Directed Differentiation of Ventral Spinal Progenitors and Motor Neurons from Human Embryonic Stem Cells by Small Molecules (original) (raw)
. Author manuscript; available in PMC: 2009 Jul 9.
Abstract
Specification of distinct cell types from human embryonic stem cells (hESCs) is key to the potential application of these naïve pluripotent cells in regenerative medicine. Determination of the nontarget differentiated populations, which is lacking in the field, is also crucial. Here, we show an efficient differentiation of motor neurons (~50%) by a simple sequential application of retinoid acid and sonic hedgehog (SHH) in a chemically defined suspension culture. We also discovered that purmorphamine, a small molecule that activates the SHH pathway, could replace SHH for the generation of motor neurons. Immunocytochemical characterization indicated that cells differentiated from hESCs were nearly completely restricted to the ventral spinal progenitor fate (NKX2.2+, Irx3+, and Pax7−), with the exception of motor neurons (HB9+) and their progenitors (Olig2+). Thus, the directed neural differentiation system with small molecules, even without further purification, will facilitate basic and translational studies using human motoneurons at a minimal cost.
Keywords: Stem cell, Motor neuron, Small molecule, Spinal cord
INTRODUCTION
Potential use of human embryonic stem cells (hESCs) in biotechnology and regenerative medicine depends upon the development of strategies for directed differentiation into functional cell/tissue types. In the past decade, since the establishment of hESCs [1, 2], protocols have been devised to differentiate hESCs to enriched populations of specialized cells, such as hematopoietic [3], cardiac [4], skeletal muscle [5], pancreatic [6], and neural [7–9] cells. With the exception of neuroepithelial cells, which can be differentiated from hESCs with more than 95% efficiency [9, 10], most differentiation protocols yield a mixed cell population. Differentiation to more specialized sub-types of neurons, such as midbrain dopamine neurons [11–14] and spinal motor neurons [15–17], becomes less efficient. Consequently, it is not known what the nontarget cells in the mixture are. These nontarget cells are often the source of aberrant tissue formation in transplants [13, 14, 18]. There is therefore a critical need to develop strategies for directed differentiation of hESCs to specialized functional cell types, such as subtypes of neural progenitors and functional motor neurons.
Developmental principles are bases for devising strategies for directed neural differentiation of hESCs. In the ventral neural tube, there are five different progenitor domains (p0, p1, p2, p3, and pMN), which give rise to motoneurons and interneuron subtypes of the ventral spinal cord [19, 20]. These progenitor domains are established mainly by interaction of class I and class II homeodomain (HD) proteins, which are inhibited or induced by the graded secreted inductive factors, such as sonic hedgehog [19, 20]. The motoneuron domain is flanked dorsally by the p2 domain (expressing Irx3) and ventrally by the p3 domain (marked by Nkx2.2) in the ventral neural tube. Expression of Olig2, a basic helix-loop-helix transcriptional factor, is a determinant factor in establishing the pMN domain [21]. Subsequently, Olig2, together with a pan-neuronal factor (Ngn2), will induce the downstream HD factors of motoneuron identity, such as HB9 [22, 23].
On the basis of the developmental principles, we have previously shown that hESCs can be differentiated to spinal motor neurons in an adherent culture by applying retinoid acid (RA) and sonic hedgehog (SHH) with 20% efficiency [15], similar to that from mouse ESCs [24–26]. However, this efficiency is not ideal for a variety of analyses, and the identity of nearly 80% of the differentiated cells in the culture remains unknown. In the present study, we have developed a simple chemically defined suspension culture for a near-complete restriction of hESCs to a ventral spinal progenitor fate, with highly efficient generation of motor neurons. We also discovered that this process can be achieved by using a small molecule, purmorphamine, instead of SHH.
MATERIALS AND METHODS
Culture of Neuroepithelial Cells and Motor Neurons
Human ES cells (lines H1 and H9, passages 19–42) were cultured and passaged weekly on a feeder layer of irradiated embryonic mouse fibroblasts as described [1]. The procedure for generating neuroepithelial cells from hESCs was essentially the same as described [9].
For motoneuron induction, hESC-derived neuroepithelial cells at day 10 were first treated with RA (0.1 µM) for caudalization in a neural medium, which consisted of Dulbecco’s modified Eagle’s medium/Ham’s F-12 medium (Gibco, Grand Island, NY, http://www.invitrogen.com), N2 supplement, heparin (2 µg/ml; Sigma-Aldrich, St. Louis, http://www.sigmaaldrich.com), and cAMP (1 µM; Sigma-Aldrich). One week later (day 17), the posteriorized neuroectodermal cells were isolated. Since neuroepithelial cells in the colony center formed neural tube-like rosettes and attached only loosely to the substrate, whereas the peripheral flat cells adhered to the Petri dish more tightly, the neuroepithelial rosettes were gently blown off by a 5-ml serological pipette. The flat cells remained attached. After isolation, the neuroepithelial clusters were suspended in the same neural medium in the presence of RA (0.1 µM) and SHH (100–200 ng/ml; 1845-SH; R&D Systems Inc., Minneapolis, http://www.rndsystems.com) for 1 week. After that (day 24), brain-derived neurotrophic factor (BDNF), glial-derived neurotrophic factor (GDNF), and insulin-like growth factor-1 (IGF1) (10 ng/ml; Peprotech, Rocky Hill, NJ, http://www.peprotech.com) were added to the culture. Purmorphamine (Calbiochem, San Diego, http://www.emdbiosciences.com) at different concentrations (0.5, 1, 2, and 5 µM) was used instead of SHH in some experiments (described in Results).
Coculture of Motoneurons and Myocytes
Motoneurons were cocultured with C2C12 myoblasts (American Type Culture Collection, Manassas, VA, http://www.atcc.org) as described previously [15]. Briefly, motoneuron-enriched clusters after purmorphamine treatment (around 4.5 weeks after differentiation) were plated on ornithine/laminin-coated coverslips in the above neural medium, supplemented with BDNF, GDNF, and IGF1 (all at 10 ng/ml) in the presence of B27 and RA (0.1 µM). After 3–5 days, the dissociated C2C12 myoblasts were plated onto the neuronal progenitor cells in the same neural differentiation medium.
Immunocytochemistry and Quantification
Immunohistochemical staining was performed according to Zhang et al. [9]. Primary antibodies used in this study included polyclonal antibodies against Olig2 (1:500; Santa Cruz Biotechnology Inc., Santa Cruz, CA, http://www.scbt.com), Ki67 (1:200; Zymed, San Francisco, http://www.zymed.com), Otx2 (1:2,000; Chemicon, Temecula, CA, http://www.chemicon.com), choline acetyltransferase (ChAT) (1:200; Chemicon), and synapsin I (1:500; Calbiochem). Antibodies against MNR2 or HB9 (81.5C10; 1:50), Pax7 (1:2,000), Nkx2.2 (1:50), and Hoxb4 (1:50) were purchased from the Developmental Studies Hybridoma Bank (Iowa City, IA, http://www.uiowa.edu/~dshbwww). Polyclonal Phox2b and Irx3 antibodies were kindly provided by J.F. Brunet and T.M. Jessell. For labeling acetylcholine receptors, coverslip cultures were incubated with Alexa Fluor 594-conjugated α-bungarotoxin (1:500; Molecular Probes, Eugene, OR, http://probes.invitrogen.com) at room temperature for 30 minutes. Images were collected using a Spot digital camera mounted onto a Nikon fluorescent microscope 600 (Nikon, Tokyo, http://www.nikon.com) or a confocal microscope (Nikon).
To quantify the efficiency of motoneuron differentiation, neuroepithelial clusters were either plated on coverslips for immunostaining or fixed directly to perform fluorescence-activated cell sorting (FACS) analysis. After immunostaining, the clusters were randomly picked up, and the population of Olig2 or HB9-expressing cells among total differentiated cells (Hoechst-labeled) was counted in two ways, as we described previously [15]. Five to 17 clusters in each group were counted, and data were expressed as mean ± SEM. Differences between different groups were compared by analysis of variance test, and statistical significance is defined as two-sided p = .05.
Fluorescence-Activated Cell Sorting
Cells were harvested using Accutase (Innovative Cell Technologies Inc., San Diego, http://www.innovativecelltech.com), gently dissociated to single cells, and washed with a FACS buffer (phosphate-buffered saline, 0.1% NaN3, 2% donkey serum). After being fixed and permeabilized with ice-cold 0.1% paraformaldehyde for 10 minutes and 90% methanol for 30 minutes, cells were incubated in primary antibody (Olig2, goat IgG; 1:500) or a goat IgG control at a concentration of 1 mg of protein per 1 million cells. Cells were then washed and incubated with the corresponding secondary antibody, Alexa 488-conjugated donkey anti-goat IgG, for 2 hours followed by washing steps. Cells were analyzed using a Becton Dickinson FACSCalibur instrument and CellQuest Pro software (BD Biosciences, San Diego, http://www.bdbiosciences.com).
Reverse Transcription-Polymerase Chain Reaction Assays
Total RNA was extracted from motoneuron differentiation cultures using RNA STAT-60 (Tel-Test, Friendswood, TX, http://www.isotexdiagnostics.com). cDNA was synthesized using the SuperScript III first-strand synthesis system (Invitrogen, Carlsbad, CA, http://www.invitrogen.com) according to the supplier’s protocol and was used as templates for the polymerase chain reaction (PCR). PCR was performed in 15 µl of mixture containing cDNA, primers, and 1 × PCR Master Mix (Promega, Madison, WI, http://www.promega.com). The following primers were used: Olig2, 5′-AAGGAGGCAGTGGCTTCAAGTC-3′, 5′-CGCTCACCAGTCGCTTCATC-3′, 315 base pairs (bp); Nkx2.2, 5′-TGCCTCTCCTTCTGAACCTTGG-3′, 5′-GCGAAATCTGCCACCAGTTG-3′, 337 bp; Irx3, 5′-AGAACGCCACCAGGGAGAG-3′, 5′-TTGGAGTCCGAAATGGGTCC-3′, 473 bp; Pax6, 5′-GGCAACCTACGCAAGATGGC-3′, 5′-TGAGGGCTGTGTCTGTTCGG-3′, 459 bp; Nkx6.1, 5′-ACACGAGACCCACTTTTTCCG-3′, 5′-TGCTGGACTTGTGCTTCTTCAAC-3′, 335 bp; glyceraldehyde-3-phosphate dehydrogenase, 5′-ACCACAGTCCATGCCATCAC-3′, 5′-TCCACCACCCTGTTGCTGTA-3′, 450 bp. HB9, 5′-GATGCCCGACTTCAACTCCC-3′, 5′-CCTTCTGTTTCTCCGCTTCCTG-3′, 269 bp; Ngn2, 5′-TGATTCCTCGGTTGTTTCTTGC-3′, 5′-AAAGCAGATGCCAGCCATTG-3′, 399 bp; Pax7, 5′-CACTGTGACCGAAGCACTGGT-3′, 5′-CCTCTGTCAGCTTGGTCCTC-3′, 352 bp; Gli1, 5′-TTCCTACCAGAGTCCCAAGT-3′, 5′-CCCTATGTGAAGCCCTATTT-3′, 185 bp.
RESULTS
RA and SHH Efficiently Restrict hESCs to Ventral Spinal Progenitors in a Suspension Culture
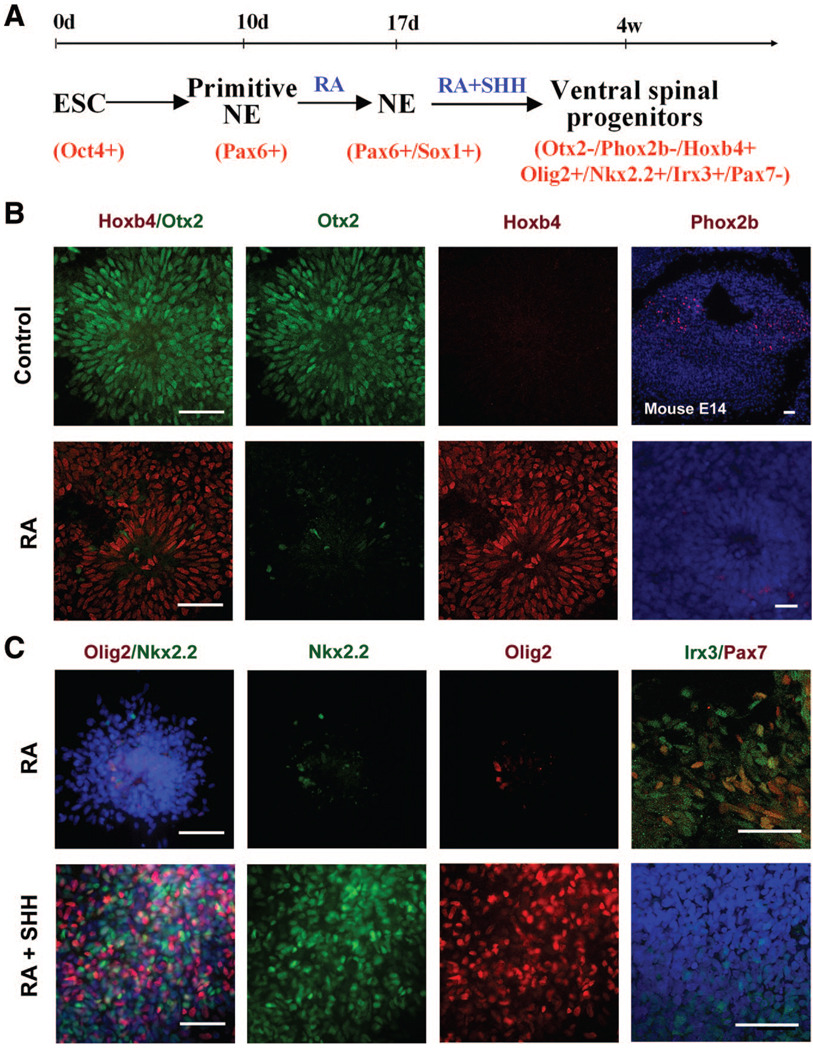
Human ESCs, following separation from feeder cells through aggregation, differentiate to neuroepithelia (NE) in an adherent colony culture [9]. Columnar epithelial cells appear at days 8–10 of hESC differentiation, and they express anterior transcription factors, such as Otx2 and Pax6, but not caudal markers, such as Hoxb4, which we refer to as primitive anterior NE [10]. For generating spinal progenitors, RA (0.1 µM) was added to the culture of primitive NE cells (day 10) (Fig. 1A). After 1 week of treatment (day 17), NE cells started to express Hoxb4 and organized into neural tube-like rosettes. These posteriorized neuroepithelial cell colonies were detached mechanically with a pipette. Unlike our previous adherent cultures, the neuroepithelial clusters were expanded in suspension in the same neural medium for an additional 10 days. Almost all the cells were positive for Hoxb4 and negative for Otx2 (Fig. 1B). This is in contrast to the control culture in which no morphogens (FGF2 or RA) were added (Fig. 1B). Hoxb4 is expressed by cells in both the hindbrain and spinal cord. Immunostaining for Phox2b, a marker positively staining for embryonic mouse hindbrain cells [27], indicated that very few cells expressed Phox2b (Fig. 1B). Thus, RA treatment under the suspension culture conditions essentially restricts hESCs to spinal progenitors.
Figure 1. Near complete specification of ventral spinal progenitors from human ESCs in suspension culture.
(A): Schematic procedure for ventral spinal progenitor differentiation.(B): Primitive NE (d10), after treatment with RA for 1 week, were isolated and cultured in suspension without (control; upper row) or with (lower row) RA for another week (total 24 d). RA induced the expressionof Hoxb4 but inhibited Otx2 expression.Very few cells expressed Phox2b in the RA-treated cultures. (C): Posteriorized neuralprogenitors (d17) were cultured in the absence(upper row) or presence (lower row)of SHH, and expression of transcriptional factors along the dorsal-ventral axis was examined at d28. In the absence of SHH, a small population of cells expressed Nkx2.2 and Olig2, whereas more cells were positive for Irx3, among which some also expressed Pax7. When SHH (100 ng/ml) was added (lower row), a large portion of cells expressedOlig2 or Nkx2.2, whereas few cells were positive for Irx3 and no cells werepositively stained for Pax7 (second row).Blue indicates Hoechst-stained nuclei. Scalebars = 50 µm. Abbreviations: d, days; E, embryonic day; NE, neuroepithelia; RA, retinoidacid; SHH, sonic hedgehog.
To ventralize the spinal progenitors, a more potent recombinant SHH (human SHH; 1845-SH; 100 ng/ml; with a mutation at Cys24; R&D Systems) was added to the culture at day 17, together with RA (0.1 µM) (Fig. 1A, 1C). Cells began to express ventral transcription factors Olig2 or Nkx2.2 after a week of treatment, and the ventral progenitor population reached a peak at 4 weeks of hESC differentiation. Approximately 40% of the cells expressed Olig2, whereas 34% ± 5% expressed Nkx2.2, and Olig2 and Nkx2.2 were not coexpressed in the same cells at this stage (Fig. 1C). Irx3 is expressed by the dorsal spinal cord and dorsal domains (p0–p2) of the ventral spinal cord [19]. Approximately 12% ± 4% of the cells expressed Irx3, but they were negative for Pax7 (Fig. 1C), a transcription factor expressed by the dorsal spinal cord [19, 20]. Thus, approximately 86% of the cells were ventral spinal progenitors (i.e., Nkx2.2+ [p3], Irx3+/Pax7− [p0–p2], and Olig2+ [pMN]) in the presence of SHH. Since some cells become postmitotic neurons, including motor neurons, at this stage (described below), almost all the differentiated progenies are restricted to the ventral spinal fate. In the absence of SHH (but with RA), only a few cells were positive for Olig2 and Nkx2.2 (4.9% ± 1.5% and 1.7% ± 0.9%, separately; Fig. 1C). Some cells were Irx3+ but Pax7− (p0–p2), and most cells were positive for Irx3 and/or Pax7 (Fig. 1C). Thus, the differentiated progenies without SHH are a mixture of ventral and dorsal spinal progenitors.
Ventral Spinal Progenitors Efficiently Differentiate to Motor Neurons in the Continual Presence of SHH
We have previously reported that motor neurons represent approximately 20% of the differentiated progenies in cultures with a reduced amount of SHH (corresponding to approximately 10 ng/ml for the current SHH; 1845-SH; R&D Systems) following the appearance of Olig2-expressing progenitors [15]. Our recent finding using genetically modified mouse ESCs indicates that the transition from Olig2-expressing progenitors to postmitotic motor neurons requires continual activation of SHH signaling [28]. We therefore cultured the Olig2-expressing progenitors in the suspension culture in the presence of 100 ng/ml of SHH in the present study. As we described above, Olig2+ cells began to appear at around 3.5 weeks, at which time there were almost no HB9+ cells. At 4 weeks of hESC differentiation, a small population of cells (~10%) was positive for HB9. At week 5, the population of HB9+ motoneurons increased to approximately 50%, whereas the Olig2+ cells decreased to 28% (Fig. 2A, 2C). The expression of Olig2 and HB9 did not overlap, as shown by confocal microscopy analysis (Fig. 2B). Thus, motor neurons (HB9+) and their progenitors (Olig2+) account for nearly 80% of the total cell population. Subsequently, as described in our previous paper [15], ChAT, an enzyme for synthesizing the transmitter acetylcholine, was expressed by HB9+ motoneurons, indicating the maturation of motoneurons. SHH, at a higher concentration (200 ng/ml) or added earlier (at primitive NE stage), generated a similar population of Olig2+ and HB9+ cells in the culture (data not shown). Thus, the ventral spinal progenitors can efficiently differentiate to postmitotic motor neurons in the continual presence of SHH and RA.
Figure 2. Highly efficient generation of motoneurons in the continual presence of sonic hedgehog.
(A): Olig2+ motoneuron progenitors peaked at approximately 4 weeks after differentiation, when HB9+ postmitotic motoneurons began to appear. Subsequently, the population of HB9+ motoneurons increased and peaked at 5 weeks. (B): A confocal image showing the separation of most Olig2- and HB9-positive cells at 5 weeks after differentiation. (C): Diagram showing the change of population of Olig2+ and HB9+ cells at 4–5 weeks after differentiation. Data are presented as mean ± SEM; n = 15–17. Blue indicates Hoechst-stained nuclei. Scale bars = 50 µm. Abbreviation: w, weeks.
Continued Presence of SHH Promotes the Division of Olig2 Progenitors
We have reported that approximately 20% HB9-expressing motor neurons were differentiated from hESCs in an adherent culture in which SHH was reduced during the MN progenitor differentiation period (after 4 weeks). In the present study, we routinely generated approximately 50% of the cells as motor neurons and nearly all the differentiated cells of ventral spinal fate in a suspension culture in which SHH was applied continuously until the production of HB9 cells. This comparison suggests that SHH may also affect the survival and/or proliferation of the Olig2 progenitors, in addition to their specification. Olig2-enriched clusters at 4 weeks were dissociated and adhered to coverslips in a neural medium (neural basal medium plus 2% B27) with or without SHH for 24 hours. In the absence of SHH, Olig2-expressing cells decreased to 20% of the total cells, whereas in the presence of SHH, the proportion of Olig2 cells (40%) was similar to that in suspension cultures (Fig. 3A, 3B). Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) analysis indicated that there was a similar population of positively labeled cells in cultures with or without SHH, and the Olig2+ cells were not labeled by TUNEL (Fig. 3A, 3B). Hence, survival of the Olig2+ progenitors does not appear to be affected within the 1st day. Immunostaining for Ki67, a protein expressed by proliferating cells, indicated that the proportion of Ki67-expressing Olig2 cells was significantly lower in the absence of SHH than in the presence of SHH (Fig. 3A, 3B). The total Ki67-expressing cell population did not exhibit a significant difference between the two groups (Fig. 3B). These findings suggest that SHH promotes the proliferation of specified Olig2+ progenitors, resulting in an increase in motor neuron progenitors and subsequently postmitotic motor neurons.
Figure 3. SHH promotes proliferation of Olig2+ progenitors.
(A): Olig2-enriched clusters were dissociated and plated on polyornithine/laminin-coated coverslips in the neural medium supplemented with B27 in the absence or presence of SHH (100 ng/ml) for 24 hours. More Olig2+ and Ki67+/ Olig2+ cells were seen with SHH than without SHH. TUNEL staining showed no difference between the SHH and non-SHH groups. Blue indicates Hoechst-stained nuclei. Scale bars = 50 µm. (B): Quantitative analyses indicated that there were more Olig2+, Ki67+/Olig2+ cells in the SHH-treated cultures than in the control cultures without SHH, whereas the numbers of Ki67+ and TUNEL+ cells in the total differentiated cells were similar between the SHH and non-SHH-treated groups. Data are presented as mean ± SEM; n = 7–8.*, analysis of variance test between SHH and non-SHH-treated groups, p < .05. Abbreviations: SHH, sonic hedgehog; TUNEL, terminal deoxynucleotidyl transferase dUTP nick-end labeling.
Purmorphamine Replaces SHH for Motor Neuron Generation
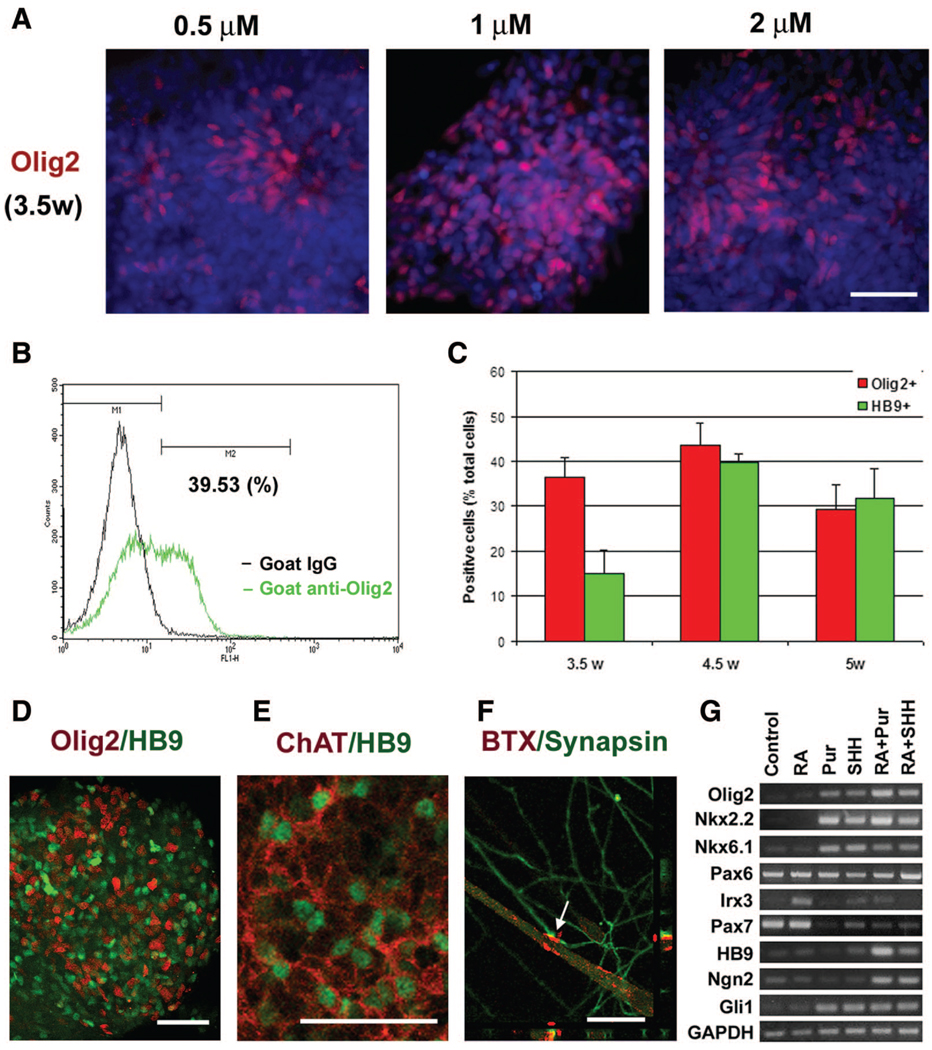
The activity of commercially available SHH has been improved through a mutation at the N terminus. However, the activity remains variable. Purmorphamine is a small molecule that activates sonic hedgehog signaling [29], possibly via Smoothened [30]. We therefore investigated whether purmorphamine can replace SHH in the generation of motoneurons. Caudalized neural progenitors were treated with different concentrations of purmorphamine instead of SHH from day 17. Purmorphamine alone was not sufficient to induce the expression of Olig2+ or HB9+ cells. Olig2 expression was induced robustly in the progenitors by all concentrations of purmorphamine (0.5, 1, and 2 µM), among which 1 µM generated the highest percentage of Olig2+ cells (approximately 40%) at 3.5 weeks in the presence of RA (Fig. 4A), as confirmed by FACS analysis (Fig. 4B). Olig2+ cells were first observed within 5 days of treatment after isolating NE cells (day 22 of hESC differentiation), which was a few days earlier than in SHH/RA-treated group. Remaining progenitor cells expressed other ventral markers, such as Nkx2.2, but not Pax7 (not shown), similar to cultures in the presence of SHH.
Figure 4. Efficient generation of spinal progenitors and motor neurons by purmorphamine.
(A): Caudalized neuroepithelia (NE) (day 17) were treated with RA and different concentrations of purmorphamine. At 3.5 weeks after human ESC differentiation, Olig2 was induced by purmorphamine in a dose-dependent manner. (B): Cell populations were quantified by fluorescence-activated cell sorting, as exemplified by Olig2-expressing cells in the purmorphamine (1 µM) group. (C): Diagram showing time-dependent change of population of Olig2+ and HB9+ cells after differentiation. Data are presented as mean ± SEM; n = 5–7. (D): At 4.5 weeks, the expression of Olig2 and HB9 increased to more than 40%. (E): After another week of differentiation in adherent cultures, most HB9+ motoneurons also expressed ChAT. (F): Synapsin-positive neurites colocalized with α-BTX-stained acetylcholine receptors on the surface of the myotube (arrow) after 2 weeks of coculture of motor neurons and C2C12 myoblasts, as shown on a 0.5-µm confocal section. (G): Reverse transcription-polymerase chain reaction analyses indicated expression of transcriptional factors by caudalized NE that were cultured with RA (0.1 µM), purmorphamine, SHH, or purmorphamine plus RA for 1 week (day 24). Blue indicates Hoechst-stained nuclei. Scale bars = 50 µm (A, D, E) and 30 µm (F). Abbreviations: ChAT, choline acetyltransferase; Pur, purmorphamine; RA, retinoid acid; SHH, sonic hedgehog; w, weeks.
Immunocytochemical analyses showed a pattern of HB9+ motor neuron differentiation similar to that in the cultures with SHH (Fig. 4C, 4D). By 4.5 weeks of hESC differentiation, Olig2+ motor neuron progenitors and HB9+ motor neurons accounted for at least 80% of the differentiated population (Fig. 4C). There were no significant differences between the SHH-treated and purmorphamine (Pur)-treated groups in the Olig2+ (Pur 4.5 weeks vs. SHH 4 weeks) and HB9+ (Pur 4.5 weeks vs. SHH 5 weeks) cells at the peak time points. Purmorphamine at 1 µM induced a higher percentage of Olig2+ and HB9+ cells than the lower concentration (0.5 µM). A higher dosage of purmorphamine (2 µM) did not further increase Olig2+ or HB9+ cells, and it also resulted in some dead cells in the culture in the long term (supplemental online Fig. 1). After attachment on the coverslips and differentiation for another week, most HB9+ motoneurons were also positive for ChAT (Fig. 4E). When coculturing with C2C12 myoblasts, the synapsin-positive nerve fibers induced acetylcholine receptor accumulation on the surface of the myotube, indicated by the clustered or patched BTX staining (Fig. 4F). These results confirm that mature motoneurons are generated in the culture, similar to the motor neuron differentiation cultures induced by SHH treatment [15]. Together, these data indicate that purmorphamine treatment results in the differentiation of ventral spinal progenitors and motor neurons at an efficiency similar to that of SHH.
Reverse transcription-PCR analysis following 1 week of purmorphamine treatment showed that purmorphamine induced an expression pattern of class II factors, such as Nkx6.1 and Nkx2.2, almost identical to that of SHH (Fig. 4G). Combination of RA and purmorphamine resulted in a high-level expression of class II genes (Nkx6.1) and a low-level expression of class I genes (Irx3 and Pax7), in addition to Olig2 and Ngn2 (Fig. 4G), which have been shown to be necessary for motor neuron specification [21–23]. In addition, purmorphamine increased levels of mRNA for Gli1, which is one of the targets in the SHH pathway. This result suggests that purmorphamine acts through a molecular pathway similar to that of SHH in inducing motor neuron specification.
DISCUSSION
On the basis of our prior success in directed neural differentiation of hESCs and identification of a signaling requirement for the in vitro motor neuron differentiation, we have now developed a chemically defined suspension culture for a near-complete generation of ventral spinal progenitors and highly efficient motor neuron generation. In this culture, ventral spinal progenitors and postmitotic (HB9+) motoneurons at 4 weeks after differentiation account for more than 96% of the total hESC-differentiated progenies. To our knowledge, this is the most efficient directed differentiation approach for producing defined classes of neurons in chemically defined systems without immunochemical selection procedures. Furthermore, we have discovered that purmorphamine can replace SHH in the entire process of ventral spinal progenitor specification and motor neuron differentiation with a similar efficiency. Thus, the complex process of motor neuron generation in the spinal cord can be mimicked by the two simple chemicals RA and purmorphamine. This paves a way for large-scale production of spinal neurons and motor neurons in industry, as well as in ordinary laboratories.
Differentiation of mouse and human ESCs using RA and SHH (or SHH agonists or SHH-producing cells) has yielded approximately 20% of the differentiated progenies being motoneurons [15, 24–26]. However, the composition of the other nearly 80% of the cells in the culture remains unknown. Our present study clearly demonstrates that nearly all the differentiated cells produced using our modified protocol are spinal cord neural cells, and they carry the ventral spinal cord characteristics but not those of the brain (fore-, mid-, and hindbrain) or dorsal spinal cord. It is thus remarkable that the pluripotent hESCs can be limited to cells with such a restricted regional identity at such a high efficiency.
In our previous protocol, we reduced the amount of SHH in the culture once the Olig2-expressing motoneuron progenitors were generated [15]. We have recently discovered that the differentiation of Olig2 progenitors to postmitotic motoneurons, as well as specification of Olig2 progenitors from the neuroectodermal cells, requires SHH [28]. It has been shown that SHH promotes the proliferation of spinal cord precursors at the early but not late stage [31], and the proliferative effect is possibly achieved through activating cyclin D1 [32]. Indeed, we have found that SHH also promotes the proliferation of the Olig2-expressing motoneuron progenitors. Thus, increased production of HB9-expressing motor neurons in the continued presence of SHH in the present study may be partly due to the proliferative effect of SHH on the motor neuron progenitors. On the other hand, SHH is also required for inducing Ngn2 expression in motor neuron progenitors, and Ngn2 antagonizes Olig2 during the differentiation of postmitotic MN [23]. Using the mouse ESC differentiation system, we confirmed that Ngn2 expression is induced by SHH and RA at an early (neurogenic) stage [28]. However, continued SHH sustained Olig2 expression and increased the Olig2 progenitor population, and these progenitors became oligodendrocytes but not motor neurons at a late (gliogenic) stage. Hence, the increase in motor neuron population in the continued presence of SHH is most likely due to the increased proliferation of the Olig2-expressing progenitors but not enhanced differentiation to HB9+ motor neurons. More motor neurons may be generated if SHH is reduced (or removed) after the maximal proliferation of Olig2 proliferation is achieved.
Following motor neuron generation at the 5th week, the HB9+ motor neuron population decreases even with the sustained presence of SHH in the culture. The decrease of motoneuron population may be due to the proliferation of other cell types. Furthermore, there is an intrinsic shift of the Olig2 progenitor cells from producing motor neurons to generating oligodendrocytes. By the 6th week in culture, many of the Olig2 cells also express Nkx2.2 (B.-Y. Hu et al., unpublished data). Coexpression of Olig2 and Nkx2.2 usually indicates the potential of the progenitor to become oligodendrocytes [33, 34].
The activity of recombinant SHH is variable, and the cost for a high dose of SHH in long-term cultures of human cells is high. Cell-permeable small molecules are a potential solution [35]. One of these small molecules is purmorphamine, which activates Gli1, the downstream target of the SHH pathway [27]. We show here that purmorphamine activates (suppresses) an almost identical set of transcription factors that are involved in the specification of ventral spinal progenitors and motor neurons, as well as stimulating Gli1. We have also found that the Olig2-expressing motoneuron progenitors appear several days earlier following purmorphamine treatment than after treatment with SHH. This may be due to the improved penetration and direct downstream effect by purmorphamine [29, 30, 36]. This may be especially helpful in our current approach using suspension culture following neuroepithelial differentiation. Continued adherent culture often results in a ring of flat, potentially nonneural cells in each colony [9, 15], which may reduce the proportion of motoneuron lineage. Suspension cultures limit the differentiation of these flat cells, together with the permeable nature of purmorphamine, may account for the high efficiency of motoneuron production. The use of purmorphamine not only achieves the high efficiency of differentiation and decreases the cost but also makes large-scale production feasible because of its stable chemical nature and easy preparation procedure.
The drastically simplified but much more efficient protocol for differentiation of ventral spinal progenitors and motor neurons enables virtually every laboratory to produce large amount of target cells for genetic and/or epigenetic analyses without the need for cell sorting [16], which is often traumatic to large projection neurons such as motoneurons. The strategy described herein is likely applicable to many other cell lineages, as we have recently found that oligodendrocytes and neuronal types in the forebrain (X.J. Li et al., unpublished data; B.-Y. Hu et al., unpublished data) can be similarly generated with high efficiencies.
Supplementary Material
suppl
ACKNOWLEDGMENTS
This study was supported by the Amyotrophic Lateral Sclerosis Association, the NIH (Grants R01-NS045926 and R21-NS055261), and partly by a core grant to the Waisman Center from the National Institute of Child Health and Human Development (P30 HD03352). We are grateful to Matthew Pankratz for the help in the FACS sorting and to Adedayo Fashoyin for technical help. We also thank T.M. Jessell and J.F. Brunet for generously providing antibodies against Irx3 and Phox2b. X.J.L. is currently affiliated with the Department of Neuroscience, MC-3401, University of Connecticut Health Center, Farmington, Connecticut.
Footnotes
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors indicate no potential conflicts of interest.
REFERENCES
- 1.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Reubinoff BE, Pera MF, Fong CY, et al. Embryonic stem cell lines from human blastocysts: Somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman DS, Hanson ET, Lewis RL, et al. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2001;98:10716–10721. doi: 10.1073/pnas.191362598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mummery C, Ward D, van den Brink CE, et al. Cardiomyocyte differentiation of mouse and human embryonic stem cells. J Anat. 2002;200:233–242. doi: 10.1046/j.1469-7580.2002.00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barberi T, Bradbury M, Dincer Z, et al. Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat Med. 2007;13:642–648. doi: 10.1038/nm1533. [DOI] [PubMed] [Google Scholar]
- 6.D’Amour KA, Bang AG, Eliazer S, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter MK, Inokuma MS, Denham J, et al. Enrichment of neurons and neural precursors from human embryonic stem cells. Exp Neurol. 2001;172:383–397. doi: 10.1006/exnr.2001.7832. [DOI] [PubMed] [Google Scholar]
- 8.Reubinoff BE, Itsykson P, Turetsky T, et al. Neural progenitors from human embryonic stem cells. Nat Biotechnol. 2001;19:1134–1140. doi: 10.1038/nbt1201-1134. [DOI] [PubMed] [Google Scholar]
- 9.Zhang SC, Wernig M, Duncan ID, et al. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 10.Pankratz MT, Li XJ, Lavaute TM, et al. Directed neural differentiation of human embryonic stem cells via an obligated primitive anterior stage. STEM CELLS. 2007;25:1511–1520. doi: 10.1634/stemcells.2006-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perrier AL, Tabar V, Barberi T, et al. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci U S A. 2004;101:12543–12548. doi: 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan Y, Yang D, Zarnowska ED, et al. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. STEM CELLS. 2005;23:781–790. doi: 10.1634/stemcells.2004-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roy NS, Cleren C, Singh SK, et al. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med. 2006;12:1259–1268. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- 14.Sonntag KC, Pruszak J, Yoshizaki T, et al. Enhanced yield of neuroepithelial precursors and midbrain-like dopaminergic neurons from human embryonic stem cells using the bone morphogenic protein antagonist noggin. STEM CELLS. 2007;25:411–418. doi: 10.1634/stemcells.2006-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li XJ, Du ZW, Zarnowska ED, et al. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol. 2005;23:215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- 16.Singh RN, Nakano T, Xuing L, et al. Enhancer-specified GFP-based FACS purification of human spinal motor neurons from embryonic stem cells. Exp Neurol. 2005;196:224–234. doi: 10.1016/j.expneurol.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 17.Lee H, Al Shamy G, Elkabetz Y, et al. Directed differentiation and transplantation of human embryonic stem cell-derived motoneurons. STEM CELLS. 2007;25:1931–1939. doi: 10.1634/stemcells.2007-0097. [DOI] [PubMed] [Google Scholar]
- 18.Brederlau A, Correia AS, Anisimov SV, et al. Transplantation of human embryonic stem cell-derived cells to a rat model of Parkinson’s disease: Effect of in vitro differentiation on graft survival and teratoma formation. STEM CELLS. 2006;24:1433–1440. doi: 10.1634/stemcells.2005-0393. [DOI] [PubMed] [Google Scholar]
- 19.Briscoe J, Pierani A, Jessell TM, et al. A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell. 2000;101:435–445. doi: 10.1016/s0092-8674(00)80853-3. [DOI] [PubMed] [Google Scholar]
- 20.Jessell TM. Neuronal specification in the spinal cord: Inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- 21.Lu QR, Sun T, Zhu Z, et al. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109:75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- 22.Mizuguchi R, Sugimori M, Takebayashi H, et al. Combinatorial roles of olig2 and neurogenin2 in the coordinated induction of pan-neuronal and subtype-specific properties of motoneurons. Neuron. 2001;31:757–771. doi: 10.1016/s0896-6273(01)00413-5. [DOI] [PubMed] [Google Scholar]
- 23.Lee SK, Lee B, Ruiz EC, et al. Olig2 and Ngn2 function in opposition to modulate gene expression in motor neuron progenitor cells. Genes Dev. 2005;19:282–294. doi: 10.1101/gad.1257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wichterle H, Lieberam I, Porter JA, et al. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 25.Soundararajan P, Lindsey BW, Leopold C, et al. Easy and rapid differentiation of embryonic stem cells into functional motoneurons using sonic hedgehog-producing cells. STEM CELLS. 2007;25:1697–1706. doi: 10.1634/stemcells.2006-0654. [DOI] [PubMed] [Google Scholar]
- 26.Shin S, Xue H, Mattson MP, et al. Stage-dependent Olig2 expression in motor neurons and oligodendrocytes differentiated from embryonic stem cells. Stem Cells Dev. 2007;16:131–141. doi: 10.1089/scd.2006.0023. [DOI] [PubMed] [Google Scholar]
- 27.Pattyn A, Morin X, Cremer H, et al. Expression and interactions of the two closely related homeobox genes Phox2a and Phox2b during neurogenesis. Development. 1997;124:4065–4075. doi: 10.1242/dev.124.20.4065. [DOI] [PubMed] [Google Scholar]
- 28.Du ZW, Li XJ, Nguyen GD, et al. Induced expression of Olig2 is sufficient for oligodendrocyte specification but not for motoneuron specification and astrocyte repression. Mol Cell Neurosci. 2006;33:371–380. doi: 10.1016/j.mcn.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Wu X, Walker J, Zhang J, et al. Purmorphamine induces osteogenesis by activation of the hedgehog signaling pathway. Chem Biol. 2004;11:1229–1238. doi: 10.1016/j.chembiol.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 30.Sinha S, Chen JK. Purmorphamine activates the Hedgehog pathway by targeting Smoothened. Nat Chem Biol. 2006;2:29–30. doi: 10.1038/nchembio753. [DOI] [PubMed] [Google Scholar]
- 31.Rowitch DH, S-Jacques B, Lee SM, et al. Sonic hedgehog regulates proliferation and inhibits differentiation of CNS precursor cells. J Neurosci. 1999;19:8954–8965. doi: 10.1523/JNEUROSCI.19-20-08954.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lobjois V, Benazeraf B, Bertrand N, et al. specific regulation of cyclins D1 and D2 by FGF and Shh signaling coordinates cell cycle progression, patterning, and differentiation during early steps of spinal cord development. Dev Biol. 2004;273:195–209. doi: 10.1016/j.ydbio.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 33.Fu H, Qi Y, Tan M, et al. Dual origin of spinal oligodendrocyte progenitors and evidence for the cooperative role of Olig2 and Nkx2.2 in the control of oligodendrocyte differentiation. Development. 2002;129:681–693. doi: 10.1242/dev.129.3.681. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Q, Choi G, Anderson DJ. The bHLH transcription factor Olig2 promotes oligodendrocyte differentiation in collaboration with Nkx2.2. Neuron. 2001;31:791–807. doi: 10.1016/s0896-6273(01)00414-7. [DOI] [PubMed] [Google Scholar]
- 35.Ding S, Schultz PG. A role for chemistry in stem cell biology. Nat Biotechnol. 2004;22:833–840. doi: 10.1038/nbt987. [DOI] [PubMed] [Google Scholar]
- 36.Riobo NA, Saucy B, Dilizio C, et al. Activation of heterotrimeric G proteins by Smoothened. Proc Natl Acad Sci U S A. 2006;103:12607–12612. doi: 10.1073/pnas.0600880103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
suppl