− IL-12−) accounts for <3% of total DCs in blood; this could explain the apparent low HCV RNA copy number per DC. Thus, even if it is impossible to pinpoint the exact levels of HCV RNA in MDCs in the current study, we estimate that there are between 3 and 20 HCV RNA molecules per cell, assuming segregation of HCV to MDCs nonfunctional for cytokine production (30 of 1,000 overall DCs in blood are defective). Clearly, a continued understanding of HCV-DC dynamics in viremic patients should allow for important insight into how DCs harboring increasing amounts of HCV RNA become functionally exhausted.
Although the molecular basis by which MDCs become unresponsive to danger stimuli in HCV infection is currently unclear, attenuation of the DC cytokine synthesis potential may be considered in the context of previous literature demonstrating that HCV is remarkably proficient at antagonizing poly(I:C)-activated signaling through direct inactivation of events proximal to the TLR3 pathway (reviewed in reference 10). TRIF, a signal bridging adapter for both TLR3 and TLR4, is a known proteolytic substrate of HCV NS3/4A protease (25), and _Trif_−/− DCs (16, 22, 44) show defective production of both IL-12 and TNF-α, but not IL-6, in response to poly(I:C) and LPS (TLR4). Considering that a 1,000- to 10,000-fold excess of viral proteins relative to positive-strand RNA is observed with full-length HCV replicon cell clones (34), it is tempting to speculate that even the presence of a few viral genomes per DC may be sufficient to produce concentrations of viral proteins required to effectively interfere with innate defense functions at the single-cell level without the need for productive infection (replication). The HCV RNA density-dependent defect in cytokine production (Fig. 2 and 3) represents an empirical basis consistent with such a hypothesis. However, since we have no evidence in this study for infection and/or NS3/4A expression in DCs, the possibility that chronic infection in some individuals induces an impaired DC response independent of direct virus-DC interaction cannot be excluded. Nonetheless, the ability of NS3/4A to interfere with TRIF-coupled danger signal processing provides a plausible mechanism by which HCV could exert its negative effect on poly(I:C)- and LPS-activated cytokine production in DCs. Whether such interference indeed occurs has yet to be established.
Recent studies have shown that HCV-like particles pseudotyped with functional E1/E2 envelope proteins bind to DCs through C-type lectins (20, 28, 33). Evidence for selective inhibition of LPS-induced IL-12 and TNF-α production by cross-linking of C-type lectin receptors expressed on MDCs has been provided for CD206 (mannose receptor) (30), CD209 (DC-SIGN) (11), and DCAL-2 (8). Hence, in the absence of direct infection, cross-linking of C-type lectins by HCV glycoproteins as a result of a quantitative virion binding effect at the cell surface could lead to inactivation of TLR3 and TLR4 innate sensing functions of MDCs in chronic infection. Genetic distinctions in HCV variants between viremic patients are also likely to impart differential levels of control and activation of these TLR-dependent pathways in DCs to evade the host's innate response to infection (10). In this context, one of the reasons that defects in DC function extending into the chronic phase of infection are not always clinically evident may be due to the lack of an effective immune response exerting selective pressure for change in the genetic makeup of HCV quasispecies. This would explain the observation that danger responsiveness is not perturbed in MDCs of CP-N patients with persistent infection. Identification at the molecular level of the mechanisms that lead to HCV-induced DC dysfunction will help to resolve the relative contributions of these evasion strategies in reducing innate activation potential as well as to determine whether the variations among different subjects are related to viral pathogenicity.
In any event, virus-induced defects in MDC innate sensing may represent an evolutionary adaptation that favors (with other factors) persistent infection in an otherwise immunocompetent host and prolonged host-parasite coexistence. Most circulating MDCs are unlikely to present HCV antigens; any MDCs that present HCV antigens will most likely have been in contact with pathogen components and therefore will have been particularly susceptible to an attack on innate immune sensing mechanisms essential for their effective antigen presentation to T cells (31, 39). This cell-specific and numerically restrained MDC defect offers a promising field of investigation with which to study and understand the HCV-restricted nature of the deficit in cellular immunity (38). Our observations lend empirical support to a currently working model in which virus-induced impairment of DC-mediated innate immunity would participate in the failure of HCV-specific CD4+ T-cell help (6); in this model, maintenance of help would be contingent on proper processing and delivery of danger signals by DCs presenting HCV antigens.
Acknowledgments
This work was supported by Canadian Institute of Health Research grant MOP-79546 (D.L.), a Université de Montréal Novartis/Canadian Liver Foundation Hepatology Research chairmanship (D.L.), an INSERM U743 doctoral scholarship (I.G.R.-G.), and a Canadian Liver Foundation doctoral scholarship (L.J.).
We thank C. Rice for the p90/HCV FL-long plasmid, C. Richardson for the pHCVrepAB12 plasmid, R. Bartenschlager for providing replicons, L. Montcalm for blood sample collection, D. Murphy for viremia quantification, and A. Lamarre and N. Shoukry for critical reviews of the manuscript. We are indebted to the patients for participating in the study and to P. Melançon, H. Rigsby, M. Lainesse, S. Reis, and J. Reis for technical assistance.
Footnotes
▿
Published ahead of print on 21 March 2007.
REFERENCES
- 1.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413**:**732-738. [DOI] [PubMed] [Google Scholar]
- 2.Anthony, D. D., N. L. Yonkers, A. B. Post, R. Asaad, F. P. Heinzel, M. M. Lederman, P. V. Lehmann, and H. Valdez. 2004. Selective impairments in dendritic cell-associated function distinguish hepatitis C virus and HIV infection. J. Immunol. 172**:**4907-4916. [DOI] [PubMed] [Google Scholar]
- 3.Auffermann-Gretzinger, S., E. B. Keeffe, and S. Levy. 2001. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood 97**:**3171-3176. [DOI] [PubMed] [Google Scholar]
- 4.Averill, L., W. M. Lee, and N. J. Karandikar. 2007. Differential dysfunction in dendritic cell subsets during chronic HCV infection. Clin. Immunol. 123**:**40-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boisvert, J., X. S. He, R. Cheung, E. B. Keeffe, T. Wright, and H. B. Greenberg. 2001. Quantitative analysis of hepatitis C virus in peripheral blood and liver: replication detected only in liver. J. Infect. Dis. 184**:**827-835. [DOI] [PubMed] [Google Scholar]
- 6.Bowen, D. G., and C. M. Walker. 2005. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature 436**:**946-952. [DOI] [PubMed] [Google Scholar]
- 7.Brenchley, J. M., D. A. Price, T. W. Schacker, T. E. Asher, G. Silvestri, S. Rao, Z. Kazzaz, E. Bornstein, O. Lambotte, D. Altmann, B. R. Blazar, B. Rodriguez, L. Teixeira-Johnson, A. Landay, J. N. Martin, F. M. Hecht, L. J. Picker, M. M. Lederman, S. G. Deeks, and D. C. Douek. 2006. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 12**:**1365-1371. [DOI] [PubMed] [Google Scholar]
- 8.Chen, C. H., H. Floyd, N. E. Olson, D. Magaletti, C. Li, K. Draves, and E. A. Clark. 2006. Dendritic-cell-associated C-type lectin 2 (DCAL-2) alters dendritic-cell maturation and cytokine production. Blood 107**:**1459-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foy, E., K. Li, C. Wang, R. Sumpter, M. Ikeda, S. M. Lemon, and M. Gale. 2003. Regulation of interferon regulatory factor-3 by the hepatitis C virus serine protease. Science 300**:**1145-1148. [DOI] [PubMed] [Google Scholar]
- 10.Gale, M., and E. M. Foy. 2005. Evasion of intracellular host defence by hepatitis C virus. Nature 436**:**939-945. [DOI] [PubMed] [Google Scholar]
- 11.Geijtenbeek, T. B., S. J. Van Vliet, E. A. Koppel, M. Sanchez-Hernandez, C. M. Vandenbroucke-Grauls, B. Appelmelk, and Y. Van Kooyk. 2003. Mycobacteria target DC-SIGN to suppress dendritic cell function. J. Exp. Med. 197**:**7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goutagny, N., A. Fatmi, V. De Ledinghen, F. Penin, P. Couzigou, G. Inchauspe, and C. Bain. 2003. Evidence of viral replication in circulating dendritic cells during hepatitis C virus infection. J. Infect. Dis. 187**:**1951-1958. [DOI] [PubMed] [Google Scholar]
- 13.Grakoui, A., N. H. Shoukry, D. J. Woollard, J. H. Han, H. L. Hanson, J. Ghrayeb, K. K. Murthy, C. M. Rice, and C. M. Walker. 2003. HCV persistence and immune evasion in the absence of memory T cell help. Science 302**:**659-662. [DOI] [PubMed] [Google Scholar]
- 14.Heil, F., H. Hemmi, H. Hochrein, F. Ampenberger, C. Kirschning, S. Akira, G. Lipford, H. Wagner, and S. Bauer. 2004. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science 303**:**1526-1529. [DOI] [PubMed] [Google Scholar]
- 15.Ho, C. S., D. Munster, C. M. Pyke, D. N. Hart, and J. A. Lopez. 2002. Spontaneous generation and survival of blood dendritic cells in mononuclear cell culture without exogenous cytokines. Blood 99**:**2897-2904. [DOI] [PubMed] [Google Scholar]
- 16.Hoebe, K., X. Du, P. Georgel, E. Janssen, K. Tabeta, S. O. Kim, J. Goode, P. Lin, N. Mann, S. Mudd, K. Crozat, S. Sovath, J. Han, and B. Beutler. 2003. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature 424**:**743-748. [DOI] [PubMed] [Google Scholar]
- 17.Irish, J. M., R. Hovland, P. O. Krutzik, O. D. Perez, O. Bruserud, B. T. Gjertsen, and G. P. Nolan. 2004. Single cell profiling of potentiated phospho-protein networks in cancer cells. Cell 118**:**217-228. [DOI] [PubMed] [Google Scholar]
- 18.Iwasaki, A., and R. Medzhitov. 2004. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5**:**987-995. [DOI] [PubMed] [Google Scholar]
- 19.Jung, S., D. Unutmaz, P. Wong, G. Sano, K. De los Santos, T. Sparwasser, S. Wu, S. Vuthoori, K. Ko, F. Zavala, E. G. Pamer, D. R. Littman, and R. A. Lang. 2002. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity 17**:**211-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaimori, A., T. Kanto, C. Kwang Limn, Y. Komoda, C. Oki, M. Inoue, H. Miyatake, I. Itose, M. Sakakibara, T. Yakushijin, T. Takehara, Y. Matsuura, and N. Hayashi. 2004. Pseudotype hepatitis C virus enters immature myeloid dendritic cells through the interaction with lectin. Virology 324**:**74-83. [DOI] [PubMed] [Google Scholar]
- 21.Kanto, T., M. Inoue, H. Miyatake, A. Sato, M. Sakakibara, T. Yakushijin, C. Oki, I. Itose, N. Hiramatsu, T. Takehara, A. Kasahara, and N. Hayashi. 2004. Reduced numbers and impaired ability of myeloid and plasmacytoid dendritic cells to polarize T helper cells in chronic hepatitis C virus infection. J. Infect. Dis. 190**:**1919-1926. [DOI] [PubMed] [Google Scholar]
- 22.Kato, H., O. Takeuchi, S. Sato, M. Yoneyama, M. Yamamoto, K. Matsui, S. Uematsu, A. Jung, T. Kawai, K. J. Ishii, O. Yamaguchi, K. Otsu, T. Tsujimura, C. S. Koh, C. Reis e Sousa, Y. Matsuura, T. Fujita, and S. Akira. 2006. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441**:**101-105. [DOI] [PubMed] [Google Scholar]
- 23.Kawai, T., and S. Akira. 2006. TLR signaling. Cell Death Differ. 13**:**816-825. [DOI] [PubMed] [Google Scholar]
- 24.Larsson, M., E. Babcock, A. Grakoui, N. Shoukry, G. Lauer, C. Rice, C. Walker, and N. Bhardwaj. 2004. Lack of phenotypic and functional impairment in dendritic cells from chimpanzees chronically infected with hepatitis C virus. J. Virol. 78**:**6151-6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li, K., E. Foy, J. C. Ferreon, M. Nakamura, A. C. Ferreon, M. Ikeda, S. C. Ray, M. Gale, and S. M. Lemon. 2005. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc. Natl. Acad. Sci. USA 102**:**2992-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Longman, R. S., A. H. Talal, I. M. Jacobson, M. L. Albert, and C. M. Rice. 2004. Presence of functional dendritic cells in patients chronically infected with hepatitis C virus. Blood 103**:**1026-1029. [DOI] [PubMed] [Google Scholar]
- 27.Longman, R. S., A. H. Talal, I. M. Jacobson, C. M. Rice, and M. L. Albert. 2005. Normal functional capacity in circulating myeloid and plasmacytoid dendritic cells in patients with chronic hepatitis C. J. Infect. Dis. 192**:**497-503. [DOI] [PubMed] [Google Scholar]
- 28.Ludwig, I. S., A. N. Lekkerkerker, E. Depla, F. Bosman, R. J. Musters, S. Depraetere, Y. van Kooyk, and T. B. Geijtenbeek. 2004. Hepatitis C virus targets DC-SIGN and L-SIGN to escape lysosomal degradation. J. Virol. 78**:**8322-8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murakami, H., S. M. Akbar, H. Matsui, N. Horiike, and M. Onji. 2004. Decreased interferon-alpha production and impaired T helper 1 polarization by dendritic cells from patients with chronic hepatitis C. Clin. Exp. Immunol. 137**:**559-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nigou, J., C. Zelle-Rieser, M. Gilleron, M. Thurnher, and G. Puzo. 2001. Mannosylated lipoarabinomannans inhibit IL-12 production by human dendritic cells: evidence for a negative signal delivered through the mannose receptor. J. Immunol. 166**:**7477-7485. [DOI] [PubMed] [Google Scholar]
- 31.Pasare, C., and R. Medzhitov. 2004. Toll-dependent control mechanisms of CD4 T cell activation. Immunity 21**:**733-741. [DOI] [PubMed] [Google Scholar]
- 32.Piccioli, D., S. Tavarini, S. Nuti, P. Colombatto, M. Brunetto, F. Bonino, P. Ciccorossi, F. Zorat, G. Pozzato, C. Comar, S. Abrignani, and A. Wack. 2005. Comparable functions of plasmacytoid and monocyte-derived dendritic cells in chronic hepatitis C patients and healthy donors. J. Hepatol. 42**:**61-67. [DOI] [PubMed] [Google Scholar]
- 33.Pohlmann, S., J. Zhang, F. Baribaud, Z. Chen, G. J. Leslie, G. Lin, A. Granelli-Piperno, R. W. Doms, C. M. Rice, and J. A. McKeating. 2003. Hepatitis C virus glycoproteins interact with DC-SIGN and DC-SIGNR. J. Virol. 77**:**4070-4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quinkert, D., R. Bartenschlager, and V. Lohmann. 2005. Quantitative analysis of the hepatitis C virus replication complex. J. Virol. 79**:**13594-13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rollier, C., E. Depla, J. A. Drexhage, E. J. Verschoor, B. E. Verstrepen, A. Fatmi, C. Brinster, A. Fournillier, J. A. Whelan, M. Whelan, D. Jacobs, G. Maertens, G. Inchauspe, and J. L. Heeney. 2003. Chronic hepatitis C virus infection established and maintained in chimpanzees independent of dendritic cell impairment. Hepatology 38**:**851-858. [DOI] [PubMed] [Google Scholar]
- 36.Schnare, M., G. M. Barton, A. C. Holt, K. Takeda, S. Akira, and R. Medzhitov. 2001. Toll-like receptors control activation of adaptive immune responses. Nat. Immunol. 2**:**947-950. [DOI] [PubMed] [Google Scholar]
- 37.Schulz, O., S. S. Diebold, M. Chen, T. I. Naslund, M. A. Nolte, L. Alexopoulou, Y. T. Azuma, R. A. Flavell, P. Liljestrom, and C. Reis e Sousa. 2005. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 433**:**887-892. [DOI] [PubMed] [Google Scholar]
- 38.Shoukry, N. H., A. G. Cawthon, and C. M. Walker. 2004. Cell-mediated immunity and the outcome of hepatitis C virus infection. Annu. Rev. Microbiol. 58**:**391-424. [DOI] [PubMed] [Google Scholar]
- 39.Spörri, R., and C. Reis e Sousa. 2005. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4(+) T cell populations lacking helper function. Nat. Immunol. 6**:**163-170. [DOI] [PubMed] [Google Scholar]
- 40.Sumpter, R., Y. M. Loo, E. Foy, K. Li, M. Yoneyama, T. Fujita, S. M. Lemon, and M. Gale. 2005. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J. Virol. 79**:**2689-2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tester, I., S. Smyk-Pearson, P. Wang, A. Wertheimer, E. Yao, D. M. Lewinsohn, J. E. Tavis, and H. R. Rosen. 2005. Immune evasion versus recovery after acute hepatitis C virus infection from a shared source. J. Exp. Med. 201**:**1725-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsubouchi, E., S. M. Akbar, N. Horiike, and M. Onji. 2004. Infection and dysfunction of circulating blood dendritic cells and their subsets in chronic hepatitis C virus infection. J. Gastroenterol. 39**:**754-762. [DOI] [PubMed] [Google Scholar]
- 43.Tsubouchi, E., S. M. Akbar, H. Murakami, N. Horiike, and M. Onji. 2004. Isolation and functional analysis of circulating dendritic cells from hepatitis C virus (HCV) RNA-positive and HCV RNA-negative patients with chronic hepatitis C: role of antiviral therapy. Clin. Exp. Immunol. 137**:**417-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamamoto, M., S. Sato, H. Hemmi, K. Hoshino, T. Kaisho, H. Sanjo, O. Takeuchi, M. Sugiyama, M. Okabe, K. Takeda, and S. Akira. 2003. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science 301**:**640-643. [DOI] [PubMed] [Google Scholar]
- 45.Yang, Y., C. T. Huang, X. Huang, and D. M. Pardoll. 2004. Persistent Toll-like receptor signals are required for reversal of regulatory T cell-mediated CD8 tolerance. Nat. Immunol. 5**:**508-515. [DOI] [PubMed] [Google Scholar]