Vitronectin is A Critical Protein Adhesion Substrate for IL-4-INDUCED Foreign Body Giant Cell Formation (original) (raw)
. Author manuscript; available in PMC: 2014 Nov 11.
Published in final edited form as: J Biomed Mater Res A. 2008 Aug;86(2):535–543. doi: 10.1002/jbm.a.31658
Abstract
An in vitro system of interleukin (IL)-4-induced foreign body giant cell (FBGC) formation was utilized to define the adhesion protein substrate(s) that promotes this aspect of the foreign body reaction on biomedical polymers. Human monocytes were cultured on cell culture polystyrene surfaces that had been pre-adsorbed with a synthetic arginine-glycine-aspartate (RGD) peptide previously found to support optimal FBGC formation, or with various concentrations of potential physiological protein substrates, i.e. complement C3bi, collagen types I or IV, fibrinogen, plasma fibronectin, fibroblast fibronectin, laminin, thrombospondin, vitronectin, or von Willebrand factor. Cultures were evaluated on days 0 (1.5 hr), 3, and 7 by May-Grünwald/Giemsa staining. Initial monocyte adhesion occurred on all adsorbed proteins. However, by day 7 of culture, only vitronectin was striking in its ability to support significant macrophage adhesion, development, and fusion leading to FBGC formation. Vitronectin supported high degrees of FBGC formation at an absorption concentration between 5 and 25 μg per ml. These findings suggest that adsorbed vitronectin is critical in the collective events that support and promote FBGC formation on biomedical polymers, and that the propensity for vitronectin adsorption may underlie the material surface chemistry dependency of FBGC formation.
Keywords: adhesion, integrins, monocyte, macrophage fusion, biomedical polymers
Introduction
Inflammatory cell interactions are known to significantly impact the function of medical devices, prostheses, and biomaterials and can contribute to their clinical failure (1-4). Adherent monocyte-derived macrophages and foreign body giant cells (FBGC) are prominent features of the chronic inflammatory cellular response and are believed to control biocompatible outcomes for both host tissue and implanted biomaterial via multiple mechanisms. These include the secretion of reactive oxygen intermediates and the production of chemokines and cytokines that promote cellular accumulation and proliferation.
Material surface property-dependent blood protein adsorption occurs immediately upon surgical implantation of a biomedical device (5), and it is therefore the blood protein-modified material surface that all inflammatory leukocytes encounter as a potential adhesion substrate (1,6). However, the specific blood protein and cell adhesion receptor interactions that mediate inflammatory cell adhesion to biomaterials have not yet been clearly demonstrated.
In previous studies, we identified the β1 and β2 integrin families as necessary and sufficient mediators of adhesion during monocyte-to-macrophage development and interleukin (IL)-4-induced FBGC formation (7). Integrins comprise a large group of heterodimeric transmembrane molecules that mediate both cell-extracellular matrix and cell-cell interactions (8). These receptors are well known as important mediators of adhesion signaling between the extracellular and intracellular environments (9). Further identification of specific α partners to these β integrins led to the following integrin expression profile for IL-4-induced FBGC: αMβ2, αXβ2, α5β1 > αVβ1 > α3β1 and α2β1 (10). Therefore, monocytes/macrophages and FBGC express a select group of adhesion receptors with potential for binding to specific blood proteins that may adsorb to biomaterials and to extracellular matrix proteins (11-13).
Through the RGD (arginine-glycine-aspartate) cell attachment sequence in protein ligands, members of the β1 integrin family can bind collagens (CNs), fibronectin (FN), laminin (LN), or vitronectin (VN); the interaction between FN and α5β1 is a classic example (14,15). Consistent with this, we have demonstrated that RGD-modified surfaces are an optimum substrate for macrophage development and IL-4-induced FBGC formation (7,16). In addition to their roles in leukocyte extravasation to inflammatory sites (17), αMβ2 and αXβ2 are capable of interactions with multiple ligands to mediate cell-particle or cell-substrate interactions and the induction of β1 integrin expression (18,19). These include blood proteins, i.e. C3bi and fibrin(ogen) (FG), adsorbed to material surfaces (11-13). Notably, VN has also been identified as an adhesion ligand for αMβ2 (20).
Based on our collective experiences with FBGC, adhesion success is required for macrophage fusion leading to FBGC formation, which is highly material surface property-dependent (21-26). Materials that do not support adhesion and macrophage morphological development, i.e. cytoplasmic spreading, are poor substrates for IL-4-induced macrophage fusion and FBGC formation. Apparently, the appropriate adsorbed protein ligands are required to engage particular integrins that initiate activation of specific adhesion signals in what is termed “outside-in signaling” (9). In phagocytic cells, integrin activation leads to integrin clustering, adhesion kinase activation, cytoskeletal responses, and the formation of podosome adhesion structures (27,28). Macrophages and FBGC, in particular, exhibit distinct peripheral rings of podosomes (29). These, in turn, support cell mobility, survival, and synthetic abilities (9).
The alternative to adhesion success is anoikis, or apoptosis specifically due to adhesion failure. Anoikis is a normal biological mechanism for the control and regulation of cell proliferation and tissue development (30,31). Cell death signaling by this mechanism is believed to be regulated by the cytoskeleton. Our in vitro observations have been that monocytes initially adhere quite well to most surfaces. However, those that fail to maintain adhesion and support cytoplasmic spreading apparently do not survive and/or cannot form FBGC. Related to this, we have also discovered that monocyte/macrophage anoikis/apoptosis is a material surface-dependent phenomenon (32-35). Thus, whether adhesion signals that promote integrin activation, cytoskeletal responses, and podosome formation are engaged such that macrophage development can proceed is evidently determined by material-dependent protein adsorption.
In this study, we sought to further illuminate specific adhesive interactions that support macrophage development and promote IL-4-induced FBGC formation. Our approach was to adsorb cell culture polystyrene with potential blood and extracellular matrix protein ligands for the identified β1 and β2 integrins on fusing macrophages and FBGC. Utilizing our in vitro system of macrophage culture and fusion, we then asked whether monocyte-to-macrophage development and IL-4-induced macrophage fusion leading to FBGC formation could proceed on these adsorbed protein substrates.
Materials and Methods
All purified human proteins were from Calbiochem-EMD Biosciences (La Jolla, CA), except LN, which was from Sigma-Aldrich (St. Louis, MO). The RGD (arginine-glycineaspartate) peptide was also from Sigma-Aldrich and was reconstituted according to manufacturer's instructions to a 1 mg per ml stock solution and stored at room temperature.
Adsorption of Cell Culture Polystyrene with Blood Proteins or RGD peptide
All proteins were reconstituted as directed by the manufacturer and diluted to working concentrations in PBS containing Ca++ and Mg++ (PBS++). Adsorption was carried out at the indicated concentrations (Figure legends) for 1 hr at 37°C in 0.1 ml volumes in 96-well Falcon culture plates (Becton Dickinson, Franklin Lakes, NJ). Unadsorbed proteins were then removed by vacuum aspiration and the wells were each washed two times with 0.2 ml PBS++ just prior to the addition of monocytes. Solutions of RGD peptide were similarly adsorbed for 20 min at room temperature, aspirated, and washed twice before adding monocytes. A protein assay (BCA, biscinchoninic acid, Pierce, Rockford, IL) was performed in separate wells according to assay instructions to confirm adsorption of proteins to cell culture polystyrene.
Monocyte/macrophage Culture and Induction of Macrophage Fusion/FBGC Formation
Human monocytes were isolated and cultured as previously described (7,12). Briefly, 2 × 105 monocytes per well were added to 96-well RGD- or protein-adsorbed culture plates in 0.l ml of serum free medium for macrophages (SFM, Invitrogen, Grand Island, NY) supplemented with 20% autologous serum. After 1.5 h at 37°C, non adherent cells were removed by washing with PBS++ at 37°C, and either fixed with methanol or recovered with 0.2 ml of unsupplemented SFM, and incubated for 3 days at 37°C in a humidified atmosphere of 95% air and 5% CO2. On day 3, the monocytes/macrophages were either fixed with methanol or the medium was replaced with 0.2 ml of SFM with 5% heat-treated autologous serum (1 hr at 56°C) and 15 ng/ml recombinant human IL-4 (R&D Systems, Minneapolis, MN). The macrophages were then incubated until day 7, when the plates were washed twice with PBS++ at 37°C, and fixed with methanol. All fixed cultures were stained with May-Grünwald/Giemsa for evaluation of cellular morphology, adhesion, and % macrophage fusion. This is an established culture system in which 15 ng per ml of IL-4 induces high degrees of human monocyte-derived macrophage fusion over a period of 7-10 days on RGD-modified surfaces (7,36 and see Figure 1).
Fig 1.
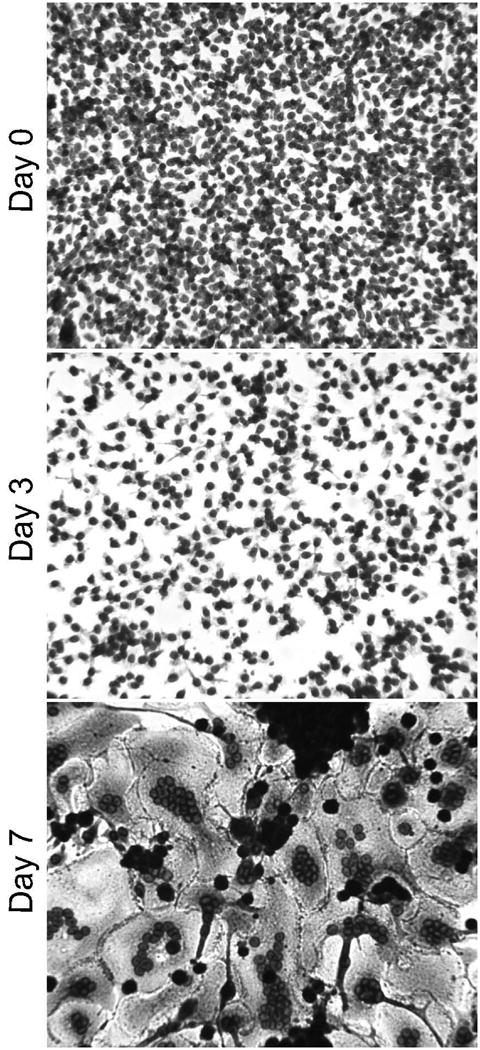
Monocyte/macrophage adhesion, morphology, and FBGC formation on RGD-modified polystyrene. RGD peptide was adsorbed at 25 μg per ml. Monocytes were plated and cultured for 1.5 hr (day 0), or until days 3 or 7. FBGC formation was induced with IL-4 on day 3. May-Grünwald/Giemsa. 20X
Determination of Adhesion and FBGC Formation (Percent Fusion)
Macrophage adhesion and fusion were simultaneously evaluated by counting the average number of adherent cells in representative low power (20X) microscopic fields (∼250 cells) and recording the number of cells per unit area and the percentage of nuclei within multinucleated cells (cells with > 2 nuclei). Results represent the mean ± SEM of data from at least three different monocyte donors. Statistical significance was assigned at the 95% confidence level using the unpaired Student's _t_-test. Photomicrograph images were obtained using a Nikon DXM-1200 digital camera (Nikon, Melville, NY) with ACT-1 software in single image acquisition mode and are representative of results from at least three different donors.
Results
Monocyte-to-Macrophage Development and FBGC Formation on Adsorbed RGD
We previously demonstrated that IL-4-induced macrophage fusion and FBGC formation occurs optimally on culture surfaces modified with the RGD integrin cell attachment sequence as a synthetic RGD peptide (7,16). Therefore, we included RGD peptide as a positive control substrate in these studies. A representative photomicrograph of monocyte-to-macrophage development and FBGC formation on adsorbed RGD peptide is shown in Figure 1, in which it can be seen that there is a high degree of initial monocyte adhesion (day 0) followed by macrophage development (day 3) and excellent IL-4-induced FBGC formation (day 7). Note the extensive cytoplasmic spreading on this material which begins around day 3 and is remarkable following macrophage fusion on day 7.
Monocyte/Macrophage/FBGC Adhesion on Adsorbed Blood or Matrix Proteins
In order to discover a physiologic protein substrate for FBGC formation, we targeted various purified blood and extracellular matrix proteins that are known ligands for the integrins previously determined to be expressed on fusing macrophages and FBGC (7,10). These proteins and their potential integrin receptors are shown in Table 1. In addition, because we previously found no role for β3 integrins in adhesion during macrophage development and fusion (7,10), we also included human plasma von Willebrand Factor (vWF), which is reported to bind to both αVβ3 and αIIbβ3 (13), as a negative control protein in these studies.
Table 1.
Adsorbed proteins to target identified macrophage/FBGC integrins (7,10,13,15,20).
| Adsorbed Protein | Abbreviation | Source | Integrin |
|---|---|---|---|
| Complement C3bi | C3bi | Human plasma | αMβ2, αXβ2 |
| Fibrino(gen) | FG | Human plasma | αMβ2, αXβ2, a5β1 |
| Collagen type I | CN I | Human placenta | α2βl, α3βl |
| Collagen type IV | CN IV | Human placenta | α2βl |
| Fibronectin, cellular | FNc | Human fibroblast | α3βl, α5βl, αVβ1 |
| Fibronectin, plasma | FN | Human plasma | α3β1, α5β1, αVβ1 |
| Laminin | LN | Human placenta | α2β1, α3β1 |
| Thrombospondin | TSP | Human platelet | α3β1 |
| Vitronectin | VN | Human plasma | α3β1, αVβ1, αMβ2 |
Proteins were adsorbed to standard cell culture polystyrene as described in Methods. Monocytes were added and the cultures continued until day 7, with IL-4 added to induce macrophage fusion on day 3. At each time point (day 0, 3, or 7), cells were washed and fixed for evaluation of adhesion. The results are shown in Figure 2, in which it can be seen that monocyte (day 0) adhesion occurs on adsorbed CN I, C3bi, FG, FN, VN, and RGD but is highest on RGD and VN. Adsorbed CN IV, fibroblast FN, LN, TSP, and vWF also support monocyte adhesion (not shown). By days 3 and 7, however, significant numbers of adherent macrophages remain only on adsorbed and VN and RGD.
Fig. 2.
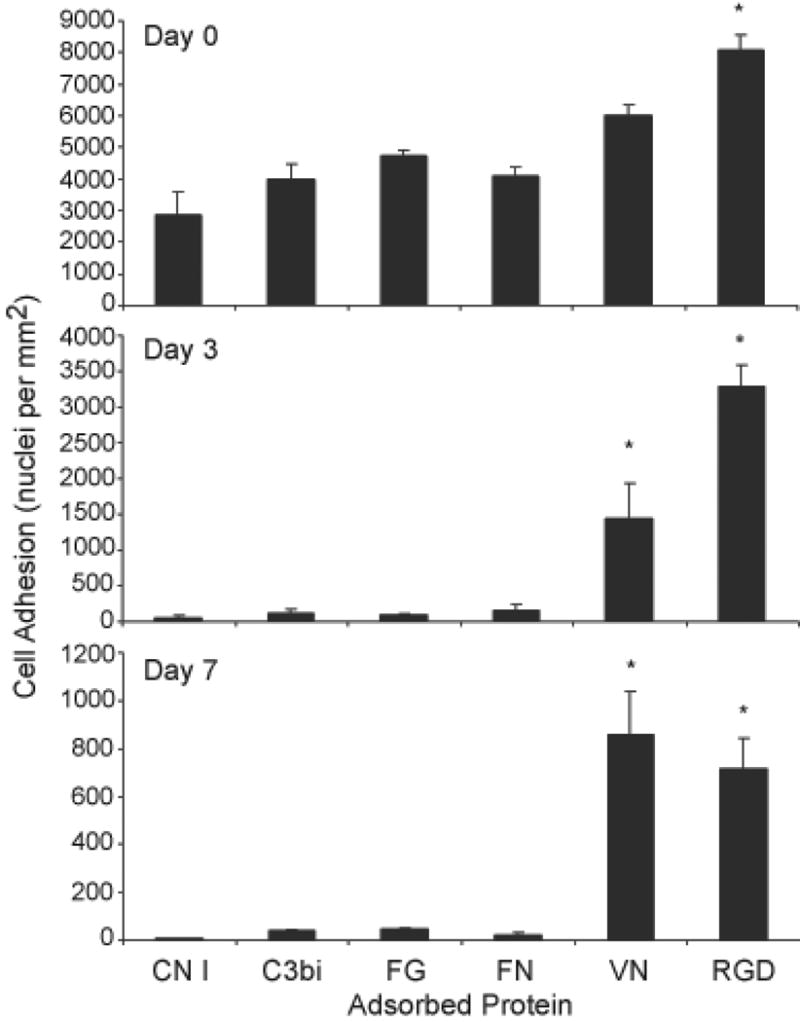
Adhesion to protein- or RGD-adsorbed polystyrene. Proteins or RGD peptide were adsorbed at a concentration of 25 μg per ml. Following removal of unadsorbed proteins by washing, monocytes were plated and cultured for 1.5 hr (day 0), or until days 3 or 7 and stained with May-Grünwald/Giemsa. FBGC formation was induced with IL-4 on day 3. Adhesion was determined as described in Methods. Results represent mean adherent cell number ± SEM, n = 3 different monocyte donors. *Significantly different from other adsorbed proteins (P<0.05).
Monocyte-to-Macrophage Development and FBGC Formation on Adsorbed Blood or Matrix Proteins
Monocyte-to-macrophage development and FBGC formation were also evaluated by morphology on days 0, 3, and 7. Representative results are depicted in Figure 3, which, as adherent cell numbers indicate in Figure 2, demonstrate considerable monocyte adhesion on day 0, with a greater degree of adhesion on adsorbed VN than on CN I, C3bi, FG, or FN. On day 3, although some adherent cells still remain on the latter proteins, essentially no macrophage morphologic development occurs on these substrates. In contrast, the many adherent cells on adsorbed VN clearly exhibit macrophage development in terms of cytoplasmic spreading at this time point. Consistent with this, adsorbed VN is the only blood or matrix protein substrate that supports IL-4-induced FBGC formation on day 7. The morphology of FBGC on adsorbed VN (Fig. 3) is not distinguishable from that on adsorbed RGD peptide (Fig. 1). In addition, at adsorption concentrations of 25 μg per ml, VN supports high degrees of macrophage fusion that are comparable to those supported by the RGD peptide-adsorbed substrate (Fig. 4).
Fig. 3.
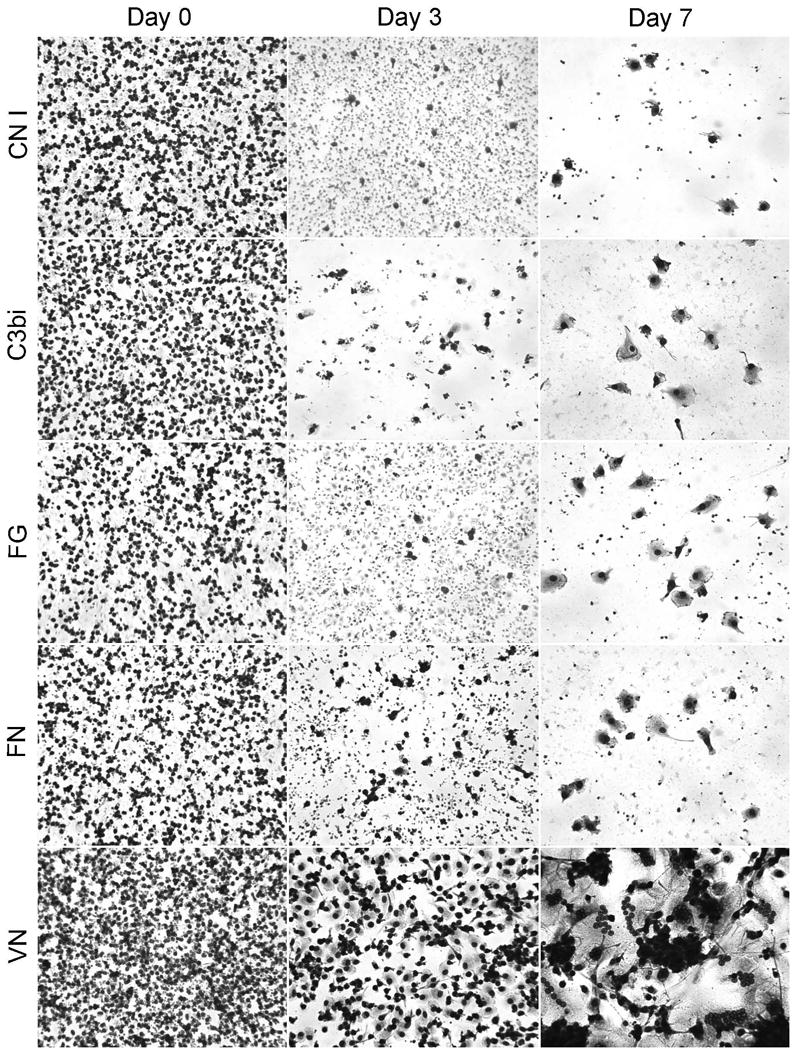
Monocyte-to-macrophage development and FBGC formation on adsorbed proteins. CN I, C3bi, FG, FN, or VN were adsorbed at a concentration of 25 μg per ml. Monocytes were plated and cultured for 1.5 hr (day 0), or until days 3 or 7, and stained with May-Grünwald/Giemsa. FBGC formation was induced with IL-4 on day 3. 20X
Fig. 4.
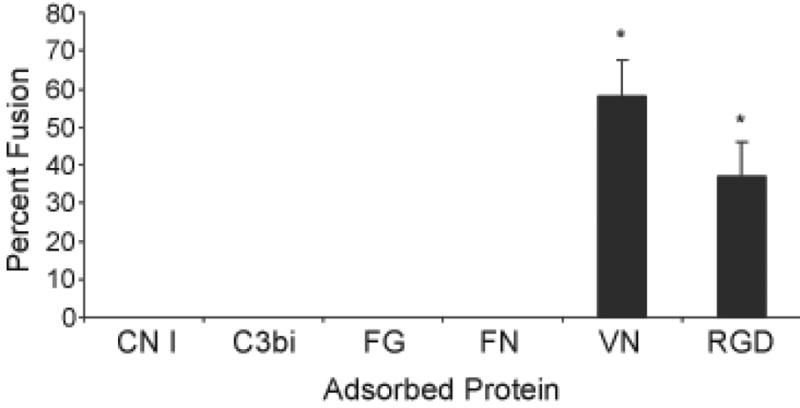
Macrophage fusion protein- or RGD-adsorbed polystyrene. Proteins or RGD peptide were adsorbed at a concentration of 25 μg per ml. Monocytes were plated and cultured for 7 days as described in Methods with the IL-4-induction of macrophage fusion on day 3. Following May-Grünwald/Giemsa staining, percent macrophage fusion was determined. Results represent mean % fusion ± SEM, n = 3 different monocyte donors. *Significantly different from other adsorbed proteins (P<0.05).
We then asked whether relative amounts of proteins adsorbed to polystyrene culture wells could account for the observed differences in macrophage adhesion, development, and fusion. In Table 2, protein assay data are shown, which demonstrate that following adsorption and washing to remove unadsorbed proteins, all tested proteins are readily detectable. The highest relative adsorption occurs with FG, plasma FN, and vWF. Therefore, the striking differences between VN and other proteins in supporting macrophage adhesion, development, and fusion are not due to marked differences in their abilities to adsorb to the culture material.
Table 2.
Relative adsorption of proteins to cell culture polystyrene.
| Adsorbed Protein | Abbreviation | μg per ml |
|---|---|---|
| Complement C3bi | C3bi | 15.8±0.8 |
| Collagen type I | CN I | 13.1±1.0 |
| Fibrinogen | FG | 24.9±0.5 |
| Fibronectin, cellular | FNc | 21.0±1.0 |
| Fibronectin, plasma | FN | 30.1±1.2 |
| Thrombospondin | TSP | 17.4±0.9 |
| Vitronectin | VN | 16.5±0.6 |
| Von Willebrand Factor | vWF | 32.2±0.7 |
Macrophage Fusion and FBGC Formation on Adsorbed VN are Concentration-Dependent
Further experiments to determine the optimum adsorption concentration of VN for FBGC formation reveal that VN supported FBGC formation is concentration-dependent and occurs well below the 200-400 μg per ml normal concentration of VN in blood plasma (37). Figures 5 and 6 demonstrate FBGC morphologies and degrees of macrophage fusion, respectively, on different concentrations of adsorbed VN. If no VN is adsorbed, there is poor macrophage adhesion and development and essentially no FBGC formation. In contrast, adsorption from only 2.5 μg per ml of VN results in extensive macrophage adhesion and cytoplasmic spreading with moderate FBGC formation. Greater extents of FBGC formation occur when culture surfaces have been exposed to a solution of 5 μg per ml VN. Increasing VN concentrations beyond 5 μg per ml does not significantly increase macrophage fusion at this day 7 time point (Fig. 6). We do note, however, apparent increases in FBGC cytoplasmic spreading with VN concentrations up to 25 μg per ml (Fig. 5), and we find that lengthening the culture period to 10 days with an additional dose of IL-4 added on day 7 also increases overall FBGC formation (A.K. McNally and J.M. Anderson, unpublished data).
Fig. 5.

VN-supported IL-4-induced FBGC formation: Concentration dependence. VN was not adsorbed or adsorbed at 2.5, 5, or 25 μg per ml, and monocytes were plated and cultured for 7 days as described in Methods with the IL-4-induction of macrophage fusion on day 3. May-Grünwald/Giemsa. 20X
Fig. 6.
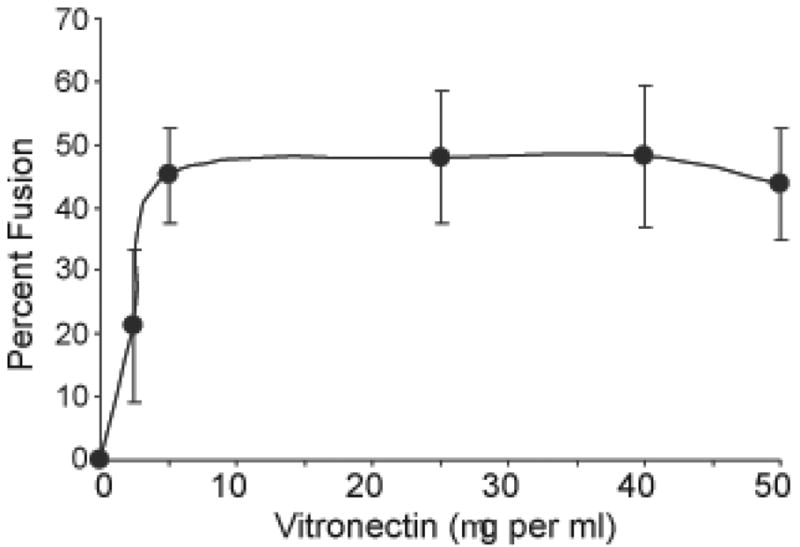
VN-supported IL-4-induced macrophage fusion: Concentration dependence. VN was not adsorbed or adsorbed at the indicated concentrations, and monocytes were plated and cultured for 7 days as described in Methods with the IL-4-induction of macrophage fusion on day 3. Following May-Grünwald/Giemsa staining, percent macrophage fusion was determined. Results represent mean % fusion ± SEM, n = 3 different monocyte donors.
Discussion
In summary, these studies identify the blood protein VN as a critical adhesion substrate for IL-4-induced FBGC formation. By targeting integrin adhesion receptors with potential adsorbed protein ligands, we find that IL-4-induced FBGC formation does not proceed in vitro on material adsorbed with complement C3bi, FG, plasma FN, cell-derived (fibroblast) FN, CN types I or IV, or LN. In striking contrast, FBGC formation readily occurs on adsorbed VN, and this effect of VN is comparable in degrees of macrophage fusion and in FBGC morphology to FBGC formation on adsorbed synthetic RGD peptide. Therefore, although fusing macrophages/FBGC express several β1 and β2 integrins and thereby possess broad ligand binding potential, they appear to selectively utilize VN to support macrophage adhesion and fusion leading to FBGC formation. Although FG, FN, CNs, and LN are well known as adhesion substrates for multiple other cell types, they do not support IL-4-induced FBGC. Therefore, these findings further suggest that a material-dependent propensity for VN adsorption may be a mechanism for the material surface dependency of FBGC formation on biomaterials (21-26).
The VN adsorption concentration that supports optimal FBGC formation in our culture system (between 5 and 25 μg per ml) is physiologically relevant, i.e. it is well below that of normal blood plasma, which is 200-400 μg per ml (37). The FBGC morphology obtained on adsorbed VN is similar to that observed on RGD-modified polystyrene material in our culture system (7,38) and on retrieved biomaterials (39) and is consistent with known utilization of the RGD motif during VN-mediated cell adhesion (40). These results do not rule out roles for other serum components in FBGC formation. However, they do point to a selective requirement for macrophage adhesive interactions with adsorbed VN in macrophage development leading to FBGC.
In addition, the present results are consistent with both our β and α integrin findings (7,10) and further suggest that VN interacts with αMβ2 (20) throughout the culture period and with αVβ1 following macrophage development and fusion. Since αXβ2 mimics multiple other αMβ2 binding characteristics (C3bi, fibrinogen), the present findings also raise the question of whether αXβ2, which is strongly expressed in fusing macrophages/FBGC (10), is able to bind VN.
The inabilities of other tested proteins to support IL-4 induced FBGC formation in vitro does not appear to stem from decreased degrees of initial monocyte adhesion to these adsorbed components. Rather, it appears be related to comparatively poor macrophage adhesion and development, which is observable on days 3 and 7 of the culture period. This suggests that successful monocyte/macrophage adhesion signaling and adhesion structure formation does not occur on these apparently unfavorable protein substrates, and apoptosis/anoikis is the outcome (30,31). Further studies of the mechanisms of adhesion failure on these adsorbed proteins are necessary to determine whether or not this is the case.
VN is known to outcompete FN for adsorption to cell culture polystyrene from fetal bovine serum (41,42). However, when allowed to adsorb to culture surfaces in the absence of other competing proteins, FN nevertheless does not support IL-4-induced FBGC formation. In addition, other potentially relevant blood proteins, e.g. C3bi and FG, or extracellular matrix components, e.g. CNs and LN, do not support FBGC formation when adsorbed alone. Therefore, in spite of the subsequent presence of serum VN during initial monocyte adhesion and during the induction of macrophage fusion, the prior adsorption of these particular proteins restricts macrophage development and FBGC formation. This raises the possibility that they may be exploited to modulate FBGC formation on biomedical materials.
In this regard, material surface chemistry likely plays a key role in the adsorption of VN from a complex mixture of blood proteins, such that material-dependent competitive adsorption of other components, e.g. FN or FG, actually undermines VN-supported FBGC formation. The present discovery that VN is a critical protein adhesion substrate for IL-4-induced FBGC formation provokes speculation that material surface chemistry-dependent VN adsorption may underlie the mechanism for the material surface dependency of FBGC formation (21-26). This is also supported by our earlier report in which, based on its adsorption strength to surfaces that supported high degrees of IL-4-induced FBGC formation, VN was postulated to play a role in FBGC formation (43).
The present study targeted selected proteins and matrix components which are potential ligands for integrins that have been demonstrated to mediate adhesion during IL-4-induced FBGC formation (7,10). It did not address roles for additional blood proteins or components, such as (heterologous) IgG, which has been reported to support macrophage adhesion in vitro (44), but does not promote FBGC formation (A.K. McNally and J.M. Anderson, unpublished data) and is not known to be an integrin ligand.
We note that these findings prompt further comparisons between the IL-4-induced FBGC and the bone-resorbing osteoclast, another type of multinucleated macrophage that also utilizes VN as an adhesion substrate. However, osteoclasts are not induced by IL-4 or IL-13 and are reported to interact with VN via αVβ3 and αVβ5 integrins (45). Although we cannot rule out participation of αVβ5, we do not detect β3 integrins on fusing macrophages/FBGC (7,10). In addition, functional blockade of β1 and β2 integrins with neutralizing antibodies is sufficient to inhibit 90% of macrophage/FBGC adhesion to RGD-modified surfaces (7).
Predictably, surface modifications that inhibit, attenuate, or alter the progression of the chronic inflammatory cell response will ultimately enhance the function of biomedical devices. These studies provide support for the concept that material-dependent adhesion mechanisms lead either to adhesion success or adhesion failure (anoikis) of inflammatory cells, and, once understood, these mechanisms may potentially be targeted for control. Further investigation of material-dependent VN adsorption and its role in the macrophage/FBGC-mediated foreign body reaction in vitro and in vivo will advance our understanding of these mechanisms.
Acknowledgments
These studies were supported by the National Institutes of Health, Devices and Technology Branch, Grant EB 000275
References
- 1.Anderson JM. Inflammatory response to implants. Am Soc Artif Intern Organs. 1988;11:101–107. doi: 10.1097/00002480-198804000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JM. Inflammatory reaction: The nemesis of implants. In: Zilla P, Greisler HP, editors. Tissue Engineering of Vascular Prosthetic Grafts. Austin TX: RG Landes Co; 1999. pp. 197–206. 1999. [Google Scholar]
- 3.Anderson JM. Biological responses to materials. Ann Rev Mater Res. 2001;31:81–11. [Google Scholar]
- 4.Wiggins MJ, Wilkoff B, Anderson JM, Hiltner A. Biodegradation of polyether polyurethane inner insulation in bipolar pacemaker leads. J Biomed Mater Res (Appl Biomater) 2001;58:302–307. doi: 10.1002/1097-4636(2001)58:3<302::aid-jbm1021>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 5.Bamford CH, Cooper SL, Tsurutta T. The Vroman Effect. The Netherlands: VSP Zeist; 1992. [Google Scholar]
- 6.Anderson JM. Chapter 4.2 Inflammation, Wound Healing, and the Foreign Body Response. In: Ratner BD, Hoffman AS, Schoen FJ, Lemons JE, editors. Biomaterials Science: An Introduction to Materials in Medicine. New York: Elsevier; 2004. pp. 296–304. [Google Scholar]
- 7.McNally AK, Anderson JM. β1 and β2 integrins mediate adhesion during macrophage fusion and multinucleated foreign body giant cell formation. Am J Pathol. 2002;160:621–630. doi: 10.1016/s0002-9440(10)64882-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzales-Amaro R, Sanchez-Madrid F. Cell adhesion molecules: Selectins and integrins. Crit Rev Immunol. 1999;19:389–429. [PubMed] [Google Scholar]
- 9.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 10.McNally AK, MacEwan SR, Anderson JM. α subunit partners to β1 and β2 integrins during IL-4-induced foreign body giant cell formation. J Biomed Mater Res. 2007 doi: 10.1002/jbm.a.31161. In press. [DOI] [PubMed] [Google Scholar]
- 11.Anderson JM, Ziats NP, Bonfield TL, McNally AK, Topham NS. Human blood protein and cell interactions with cardiovascular materials. In: Akutsu T, Koyanagi H, editors. Artificial Heart 3. New York: Springer-Verlag; 1991. pp. 45–55. [Google Scholar]
- 12.McNally AK, Anderson JM. Complement C3 participation in monocyte adhesion to different surfaces. Proc Natl Acad Sci USA. 1994;91:10119–10123. doi: 10.1073/pnas.91.21.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harbers G, Grainger DW. Cell-materials interactions: Fundamental design issues for tissue engineering and clinical considerations. In: Hollinger JO, Guelcher S, editors. An introduction to biomaterials. New York: CRC Press; 2006. pp. 15–45. [Google Scholar]
- 14.Ruoslahti E. RGD and other recognition sequences for integrins. Ann Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 15.Tsuji T. Physiological and pathological roles of α3β1 integrin. J Membrane Biol. 2004;200:115–132. doi: 10.1007/s00232-004-0696-5. [DOI] [PubMed] [Google Scholar]
- 16.Anderson JM, Brodbeck WG, MacEwan M, McNally AK. Monocyte, macrophage, and foreign body giant cell interactions with molecularly engineered surfaces. J Mater Sci Mater Med. 1999;10:579–588. doi: 10.1023/a:1008976531592. [DOI] [PubMed] [Google Scholar]
- 17.Luscinskas FW, Kansas GS, Ding H, Pizcueta P, Schleiffenbaum BE, Tedder TF, Gimbrone MA. Monocyte rolling, arrest, and spreading on IL-4-activated endothelium under flow is mediated via sequential action of L-selectin, beta 1-integrins, and beta 2-integrins. J Cell Biol. 1994;125:1417–1427. doi: 10.1083/jcb.125.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berton G, Lowell CA. Integrin signaling in neutrophils and macrophages. Cell Signal. 1999;11:621–635. doi: 10.1016/s0898-6568(99)00003-0. [DOI] [PubMed] [Google Scholar]
- 19.Werr J, Eriksson AA, Hedqvist P, Lindbom L. Engagement of beta2 integrins induces surface expression of beta1 integrin receptors in human neutrophils. J Leuko Biol. 2000;68:553–560. [PubMed] [Google Scholar]
- 20.Kanse SM, Matz RL, Preissner KT, Peter K. Promotion of leukocyte adhesion by a novel interaction between vitronectin and the β2 integrin Mac-1 (αMβ2, CD11b/CD18) Arterioscler Thromb Vasc Biol. 2004;24:2251–2256. doi: 10.1161/01.ATV.0000146529.68729.8b. [DOI] [PubMed] [Google Scholar]
- 21.McNally AK, Anderson JM. Fifth World Biomaterials Congress. Toronto: Canada; 1996. May 29 2, Jun 29 2, The lymphokine interleukin-4 induces foreign body giant cell formation from human macrophages in a material surface property-dependent manner in vitro. [Google Scholar]
- 22.Jenney CR, DeFife KM, Colton E, Anderson JM. Human monocyte/macrophage adhesion, macrophage motility, and IL-4 induced foreign body giant cell formation on silane modified surfaces in vitro. J Biomed Mater Res. 1998;41:171–184. doi: 10.1002/(sici)1097-4636(199808)41:2<171::aid-jbm1>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 23.Jenney CR, Anderson JM. Effects of surface-coupled polyethylene oxide on human macrophage adhesion and foreign body giant cell formation in vitro. J Biomed Mater Res. 1999;44:206–216. doi: 10.1002/(sici)1097-4636(199902)44:2<206::aid-jbm11>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 24.Jenney CR, Anderson JM. Alkylsilane-modified surfaces: Inhibition of human macrophage adhesion and foreign body giant cell formation. J Biomed Mater Res. 1999;46:11–21. doi: 10.1002/(sici)1097-4636(199907)46:1<11::aid-jbm2>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 25.Brodbeck WG, Nakayama Y, Matsuda T, Colton E, Ziats NP, Anderson JM. Biomaterial surface chemistry dictates adherent monocyte/macrophage cytokine expression in vitro. Cytokine. 2002;18:311–319. doi: 10.1006/cyto.2002.1048. [DOI] [PubMed] [Google Scholar]
- 26.Collier TO, Anderson JM, Brodbeck WG, Barber T, Healy KE. Inhibition of macrophage development and foreign body giant cell formation by hydrophilic interpenetrating polymer network. J Biomed Mater Res A. 2004;69:644–650. doi: 10.1002/jbm.a.30030. [DOI] [PubMed] [Google Scholar]
- 27.Duong LT, Rodan GA. PYK2 is an adhesion kinase in macrophages, localized in podosomes and activated by β2-integrin ligation. Cell Motil Cytoskel. 2000;47:174–188. doi: 10.1002/1097-0169(200011)47:3<174::AID-CM2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 28.Calle Y, Burns S, Thrasher AJ, Jones GE. The leukocyte podosome. Eur J Cell Biol. 2006;85:151–157. doi: 10.1016/j.ejcb.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 29.DeFife KM, Jenney CR, Colton E, Anderson JM. Cytoskeletal and adhesive structural polarizations accompany IL-13-induced human macrophage fusion. J Histochem Cytochem. 1999;47:65–74. doi: 10.1177/002215549904700107. [DOI] [PubMed] [Google Scholar]
- 30.Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13:555–562. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 31.Reddig PJ, Juliano RL. Clinging to life: cell to matrix adhesion and cell survival. Cancer and Metastasis Reviews. 2005;24:425–439. doi: 10.1007/s10555-005-5134-3. [DOI] [PubMed] [Google Scholar]
- 32.Brodbeck W, Shive M, Colton E, Nakayama Y, Matsuda T, Anderson JM. Influence of biomaterial surface chemistry on the apoptosis of adherent cells. J Biomed Mater Res. 2001;55:661–668. doi: 10.1002/1097-4636(20010615)55:4<661::aid-jbm1061>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 33.Brodbeck WG, Patel J, Voskerician G, Christenson E, Shive MS, Nakayama Y, Matsuda T, Ziats NP, Anderson JM. Biomaterial adherent macrophage apoptosis is increased by hydrophilic and anionic substrates in vitro. Proc Natl Acad Sci USA. 2002;99:10287–10292. doi: 10.1073/pnas.162124199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brodbeck WG, Colton E, Anderson JM. Effects of adsorbed heat labile serum proteins and fibrinogen on adhesion and apoptosis of monocytes/macrophages on biomaterials. J Mater Sci Mater Med. 2003;14:671–675. doi: 10.1023/a:1024951330265. [DOI] [PubMed] [Google Scholar]
- 35.Jones JA, Dadsetan M, Collier TO, Ebert M, Stokes KS, Ward RS, Hiltner AP, Anderson JM. Macrophage behavior on surface-modified polyurethanes. J Biomat Sci Polymer Edn. 2004;15:576–584. doi: 10.1163/156856204323046843. [DOI] [PubMed] [Google Scholar]
- 36.McNally AK, Anderson JM. Interleukin-4 induces foreign body giant cells from human monocytes/macrophages. Differential lymphokine regulation of macrophage fusion leads to morphological variants of multinucleated giant cells. Am J Pathol. 1995;147:1487–1499. [PMC free article] [PubMed] [Google Scholar]
- 37.Schvartz I, Seger D, Shaltiel S. Vitronectin. Int J Biochem Cell Biol. 1999;31:539–544. doi: 10.1016/s1357-2725(99)00005-9. [DOI] [PubMed] [Google Scholar]
- 38.McNally AK, Anderson JM. Foreign body-type multinucleated giant cell formation is potently induced by a-tocopherol and prevented by the diacylglycerol kinase inhibitor R59022. Am J Pathol. 2003;163:1147–1156. doi: 10.1016/s0002-9440(10)63474-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao Q, Topham NS, Anderson JM, Hiltner A, Lodoen G, Payet CR. Foreign body giant cells and polyurethane biostability: in vivo correlation of cell adhesion and surface cracking. J Biomed Mater Res. 1991;25:177–183. doi: 10.1002/jbm.820250205. [DOI] [PubMed] [Google Scholar]
- 40.Cherny RC, Honan MA, Thiagarajan P. Site-directed mutagenesis of the arginine-glycine-aspartic acid in vitronectin abolishes cell adhesion. J Biol Chem. 1993;268:9725–9729. [PubMed] [Google Scholar]
- 41.Underwood PA, Bennett FA. A comparison of the biological activities of the cell-adhesive proteins vitronectin and fibronectin. J Cell Sci. 1989;93:641–649. doi: 10.1242/jcs.93.4.641. [DOI] [PubMed] [Google Scholar]
- 42.Fabrizius-Homan DJ, Cooper SL. Competitive adsorption of vitronectin with albumin, fibrinogen, and fibronectin on polymeric biomaterials. J Biomed Mater Res. 1991;25:953–971. doi: 10.1002/jbm.820250804. [DOI] [PubMed] [Google Scholar]
- 43.Jenney CR, Anderson JM. Adsorbed serum proteins responsible for surface dependent human macrophage behavior. J Biomed Mater Res. 1999;49:435–437. doi: 10.1002/(sici)1097-4636(20000315)49:4<435::aid-jbm2>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 44.Jenney CR, Anderson JM. Adsorbed IgG: A potent adhesive substrate for human macrophages. J Biomed Mater Res. 1999;50:281–290. doi: 10.1002/(sici)1097-4636(20000605)50:3<281::aid-jbm1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 45.Teitelbaum SL. Osteoclasts: What do they do and how do they do it? Am J Pathol. 2007;170:427–435. doi: 10.2353/ajpath.2007.060834. [DOI] [PMC free article] [PubMed] [Google Scholar]